Abstract
Breast reconstruction is a type of surgery for women who have had a mastectomy, and involves using autologous tissue or prosthetic material to construct a natural-looking breast. Adipose tissue is the major contributor to the volume of the breast, whereas epithelial cells comprise the functional unit of the mammary gland. Adipose-derived stem cells (ASCs) can differentiate into both adipocytes and epithelial cells and can be acquired from autologous sources. ASCs are therefore an attractive candidate for clinical applications to repair or regenerate the breast. Here we review the current state of adipose tissue engineering methods, including the biomaterials used for adipose tissue engineering and the application of these techniques for mammary epithelial tissue engineering. Adipose tissue engineering combined with microfabrication approaches to engineer the epithelium represents a promising avenue to replicate the native structure of the breast.
Keywords: patterning, mammary epithelium, morphogenesis, 3D, organotypic
Anatomy of the Breast
The human breast is comprised of glandular, ductal, connective, and adipose tissues (Fig. 1). The functional unit of the breast is the mammary gland, a tree-like structure of epithelial ducts surrounded by adipose tissue.1 The glandular and adipose tissues are held together by connective tissue, including Cooper’s ligaments which attach the breast to the dermis of the overlying skin.2 Each breast has 15–20 sections (lobes) that branch out from the nipple. Each lobe is further divided into many smaller lobules, at the end of which are tiny bulb-like glands, known as terminal ductal lobular units (TDLUs), wherein milk is produced in response to hormonal signals. The lobes, lobules, and glands are connected by ducts, which deliver milk to openings in the nipple.3,4
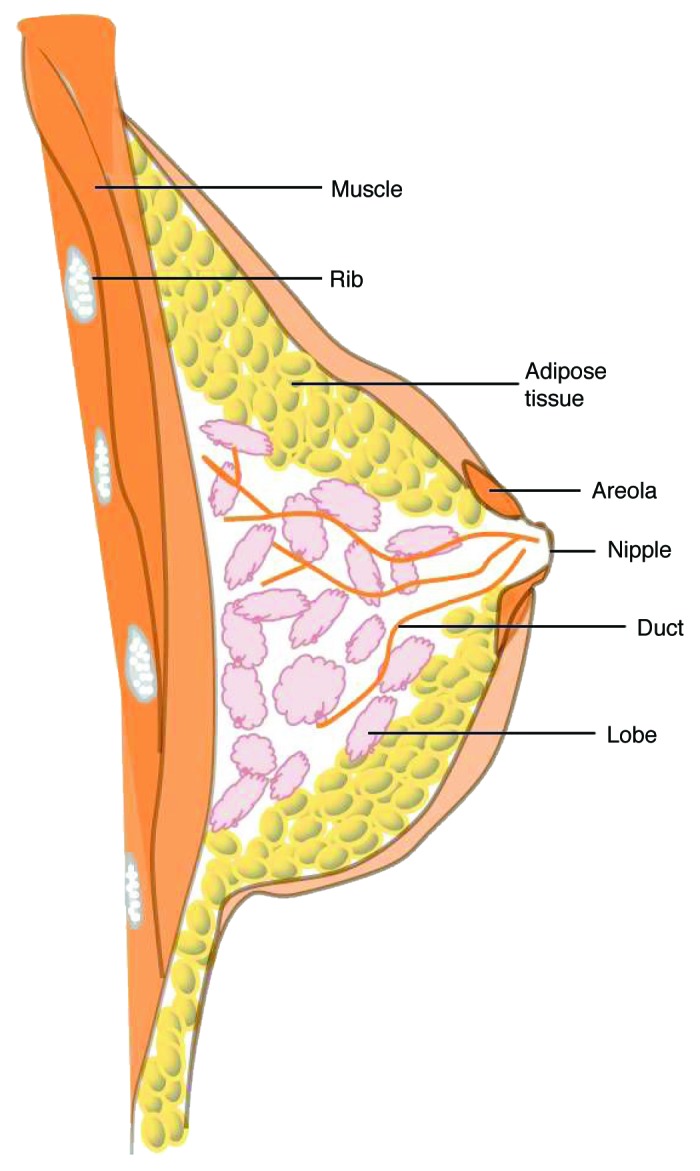
Figure 1. Anatomy of the breast. The female breast consists of glandular, ductal, connective, and adipose tissues.
The breasts of both women and men develop from the same embryonic tissues and are morphologically indistinguishable until the onset of puberty,5 at which time ovarian estrogens promote the sprouting, growth, and development of the mammary gland. In men, high levels of testosterone inhibit this development. As a result, the breasts of human males are much less prominent than those of females.5
Approximately 1 in 8 women will develop invasive breast cancer in the United States, and up to 40% will require a mastectomy.6 Breast reconstruction is a type of plastic surgery that aims to restore the shape, appearance, and size of a breast following its removal by mastectomy. Breast augmentation surgery, also known as augmentation mammoplasty, uses implants to increase the size of the breast or to restore its volume after weight loss or pregnancy. Saline-filled and silicone gel-filled implants are the most common. However, complications derived from the foreign body, such as capsular contracture, malposition, implant rupture, and infection, occur at a relatively high rate and frequently result in the need for implant removal.7
Breast reconstruction using the patient’s own tissues, rather than implantable devices, tends to produce better results with fewer complications and better approximates the shape, contour, softness, and fullness of the natural breast.4 The softness and suppleness which give the breasts their shape are mainly due to the presence of adipose tissue. Recent studies suggest the use of autologous fat tissue as an alternative implant material for breast augmentation.7-11 Stem cells are collected from the patient’s own adipose tissue and then placed, along with appropriate angiogenic and adipogenic growth factors, within a biodegradable scaffold. The transplanted stem cells are able to differentiate into new adipose tissue or vascular endothelial cells.12 Adipose tissue engineering is an emerging field that combines expertise in areas such as cell culture, cell differentiation, angiogenesis, tissue transfer, and polymer chemistry to regenerate adipose tissue de novo for breast reconstruction.
Adipose Tissue and Adipose Tissue Engineering
Adipose tissue, also known as fat, is the anatomical term for loose connective tissue composed of adipocytes.12 Adipose tissue is primarily located beneath the skin and is also found around internal organs, in bone marrow, and as described above, is a major component of the human breast.13 There are two types of adipose tissue, white adipose tissue (WAT) and brown adipose tissue (BAT), which have essentially antagonistic functions. WAT stores excess energy as triglycerides and BAT is specialized to dissipate energy via the production of heat.14 BAT is especially abundant in newborns and in hibernating mammals to enable survival in cold temperatures.15 As an animal ages, BAT is gradually replaced by WAT.15 Adipose tissue contains several cell types in addition to adipocytes, including fibroblasts, macrophages, and endothelial cells.16,17
Tissue engineering involves the use of living cells, biomaterials, and suitable biochemical and physico-chemical factors to improve or replace biological function.18 Most patients possess excess adipocytes that can be harvested without creating deformities.19 The therapeutic use of adipocytes in preclinical studies and clinical trials has been well documented.20 Adipose tissue has also been identified as a source of multipotent cells that have the capacity to differentiate into myogenic, osteogenic, and neurogenic lineages when cultured with the appropriate lineage-specific stimuli.21
Adipose-Derived Stem Cells for Tissue Engineering
Adipose-derived stem cells (ASCs) represent a readily available source for isolation of potentially useful stem cells.20 Stem cells are distinguished from other cell types by two properties. First, they have the ability to renew themselves through cell division while maintaining the undifferentiated state. Second, they have the capacity to differentiate into specialized cell types under certain physiologic or experimental conditions. There are primarily two kinds of stem cells that can be isolated from animals and humans: embryonic stem cells and adult stem cells. In 2006, researchers identified a new type of stem cell, called induced pluripotent stem cells (iPSCs),22 which are generated from somatic cells by the transgenic expression of three transcription factors referred to as OSK: Oct3/4, Sox2 and Klf4.22,23 The use of ASCs circumvents ethical issues associated with embryonic stem cells and the potential for oncogenic issues associated with iPSCs.
Ideally, a stem cell used for applications in regenerative medicine should meet the following criteria24: (1) available in abundant quantities (millions to billions of cells); (2) harvested using minimally invasive procedures; (3) able to differentiate into multiple cell lineages in a regulatable and reproducible manner; (4) safely and effectively transplanted to either an autologous or allogeneic host; (5) manufactured in accordance with current Good Manufacturing Practice guidelines.
Adipose stem cells can fulfill all of these criteria. ASCs are localized near the vasculature in adipose tissue,25 and can be retrieved in high number from either liposuction aspirates or fragments of subcutaneous tissue. Furthermore, ASCs are easily expanded in culture,26 with one gram of adipose tissue yielding approximately 5000 stem cells,27 500-fold greater than the yield from the same volume of bone marrow.28 ASCs have similar properties to bone marrow stem cells and are capable of osteogenic, chondrogenic, adipogenic, and neurogenic differentiation in culture. ASCs have been shown to be immunoprivileged, to prevent severe graft-vs.-host disease in culture and in vivo, and to be genetically stable in long-term culture.29 The potential of ASCs to differentiate into cells derived from all three germ layers has been shown in a variety of studies.30
Rodbell and colleagues pioneered the original methods in the 1960s to isolate ASCs from adipose tissue using fat from rats.31-33 Several other groups further adapted these methods for human fat.34-36 Briefly, raw liposuction aspirate or finely minced adipose tissue is washed, digested with collagenase, and centrifuged to remove blood cells, saline, and local anesthetics.24 Undifferentiated ASCs can be characterized by several cell-surface markers including CD29, CD44, CD71, CD90 and CD105.37-39 One of the most important uses of ASCs is to replace fat tissue itself. ASCs are able to undergo adipogenic differentiation in response to inductive stimuli including dexamethasone, insulin, forskolin, and peroxisome proliferator-activated receptor-γ (PPARγ).39-42 During this process, ASCs decrease their proliferation and change in morphology from an elongated fibroblast-like appearance to a rounded shape.43 In addition, these cells start accumulating intracellular lipid droplets, secrete increased amounts of the adipocyte protein leptin, and express adipogenic proteins including fatty acid-binding protein and lipoprotein lipase.41,43-45 Large soft tissue defects are common following trauma, burns, and oncological resections including mastectomy, as described above. The ability of ASCs to produce fat tissue definitely represents a promising avenue to reconstruct these various tissue defects.
Biomaterials for Adipose Tissue Engineering
Tissue-specific scaffolds are essential to differentiate ASCs and effectively construct three-dimensional (3D) tissues. Ultimately, the scaffold must degrade as it is replaced by healthy host tissue. A number of scaffold biomaterials have been investigated for the purpose of engineering adipose tissue, including synthetic scaffolds and naturally derived materials.46 Several factors must be considered when designing a scaffold, including its mechanical properties, degradation characteristics, immunogenicity, cellular response to the material, ease of handing in the clinic, and cost.47
Synthetic scaffolds have been used widely for adipose tissue engineering. The advantages of synthetic polymers include the ability to specifically tailor their mechanical, chemical, and degradation properties.48 Considerable work has been performed using synthetic polymers such as poly(lactic acid) (PLA), polyglycolic acid (PGA), polyethylene terephthalate (PET), and poly(lactic-co-glycolic acid) (PLGA).48
PLA and PGA have been used for studies both in culture and in vivo and have the potential to support regenerated adipose tissue.49-52 When ASCs were cultured on PLA-based scaffolds in the presence of adipogenic stimulants, they showed significant upregulation of adipogenic transcript levels and substantial lipid accumulation. However, the scaffolds rapidly degraded within 4 weeks after implantation in a rat muscle pouch defect model.52 Likewise, PGA, while showing promise to support adipogenesis in culture, also degrades rapidly in vivo.49-51,53,54 When mouse 3T3-L1 cells, a preadipocyte cell line derived from disaggregated Swiss 3T3 mouse embryos,55 were seeded on fibrous PET matrices, they acquired morphological and biological features of mature adipocytes.56 However, long-term studies have yet to be performed.
Newly formed adipose tissue was obtained when a combination of ASCs and PLGA spheres was injected into subcutaneous tissue of immune-deficient mice.57 Unfortunately, the new adipose tissue could not retain a specific shape because the implanted PLGA support rapidly disappeared after degradation. A separate study used PLGA scaffolds seeded with rat preadipocytes prior to implantation and found maximum formation of adipose tissue at 2 months, followed by a decrease at 3 months, and complete absence of adipose tissue and PLGA at 5–12 mo.58 Other synthetic materials have also been explored for adipose tissue engineering applications, and some show promise for potential soft tissue replacement. These include polytetrafluoroethylene (PTFE),59 polyethylene glycol diacrylate (PEGDA),60 and polyethylene glycol (PEG).60,61 Whereas adipose tissue can be formed in vivo using synthetic scaffold-based tissue engineering strategies, the long-term maintenance of adipose tissue remains elusive.
Natural polymers are found as part of the native extracellular matrix (ECM).62 Compared with synthetic materials, natural polymers tend to be more biocompatible, and their mechanical and biological properties tend to match those found in vivo. The most common natural polymers used recently for adipose tissue engineering include collagen, derivatives of hyaluronic acid (HA), adipose-derived ECM, matrigel, gelatin, and decellularized human placenta.46,48
Collagen is the most prevalent natural polymer used in current adipose tissue engineering research due to its biodegradability, biocompatibility, weak antigenicity, and the ability to be shaped to fill a specific defect.63 Several studies have demonstrated that 3D collagen sponges can support adipogenesis from a variety of cell sources,64-67 as well as promote the development of new adipose tissue in vivo after 12 weeks.68 Mauney et al. studied the ability of collagen and PLA matrices to support adipogenesis both in culture and in vivo, and found that although collagen scaffolds supported greater cell attachment upon seeding and greater lipid accumulation in culture, both collagen and PLA scaffolds were undetectable after 4 weeks in vivo due to rapid degradation.48,52 Human preadipocytes could be successfully and reproducibly inoculated and cultured on 3D HA-based scaffolds, and were able to differentiate into adipocytes in culture,69 but their properties in vivo remain to be investigated. Adipose-derived ECM promotes a favorable microenvironment for adipogenesis, but has yet to be formulated as a 3D porous scaffold.70-72 The placenta is also a rich source of ECM and basement membrane components, and contains similar types of collagen as does adipose tissue,73 and therefore has great potential for use as a scaffold for adipose tissue engineering. Mature adipocytes were observed 8 weeks after seeding within a decellularized human placenta scaffold.74 However, the isolation and decellularization procedure for the placenta is both expensive and time-consuming.74
In summary, several studies have demonstrated adipose tissue formation using both synthetic and natural polymers. On the one hand, synthetic materials offer consistent control of material properties. On the other hand, natural materials offer considerable advantages with respect to biocompatibility and degradation properties. Additional studies are needed to further demonstrate and compare long-term in vivo functionality of each material for clinical applications in soft tissue replacement.
Epithelial Tissue Engineering
Epithelial tissues line the cavities and surfaces of structures throughout the body and also form many glands, including the mammary gland. Epithelial cells can arise from each of the three germ layers of the embryo. The epidermis and its appendages, including the mammary gland, originate from the ectoderm. In contrast, the lining of the gastrointestinal tract derives from the endoderm, and the inner linings of body cavities derive from the mesoderm.75
In 2004, two groups demonstrated the capacity of ASCs to differentiate into endothelial cells, a specialized epithelium.76,77 Subsequent studies have demonstrated the differentiation potential of ASCs into an epithelial lineage. Human ASCs were able to undergo epithelial differentiation in culture in the presence of all-trans retinoic acid. The differentiated cells displayed several epithelial characteristics including monolayer formation, the expression of the epithelial-specific marker cytokeratin 18, and the formation of keratin fibers. The percentage of ASCs able to undergo epithelial differentiation as quantified by flow cytometry analysis was greater than 80%.78 Studies by several other groups suggest that the epithelial differentiation of ASCs can also be initiated by direct cell-cell or cell-matrix contacts,79,80 or by secreted factors such as cytokines, interleukins, or growth factors present in conditioned media.75
Cells typically reside in a 3D microenvironment in vivo. Several recently developed techniques for microfabricating 3D epithelial tissues hold promise for engineering these structures with a higher level of physiological and histological realism.81-83 Our lab has developed an engineered tissue model of the mammary epithelial duct comprised of murine mammary epithelial tubules of arbitrary geometry embedded within a 3D type I collagen gel.81 A concentrated suspension of mammary epithelial cells is placed within micro-scale collagen cavities prepared by replica micro-molding. Over a 24-h period, the cells form contacts with their neighbors, synthesize and assemble a basement membrane, and rearrange into a polarized epithelial tubule. When induced with growth factors, such as those that act downstream of ovarian hormones in vivo, these 3D epithelial tissues undergo branching morphogenesis, thus expanding the epithelial tree.81 Importantly, these epithelial tissues can also be engineered within gels containing adipocytes,84 thus more closely approximating the native structure of the mammary gland (Fig. 2). This model has been used to analyze quantitatively the spatial and temporal dynamics of gene expression85,86 and the mechanical stress profile during branching morphogenesis,87,88 as well as how the biophysical characteristics of the host microenvironment affect the invasive phenotype of breast tumor cells.89 Given that the mammary gland is composed of multiple cell types, one of the major challenges of the microfabrication approach is to incorporate epithelial cells, fibroblasts, endothelial cells, adipocytes, and macrophages into a single platform. This, together with incorporation of adequate matrix scaffolds, will enable the generation of more complex, realistic mammary tissues.90
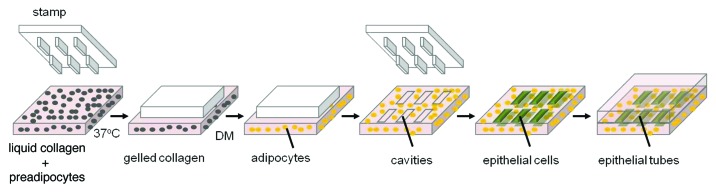
Figure 2. Reconstructing the breast. Schematic of 3D microfabrication procedure used to build the mammary epithelial tissues. Preadipocytes are seeded in unpolymerized collagen. Cavities of collagen gel are generated by molding the cell-gel mixture around a patterned elastomeric stamp. In the presence of differentiation medium (DM), preadipocytes are induced to differentiate into adipocytes. Epithelial cells are then embedded into the cavities, which form hollow tubules conforming to the size and shape of the collagen cavities.
Conclusions
The field of tissue engineering offers great potential for abrogating the current limitations of breast reconstruction following tumor resection. The primary basis for any tissue-engineered construct is the cellular source that is used to initiate new tissue growth. ASCs provide an abundant and readily accessible source of multipotent stem cells. The use of stem cells expanded in culture and combined with novel biomaterials for organ reconstruction offers a potential solution for tissue replacement. ASCs have several advantages over other sources of stem cells, the most important being their ease of availability. The ability of ASCs to differentiate into epithelial cells makes them a promising tool for breast reconstruction. With the evolution of biological microfabrication, it is plausible to construct tissue models in which the biology, chemistry, geometry, and mechanics can be controlled at every length scale. Future studies are needed to demonstrate the safety and efficacy of adipocytes and epithelial cells derived from ASCs in animal models or clinically, either alone or in combination with novel biomaterial scaffolds.
Acknowledgments
Work from the authors’ lab was supported in part by the NIH (GM083997 and HL110335), the David and Lucile Packard Foundation, the Alfred P. Sloan Foundation, the Camille and Henry Dreyfus Foundation and Susan G. Komen for the Cure. CMN holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund.
Glossary
Abbreviations:
- ASC
adipose-derived stem cell
- BAT
brown adipose tissue
- ECM
extracellular matrix
- iPSC
induced pluripotent stem cell
- TDLU
terminal ductal lobular unit
- WAT
white adipose tissue
- 3D
three-dimensional
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/24630
References
- 1.Nickell WB, Skelton J. Breast fat and fallacies: more than 100 years of anatomical fantasy. J Hum Lact. 2005;21:126–30. doi: 10.1177/0890334405276471. [DOI] [PubMed] [Google Scholar]
- 2.Howard BA, Gusterson BA. Human breast development. J Mammary Gland Biol Neoplasia. 2000;5:119–37. doi: 10.1023/A:1026487120779. [DOI] [PubMed] [Google Scholar]
- 3.Cooper AP. (1840). On the anatomoy of the breast. Longman, Orme, Green, Brown and Longmans, London. [Google Scholar]
- 4.Patrick CW. Breast tissue engineering. Annu Rev Biomed Eng. 2004;6:109–30. doi: 10.1146/annurev.bioeng.6.040803.140032. [DOI] [PubMed] [Google Scholar]
- 5.Levin RJ. The breast/nipple/areola complex and human sexuality. Sex Relationship Ther. 2006;21:237–49. doi: 10.1080/14681990600674674. [DOI] [Google Scholar]
- 6.Malata CM, McIntosh SA, Purushotham AD. Immediate breast reconstruction after mastectomy for cancer. Br J Surg. 2000;87:1455–72. doi: 10.1046/j.1365-2168.2000.01593.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura K, Asano Y, Aoi N, Kurita M, Oshima Y, Sato K, et al. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J. 2010;16:169–75. doi: 10.1111/j.1524-4741.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 8.Amar O, Bruant-Rodier C, Lehmann S, Bollecker V, Wilk A. Greffe de tissu adipeux: restauration du volume mammaire après traitement conservateur des cancers du sein, aspect clinique et radiologique. [Fat tissue transplant: restoration of the mammary volume after conservative treatment of breast cancers, clinical and radiological considerations] Ann Chir Plast Esthet. 2008;53:169–77. doi: 10.1016/j.anplas.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg. 2007;119:775–85, discussion 786-7. doi: 10.1097/01.prs.0000252001.59162.c9. [DOI] [PubMed] [Google Scholar]
- 10.Spear SL, Wilson HB, Lockwood MD. Fat injection to correct contour deformities in the reconstructed breast. Plast Reconstr Surg. 2005;116:1300–5. doi: 10.1097/01.prs.0000181509.67319.cf. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55, discussion 56-7. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patrick CW., Jr. Adipose tissue engineering: the future of breast and soft tissue reconstruction following tumor resection. Semin Surg Oncol. 2000;19:302–11. doi: 10.1002/1098-2388(200010/11)19:3<302::AID-SSU12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 14.Saely CH, Geiger K, Drexel H. Brown versus white adipose tissue: a mini-review. Gerontology. 2012;58:15–23. doi: 10.1159/000321319. [DOI] [PubMed] [Google Scholar]
- 15.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–56. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Eto H, Suga H, Matsumoto D, Inoue K, Aoi N, Kato H, et al. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast Reconstr Surg. 2009;124:1087–97. doi: 10.1097/PRS.0b013e3181b5a3f1. [DOI] [PubMed] [Google Scholar]
- 17.Rink JD, Simpson ER, Barnard JJ, Bulun SE. Cellular characterization of adipose tissue from various body sites of women. J Clin Endocrinol Metab. 1996;81:2443–7. doi: 10.1210/jc.81.7.2443. [DOI] [PubMed] [Google Scholar]
- 18.Nerem RM, Sambanis A. Tissue engineering: from biology to biological substitutes. Tissue Eng. 1995;1:3–13. doi: 10.1089/ten.1995.1.3. [DOI] [PubMed] [Google Scholar]
- 19.Patrick CW., Jr. Tissue engineering strategies for adipose tissue repair. Anat Rec. 2001;263:361–6. doi: 10.1002/ar.1113. [DOI] [PubMed] [Google Scholar]
- 20.Sterodimas A, de Faria J, Nicaretta B, Pitanguy I. Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. J Plast Reconstr Aesthet Surg. 2010;63:1886–92. doi: 10.1016/j.bjps.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Gomillion CT, Burg KJ. Stem cells and adipose tissue engineering. Biomaterials. 2006;27:6052–63. doi: 10.1016/j.biomaterials.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Maekawa M, Yamaguchi K, Nakamura T, Shibukawa R, Kodanaka I, Ichisaka T, et al. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature. 2011;474:225–9. doi: 10.1038/nature10106. [DOI] [PubMed] [Google Scholar]
- 24.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin G, Banie L, Ning H, Bella AJ, Lin CS, Lue TF. Potential of adipose-derived stem cells for treatment of erectile dysfunction. J Sex Med. 2009;6(Suppl 3):320–7. doi: 10.1111/j.1743-6109.2008.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tremolada C, Palmieri G, Ricordi C. Adipocyte transplantation and stem cells: plastic surgery meets regenerative medicine. Cell Transplant. 2010;19:1217–23. doi: 10.3727/096368910X507187. [DOI] [PubMed] [Google Scholar]
- 27.Kitagawa Y, Korobi M, Toriyama K, Kamei Y, Torii S. History of Discovery of Human Adipose-Derived Stem Cells and Their Clinical Application. Jpn J Plast Reconstr Surg. 2006;49:1097–104. [Google Scholar]
- 28.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–4. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011;7:269–91. doi: 10.1007/s12015-010-9193-7. [DOI] [PubMed] [Google Scholar]
- 30.Baer PC. Adipose-derived stem cells and their potential to differentiate into the epithelial lineage. Stem Cells Dev. 2011;20:1805–16. doi: 10.1089/scd.2011.0086. [DOI] [PubMed] [Google Scholar]
- 31.Rodbell M. Metabolism of isolated fat cells. II. The similar effects of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on glucose and amino acid metabolism. J Biol Chem. 1966;241:130–9. a. [PubMed] [Google Scholar]
- 32.Rodbell M. The metabolism of isolated fat cells. IV. Regulation of release of protein by lipolytic hormones and insulin. J Biol Chem. 1966;241:3909–17. b. [PubMed] [Google Scholar]
- 33.Rodbell M, Jones AB. Metabolism of isolated fat cells. 3. The similar inhibitory action of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. J Biol Chem. 1966;241:140–2. [PubMed] [Google Scholar]
- 34.Deslex S, Negrel R, Vannier C, Etienne J, Ailhaud G. Differentiation of human adipocyte precursors in a chemically defined serum-free medium. Int J Obes. 1987;11:19–27. [PubMed] [Google Scholar]
- 35.Engfeldt P, Arner P, Ostman J. Influence of adipocyte isolation by collagenase on phosphodiesterase activity and lipolysis in man. J Lipid Res. 1980;21:443–8. [PubMed] [Google Scholar]
- 36.Ho JH, Ma WH, Tseng TC, Chen YF, Chen MH, Lee OK. Isolation and characterization of multi-potent stem cells from human orbital fat tissues. Tissue Eng Part A. 2011;17:255–66. doi: 10.1089/ten.tea.2010.0106. [DOI] [PubMed] [Google Scholar]
- 37.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 38.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 40.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–73. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, et al. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest. 1989;84:1663–70. doi: 10.1172/JCI114345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazar MA. PPAR gamma, 10 years later. Biochimie. 2005;87:9–13. doi: 10.1016/j.biochi.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Ogawa R, Mizuno H, Watanabe A, Migita M, Hyakusoku H, Shimada T. Adipogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice-including relationship of sex differences. Biochem Biophys Res Commun. 2004;319:511–7. doi: 10.1016/j.bbrc.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Halvorsen YD, Franklin D, Bond AL, Hitt DC, Auchter C, Boskey AL, et al. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001;7:729–41. doi: 10.1089/107632701753337681. [DOI] [PubMed] [Google Scholar]
- 45.Sen A, Lea-Currie YR, Sujkowska D, Franklin DM, Wilkison WO, Halvorsen YD, et al. Adipogenic potential of human adipose derived stromal cells from multiple donors is heterogeneous. J Cell Biochem. 2001;81:312–9. doi: 10.1002/1097-4644(20010501)81:2<312::AID-JCB1046>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 46.Flynn L, Prestwich GD, Semple JL, Woodhouse KA. Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials. 2007;28:3834–42. doi: 10.1016/j.biomaterials.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Kim BS, Baez CE, Atala A. Biomaterials for tissue engineering. World J Urol. 2000;18:2–9. doi: 10.1007/s003450050002. [DOI] [PubMed] [Google Scholar]
- 48.Choi JH, Gimble JM, Lee K, Marra KG, Rubin JP, Yoo JJ, et al. Adipose tissue engineering for soft tissue regeneration. Tissue Eng Part B Rev. 2010;16:413–26. doi: 10.1089/ten.teb.2009.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischbach C, Seufert J, Staiger H, Hacker M, Neubauer M, Göpferich A, et al. Three-dimensional in vitro model of adipogenesis: comparison of culture conditions. Tissue Eng. 2004;10:215–29. doi: 10.1089/107632704322791862. a. [DOI] [PubMed] [Google Scholar]
- 50.Fischbach C, Spruss T, Weiser B, Neubauer M, Becker C, Hacker M, et al. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp Cell Res. 2004;300:54–64. doi: 10.1016/j.yexcr.2004.05.036. b. [DOI] [PubMed] [Google Scholar]
- 51.Lee JA, Parrett BM, Conejero JA, Laser J, Chen J, Kogon AJ, et al. Biological alchemy: engineering bone and fat from fat-derived stem cells. Ann Plast Surg. 2003;50:610–7. doi: 10.1097/01.SAP.0000069069.23266.35. [DOI] [PubMed] [Google Scholar]
- 52.Mauney JR, Nguyen T, Gillen K, Kirker-Head C, Gimble JM, Kaplan DL. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28:5280–90. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin SD, Wang KH, Kao AP. Engineered adipose tissue of predefined shape and dimensions from human adipose-derived mesenchymal stem cells. Tissue Eng Part A. 2008;14:571–81. doi: 10.1089/tea.2007.0192. [DOI] [PubMed] [Google Scholar]
- 54.Weiser B, Prantl L, Schubert TE, Zellner J, Fischbach-Teschl C, Spruss T, et al. In vivo development and long-term survival of engineered adipose tissue depend on in vitro precultivation strategy. Tissue Eng Part A. 2008;14:275–84. doi: 10.1089/tea.2007.0130. [DOI] [PubMed] [Google Scholar]
- 55.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–33. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 56.Kang X, Xie Y, Kniss DA. Adipose tissue model using three-dimensional cultivation of preadipocytes seeded onto fibrous polymer scaffolds. Tissue Eng. 2005;11:458–68. doi: 10.1089/ten.2005.11.458. [DOI] [PubMed] [Google Scholar]
- 57.Choi YS, Cha SM, Lee YY, Kwon SW, Park CJ, Kim M. Adipogenic differentiation of adipose tissue derived adult stem cells in nude mouse. Biochem Biophys Res Commun. 2006;345:631–7. doi: 10.1016/j.bbrc.2006.04.128. [DOI] [PubMed] [Google Scholar]
- 58.Patrick CW, Jr., Zheng B, Johnston C, Reece GP. Long-term implantation of preadipocyte-seeded PLGA scaffolds. Tissue Eng. 2002;8:283–93. doi: 10.1089/107632702753725049. [DOI] [PubMed] [Google Scholar]
- 59.Kral JG, Crandall DL. Development of a human adipocyte synthetic polymer scaffold. Plast Reconstr Surg. 1999;104:1732–8. doi: 10.1097/00006534-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 60.Alhadlaq A, Tang M, Mao JJ. Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimension: implications in soft tissue augmentation and reconstruction. Tissue Eng. 2005;11:556–66. doi: 10.1089/ten.2005.11.556. [DOI] [PubMed] [Google Scholar]
- 61.Vashi AV, Keramidaris E, Abberton KM, Morrison WA, Wilson JL, O’Connor AJ, et al. Adipose differentiation of bone marrow-derived mesenchymal stem cells using Pluronic F-127 hydrogel in vitro. Biomaterials. 2008;29:573–9. doi: 10.1016/j.biomaterials.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 62.Lavik E, Langer R. Tissue engineering: current state and perspectives. Appl Microbiol Biotechnol. 2004;65:1–8. doi: 10.1007/s00253-004-1580-z. [DOI] [PubMed] [Google Scholar]
- 63.Vashi AV, Abberton KM, Thomas GP, Morrison WA, O’Connor AJ, Cooper-White JJ, et al. Adipose tissue engineering based on the controlled release of fibroblast growth factor-2 in a collagen matrix. Tissue Eng. 2006;12:3035–43. doi: 10.1089/ten.2006.12.3035. [DOI] [PubMed] [Google Scholar]
- 64.Hiraoka Y, Yamashiro H, Yasuda K, Kimura Y, Inamoto T, Tabata Y. In situ regeneration of adipose tissue in rat fat pad by combining a collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. Tissue Eng. 2006;12:1475–87. doi: 10.1089/ten.2006.12.1475. [DOI] [PubMed] [Google Scholar]
- 65.Kimura Y, Ozeki M, Inamoto T, Tabata Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials. 2003;24:2513–21. doi: 10.1016/S0142-9612(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 66.Lu F, Gao JH, Ogawa R, Mizuro H, Hykusoku H. Adipose tissues differentiated by adipose-derived stem cells harvested from transgenic mice. Chin J Traumatol. 2006;9:359–64. [PubMed] [Google Scholar]
- 67.Neuss S, Stainforth R, Salber J, Schenck P, Bovi M, Knüchel R, et al. Long-term survival and bipotent terminal differentiation of human mesenchymal stem cells (hMSC) in combination with a commercially available three-dimensional collagen scaffold. Cell Transplant. 2008;17:977–86. doi: 10.3727/096368908786576462. [DOI] [PubMed] [Google Scholar]
- 68.von Heimburg D, Kuberka M, Rendchen R, Hemmrich K, Rau G, Pallua N. Preadipocyte-loaded collagen scaffolds with enlarged pore size for improved soft tissue engineering. Int J Artif Organs. 2003;26:1064–76. doi: 10.1177/039139880302601204. [DOI] [PubMed] [Google Scholar]
- 69.Halbleib M, Skurk T, de Luca C, von Heimburg D, Hauner H. Tissue engineering of white adipose tissue using hyaluronic acid-based scaffolds. I: in vitro differentiation of human adipocyte precursor cells on scaffolds. Biomaterials. 2003;24:3125–32. doi: 10.1016/S0142-9612(03)00156-X. [DOI] [PubMed] [Google Scholar]
- 70.Uriel S, Huang JJ, Moya ML, Francis ME, Wang R, Chang SY, et al. The role of adipose protein derived hydrogels in adipogenesis. Biomaterials. 2008;29:3712–9. doi: 10.1016/j.biomaterials.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 71.Vallée M, Côté JF, Fradette J. Adipose-tissue engineering: taking advantage of the properties of human adipose-derived stem/stromal cells. Pathol Biol (Paris) 2009;57:309–17. doi: 10.1016/j.patbio.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 72.Vermette M, Trottier V, Ménard V, Saint-Pierre L, Roy A, Fradette J. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials. 2007;28:2850–60. doi: 10.1016/j.biomaterials.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 73.Flynn L, Semple JL, Woodhouse KA. Decellularized placental matrices for adipose tissue engineering. J Biomed Mater Res A. 2006;79:359–69. doi: 10.1002/jbm.a.30762. [DOI] [PubMed] [Google Scholar]
- 74.Flynn L, Prestwich GD, Semple JL, Woodhouse KA. Adipose tissue engineering in vivo with adipose-derived stem cells on naturally derived scaffolds. J Biomed Mater Res A. 2009;89:929–41. doi: 10.1002/jbm.a.32044. [DOI] [PubMed] [Google Scholar]
- 75.Baer PC, Bereiter-Hahn J, Missler C, Brzoska M, Schubert R, Gauer S, et al. Conditioned medium from renal tubular epithelial cells initiates differentiation of human mesenchymal stem cells. Cell Prolif. 2009;42:29–37. doi: 10.1111/j.1365-2184.2008.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miranville A, Heeschen C, Sengenès C, Curat CA, Busse R, Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–55. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 77.Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–63. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 78.Brzoska M, Geiger H, Gauer S, Baer P. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun. 2005;330:142–50. doi: 10.1016/j.bbrc.2005.02.141. [DOI] [PubMed] [Google Scholar]
- 79.Ebrahimian TG, Pouzoulet F, Squiban C, Buard V, André M, Cousin B, et al. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol. 2009;29:503–10. doi: 10.1161/ATVBAHA.108.178962. [DOI] [PubMed] [Google Scholar]
- 80.Long JL, Zuk P, Berke GS, Chhetri DK. Epithelial differentiation of adipose-derived stem cells for laryngeal tissue engineering. Laryngoscope. 2010;120:125–31. doi: 10.1002/lary.20719. [DOI] [PubMed] [Google Scholar]
- 81.Nelson CM, Inman JL, Bissell MJ. Three-dimensional lithographically defined organotypic tissue arrays for quantitative analysis of morphogenesis and neoplastic progression. Nat Protoc. 2008;3:674–8. doi: 10.1038/nprot.2008.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nelson CM, Tien J. Microstructured extracellular matrices in tissue engineering and development. Curr Opin Biotechnol. 2006;17:518–23. doi: 10.1016/j.copbio.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 83.Tang MD, Golden AP, Tien J. Molding of three-dimensional microstructures of gels. J Am Chem Soc. 2003;125:12988–9. doi: 10.1021/ja037677h. [DOI] [PubMed] [Google Scholar]
- 84.Pavlovich AL, Manivannan S, Nelson CM. Adipose stroma induces branching morphogenesis of engineered epithelial tubules. Tissue Eng Part A. 2010;16:3719–26. doi: 10.1089/ten.tea.2009.0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee K, Gjorevski N, Boghaert E, Radisky DC, Nelson CM. Snail1, Snail2, and E47 promote mammary epithelial branching morphogenesis. EMBO J. 2011;30:2662–74. doi: 10.1038/emboj.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gjorevski N, Nelson CM. Endogenous patterns of mechanical stress are required for branching morphogenesis. Integr Biol (Camb) 2010;2:424–34. doi: 10.1039/c0ib00040j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gjorevski N, Nelson CM. Mapping of mechanical strains and stresses around quiescent engineered three-dimensional epithelial tissues. Biophys J. 2012;103:152–62. doi: 10.1016/j.bpj.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boghaert E, Gleghorn JP, Lee K, Gjorevski N, Radisky DC, Nelson CM. Host epithelial geometry regulates breast cancer cell invasiveness. Proc Natl Acad Sci U S A. 2012;109:19632–7. doi: 10.1073/pnas.1118872109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X, Reagan MR, Kaplan DL. Synthetic adipose tissue models for studying mammary gland development and breast tissue engineering. J Mammary Gland Biol Neoplasia. 2010;15:365–76. doi: 10.1007/s10911-010-9192-y. [DOI] [PubMed] [Google Scholar]


