Abstract
Understanding the effects to human health resulting from exposure to low doses of ionizing radiation is a persisting challenge. No one questions the deleterious consequences for humans following exposure to high radiation doses; however, in the low dose range, the complex and to some extent unknown cellular responses raise important misgivings about the resulting protective or potentially detrimental effects. Bystander effects are involved in low dose exposures, being characterized by the appearance in unirradiated cells of a cellular damage associated with direct radiation exposure. The purpose of our work was to assess, by using clonogenic and micronuclei assays, the dose and time dependence of the bystander response after cells exposure to very low doses of α-particles and to evaluate its importance in the overall induced damage. The study includes an irradiated cells culture, a medium transfer culture with non-irradiated cells and a culture with irradiated cells after centrifugation. We observed a non-negligible contribution of the bystander effects in the overall cellular damage. Low-dose hyper-sensitivity was observed for medium transfer and irradiated cells after centrifugation cultures. Delayed and earlier cellular damage were similar in almost all experiments, suggesting an effectiveness of irradiated medium to induce a bystander response soon after irradiation.
Keywords: bystander effects, very low doses, MN assay, early and delayed cellular damage
1. INTRODUCTION
It is rather consensual in the scientific community the need to reconsider the deterministic ‘hit-effect” model used to estimate the risks attributable from high to low doses of radiation. One of the most successful approaches to model these effects is the linear no-threshold (LNT) model in which risk assessment (<0.2 Sv) is predicted from the epidemiologic studies of accidental exposures to radiation (Tubiana et al. 2006; Brenner and Sachs 2010). However, these exposures values, in the range of 0.2 to 2.5 Sv, are much higher when compared with the worldwide annual exposures to natural radiation sources estimated in the range 1 to 10 mSv/year, with 2.4 mSv being the present estimate of the central dose (UNSCEAR 2000). One of the major concerns about the human exposure to natural radiation sources is associated to the radon gas. Most 222Rn gas inhaled is immediately exhaled, however if decay occurs the particles would be deposited onto bronchial epithelial cells. According to the National Research Council (1999) the high density of ionizations than can occur along the path length of α-particles could deliver localized energy of about 10 – 50 cGy.
The validity of the aforementioned models has been challenged (Jenkins et al. 2010; Little 2010) due to i) the observation of mechanisms for safeguarding the genome (essentially involving DNA repair), ii) the elimination of cells whose DNA has been damaged via cellular death and iii) the evidence of radiation-similar effects in bystander cells that have not themselves been exposed to radiation. Additionally to these, several others factors, such as genomic instability, adaptive response, low-dose hyper-radiosensitivity, delayed reproductive death and the induction of genes by radiation effects, have challenged what we know about the radiation induced cellular damage (Mothersill and Seymour 1998a; Wolff 1998; Joiner et al. 2001; Amundson et al. 2001).
Bystander effects can be observed by medium transfer (radiation induced genomic instability) (Iyer et al. 2000; Mothersill et al. 2001; Grifalconi et al. 2007) or by intercellular communication of irradiated and non-irradiated cells (radiation induced bystander effects) (Mothersill and Seymour 1998b; Azzam et al. 2001; Azzam et al. 2003). The major adverse consequences observed are attributed to the oxidative stress effect induced by reactive oxygen species (ROS) (Azzam et al. 2003). Additionally, some studies showed that irradiated cells may release into the medium soluble factors which are toxic to non-irradiated cells (Hu et al. 2006). Differences in DNA damage quantification, among various cell types, are endorsed to the different metabolic repair mechanisms, suggesting a fundamental role of the DNA in inducing bystander effects (Nagasawa et al. 2003). Grifalconi et al. (2007) demonstrated that TK6 cells, when exposed to 0.5 – 1 Gy of γ-rays, release into the cell culture medium soluble molecules which maintain cell mortality high in bystander cells for at least 48h. Other study performed by Bowler et al. (2006) showed the appearance of delayed aberrations (genomic instability) induced by medium transfer technique in bystander culture, being the irradiation doses from 0.1 to 2 Gy.
In our study, using the same methodology of the Bowler et al. (2006), we investigated the time and dose dependence of targeted and untargeted effects in the region of very low doses (<100 mGy). Our study includes three distinct cell culture conditions: a culture of irradiated cells, a medium transfer culture with non-irradiated cells and a culture with irradiated cells after centrifugation.
Lung epithelial cells, A549 cell line, were chosen as the epithelial cells which respond directly to the toxic agents that are inhaled in the air (Fujii et al. 2001). Some of the deleterious effects induced in these cells include changes in cell morphology (Bayram et al. 1998), release of inflammatory cytokines (Ohtoshi et al. 1998) and alterations in cellular functions (Stringer and Kobzik 1998). Since, α-particles were the radiation type used in this work; the epithelial cells are of extreme relevance to evaluate the cellular damage and survival induced at low doses, namely due to natural sources exposures.
Through the cytokinesis blocked micronuclei assay, we provide evidence that human A549 cells display a dependence of bystander effects with dose values, in the region of very low doses. Moreover, in this region, the induced cellular damage could not be negligible because it is similar to that obtained in cells directly irradiated. This trend persists in time, since after 6–7 population doublings the bystander effect remains in the culture leading to a cellular damage similar to that of directly irradiated cells. It has been reported that post-irradiation instability is not universally expressed in mammalian cells in vitro or in vivo (Kadhim et al. 1995, Dugan and Bedford 2003 and Whitehouse and Tawn 2001). Our study reveals that A549 cells express radiation-induced genomic instability after low doses of α-radiation, both in direct and bystander cells.
A set of in vitro studies have revealed the existence of hyper-radiosensitivity (HRS) to doses below 0.3 Gy in several mammalian normal and tumour cell lines. Indeed, Mothersill et al (2002) studied the relationship between the bystander effect and the low-dose HRS, concluding that a considerable variation in the expression of both phenomena suggests that cell lines with a large bystander effect do not show HRS. On the other hand, Nuta and Darroudi (2008) concluded that the HRS might be causally related to bystander factors in the low-dose region. Our study revealed a low-dose HRS effect at 10 mGy for bystander cells. Although our results suggest that the bystander signal has a prominent effect in the overall cellular damage induced, we cannot conclude about the influence of this in the HRS phenomenon. Analyzing the trend of the dose-response curves obtained our results provide some evidence, with no statistical relevance, for a higher sensitive effect of cellular response at doses lower than 10 mGy, both in irradiated and bystander cells.
Summarizing, our results emphasize that the risks attributable to very low dose radiations encompass a complex cellular response and cannot simply be extrapolated from higher doses. Hu et al. (2006) showed that the bystander-signal derived from irradiated cells could be transferred to anywhere in the culture dish, so, the observed bystander effects described in our work show that a cellular lesion could be induced in the progeny of irradiated cells. These results raise important questions about potentially detrimental effects associated with low dose exposures, which are not included in a simply linear extrapolation.
2. MATERIALS AND METHODS
2.1. Cell line and reagents
The cell line and main reagents used in this study included human lung adenocarcinoma cell line A549 (kindly provided by University of Porto, Portugal), Dulbecco’s Modified Eagle’s medium (DMEM), foetal bovine serum (FBS), penicillin-streptomycin solution, cytochalasin B, trypsin and Giemsa dye (Sigma-Aldrich, USA), Thiazolyl Blue Tetrazolium Bromide (Alfa Aescar, Germany), and other reagents such as methanol and acetic acid (Merck, Germany).
2.2. Cell culture and 210Po irradiation
A549 cells were cultured at 37°C with 5% CO2 in DMEM medium containing 10% FBS and 1% penicillin-streptomycin solution. Log-phase cells were seeded onto 3.5 cm culture dishes with 6.3 μm of Mylar base 24 hours before irradiation. Cells at exponential growth were exposed to 100, 50, 10 and 5 mGy using a monoenergetic 210Po source developed by Szabó et al. (2002) (see Figure 1), characterized by emitting 5.297 MeV α-particles with an average LET value of 156 keV/μm (Belchior et al. 2010). The cells were then returned to the incubator for harvesting at the appropriate later time, 2 and 7–8 population doublings after irradiation for early and delayed studies, respectively. The cells cultured in 6.3 μm Mylar are positioned at the exposure windows paced a few millimeters above α-particles source, as shown in Figure 1.
FIGURE 1.
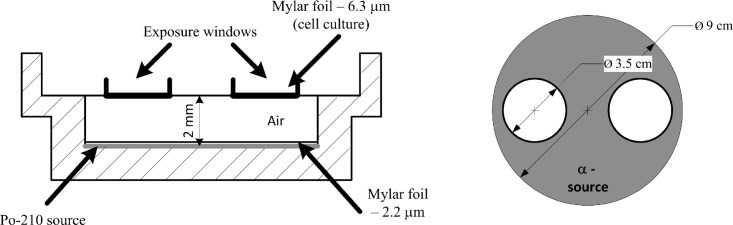
Scheme of the irradiation apparatus, cross-section (left) and top view (right) of the irradiation device. Briefly, the α-particles cross a 2.2 μm Mylar membrane before reaching the 2mm air layer, and then reach the cell monolayer after cross the 6.3 μm of Mylar where cells are cultured.
2.3. Medium transfer study
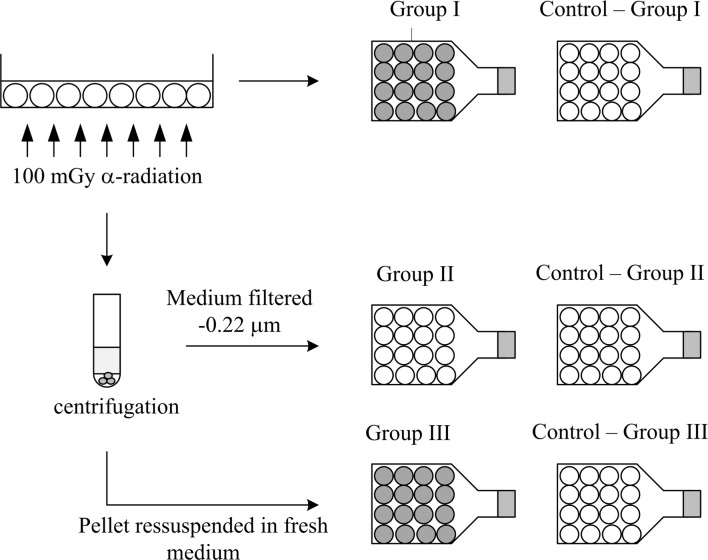
Immediately after the irradiation, cells were recovered from the Mylar dish using 3 ml of total fresh DMEM medium. The pooled cells were counted and divided into separate groups each containing approximately the same number of cells; 1 × 105 cells/flask in 5 ml of supplemented DMEM medium. The different groups used in this study are illustrated in Figure 2 and can be described as follows;
GROUP I – Irradiated cells, collected in supplemented fresh medium, are re-cultured with an appropriate cell concentration for cytogenetic studies at 2 and 6 days post-irradiation. The re-culture implies that a small portion of irradiated medium, in contact with irradiated cells, coming from the Mylar dish remains in the culture.
GROUP II – On replicate Mylar dishes, cells were irradiated and collected in fresh medium as in Group I. These cells were collected by centrifugation at 1200 rpm for 5 minutes; the medium was filtered through a 2. 2 μm membrane filter (Millipore). The filtered medium was transferred to non-irradiated cells and cytogenetic studies were performed 2 and 6 days after irradiation. During this article this medium will be denominated as irradiated medium.
GROUP III – The irradiated cells collected from Group II were cultured in an appropriate concentration for cytogenetic studies 2 and 6 days post-irradiation. The main difference between this and the group I is that in this case the radiation induced bystander effect is minimized due to the re-suspension in supplemented fresh medium after centrifugation.
FIGURE 2.
Medium transfer study for 100 mGy of exposure; the same methodology was used for the others values of dose. In group I irradiated cells were cultured with fresh medium after exposure to the aforementioned radiation doses. In group II, non-irradiated cells received irradiated medium. Finally, group III corresponds to irradiated cells cultured after centrifugation with supplemented fresh medium. White color refers to non-irradiated cells and dark grey refers to irradiated ones.
In all groups, in order to maintain a non-confluent monolayer the referred appropriate concentration of cells denotes to approximately 1000 cells/culture for studies after 2 days of irradiation, and approximately 200 for delayed studies. In Figure 2, the label control, refers to non-irradiated cells maintained in the same conditions as the irradiated ones.
2.4. The clonogenic assay
For both time points’ experiments, the clonogenic assay (Franken et al. 2006) was used to assess the cell survival in all described groups. To evaluate the survival fraction after 2 and 6 days of irradiation, 200 or 400 cells were plated, depending of the dose value. Briefly, a higher cell density was used for higher doses and the opposite is applicable for control and lower doses. Surviving fractions were expressed in terms of platting efficiency and averaged over three independent experiments for each treatment group.
2.5. The cytokinesis blocked micronuclei assay
In this study, the number of micronuclei (MN) was assayed by the cytokinesis blocked micronuclei assay. Briefly, 2 μg/ml cytochalasin-B was added to the medium 20h after irradiation to arrest cytokinesis and cells were cultured for more 24 hours. After, cells were harvested and centrifuged (800 rpm, 10 min). After being re-suspended two times in a wash solution (RPMI medium and foetal bovine serum solution) and centrifuged (800 rpm, 8 min) cells were subjected to a hypotonic treatment (RPMI medium, water and foetal bovine serum solution). The cells were fixed during 20 minutes in cold methanol: acetic acid (3:1). Slides were prepared and stained in a solution of 4% Giemsa dye in phosphate buffer (pH 6.8) for 8 min. The slides were coded and scored under a light microscopy at 400× magnification. MNs were identified according to the criteria previously published by Fenech (2000). The frequency of binu-cleated (BN) cells containing one or more MN was also scored.
2.6. Statistical analysis
Analysis of variance was performed using the ANOVA method (Origin 7.5 for windows statistical package). To analyze the significance of the results at 2 and 6 days post irradiation the t-student test was applied. The MN distributions were analyzed by Papworth’s u test (Edwards et al. 1979). The test used the relative variance (σ2/y) and the dispersion index (u) of the mean number of observed MN per BN cell (y) in order to judge whether they are significantly different. The variance was calculated by equation 1 and dispersion index by equation 2.
| (1) |
| (2) |
where, N is the total number of cells scored, N0, N1, N2…Ni is the number of cells carrying 1, 2 … i micronuclei, respectively. Positive or negative values of u refer an over or under-dispersion, respectively. If the value of u is greater than ± 1.96, the dispersion is significant at 95% confidence level (Edwards et al. 1979).
3. RESULTS
3.1. Radiation and bystander – induced cellular damage
Early cellular damage was quantified 2 days post-irradiation and genomic instability was evaluated by cytogenetic analysis 6 days after irradiation (referred to as “early cellular damage” and “delayed cellular damage” in the sequence, respectively). All groups were compared to their own controls.
3.1.1. Survival fraction
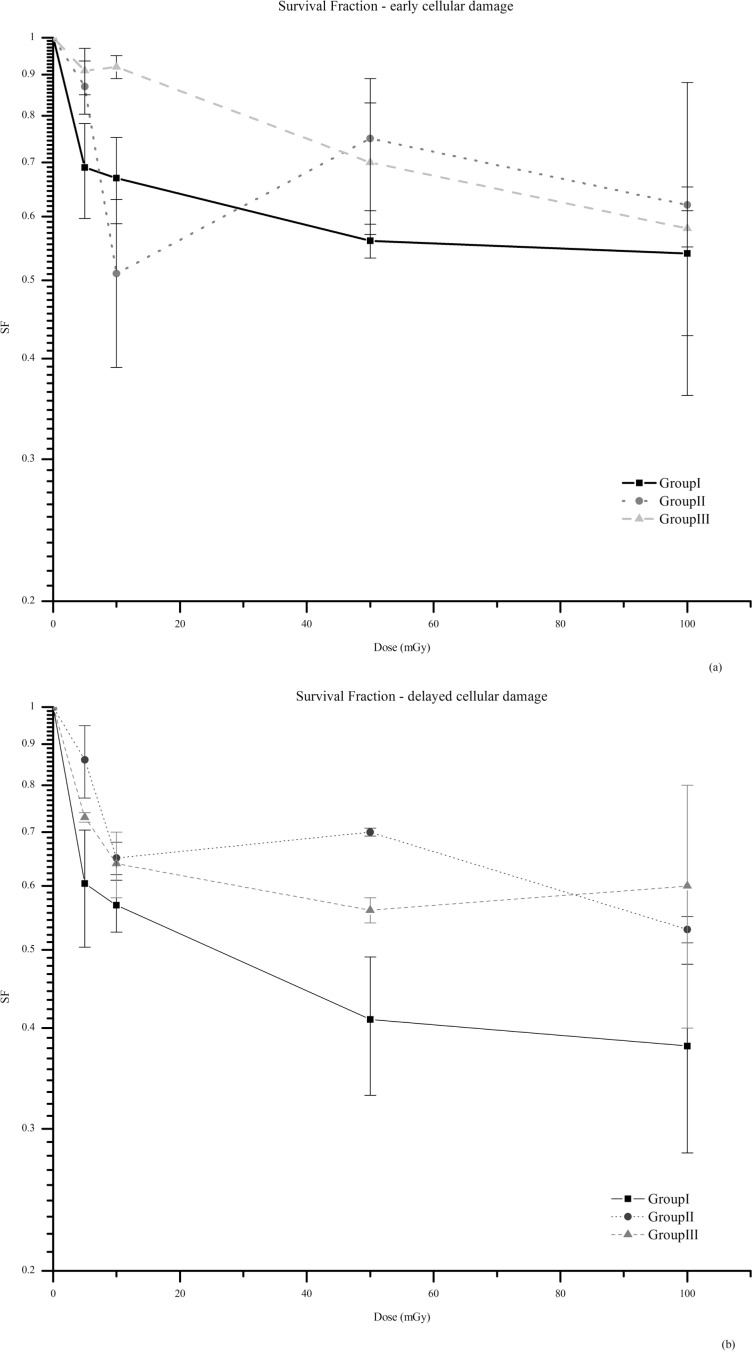
The survival fraction (SF), performed by clonogenic assay, is presented in Figure 3. At both time points, early and delayed cellular damage (2 and 6 days after irradiation, respectively) analyzed, the survival fraction was reduced in irradiated and bystander cells at all irradiation doses compared with its own controls. The exception is for group III, irradiated cells expanded with fresh media, at day 2 the survival fraction is very similar to the matched control (p=0.84) at 10 mGy. For each Group, when both time points were compared by dose values, the difference of SF was not significant, however a higher survival fraction is observed at day 2 post-irradiation.
FIGURE 3.
Survival fraction (SF), obtained by clonogenic assay, at day 2 (a) and at day 6 (b). The results represent the mean of three independent experiments ± standard error of the mean (SEM). At both time points and for all groups, survival is significantly reduced compared to its own controls. The only exception is observed for Group III at day 2, at 10 mGy, being the survival fraction similar to unirradiated control (p=0.84). In the media transfer experiment, group II a lower survival fraction is observed at 10 mGy, which corroborates with the HRS observed, by means of MN assay, at this dose value. Note: The lines are purely eye guided.
At day 2, when irradiated and bystander cells were compared for each dose value, survival was lower in irradiated cells, group I, but was significant only at 5 mGy (p<0.2) (Figure 3a). At 10 mGy the survival fraction for group II is significantly lower than group III (p<0.05) and similar to group I (p=0.34). This result is in agreement with the HRS, described below, at this dose value, by means of MN assay.
At day 6, comparing the irradiated cells with bystander ones, it is also noticeable that cell survival is lower at group I, with a higher significance between groups for each dose value. For 100 mGy, a moderate difference was observed (p=0.28), but for 5, 10 and 50 mGy the difference was statistically relevant (p<0.2 for 5 and 10 mGy and p<0.05 at 50 mGy). The comparison of the result obtained for 50 mGy with 10 mGy (p=0.27) and 100 mGy (p<0.05) provides some evidence for a HRS phenomena. However, the cellular damage, quantified by the MN assay, doesn t corroborates this finding.
3.1.2. Micronuclei frequency – Early cellular damage
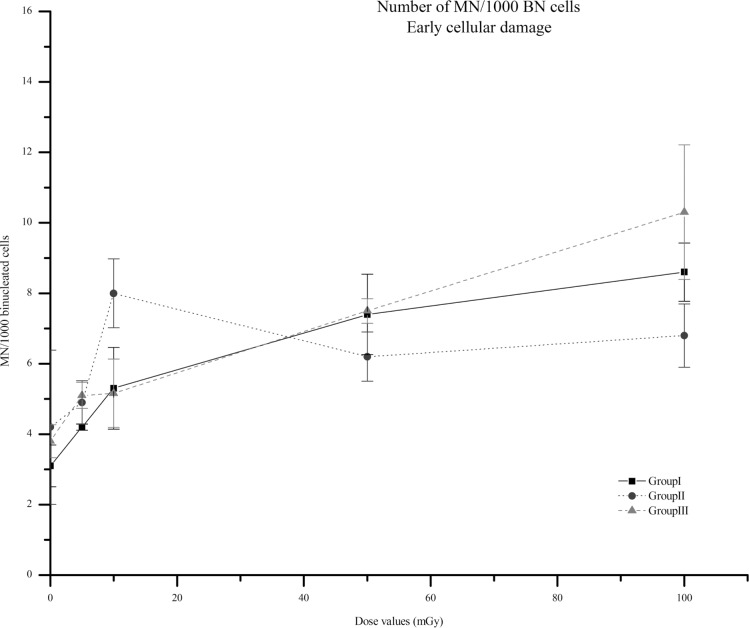
Figure 4 shows the results obtained, at day 2, for the aforementioned three experimental groups (Group I to III) and different dose values.
FIGURE 4.
Number of MN per 1000 BN cells for each value of dose, i.e. 5, 10 50 and 100 mGy at day 2 after irradiation for groups I to III. In group I irradiated cells were cultured with fresh medium after exposure to the aforementioned radiation doses. In group II, non-irradiated cells received irradiated medium. Finally, group III corresponds to irradiated cells cultured after centrifugation with supplemented fresh medium. The non-irradiated cell cultures are marked as 0 mGy. Data represent means of 3 independent experiments, ± SEM. The fraction of cells with MN was significantly increased when compared to its own controls (p<0.05). In group II, at lower doses < 10 mGy, the inducible cellular lesion appears to be higher and then reaches a plateau. In groups I and III, the cellular lesion increases with doses, but a higher increase is observable for lower doses. Note: the lines are purely eye guided.
Analyzing Figure 4, one can observe an increase, with dose values, in the number of MN per 1000 BN cells for all groups, comparing with its own control (p<0.05). However, none of the pair wise dose comparisons between groups I to III were significant (p=0.24, p=0.5, p=0.87, p=0.35, for 100, 50, 10 and 5 mGy, respectively). The trend of the dose response curve is to increase the cellular damage with dose values, but, for group II this dose dependence is more moderate, namely in the range of 50 up to 100 mGy. Our results provide some evidence for a plateau of bystander effects after 50 mGy. Also, in group II a HRS is observed at 10 mGy, since at this dose value the number of MN significantly increase when compared with 5 (p<0.1) and 50 mGy (p=0.21). This phenomenon was also observed by clonogenic assay with the evidence for a lower survival fraction at this dose value.
3.1.3. Micronuclei frequency – delayed cellular damage
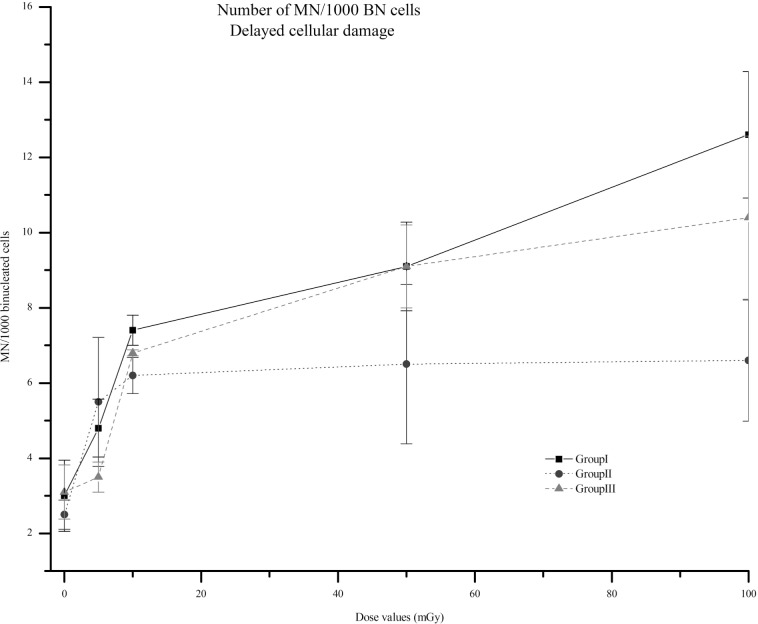
Radiation significantly increased the number of MN, at day 6, in all groups compared to their non-irradiated controls (Figure 5), with the exception in group III for 5 mGy were the increasing of MN was almost similar (p=0.68).
FIGURE 5.
Number of MN, 6 days after irradiation for groups I to III, per 1000 BN cells for each value of dose, i.e., 5, 10, 50 and 100, mGy. In group I irradiated cells were cultured with fresh medium after exposure to the aforementioned radiation doses. In group II, non-irradiated cells received irradiated medium. Finally, group III corresponds to irradiated cells cultured after centrifugation with supplemented fresh medium. The non-irradiated cell cultures are marked as 0 mGy. Data represent means of 3 independent experiments, ± SEM. In all groups, radiation significantly increased the number of MN when compared with its own controls (p<0.05), with exception in group III for 5 mGy where the increasing of MN was almost the same (p=0.68). Similarly to earlier effects, the trend to reach a plateau after 10 mGy for group II is observed. Note: the lines are purely eye guided.
At this time point, we also observe an increase in the number of MN compared to the matched controls (see Figure 5) (p<0.05). Also, comparing the results obtained for each group, by dose value, a non-significant difference was found. However, at a delayed time, the trend of the dose response curve is different, for each group, when comparing with the cellular damage induced after 2 days. Between 10 and 50 mGy, our results give some evidence for a plateau, more evident for bystander, but also notable for irradiated cells. After this dose value, the trend of the cellular damage for irradiated cells is to slightly increase, but for bystander the plateau remains.
3.2. Micronuclei distribution
Table 1 and 2 shows the MN yield and their distribution, 2 and 6 days after irradiation, respectively, in all groups. There is clear evidence that BN cells with only one MN are the most frequent. For a higher radiation exposure, in both day 2 and 6 post irradiation, the occurrence of 2, 3 or more than 3 MN per BN cell are more relevant than for lower dose values.
TABLE 1.
The distribution and yield of MN in the aforementioned groups and dose values, 2 days after irradiation.
| MN distribution
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mGy) | Cells scored | Total MN | 0MN | 1MN | 2MN | 3MN | 4MN | MN yield (y) ± SEM | σ2/y | u |
| Group I | ||||||||||
| 0 | 8000 | 296 | 7751 | 210 | 35 | 0 | 4 | 0.037±0.004 | 1.362 | 22.88 |
| 5 | 3000 | 128 | 2875 | 122 | 3 | 0 | 0 | 0.044±0.001 | 0.974 | −1.00 |
| 10 | 3000 | 167 | 2840 | 153 | 7 | 0 | 0 | 0.056±0.016 | 1.029 | 1.10 |
| 50 | 3000 | 255 | 2777 | 196 | 23 | 3 | 1 | 0.085±0.020 | 1.213 | 8.26 |
| 100 | 3000 | 295 | 2743 | 226 | 26 | 3 | 2 | 0.098±0.011 | 1.221 | 8.59 |
| Group II | ||||||||||
| 0 | 7000 | 247 | 6783 | 189 | 27 | 0 | 1 | 0.035±0.006 | 1.232 | 13.73 |
| 5 | 3000 | 202 | 2819 | 160 | 21 | 0 | 0 | 0.067±0.014 | 1.141 | 5.46 |
| 10 | 3000 | 276 | 2760 | 204 | 36 | 0 | 0 | 0.092±0.012 | 1.169 | 6.55 |
| 50 | 3000 | 214 | 2814 | 162 | 22 | 3 | 1 | 0.071±0.009 | 1.246 | 9.56 |
| 100 | 3000 | 239 | 2796 | 176 | 23 | 3 | 2 | 0.079±0.016 | 1.289 | 11.18 |
| Group III | ||||||||||
| 0 | 6000 | 255 | 5775 | 187 | 38 | 0 | 0 | 0.043±0.005 | 1.284 | 15.58 |
| 5 | 3000 | 171 | 2847 | 137 | 14 | 2 | 0 | 0.057±0.003 | 1.177 | 6.86 |
| 10 | 3000 | 170 | 2845 | 140 | 15 | 0 | 0 | 0.057±0.007 | 1.120 | 4.65 |
| 50 | 3000 | 255 | 2775 | 198 | 25 | 1 | 1 | 0.085±0.006 | 1.182 | 7.05 |
| 100 | 3000 | 382 | 2691 | 258 | 38 | 4 | 9 | 0.127±0.023 | 1.418 | 16.17 |
TABLE 2.
The distribution and yield of MN in the aforementioned groups and dose values, 6 days after irradiation.
| MN distribution
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mGy) | Cells scored | Total MN | 0MN | 1MN | 2MN | 3MN | 4MN | MN yield (y) ± SEM | σ2/y | u |
| Group I | ||||||||||
| 0 | 7000 | 233 | 6791 | 191 | 15 | 0 | 3 | 0.033±0.006 | 1.250 | 14.79 |
| 5 | 3000 | 148 | 2860 | 132 | 8 | 0 | 0 | 0.049±0.005 | 1.059 | 2.29 |
| 10 | 3000 | 231 | 2779 | 213 | 7 | 0 | 1 | 0.077±0.003 | 1.036 | 1.39 |
| 50 | 3000 | 279 | 2739 | 247 | 12 | 0 | 2 | 0.093±0.013 | 1.079 | 3.07 |
| 100 | 3000 | 487 | 2622 | 311 | 41 | 10 | 16 | 0.162±0.026 | 1.524 | 20.29 |
| Group II | ||||||||||
| 0 | 7000 | 166 | 6810 | 138 | 14 | 0 | 0 | 0.029±0.003 | 0.919 | −4.73 |
| 5 | 3000 | 175 | 2839 | 149 | 11 | 0 | 1 | 0.058±0.012 | 1.136 | 5.28 |
| 10 | 3000 | 199 | 2816 | 169 | 15 | 0 | 0 | 0.066±0.005 | 1.085 | 3.28 |
| 50 | 3000 | 226 | 2800 | 174 | 25 | 1 | 0 | 0.076±0.016 | 1.171 | 6.64 |
| 100 | 3000 | 189 | 2826 | 164 | 7 | 1 | 2 | 0.063±0.011 | 1.170 | 6.59 |
| Group III | ||||||||||
| 0 | 6000 | 184 | 5816 | 166 | 18 | 0 | 0 | 0.031±0.004 | 1.257 | 14.08 |
| 5 | 3000 | 116 | 2891 | 102 | 7 | 0 | 0 | 0.039±0.002 | 1.082 | 3.19 |
| 10 | 3000 | 208 | 2797 | 198 | 1 | 0 | 0 | 0.069±0.002 | 0.979 | −0.81 |
| 50 | 3000 | 296 | 2735 | 241 | 20 | 1 | 3 | 0.098±0.013 | 1.178 | 6.92 |
| 100 | 3000 | 375 | 2688 | 266 | 33 | 9 | 4 | 0.125±0.028 | 1.323 | 12.52 |
The yield of MN, MN yield (y), was calculated as the ratio of the total number of MN to the scored BN cells. As a result of cellular damage, MN was produced in irradiated cells and its yield increased in a dose-dependent manner in almost all doses values and groups.
At 10 mGy a HRS effect is observed in group II (see table 2), i.e., there is a low dose sensitive effect of MN induction. However, this trend inverts after 50 mGy, being the MN yield for group II lower than for group I and III. Also, at low doses, unexpectedly group I showed a lower MN yield when compared to group III (p<0.05). This could indicate, that the bystander contribution to the overall cellular lesion, at doses lower than 10 mGy, could be namely due to intercellular gap-junction contact. Since, due to centrifugation released in group III any bystander signal, presented in the culture medium, is removed from the culture.
The results obtained at 6 days post-irradiation show a similar trend of response when compared to the earlier effects of radiation. At group II, the low dose sensitive effect is no more observed at 10 mGy although slightly occurs at 50 mGy. This result is not in agreement with the higher survival fraction observed at this dose value by clonogenic assay.
4. DISCUSSION
The appraisal of how the risks associated to a low-dose exposure could be exactly determined remains unclear. Some authors claim that a revision of the implemented models, such as those based on the LNT hypothesis is needed, namely in the dose range up to 100 mGy. However, in this dose range, there are no epidemiologic data and in vitro studies include mechanisms such as, apoptosis, bystander effects, genomic instability, among others, which sometimes reveal a different outcome according to cell lines. So far, it is not clear how the assessment of health risks associated to low-dose radiation exposure could be correctly estimated and evaluated.
In this study, we investigated if bystander effects are induced in A549 cells after irradiation to very low doses of α-particle, and its dependence with dose and time. Also, the trend of cellular response of A549 cells exposed directly to α-particles irradiation was studied. Previous studies using medium transferred have shown that medium from irradiated cells can induce bystander effects in non-irradiated cells at low doses and in a time-dependent manner (Mothersill and Seymour 1998b; Mothersill et al. 2001). However, these studies included only dose dependent effects few minutes after irradiation and with doses higher than 100 mGy. We have extended our assessment to a time interval up to six days, in order to understand the earlier and delayed induced cellular damage, not only in bystander but also for direct effects, and for doses lower than 100 mGy.
We assessed the cellular damage induced and survival in lung epithelial cell line (A549) at very low doses of α-particles, in order to understand the trend of the dose-response curve not only for irradiated but also for bystander cells.
The obtained dose-response curves for both early and delayed times pinpoint, for each value of the dose and for all groups, an increase of cellular damage, compared with the matched controls. Regarding the trend of the curves it should be highlighted the non-linear pattern of the curves at all groups. It seems that up to 10 mGy the cells are more sensitive to radiation being the increase of the MN more evident.
The studies of Shao et al. (2006) suggested that the bystander effect is not dose dependent, but our study provides evidence for a dose-dependent behavior at the region of very low doses, up to 10 mGy. Moreover, the results obtained for all groups suggest that at very low doses the bystander effects are not be negligible since they result in a cellular damage similar to those obtain by direct irradiation. Ojima et al. (2008) concluded that DNA double strand breaks induced by very low X-ray doses (1.2 to 200 mGy) are largely due to bystander effects. The study included the inhibition of cell-to-cell contact in order to test the supralinear dose-response relationship obtained without treatment. In our study, comparing group I and III, Figure 3 and 5, it can be noticeable that irradiated cells at group I show a higher induced lesion than cells in group III. This corroborates the assumption that bystander effects have an important contribution to the overall lesion induced by radiation.
Data obtained in other cell lines show that the induction of cellular damage in bystander cells persists with time, probably as a consequence of the formation of bystander factors that themselves generate ROS, leading to a self sustaining system responsible for delayed effects (Yang et al. 2005). Our results are in agreement with these evidences showing a persistent bystander signal at a delayed time. As in earlier induced cellular damage, one important remark of this study is the similar evidence for bystander effects when compared to directly irradiated cells. As a consequence of an environmental exposure to α-radiation, from radon for example, the deposition of such particles onto bronchial epithelial cells will unavoidably induce a cellular damage. This study suggests that the quantification of the possible cellular damage induced should be quantified considering its time dependence.
Some studies showed that, for doses below 0.5 Gy, the determinant factor for the observed HRS in bystander effects is not the DNA damage (Mothersill and Seymour 2000 and Seymour and Mothersill 2000). Moreover, Wykes et al. (2006) found that the prevalence of low-dose hypersensitivity is not related to DNA DSBs. Our results endorse these outcomes since the difference in the magnitude of cell survival between groups (see Figure 3), suggests that the cell irradiation itself cannot be the unique mechanism to induce cell damage/killing. Comparing group I and III (Figure 3) it is noticeable that group I shows a lower cell survival fraction which could indicate that the clastogenic factors, including free radicals, release immediately after and a few minutes after irradiation could be involved in the magnitude of cellular response. Moreover, in group II, the survival fraction at each dose value decreased when compared to the matched controls. At day 2, it is observed an HRS effect at 10 mGy for group II, which is in accordance with the induced cellular damage. These results put in evidence the importance of well quantifying the low dose exposure. It is notable, Figure 3a), that the survival fraction at 10 mGy is lower than the one observed for irradiated cells. This pattern could suggest that also the irradiated cells are more sensitive at this dose value producing more detrimental “bystander signals” that would impart deleterious effects in non-irradiated cells. In fact, the magnitude of survival fraction reveal that group III (without any bystander signal produced a few minutes post-irradiation) discloses a slightly decrease of cell survival when groups I and III are compared. Lorimore et al. (1998) found no increase in cell killing that could be attributed to bystander cells. While this pattern is similar to our results, mainly after 50 mGy, where exists a plateau, we observed a prominent decrease of cell survival namely up to 10 mGy, which suggested that cell killing is affected by bystander signals.
It can be stated that the response of lung epithelial cells exposed to low doses of α-particles exhibit dynamic effects and the interaction of different cellular processes, such as DNA damage, cell killing and HRS.
The results here reported emphasize that the risks attributable to the exposure to low dose radiations encompass a complex variable cellular response and cannot simply be extrapolated from higher doses. Moreover, they raise important questions about the potentially detrimental effects associated with very low doses exposures.
Acknowledgments
This work was developed in the radiobiology laboratories of Radiological Protection and Safety Unit (UPSR) at Instituto Superior Técnico/Instituto Tecnológico e Nuclear (IST/ITN). The work was partially supported by FCT/MCTES grant No. SFRH/BD/42172/2007 and by ITN. A special thanks those who have contributed directly to the successful outcome of this research, namely; Dr. Imre Balashazy (KFKI, Hungary) who has kindly sent us the device for cell irradiation and the 210Po sources used in this work. Prof. Sebastião Rodrigues (FCM-UNL) who helped in the development and validation of the cytogenetic techniques applied in this work. Prof. Luis Peralta (FC-UL) who helped in the measurements of alpha particles energy spectrum performed in the Faculty of Sciences, University of Lisbon.
REFERENCES
- Amundson SA, Bittner M, Meltzer P, Trent J, Fornace AJ. Induction of gene expression as a monitor of exposure to ionizing radiation. Radiat Res. 2001;133:41–51. doi: 10.1667/0033-7587(2001)156[0657:iogeaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Azzam EI, Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam EI, Toledo SM, Little JB. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene. 2003;22:7050–7057. doi: 10.1038/sj.onc.1206961. [DOI] [PubMed] [Google Scholar]
- Bayram H, Devalia JL, Sapsford RJ, Ohtoshi T, Miyabara Y, Sagai M, Davies RJ. The effect of diesel exhaust particles on cell function and release of inflammatory madiators from human bronchial epithelial cells in vitro. Am. J. Respir. Cell. Mol. Biol. 1998;18:441–448. doi: 10.1165/ajrcmb.18.3.2882. [DOI] [PubMed] [Google Scholar]
- Belchior A, Peralta L, Almeida P, Vaz P. Calibration of an alpha particle irradiator for in vitro cells irradiation. International Journal of Low Radiation. 2010;7(6):500–510. [Google Scholar]
- Bowler D, Moore S, Macdonald D, Smyth S, Clapman P. Bystander-mediated genomic instability after high LET radiation in murine primary haemopoietic stem cells. Mutation Research. 2006;597:50–61. doi: 10.1016/j.mrfmmm.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Brenner D, Sachs R. Estimating radiation-induced cancer risks at very low doses: rationale for using a linear no-threshold approach. Radiat. Environ Biophys. 2010;44:253–256. doi: 10.1007/s00411-006-0029-4. [DOI] [PubMed] [Google Scholar]
- Dugan LC, Bedford JS. Are chromosomal instabilities induced by exposure of cultured normal human cells to low-or high-LET radiation? Radiat. Res. 2003;159:301–311. doi: 10.1667/0033-7587(2003)159[0301:aciibe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Edwards AA, Lloyd DC, Purrott RJ. Radiation induced chromossome aberrations and the poisson distribution. Rad. And Environm. Biophys. 1979;16:89–100. doi: 10.1007/BF01323216. [DOI] [PubMed] [Google Scholar]
- Fenech M. The in vitro micronucleus technique. Mutat. Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Franken N, Rodermond H, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nature Protocols Vol. 1. 2006;5:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- Fujii T, Hayashi S, Hogg JC, Vincent R, Vincent SF. Particulate matter induces cytokine expression in human bronchial epithelial cells. Am. J. Respir. Cell. Mol. Biol. 2001;25:265–271. doi: 10.1165/ajrcmb.25.3.4445. [DOI] [PubMed] [Google Scholar]
- Grifalconi M, Celloti L, Mognato M. Bystander response in human lymphoblastoid TK6 cells. Mutat Res. 2007;625:102–111. doi: 10.1016/j.mrfmmm.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Hu B, Wu L, Han W, Zhang L, Chen S. The time and spatial effects of bystander response in mammalian cells induced by low dose radiation. Carcinogenesis. 2006;27:245–251. doi: 10.1093/carcin/bgi224. [DOI] [PubMed] [Google Scholar]
- Iyer R, Lehnert BE, Svensson R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000;60:1290–1298. [PubMed] [Google Scholar]
- Jenkins G, Zair Z, Johnson G, Doak S. Genotoxic thresholds, DNA repair, and susceptibility in human populations. Toxicology. 2010;10:305–310. doi: 10.1016/j.tox.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Joiner MC, Marples B, Lambin P, Short SC, Turesson I. Low-dose hypersensivity: current status and possible mechanisms. Int J Radiat Oncol Biol Phys. 2001;49:379–389. doi: 10.1016/s0360-3016(00)01471-1. [DOI] [PubMed] [Google Scholar]
- Kadhim MA, Lorimore SA, Townsend KM, Goodhead DT, Buckle VJ, Wright EG. Radiation-induced genomic instability: delayed cytogenetic aberrations and apoptosis in primary human bone narrow cells. Int. J. Radiat. Biol. 1995;76:31–42. doi: 10.1080/09553009514550341. [DOI] [PubMed] [Google Scholar]
- Little M. Do non-targeted effects increase or decrease low dose risk in relation to the linear-non-threshold (LNT) model? Mutation Research. 2010;687:17–27. doi: 10.1016/j.mrfmmm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimore SA, Kadhim MA, Pocock DA, Papworth D, Stevens DL, Goodhead DT, Wright EG. Chromossomal instability in the descendents of unirradiated surviving cells after alpha-particle irradiation. Proc. Natl. Acad. Sci. USA. 1998;95:5730–5733. doi: 10.1073/pnas.95.10.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Mechanisms and implications of genomic instability and other delayed effects of ionizing radiation exposure. Mutagenesis. 1998a;13:421–426. doi: 10.1093/mutage/13.5.421. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Cell-cell contact during gamma irradiation is not required to induce a bystander effect in normal human kerotinocytes: evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res. 1998b;149:256–262. [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Genomic instability, bystander effects and radiation risks: implications for development of protection strategies for man and the environment. Radiats Biol Radioecol. 2000;40:615–620. [PubMed] [Google Scholar]
- Mothersill C, Rea D, Wright EG, Lorimore SA, Murphy D. Individual variation in the production of a ‘bystander signal’ following irradiation of primary cultures of normal human urothelium. Carcinogenesis. 2001;22:1465–1471. doi: 10.1093/carcin/22.9.1465. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB, Joiner MC. Relationship between radiation-induced low-dose hypersensitivity and the bystander effect. Radiat Res. 2002;157:526–532. doi: 10.1667/0033-7587(2002)157[0526:rbrild]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Huo L, Little JB. Increased bystander mutagenic effect in DNA double-strand break repair-deficient mammalian cells. Int. J. Radiat. Biol. 2003;79:35–41. [PubMed] [Google Scholar]
- National Research Council . Health Effects of Exposure to Radon (BEIR VI) National Academy Press; Washington, DC: 1999. [PubMed] [Google Scholar]
- Nuta O, Darroudi F. The impact of the bystander effect on the low-dose hypersensitivity phenomenon. Radiat Environ Biophys. 2008;47:265–274. doi: 10.1007/s00411-007-0145-9. [DOI] [PubMed] [Google Scholar]
- Ohtoshi T, Takizawa H, Okazaki H, Kawasaki S, Takeuchi N, Ohta K, Ito K. Diesel exhaust particles stimulate human airway epithelial cells to produce cytokines relevant to airway inflammation in vitro. J. Allergy Clin. Immunol. 1998;101:778–785. doi: 10.1016/S0091-6749(98)70307-0. [DOI] [PubMed] [Google Scholar]
- Ojima M, Ban N, Kai M. DNA double-strand breaks induced by very low X-ray doses are largely due to bystander effects. Radiat Res. 2008;170(3):365–71. doi: 10.1667/RR1255.1. [DOI] [PubMed] [Google Scholar]
- Seymour C, Mothersill C. Relative contribution of bystander and targeted cell killing to the low-dose region of the radiation dose-response curve. Radiat Res. 2000;153:508–511. doi: 10.1667/0033-7587(2000)153[0508:rcobat]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Shao C, Furusawa Y, Kobayashi Y, Funayama T. Involvement of gap junctional intercellular communication in the bystander effect induced by broad-beam or microbeam heavy ions. Nuclear Instruments and Methods in Physics Research B. 2006;251:177–181. [Google Scholar]
- Stringer B, Kobzik L. Environmental particulate-mediated cytokine production in lung epithelial cells (A549): role of preexisting inflammation and oxidant stress. J. Toxicol. Environ. 1998;55:31–44. doi: 10.1080/009841098158601. [DOI] [PubMed] [Google Scholar]
- Szabo J, Feher I, Palfalvi J. In vitro cell irradiation systems based on 210Po alpha source: construction and characterization. Radiation Measurements. 2002;35:575–578. doi: 10.1016/s1350-4487(02)00089-6. [DOI] [PubMed] [Google Scholar]
- Tubiana M, Aurengo A, Averbeck D, Masse R. The debate on the use of a linear no threshold for assessing the effects of low doses. J. Radiol. Prot. 2006;26:317–324. doi: 10.1088/0952-4746/26/3/N01. [DOI] [PubMed] [Google Scholar]
- UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) Sources and effects of ionizing radiation Report to the General Assembly with scientific annexes. New York: United Nations; 2000. [Google Scholar]
- Whitehouse CA, Tawn EJ. No evidence for chromosomal instability in radiation workers with in vivo exposure to plutonium. Radiat. Res. 2001;156:467–475. doi: 10.1667/0033-7587(2001)156[0467:nefcii]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wykes SM, Piasentin E, Joiner MC, Wilson GD, Marples B. Low-dose hyper-sensitivity is not caused by a failure to recognize DNA double-strand breaks. Radiat Res. 2006;165:516–524. doi: 10.1667/RR3553.1. [DOI] [PubMed] [Google Scholar]
- Wolff S. The adaptive response in radiobiology: evolving insights and implications. Environ Health Perspect. 1998;106(Suppl 1):277–283. doi: 10.1289/ehp.98106s1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Assad N, Held KD. Medium-mediated intercellular communication is involved in bystander responses of X-ray – irradiated normal human fibroblasts. Oncogene. 2005;24:2096–2103. doi: 10.1038/sj.onc.1208439. [DOI] [PubMed] [Google Scholar]