SUMMARY
We assessed the distribution of Trypanosoma cruzi Discrete Typing Units (DTUs) in domestic and peridomestic Triatoma infestans and Triatoma sordida specimens collected in a well-defined rural area in Pampa del Indio, northeastern Argentina. Microscopically-positive bugs were randomly selected with a multi-level sampling design, and DTUs were identified using direct PCR strategies. TcVI predominated in 61% of 69 T. infestans and in 56% of 9 T. sordida. TcV was the secondary DTU in T. infestans (16%) and was found in one T. sordida specimen (11%). Three T. sordida (33%) were found infected with TcI, a DTU also identified in local Didelphis albiventris opossums. Mixed DTU infections occurred rarely (5%) and were detected both directly from the bugs’ rectal ampoule and parasite cultures. The identified DTUs and bug collection sites of T. infestans were significantly associated. Bugs infected with TcV were almost exclusively captured in domiciles whereas those with TcVI were found similarly in domiciles and peridomiciles. All mixed infections occurred in domiciles. TcV-infected bugs fed more often on humans than on dogs, whereas TcVI-infected bugs showed the reverse pattern. T. sordida is a probable sylvatic vector of TcI linked to D. albiventris, and could represent a secondary vector of TcVI and TcV in the domestic/peridomestic cycle.
Keywords: Trypanosoma cruzi, Discrete Typing Units, PCR, Triatoma infestans, Triatoma sordida Chagas disease
INTRODUCTION
American Trypanosomiasis (Chagas disease) is the most important parasitic infection in Latin America in terms of public health and economic impact. Ten to 15 million people are infected by Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) and 28 million people remain at risk of infection (WHO, 2007). Natural infections by T. cruzi are constituted by multiple clones with different biological properties such as virulence and tissue tropism (Macedo and Pena, 1998). T. cruzi is currently classified into six Discrete Typing Units (DTU), TcI – TcVI (Zingales et al. 2009), defined as “sets of stocks that are genetically more related to each other than to any other stock and that are identifiable by common genetic, molecular or immunological markers” (Tibayrenc et al. 1998). Considerable genetic diversity at sub-DTU level has been revealed within TcI and TcIII parasite isolates (Herrera et al. 2007; Llewellyn et al. 2009, 2011; Miles et al. 2009; Cura et al. 2010).
The DTUs of T. cruzi are distributed differentially among triatomine bugs, vertebrate host species and habitats in different geographical areas (Higo et al. 2004, Noireau et al. 2009). The Gran Chaco is a biogeographical region that stretches mostly over Argentina, Paraguay and Bolivia. With 4 million people living under conditions of poverty and weak health-care systems, this region is hyperendemic for Chagas disease and other preventable diseases (Gürtler et al. 2007a). In the Argentine Chaco, Triatoma infestans constitutes the main domestic vector of T. cruzi and humans, dogs and cats are the most important domestic hosts. Natural infection by T. cruzi has been found in local sylvatic mammals such as Didelphis albiventris opossums and Dasypus novemcinctus armadillos (Yeo et al. 2005; Ceballos et al. 2006), and in sylvatic triatomine bugs mostly within the Triatoma sordida complex (Bar and Wisnivesky-Colli, 2001). However, the role of these species as vectors of T. cruzi in the sylvatic cycle remains unclear (Marcet et al. 2006; Alvarado-Otegui et al. in revision). We hypothesized that T. sordida is a putative sylvatic vector of T. cruzi and may represent a putative (peri)domestic vector in the study area. In the Argentine Chaco, T. infestans has been found infected with TcV and TcVI, and the frequency of these DTUs differed between study areas (Diosque et al. 2003; Cardinal et al. 2008). Understanding the complex epidemiology of T. cruzi and the variety of transmission cycles and pathogenic behaviors requires a representative, clearer picture of parasite genetic diversity (Brisse et al. 2001; Miles et al. 2009). Convenience sampling of insect vectors or patient samples have usually been performed to characterize the genetic diversity of T. cruzi at a local level (Diosque et al. 2003; Marcet et al. 2006; Burgos et al. 2007). In addition, most parasite typing studies required isolation by culture expansion (de Luca d’Oro et al. 1993; Montamat et al. 1987, 1992; Diosque et al. 2003; Cardinal et al. 2008; Lewis et al., 2009) at the possible expense of selecting certain strains, as was previously described in other studies (Bosseno et al. 2000).
Here we investigated the distribution of T. cruzi DTUs in T. infestans and T. sordida in a well-defined rural area in the Argentine Chaco using direct PCR techniques. For T. infestans, we tested the association between identified DTU and bloodmeal source, and we compared our results with the ones obtained in previous studies from areas with dissimilar epidemiological backgrounds (Diosque et al. 2003; Cardinal et al. 2008). Regarding T. sordida, we hypothesized that this species may constitute one of the vectors in the sylvatic cycle of transmission and could also represent a secondary vector in the domestic/peridomestic cycle.
MATERIALS AND METHODS
Study Area
Field studies were carried out in a section (450 km2) of the Municipality of Pampa del Indio (25° 5’S 56° 58’W), Province of Chaco, Argentina, located in the humid (east) Chaco, close to the transition to the dry (west) Chaco. The study area has been described elsewhere (Gurevitz et al. 2011). It included 353 houses and several public buildings clustered in 13 neighboring rural villages. The last community-wide insecticide spraying campaign conducted by vector control personnel was carried out in 1996, except for a few houses treated by villagers or hospital staff in 2006.
Entomological survey
A total of 327 inhabited house compounds was visited for an entomological survey between September and November, 2007. All sites within each household were searched for triatomine bugs by timed manual collections conducted by two skilled bug collectors from the national or provincial vector control programs using 0.2% tetramethrin (Espacial, Argentina) as a flushing-out agent. Domiciles were inspected by one person during 20 min whereas all peridomestic sites were searched by one person during 15 min (Gurevitz et al. 2011). Infestation by T. infestans was determined in 39.8% of inhabited house compounds, and T. sordida was found in 18.3% of them, mainly in peridomestic sites (Gurevitz et al. 2011). Immediately after the baseline survey, a community-wide insecticide spraying campaign was conducted and all sites from each house compound were sprayed with suspension concentrate deltamethrin (K-Othrina, Bayer) at standard dose (25 mg/m2) in December 2007 by vector control personnel (Gurevitz et al. 2011). During the next three years all houses were regularly inspected for infestation and selectively sprayed with insecticides if found reinfested.
All collected bugs were identified to species and stage at the field laboratory and counted as described elsewhere (Cardinal et al. 2006). All live or moribund third to fifth-instar nymphs and adult bugs were individually examined for T. cruzi infection by optic microscopy (OM) at 400× within 10 days of capture as described (Cardinal et al. 2006). The overall prevalence of T. cruzi infection was 22.1% (n, number of examined bugs = 2,138) for T. infestans and 1.0% (n = 290) for T. sordida (Cardinal et al. unpublished results).
Sampling design
Multi-level sampling was used to select OM-positive T. infestans and establish the overall distribution of DTUs. The 13 rural villages were divided in four strata according to the village-specific prevalence of houses with T. cruzi-infected T. infestans bugs: high (over 60%), medium (between 40% and 60%), low (between 10% and 30%), and very low (below 5%). The village-specific prevalence of houses with infected bugs was defined as the number of houses with at least one T. infestans positive for T. cruzi (as determined by OM) divided by the total number of houses positive for T. infestans. The very-low prevalence strata included the rural villages of Los Ciervos and La Herradura which had a bug infection prevalence ≤1% and were therefore ruled out of the sampling frame. Among the rest of study villages, 35% of all houses were randomly selected to enhance the power of subsequent statistical tests.
Within the randomly-selected houses, all T. infestans collection sites were sampled. To define the total number of bugs to sample within the house-compound level, bug collection sites were divided in three abundance strata (i.e., less than 5, between 5 and 15, and more than 15 infected bugs). All bugs from the first stratum were analyzed; 40% of bugs from the second one were selected, and 20% of bugs from the third one were used for DTU identification. Upper and lower strata boundaries were defined according to the outcome of a preliminary sampling exercise showing that: 1) In sites with low bug abundance, it was necessary to examine all OM-positive bugs to display the whole DTU diversity; 2) In sites with high bug abundance, there was an upper number of examined bugs above which no new DTU was identified (i.e., 6 bugs). In this regard, all sites with high bug abundance presented over 30 specimens; thus the minimum of 6 bugs was always ensured. The described sampling protocol yielded a total of 114 OM-positive T. infestans from 25 house compounds in 10 rural villages.
For T. sordida, all domestic and peridomestic bugs infected with T. cruzi were analyzed because of its very low infection prevalence. Therefore, we included the total number of OM-positive bugs from surveys conducted before (September-November 2007) and after residual spraying with insecticides (April and November, 2008; May and September, 2009). In total, we analyzed nine OM-positive T. sordida collected from seven house compounds located in seven villages.
DNA extraction
T. cruzi DNA samples were extracted from the bugs’ rectal ampoule of all selected insects by cutting the abdomen below the third tergite and then storing it in microtubes containing 25 ul of sterile saline solution. Forceps were rinsed in 10% bleach and 70% ethanol and flamed between dissections of successive bugs. Negative controls of this procedure were obtained by systematically rinsing forceps in saline solution on a slide and storing the wet preparation in sterile microtubes. The rectal ampoules were boiled for 15 minutes and DNA from 25 μl of each fecal sample was purified using DNAzol® (Invitrogen, USA) reagent as described previously (Marcet et al. 2006).
Parasite culture
Isolation of T. cruzi from feces of a subset of OM-positive bugs (20 T. infestans and 9 T. sordida) and cultures in biphasic medium (Nutrient agar defibrinated rabbit blood/Brain Heart Infusion) were conducted at the National Institute of Parasitology “Dr. Mario Fatala Chabén”-ANLIS. Cultures were kept at 28°C and 50% relative humidity and microscopically monitored for parasite growth bimonthly for four months. Cultures were then stored in liquid nitrogen and defrosted for genotyping as described elsewhere (Lauricella et al. 2005). T. cruzi DNA was then extracted from culture isolates as before (Marcet et al. 2006).
DTU identification
Trypanosoma cruzi DTUs were identified using a combination of PCR strategies targeted to nuclear genomic markers which had been previously optimized for direct identification from blood samples (Burgos et al., 2007). Consequently, we assumed the protocol proposed by Burgos et al. (2007) could be applied to direct identification of DTUs from samples obtained from the bugs’ rectal ampoules. Given that for most samples we did not use amplification of parasites by culture, our main concern was to choose a set of PCR strategies that would not require large amounts of T. cruzi DNA to identify DTUs. The selected protocol allowed successful DTU typing using a range of DNA (100 fg-10 pg) (Burgos et al., 2007) that may be obtained from rectal ampoules (results not shown), and was smaller than the ones required by other protocols (Lewis et al., 2009). In all rectal ampoule samples we incorporated Taq platinum polymerase (Invitrogen, USA) to augment sensitivity.
As detailed elsewhere (Burgos et al., 2007), the selected protocol targeted three different genomic markers: the intergenic region of spliced leader genes (SL-IR), the D7 domain of the 24Sα ribosomal RNA genes, and the genomic marker A10. Amplification of the SL-IR using three independent hot-start PCR reactions, named SL-IR I, SL-IRac and SL-IR II, were carried out for a first classification of T. cruzi populations in three groups of DTUs: Tc I, Tc IV/III and Tc II/V/VI, respectively. Regarding the 24Sα ribosomal RNA genes, a dimorphic region within the D7 domain was amplified by hot-start heminested PCR to distinguish between Tc V and TcII/TcVI groups. The first round PCR was performed using D75 and D76 primers. The heminested round was carried out using 1 ul of the first round PCR in a 30 ul vol. reaction using primers D71-D76. Finally genomic marker A10 was used in two rounds of PCRs to separate TcII from TCVI. The first round was carried out using Pr1 and P6 primers whereas the heminested round was performed with primers Pr1 and Pr3 (Burgos et al., 2007). PCR products were analyzed in 3% agarose gels (Invitrogen, USA) and UV visualization after staining with Gel Red (GenBiotech).
It is necessary to point out that some samples infected with TcV amplified both 125 and 140 bp ribosomal DNA bands in the 24Sα DNA-PCR (D71 and D76) (Burgos et al. 2007). When this pattern appears, it is not possible to differentiate infections with TcV from mixed infections with TcV+TcVI. When possible, we considered the results from the first round of the 24s alpha rDNA-PCR (D75 and D76) to distinguish infections with only TcV from those with TcV+TcVI. However, some of the rectal-ampoule samples presented less DNA content and therefore differentiation between these DTUs could not be achieved. For comparative purposes, these bugs were considered as only infected with TcV because (i) TcV infected the bugs beyond any doubt, and (ii) when we were able to use results from the first round of the 24s alpha rDNA-PCR, only three bugs that showed the double-band pattern presented a mixed infection with TcV+TcVI. Due to the weak sensitivity of the A10 genomic marker, some rectal ampoule samples could not be resolved as TcII or TcVI; these cases were identified as TcII/VI. For statistical analysis we considered them as TcVI, as no TcII infection has been detected in domestic or sylvatic hosts or vectors from our study area so far (unpublished results).
Identification of bloodmeal sources
A direct ELISA assay was used to test bloodmeal contents against human, dog, cat, chicken and goat antisera as described elsewhere (Gürtler et al. 2009).
Data analysis
The associations between parasite DTU and other attributes (bug collection site, bloodmeal sources, and study areas) were assessed by means of Fisher’s exact test. The degree of agreement between paired results of DTU identification based on DNA samples obtained by parasite culture and from rectal-ampoule material was assessed by the exact McNemar’s test. All tests were made using Stata 10.1 (StataCorp 2007).
RESULTS
Triatoma infestans
In a preliminary sample of 15 OM-positive T. infestans collected in the study area and from other villages within the district (not included in later analyses) that were cultured, TcVI was identified in 93% of the bugs whereas TcV was found only in one specimen.
We analyzed 114 (peri)domestic OM-positive T. infestans from 25 selected house compounds (Fig. 1). Identification of parasite DTUs from the bugs’ rectal ampoule by means of direct PCR strategies was successful in 59 (52%) insects. Identification of DTUs from parasite cultures was successful in all samples. Overall, TcVI was found in 61% (n = 69) of the bugs, and TcV in 16% of them (Table 1). Three specimens showed mixed infections of TcV+TcVI. Figure 2 shows the amplification results obtained with the 24Sα DNA-PCRs. Thirteen T. infestans that were identified as TcV/TcV+TcVI were considered as infected only with TcV for further analyses.
Figure 1.
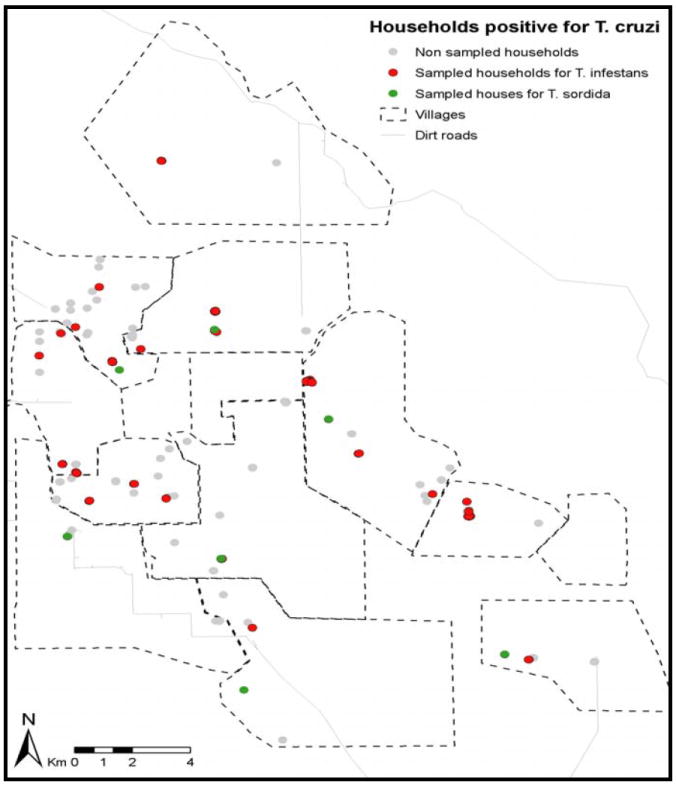
Map of the study area in Pampa del Indio showing villages (polygons) and houses positive for T. cruzi-infected triatomine bugs (dots). Dots identify positive houses sampled for DTU identification in T. infestans (red dots) and T. sordida specimens (green dots).
Table 1.
Identification of T. cruzi DTUs in domestic and peridomestic vector species, Pampa del Indio, Chaco Province, Argentine, 2007-2009.
| Species | Identified DTU
|
|||||
|---|---|---|---|---|---|---|
| TcV | TcVI | TcV+TcVI | TcV / TcV+TcVIa | TcI | Total | |
| T. infestans | 11 | 42 | 3 | 13 | 0 | 69 |
| T. sordida | 0 | 5 | 0 | 1 | 3 | 9 |
Single TcV infections or mixed infections with TcV+TcVI could not be distinguished.
Figure 2.
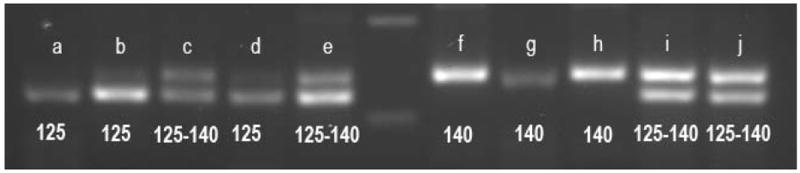
Example of the heminested 24Sα rDNA PCR using primers D71-D76 product size polymorphism in DNA samples from parasites cultures and bugs’ rectal ampoules. Bands a-e show the two patterns that individuals infected with TcV display: a single band of 125 bp or two bands of 125 + 140 bp, (bands a and d are samples obtained from bugs’ rectal ampoules; bands b and e from parasite cultures; band c: TcV reference stock, PAH 265). Central lane: 100 bp ladder. Bands f-h show the unique pattern for TcVI, a single band of 140 bp (band f: parasite culture sample, band g: rectal ampoule sample, band h: TcVI reference stock CL-Brener). Finally, bands i (parasite culture sample) and j (rectal ampoule sample) show the pattern observed for mixed infections TcV+TcVI that correspond also to a double band of 125 + 140 bp.
Triatoma sordida
DTU identification was achieved in the nine OM-positive T. sordida detected (Table 1). TcVI was identified in 56% of the insects, and was also the main DTU. T. sordida bugs infected with TcVI were collected both in domestic and peridomestic sites: two of them were collected in a pig corral; one in a tree where chickens roosted at night, and two in different domiciles. An adult T. sordida infected with TcV was found in a kitchen near human sleeping quarters. Three adult T. sordida were found infected with TcI; two of them were captured in a chicken coop and the remainder in a tree where chickens roosted at night.
Identification of DTUs from parasite culture and rectal ampoule samples
The extraction of parasite DNA from rectal ampoules led to successful DTU identification in 52% of 114 T. infestans and in 100% of 9 T. sordida. The remaining samples tested negative or were identified incompletely mainly due to scarce fecal material. Samples from parasite culture always allowed identification of DTUs in both species of triatomine bugs. A subset of 20 T. infestans and 4 T. sordida was typified using both types of DNA samples. Paired results of DTU identification agreed in 18 bugs whereas for 6 specimens we could not identify the DTUs from rectal-ampoule material (exact McNemar’s test, p = 0.031). All 3 mixed infections with TcV+TcVI detected directly from fecal material were also detected from parasite cultures.
DTU distribution among ecotopes
Most of the OM-positive T. infestans analyzed for DTUs were collected in domestic sites (68%). T. cruzi-infected bugs from peridomestic sites such as storerooms and corrals were much less frequent (29%). A highly significant association between bug collection site and parasite DTU was detected (Fisher’s exact test, p = 0.001) (Table 2). TcV was found almost exclusively in domestic bugs (95%) and was the main DTU in human habitations. TcVI was detected with rather similar frequency in domestic (57%) and peridomestic bugs (43%) (Table 2). All of the mixed infections with TcV+TcVI occurred in domiciles and were excluded from this analysis.
Table 2.
Distribution of T. cruzi DTUs according to the collection sites of infected T. infestans, Pampa del Indio, Chaco Province, Argentine, 2007.
Three bugs with unreliable information regarding collection site were excluded.
Three bugs with mixed infections of TcV+TcVI were excluded.
DTU distribution and bloodmeal sources
The association between identified DTUs and bloodmeal sources was investigated (Table 3). Of 50 T. infestans with identified DTUs, only 21 were ELISA-reactive. The rest of the bugs lacked bloodmeal contents on dissection and later were not reactive; all T. sordida specimens also lacked bloodmeal contents and were not tested by ELISA. Blood meals were identified in 10 (20%) T. infestans infected with TcV, 10 (20%) infected with TcVI, and in 1 (2%) having a mixed infection with TcV+TcVI. Bugs infected with TcV had fed mainly on chickens only (40%) and humans only (33%); one insect had fed on dog only, and two bugs had mixed blood meals on human and chicken or dog. Among 10 ELISA-reactive bugs infected with TcVI, 60% had fed on dogs only, 20% on chickens only, and 20% on humans only. The insect with a mixed TcV+TcVI infection was positive for human blood only. All human-fed bugs were captured in domiciles. Disregarding chicken blood meals (because they cannot be a source of T. cruzi infection) and assuming independence between each identified dog or human blood meal and each identified DTU (i.e., each meal and each DTU counts separately), the relative frequency of dog:human meals was not statistically associated to infection with TcV (2:6) and TcVI (6:3) (Fisher’s exact test, p = 0.153).
Table 3.
Association between identified DTUs and bloodmeal sources of T. infestans, Pampa del Indio, Chaco Province, Argentine, 2007.
| Bloodmeal source | Identified DTU
|
Total | ||
|---|---|---|---|---|
| TcV | TcVI | TcV-VI | ||
| Dog | 1 | 6 | 0 | 7 |
| Chicken | 4 | 2 | 0 | 6 |
| Human | 3 | 2 | 1 | 6 |
| Human–Chicken | 1 | 0 | 0 | 1 |
| Human–Dog | 1 | 0 | 0 | 1 |
| Not reactive | 7 | 20 | 2 | 29 |
| Total | 17 | 30 | 3 | 50 |
Comparative distribution of DTUs in the Argentine Chaco
To assess the occurrence of geographic variation in the distributions of parasite DTUs in T. infestans in the Argentine Chaco, we compared the data recorded at Pampa del Indio with those recorded in the Department of Chacabuco (SE of Chaco Province) (Diosque et al. 2003) and in the Department of Moreno (E of Santiago del Estero Province) (Cardinal et al. 2008) (Fig. 3). Pampa del Indio and Chacabuco lacked recent vector control actions and showed high levels of house infestation and prevalence of infection by T. cruzi both in vectors and hosts. The Moreno study area had been under sustained or more sporadic control efforts, and had low to very low levels of house infestation and prevalence of bug or host infection with T. cruzi. For statistical analysis, we included T. infestans bugs infected with TcV and TcVI, and excluded bugs with TcI to avoid having contingency tables with very sparse data. The frequency distribution of DTUs differed in a highly significant fashion between Pampa del Indio and Moreno (Fisher’s exact test, p = 0.0001); in the latter almost all bugs were found infected with TcVI and the prevalence of TcV was marginal. Pampa del Indio and Chacabuco presented similar prevalence of bug infection with TcV and TcVI (Fisher’s exact test, p = 0.117). TcI was detected in T. infestans from Chacabuco and Moreno but not in Pampa del Indio.
Figure 3.
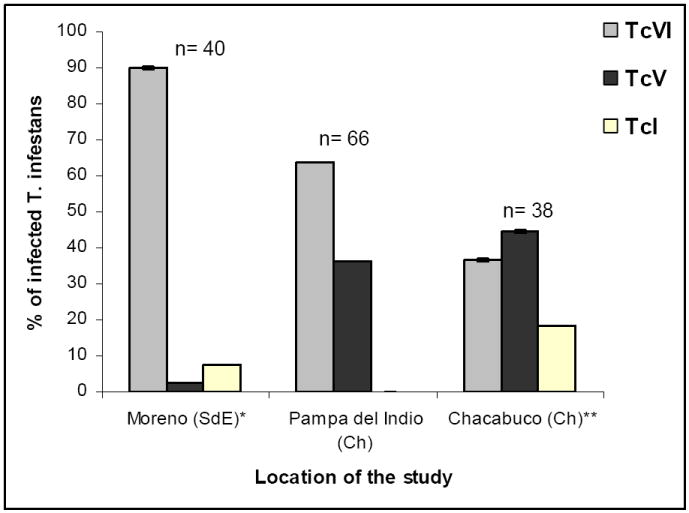
Geographical variation of T. cruzi DTU distribution in T. infestans from three study areas in the Argentine Chaco. Numbers on top of the bars represent the total number of bugs with identified DTUs.
DISCUSSION
Our study shows that at least three DTUs (TcI, TcV and TcVI) were present in T. infestans bugs collected in domestic or peridomestic habitats in the study area. TcVI and TcV infected domestic or peridomestic T. infestans, with predominance of TcVI. This may be the first study in which the genotypic diversity of natural populations of T. cruzi associated with triatomine bugs is studied in a carefully selected, representative sample of triatomine bugs in a well-defined area. The number of parasite samples from bugs more than doubled the size of previous studies in the Argentine Chaco (Diosque et al. 2003; Marcet et al. 2006; Cardinal et al. 2008).
A novel finding of our study is the strong association between the distribution of identified DTUs and the individual collection sites of T. infestans. Bugs infected with TcV were almost exclusively collected in human sleeping quarters, where they most likely contracted the infection, whereas TcVI-infected bugs occurred indistinctively in peridomestic or domestic ecotopes. Whether the latter might have become infected in domiciles and then dispersed to peridomestic habitats or vice versa is also uncertain. For example, in Santiago del Estero Province adult T. infestans infected with TcVI were collected with light traps while dispersing by flight most likely out of a human habitation toward peridomestic habitats (Vazquez-Prokopec et al. 2006, Cardinal et al. 2008). Another possibility is that bug infections by TcVI originated in domestic and peridomestic ecotopes from dogs or cats infected with TcVI that used both habitats.
Our results do not provide sufficient evidence on the association between bloodmeal sources (dog or human) and DTUs (TcVI and TcV, respectively). Unfortunately, most of the bugs with identified DTUs lacked bloodmeal contents, and therefore the final sample size of reactive bugs was very small. A second limitation is that bloodmeal identification tests fail to detect the old blood meals that may have originated the detected infection (e.g., infected bugs with chicken-only blood meals). However, TcVI-infected bugs were more often fed on dogs than on humans whereas TcV-infected bugs tended to show the reverse pattern. These results are consistent with the important role of dogs as domestic reservoir hosts of T. cruzi in northern Argentina and probably elsewhere (Gürtler et al. 2007a, 2007b, Cohen and Gürtler, 2001; Cardinal et al. 2007, 2008). A larger survey of blood-feeding sources of T. infestans in Pampa del Indio showed that domestic bugs fed mainly on humans followed by chickens and dogs, whereas peridomestic bugs blood-fed on dogs and chickens (Ordóñez-Krasnowski et al. unpublished results). Consistent with these patterns, most human cases throughout the Argentine Chaco have been found infected with TcV (De Luca D’Oro et al. 1993; Diosque et al. 2003; Cardinal et al. 2008, Cura et al. 2011), whereas elsewhere in the Gran Chaco humans are also infected with TcI and TcII (Brenière et al. 2002; Gomes Abolis et al. 2011; Cura et al. 2011). Ongoing efforts seeking to identify DTUs from humans in Pampa del Indio could shed light on the source of infection of TcV-infected bugs.
Although frequent elsewhere in the Gran Chaco region (Bosseno et al. 2000; Breniére et al. 2002, Yeo et al. 2007), in our study area mixed infections were rare, only found in 5% of the bugs. This could be explained by the occurrence of differential DTU selection processes during culture. However, all mixed infections with TcV+TcVI were identified from both rectal ampoule and culture samples and DTU identification were predominantly performed directly from rectal ampoule samples. Previous studies showed that initially mixed infections with TcI and TcV displayed high degrees of selection at DTU level during culture in liquid medium (Bosseno et al. 2000). Groups of clones from these DTUs also presented different growth rates. TcI had a faster growth rate than TcV in LIT monophasic medium (Laurent et al. 1997) whereas TcVI had faster growth rates than TcII in liquid medium culture (Yeo et al. 2007). Our results might be explained by the fact that infections were composed by TcV and TcVI and by the type of culture medium used. Events of selection at the infra-DTU level during DNA extraction from rectal ampoules, culture or when performing PCR were not assessed and cannot be discarded (Llewellyn et al. 2011).
The observed distribution of DTUs in Pampa del Indio differed significantly from the one in Moreno, where TcVI predominated and TcV was rare (Cardinal et al. 2008), and resembled the pattern recorded elsewhere in Chaco Province where TcV and TcVI presented similar frequencies (Diosque et al. 2003). In areas with sustained vector control actions such as in Moreno, house infestation and prevalence levels of T. cruzi are low so domestic transmission is depressed or interrupted and human prevalence of T. cruzi declines to low levels over extended time periods (Gürtler et al. 2007). T. cruzi infection in the household is then focused on dogs and cats, which in the Argentine Chaco are generally infected with TcVI (Diosque et al. 2003, 3004; Cardinal et al. 2008). In such context, dogs and cats would act as the primary sources of parasite infection for T. infestans and the DTUs in bugs and dogs/cats would agree as recorded by Cardinal et al. (2006, 2008) in Moreno.
In contrast, in areas with no regular vector control actions, higher infestation and host and bug infection levels are observed and domestic transmission is intense. In such scenario, the possible sources of infection for T. infestans are more diverse and include TcV in humans and TcVI and TcI in dogs and cats, as in Chacabuco (Diosque et al. 2003, 2004). The epidemiological background of Pampa del Indio and Chacabuco were similar, with no recent history of vector control interventions. TcI was identified in both areas infecting D. albiventris opossums (Diosque et al. 2003; Alvarado Otegui et al. in revision). TcI was also detected in T. infestans and dogs in Chacabuco, whereas in Pampa del Indio it was only found in three adult specimens of T. sordida despite a large sampling effort of bugs and hosts.
Our study also shows that T. sordida may have been partially implicated in local domestic transmission cycles, as suggested by the finding of TcV and TcVI in five specimens collected in peridomestic or domestic habitats. To our knowledge, this is the first such finding in Argentina. However, previous studies in Bolivia suggested that T. sordida posed a low risk of human infection with T. cruzi, with transmission mostly confined to synanthropic mammals (Noireau et al. 1997). Experimental studies showed that the vector competence of T. sordida may lag behind that from other vector species such as T. guasayana (Loza-Murguía and Noireau, 2010); thus the role of this species as a putative domestic vector still needs clarification.
Three adult T. sordida captured in peridomestic habitats associated with chickens were infected with TcI. So far TcI has only been detected in local D. albiventris opossums trapped in sylvatic habitats (Alvarado-Otegui et al. in revision). Didelphis opossums have been found infected almost exclusively with TcI throughout the Americas (Wisnivesky-Colli et al. 1992; Diotaiuti et al. 1995; Diosque et al. 2003; Yeo et al. 2005; Ceballos et al. 2006; Alvarado Otegui et al. in revision). The absence of TcI in local T. infestans, domestic dogs and cats (Enríquez et al. unpublished results), combined with the ability of adult T. sordida to disperse by flight (Schofield et al. 1991), suggests that the TcI-infected T. sordida may have become infected from opossums and then invaded peridomestic habitats. However, it is uncertain whether parasite transmission events occurred at the bugs’ collection sites or elsewhere in the forest, given that opossums frequently approach human dwellings and may serve as a bridge host between sylvatic and domestic habitats (Diotaiuti et al. 1995; Schweigmann et al. 1999). The available evidence suggests that T. sordida may be a local sylvatic vector of TcI associated with D. albiventris. The use of microsatellite markers and RFLP-PCR to assess the genetic diversity of TcI in local T. sordida and D. albiventris may shed light on these putative associations.
Acknowledgments
The authors are grateful to Julián Alvarado-Otegui, Sol Gaspe, Juan Gurevitz and Leonardo Ceballos for field and laboratory assistance. To Gustavo Enríquez, Fernando Garelli, Sol Gaspe, Juan Gurevitz, Yael Provecho, Marcela Orozco, Jimena Gronzo, Marina Leporace, Carla Cecere and Romina Piccinali for valuable comments during the study. To the villagers of Pampa del Indio, for kindly welcoming us into their homes and cooperating with the investigation. Reference strains of TcI-TcVI were kindly provided by Patricio Diosque, Miguel A. Basombrío and Michel Tibayrenc to AGS.
FINANCIAL SUPPORT
This study received financial support from International Development Research Center (EcoHealth Program); the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR); the National Institutes of Health/National Science Foundation Ecology of Infectious Disease program award R01 TW05836 funded by the Fogarty International Center and the National Institute of Environmental Health Sciences (to Uriel Kitron and REG), and University of Buenos Aires. REG, MVC and AGS are members of CONICET Researcher’s Career.
References
- Bar ME, Wisnivesky-Colli C. Triatoma sordida Stål 1859 (Hemiptera, Reduviidae: Triatominae) in palms of Northeastern Argentina. Memorias do Instituto Oswaldo Cruz. 2001;96:895–899. doi: 10.1590/s0074-02762001000700002. [DOI] [PubMed] [Google Scholar]
- Bosseno MF, Yacsik N, Vargas F, Brenière SF. Selection of Trypanosoma cruzi clonal genotypes (Clonet 20 and 39) isolated from Bolivian triatomines following subculture in liquid medium. Memorias do Instituto Oswaldo Cruz. 2000;95:601–607. doi: 10.1590/s0074-02762000000500002. [DOI] [PubMed] [Google Scholar]
- Brenière SF, Bosseno MF, Noireau F, Yacsik N, Liegeard P, Aznar C, Hontebeyrie M. Integrated study of a Bolivian population infected by Trypanosoma cruzi, the agent of Chagas Disease. Memorias do Instituto Oswaldo Cruz. 2002;97:289–295. doi: 10.1590/s0074-02762002000300002. [DOI] [PubMed] [Google Scholar]
- Brisse S, Verhoef J, Tibayrenc M. Characterization of large and small subunit rRNA and mini-exon genes further supports the distinction of six Trypanosoma cruzi lineages. International Journal for Parasitology. 2001;31:1218–1226. doi: 10.1016/s0020-7519(01)00238-7. [DOI] [PubMed] [Google Scholar]
- Burgos JM, Altcheh J, Bisio M, Duffy T, Valadares HMS, Seidenstein ME, Piccinali R, Freitas JM, Levin MJ, Machi L, Macedo AM, Freilij H, Schijman AG. Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations cuasing congenital Chagas disease. International Journal for Parasitology. 2007;37:1319–1327. doi: 10.1016/j.ijpara.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Cardinal MV, Castañera MB, Lauricella MA, Cecere MC, Ceballos AL, Vazquez-Prokopec GM, Kitron U, Gürtler RE. A prospective study of the effects of sustained vector surveillance following community-wide insecticide application on Trypanosoma cruzi of dogs and cats in rural northwestern Argentina. American Journal of Tropical Medicine and Hygiene. 2006;75:753–761. [PMC free article] [PubMed] [Google Scholar]
- Cardinal MV, Lauricella MA, Marcet PL, Orozco MM, Kitron U, Gürtler RE. Impact of community-based vector control on house infestation and Trypanosoma cruzi infection in Triatoma infestans, dogs and cats in the Argentine Chaco. Acta Tropica. 2007;103:201–211. doi: 10.1016/j.actatropica.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal MV, Lauricella MA, Ceballos LA, Lanati L, Marcet PL, Levin MJ, Kitron U, Gürtler RE, Schijman AG. Molecular epidemiology of domestic and sylvatic Trypanosoma cruzi infection in rural northwestern Argentina. International Journal for Parasitology. 2008;38:1533–1543. doi: 10.1016/j.ijpara.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos LA, Cardinal MV, Vazquez-Prokopec GM, Lauricella MA, Orozco MM, Cortinas R, Schijman AG, Levin MJ, Kitron U, Gürtler RE. Long-term reduction of Trypanosoma cruzi infection in sylvatic mammals following deforestation and sustained vector surveillance in northwestern Argentina. Acta Tropica. 2006;98:286–296. doi: 10.1016/j.actatropica.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JE, Gürtler RE. Modeling household transmission of American Trypanosomiasis. Science. 2001;293:694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- Cura CI, Mejía-Jaramillo AM, Duffy T, Burgos JM, Rodriguero M, Cardinal MV, Kjos S, Gurgel-Gonçalves R, Blanchet D, De Pablos LM, Tomasini N, da Silva A, Russomando G, Cuba CA, Aznar C, Abate T, Levin MJ, Osuna A, Gürtler RE, Diosque P, Solari A, Triana-Chávez O, Schijman AG. Trypanosoma cruzi I genotypes in different geographical regions and transmission cycles based on a microsatellite motif of the intergenic spacer of spliced-leader genes. International Journal for Parasitology. 2010;40:1599–1607. doi: 10.1016/j.ijpara.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cura CI, Lucero RH, Bisio M, Oshiro E, Formicelli LB, Burgos JM, Lejonas S, Brusés BL, Hernández DO, Severini GV, Velázquez E, Duffy T, Anchart E, Latte R, Altcheh J, Freilij H, Diez M, Nagel C, Vigliano C, Favaloro L, Favaloro RR, Merino DE, Sosa-Estani S, Schijman AG. Trypanosoma cruzi Discrete Typing Units in Chagas disease patients from endemic and non endemic regions of Argentina. Parasitology. doi: 10.1017/S0031182011002186. in press. [DOI] [PubMed] [Google Scholar]
- De Luca D’Oro GM, Gardenal CN, Perret B, Crisci JV, Montamat EE. Genetic structure of Trypanosoma cruzi populations from Argentina estimated from enzyme polymorphism. Parasitology. 1993;107:405–410. doi: 10.1017/s0031182000067755. [DOI] [PubMed] [Google Scholar]
- Diosque P, Barnabe C, Padilla AM, Marco JD, Cardozo RM, Cimino RO, Nasser JR, Tibayrenc M, Basombrio MA. Multilocus enzyme electrophoresis analysis of Trypanosoma cruzi isolates from a geographically restricted endemic area for Chagas’ disease in Argentina. International Journal for Parasitology. 2003;33:997–1003. doi: 10.1016/s0020-7519(03)00139-5. [DOI] [PubMed] [Google Scholar]
- Diosque P, Padilla AM, Cimino RO, Marino Cardozo R, Sanchez Negrette O, Marco JD, Zacca R, Meza C, Juarez A, Rojo H, Rey R, Corrales RM, Nasser JR, Basombrío MA. Chagas disease in rural areas of Chaco province, Argentina: epidemiologic survey in humans, reservoirs, and vectors. American Journal for Tropical Medicine and Hygiene. 2004;71:590–593. [PubMed] [Google Scholar]
- Diotaiuti L, Pereira AS, Loiola CF, Fernandes AJ, Schofield JC, Dujardin JP, Dias JC, Chiari E. Inter-relation of sylvatic and domestic transmission of Trypanosoma cruzi in areas with and without domestic vectorial transmission in Minas Gerais, Brazil. Memorias do Instituto Oswaldo Cruz. 1995;90:443–448. doi: 10.1590/s0074-02761995000400002. [DOI] [PubMed] [Google Scholar]
- Gurevitz JM, Ceballos LA, Gaspe MS, Alvarado Otegui JA, Enríquez GF, Kitron U, Gürtler RE. Factors affecting infestation by Triatoma infestans in a rural area of the humid Chaco in Argentina: a multi-model inference approach. PLoS Neglected Tropical Diseases. 2011;5:e1349. doi: 10.1371/journal.pntd.0001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE, Kitron U, Cecere MC, Segura EL, Cohen JE. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proceedings of the National Academy of Sciences. 2007a;104:16194–16199. doi: 10.1073/pnas.0700863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Lauricella MA, Cardinal MV, Kitron U, Cohen JE. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007b;134:69–82. doi: 10.1017/S0031182006001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE, Ceballos LA, Ordóñez Krasnowski PC, Lanati LA, Stariolo R, Kitron U. Strong host-feeding preferences of the vector Triatoma infestans modified by vector density: implications for the epidemiology of Chagas Disease. PLoS Neglected Tropical Diseases. 2009;3:e447. doi: 10.1371/journal.pntd.0000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C, Bargues MD, Fajardo A, Montilla M, Triana O, Vallejo GA, Guhl F. Identifying four Trypanosoma cruzi I isolate haplotypes from different geographic regions in Colombia. Infection, Genetics and Evolution. 2007;7:535–539. doi: 10.1016/j.meegid.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Higo H, Miura S, Horio M, Mimori T, Hamano S, Agatsuma T, Yanagi T, Cruz-Reyes A, Uyema N, Rojas de Arias A, Matta V, Akahane H, Hirayama K, Takeuchi T, Tada I, Himeno K. Genotypic variation among lineages of Trypanosoma cruzi and its geographic aspects. Parasitology International. 2004;53:337–344. doi: 10.1016/j.parint.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Laurent JP, Barnabé C, Quesney V, Noel S, Tibayrenc M. Impact of clonal evolution on the biological diversity of Trypanosoma cruzi. Parasitology. 1997;114:213–218. doi: 10.1017/s0031182096008414. [DOI] [PubMed] [Google Scholar]
- Lauricella MA, Stariolo RL, Riarte AR, Segura EL, Gürtler RE. Distribution and pathogenicity of Trypanosoma cruzi isolated from peridomestic populations of Triatoma infestans and Triatoma guasayana from rural western Argentina. Memorias do Instituto Oswaldo Cruz. 2005;100:123–129. doi: 10.1590/s0074-02762005000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn MS, Miles MA, Carrasco HJ, Lewis MD, Yeo M, Vargas J, Torrico F, Diosque P, Valente V, Valente SA, Gaunt MW. Genome-scale multilocus microsatellite typing of Trypanosoma cruzi discrete typing unit I reveals phylogeographic structure and specific genotypes linked to human infection. PLoS Pathogens. 2009;5:e1000410. doi: 10.1371/journal.ppat.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn MS, Rivett-Carnac JB, Fitzpatrick S, Lewis MD, Yeo M, Gaunt MW, Miles MA. Extraordinary Trypanosoma cruzi diversity within single mammalian reservoir hosts implies a mechanism of diversifying selection. International Journal for Parasitology. 2011;41:609–614. doi: 10.1016/j.ijpara.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MD, Ma J, Yeo M, Carrasco HJ, Llewellyn MS, Miles MA. Genotyping of Trypanosoma cruzi: systematic selection of assays allowing rapid and accurate discrimination of all known lineages. American Journal of Tropical Medicine and Hygiene. 2009;81:1041–1049. doi: 10.4269/ajtmh.2009.09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loza-Murguía M, Noireau F. Vectorial capacity of Triatoma guasayana (Wygodzinsky & Abalos) (Hemiptera: Reduviidae) compared with two other species of epidemic importance. Neotropical Entomology. 2010;39:799–809. doi: 10.1590/s1519-566x2010000500020. [DOI] [PubMed] [Google Scholar]
- Macedo AM, Pena SD. Genetic variability of Trypanosoma cruzi: implications for the pathogenesis of Chagas disease. Parasitology Today. 1998;14:119–123. doi: 10.1016/s0169-4758(97)01179-4. [DOI] [PubMed] [Google Scholar]
- Marcet PL, Duffy T, Cardinal MV, Burgos JM, Lauricella MA, Levin MJ, Kitron U, Gurtler RE, Schijman AG. PCR-based screening and lineage identification of Trypanosoma cruzi directly from faecal samples of triatomine bugs from northwestern Argentina. Parasitology. 2006;132:57–65. doi: 10.1017/S0031182005008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles MA, Llewellyn MS, Lewis MD, Yeo M, Baleela R, Fitzpatrick S, Gaunt MW, Mauricio IL. The molecular epidemiology and phylogeography of Trypanosoma cruzi and parallel research on Leishmania: looking back and to the future. Parasitology. 2009;136:1509–1528. doi: 10.1017/S0031182009990977. [DOI] [PubMed] [Google Scholar]
- Montamat EE, Arauzo S, Cazzulo JJ, Subias E. Characterization by electrophoretic zymograms of 19 Trypanosoma cruzi clones derived from two chronic chagasic patients. Comparative Biochemistry and Physiology. 1987;87:417–422. doi: 10.1016/0305-0491(87)90161-1. [DOI] [PubMed] [Google Scholar]
- Noireau F, Brenière F, Ordoñez J, Cardozo L, Morochi W, Gutierrez T, Bosseno MF, Garcia S, Vargas F, Yaksic N, Dujardin JP, Peredo C, Wisnivesky-Colli C. Low probability of transmission of Trypanosoma cruzi to humans by domiciliary Triatoma sordida in Bolivia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1997;91:653–656. doi: 10.1016/s0035-9203(97)90508-3. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Lehane MJ, McEwan P, Catalá SS, Gorla DE. Dispersive flight by Triatoma sordida. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1991;85:676–678. doi: 10.1016/0035-9203(91)90392-c. [DOI] [PubMed] [Google Scholar]
- Schweigmann NJ, Pietrokovsky S, Bottazzi V, Conti O, Bujas MA, Wisnivesky-Colli C. Estudio de la prevalencia de infección por Trypanosoma cruzi en zarigüeyas (Didelphis albiventris) en Santiago del Estero, Argentina. Revista Panamericana de Salud Pública. 1999;6:371–377. doi: 10.1590/s1020-49891999001100001. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 10.0. Stata Corporation; College Station, TX: 2007. [Google Scholar]
- Tibayrenc M. Genetic epidemiology of parasitic protozoa and other infectious agents: the need for an integrated approach. International Journal for Parasitology. 1998;28:85–104. doi: 10.1016/s0020-7519(97)00180-x. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prokopec GM, Ceballos LA, Marcet PL, Cecere MC, Cardinal MV, Kitron U, Gürtler RE. Seasonal variations in active dispersal of natural populations of Triatoma infestans in rural north-western Argentina. Medical and Veterinary Entomology. 2006;20:273–279. doi: 10.1111/j.1365-2915.2006.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnivesky-Colli C, Schweigmann NJ, Alberti A, Pietrokovsky SM, Conti O, Montoya S, Riarte A, Rivas C. Sylvatic American trypanosomiasis in Argentina. Trypanosoma cruzi infection in mammals from the Chaco forest in Santiago del Estero. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1992;86:38–41. doi: 10.1016/0035-9203(92)90433-d. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Report of the Scientific Working Group on Chagas Disease. TDR/SWH/09. World Health Organization; Geneva, Switzerland: 2007. [Google Scholar]
- Yeo M, Acosta N, Llewellyn M, Sánchez H, Adamson S, Miles GAJ, López E, González N, Patterson JS, Gaunt MW, Rojas de Arias A, Miles MA. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. International Journal for Parasitology. 2005;35:225–233. doi: 10.1016/j.ijpara.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Yeo M, Lewis MD, Carrasco HJ, Acosta N, Llewellyn M, Aldo S, Vera de Costa V, Rojas de Arias A, Miles MA. Resolution of multiclonal infections of Trypanosoma cruzi from naturally infected triatomine bugs and from experimentally infected mice by direct plating on a sensitive solid medium. International Journal for Parasitology. 2007;37:111–120. doi: 10.1016/j.ijpara.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Zingales B, Andrade SG, Briones MRS, Campbe DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Memorias do Instituto Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]


