Abstract
CCR5 blockers inhibit CCR5-tropic (R5) HIV-1, including strains resistant to other antiretrovirals. We demonstrate that the CCR5 antibody HGS004 and the CCR5 antagonist Maraviroc have potent antiviral synergy against R5 HIV-1, translating into dose reductions of >10-fold for Maraviroc and >150-fold for HGS004. These data, together with the high barrier of resistance to HGS004, suggest that combinations of Maraviroc and HGS004 could provide effective preventive and therapeutic strategies against R5 HIV-1.
CCR5 serves as the main coreceptor for the predominantly transmitted and most persistent HIV-1, the R5 strains. Maraviroc (MVC) is the only licensed CCR5 inhibitor [1, 2]. CCR5 antibodies PRO 140 and HGS004 have significantly reduced HIV-1 RNA in clinical trials of patients infected with R5 HIV-1 only [3-6]. However, HGS004 reduced HIV-1 RNA in only 54% of patients. Retrospectively, it was found that sensitivity of the patients’ viruses to HGS004 predicted antiviral responses [6]. We tested the hypothesis that the combination of HGS004 and MVC has antiviral synergy, thereby increasing the potency of both drugs and reducing their effective doses. This hypothesis is based on the observation that HGS004 and MVC recognize separate regions of CCR5, with HGS004 binding to the second extracellular loop [6], and MVC to the transmembrane region [7-9].
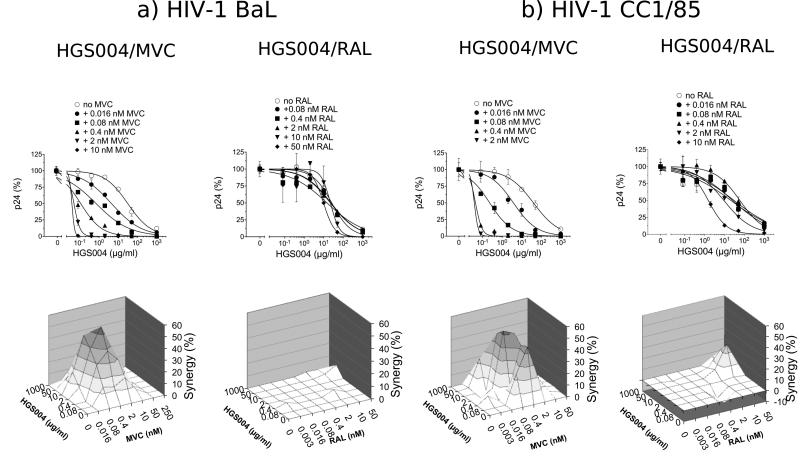
We tested the antiviral activity of HGS004 alone and in the presence of varying concentrations of MVC. For comparison, we evaluated HGS004 in combination with the integrase inhibitor Raltegravir (RAL). Both MVC and RAL were used at concentrations spanning their EC50s. We performed these assays using PHA-activated peripheral blood mononuclear cells (PBMCs) infected with the R5 HIV-1 strains BaL or CC1/85. Data for HIV-1 BaL are shown in Fig 1a (upper panels). In the absence of MVC or RAL, HGS004 inhibited HIV-1 BaL with an EC50 value of 31.3 µg/ml. However, in the presence of increasing concentrations of MVC, HGS004 EC50s were successively lowered to 4.4, 0.7, 0.1, 0.05 and 0.03 μg/ml. In contrast, RAL had little effect on HGS004 potency, with HGS004 EC50s of 26, 24, 22, 15 and 9.8 μg/ml in the presence of increasing RAL. Neither the drug alone nor the drug combinations treatments were toxic to cells as demonstrated by MTT assays (not shown). Together, the marked leftwards shift of the HGS004 viral inhibition curves in the presence of MVC compared to RAL suggested that HGS004 and MVC are synergistic against HIV-1 BaL.
Figure 1. Antiviral interactions between HGS004 and MVC versus HGS004 and RAL against R5 HIV-1 in primary cells.
PHA-activated PBMCs infected with HIV-1 BaL (a) or HIV-1 CC1/85 (b) using an MOI of 0.001 were cultured in the presence of the indicated drug concentrations. Virus production was measured on day 7 by p24 ELISA. Inhibition data from replicates were plotted using GraphPad Prism software and EC50 values determined using variable slope non-linear regression analysis. MVC EC50s for BaL and CC1/85 were 0.74 and 0.34 nM, respectively; whereas RAL EC50s for BaL and CC1/85 were 4.3 and 1.1 nM, respectively (not shown). Upper panels: Dose response curves for HGS004, alone and in combination with MVC or RAL. At each MVC or RAL concentration, p24 inhibition by HGS004 was normalized to inhibition by MVC or RAL alone. Data points are means ± S.D. of duplicates. Lower panels: Three-dimensional analysis of viral inhibition by drug combinations. Percentage synergy obtained by each combination was plotted against HGS004 and MVC or RAL concentrations according to Prichard's synergy model. The 96% confidence interval synergy plots are shown. Percentage synergies at each 10% increment are shown in different color intensities. Note that the synergy plots included more drug concentrations than those shown in dose response curves. Data are representative of three independent experiments for BaL and two independent experiments for CC1/85, with PBMCs from different donors in each experiment.
To determine whether inhibition by HGS004 and MVC or RAL might indeed be synergistic, we performed a three-dimensional analysis using the method of Prichard and Shipman [10]. For the HGS004/MVC and HGS004/RAL combinations, the 96% confidence synergy plots, after Bonferroni adjustment, are shown in Fig 1a (lower panels). The combination of HGS004 and MVC had antiviral synergy across the entire concentration grid (synergy volume of 522). In contrast, the combination of HGS004 and RAL was mainly additive (synergy volume of only 19).
Similar results to those with HIV-1 BaL were obtained with HIV-1 CC1/85, a R5 HIV-1 primary patient isolate [11] (Fig 1b, upper panels). HGS004 EC50s were 40 μg/ml in antibody alone treatment, but only 3.75, 0.22, 0.04 and 0.03 μg/ml in combinations containing the indicated MVC concentrations. HGS004 EC50s were 30, 26, 59, 9.2 and 1.5 nM in combinations containing RAL, suggesting some increased antibody potency at high concentrations of RAL (Fig 1b, upper panels). Inhibition of CC1/85 by the HGS004/MVC and HGS004/RAL combinations gave synergy volumes of 502 and 54, respectively (Fig 1b, lower panels).
To confirm the synergy data, we analyzed the drug combinations by the Median Effect Principle [12], which evaluates drug combinations at fixed ratios and allows calculation of Combination Indices (CI). CI=1 indicates additivity, CI>1 indicates antagonism, and CI<1 indicates synergy. At 50% viral inhibition, the CI values for the HGS004/MVC combinations ranged 0.070-0.154 for HIV-1 BaL, and 0.060-0.117 for HIV-1 CC1/85. In contrast, CI values for the HGS004/RAL combination ranged 0.898-1.230. As CI values are proportional to the amount of synergy, the obtained CIs for the HGS004/MVC combinations demonstrate a quite potent synergistic interaction. These CI values translated into 10-fold lower doses of MVC and 230-fold lower doses of HGS004 against BaL, and 14-fold lower doses of MVC and 155-fold lower doses of HGS004 against CC1/85. Overall, these data are consistent with those obtained by Three-dimensional modeling analysis, demonstrating that HGS004 has antiviral synergy with MVC, but mostly additivity with RAL. The low level synergy between HGS004 and RAL identified by Three-dimensional analysis was not identified by the Median Effect Principle analysis, likely reflecting that synergy occurs only at a restricted concentration range (see synergy plots in Fig 1).
We also evaluated the HGS004/MVC combination against R5 primary isolates 92BR020 (Subtype B, from Brazil) and 92UG031 (Subtype A, from Uganda). Antiviral synergy was higher than for BaL and CC1/85, with synergy volumes of 811 and 1100 for 92BR020 and 92UG031, respectively. We could not calculate CI values because drugs were not used at fixed ratios. We also evaluated HGS004 and MVC against R5X4 dual tropic HIV-1 89.6. Neither HGS004 nor MVC, alone or in combination, inhibited 89.6, in agreement with previous data suggesting that this virus primarily uses CXCR4 in PBMCs [7].
In summary, we demonstrate that MVC and HGS004 have potent synergy against R5 HIV-1 in PBMCs, significantly reducing HGS004 and MVC effective doses. Our results are consistent with previously described synergies between MVC and CCR5 antibodies PRO 140 [13] or RoAb13 [14]. These data, together with HGS004 long-acting activity, high barrier to drug resistance, and favorable antiviral interactions with reverse transcriptase, protease and integrase inhibitors ([6, 15]; this report), suggest that combinations of MVC and HGS004 could provide effective and durable treatment options for patients infected with R5 HIV-1. In addition, since viral transmission is mainly due to R5 HIV-1 [16], HGS004 could improve the potency of MVC in HIV-1 prevention strategies [17].
In flow cytometry studies, binding of HGS004 to CCR5 was higher in the presence than in the absence of MVC, suggesting that MVC induces conformational changes increasing exposure of the HGS004 epitope [18]. As such, HGS004 and MVC may act synergistically by reducing the number of unoccupied CCR5 molecules available to the virus [19, 20]. Consistent with this, HGS004 restored MVC sensitivity of MVC-resistant R5 HIV-1 [18], whose reduced affinity for MVC-bound CCR5 presumably requires the engagement of more coreceptor molecules for formation of a fusion pore [18, 21-23]. Thus, the combination of HGS004 and MVC may also reduce the risk of developing drug resistance.
Acknowledgments
We thank Mark Prichard (University of Alabama, Birmingham, Alabama) for providing the MacSynergy software, Thi Migone (Human Genome Sciences, Rockville, Maryland) for the HGS004 antibody, John Moore (Weill Medical College, Cornell University, New York) for the primary isolate HIV-1 CC1/85. We thank the NIH Aids Research and Reference Reagent Program (Germantown, Maryland) for Maraviroc and Raltegravir, and for HIV-1 isolates 92BR020 and 92UG031.
This project was funded by NIH NIAID grant AI084417.
REFERENCES
- 1.FDA notifications Maraviroc approved as a CCR5 co-receptor antagonist. AIDS Alert. 2007;22:103. [PubMed] [Google Scholar]
- 2.FDA Approves Expanded Use of Selzentry® for Appropriate Patients Starting HIV Antiretroviral Therapy for the First Time. 2009 Availalble at: http://www.viivhealthcare.com/media-room/press-releases/2009-11-20.aspx.
- 3.Jacobson JM, Lalezari JP, Thompson MA, Fichtenbaum CJ, Saag MS, Zingman BS, et al. Phase 2a Study of the CCR5 Monoclonal Antibody PRO 140 Administered Intravenously to HIV-infected Adults. Antimicrob Agents Chemother. 2010 doi: 10.1128/AAC.00086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson JM, Saag MS, Thompson MA, Fischl MA, Liporace R, Reichman RC, et al. Antiviral activity of single-dose PRO 140, a CCR5 monoclonal antibody, in HIV-infected adults. J Infect Dis. 2008;198:1345–1352. doi: 10.1086/592169. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson JM, Thompson MA, Lalezari JP, Saag MS, Zingman BS, D'Ambrosio P, et al. Anti-HIV-1 activity of weekly or biweekly treatment with subcutaneous PRO 140, a CCR5 monoclonal antibody. J Infect Dis. 2010;201:1481–1487. doi: 10.1086/652190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lalezari J, Yadavalli GK, Para M, Richmond G, Dejesus E, Brown SJ, et al. Safety, pharmacokinetics, and antiviral activity of HGS004, a novel fully human IgG4 monoclonal antibody against CCR5, in HIV-1-infected patients. J Infect Dis. 2008;197:721–727. doi: 10.1086/527327. [DOI] [PubMed] [Google Scholar]
- 7.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondru R, Zhang J, Ji C, Mirzadegan T, Rotstein D, Sankuratri S, Dioszegi M. Molecular interactions of CCR5 with major classes of small-molecule anti-HIV CCR5 antagonists. Mol Pharmacol. 2008;73:789–800. doi: 10.1124/mol.107.042101. [DOI] [PubMed] [Google Scholar]
- 9.Seibert C, Ying W, Gavrilov S, Tsamis F, Kuhmann SE, Palani A, et al. Interaction of small molecule inhibitors of HIV-1 entry with CCR5. Virology. 2006;349:41–54. doi: 10.1016/j.virol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Prichard MN, Shipman C., Jr. A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 1990;14:181–205. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- 11.Trkola A, Kuhmann SE, Strizki JM, Maxwell E, Ketas T, Morgan T, et al. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc Natl Acad Sci U S A. 2002;99:395–400. doi: 10.1073/pnas.012519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 13.Murga JD, Franti M, Pevear DC, Maddon PJ, Olson WC. Potent antiviral synergy between monoclonal antibody and small-molecule CCR5 inhibitors of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2006;50:3289–3296. doi: 10.1128/AAC.00699-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji C, Zhang J, Dioszegi M, Chiu S, Rao E, Derosier A, et al. CCR5 small-molecule antagonists and monoclonal antibodies exert potent synergistic antiviral effects by cobinding to the receptor. Mol Pharmacol. 2007;72:18–28. doi: 10.1124/mol.107.035055. [DOI] [PubMed] [Google Scholar]
- 15.Giguel F, Beebe L, Migone T, Kuritzkes DR. The anti CCR5 mAb004 inhibits HIV-1 replication synergistically in combination with other antiretroviral agents but does not select for resistance during in vitro passage [abstract 505]. Program and abstracts of the 13th Conference on Retroviruses and Opportunistic Infections (Denver) 2006 [Google Scholar]
- 16.Hughes A, Nelson M. HIV entry: new insights and implications for patient management. Curr Opin Infect Dis. 2009;22:35–42. doi: 10.1097/QCO.0b013e3283213093. [DOI] [PubMed] [Google Scholar]
- 17.Veazey RS, Ketas TJ, Dufour J, Moroney-Rasmussen T, Green LC, Klasse PJ, Moore JP. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis. 2010;202:739–744. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latinovic O, Reitz M, Le NM, Foulke JS, Fatkenheuer G, Lehmann C, et al. CCR5 antibodies HGS004 and HGS101 preferentially inhibit drug-bound CCR5 infection and restore drug sensitivity of Maraviroc-resistant HIV-1 in primary cells. Virology. 2011;411:32–40. doi: 10.1016/j.virol.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhmann SE, Platt EJ, Kozak SL, Kabat D. Cooperation of multiple CCR5 coreceptors is required for infections by human immunodeficiency virus type 1. J Virol. 2000;74:7005–7015. doi: 10.1128/jvi.74.15.7005-7015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sougrat R, Bartesaghi A, Lifson JD, Bennett AE, Bess JW, Zabransky DJ, Subramaniam S. Electron tomography of the contact between T cells and SIV/HIV-1: implications for viral entry. PLoS Pathog. 2007;3:e63. doi: 10.1371/journal.ppat.0030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heredia A, Latinovic O, Gallo RC, Melikyan GB, Reitz M, N L, Redfield R. Reduction of CCR5 with low-dose Rapamycin enhances the antiviral activity of Vicriviroc against both sensitive and drug-resistant HIV-1. Proc Natl Acad Sci U S A. 2008;105:20476–20481. doi: 10.1073/pnas.0810843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pugach P, Ray N, Klasse PJ, Ketas TJ, Michael E, Doms RW, et al. Inefficient entry of vicriviroc-resistant HIV-1 via the inhibitor-CCR5 complex at low cell surface CCR5 densities. Virology. 2009;387:296–302. doi: 10.1016/j.virol.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westby M, Smith-Burchnell C, Mori J, Lewis M, Mosley M, Stockdale M, et al. Reduced maximal inhibition in phenotypic susceptibility assays indicates that viral strains resistant to the CCR5 antagonist maraviroc utilize inhibitor-bound receptor for entry. J Virol. 2007;81:2359–2371. doi: 10.1128/JVI.02006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]