Abstract
The MNT1 gene of the human fungal pathogen Candida albicans is involved in O-glycosylation of cell wall and secreted proteins and is important for adherence of C. albicans to host surfaces and for virulence. Here we describe the molecular analysis of CaMNT2, a second member of the MNT1-like gene family in C. albicans. Mnt2p also functions in O-glycosylation. Mnt1p and Mnt2p encode partially redundant α-1,2-mannosyltransferases that catalyze the addition of the second and third mannose residues in an O-linked mannose pentamer. Deletion of both copies of MNT1 and MNT2 resulted in reduction in the level of in vitro mannosyltransferase activity and truncation of O-mannan. Both the mnt2Δ and mnt1Δ single mutants were significantly reduced in adherence to human buccal epithelial cells and Matrigel-coated surfaces, indicating a role for O-glycosylated cell wall proteins or O-mannan itself in adhesion to host surfaces. The double mnt1Δmnt2Δ mutant formed aggregates of cells that appeared to be the result of abnormal cell separation. The double mutant was attenuated in virulence, underlining the importance of O-glycosylation in pathogenesis of C. albicans infections.
Candida albicans is the major fungal pathogen of humans. This opportunistic pathogen can cause irritating superficial infections of the mucosa and serious life threatening systemic infections in the immunocompromised patient (1, 2). Invasive candidosis in hospitals now represents the third or fourth most common form of septicaemia (3, 4). The cell surface of C. albicans is the immediate point of contact between the fungus and host and plays vital roles in adhesion and immunomodulation of host responses, and it is a source of antigens (5–8). The outer cell wall layer is enriched in mannoproteins, which are embedded in a matrix of structural polysaccharides consisting of β-1,3- and β-1,6-linked glucan and chitin (9). This layer is important in adhesion to host surfaces and their subsequent colonization (10–12). Both the protein and carbohydrate components of mannoproteins have been implicated in adhesion to the host (10, 13–15), although details of the nature of the ligands and receptors are still lacking. Hence, glycosylation of cell wall proteins is critical for host-fungal interactions and pathogenicity.
Knowledge of glycosylation in Saccharomyces cerevisiae (16–28) and information from the C. albicans genome data base has provided significant resources for the identification and analysis of glycosylation genes in C. albicans. Mannoproteins of S. cerevisiae and C. albicans contain both N- and O-linked oligosaccharides. The N-linked glycans, attached to asparagine residues of proteins, contain a conserved core structure and an elaborate, highly branched outer mannose chain that is specific to fungi and contains both acid-stable and acid-labile components (17, 29, 30). Glycosylation in C. albicans has its own relevance in investigations of the role of specific oligosaccharide moieties in host-fungal interactions. The acid-labile mannosylphosphate component, containing β-1,2-linked mannose, has been implicated in adhesion and recognition of phagocytic leukocytes, although mutants lacking this component have been shown to have normal interactions with macrophages (31). Both β-1,2- and α-1,2-linked mannan oligosaccharides have been implicated directly in adhesion functions (12, 32).
In C. albicans, O-glycans are linear oligosaccharides of one to five α-1,2-linked mannose residues (32–34). In S. cerevisiae an α-1,2-linked O-linked glycan is capped with one or two α-1,3-linked mannose residues (27). O-Glycosylation in S. cerevisiae is initiated in the endoplasmic reticulum where at least four of the seven-membered PMT gene family act to transfer mannose from dolichyl phosphate-activated mannose to serine or threonine (18, 35, 36). Evidently this step is essential, as certain combinations of pmtΔ mutants are non-viable (35). Five C. albicans PMT genes have been identified through the C. albicans sequencing project, of which two have been characterized (37, 38). Strains lacking CaPmt1p and CaPmt6p underglycosylate certain proteins in the cell wall, have reduced growth rates, are hypersensitive to a range of cell wall perturbing agents, exhibit defective yeast-hyphal morphogenesis and are attenuated in virulence (37, 38). Although these studies on pmtΔ mutants confirmed that O-mannosylation is important for C. albicans virulence, it is not clear if these phenotypes are caused by misfolding of O-glycosylated proteins, whose structure may be affected by the first O-linked mannose residue or the absence of the mannose epitope.
The addition of further mannose residues to the first O-linked mannose occurs in the Golgi and involves a range of mannosyltransferases. In S. cerevisiae Mnt1p (Kre2p), Ktr1p, and Ktr3p belong to a nine-membered gene family that have partially redundant roles in the addition of the second and third α-1,2-linked mannose residues.
We previously isolated and analyzed CaMNT1, a gene that is part of a five-membered ScMNT1-like gene family in C. albicans (32, 39). CaMnt1p is an α-1,2-mannosyltransferase that adds the second mannose residue to the O-linked glycan (32). Camnt1Δ-null mutants display truncated O-glycans, altered cellular distribution of chitinase, reduced adherence to human buccal epithelial cells, are hypersensitive to the cell wall perturbing agent Calcofluor White and are attenuated in virulence in both guinea pig and mouse models of systemic infection (32). Heterologous expression of site-specific mutants of CaMnt1p in Pichia pastoris confirmed the nature of the nucleophilic reaction center and the conserved residues required for coordination of the metal ion cofactors essential for activity (39). The structure and mechanism of catalysis of this enzyme have recently been examined by crystallography revealing a novel mechanism for the interactions between the metal ion and the coordinating atypical DXD motif (40).
Here we describe a second member of the CaMNT gene family, MNT2, which encodes a protein with significant sequence similarity to the α-1,2-mannosyltransferases CaMnt1p, ScMnt1p, ScKtr1p, and ScKtr3p. We demonstrate that CaMnt2p is also an α-1,2-mannosyltransferase which, along with Mnt1p, is responsible for the addition of the second and third mannose residues in C. albicans O-glycans. Elimination of both Mnt1p and Mnt2p resulted in truncation of O-linked glycans and reduction in virulence, indicating that these enzymes are important for normal interactions of C. albicans with its host.
EXPERIMENTAL PROCEDURES
Strains, Media, and Culture Conditions
The C. albicans strains used in this study are listed in Table I. All strains were grown at 30 °C in YEPD (1% w/v yeast extract, 2% w/v mycological peptone, 2% w/v glucose) or S.D. (0.67% (w/v) yeast nitrogen base without amino acids, 2% glucose) supplemented where necessary with 50 μg/ml uridine. Solid media contained 2% (w/v) technical agar number 3 (Oxoid Ltd, Basingstoke, UK). For adherence assays, cells were grown to stationary phase in 0.67% (w/v) yeast nitrogen base without amino acids and either 50 mm glucose or 500 mm galactose as a carbon source. Hyphal cells were grown either in 20% (v/v) newborn calf serum (Invitrogen, Life Technologies, Inc.) at 37 °C (41) or on Spider plates (32) at 30 °C.
Table I. Strains used in this project.
| Strain | Parental strain | Genotype | Source |
|---|---|---|---|
| CAF2–1 | SC5314 | URA3/ura3Δ::imm434 | (42) |
| CAI-4 | CAF2–1 | ura3Δ::imm434/ura3Δ::imm434 | (42) |
| NGY23 | 32.16.4 | As CAI-4 but mnt1Δ::hisG/mnt1Δ::hisG-URA3-hisG | (32) |
| NGY24 | NGY23 | As CAI-4 but mnt1Δ::hisG/mnt1Δ::hisG | (32) |
| NGY103 | CAI-4 | As CAI-4 but MNT2/mnt2Δ::hisG-URA3-hisG | This study |
| NGY104 | NGY103 | As CAI-4 but MNT2/mnt2Δ::hisG | This study |
| NGY105 | NGY104 | As CAI-4 but mnt2Δ::hisG/mnt2Δ::hisG-URA3-hisG | This study |
| NGY106 | NGY105 | As CAI-4 but mnt2Δ::hisG/mnt2Δ::hisG | This study |
| NGY109a | CAI-4 | As CAI-4 but MNT1-MNT2/mnt1-mnt2Δ::hisG-URA3-hisG | This study |
| NGY110a | NGY109 | As CAI-4 but MNT1-MNT2/mnt1-mnt2Δ::hisG | This study |
| NGY111a | NGY110 | As CAI-4 but mnt1-mnt2Δ::hisG/mnt1-mnt2Δ::hisG-URA3-hisG | This study |
| NGY112a | NGY111 | As CAI-4 but mnt1-mnt2Δ::hisG/mnt1-mnt2Δ::hisG | This study |
| NGY145 | NGY106 | As NGY106 but RPS10/rps10Δ::CIp10 | This study |
| NGY148 | NGY24 | As NGY24 but RPS10/rps10Δ::CIp10-MNT1 | This study |
| NGY149 | NGY106 | As NGY106 but RPS10/rps10Δ::CIp10-MNT2 | This study |
| NGY152 | CAI-4 | As CAI-4 but RPS10/rps10Δ::CIp10 | This study |
| NGY158 | NGY24 | As NGY24 but RPS10/rps10Δ::CIp10 | This study |
| NGY335 | NGY112 | As NGY112 but RPS10/rps10Δ::CIp10-MNT1 | This study |
| NGY336 | NGY112 | As NGY112 but RPS10/rps10Δ::CIp10-MNT2 | This study |
Strains in which the deletion spans MNT1, the intervening β-glucosidase ORF and MNT2.
Identification of CaMNT2
The CaMNT2 gene was originally identified before the C. albicans genome data base was available by PCR using degenerate oligonucleotides (5′-CCCGAATTCGCRTCNCCCCANCKYTCRTA-3′ and 5′-CCCGAATTCTAYMGNMAYATGGYMG-3′) that were designed to conserved regions of CaMNT1, ScMNT1, ScYUR1, and ScKTR1. These generated two distinct products from genomic DNA, one from CaMNT1 and the other from CaMNT2. The cloned CaMNT2 fragment was used as a probe in colony hybridizations against partial EcoRI and HindIII genomic libraries in pBlueScript-KS (Stratagene). Two HindIII clones (2.2 kb, pEB101 and pEB102) were obtained, representing two distinct alleles distinguishable by the presence of a polymorphism at an EcoRI site, and one EcoRI clone (3.1 kb, pEB103). All three clones were sequenced completely on both strands and found to contain the entire CaMNT2 open reading frame.
Disruption of CaMNT Genes
Disruption of CaMNT1 was described previously (32). Further disruptions were achieved using the Urablaster protocol (42) after which Southern analysis of genomic DNA was used to confirm correct integration of the disruption cassette. The HindIII and EcoRI library clones pEB101 and pEB103 were combined to generate plasmid pEB136, which contains a 3.8-kb fragment spanning from the HindIII site upstream of the CaMNT2 ORF1 to the EcoRI site downstream (Fig. 1). The 1.8-kb BglII/PstI fragment of CaMNT2 was replaced with the hisG-URA3-hisG Ura-blaster cassettes from pMB-7 and p5921 (42).
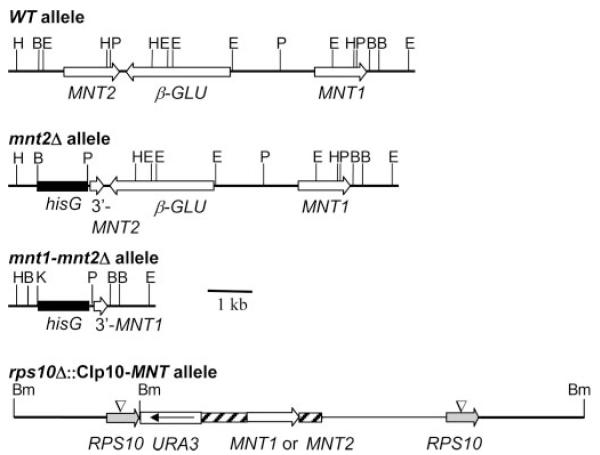
Fig. 1. CaMNT1 and CaMNT2 region of chromosome 3 with intervening β-glucosidase gene (β-GLU) (top line) and the structure of the Camnt2Δ, Camnt1-Camnt2Δ and reconstituted heterozygous alleles with MNT1 or MNT2 integrated at the CIp10 locus.
Hatched bars represent putative MNT promoter and terminator sequences. Restriction sites are: B, BglII; Bm, BamHI; E, EcoRI; H, HindIII, K, KpnI, and P, PstI.
To disrupt the entire region containing MNT1 and MNT2 the PstI/KpnI fragment containing these genes, and the intervening sequences was replaced with the PstI/KpnI fragment (32) containing CaMNT1 3′-sequences (from the PstI site within CaMNT1 to the EcoRI site downstream). CaMNT1, CaMNT2, and the intergenic region were disrupted by two successive rounds of transformation (42). To control for possible artifacts resulting from ectopic expression of URA3 (43), the URA3 gene was integrated at the RPS10 locus in the Ura−-null mutants of Camnt1Δ, Camnt2Δ, and Camnt1-Camnt2Δ mutants using the CIp10 vector as described previously (31, 43). The CaMNT1 and CaMNT2 genes under the control of 1000 bp of upstream promoter sequence were subcloned into CIp10 and transformed individually into the Camnt1-Camnt2Δ double mutant to reconstitute heterozygous strains as controls.
Isolation and Analysis of Nucleic Acids
Isolation of C. albicans genomic DNA and RNA was adapted from published protocols (44, 45). The MNT2-specific primers were: 5′-TCAATGATGTCACCACTAAGC-3′ and 5′-CAACATTACGAATAGATTTGATC-3′. To test for the presence of contaminating genomic DNA in the cDNA, PCR reactions were multiplexed using primers spanning the intron of the CaEFB1 (45, 46).
In Vitro Assay of Mannosyltransferase Activity
Mannosyltransferase enzyme activities were assayed in mixed membrane preparations of mid-exponential phase yeast cells as described previously (26, 39). Assay mixtures contained 50 mm Tris-HCl pH 7.2, 10 mm MnCl2, 64 nm GDP-[3H]mannose (0.02 μCi; specific activity 6.4 Ci/mmol) (Amersham Biosciences), 0.1% (v/v) Triton X-100, 50 μg of membrane protein, and 25 mm of either methyl-α-mannoside or methyl-α1,2-mannobiose as an acceptor. Triplicate reactions were performed for 1 h at 30 °C and were then passed through 0.6 ml of QAE-Sephadex anion exchange resin to remove unincorporated GDP-mannose. Radioactivity in eluted neutral products was counted and specific activities calculated.
Metabolic Labeling of Glycans and Non-reductive β-Elimination
Yeast cells growing exponentially in YP 0.5% sucrose were labeled with d-[2-3H]mannose according to the method of Häusler et al. (20). A 2.0-ml sample was inoculated into medium containing 1.85 MBq of d-[2-3H]-mannose (555 GBq mmol−1; PerkinElmer Life Sciences, Beaconsfield, UK) and incubated at 30 °C in a shaking incubator at 200 rpm for 90 min. O-linked glycans were β-eliminated with 100 mm NaOH for 16 h at room temperature. The reaction was neutralized with 150 mm HCl. Cells were removed by centrifugation at 11,600 × g for 5 min, and the supernatant containing the radiolabeled O-glycans removed and stored at −20 °C. Samples (50 μl) were analyzed by TLC and autofluorography.
Thin Layer Chromatography
Samples for TLC were spotted onto Silica G-60 TLC plates (Whatman) and allowed to dry. TLC plates were developed by running twice in 6:8:5:8 ethyl acetate/butan-1-ol/acetic acid/water. For detection of d-[2-3H]mannose-labeled carbohydrates, plates were sprayed with En3Hance (PerkinElmer Life Sciences) and labeled sugars visualized by autofluorography (Kodak BioMax XLS). To detect non-radiolabeled carbohydrates, plates were dried and stained by spraying with 0.2% (w/v) orcinol (Sigma) in 20% (v/v) aqueous sulfuric acid and baking at 150 °C for 5–10 min. To detect mannitol, plates were resprayed with 20% (v/v) aqueous sulfuric acid and visualized by charring at 200 °C. Standards for running alongside reductively β-eliminated glycans on TLC plates were prepared by reduction of 5 mg of α-1,2-mannobiose and α-1,3α-1,6-mannotriose (Dextra Laboratories Ltd, Reading, UK) with 0.25 m NaBH4 for 4 h followed by addition of 1 ml of 0.25 m acetic acid in methanol to destroy excess borohydride. The reduced standards were then desalted on a 1.25-ml column of AG50-X12 (Bio-Rad). Mannitol, Manα-1,2-mannitol, and Manα-1,3-Manα-1,6-mannitol (Sigma) were dissolved in 40% (v/v) propan-1-ol and used as standards.
Preparation of Yeast Cell Walls
Cell walls were prepared by a method modified from Hughes et al. (47). Cells from a 24-h culture (50 ml) grown at 30 °C in YPD were washed twice by centrifugation in water. The cell pellet was resuspended in 25 ml of 0.2 m sucrose, 20 mm MOPS, pH 7.2 containing protease inhibitor (Complete Mini, Roche Applied Science), and frozen overnight at −80 °C. The cells were then disrupted by three passages through an X-Press homogenizer (AB Biox, Järfälla, Sweden) cooled to −80 °C. The disrupted sample was defrosted and cell breakage checked microscopically. Cell walls were recovered by centrifugation at 1500 × g for 10 min at room temperature and resuspended in 50 ml of 0.1% Triton X-100 in 20 mm MOPS, pH 7.2, to remove membranes. Cell walls were then washed twice in 50 ml of milliQ water and stored as pellets at −20 °C.
Reductive β-Elimination of O-Linked Carbohydrate from C. albicans Cell Walls
Cell walls were washed three times in 10 ml of milliQ water. O-glycans were reductively β-eliminated in 5 ml of 100 mm NaOH, 0.5 m NaBH4 in milliQ water for 16 h at room temperature in the dark on a reciprocating shaker. Cell wall material was removed by centrifugation at 4,600 × g, and the supernatant recovered. Excess borohydride was destroyed by the addition of 5 ml of 0.5 m acetic acid in methanol. PIR proteins (proteins with internal repeats) released by alkaline treatment (48) were removed by precipitation overnight at −20 °C followed by centrifugation at 4,600 × g and passage of the supernatant through a 0.2-μm filter (Millipore). Samples were desalted by passing through 5 ml of Bio-Rad AG50W-X12 (Bio-Rad 142-1641, H+ form, preconditioned with 50% methanol) and eluted with 10 ml of milliQ water. Samples were frozen at −80 °C and lyophilized. Samples were resuspended in 2.5 ml of 5% (v/v) acetic acid in methanol and dried down twice at 60 °C under nitrogen and then extracted twice with 100% methanol to remove boric acid. Residual acetic acid was then removed by co-evaporation with 250 μl of toluene in a freeze-drier. The desalted O-glycans were resuspended in 1 ml of milliQ water and passed through a 1.25-ml column of Bio-Rad AG4-X4 (Bio-Rad 143-3341, converted to OH− form) followed by elution with 5 ml of milliQ water to remove any remaining anionic contaminants. The purified glycans were frozen, lyophilized, and finally resuspended in 1 ml of milliQ water. For TLC analysis, glycans were diluted to 40% (v/v) with propan-1-ol and samples (10 μl) applied to TLC plates. Following separation, carbohydrates were visualized by orcinol staining as described above.
Mannosidase Digestion of Glycans
Approximately 10 μg of reductively β-eliminated O-linked glycan was lyophilized and resuspended in 20 μl of the appropriate buffer for digestion. Jack Bean mannosidase (11 milliunits) or Aspergillus satoi α1,2-mannosidase (10 μU) (Glyko) was added, and the reactions incubated at 37 °C for 16 h. Reactions were then diluted with 80 μl of milliQ water and desalted by passage through a column of 200 μl of Bio-Rad AG50W-X12 over 200 μl of Bio-Rad AG4-X4 and eluting with 2 ml of milliQ water. The samples were lyophilized and resuspended in 50 μl of 40% (v/v) propan-1-ol. Aliquots (10 μl) were analyzed by TLC and orcinol staining.
Glycan Purification by Dionex HPAEC
Lipids were removed from the sample of reductively β-eliminated O-glycan using butyl alcohol and glycans were then dried and dissolved in milliQ water. Glycans were fractioned by Dionex HPAEC on a CarboPac PA-1 column (4 × 250 mm). The column was equilibrated with 100% 100 mm NaOH for 20 min at a flow rate of 0.5 ml/min. To separate the glycans, a linear gradient of 0–50% 200 mm sodium acetate in 100 mm NaOH was applied over a period of 40 min at a constant flow rate of 0.5 ml/min. Glycans were detected by a pulsed-amperometric detector, and sodium ions were removed from the eluate on-line with a Dionex ASRS unit (49). Glycans were collected individually at the detector outlet and fully desalted on a small column containing AG50 (H+) over AG3 (OH−). The glycans were dried and dissolved in milliQ water.
Monosaccharide Composition and Methylation Linkage Analysis
Monosaccharide analysis was carried out by GC-MS on cell wall O-glycan from wild type and mutant strains and purified glycans resolved by Dionex HPAEC (49). Methylation linkage analysis was carried out on each product resolved by Dionex HPAEC. Approximately 4 nmol of material was used for each methylation analysis as previously described (49). The partially methylated alditol acetates (PMMAs) were analyzed by GC-MS, using a HP-5 (30 m × 0.25 mm, Agilent) column.
Mass Spectrometric Analysis of Glycans
Samples of permethylated glycans in 80% acetonitrile/0.5 mm sodium acetate were loaded into nanospray tips (Micromass type F) for electrospray mass spectrometry (ES-MS). Samples were analyzed in positive ion mode with capillary and cone voltages of 0.8 kV and 30 V, respectively, using a micromass Q-Tof2 orthogonal quadrupole-time of flight mass spectrometer (Micromass). All spectra were collected and processed with Masslynx software.
Quantification of Total Cell Wall Carbohydrates by HPAE-PAD
Cell walls were hydrolyzed with sulfuric acid and monosaccharides released were measured by a modification of the method of Dallies et al. (50) as described previously (51).
Sensitivities of Mutants to Inhibitors
Stationary phase yeast cells were diluted to 5×105 cells/ml, and 10 μl of a series of 10-fold dilutions was spotted onto YEPD agar plates and supplemented with various concentrations of Calcofluor White, SDS, Hygromycin B, and Congo Red. Growth was scored after incubation for 3 days at 30 °C. The concentration of inhibitors used was 50 and 100 μg/ml Calcofluor White, 0.05% SDS, 50 and 100 μg/ml hygromycin B, and 50 and 100 μg/ml Congo Red. Sensitivity to high salt concentrations and caffeine was scored by streaking strains out onto YEPD plates and YEPD plates containing 0.5 m or 1 m NaCl or 8 mm or 15 mm caffeine. Plates were incubated at 30 °C for 24 h.
Adherence Assays
Tests for in vitro adherence to buccal epithelial cells and Matrigel were based on published protocols (52–54). For Matrigel adherence assays, C. albicans cells were grown as above, washed three times with PBS and suspended to a final concentration of 1 × 106 cells/ml. Aliquots of 0.1 ml were applied to wells of a 96-well microdilution plate, pre-coated with 100 μg/cm2 Matrigel (Biocoat Cellware, BD Biosciences). Plates were incubated for 30 min at 37 °C without shaking. Unattached cells were removed by applying 200-μl volumes of phosphate-buffered saline to wells and draining onto absorbent paper (repeated five times). For each well, the number of cells within ten fields of view were counted at ×400 magnification. Adherence values were based on triplicate samples repeated on different days.
Virulence Assays
Virulence of strains was tested with a model of systemic C. albicans infection in immunocompetent, female BALB/c mice (Harlan Sera-lab, Loughborough, UK), as described previously (31, 43). Briefly, C. albicans strains were grown by rotation for 18–24 h in NGY medium (43) at 30 °C. Cells were harvested, washed twice with sterile distilled water, and resuspended in sterile, physiological saline. Cell suspensions were adjusted to produce intravenous challenge inocula with 2.6 × 104 cfu/g mouse body weight. For each strain, 6 mice were infected. Animals were provided with food and water ad libitum. Mice showing signs of distress or illness were humanely terminated, the kidneys and brain aseptically removed, and the day of death recorded as the following day. Organs were homogenized in 0.5 ml of sterile water, and dilutions plated out on agar plates to determine fungal burdens. Burdens were expressed as cfu/g tissue. All experimentation was carried out under the terms of the UK Home Office licenses for research on animals.
Electron Microscopy
Yeast cells were grown in YEPD, 30 °C for 4 h, and fixed in 2.5% glutaraldehyde in 0.1 m phosphate buffer overnight then post-fixed in 1% osmium tetroxide for 1 h, dehydrated with ethanol, and embedded in Taab premix medium grade resin. Ultrathin sections were cut using a Reichert-Jung Ultracut ultramicrotome and mounted on 200 mesh copper grids and stained with uranyl acetate and lead citrate before being viewed with a Philips 301 TEM at 80 kV.
RESULTS
Isolation and Analysis of CaMNT2
In a previous study we isolated CaMNT1 by homologous hybridization to ScMNT1 (32). Although these two genes displayed high sequence similarity, CaMnt1p was found to add predominantly the second, not the third, mannose residue to the O-mannan chain. Therefore CaMnt1p may not be the true biochemical homolog of ScMnt1p. Further, a fraction of O-linked mannan was not truncated in the mnt1Δ-null mutant, and this strain retained 25% of the wild-type α-methyl mannoside-dependent mannosyltransferase activity. Hence, it was likely that C. albicans has additional mannosyltransferases that act in the extension of O-linked oligosaccharides.
Degenerate oligonucleotide primers designed to conserved regions in CaMNT1 and members of the ScMNT1-like gene family identified a second member of this family termed CaMNT2. The CaMNT2 ORF of 1386 bp is predicted to encode a protein of 461 amino acids (GenBank™/EMBL accession number X89263). The deduced amino acid sequence displayed 56% identity (73% similarity) to CaMnt1p and high homology to ScMnt1p, ScKtr1p and ScKtr3p, all of which are known to be α-1,2-mannosyltransferases involved in O-glycosylation (20, 27). CaMnt2p, like other mannosyltransferases, is predicted to encode a type II membrane protein with a 15 residue N-terminal cytosolic tail, a single short 19-residue membrane spanning region, a stem region of 104 amino acids and a catalytic domain of 323 residues that is likely to reside in the Golgi lumen. Both CaMNT1 and CaMNT2 are closely linked on chromosome 3. Scrutiny of the C. albicans genome sequence revealed that the two genes were ~5 kb apart and separated by an ORF encoding a potential β-glucosidase (Fig. 1). Analysis by reverse transcription PCR of C. albicans total RNA with CaMNT2 specific oligonucleotide primers confirmed that CaMNT2 was expressed in both yeasts and hyphae (not shown).
Deletion of MNT2
Both alleles of the CaMNT2 gene was disrupted by the “ura-blaster” method and the genotype of transformants (Fig. 1) was confirmed by Southern analysis. RT-PCR analysis confirmed that no MNT2 transcript could be detected in the Camnt2Δ-null mutant whereas the CaMNT1 transcript was still present (not shown). Functional redundancy exists among mannosyltransferases in S. cerevisiae that are involved in O-glycosylation (22, 24, 55). Therefore attempts were made to disrupt CaMNT2 in the Camnt1Δ-null mutant background. Disruption of CaMNT2 in a Camnt1Δ background (and vice versa) was unsuccessful despite screening of over 100 transformants. Because CaMNT1 and CaMNT2 are closely linked on chromosome 3 the entire region containing CaMNT1, CaMNT2 and the intergenic region were deleted simultaneously (Fig. 1). This established that CaMNT1 and CaMNT2 are not essential, either singly or in combination. To control for the loss of the putative β-glucosidase and the URA3 position effects, CaMNT1 and CaMNT2 were reintroduced into the double Camnt1-Camnt2Δ mutant under the control of their own promoters using the integrative plasmid CIp10 (43) (Fig. 1). Again the genotype of these reconstituted strains was confirmed by Southern analysis.
Morphology and Growth of the Camnt2Δ and Camnt1-Camnt2Δ Mutants
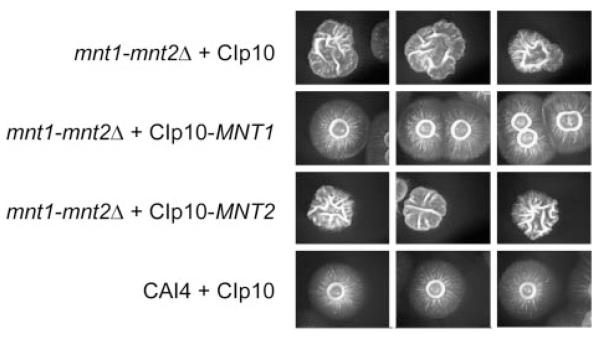
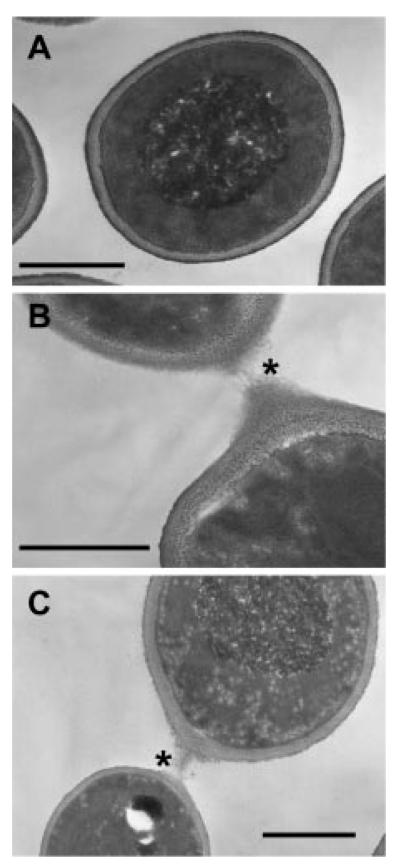
No detectable defects were displayed in the growth rate of yeast cells in YEPD at 30 °C or S.D. medium at 37 °C, or in hyphal morphogenesis in liquid serum medium or on solid Spider medium at 30 °C for the single Camnt1Δ and Camnt2Δ mutants. The Camnt1-Camnt2Δ mutant was less filamentous on Spider medium and filamentation was restored in the mutant that was complemented by addition of CaMNT1 but not CaMNT2 (Fig. 2). The double mutant was also less able to invade the agar surface on Spider medium and serum agar plates (not shown). Yeast cells of the Camnt1-Camnt2Δ were more flocculent and formed muticellular aggregates. Thin sections of these aggregated Camnt1-Camnt2Δ cells examined by transmission electron microscopy revealed a bridging matrix between adhered cells (Fig. 3). Aggregation of cells did not occur in strains in which either CaMNT1 or CaMNT2 were transformed into the double mutant background (not shown). Hence this phenotype was caused by loss of both CaMnt1p and CaMnt2p rather than the loss of the putative β-glucosidase, which was also deleted in this mutant.
Fig. 2.
Morphology of colonies on Spider medium plates after 5 days at 30 °C showing reduction in filamentation in the Camnt1-Camnt2Δ mutant and complementation of this defect by addition of CaMNT1, but not CaMNT2.
Fig. 3. Thin-sections of wild type (A, CAF2-1) and the Camnt1-Camnt2Δ double mutant (B, C) showing extracellular matrix material between flocculating yeast cells (asterisks).
Scale bar, 3 μm, for all micrographs.
Camnt1Δ and Camnt1-Camnt2Δ Mutants Are α-1,2 Mannosyltransferase-deficient
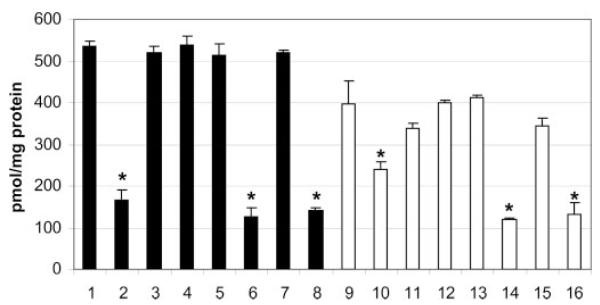
The single and double mutants were assessed for in vitro mannosyltransferase activity in mixed membrane preparations (Fig. 4). Deletion of CaMNT1 significantly affected in vitro mannosyltransferase activity with both methyl-α-mannoside and methyl-α1,2-mannobiose as acceptors. Deletion of CaMNT2 alone had little effect on specific activity with these in vitro acceptors although the Camnt1-Camnt2Δ double mutant had significantly reduced activity compared with the Camnt1Δ mutant with methyl-α-1,2-mannobiose as acceptor. This reduced activity toward methyl-α-1,2-mannobiose, indicative of addition of the third mannose residue, suggests that CaMnt2p contributed only fractionally to the total transferase activity measured in this assay. Reintroduction of CaMNT1, but not CaMNT2, restored normal enzyme activity to the Camnt1Δ and to the Camnt1-Camnt2Δ double mutant. Therefore transferase activity assays suggest that Mnt1p and Mnt2p are both α-1,2 mannosyltransferases but Mnt2p is less efficient than Mnt1p at transferring substrate to these acceptors in vitro.
Fig. 4. Mannosyltransferase activity of mntΔ deletant and control strains.
Membrane fractions were isolated from exponential phase C. albicans yeast cells and tested for the ability to transfer mannose from GDP-[3H]mannose to the acceptors methyl α-mannoside (solid bars) and methyl-α1,2-mannobiose (clear bars). Units are pmol of mannose transferred per mg of protein; error bars are S.D., n = 3. Bars marked with asterisks are statistically different from the control strain (CAI4 + CIp10) by Student’s t test (p ≤ 0.05). Samples are: 1 and 9, CAI-4 + CIp10; 2 and 10, Camnt1Δ+ CIp10; 3 and 11, Camnt1Δ + CIp10-MNT1; 4 and 12, Camnt2Δ + CIp10; 5 and 13, Camnt2Δ+ CIp10-MNT2; 6 and 14, Camnt1-Camnt2Δ + CIp10; 7 and 15, Camnt1-Camnt2Δ + CIp10-MNT1; 8 and 16, Camnt1-Camnt2Δ + CIp10-CaMNT2.
TLC Analysis of mntΔ Mutants Suggests That Mnt1p and Mnt2p Are Functionally Redundant
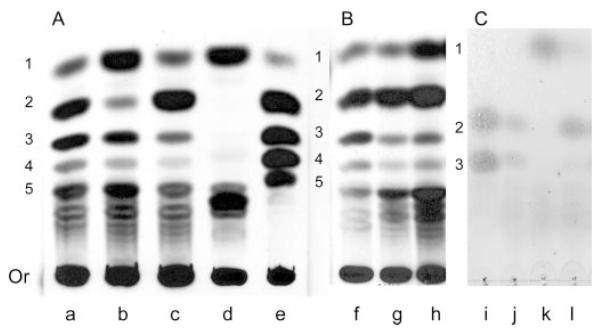
To assess the consequences of the disruption of CaMNT1 and/or CaMNT2 on O-mannan structure, the glycans in the various mutant strains were analyzed by TLC (Fig. 5). O-Glycans were labeled by growing cultures in d-[2-3H]mannose and isolated after β-elimination. The TLC autofluorogram suggested that up to five mannose sugars exist in the fully extended O-linked oligosaccharide. The Camnt1Δ mutant had reduced Man2 compared with Man3, while Camnt2Δ had reduced Man3 compared with Man2. With standard exposures the double mutant lacked any visible Man2-Man5 oligosaccharides (Fig. 5A, lane d). These observations suggest that Mnt1p adds predominantly the second mannose sugar and Mnt2p adds predominantly the third mannose sugar to the O-linked glycan, but that Mnt1p and Mnt2p form a partially redundant pair of enzymes adding the second and third mannose residues. With longer exposures some minimal label remained at the Man2-Man5 positions suggesting the possibility that at least one more functionally redundant transferase activity may contribute to the elaboration of Man2 and Man3.
Fig. 5. TLC analysis of β-eliminated O-glycans of mntΔ mutant strains.
A, cell walls were labeled by growing whole exponentially growing yeast cells in d-[2-3H]mannose and removing O-glycans by β-elimination then separating them by TLC. Labeled products then visualized by autofluorography were from: lane a, wild-type C. albicans CAF2-1; lane b, Camnt1Δ; lane c, Camnt2Δ; lane d, Camnt1-Camnt2Δ; and lane e, wild-type S. cerevisiae serving as a labeled Man1-Man5 control. B, control strains with: lane f, the parent CAI4+CIp10; lane g, Camnt1-Camnt2Δ + CIp10-MNT1; and lane h, Camnt1-Camnt2Δ + CIp10-MNT2. C, confirmation of α-1,2 linkages in O-glycans by mannosidase digestion. Standards (lane i), and untreated (lane j) purified cell walls of CAF-2 were used and were extracted by reductive β-elimination. Cell walls were then digested with Jack Bean mannosidase (lane k) and A. satoi mannosidase (lane l). Positions of Man-OH, Man1, Man2, and Man3 are shown. Or, origin.
In the double mutant, labeled material with lower mobility than Man5 included a labeled band that was not present in the parental strain or in any of the single Camnt1Δ or Camnt2Δ-null mutants. Further tests showed that this band was not GDP-mannose or a phosphorylated sugar and was present in the cell wall fraction. This band was not present when CaMNT1 was re-introduced into the double mutant. This band remained when CaMNT2 was reconstituted into the double mutant, but subtle changes were observed in the intensities of the bands corresponding to Man2 to Man5. This band was also released by acid hydrolysis of whole cells again suggesting it was not an O-linked component of the wall. The nature of the lower mobility material is not known, but is not likely to be a component of the O-linked manno-oligosaccharide since it was largely unaffected in the Camnt1-Camnt2Δ double mutant that lacked all but a trace of Man2-Man5. The above findings were also confirmed by TLC using O-linked glycans isolated from cell walls prepared from labeled and non-labeled cells (not shown). The wild-type structure of O-mannan was restored when either CaMNT1 or CaMNT2 was transformed into the double mutant confirming their functional redundancy in vivo (Fig. 5B). The structure of the O-linked glycans was also supported by experiments in which the O-linked glycans were digested with Jack Bean mannosidase (JBM), which cleaves terminal α-1,2-, α-1,3-, and α-1,6-linked mannose, and A. satoi mannosidase (ASM), which cleaves α-1,2-linked mannose at the non-reducing terminus (Fig. 5C). JBM cleaved the di- and trisaccharides resulting in the accumulation of mannose, whereas ASM cleaved the trisaccharide, accumulating disaccharide and mannose. This again suggested that the first three sugars in the O-linked oligosaccharide were α-1,2-linked.
Further analyses were carried out to determine the effect of the Camnt1-Camnt2Δ on the cell wall composition grown under different conditions. TLC analyses of O-glycans isolated from hyphae and from yeast cells grown on medium containing 500 mm galactose, that proliferate mannan-rich fimbriae-like fibers (52), had the same overall pattern of O-mannosylation (not shown). The structure of the O-glycan in the mutant strains was also confirmed by fluorophore-assisted carbohydrate electrophoresis (FACE) (not shown).
Analysis of Cell Wall by Dionex HPAEC, Methylation, and Mass Spectrometric Analyses
Analysis of the total cell wall composition using Dionex HPAE-PAD indicated that the ratio of chitin:glucan:mannan was (1.0:59.3:39.4) (%) in the CAF2-1 parent, (1.1:56.3:42.5) for Camnt1Δ, (1.1:59.1:39.8) for Camnt2Δ and (1.2:61.9:36.9) for the Camnt1-Camnt2Δ double mutant. The differences in composition between the strains were not significant (p > 0.05).
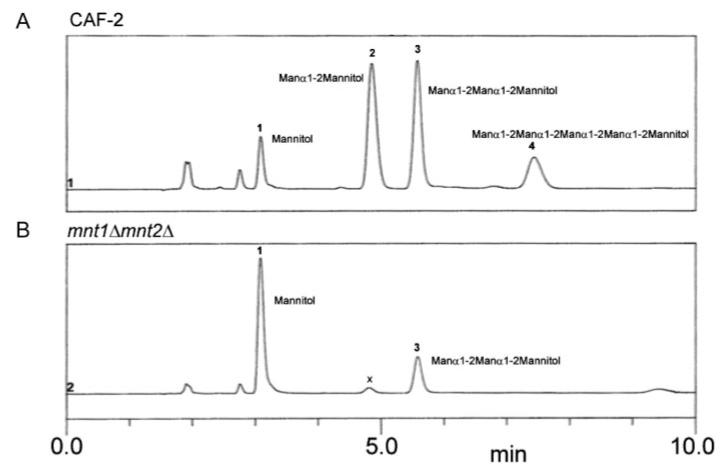
O-Linked glycans of wild-type and the Camnt1-Camnt2Δ mutant were fractionated by Dionex HPAEC (Fig. 6). Monosaccharide analysis, methylation linkage analysis and mass spectrometry were performed on all peak fractions from Dionex HPAEC of reductively β-eliminated glycans. The material in peak fractions all contained exclusively α-1,2-linked glycans up to Man5 (Table II). Monosaccharide analysis revealed that peak 1 contained only mannitol, whereas all the remaining glycans contained mannitol and mannose in different ratios. Peaks 3 and 4 showed the presence of terminal mannose, 2-O-substituted mannose and 2-O-substituted mannitol, while peak 2 contained terminal mannose and 2-O-substituted mannitol. In the Camnt1-Camnt2Δ mutant more than 90% of material isolated was mannitol and peaks corresponding to Man2 and Man5 were absent. Glycans were further analyzed by ES-MS and ES-MS-MS in positive ion mode. Glycans 2, 3, and 4 gave [M + Na]+ ions at m/z 493, 697, and 1105 Da, respectively, and daughter ion spectra were consistent with linear structures. These ions correspond to methylated disaccharide, trisaccharide, and pentasaccharide alditols (Table II).
Fig. 6. Dionex HPAEC glycan profile of the wild type (A) and the mutant (B) strain.
Separation of the glycan was performed on a CarboPac PA-100 column eluted with 200 mm sodium acetate as described under “Experimental Procedures.” The small peak labeled (x) with a retention time slightly lower than Man2 was found not to be a glycan.
Table II. Mass spectrometric analysis and identification of the O-linked glycans from wild type and Camnt1- Camnt2Δ mutant strains.
Glycans were separated on a Dionex CarboPac PA-1 column. Peaks are numbered from material identified in wild type (CAF2–1) and Camnt1-Camnt2Δ mutant cells.
| Strain/Peak | Composition Man-Mannitol |
Linkage composition |
ES-MS [M + Na]+ | |||
|---|---|---|---|---|---|---|
| t-Man | 2-O-Man | 2-O-Mannitol | Mannitol | |||
| CAF2–1/1 (Man1) | 0–1 | 0 | 0 | 0 | 1 | nda |
| CAF2–1/2 (Man2) | 1–1.3 | 1 | 0 | 0.7 | 0 493 | Da |
| CAF2–1/3 (Man3) | 3.4–1 | 1 | 1 | 1 | 0 | 697 Da |
| CAF2–1/4 (Man5) | 4.5–1 | 1 | 2.7 | 0.8 | 0 | 1105 Da |
| Camnt1-Camnt2Δ/1 (Man1) | 0–1 | 0 | 0 | 0 | 1 | nd |
| Camnt1-Camnt2Δ/3 (Man3) | 3.5–1 | 0.5 | 1 | 0.7 | 0 | 697 Da |
Not determined.
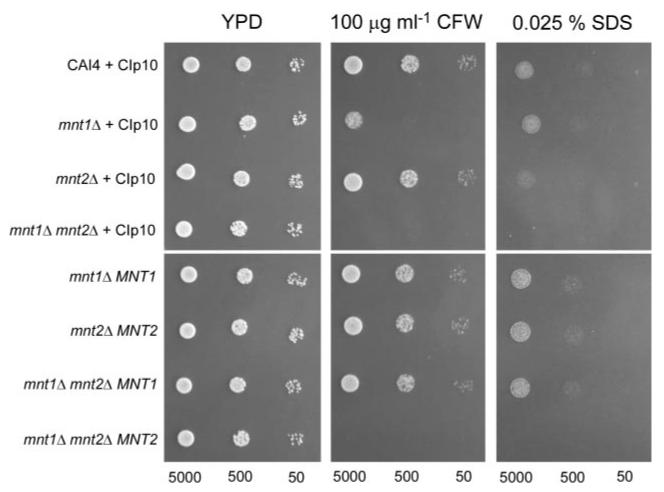
Sensitivity to Wall Damaging Agents and Adhesion of CamntΔ-null Strains
In order to assess the consequences of deletion of CaMNT1 and CaMNT2 on the robustness of the cell wall, the single, double, and reconstituted mutant strains were tested for sensitivity to Calcofluor White and to SDS in plate assays (Fig. 7). Deletion of CaMNT1 increased sensitivity to Calcofluor White whereas deletion of CaMNT2 did not. However, the double Camnt1-Camnt2Δ mutant was more sensitive than the single mnt1Δ-null mutant to Calcofluor White and was also sensitive to Congo Red. Re-integration of CaMNT1 but not CaMNT2 at the RPS10 locus into the double mutant restored the original sensitivities. The Camnt2Δ and the Camnt1-Camnt2Δ mutants were slightly more sensitive to SDS than the wild type. Re-introduction of CaMNT2 restored SDS tolerance in the Camnt2Δ mutant but not in the Camnt1-Camnt2Δ double null mutant. The Camnt1-Camnt2Δ mutant was unchanged in its sensitivity to hygromycin B and NaCl (not shown).
Fig. 7. Sensitivity of wild-type, Camnt1Δ, and Camnt2Δ, mutant strains and re-integrant strains to cell wall disruptive agents.
YPD agar plates with and without Calcofluor White (100 μg ml−1) or SDS (0.025% (w/v)) are shown after incubation of 5000, 500, and 50 cells at 30 °C for 24 h.
Cell wall mannoproteins are important for the adhesion of C. albicans to a number of surfaces (13). It has been demonstrated that Capmt1Δ, Capmt6Δ, and Camnt1Δ mutants, all of which are affected in O-glycosylation, are less adherent to a variety of mammalian cell types (32, 37, 38). The ability of wild type, Camnt2Δ and Camnt1Δ mutants to adhere to human buccal epithelial cells and to Matrigel-coated plastic after growth on glucose or galactose was compared (Table III). Galactose-grown wild-type cells were more adherent to BECs and to Matrigel (data not shown), as described previously (52, 54). Both the Camnt1Δ and the Camnt2Δ strains were affected in adhesion to both BECs and Matrigel when grown on media containing glucose or galactose. However, in both media the adhesion of the Camnt1Δ mutant was reduced more than that of the Camnt2Δ mutant. This suggests a role for O-mannosylation in adhesion. Despite numerous attempts to separate clumped yeast cells or to devise conditions that prevented clumping, aggregation of cells of the double Camnt1-Camnt2Δ mutant (Fig. 3) prevented meaningful adhesion data being obtained for this strain.
Table III. Adherence of strains relative to the parent strain.
Parent strains are CAF2–1 (Ura+) and CAI-4; NGY24 (Camnt1Δ/Camnt1Δ); NGY104 (CaMNT2/Camnt2Δ) and NGY106 (Camnt2Δ/Camnt2Δ)-all Ura−.
| Strain | Buccal epithelial cellsa adhesion (% of CAF2–1) |
Matrigela adhesion (% of CAF2–1) |
||
|---|---|---|---|---|
| Glucose | Galactose | Glucose | Galactose | |
| CAF2–1 | 100 ± 6 | 100 ± 11 | 100 ± 35 | 100 ± 29 |
| CAI-4 | 71 ±7 | 24 ±6 | 61 ± 16 | 38 ± 13 |
| NGY24 | 25 ±3 | 10 ±6 | 19 ± 20 | 16 ± 7 |
| NGY106 | 42 ±9 | 50 ± 12 | 61 ± 32 | 34 ± 32 |
Data are average values ± S.D. expressed as the percentage adherence of the parental strain CAF2–1 from a total of six replicates.
Virulence of Mnt-deficient Mutants
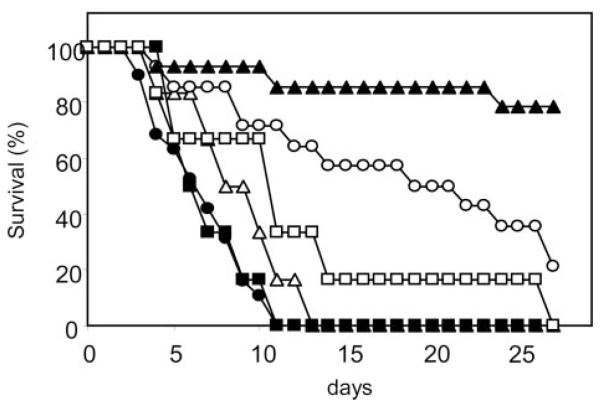
The virulence of the mntΔ mutants was tested in a mouse systemic infection model. We showed previously that a Camnt1Δ mutant was attenuated in virulence (32) but found no significant attenuation when comparing strains in which URA3 was placed at the RPS10 locus (Fig. 8). The Camnt2Δ mutants with URA3 either at the CaMNT2 or RPS10 loci were not significantly different in a logrank test of survival from the heterozygous CaMNT2 mutant or CAI-4 parent with URA3 at the RPS10 locus (Fig. 8). The double Camnt1-Camnt2Δ mutant was, however, significantly attenuated in virulence (logrank test; p < 0.05) compared with CAI-4 with URA3 introduced at RPS10 (Fig. 8). Introduction of a single copy of CaMNT1 into RPS10 allele restored wild type virulence while introduction of MNT2 at the same locus did not restore virulence. Paradoxically, this strain appeared less virulent than the Camnt1-Camnt2Δ double mutant. Counts of viable C. albicans cells recovered from the organs of infected animals showed that all mice inoculated with the various mutant and control strains had similar organ burdens in the kidneys and brain except for the double mutant strain and the double mutant strain reconstituted with MNT2, where there was a ~2-log reduction in the cfu/g of brain tissue and a greater number of C. albicans-negative kidney and brain. Therefore the Camnt1-Camnt2Δ double mutant that lacks O-linked Man2-Man5 oligosaccharides was attenuated in virulence.
Fig. 8. Relative virulence of control strains and Mnt-deficient mutants, in a systemic mouse model of infection.
Six mice were used per strain, with mice being infected intravenously with 2.6 ×104 cfu/g body weight. The control parental strain (CAI-4 + CIp10) is shown as closed circles, the Camnt1-Camnt2Δ double mutant as open circles, Camnt1-Camnt2Δ + CIp10-MNT1 as closed squares, and Camnt1-Camnt2Δ + CIp10-CaMNT2 as closed triangles. The single mutant Camnt1Δ + CIp10 is shown as open squares and Camnt2Δ + CIp10 as open triangles.
DISCUSSION
We report the characterization of two mannosyltransferases of the MNT1 gene family of C. albicans. We show that Mnt1p and Mnt2p are partially functionally redundant transferases that participate in adjacent steps of O-linked glycosylation (Fig. 9). We cannot exclude the possibility that they are also involved in N-glycosylation although no positive evidence was found supporting this possibility. For example, the electrophoretic mobility of the highly N-glycosylated secreted acid phosphatase isolated from the Camnt1-Camnt2Δ double mutant was not affected (not shown). The CaMNT2 gene was isolated by virtue of two conserved sequences in members of the CaMNT1/ScMNT1/KRE2 gene family. It has been shown subsequently that this gene belongs to a five-membered gene family in C. albicans (55) analogous to the nine-membered MNT1/KRE2 gene family of S. cerevisiae (23). Analysis of the homology in the catalytic domain of the C. albicans and S. cerevisiae gene families shows that CaMNT2 groups with CaMNT1, ScMNT1, ScKTR1, and ScKTR3 all of which encode α-1,2-mannosyltransferases (55). The finding that CaMNT2 and CaMNT1 are closely linked on chromosome 3 suggests that these genes may be the result of an ancestral duplication and translocation event (56).
Fig. 9. Structure of O-linked mannan and the enzymes involved in O-glycosylation in C. albicans as described in the text.
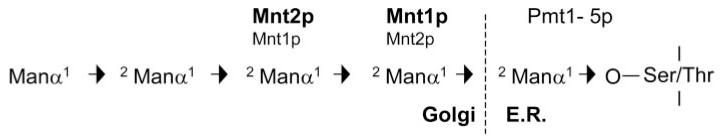
Arrows indicate the linkage between each residue. The major enzyme involved in each step is shown in bold.
Attempts to construct a mutant lacking both CaMNT1 and CaMNT2 by consecutive disruption were unsuccessful despite numerous attempts. Instead we constructed a triple mutant that deleted CaMNT1, CaMNT2, and the intergenic region that contained a gene encoding a β-glucosidase. Several independent lines of evidence suggested that the disruption of the β-glucosidase did not affect the described phenotype of this mutant because restoration of CaMNT1 into this mutant restored O-mannan structure, complemented sensitivity to CFW, Congo Red and SDS, ameliorated aggregation of the Camnt1-Camnt2Δ mutant and restored hypha formation on Spider medium and full virulence in a systemic infection model. However, although complementation of the Camnt1-Camnt2Δ mutant with CaMNT2 restored the O-mannan structure resolved by TLC, this failed to resolve other induced alterations to mannose-containing components (Fig. 5, lane h) or to complement most other phenotypes of the double mutant. CaMnt1p is therefore the critical component for elongation of O-mannan in C. albicans.
Several lines of evidence suggested that the Camnt1Δ-null mutant had an altered cell wall. Yeast cells of the Camnt1Δ-null strain and the Camnt1-Camnt2Δ mutant were sensitive to the cell wall perturbing agent Calcofluor White (55). However, the Camnt2Δ-null mutant was not sensitive to Calcofluor White and in general had a less marked phenotype than Camnt1Δ. The Camnt1Δ-, Camnt2Δ-, and Camnt1-Camnt2Δ-null strains were not altered in sensitivity to high salt concentrations or hygromycin B (not shown), indicating that these strains are not osmotically fragile nor more permeable to large compounds. The Camnt1-Camnt2Δ mutant was also sensitive to the dye Congo Red, which, like Calcofluor White binds cell wall polysaccharides but has a higher affinity for β-1,3-glucan (57) (not shown). Despite these differences in sensitivity to cell wall perturbing agents there were no marked changes in the ratio of chitin, glucan, and mannan in the single or double mntΔ mutants, indicating that the truncation of O-mannan did not induce a cell wall salvage pathway response (58). This is supported by microarray analyses that did not show activation of any signature genes associated with the cell wall compensatory mechanism (59, 60).2
Our analysis, based on whole cell β-elimination, supports in part the O-mannan structure published previously and the role of Mnt1p in O-mannosylation (32). However, we show here that the O-linked oligosaccharide can extend to five mannose sugars as reported elsewhere for C. albicans and S. cerevisiae (33, 34). However, unlike in S. cerevisiae (27) there appears to be no capping of the O-linked mannan with α-1,3-linked mannose. Also the two terminal mannose residues of O-glycans of C. albicans have been suggested elsewhere to be β-linked (34).
The major gene products involved in O-glycosylation in C. albicans have now been identified (Fig. 9). CaPmt1p is the major enzyme involved in the addition of the first mannose residue (38), with CaPmt6p potentially acting on a smaller subset of proteins (37). The functions of CaPMT2, CaPMT4 and CaPMT5, which can be recognized in the C. albicans genome, remain to be investigated. Mnt1p adds the majority of the second mannose residue to the first O-linked mannose (32), although Mnt2p can also act at this step. The major role of Mnt2p is in addition of the third mannose residue.
The cell wall of the Camnt1-Camnt2Δ double mutant showed a large increase of Man1 O-linked glycans and a lack of Man2-Man5. However, the presence of small but significant amounts of extracted Man3 of identical structure to the wild type (i.e. Manα1–2Manα1–2Man) in this mutant suggests that another mannosyltransferase can compensate, in part, for the lack of Mnt1p and Mnt2p and, presumably, the same or another mannosyltransferase can then extend the chain to Man3. The fact that these mutant cells cannot extend Man3 to Man4 or Man5 might also suggest that Mnt2p is required for the efficient addition of the fourth and fifth α-1,2 linked mannose residues. We hypothesized that other CaMntp mannosyltransferases may perform these remaining steps, however, preliminary experiments with mutants lacking CaMnt3p, CaMnt4p, and CaMnt5p showed that these strains had normal O-glycans and therefore they may not perform this role (not shown).
We have shown previously that strains deleted in CaMNT1 have reduced adherence to buccal epithelial cells and rat vaginal epithelial cells (32). We confirmed that this mutant is reduced in adherence to BECs and also show that the Camnt1Δ-null strain was less adherent to Matrigel, a cell free preparation of human basement membrane components. This suggests that O-mannosylation of cell wall proteins may be important in adhesion both to endothelial and epithelial cell layers.
The Camnt2Δ-null mutant strain was also altered in adherence to BECs and Matrigel, although to a lesser extent than for the Camnt1Δ mutant. Therefore, changes in adhesion were affected by the deletion of CaMNT1 and CaMNT2 and, to a lesser extent, the Ura-status of the cells (43, 54). The self-flocculent phenotype of the Camnt1-Camnt2Δ double mutant prevented its analysis using conventional adhesion assays that require a dispersed inoculum. The reason for this flocculation of the yeast cells is not known. It could be that alteration of O-mannosylation affects the process of cytokinesis because chitinase is underglycosylated in this mutant or that novel adhesins are induced in the Camnt1-Camnt2Δ double mutant. Whether there is any relationship between the matrix seen in TEMs and the induced mannose-containing material seen on TLC plates is not known.
Thus far, deletion of genes involved in O-glycosylation in C. albicans has led to a marked reduction in adherence to a number of substrata (32, 37, 38) and α-1,2 oligosaccharides have been shown to directly mediate adherence of C. albicans yeast cells to human enterocytes (12). A specific requirement for O-linked glycosylation in adhesion has also been reported in the binding of the cell surface mannoprotein mp58 to human fibrinogen (61). O-Glycosylation has a profound effect on protein structure, which is mainly mediated by steric interactions between the first amino acid-linked sugar and adjacent amino acids (62). This results, for example, in stabilization of rod-like serine/threonine-rich regions of extracellular glycoproteins that include classes of fungal cell wall adhesins (14). However, most of this stabilization is achieved by addition of the first O-linked mannose and therefore deletion of CaMNT1 or CaMNT2 would not be expected to result in marked changes in the tertiary structure of cell surface mannoproteins. It therefore seems likely that the O-linked epitopes may be involved directly in adherence, perhaps in a lectin-like interaction with host cells.
The attenuation of virulence reported previously in a Camnt1Δ mutant (32) was shown here to be due mainly to the consequence of the ectopic expression of URA3. Loss of CaMNT2 alone also did not affect virulence; however, the Ura-compensated Camnt1-Camnt2Δ double mutant was significantly attenuated in virulence and fewer yeast cells reached the brains of infected mice. Interestingly the introduction of an ectopic copy of MNT2 into the double mutant background did not restore virulence but instead resulted in significant further attenuation of virulence. In vitro mannosyltransferase assays also demonstrated that the MNT2 reconstituted strain failed to compensate for the lack of Mnt1p (and Mnt2p) (Fig. 4) although the strain was able to manufacture a normal Man1-Man5 penta-oligosaccharide (Fig. 5, lane b). A possible explanation for these observations is that the substrate specificities of Mnt1p and Mnt2p are sufficiently different that different proteins or different oligosaccharides are glycosylated when individual Mnt enzymes are deleted. Alternatively Mnt2p may reside in a Golgi compartment that was depleted in our cell extracts. The existence of a novel extracellular matrix material and identification of a novel mannose-containing molecule in the double mutant suggests that glycosylation defects may induce production of novel products in the cell wall.
In summary, our studies demonstrate that Mnt1p and Mnt2p represent partially redundant enzymes that together synthesize addition of the second and third mannose residues in the O-linked mannose pentamer. The structure of C. albicans O-mannan differs from S. cerevisiae in that it lacks terminal α1,3 capping. Mutants that lack CaMNT1 and CaMNT2 have truncated O-mannan and are attenuated in virulence. Evidently, O-glycosylation is necessary for virulence and normal interactions with host surfaces.
Acknowledgments
We thank Kevin McKenzie for assistance with EM and Tamaki Cho for input into the analysis of MNT2. We thank Dr Jean Marie François and Blanca Aguilar-Uscanga (Institut National des Sciences Appliquées de Toulouse) for their help with the cell wall composition analysis.
Footnotes
This work was supported by Grants 063204 and 72263 from the Wellcome Trust.
The abbreviations used are: ORF, open reading frame; MOPS, 4-morpholinepropanesulfonic acid; FACE, fluorophore-assisted carbohydrate electrophoresis; ES-MS, electrospray mass spectrometry.
C. A. Munro, S. Hamilton, A. J. P. Brown, and N. A. R. Gow, unpublished experiments.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBank™/EBI Data Bank with accession number(s) X89263.
REFERENCES
- 1.Odds FC. Candida and Candidosis. 2nd Ed Bailliere-Tindall; London: 1988. [Google Scholar]
- 2.Calderone RA. Candida and Candidiasis. ASM Press; Washington: 2002. [Google Scholar]
- 3.Sandven P. Rev. Iberoam. Micol. 2000;17:73–81. [PubMed] [Google Scholar]
- 4.Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, Kauffman CA, Hyslop N, Mangino JE, Chapman S, Horowitz HW, Edwards JE, Dismukes WE. Clin. Infect. Dis. 2003;37:634–643. doi: 10.1086/376906. [DOI] [PubMed] [Google Scholar]
- 5.Sundstrom P. Curr. Opin. Microbiol. 1999;2:353–357. doi: 10.1016/S1369-5274(99)80062-9. [DOI] [PubMed] [Google Scholar]
- 6.Romani L. Candida and Candidosis. ASM Press; Washington: 2002. pp. 223–241. [Google Scholar]
- 7.Wang Y, Shaokang P, Moser SA, Bost KL, Domer JE. Infect. Immun. 1998;66:1384–1391. doi: 10.1128/iai.66.4.1384-1391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steel C, Marrero L, Swain S, Harmsen AG, Zheng M, Brown GD, Gordon GD, Shlellito JE, Kolls JK. J. Exp. Med. 2003;198:1677–1688. doi: 10.1084/jem.20030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapteyn JC, Hoyer LL, Hecht JE, Müller MH, Andel A, Verkleij AJ, Makarow M, Van Den Ende H, Klis JM. Mol. Microbiol. 2000;35:601–611. doi: 10.1046/j.1365-2958.2000.01729.x. [DOI] [PubMed] [Google Scholar]
- 10.Calderone RA. Trends Microbiol. 1993;1:55–58. doi: 10.1016/0966-842x(93)90033-n. [DOI] [PubMed] [Google Scholar]
- 11.Kanbe T, Cutler JE. Infect. Immun. 1994;62:1662–1668. doi: 10.1128/iai.62.5.1662-1668.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalle F, Jouault T, Trinel PA, Esnault J, Mallet JM, d’Athis P, Poulain D, Bonnin A. Infect. Immun. 2003;71:7061–7068. doi: 10.1128/IAI.71.12.7061-7068.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukazawa Y, Kagaya K. J. Med. Vet. Mycol. 1997;35:87–99. doi: 10.1080/02681219780000971. [DOI] [PubMed] [Google Scholar]
- 14.Frieman MB, McCaffery JM, Cormack BP. Mol. Microbiol. 2002;46:479–492. doi: 10.1046/j.1365-2958.2002.03166.x. [DOI] [PubMed] [Google Scholar]
- 15.Gaur NK, Klotz SA. Microbiology. 2004;150:277–284. doi: 10.1099/mic.0.26738-0. [DOI] [PubMed] [Google Scholar]
- 16.Dean N. Biochim. Biophys. Acta. 1999;1426:309–322. doi: 10.1016/s0304-4165(98)00132-9. [DOI] [PubMed] [Google Scholar]
- 17.Gemmill TR, Trimble RB. Biochim. Biophys. Acta. 1999;1426:227–237. doi: 10.1016/s0304-4165(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 18.Gentzsch M, Tanner W. Glycobiology. 1997;7:481–486. doi: 10.1093/glycob/7.4.481. [DOI] [PubMed] [Google Scholar]
- 19.Häusler A, Robbins PW. Glycobiology. 1992;2:77–84. doi: 10.1093/glycob/2.1.77. [DOI] [PubMed] [Google Scholar]
- 20.Häusler A, Ballou L, Ballou CE, Robbins PW. Proc. Natl. Acad. Sci. U. S. A. 1992;89:6846–6850. doi: 10.1073/pnas.89.15.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill K, Boone C, Gobel M, Puccia R, Sdicu AM, Bussey H. Genetics. 1992;130:273–283. doi: 10.1093/genetics/130.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lussier M, Sdicu AM, Bussereau F, Jacquet M, Bussey H. J. Biol. Chem. 1997;272:15527–15531. doi: 10.1074/jbc.272.24.15527. [DOI] [PubMed] [Google Scholar]
- 23.Lussier M, Sdicu AM, Bussey H. Biochim. Biophys. Acta. 1999;1426:323–334. doi: 10.1016/s0304-4165(98)00133-0. [DOI] [PubMed] [Google Scholar]
- 24.Lussier M, Sdicu AM, Camirand A, Bussey H. J. Biol. Chem. 1996;271:11001–11008. doi: 10.1074/jbc.271.18.11001. [DOI] [PubMed] [Google Scholar]
- 25.Lussier M, Sdicu AM, Winnett E, Vo DH, Sheraton J, Dusterhoft A, Storms RK, Bussey H. Yeast. 1997;13:267–274. doi: 10.1002/(SICI)1097-0061(19970315)13:3<267::AID-YEA72>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 26.Nakijama T, Ballou C. Proc. Natl. Acad. Sci. U. S. A. 1977;72:3912–3916. doi: 10.1073/pnas.72.10.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero PA, Lusssier M, Veroneau S, Sdidu AM, Herscovics A, Bussey H. Glycobiology. 1999;9:1045–1051. doi: 10.1093/glycob/9.10.1045. [DOI] [PubMed] [Google Scholar]
- 28.Romero PA, Lussier M, Sdicu AM, Bussey H, Herscovics A. Biochem. J. 1997;321:289–295. doi: 10.1042/bj3210289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hercovics A. Biochim. Biophys. Acta. 1999;1426:275–285. doi: 10.1016/s0304-4165(98)00129-9. [DOI] [PubMed] [Google Scholar]
- 30.Cutler JE. Med. Mycol. 2001;39S:75–86. [PubMed] [Google Scholar]
- 31.Hobson RP, Munro CA, Bates S, MacCallum DM, Cutler JE, Heinsbroek SEM, Brown GD, Odds FC, Gow NAR. J. Biol. Chem. 2004;279:39628–39635. doi: 10.1074/jbc.M405003200. [DOI] [PubMed] [Google Scholar]
- 32.Buurman ET, Westwater C, Hube B, Brown AJP, Odds FC, Gow NAR. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7670–7675. doi: 10.1073/pnas.95.13.7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibata N, Ichikawa T, Tojo M, Takahasi M, Ito N, Okubo Y, Suzuki S. Arch. Biochem. Biophys. 1985;243:338–348. doi: 10.1016/0003-9861(85)90511-9. [DOI] [PubMed] [Google Scholar]
- 34.Herrero AB, Uccelletti D, Hirschberg CB, Dominguez A, Abeijon C. Euk. Cell. 2002;1:420–431. doi: 10.1128/EC.1.3.420-431.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gentzsch M, Tanner W. EMBO J. 1996;15:5752–5759. [PMC free article] [PubMed] [Google Scholar]
- 36.Strahl-Bolsinger S, Gentzsch M, Tanner W. Biochim. Biophys. Acta. 1999;1426:297–307. doi: 10.1016/s0304-4165(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 37.Timpel C, Strahl-Bolsinger S, Ziegelbauer K, Ernst JF. J. Biol. Chem. 1998;273:20837–20846. doi: 10.1074/jbc.273.33.20837. [DOI] [PubMed] [Google Scholar]
- 38.Timpel C, Zink S, Strahl-Bolsinger S, Schroppel K, Ernst J. J. Bacteriol. 2000;182:3063–3071. doi: 10.1128/jb.182.11.3063-3071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson LM, Bates S, Yamazaki S, Arisawa M, Aoki Y, Gow NAR. J. Biol. Chem. 2000;275:18933–18938. doi: 10.1074/jbc.M909699199. [DOI] [PubMed] [Google Scholar]
- 40.Lobsanov YD, Romero PA, Sleno B, Yu B, Yip P, Herscovics A, Howell PL. J. Biol. Chem. 2004;279:17921–17931. doi: 10.1074/jbc.M312720200. [DOI] [PubMed] [Google Scholar]
- 41.Gow NAR, Gooday GW. J. Gen. Microbiol. 1982;128:2187–2194. doi: 10.1099/00221287-128-9-2187. [DOI] [PubMed] [Google Scholar]
- 42.Fonzi WA, Irwin MY. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brand A, MacCallum DM, Brown AJP, Gow NAR, Odds FC. Euk. Cell. 2004;3:900–909. doi: 10.1128/EC.3.4.900-909.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffman CS, Winston F. Gene (Amst.) 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 45.Schaller M, Schafer W, Korting HC, Hube B. Mol. Microbiol. 1998;29:605–615. doi: 10.1046/j.1365-2958.1998.00957.x. [DOI] [PubMed] [Google Scholar]
- 46.Maneu V, Cervera AM, Martinez JP, Gozalbo D. FEMS Microbiol. Lett. 1996;145:157–162. doi: 10.1111/j.1574-6968.1996.tb08571.x. [DOI] [PubMed] [Google Scholar]
- 47.Hughes HB, Carzaniga R, Rawlings SL, Green JR, O’Connell RJ. Microbiology. 1999;145:1927–1936. doi: 10.1099/13500872-145-8-1927. [DOI] [PubMed] [Google Scholar]
- 48.Kapteyn JC, Van Egmond P, Sievi E, Van Den Ende H, Makarow M, Klis FM. Mol. Microbiol. 1999;31:1835–1844. doi: 10.1046/j.1365-2958.1999.01320.x. [DOI] [PubMed] [Google Scholar]
- 49.Ferguson MAJ. Lipid Modification of Proteins: A Practical Approach. IRL Press; Oxford: 1992. pp. 191–230. [Google Scholar]
- 50.Dallies N, Francois J, Paquet V. Yeast. 1998;14:1297–1306. doi: 10.1002/(SICI)1097-0061(1998100)14:14<1297::AID-YEA310>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 51.Munro CA, Whitton RK, Hughes HB, Rella M, Selvaggini S, Gow NAR. Fung. Gen. Biol. 2003;40:146–158. doi: 10.1016/s1087-1845(03)00083-5. [DOI] [PubMed] [Google Scholar]
- 52.Douglas LJ, Houston JG, McCourtie J. FEMS Microbiol. Lett. 1981;12:241–243. [Google Scholar]
- 53.Kimura LH, Pearball NN. Infect. Immun. 1978;21:64–68. doi: 10.1128/iai.21.1.64-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watts HJ, Cheah FSH, Hube B, Sanglard D, Gow NAR. FEMS Microbiol. Lett. 1998;159:129–135. doi: 10.1111/j.1574-6968.1998.tb12851.x. [DOI] [PubMed] [Google Scholar]
- 55.Gow NAR, Bates S, Brown AJP, Buurman ET, Thomson LM, Westwater C. Biochem. Soc. Trans. 1999;27:512–516. doi: 10.1042/bst0270512. [DOI] [PubMed] [Google Scholar]
- 56.Seoighe C, Federspiel N, Jones T, Hansen N, Bivolarovic V, Surzycki R, Tamse R, Komp C, Huizar L, Davis RW, Scherer S, Tait E, Shaw DJ, Harris D, Murphy L, Oliver K, Taylor K, Rajandream M-A, Barell BG, Wolfe KH. Proc. Natl. Acad. Sci. U. S. A. 2000;97:14433–14437. doi: 10.1073/pnas.240462997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wood PJ. Carbohydr. Res. 1980;85:271–287. [Google Scholar]
- 58.Popolo L, Gualtieri T, Ragni E. Med. Mycol. 2001;39S:111–122. [PubMed] [Google Scholar]
- 59.Lagorce A, Hauser NC, Labourdette D, Rodriguez C, Martin-Yken H, Arroyo J, Hoheisel JD, François J. J. Biol. Chem. 2003;278:20345–20357. doi: 10.1074/jbc.M211604200. [DOI] [PubMed] [Google Scholar]
- 60.Garćia R, Bermejo C, Grau C, Pérez R, Rodríguez-Peńa JM, Francois J, Nombela C, Arroyo J. J. Biol. Chem. 2004;279:15183–15195. doi: 10.1074/jbc.M312954200. [DOI] [PubMed] [Google Scholar]
- 61.Casanova M, Lopez Ribot JL, Monteagudo C, Llombartbosch A, Sentan-dreu R, Martinez JP. Infect. Immun. 1992;60:4221–4229. doi: 10.1128/iai.60.10.4221-4229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jentoft N. Trends Biochem. Sci. 1990;15:291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]