Abstract
The mammary gland is a unique organ that undergoes extensive and profound changes during puberty, menstruation, pregnancy, lactation and involution. The changes that take place during puberty involve large-scale proliferation and invasion of the fat-pad. During pregnancy and lactation, the mammary cells are exposed to signaling pathways that inhibit apoptosis, induce proliferation and invoke terminal differentiation. Finally, during involution the mammary gland is exposed to milk stasis, programed cell death and stromal reorganization to clear the differentiated milk-producing cells. Not surprisingly, the signaling pathways responsible for bringing about these changes in breast cells are often subverted during the process of tumorigenesis. The STAT family of proteins is involved in every stage of mammary gland development, and is also frequently implicated in breast tumorigenesis. While the roles of STAT3 and STAT5 during mammary gland development and tumorigenesis are well studied, others members, e.g. STAT1 and STAT6, have only recently been observed to play a role in mammary gland biology. Continued investigation into the STAT protein network in the mammary gland will likely yield new biomarkers and risk factors for breast cancer, and may also lead to novel prophylactic or therapeutic strategies against breast cancer.
Keywords: STATs, mammary gland development, breast cancer, pregnancy, lactation, involution
Introduction
Cells exist in a complex, fluid environment. They adapt to their changing environment via membrane receptors that respond to extracellular stimuli in the form of growth factors and cytokines. Ligand-bound receptors then recruit and activate mediatory molecules. Mediatory molecules are generally kinases and when activated are able to phosphorylate, and thereby, activate specific downstream factors that are latent in the cytoplasm. These activated effector molecules can then enter the nucleus and institute a specific transcriptional program that allows the cell to respond to its new surroundings. The Signal Transducers and Activators of Transcription (STAT) family of proteins is one such set of latent cytoplasmic factors that enable the cell to have a fluid, adaptive, highly specific mode of responding to a constantly changing ethos.
All STAT family members have similar protein structures including an N terminal domain, a coiled-coil domain, a DNA-binding domain, SH3 and SH2 domains, and a trans-activating domain at the C terminal end of the protein (Darnell, 1997). The SH2 and SH3 domains harbor phosphorylation sites at their tyrosine residues. Phosphorylation at these tyrosine residues is a critical posttranslational modifier allowing activation of STATs by various tyrosine kinases although phosphorylation at Ser/Thr residues can also modulate activity of some STATs (Bromberg, 2000). STAT proteins frequently exist as dimers or sometimes, tetramers. The dimerization of STATs appears to be required for their translocation to the nucleus and for their binding to specific DNA sequences (Horvath et al., 1995). The means by which dimerization is achieved is likely through the binding of the tyrosine residue of one molecule with the SH2 domain of another (Heim et al., 1995), suggesting the possibility of heterodimerization between various conserved STAT family members (Li et al., 1996).
STATs were thought to dimerize only upon phosphorylation; however, unphosphorylated STATs were later found to also exist as stable dimers in the cytoplasm (Braunstein et al., 2003). Further, non-phosphorylated STAT dimers can shuttle between the nucleus and the cytoplasm, and bind non-specifically to DNA (Meyer et al., 2003). Phosphorylation appears important mainly for the retention of a STAT protein in the nucleus long enough for it to initiate a transcriptional program (Meyer et al., 2003; Pranada et al., 2004). Phosphorylated STATs in the nucleus are dephosphorylated by various phosphatases and then degraded by the ubiquitin-proteasome pathway (Kim and Maniatis, 1996). By binding to specific DNA sequences, usually marked by the GAS element (Darnell, 1997), phosphorylated STAT molecules are protected from these phosphatases and therefore, remain in the nucleus (Meyer et al., 2003).
So far seven STAT proteins have been identified in mammalian cells. They were numbered based on the order of discovery—STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6. STAT1 and STAT2 proteins were discovered as acute phase proteins that responded to extracellular stimuli in the form of cytokines, interferon (IFN)α and IFNγ (Darnell et al., 1994). Later STAT1 was found to be also activated by growth factors like fibroblast growth factor (FGF) (Sahni et al., 1999). STAT4 and STAT6 are activated chiefly by cytokines like interleukin (IL)-4 and IL-12, while STAT3 and STAT5 are activated by a wide spectrum of external stimuli including cytokines, growth factors and hormones like epidermal growth factor (EGF) and prolactin (PRL), respectively (Darnell, 1996). Overall, STAT2, STAT4 and STAT6 appear to be stimulated by a small group of cytokines; while STAT1, STAT3 and STAT5 are activated by a variety of extracellular stimuli including growth factors, hormones and cytokines.
The presence of a fleet of STAT proteins in a cell suggests that each family member bestows a certain amount of specificity on cellular response to external stimuli. Different stimuli activate a variety of different receptors, either growth factor receptors or cytokine receptors. Cytokine receptors frequently mediate their effects by associating with the Janus kinase (Jak) family of kinases (Jak1, Jak2, Jak3, Tyk2), which become catalytically active upon binding to the receptors (Bromberg, 2000). A specific STAT is then recruited to the particular receptor-Jak complex formed, which subsequently phosphorylates and activates the STAT (Darnell et al., 1994). The activated homo/heterodimerized STAT then translocates to the nucleus where it activates a wide spectrum of downstream targets depending on the cellular context. While the GAS element has been described as a common consensus sequence for all known STATs to regulate transcription of their target genes, the exquisite specificity of STAT downstream pathway activation implies that there are other factors at play. For example, co-binding of estrogen receptor (ER) or progesterone receptor (PR) might alter the regions of the genome to which STATs bind as well as define the genes that can be transcribed on STAT binding (Faulds et al., 2001; Wang and Cheng, 2004). Additionally, the configuration of the phosphorylated SH2 domain and/or CTD of a given STAT may regulate the specificity with which it binds to DNA motifs (Chen et al., 1998; Hakim et al., 2012).
The remarkable specificity of the STAT signaling cascade which allows a cell to respond to subtle extracellular changes is further enhanced by the cell type specificity of STATs. STAT1 and STAT2 are largely localized to immune cells where they mediate immune response via interferons (Durbin et al., 1996; Meraz et al., 1996; Park et al., 2000). Of interest to mammary biologists, STAT1 is also constitutively expressed in the mature virgin mammary gland before pregnancy and after involution, while STAT2 expression has not yet been observed in the mammary gland (Philp et al., 1996). STAT4 is also associated mainly with the immune response to infections (Thierfelder et al., 1996). Interestingly, STAT4 mRNA has been identified in the virgin mammary gland as well as on pregnancy day 7, potentially in non-epithelial cells (Philp et al., 1996). However, STAT4 protein levels are very low and transient, and STAT4 ablation does not appear to affect mammary gland development, indicating that STAT4 likely does not play a critical role in mammary gland development. STAT6 is required for T helper (Th) cell regulation during immune response (Shimoda et al., 1996; Takeda et al., 1996) and has also been identified as a regulator of mammary gland differentiation (Khaled et al., 2007).
STAT3 and STAT5 are the family members that are most promiscuous in expression. STAT3 is essential for early embryogenesis and the regulation of embryonic stem cells, myeloid cells, neuroepithelial cells, mammary epithelial cells, T-cells, macrophages, neutrophils and wound healing in epidermal cells (Akira, 1999). STAT5a is functionally important for the mammary gland, macrophages and Th cells, while STAT5b is essential for normal sexually dimorphic functioning of the liver in response to growth hormone (GH) and for proliferation of natural killer cells (Akira, 1999). STAT5a and STAT5b are functionally redundant in the corpus luteum but play an essential role in regulating and ensuring female fertility (Akira, 1999). It is interesting to note that the cellular context has an impact on the functional and biological consequences of a STAT protein. For instance, STAT3 activation during mammary involution induces apoptosis, while it is a potent prosurvival factor in T cells (Chapman et al., 1999; Takeda et al., 1998).
Since STAT family members play important roles in regulating cell proliferation and apoptosis, it is not surprising that these proteins modulate mammary gland development during pregnancy, lactation and involution. These proteins and the signaling pathways they participate in are also frequently subverted during tumorigenesis. A perspective on the recent advances in the understanding of these STATs in mammary gland differentiation, cell survival and tumorigenesis is presented below.
STATs in mammary gland differentiation and cell survival
The mammary gland is a unique organ in that the majority of its development occurs postnatally. At birth, the mammary gland consists of a rudimentary ductal tree. Prepubertal growth involves elongation of this tree induced principally by EGF and the parathyroid hormone-related peptide, and their receptors. At the onset of puberty, mammary ductal development becomes hormone-dependent, primarily controlled by systemic estrogen and progesterone binding to their cognate receptors, ER and PR. These hormones activate a program of active proliferation, side branching and invasion of the ductal tree into the surrounding fat pad, resulting in the adult virgin mammary gland. During this pubertal epithelial expansion, there appears to be little or no involvement of the STAT family of proteins.
Several STATs are expressed in the mammary glands in adult virgins, but their functional contributions to mammary gland development at this stage are as yet unclear (Philp et al., 1996; Watson, 2001). STAT1 is phosphorylated in virgin glands and again after involution day 3, but not during pregnancy and lactation (Philp et al., 1996). A study in dairy cattle also suggests a role for STAT1 in dictating normal adult mammary gland development (Cobanoglu et al., 2006). However, a detailed examination of the effect of STAT1 on mammary gland development in the virgin mouse has not yet been conducted. Another STAT family member that is activated during postpubertal mammary gland development is STAT5. STAT5a affects the establishment of a luminal progenitor cell population in the adult virgin mammary gland (Vafaizadeh et al., 2010; Yamaji et al., 2009). STAT5 also appears to regulate secondary and side branching during estrus potentially mediating the effects of estrogen, progesterone, EGF and/or GH (Bromberg, 2000; Liu et al., 1996; Santos et al., 2010; Teglund et al., 1998).
It is only during pregnancy that the mammary gland develops fully as the mammary epithelium differentiates into alveolar-lobular units, which produce milk during lactation. Mammary gland development during pregnancy can be divided into four stages: early pregnancy, mid-late pregnancy, lactation and involution (Figure 1). The first three stages are characterized by expansion of the mammary epithelial compartment to invade the fat pad and the production of milk droplets, while the final stage is marked by the clearance of excess mammary epithelium and its return to a state grossly similar, but not identical, to the adult virgin mammary gland. To distinguish between the primary roles played by these stages in mammary gland development, we have categorized them as mammary gland differentiation, which includes pregnancy and lactation, and mammary cell survival, which focuses on involution. The STATs appear to play important, largely non-redundant, and interactive roles in every stage of this reproductive cycle.
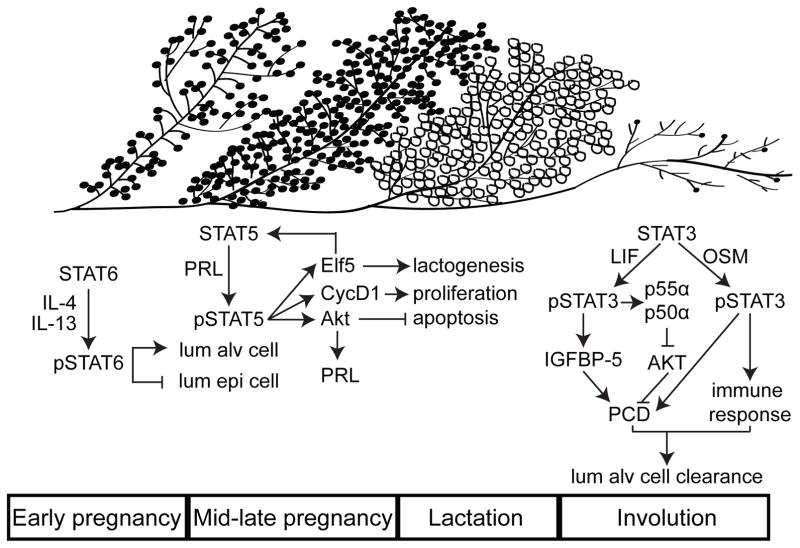
Figure 1. The STAT family of proteins regulates every stage of mammary gland development during a reproductive cycle.
During early pregnancy, STAT6, activated by cytokines IL-4 and IL-13, initiates alveolar lineage commitment. These alveolar cells activate STAT5 primarily via PRL-PRLR. pSTAT5 institutes a transcriptional program that results in lactation. At the end of lactation, the loss of the suckling triggers a STAT3-dependent apoptotic program to remove the differentiated alveolar cells. STAT3 is activated by LIF during the initial stages of involution, and by OncostatinM in the later stages. While the expression patterns of the various STATs often overlaps, in this diagram each STAT has been placed in the reproductive cycle according to its maximal impact on mammary gland development. Key activating and downstream proteins are included.
Mammary gland differentiation
During early pregnancy, STAT6 is important for the regulation of mammary cell differentiation. Activation of STAT6 occurs around day 5 of pregnancy and remains upregulated until the onset of lactation (Clarkson et al., 2004). This activation is in response to the upregulation of Th2 cytokines, IL-4 and IL-13, at early pregnancy and the resultant activation of STAT6, which is essential for inducing proliferation and luminal cell commitment to an alveolar lineage (Khaled et al., 2007). STAT6 stimulates alveolar differentiation and proliferation potentially by transcriptionally inducing GATA3 (Khaled et al., 2007). STAT6 and GATA3 together repress Zfp157 at early pregnancy, thereby inducing commitment of luminal cells to an alveolar lineage (Oliver et al., 2012). Genetic ablation of Zfp157 increases the population of STAT5+ alveolar cells in the early pregnancy mammary gland (Oliver et al., 2012). The genetic ablation of both Zfp157 and GATA3 completely rescues alveogenesis indicating the role played by GATA3 and Zfp157 in regulating the activity of each other during pregnancy (Oliver et al., 2012).
During early pregnancy, STAT5 has also been shown to be essential for the generation and proliferation of alveolar progenitor cells from the mammary stem cell compartment (Gallego et al., 2001; Yamaji et al., 2009). Ablation of STAT5 prevents the development of immature alveoli at pregnancy day 6 (Yamaji et al., 2012). Of interest, STAT3 and STAT5 appear to have reciprocal interactions during early to mid-pregnancy. STAT3 mRNA levels are high in the mammary epithelium of adult virgins and remain elevated till pregnancy day 5, when they begin to drop and, concomitantly, STAT5 levels begin to rise (Philp et al., 1996). STAT5 activation, however, does not occur maximally until mid to late pregnancy (Bednorz et al., 2011). Interestingly, Shp2, a phosphatase known to inhibit STAT activation, appears to stabilize STAT5 activation in the pregnant/lactating mammary gland while simultaneously preventing the activation of STAT3 (Ke et al., 2006). It remains to be determined whether Shp2 is the principal protein that balances the relative activities of STAT3 and STAT5 at this stage of mammary gland development.
During late pregnancy and lactation, STAT5a is both necessary and sufficient for alveogenesis and lactogenesis (Cui et al., 2004; Dong et al., 2010; Liu et al., 1997). Moreover, activation of STAT5a is sufficient for side-branching (Dong et al., 2010; Vafaizadeh et al., 2010), and can drive alveolar differentiation even in the absence of ovarian hormones (Dong et al., 2010). Prolactin-mediated stimulation of prolactin receptor (PRLR) appears to be the canonical and primary means of activating STAT5 at this stage (Liu et al., 1997). PRLR is believed to activate STAT5 through Jak2, which is supported by the observation that genetic ablation of PRLR, Jak2 and STAT5 results in similar mammary phenotypes during pregnancy and lactation (Gallego et al., 2001; Shillingford et al., 2002; Wagner et al., 2004).
PRLR and Jak2-initited STAT5 signaling in the breast is modulated by several additional factors. ErbB4 is essential for STAT5 activation by PRLR and Jak2 during late pregnancy and lactation (Long et al., 2003). The prosurvival protein AKT is an inducer of autocrine prolactin secretion in the mammary epithelium, and it is essential for the activation of STAT5 and the development of a lactating mammary gland (Chen et al., 2012). STAT5 was also found to transcriptionally activate AKT (Creamer et al., 2010). Together, these studies indicate a positive feedback mechanism for maintaining STAT5 activation in the mammary gland during pregnancy and lactation. Further, PIKE-A, which is part of the AKT signaling pathway also appears essential for STAT5 activation and lactogenesis in the mammary gland (Chan et al., 2010). SnoN, a negative regulator of the TGF-β signaling pathway, is another inducer of prolactin-STAT5 signaling (Jahchan et al., 2012).
While the PRLR-Jak2 pathway appears essential for activation of STAT5 in the epithelium, in the stromal compartment of the mammary gland, growth hormone receptor (GHR) and epidermal growth factor receptor (EGFR) seem to activate STAT5 (Gallego et al., 2001). The functional consequence of STAT5 activation in stromal cells is yet unclear. GH has been reported to regulate alveogenesis and ductal growth (Gallego et al., 2001), but whether GH exerts its effect through stromal cells is unknown.
During pregnancy and lactation, STAT5 signaling is also controlled by several negative regulators. Genetic experiments have identified several inhibitors of STAT5 in the mammary gland including Caveolin-1, SOCS1, and PTP1B (Lindeman et al., 2001; Milani et al., 2012; Sotgia et al., 2006). Interestingly, Myc, when activated during a specific 72 hour window during mid-pregnancy, downregulates Caveolin-1, prematurely activates STAT5, and causes precocious lactation and involution (Blakely et al., 2005). These data further emphasize the finesse with which the activation and inactivation of various STATs during the course of pregnancy and lactation orchestrate the growth and differentiation of the mammary gland (Figure 1).
STAT5 executes its differentiation functions through its transcriptional activity. A number of transcriptional targets of STAT5 have been identified, and their protein products chiefly include those responsible for alveogenesis and lactogenesis like Elf5, SOCS2, β-casein and whey acidic protein (WAP). A set of 400 genes appear to be at least partially under the transcriptional control of STAT5 at parturition, and mainly encode milk proteins and proteins regulating cellular metabolism and secretion (Yamaji et al., 2012). It is likely that increasing levels of pSTAT5 in the mammary gland during the progression of pregnancy can trigger distinct transcriptional programs. Elf5, for instance, is transcribed even when only one allele of either STAT5a or STAT5b is present in a mammary epithelial cell, while WAP is not (Yamaji et al., 2012). Interestingly, Elf5 also transcriptionally activates STAT5 (Choi et al., 2009; Harris et al., 2006), suggesting a positive feedback regulatory loop. Elf5 has been shown to be one of the primary effectors of the alveogenic and lactogenic program instituted by pSTAT5 (Choi et al., 2009).
STATs in mammary cell survival
During pregnancy and lactation, the mammary gland sustains the growth and establishment of a large number of alveolar cells in order to produce sufficient quantities of milk. At these stages, one of the functions of STAT5 is to transactivate prosurvival and proliferative genes like AKT and CCND1 (Creamer et al., 2010; Sakamoto et al., 2007). There are probably other downstream targets of STAT5 that are instrumental in maintaining cell survival and division during these two stages of mammary gland development. For example, in some other tissue types and during mammary tumorigenesis, additional STAT5 targets have been identified including the Bcl family of prosurvival genes (Bcl2, BclXL, and MCL-1), Id1, and Survivin (Socolovsky et al., 1999; Xu et al., 2003; Yoshimoto et al., 2009; Zhou et al., 2009). Further investigation is warranted to elucidate the role of these other candidates in mediating STAT5 functions and to establish the relative contributions of each of these targets.
After the young are weaned, it is no longer necessary for the mammary gland to sustain this large population of terminally differentiated lactating mammary cells. Therefore, at weaning, STAT5 is rapidly inactivated, and an apoptotic program mediated by STAT3 is initiated to clear the mammary gland of its excess cellular burden. The inactivation of STAT5 at involution is attributed to various mechanisms including milk stasis, decrease in circulating levels of PRL, degradation of PRLR by GSK3β, increase in TGFβ3 levels, upregulation of IL-6 and the related activation of oncostatin M receptor (OSMR), and downregulation of glucocorticoids (Bertucci et al., 2010; Li et al., 1997; Li et al., 2004; Quarrie et al., 1996; Tiffen et al., 2008). The relative contribution of each of these pathways and potential interactions between them is as yet unsubstantiated.
Involution is characterized by two phases – an initial 24 hour phase involving cell death and a second phase of phagocytosis and immune cell mobilization to clear away cellular debris (Clarkson et al., 2004). During the first phase of involution, pSTAT3 levels in the mammary gland are elevated, and this rapid activation of STAT3 is essential for involution to proceed (Chapman et al., 1999; Humphreys et al., 2002). STAT3 induces apoptosis by either directly or indirectly influencing IGF-binding protein-5 levels in mammary cells (Chapman et al., 1999). During the second phase of involution, STAT3 is also responsible for inducing an immune response and for polarizing macrophages and mast cells into an alternate state required for epithelial cell clearing (Hughes et al., 2012; Kreuzaler et al., 2011). There appears to be a population of alveolar cells designated as PI-MECs in the mammary gland that avoid post-lactational cell death (Wagner et al., 2002); these cells have regenerative potential and can prepare the mammary gland for subsequent pregnancies (Boulanger et al., 2005; Matulka et al., 2007). It is unclear how much of a role STAT3 plays in maintaining and/or regulating this population.
Concomitant with its primary function of inducing apoptosis and immune response, the main transcriptional targets of STAT3 in these cells are genes encoding CBPδ, purine nucleoside phosphorylase, c-fos and two PI3K regulatory subunits (p55α and p50α) (Clarkson et al., 2006). In fact, during involution, p55α and p50α-mediated inhibition of PI3K-AKT signaling is essential for STAT3-initiated apoptosis (Abell et al., 2005). This STAT3-induced AKT deactivation might potentially constitute another mechanism by which STAT3 inhibits STAT5 at the onset of involution, since AKT can activate STAT5 (Chen et al., 2012).
There are several posited mechanisms by which STAT3 is activated at involution. During the first phase of involution, milk stasis and glucocorticoids seem to play primary roles in activating STAT3. Glucocorticoids have been shown to activate STAT3 (Bertucci et al., 2010), via an unknown mechanism. Milk stasis due to the lack of suckling rapidly induces the production of leukemia inhibitory factor (LIF) (Kritikou et al., 2003; Schere-Levy et al., 2003), which activates the gp130/Jak complex, leading to the phosphorylation and activation of STAT3 (Zhao et al., 2004). During the second phase of involution, as LIF levels decline, Oncostatin M (OSM), a member of the IL-6 cytokine family that has the closest homology to LIF, binds to its cognate receptor OSMR and seems to become the principal activator of STAT3 (Tiffen et al., 2008). Of interest, STAT3 transactivates OSMR in a positive feedback loop, thereby sustaining its own activation until involution is completed (Tiffen et al., 2008). OSM signaling is distinct from LIF-induced STAT3 activation in that it also instigates the dephosphorylation of STAT5 even in the presence of PRL (Tiffen et al., 2008). This OSM-OSMR complex, therefore, provides yet another signaling axis which both activates STAT3 and inhibits STAT5 at the onset of involution.
STATs in tumorigenesis
The STAT family of proteins is present at almost every stage of postnatal mammary gland development. Some STATs plays important roles in promoting cell growth and survival as well as inducing inflammation, while others suppress cell proliferation. It is therefore, not surprising that most STATs are also implicated in mammary tumorigenesis either as tumor-promoting or tumor-suppressive factors. Thus far, there is a substantial body of evidence indicating the involvement of STAT1, STAT3, STAT5 and STAT6 in breast cancer formation, progression, prognosis and prediction. STAT2 and STAT4 have not yet been associated with breast cancer.
STAT1
A preponderance of in vivo data suggests that STAT1 acts as a tumor suppressor in preventing breast tumor initiation (Figure 2). STAT1−/− mice are prone to the spontaneous formation of ER+ breast tumors that display a molecular signature similar to that of human luminal breast cancer (Chan et al., 2012). The addition of wild type STAT1, but not of STAT1 mutants with compromised transcriptional activity, to primary cultures made from the breast tumors generated in STAT1−/− mice results in tumor cell apoptosis, indicating that the tumor suppressive effects of STAT1 are cell autonomous and dependent on STAT1-mediated transcription (Chan et al., 2012). Moreover, compared to wild type mice, STAT1−/− mice are at increased susceptibility to tumorigenesis initiated by ErbB2 (Klover et al., 2010; Raven et al., 2011) and chemical carcinogenesis both in the presence and absence of p53 (Kaplan et al., 1998).
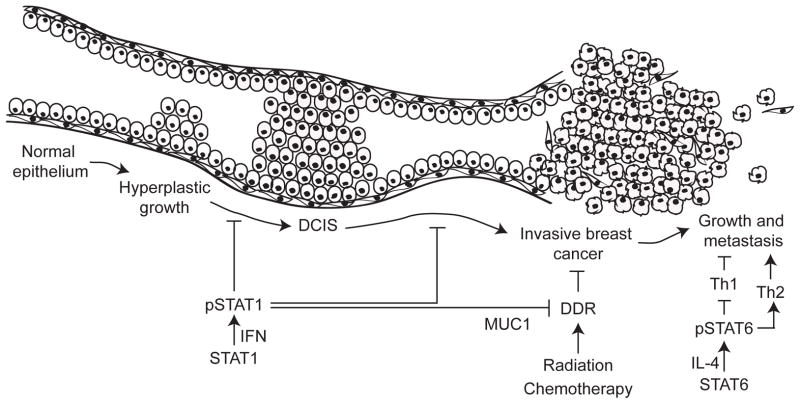
Figure 2. STAT1 and STAT6 may be important regulators of breast tumorigenesis.
The various stages during breast tumor development and progression are depicted along a single duct, going from normal epithelium, through hyperplasia, DCIS, tumor and finally tumor growth and metastasis. STAT1, which is probably activated by interferons, is a tumor suppressor. However, STAT1 also promotes tumor resistance to therapy by suppressing the DNA damage response (DDR) pathway. STAT6, which is activated by IL-4, induces the differentiation of T-helper type 2 cells, which promotes tumor establishment and metastasis.
Studies of human breast cancer provide additional support for the role of STAT1 as a tumor suppressor and, importantly, as a good prognostic factor. According to one study, 45% of human ERα+ (n=83) and 22% of ERα− (n=78) breast cancers display low levels of STAT1 in neoplastic cells while exhibiting high levels of STAT1 in the tumor adjacent histopathologically benign breast tissue (Chan et al., 2012). These data could be interpreted to mean that STAT1 has to be inactivated in breast tumor evolution. Another large-scale analysis of gene expression data from public databases correlates STAT1 expression with better prognosis in ER−/HER2− and in HER2+ breast cancers (Desmedt et al., 2008). A smaller retrospective study (n=73) of STAT1 DNA binding activity and phosphorylation status in invasive breast carcinomas also found a strong correlation between STAT1 activation and longer overall and relapse-free survival (Widschwendter et al., 2002). The majority of the patients in this study were postmenopausal (46 of the 73) (Widschwendter et al., 2002).
Paradoxically, when expressed at very high levels, STAT1 may also promote metastasis and drug-resistance. One study of breast tumors from 295 patients found an association of STAT1 with a DNA damage resistance signature, which correlated with a prosurvival phenotype and drug resistance (Weichselbaum et al., 2008). Another study from the same group used in vitro experiments in breast cancer cell lines that expressed STAT1 to reveal a constitutive interaction between STAT1 and MUC1 which contributed to the activation of STAT1 target genes (Khodarev et al., 2010). Moreover, the same study analyzed coexpression of STAT1 and MUC1 in two independent databases of primary breast tumors (Khodarev et al., 2010). They identified that 15% and 16% respectively in each database of primary breast tumors (n=327 and n=155) coexpressed STAT1 and MUC1, and the coexpression correlated with decreased recurrence-free and overall survival (Khodarev et al., 2010). However, this study did not address the type of breast cancer and the menopausal status of the patients.
Evidence suggests that menopausal status affects STAT1 functions as a tumor suppressor. Among breast cancers in premenopausal women, STAT1 was associated with worse overall and shorter disease-free survival (Khodarev et al., 2012; Magkou et al., 2012). However, among ER+ breast cancers in postmenopausal women from the same dataset, high levels of pSTAT1 was associated with longer disease-free survival (Magkou et al., 2012). Therefore, it appears that STAT1 might be a tumor suppressor in ER+ breast tumors diagnosed postmenopausally and promote the progression of tumors that are either ER− or diagnosed premenopausally. Investigating the effect of estrogen and progesterone on STAT1 activity might shed some light on this context-dependent ability of STAT1 to act as both a tumor promoter and a tumor suppressor.
STAT3
Even though in normal mammary gland development, STAT3 is most widely studied for its role to induce apoptosis and cell clearance during involution, aberrant activation of STAT3 can also promote breast cancer formation and progression (Figure 3). In tumor cells, pSTAT3 transactivates proliferative genes like cMyc and cyclinD1, prosurvival genes like Bcl-xL and Survivin, and angiogenesis and invasion genes like VEG-f and Klf-8 (Bromberg et al., 1999). The transcriptional program instituted by pSTAT3 in tumors results in the formation of rapidly growing tumors that are highly metastatic. For instance, MMTV-Neu transgenic mice with a constitutively active STAT3 allele develop significantly more aggressive and metastatic tumors than those without (Barbieri et al., 2010).
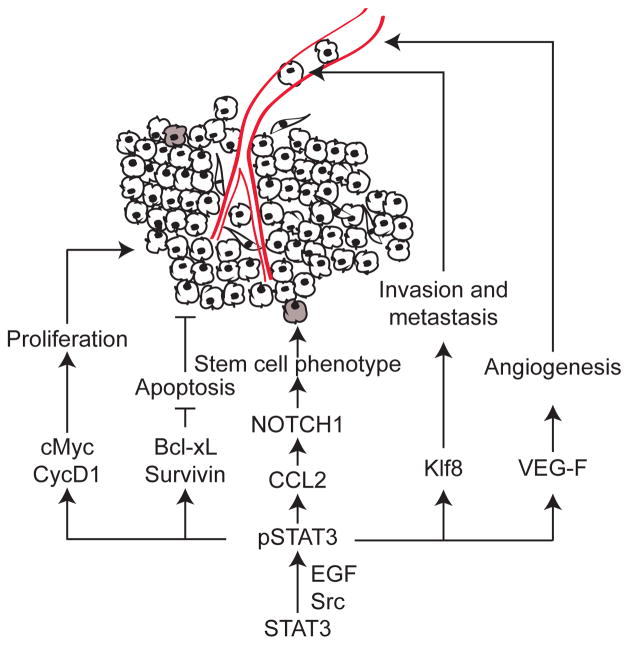
Figure 3. STAT3 stimulates breast cancer cell survival, proliferation, invasiveness and metastasis.
STAT3 is a known oncogene with several diverse tumorigenic functions. The downstream effectors of STAT3 in promoting tumor growth, stemness, metastasis and angiogenesis are depicted. Grey cells represent stem cell-like cells in the tumor. The blood vessel is drawn in red.
In addition to its function as a promoter of tumor invasiveness and metastasis, STAT3 also regulates the inflammatory response in breast tumorigenesis. An example of the effect of STAT3 on inflammation is its transactivation of microRNAs, which initiate an inflammatory signal that epigenetically transforms normal cells into cancer cells (Iliopoulos et al., 2010). Furthermore, stromal-epithelial crosstalk can induce cancer stem cell-like phenotypes via STAT3 signaling. For example, growth factors and cytokines secreted by tumor cells activate STAT3 in tumor-associated fibroblasts (Tsuyada et al., 2012). Activated STAT3 in these fibroblasts then transactivates the gene encoding CCL2, which is then secreted and activates NOTCH1 signaling in the tumor cells, thereby triggering a stem cell-like phenotype (Tsuyada et al., 2012). In addition, tumor-associated macrophages can induce a stem cell-like phenotype in breast tumor cells by secreting EGF, which binds to EGFR on the tumor cell surface and phosphorylates and activates STAT3 (Yang et al., 2012). pSTAT3 then transcriptionally upregulates the gene encoding Sox2, which subsequently activates a stem cell-like phenotype (Yang et al., 2012).
In humans, STAT3 activation is frequently observed in primary breast cancers and is associated with poor prognosis (Charpin et al., 2009; Watson and Miller, 1995) and invasiveness (Diaz et al., 2006). Moreover, inhibition of STAT3 with various pharmacological agents including small molecular inhibitors suppresses tumor growth, recurrence and invasion in breast cancer cell lines as well as in a human-xenograft model (Liu et al., 2012; Zhang et al., 2012). A more detailed analysis of the effect of STAT3 on breast tumors is also presented elsewhere in this issue. It remains to be seen how well these preclinical strategies will translate to clinical use.
STAT5
Aberrant activation of STAT5 has been found to be weakly oncogenic in several mouse models of breast cancer. Transgenic mice expressing the gene encoding a constitutively activated mutant STAT5 develop occasional mammary adenocarcinomas with long latencies (Iavnilovitch et al., 2004; Vafaizadeh et al., 2012). Similarly, transgenic overexpression of genes encoding upstream activators of STAT5, like Jak2, in mouse mammary epithelial cells predisposes the cells to tumorigenesis (Caffarel et al., 2012). Targeted expression of a constitutively active STAT5a mutant into a small subset of luminal cells in the mouse mammary gland induces a state of alveolar hyperproliferation, but does not result in tumor formation within one year (Cui et al., 2004; Dong et al., 2010; Liu et al., 1997). Collectively, these observations suggest that activated STAT5 may promote tumorigenesis but is not a bona-fide proto-oncogene, at least in the mammary gland. One possible means by which pSTAT5 promotes tumorigenesis is by expanding the population of mammary alveolar cells, which have been suggested to be especially susceptible to tumorigenesis (Henry et al., 2004). It is also possible that activation of STAT5 in breast epithelium that has suffered an oncogenic insult will proffer a survival advantage, predisposing the cells to tumorigenesis. When epithelial cells in the mammary gland incur an oncogenic insult, they activate a DNA damage response that can result in apoptosis (Bartkova et al., 2005; Reddy et al.). Epithelial cells that overcome this apoptotic response proceed to form frank tumors. Since the transcriptional targets of pSTAT5 in normal mammary cells and in breast tumor cells include prosurvival genes like CCND1 and IGF1 (Lim et al., 2010), it is possible that activation of STAT5 in oncogene-activate cells may circumvent the apoptotic barrier to tumorigenesis.
pSTAT5 is detected in 20 to 70% of human breast cancers (Cotarla et al., 2004; Nevalainen et al., 2004; Peck et al., 2011). It is not yet known whether there are tumor-specific mechanisms that aberrantly activate STAT5 or maintain pSTAT5 levels, but it appears that growth factors and hormones (e.g. FGF-2, progesterone, GH, and PRL) that are stimulators of STAT5 activation in normal breast cells also turn on STAT5 in breast cancer cells (Cerliani et al., 2011; Xu et al., 2011). Of note, STAT5 has not been found to be mutationally activated in human breast cancer although activating mutations in PRLR, its principal upstream activator in the mammary gland, have been associated with some breast cancer histopathological subtypes (Bogorad et al., 2008; Courtillot et al., 2010; Goffin et al., 2010). pSTAT5 is found primarily in ER+ tumors (Cotarla et al., 2004), but also in a subset of HER2+ (i.e., ErbB2+) tumors as well as in tumors lacking ER, PR, and HER2 (triple-negative or basal) (Cotarla et al., 2004; Nevalainen et al., 2004; Peck et al., 2011). pSTAT5 in ER+ breast cancers is associated with a higher differentiation status, favorable response to endocrine therapy and longer over-all survival (Cotarla et al., 2004; Peck et al., 2011; Yamashita et al., 2006). These data suggest that STAT5—even when subverted by the tumorigenic process—is able to maintain some of its differentiating functions (Figure 4). In support of this hypothesis, Bcl6, an inhibitor of differentiation, is transcriptionally repressed in normal cells and in breast cancer by STAT5 (Tran et al., 2010).
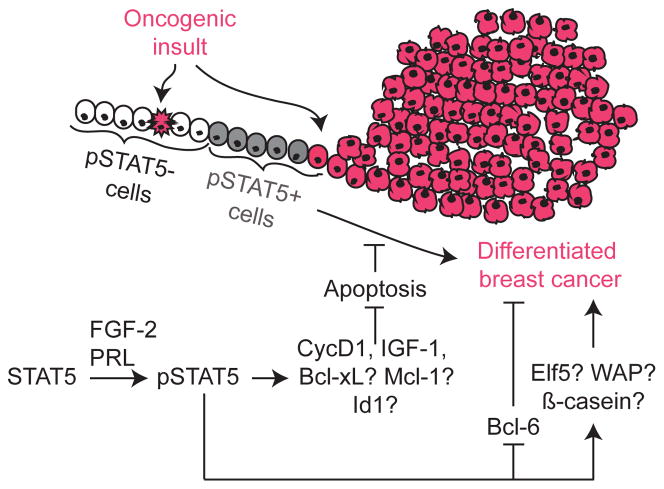
Figure 4. STAT5 plays distinct roles during tumor initiation and tumor progression.
STAT5 promotes tumor initiation, while it also promotes differentiation of established cancer cells. Cells that have sustained an oncogenic insult are depicted pink. On sustaining an oncogenic insult, pSTAT5− cells (white) may undergo cell death (pink with jagged edge), but pSTAT5+ cells (grey) are able to overcome oncogene-induced apoptosis, thereby evolving into a differentiated tumor (pink) with good prognosis. Known downstream mediators are depicted in the accompanying pathways, while potential effectors are noted with a question mark.
Besides being associated with better prognosis, pSTAT5 is also detected less often in invasive cancer, irrespective of cancer subtype, than in in situ tumors, and even less frequently in lymph node metastases (Nevalainen et al., 2004; Peck et al., 2011). This pattern of expression, along with its known function in causing cell differentiation in normal mammary gland, was sometimes interpreted to suggest that STAT5 may function as a tumor suppressor. However, ER has a nearly identical expression pattern, is associated with cellular differentiation, and is also a good prognostic marker; but it is a proven cancer prevention and treatment target (Albrektsen et al.). Perhaps, pSTAT5 plays an important tumorigenic role during the initial phases of tumor evolution but as precancerous cells accumulate more mutations and morph into cancer, pSTAT5 becomes dispensable (Figure 4). In support of this possibility, conditional ablation of Jak2 in the mammary gland of mice prevents the initiation of tumors but does not affect tumor progression once the tumors are formed (Sakamoto et al., 2010). Therefore, prophylactic suppression of STAT5 activity may prevent breast cancer, while anti-STAT5 therapy may only benefit a small number of patients whose tumors produce pSTAT5 and depend on it for growth or survival.
STAT6
The best studied function of STAT6 relates to its role in the immune system where it is essential for the IL4-mediated differentiation of T lymphocytes into Th2 cells (Kaplan et al., 1996). T lymphocytes can differentiate into either Th1 or Th2 cells based on the cytokines secreted in their environment. Th1 cells recognize tumor cell antigens and then mobilize an immune response against them, while Th2 cells promote tumor invasiveness and metastasis (Albrektsen et al.). The ratio of Th1/Th2 cells is affected by several factors including the tumor microenvironment (Albrektsen et al.). For instance, cancer-associated fibroblasts help maintain a pro-tumor Th2, rather than a tumor-suppressive Th1-dominated T cell profile in the tumor microenvironment (Liao et al., 2009). Another immune cell subset, the T regulatory cells, also affects the ratio of Th1/Th2 cells by secreting cytokines, and the T regulatory cell number is a prognostic factor in breast cancer (Yan et al., 2011).
As STAT6 is instrumental in regulating the balance between Th1 and Th2 cells, it is not surprising that STAT6 affects tumor progression (Figure 2). In a xenograft mouse model, STAT6 effectively inhibits immune clearance of nonimmunogenic, metastatic 4T1 breast tumor cells (Ostrand-Rosenberg et al., 2000). Inhibition of tumor cells by STAT6 is at least partially dependent on hemopoietic components (Ostrand-Rosenberg et al., 2002). STAT6 likely induces a tumor-promoting Th2 immune cell response in these mice, while also suppressing the host innate immunity against tumor antigens (Jensen et al., 2003; Norton et al., 2006). In addition, IL4-expressing T lymphocytes promote tumor invasiveness and metastasis in a MMTV-PyMT mouse model of breast cancer (DeNardo et al., 2009). Together, these data suggest a biphasic role for STAT6 in tumor initiation and metastasis.
Conclusion
The mammary gland is a unique tissue in that it is cyclically exposed to a battery of hormones and growth factors that promote cellular expansion and regression. During puberty and pregnancy, a sustained program of proliferation, invasion and cell survival is instituted by a complex system of regulatory molecules. It is not surprising therefore, that breast cancer is one of the most common malignancies among women. Epidemiological studies have implicated the effect of STAT-regulated mammary developmental stages, e.g. pregnancy, lactation and involution, on breast cancer risk (Borges and Schedin, 2012; MacMahon et al., 1970). In fact, one of the most consistently identified and profound risk factors for breast cancer incidence is pregnancy (Turkoz et al., 2012). Therefore, an understanding of the Jak-STAT signaling network that regulates these complex processes in the mammary gland is important for therapeutic and prophylactic strategies against breast cancer (Figure 1).
The STAT proteins form a highly conserved, evolutionarily important regulatory network, which is crucial for the normal development of the mammary gland. Elucidation of the various molecules involved in the activation and inactivation of this pathway in normal cells will help understand how the pathway is deregulated in tumor cells. The best studied components are STAT3 and STAT5 along with their upstream and downstream factors. However, recent research has expanded our understanding, and emphasized the importance, of the roles of the other STAT family members in the mammary gland. Continued investigation into the cross-talk between STATs, their expression patterns, and downstream functions will expand our knowledge of mammary cell regulation and will likely lead to more effective therapeutic and prophylactic strategies against breast cancer.
Highlights.
STATs are essential modulators of cellular response to the external environment
STATs mediate breast cell response to pregnancy, lactation and involution stimuli
STATs are important regulators of breast tumor initiation, growth and metastasis
Studying STATs in the normal breast is essential for understanding breast cancer
Acknowledgments
We thank Drs. Jeffrey Rosen and Kim Holloway for critical review of this manuscript. This work was supported in part by funds from CDMRP BC073703 (to Y.L) and BC085050 (to Y.L); and from NIH CA124820 (to Y.L), U54CA149196 (to Y. L; PI: Stephan Wong).
Footnotes
Disclosure summary
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abell K, Bilancio A, Clarkson RW, Tiffen PG, Altaparmakov AI, Burdon TG, Asano T, Vanhaesebroeck B, Watson CJ. Stat3-induced apoptosis requires a molecular switch in PI(3)K subunit composition. Nat Cell Biol. 2005;7:392–398. doi: 10.1038/ncb1242. [DOI] [PubMed] [Google Scholar]
- Akira S. Functional roles of STAT family proteins: lessons from knockout mice. Stem Cells. 1999;17:138–146. doi: 10.1002/stem.170138. [DOI] [PubMed] [Google Scholar]
- Albrektsen G, Heuch I, Thoresen S, Kvale G. Family history of breast cancer and short-term effects of childbirths on breast cancer risk. Int J Cancer. 2006;119:1468–1474. doi: 10.1002/ijc.22003. [DOI] [PubMed] [Google Scholar]
- Barbieri I, Pensa S, Pannellini T, Quaglino E, Maritano D, Demaria M, Voster A, Turkson J, Cavallo F, Watson CJ, et al. Constitutively active Stat3 enhances neu-mediated migration and metastasis in mammary tumors via upregulation of Cten. Cancer research. 2010;70:2558–2567. doi: 10.1158/0008-5472.CAN-09-2840. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- Bednorz NL, Brill B, Klein A, Gabel K, Groner B. Tracking the activation of Stat5 through the expression of an inducible reporter gene in a transgenic mouse line. Endocrinology. 2011;152:1935–1947. doi: 10.1210/en.2011-0053. [DOI] [PubMed] [Google Scholar]
- Bertucci PY, Quaglino A, Pozzi AG, Kordon EC, Pecci A. Glucocorticoid-induced impairment of mammary gland involution is associated with STAT5 and STAT3 signaling modulation. Endocrinology. 2010;151:5730–5740. doi: 10.1210/en.2010-0517. [DOI] [PubMed] [Google Scholar]
- Blakely CM, Sintasath L, D’Cruz CM, Hahn KT, Dugan KD, Belka GK, Chodosh LA. Developmental stage determines the effects of MYC in the mammary epithelium. Development. 2005;132:1147–1160. doi: 10.1242/dev.01655. [DOI] [PubMed] [Google Scholar]
- Bogorad RL, Courtillot C, Mestayer C, Bernichtein S, Harutyunyan L, Jomain JB, Bachelot A, Kuttenn F, Kelly PA, Goffin V, Touraine P. Identification of a gain-of-function mutation of the prolactin receptor in women with benign breast tumors. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14533–14538. doi: 10.1073/pnas.0800685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges VF, Schedin PJ. Pregnancy-associated breast cancer: an entity needing refinement of the definition. Cancer. 2012;118:3226–3228. doi: 10.1002/cncr.26643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene. 2005;24:552–560. doi: 10.1038/sj.onc.1208185. [DOI] [PubMed] [Google Scholar]
- Braunstein J, Brutsaert S, Olson R, Schindler C. STATs dimerize in the absence of phosphorylation. The Journal of biological chemistry. 2003;278:34133–34140. doi: 10.1074/jbc.M304531200. [DOI] [PubMed] [Google Scholar]
- Bromberg J. Signal transducers and activators of transcription as regulators of growth, apoptosis and breast development. Breast Cancer Res. 2000;2:86–90. doi: 10.1186/bcr38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Caffarel MM, Zaragoza R, Pensa S, Li J, Green AR, Watson CJ. Constitutive activation of JAK2 in mammary epithelium elevates Stat5 signalling, promotes alveologenesis and resistance to cell death, and contributes to tumourigenesis. Cell Death Differ. 2012;19:511–522. doi: 10.1038/cdd.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerliani JP, Guillardoy T, Giulianelli S, Vaque JP, Gutkind JS, Vanzulli SI, Martins R, Zeitlin E, Lamb CA, Lanari C. Interaction between FGFR-2, STAT5, and progesterone receptors in breast cancer. Cancer research. 2011;71:3720–3731. doi: 10.1158/0008-5472.CAN-10-3074. [DOI] [PubMed] [Google Scholar]
- Chan CB, Liu X, Ensslin MA, Dillehay DL, Ormandy CJ, Sohn P, Serra R, Ye K. PIKE-A is required for prolactin-mediated STAT5a activation in mammary gland development. The EMBO journal. 2010;29:956–968. doi: 10.1038/emboj.2009.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SR, Vermi W, Luo J, Lucini L, Rickert C, Fowler AM, Lonardi S, Arthur C, Young LJ, Levy DE, et al. STAT1-deficient mice spontaneously develop estrogen receptor alpha-positive luminal mammary carcinomas. Breast cancer research: BCR. 2012;14:R16. doi: 10.1186/bcr3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RS, Lourenco PC, Tonner E, Flint DJ, Selbert S, Takeda K, Akira S, Clarke AR, Watson CJ. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes & development. 1999;13:2604–2616. doi: 10.1101/gad.13.19.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpin C, Secq V, Giusiano S, Carpentier S, Andrac L, Lavaut MN, Allasia C, Bonnier P, Garcia S. A signature predictive of disease outcome in breast carcinomas, identified by quantitative immunocytochemical assays. International journal of cancer Journal international du cancer. 2009;124:2124–2134. doi: 10.1002/ijc.24177. [DOI] [PubMed] [Google Scholar]
- Chen CC, Stairs DB, Boxer RB, Belka GK, Horseman ND, Alvarez JV, Chodosh LA. Autocrine prolactin induced by the Pten-Akt pathway is required for lactation initiation and provides a direct link between the Akt and Stat5 pathways. Genes & development. 2012;26:2154–2168. doi: 10.1101/gad.197343.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Vinkemeier U, Zhao Y, Jeruzalmi D, Darnell JE, Jr, Kuriyan J. Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell. 1998;93:827–839. doi: 10.1016/s0092-8674(00)81443-9. [DOI] [PubMed] [Google Scholar]
- Choi YS, Chakrabarti R, Escamilla-Hernandez R, Sinha S. Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: failure of Stat5 activation and functional differentiation in the absence of Elf5. Dev Biol. 2009;329:227–241. doi: 10.1016/j.ydbio.2009.02.032. [DOI] [PubMed] [Google Scholar]
- Clarkson RW, Boland MP, Kritikou EA, Lee JM, Freeman TC, Tiffen PG, Watson CJ. The genes induced by signal transducer and activators of transcription (STAT)3 and STAT5 in mammary epithelial cells define the roles of these STATs in mammary development. Molecular endocrinology. 2006;20:675–685. doi: 10.1210/me.2005-0392. [DOI] [PubMed] [Google Scholar]
- Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast cancer research: BCR. 2004;6:R92–109. doi: 10.1186/bcr754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobanoglu O, Zaitoun I, Chang YM, Shook GE, Khatib H. Effects of the signal transducer and activator of transcription 1 (STAT1) gene on milk production traits in Holstein dairy cattle. J Dairy Sci. 2006;89:4433–4437. doi: 10.3168/jds.S0022-0302(06)72491-2. [DOI] [PubMed] [Google Scholar]
- Cotarla I, Ren S, Zhang Y, Gehan E, Singh B, Furth PA. Stat5a is tyrosine phosphorylated and nuclear localized in a high proportion of human breast cancers. Int J Cancer. 2004;108:665–671. doi: 10.1002/ijc.11619. [DOI] [PubMed] [Google Scholar]
- Courtillot C, Chakhtoura Z, Bogorad R, Genestie C, Bernichtein S, Badachi Y, Janaud G, Akakpo JP, Bachelot A, Kuttenn F, et al. Characterization of two constitutively active prolactin receptor variants in a cohort of 95 women with multiple breast fibroadenomas. J Clin Endocrinol Metab. 2010;95:271–279. doi: 10.1210/jc.2009-1494. [DOI] [PubMed] [Google Scholar]
- Creamer BA, Sakamoto K, Schmidt JW, Triplett AA, Moriggl R, Wagner KU. Stat5 promotes survival of mammary epithelial cells through transcriptional activation of a distinct promoter in Akt1. Molecular and cellular biology. 2010;30:2957–2970. doi: 10.1128/MCB.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol. 2004;24:8037–8047. doi: 10.1128/MCB.24.18.8037-8047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE., Jr Reflections on STAT3, STAT5, and STAT6 as fat STATs. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6221–6224. doi: 10.1073/pnas.93.13.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz N, Minton S, Cox C, Bowman T, Gritsko T, Garcia R, Eweis I, Wloch M, Livingston S, Seijo E, et al. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:20–28. doi: 10.1158/1078-0432.CCR-04-1749. [DOI] [PubMed] [Google Scholar]
- Dong J, Tong T, Reynado AM, Rosen JM, Huang S, Li Y. Genetic manipulation of individual somatic mammary cells in vivo reveals a master role of STAT5a in inducing alveolar fate commitment and lactogenesis even in the absence of ovarian hormones. Dev Biol. 2010;346:196–203. doi: 10.1016/j.ydbio.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- Faulds MH, Pettersson K, Gustafsson JA, Haldosen LA. Cross-talk between ERs and signal transducer and activator of transcription 5 is E2 dependent and involves two functionally separate mechanisms. Molecular endocrinology. 2001;15:1929–1940. doi: 10.1210/mend.15.11.0726. [DOI] [PubMed] [Google Scholar]
- Gallego MI, Binart N, Robinson GW, Okagaki R, Coschigano KT, Perry J, Kopchick JJ, Oka T, Kelly PA, Hennighausen L. Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev Biol. 2001;229:163–175. doi: 10.1006/dbio.2000.9961. [DOI] [PubMed] [Google Scholar]
- Goffin V, Bogorad RL, Touraine P. Identification of gain-of-function variants of the human prolactin receptor. Methods Enzymol. 2010;484:329–355. doi: 10.1016/B978-0-12-381298-8.00017-4. [DOI] [PubMed] [Google Scholar]
- Hakim O, Sung MH, Nakayamada S, Voss T, Baek S, Hager G. Spatial congregation of STAT binding directs selective nuclear architecture during T cell functional differentiation. Genome Res. 2012 doi: 10.1101/gr.147652.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, Stanford PM, Sutherland K, Oakes SR, Naylor MJ, Robertson FG, Blazek KD, Kazlauskas M, Hilton HN, Wittlin S, et al. Socs2 and elf5 mediate prolactin-induced mammary gland development. Mol Endocrinol. 2006;20:1177–1187. doi: 10.1210/me.2005-0473. [DOI] [PubMed] [Google Scholar]
- Heim MH, Kerr IM, Stark GR, Darnell JE., Jr Contribution of STAT SH2 groups to specific interferon signaling by the Jak-STAT pathway. Science. 1995;267:1347–1349. doi: 10.1126/science.7871432. [DOI] [PubMed] [Google Scholar]
- Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene. 2004;23:6980–6985. doi: 10.1038/sj.onc.1207827. [DOI] [PubMed] [Google Scholar]
- Horvath CM, Wen Z, Darnell JE., Jr A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes & development. 1995;9:984–994. doi: 10.1101/gad.9.8.984. [DOI] [PubMed] [Google Scholar]
- Hughes K, Wickenden JA, Allen JE, Watson CJ. Conditional deletion of Stat3 in mammary epithelium impairs the acute phase response and modulates immune cell numbers during post-lactational regression. The Journal of pathology. 2012;227:106–117. doi: 10.1002/path.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys RC, Bierie B, Zhao L, Raz R, Levy D, Hennighausen L. Deletion of Stat3 blocks mammary gland involution and extends functional competence of the secretory epithelium in the absence of lactogenic stimuli. Endocrinology. 2002;143:3641–3650. doi: 10.1210/en.2002-220224. [DOI] [PubMed] [Google Scholar]
- Iavnilovitch E, Cardiff RD, Groner B, Barash I. Deregulation of Stat5 expression and activation causes mammary tumors in transgenic mice. International journal of cancer Journal international du cancer. 2004;112:607–619. doi: 10.1002/ijc.20484. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Molecular cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahchan NS, Wang D, Bissell MJ, Luo K. SnoN regulates mammary gland alveologenesis and onset of lactation by promoting prolactin/Stat5 signaling. Development. 2012;139:3147–3156. doi: 10.1242/dev.079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SM, Meijer SL, Kurt RA, Urba WJ, Hu HM, Fox BA. Regression of a mammary adenocarcinoma in STAT6−/− mice is dependent on the presence of STAT6-reactive T cells. Journal of immunology. 2003;170:2014–2021. doi: 10.4049/jimmunol.170.4.2014. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- Ke Y, Lesperance J, Zhang EE, Bard-Chapeau EA, Oshima RG, Muller WJ, Feng GS. Conditional deletion of Shp2 in the mammary gland leads to impaired lobulo-alveolar outgrowth and attenuated Stat5 activation. The Journal of biological chemistry. 2006;281:34374–34380. doi: 10.1074/jbc.M607325200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled WT, Read EK, Nicholson SE, Baxter FO, Brennan AJ, Came PJ, Sprigg N, McKenzie AN, Watson CJ. The IL-4/IL-13/Stat6 signalling pathway promotes luminal mammary epithelial cell development. Development. 2007;134:2739–2750. doi: 10.1242/dev.003194. [DOI] [PubMed] [Google Scholar]
- Khodarev N, Ahmad R, Rajabi H, Pitroda S, Kufe T, McClary C, Joshi MD, MacDermed D, Weichselbaum R, Kufe D. Cooperativity of the MUC1 oncoprotein and STAT1 pathway in poor prognosis human breast cancer. Oncogene. 2010;29:920–929. doi: 10.1038/onc.2009.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodarev NN, Roizman B, Weichselbaum RR. Molecular pathways: interferon/stat1 pathway: role in the tumor resistance to genotoxic stress and aggressive growth. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:3015–3021. doi: 10.1158/1078-0432.CCR-11-3225. [DOI] [PubMed] [Google Scholar]
- Kim TK, Maniatis T. Regulation of interferon-gamma-activated STAT1 by the ubiquitin-proteasome pathway. Science. 1996;273:1717–1719. doi: 10.1126/science.273.5282.1717. [DOI] [PubMed] [Google Scholar]
- Klover PJ, Muller WJ, Robinson GW, Pfeiffer RM, Yamaji D, Hennighausen L. Loss of STAT1 from mouse mammary epithelium results in an increased Neu-induced tumor burden. Neoplasia. 2010;12:899–905. doi: 10.1593/neo.10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzaler PA, Staniszewska AD, Li W, Omidvar N, Kedjouar B, Turkson J, Poli V, Flavell RA, Clarkson RW, Watson CJ. Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol. 2011;13:303–309. doi: 10.1038/ncb2171. [DOI] [PubMed] [Google Scholar]
- Kritikou EA, Sharkey A, Abell K, Came PJ, Anderson E, Clarkson RW, Watson CJ. A dual, non-redundant, role for LIF as a regulator of development and STAT3-mediated cell death in mammary gland. Development. 2003;130:3459–3468. doi: 10.1242/dev.00578. [DOI] [PubMed] [Google Scholar]
- Li M, Liu X, Robinson G, Bar-Peled U, Wagner KU, Young WS, Hennighausen L, Furth PA. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3425–3430. doi: 10.1073/pnas.94.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Leung S, Qureshi S, Darnell JE, Jr, Stark GR. Formation of STAT1-STAT2 heterodimers and their role in the activation of IRF-1 gene transcription by interferon-alpha. The Journal of biological chemistry. 1996;271:5790–5794. doi: 10.1074/jbc.271.10.5790. [DOI] [PubMed] [Google Scholar]
- Li Y, Kumar KG, Tang W, Spiegelman VS, Fuchs SY. Negative regulation of prolactin receptor stability and signaling mediated by SCF(beta-TrCP) E3 ubiquitin ligase. Molecular and cellular biology. 2004;24:4038–4048. doi: 10.1128/MCB.24.9.4038-4048.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4:e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EJ, Joung YH, Jung SM, Park SH, Park JH, Kim SY, Hwang TS, Hong DY, Chung SC, Ye SK, et al. Hemin inhibits cyclin D1 and IGF-1 expression via STAT5b under hypoxia in ERalpha-negative MDA-MB 231 breast cancer cells. International journal of oncology. 2010;36:1243–1251. doi: 10.3892/ijo_00000608. [DOI] [PubMed] [Google Scholar]
- Lindeman GJ, Wittlin S, Lada H, Naylor MJ, Santamaria M, Zhang JG, Starr R, Hilton DJ, Alexander WS, Ormandy CJ, Visvader J. SOCS1 deficiency results in accelerated mammary gland development and rescues lactation in prolactin receptor-deficient mice. Genes Dev. 2001;15:1631–1636. doi: 10.1101/gad.880801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Liu Y, Jin Z, Hu Q, Lin L, Jou D, Yang J, Xu Z, Wang H, Li C, Lin J. XZH-5 Inhibits STAT3 Phosphorylation and Enhances the Cytotoxicity of Chemotherapeutic Drugs in Human Breast and Pancreatic Cancer Cells. PLoS One. 2012;7:e46624. doi: 10.1371/journal.pone.0046624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Hennighausen L. Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol Endocrinol. 1996;10:1496–1506. doi: 10.1210/mend.10.12.8961260. [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- Long W, Wagner KU, Lloyd KC, Binart N, Shillingford JM, Hennighausen L, Jones FE. Impaired differentiation and lactational failure of Erbb4-deficient mammary glands identify ERBB4 as an obligate mediator of STAT5. Development. 2003;130:5257–5268. doi: 10.1242/dev.00715. [DOI] [PubMed] [Google Scholar]
- MacMahon B, Cole P, Lin TM, Lowe CR, Mirra AP, Ravnihar B, Salber EJ, Valaoras VG, Yuasa S. Age at first birth and breast cancer risk. Bull World Health Organ. 1970;43:209–221. [PMC free article] [PubMed] [Google Scholar]
- Magkou C, Giannopoulou I, Theohari I, Fytou A, Rafailidis P, Nomikos A, Papadimitriou C, Nakopoulou L. Prognostic significance of phosphorylated STAT-1 expression in premenopausal and postmenopausal patients with invasive breast cancer. Histopathology. 2012;60:1125–1132. doi: 10.1111/j.1365-2559.2011.04143.x. [DOI] [PubMed] [Google Scholar]
- Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- Meyer T, Marg A, Lemke P, Wiesner B, Vinkemeier U. DNA binding controls inactivation and nuclear accumulation of the transcription factor Stat1. Genes & development. 2003;17:1992–2005. doi: 10.1101/gad.268003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani ES, Brinkhaus H, Dueggeli R, Klebba I, Mueller U, Stadler M, Kohler H, Smalley MJ, Bentires-Alj M. Protein tyrosine phosphatase 1B restrains mammary alveologenesis and secretory differentiation. Development. 2012 doi: 10.1242/dev.082941. [DOI] [PubMed] [Google Scholar]
- Nevalainen MT, Xie J, Torhorst J, Bubendorf L, Haas P, Kononen J, Sauter G, Rui H. Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J Clin Oncol. 2004;22:2053–2060. doi: 10.1200/JCO.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Norton JA, Li M, Lee NC, Tsung K. Inhibition of host signal transducer and activator of transcription factor 6 results in cure with cyclophosphamide and interleukin 12 immunotherapy. Ann Surg Oncol. 2006;13:118–124. doi: 10.1245/ASO.2006.03.514. [DOI] [PubMed] [Google Scholar]
- Oliver CH, Khaled WT, Frend H, Nichols J, Watson CJ. The Stat6-regulated KRAB domain zinc finger protein Zfp157 regulates the balance of lineages in mammary glands and compensates for loss of Gata-3. Genes & development. 2012;26:1086–1097. doi: 10.1101/gad.184051.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Clements VK, Terabe M, Park JM, Berzofsky JA, Dissanayake SK. Resistance to metastatic disease in STAT6-deficient mice requires hemopoietic and nonhemopoietic cells and is IFN-gamma dependent. Journal of immunology. 2002;169:5796–5804. doi: 10.4049/jimmunol.169.10.5796. [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Grusby MJ, Clements VK. Cutting edge: STAT6-deficient mice have enhanced tumor immunity to primary and metastatic mammary carcinoma. Journal of immunology. 2000;165:6015–6019. doi: 10.4049/jimmunol.165.11.6015. [DOI] [PubMed] [Google Scholar]
- Park C, Li S, Cha E, Schindler C. Immune response in Stat2 knockout mice. Immunity. 2000;13:795–804. doi: 10.1016/s1074-7613(00)00077-7. [DOI] [PubMed] [Google Scholar]
- Peck AR, Witkiewicz AK, Liu C, Stringer GA, Klimowicz AC, Pequignot E, Freydin B, Tran TH, Yang N, Rosenberg AL, et al. Loss of nuclear localized and tyrosine phosphorylated Stat5 in breast cancer predicts poor clinical outcome and increased risk of antiestrogen therapy failure. J Clin Oncol. 2011;29:2448–2458. doi: 10.1200/JCO.2010.30.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp JA, Burdon TG, Watson CJ. Differential activation of STATs 3 and 5 during mammary gland development. FEBS letters. 1996;396:77–80. doi: 10.1016/0014-5793(96)01069-1. [DOI] [PubMed] [Google Scholar]
- Pranada AL, Metz S, Herrmann A, Heinrich PC, Muller-Newen G. Real time analysis of STAT3 nucleocytoplasmic shuttling. The Journal of biological chemistry. 2004;279:15114–15123. doi: 10.1074/jbc.M312530200. [DOI] [PubMed] [Google Scholar]
- Quarrie LH, Addey CV, Wilde CJ. Programmed cell death during mammary tissue involution induced by weaning, litter removal, and milk stasis. J Cell Physiol. 1996;168:559–569. doi: 10.1002/(SICI)1097-4652(199609)168:3<559::AID-JCP8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Raven JF, Williams V, Wang S, Tremblay ML, Muller WJ, Durbin JE, Koromilas AE. Stat1 is a suppressor of ErbB2/Neu-mediated cellular transformation and mouse mammary gland tumor formation. Cell Cycle. 2011;10:794–804. doi: 10.4161/cc.10.5.14956. [DOI] [PubMed] [Google Scholar]
- Reddy JP, Peddibhotla S, Bu W, Zhao J, Haricharan S, Du YC, Podsypanina K, Rosen JM, Donehower LA, Li Y. Defining the ATM-mediated barrier to tumorigenesis in somatic mammary cells following ErbB2 activation. Proc Natl Acad Sci U S A. 107:3728–3733. doi: 10.1073/pnas.0910665107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni M, Ambrosetti DC, Mansukhani A, Gertner R, Levy D, Basilico C. FGF signaling inhibits chondrocyte proliferation and regulates bone development through the STAT-1 pathway. Genes & development. 1999;13:1361–1366. doi: 10.1101/gad.13.11.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Creamer BA, Triplett AA, Wagner KU. The Janus kinase 2 is required for expression and nuclear accumulation of cyclin D1 in proliferating mammary epithelial cells. Molecular endocrinology. 2007;21:1877–1892. doi: 10.1210/me.2006-0316. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Triplett AA, Schuler LA, Wagner KU. Janus kinase 2 is required for the initiation but not maintenance of prolactin-induced mammary cancer. Oncogene. 2010;29:5359–5369. doi: 10.1038/onc.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SJ, Haslam SZ, Conrad SE. Signal transducer and activator of transcription 5a mediates mammary ductal branching and proliferation in the nulliparous mouse. Endocrinology. 2010;151:2876–2885. doi: 10.1210/en.2009-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schere-Levy C, Buggiano V, Quaglino A, Gattelli A, Cirio MC, Piazzon I, Vanzulli S, Kordon EC. Leukemia inhibitory factor induces apoptosis of the mammary epithelial cells and participates in mouse mammary gland involution. Exp Cell Res. 2003;282:35–47. doi: 10.1006/excr.2002.5666. [DOI] [PubMed] [Google Scholar]
- Shillingford JM, Miyoshi K, Robinson GW, Grimm SL, Rosen JM, Neubauer H, Pfeffer K, Hennighausen L. Jak2 is an essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Molecular endocrinology. 2002;16:563–570. doi: 10.1210/mend.16.3.0805. [DOI] [PubMed] [Google Scholar]
- Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- Sotgia F, Schubert W, Pestell RG, Lisanti MP. Genetic ablation of caveolin-1 in mammary epithelial cells increases milk production and hyper-activates STAT5a signaling. Cancer biology & therapy. 2006;5:292–297. doi: 10.4161/cbt.5.3.2390. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. Journal of immunology. 1998;161:4652–4660. [PubMed] [Google Scholar]
- Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- Tiffen PG, Omidvar N, Marquez-Almuina N, Croston D, Watson CJ, Clarkson RW. A dual role for oncostatin M signaling in the differentiation and death of mammary epithelial cells in vivo. Molecular endocrinology. 2008;22:2677–2688. doi: 10.1210/me.2008-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TH, Utama FE, Lin J, Yang N, Sjolund AB, Ryder A, Johnson KJ, Neilson LM, Liu C, Brill KL, et al. Prolactin inhibits BCL6 expression in breast cancer through a Stat5a-dependent mechanism. Cancer research. 2010;70:1711–1721. doi: 10.1158/0008-5472.CAN-09-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyada A, Chow A, Wu J, Somlo G, Chu P, Loera S, Luu T, Li AX, Wu X, Ye W, et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer research. 2012;72:2768–2779. doi: 10.1158/0008-5472.CAN-11-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkoz FP, Solak M, Petekkaya I, Keskin O, Kertmen N, Sarici F, Arik Z, Babacan T, Ozisik Y, Altundag K. Association between common risk factors and molecular subtypes in breast cancer patients. Breast. 2012 doi: 10.1016/j.breast.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Vafaizadeh V, Klemmt P, Brendel C, Weber K, Doebele C, Britt K, Grez M, Fehse B, Desrivieres S, Groner B. Mammary epithelial reconstitution with gene-modified stem cells assigns roles to Stat5 in luminal alveolar cell fate decisions, differentiation, involution, and mammary tumor formation. Stem Cells. 2010;28:928–938. doi: 10.1002/stem.407. [DOI] [PubMed] [Google Scholar]
- Vafaizadeh V, Klemmt PA, Groner B. Stat5 assumes distinct functions in mammary gland development and mammary tumor formation. Front Biosci. 2012;17:1232–1250. doi: 10.2741/3983. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377–1386. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Krempler A, Triplett AA, Qi Y, George NM, Zhu J, Rui H. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Molecular and cellular biology. 2004;24:5510–5520. doi: 10.1128/MCB.24.12.5510-5520.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cheng CH. ERalpha and STAT5a cross-talk: interaction through C-terminal portions of the proteins decreases STAT5a phosphorylation, nuclear translocation and DNA-binding. FEBS letters. 2004;572:238–244. doi: 10.1016/j.febslet.2004.06.098. [DOI] [PubMed] [Google Scholar]
- Watson CJ. Stat transcription factors in mammary gland development and tumorigenesis. Journal of mammary gland biology and neoplasia. 2001;6:115–127. doi: 10.1023/a:1009524817155. [DOI] [PubMed] [Google Scholar]
- Watson CJ, Miller WR. Elevated levels of members of the STAT family of transcription factors in breast carcinoma nuclear extracts. British journal of cancer. 1995;71:840–844. doi: 10.1038/bjc.1995.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichselbaum RR, Ishwaran H, Yoon T, Nuyten DS, Baker SW, Khodarev N, Su AW, Shaikh AY, Roach P, Kreike B, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18490–18495. doi: 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widschwendter A, Tonko-Geymayer S, Welte T, Daxenbichler G, Marth C, Doppler W. Prognostic significance of signal transducer and activator of transcription 1 activation in breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2002;8:3065–3074. [PubMed] [Google Scholar]
- Xu J, Zhang Y, Berry PA, Jiang J, Lobie PE, Langenheim JF, Chen WY, Frank SJ. Growth hormone signaling in human T47D breast cancer cells: potential role for a growth hormone receptor-prolactin receptor complex. Molecular endocrinology. 2011;25:597–610. doi: 10.1210/me.2010-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Nie L, Kim SH, Sun XH. STAT5-induced Id-1 transcription involves recruitment of HDAC1 and deacetylation of C/EBPbeta. The EMBO journal. 2003;22:893–904. doi: 10.1093/emboj/cdg094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji D, Kang K, Robinson GW, Hennighausen L. Sequential activation of genetic programs in mouse mammary epithelium during pregnancy depends on STAT5A/B concentration. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji D, Na R, Feuermann Y, Pechhold S, Chen W, Robinson GW, Hennighausen L. Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes & development. 2009;23:2382–2387. doi: 10.1101/gad.1840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Nishio M, Ando Y, Zhang Z, Hamaguchi M, Mita K, Kobayashi S, Fujii Y, Iwase H. Stat5 expression predicts response to endocrine therapy and improves survival in estrogen receptor-positive breast cancer. Endocrine-related cancer. 2006;13:885–893. doi: 10.1677/erc.1.01095. [DOI] [PubMed] [Google Scholar]
- Yan M, Jene N, Byrne D, Millar EK, O’Toole SA, McNeil CM, Bates GJ, Harris AL, Banham AH, Sutherland RL, Fox SB. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast cancer research: BCR. 2011;13:R47. doi: 10.1186/bcr2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liao D, Chen C, Liu Y, Chuang TH, Xiang R, Markowitz D, Reisfeld RA, Luo Y. Tumor associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells. 2012 doi: 10.1002/stem.1281. [DOI] [PubMed] [Google Scholar]
- Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, Iino T, Rocnik JL, Kikushige Y, Mori Y, Shima T, Iwasaki H, Takenaka K, et al. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood. 2009;114:5034–5043. doi: 10.1182/blood-2008-12-196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yue P, Page BD, Li T, Zhao W, Namanja AT, Paladino D, Zhao J, Chen Y, Gunning PT, Turkson J. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9623–9628. doi: 10.1073/pnas.1121606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Hart S, Cheng J, Melenhorst JJ, Bierie B, Ernst M, Stewart C, Schaper F, Heinrich PC, Ullrich A, et al. Mammary gland remodeling depends on gp130 signaling through Stat3 and MAPK. The Journal of biological chemistry. 2004;279:44093–44100. doi: 10.1074/jbc.M313131200. [DOI] [PubMed] [Google Scholar]
- Zhou J, Bi C, Janakakumara JV, Liu SC, Chng WJ, Tay KG, Poon LF, Xie Z, Palaniyandi S, Yu H, et al. Enhanced activation of STAT pathways and overexpression of survivin confer resistance to FLT3 inhibitors and could be therapeutic targets in AML. Blood. 2009;113:4052–4062. doi: 10.1182/blood-2008-05-156422. [DOI] [PubMed] [Google Scholar]