Abstract
Fecal and serologic biomarkers can be used in the diagnosis and management of inflammatory bowel disease (IBD). Fecal markers such as calprotectin and lactoferrin have been studied for their ability to identify patients with IBD, assess disease activity, and predict relapse. Antibodies against Saccharomyces cerevisiae and perinuclear antineutrophil cytoplasmic proteins have been used in diagnosis of IBD, to distinguish Crohn's disease (CD) from ulcerative colitis, and to predict the risk of complications of CD. Tests for c-reactive protein and erythrocyte sedimentation rate have been used to assess inflammatory processes and predict the course of IBD progression. Levels of drug metabolites and antibodies against therapeutic agents might be measured to determine why patients do not respond to therapy and to select alternative treatments. This review addresses the potential for biomarker assays to improve treatment strategies and challenges to their use and development.
Keywords: Crohn's disease, ulcerative colitis, biomarker, prognosis
Physicians guide management of patients with inflammatory bowel disease (IBD) using blood tests, radiology and endoscopy studies, and other tests. These diagnostic tests can be used to identify patients with IBD, determine prognosis, assess disease activity, and determine optimal therapeutic strategies (Figure 1 and Table 1).
Figure 1.
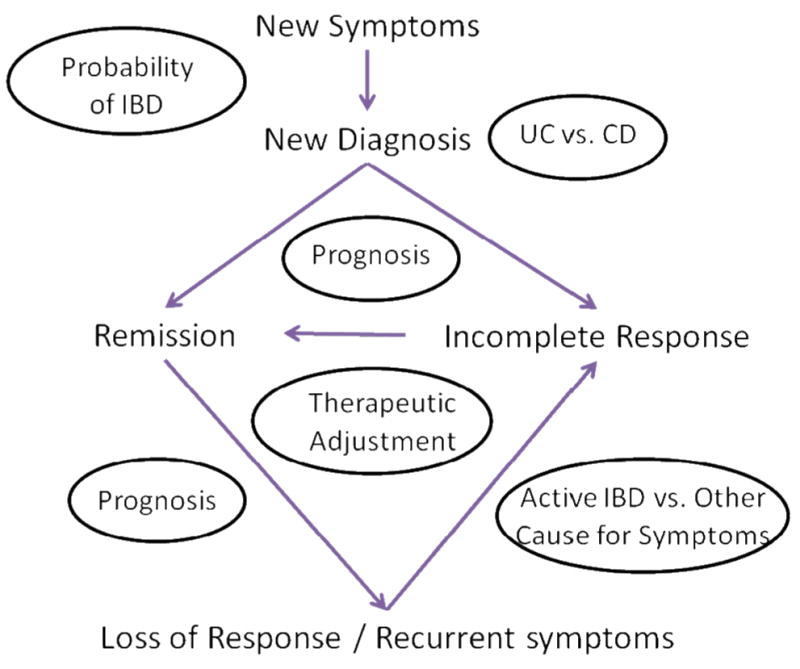
The potential role of biomarker assays in the care of patients with suspected or established IBD. Biomarkers might be used in all phases of the care. For patients with suspected IBD, biomarkers can be used to select which patients are unlikely to have IBD and could forgo further testing. Once patients are diagnosed, biomarkers can be used to determine which patients have CD or UC and to predict disease course. Biomarkers might be used to determine which patients are most likely to respond to therapies and determine prognosis, to identify those that require more aggressive therapies. In patients with recurrent symptoms, biomarkers can differentiate patients with active inflammation from those likely to have symptoms from other causes.
Table 1. Applications for selected biomarkers*.
| Test | IBD vs. Other Disease | UC vs. CD | Risk of Complications | Active Disease vs. Remission | Assess Mucosal Healing | Predict Relapse | Predict Response to Therapy | Therapeutic Adjustment |
|---|---|---|---|---|---|---|---|---|
| Calprotectin | + | + | + | + | + | |||
| Lactoferrin | + | + | + | + | + | |||
| S100A12 | + | + | + | + | + | |||
| CRP | + | + | + | + | + | |||
| ESR | + | + | + | |||||
| Serologies | + | + | + | + | ||||
| 6MP metabolite levels | + | + | + | |||||
| Anti-TNF drug levels | + | + | + | |||||
| Antibodies against biologic therapies | + | + | + |
The table includes both confirmed and theoretical roles for these biomarkers.
Tests Used to Evaluate Patients with Symptoms of IBD
Numerous fecal markers can potentially be used to determine the likelihood that a patient has IBD.1-18 The 2 most commonly utilized are calprotectin and lactoferrin. Calprotectin is 36 kDa calcium- and zinc-binding protein that represents 60% of cytosolic proteins in granulocytes.19 It is stable in feces when stored at room temperature for up to 1 week.20 The concentration of calprotectin in feces is an indirect measure of neutrophil infiltrate in the bowel mucosa. In patients with Crohn's disease (CD), the 4-day fecal excretion of 111indium-labeled white blood cells (WBCs) correlates with 4-day excretion of calprotectin and even the concentration of calprotectin in a single fecal specimen .21
Numerous studies have addressed whether fecal calprotectin could be used to select patients with symptoms of IBD that warrant endoscopic or radiologic evaluation. Von Roon et al. summarized data from 30 studies that included 5,983 patients (1210 had IBD).22 The estimated sensitivity and specificity values for the identification of patients with IBD, compared to those without, were 89% and 81%, respectively in studies that used a threshold fecal calprotectin concentration of 50 μg/g; these values were 98% and 91% in studies that used a threshold fecal calprotectin concentration of 100 μg/g. However, these estimates come from combinations of different studies, rather than tests of different threshold levels in a single study. In fact, it is implausible for the fecal calprotectin assay to have higher sensitivity when using a higher threshold to define a positive test. Therefore, these data cannot be used to select an optimal cut point.
van Rheenen et al. performed a similar analysis that was limited to studies that included only patients suspected to have IBD based on signs and symptoms.23 Among the 6 studies, the sensitivity and specificity for identification of IBD in adults were 93% and 96%, respectively. In children, the test's sensitivity was 92% but the specificity was only 76%. The authors conclude that using these tests to choose what patients require further testing reduces the need for endoscopy or radiology tests in a large portion of patients but would delay the diagnosis of IBD in 6% and 8% of the adults and children with disease, respectively.
Lactoferrin is an iron-binding protein found in neutrophil granules and serum and is secreted by mucosal membranes. It is resistant to degradation and proteolysis (although less so than calprotectin), making it a useful marker of intestinal inflammation24. Pooling data from multiple studies and 1001 patients, Gisbert et al. estimated that the lactoferrin test identified patients with IBD with a mean sensitivity of 80% and specificity of 82%25. Most but not all studies reported similar performance of calprotectin and lactoferrin tests26-28.
100A12 is a S100 protein that is similar to calprotectin. In a study of children, fecal levels of S100A12 greater than 10 mg/kg identified IBD with a sensitivity of 96% and a specificity 92%.29 In a subsequent study of adults, S100A12 distinguished patients with IBD from those with irritable bowel syndrome with sensitivity and specificity values of 86% and 96%, respectively.30 S100A12 can be measured in serum; although serum levels are increased in patients with IBD, this test does not distinguish IBD from irritable bowel syndrome with the same levels of sensitivity and specificity as the fecal assay.31
For many reasons, blood-based biomarkers might be preferred to stool-based tests. C-reactive protein (CRP) is one of many acute phase proteins that increase in the serum of patients with acute-phase IBD. Studies dating back several decades identified increased levels of CRP in nearly 100% of patients with CD and approximately 50% of those with UC32-34. The reason for the higher rates of increased levels of CRP in patients with CD, compared with UC, is unknown. Furthermore, many patients with established CD do not have increased levels of CRP, despite evidence of active disease, so these studies probably overestimated the sensitivity of this test in detecting CD35. Because of the relatively low sensitivity of the CRP test in detection of UC, use of this marker alone to identify patients with symptoms compatible with IBD that should undergo further evaluation would delay diagnosis for many.
Various serologic tests have been used in attempts to improve the diagnosis of IBD and to distinguish CD from UC, such as tests for perinuclear antineutrophil cytoplasmic antibodies (pANCAs) and anti–Saccharomyces cerevisiae antibodies (ASCAs)36. Increased titers of ASCA were reported to identify patients with CD with high levels of specificity (96%–100%) but low sensitivity (approximately 50%).36 In contrast, increased levels of pANCA were more common in patients with UC or those with CD that had UC-like pancolitis.36 A meta-analysis of 60 studies estimated the sensitivity and specificity of ASCA+/pANCA- for detection of CD to be 55% and 93%, respectively, and 63% and 93% for any form of IBD 37. In pediatric patients, the test for pANCA+/ASCA- performed particularly well, identifying patients with CD with 70% sensitivity and 93% specificity 37.
Other serologic markers of CD include antibodies to Escherichia coli outer membrane porin (OmpC), Pseudomonas fluorescens-associated sequence I2, and flagellin CBir1. A recent study of more than 300 children with suspected IBD found that a test for this panel of antibodies identified patients with IBD with 67% sensitivity and 76% specificity.38 However, studies have demonstrated comparable sensitivity and specificity using routine tests for anemia, thrombocytosis, and increased erythrocyte sedimentation rate (ESR) in the same population. 38, 39
ASCA is believed to interact with mannose residues on mannan in the cell walls of S cerevisiae, although data indicate that C albicans also produces the ASCA-binding epitope 40, 41. Antibodies against other sugars (particularly glycans on the surface of cells) and microorganisms have also been studied. Antibodies against laminaribioside (ALCA) and chitobioside (ACCA) have been associated with CD42. Tests for ALCA, ASCA, and antibodies against a covalently immobilized mannan from S cerevisiae (gASCA) distinguish patients with CD from healthy controls with similar operating characteristics as ASCA. Interestingly, 34%–44% of ASCA-negative patients with CD had positive results in tests for ALCA or ACCA42, 43. Others studies have shown that the combination of gASCA, pANCA, and ALCA is more accurate than other combinations of these serologic markers, or ACCA, antibodies to mannobioside (AMCA), and Omp, in distinguishing individuals with IBD from healthy controls44.
In considering results from these studies, it is important to assess the sensitivity, specificity, and predictive values of diagnostic tests. The cut point for a test determines its sensitivity and specificity; higher sensitivity results in lower specificity. In comparing results between studies, it is important to assess whether comparable cut points were used to define the test operating characteristics. Similarly, because positive and negative predictive values are determined based on the prevalence of disease in the population, one must compare the study populations before drawing conclusions about predictive values.
Tests Used to Evaluate Patients Diagnosed with IBD
Differentiating between CD and UC
ASCA is associated with CD whereas increased levels of pANCA are more common among patients with UC36. In a meta-analysis, combinations of tests for ASCA and pANCA distinguished patients with CD from those with UC with 40%–50% sensitivity and specificity of >90%37. However, when the population was limited to those with colonic disease, for whom the diagnostic question is most relevant, the ASCA test was less sensitive for CD and discriminated less well between CD and UC37.
The need for such a test is greatest in patients with IBD type unclassified (indeterminate colitis). One prospective study found that nearly half of the patients with IBD type unclassified had negative results from the ASCA and pANCA tests and that most continued to have clinical characteristics that precluded a definitive diagnosis of CD or UC45. Interestingly, of the patients who had a positive result from the pANCA or ASCA test, 44% developed CD or UC over a mean follow-up period of 9.9 years. Among 26 patients that had ASCA+/pANCA- results at baseline, 8 were later diagnosed with CD and 2 with UC. Among 20 patients that had ASCA-/pANCA+ results at baseline, 4 were later diagnosed with CD and 7 with UC. Thus, among the patients with positive results from serology analyses, ASCA and pANCA were predictive of disease type, but did not have 100% accuracy45. Addition of the tests for I2 and anti-OmpC to the tests for ASCA and pANCA has only a marginal incremental benefit in determining which patients with IBD type unclassified have CD and which have UC46. Similarly, addition of tests for the anti-glycan antibodies to tests for ASCA and pANCA does not appreciably increase the ability to distinguish CD from UC in cross-sectional studies44. An assay that used 2 different synthetic oligomannose blocks to detect anti-mannose antibodies (antibodies to the synthetic mannose blocks known as AΣMA) demonstrated better sensitivity (45% vs. 27%) and specificity (100% vs. 71%) than the ASCA test in predicting development of CD in patients with IBD type unclassified47.
Differentiating quiescent from active disease
Concentrations of fecal calprotectin, lactoferrin and CRP have each been correlated with histologic and endoscopic disease activity in patients with UC and CD (Table 2).48-58 In general, calprotectin and lactoferrin correlate better with colonic than ileal disease activity, although extent of colonic disease in does not appear to be important in patients with UC48, 50, 53. The sensitivities of tests for calprotectin to detect any active mucosal disease range from 70%–100%, with a specificity range of 44%–100%, depending on the cut point used49, 51, 59-61. Sensitivities and specificities of tests for lactoferrin are similar (Table 3).
Table 2.
Correlation of biomarkers with disease activity, determined by endoscopy.
| Author | Patient population | Assessment of endoscopic disease activity | Lactoferrin (correlation coefficient) | Calprotectin (correlation coefficient) | CRP (correlation coefficient) |
|---|---|---|---|---|---|
| Sipponen50 (2008) | CD | CDEIS | 0.77 | 0.73 | 0.55 |
| D'Inca51 (2007) | CD | SES-CD | 0.19 | 0.48 | |
| D'Inca51 (2007) | UC | Mayo score | 0.35 | 0.51 | |
| Hanai 200452 | UC | Matt's Index | 0.81 | ||
| Siponnen53 (2008) | CD | SES-CD | 0.63 | 0.64 | 0.52 |
| Fagerberg54 (2007) | IBD | Saverymuttu114 | 0.52 | ||
| Roseth48 (1997) | UC | Mayo score | 0.57 | ||
| Jones55 (2008) | CD | SES-CD | 0.76 | 0.72 | 0.46 |
| Sipponen56 (2008) | CD | CDEIS | 0.87 | 0.83 | 0.61 |
| Schoepfer58 (2009) | UC | Rachmilewitz Index | 0.83 | 0.50 | |
| Schoepfer57 (2010) | CD | CDEIS | 0.75 | 0.53 |
CDEIS – Crohn's Disease Endoscopic Index of Severity; SES-CD - Simple Endoscopic Score for Crohn's Disease
Table 3.
Sensitivity and specificity of biomarkers to identify active disease, based on endoscopy.
| Author | Patient population | Assessment of endoscopic disease activity | Lactoferrin (Sensitivity / Specificit) | Calprotectin (Sensitivity / Specificity) | CRP (Sensitivity / Specificit) |
|---|---|---|---|---|---|
| Roseth#49 (2004) | CD and UC | Farup method115 | 100% / 100% | ||
| Solem62 (2005) | CD | Study specific index## | 54% / 75% | ||
| Siponnen* (2008)59 | CD | CDEIS | 77% / 100% | 87% / 100% | |
| Siponnen60† (2008) | CD | CDEIS | 66%-71% / 83%-92% | 70%-91% / 44%-92% | 48% / 91% |
| Siponnen61‡ (2010) | CD | SES-CD | 80% / 67% | 80% / 89% | |
| D'Inca**51 (2007) | CD | SES-CD | 77% / 80% | 81% / 80% | |
| D'Inca**51 (2007) | UC | Mayo score | 75% / 60% | 78% / 70% | |
| Schoepfer††58 (2009) | UC | Rachmilewitz Index | 86% - 93% / 71% - 88% | ||
| Schoepfer#57 (2010) | CD | CDEIS | 89% / 58% | 68%/58% |
CDEIS – Crohn's Disease Endoscopic Index of Severity
SES-CD - Simple Endoscopic Score for Crohn's Disease
Threshold concentrations - calprotectin 50 μg/g
Threshold concentrations - calprotectin 200 μg/g; lactoferrin 10 μg/g
Threshold concentrations - calprotectin 50-200 μg/g; lactoferrin 7.25 – 10 μg/g
Threshold concentrations - calprotectin 100 μg/g; lactoferrin 7.25 μg/g
Findings of erosions or ulcerations; spontaneous bleeding; exudate; inflammatoryappearing nodularity, masses, or fistulas; friability; granularity; cobblestoning; extensive erythema; and concomitant erythema with edema.
Threshold concentrations - calprotectin 80 μg/g; lactoferrin not specified in μg/g
Threshold concentrations - calprotectin 50-100 μg/g
Serum biomarkers, particularly CRP, have also been used to distinguish quiescent from active disease. In general, the correlation between CRP and endoscopic activity is lower than that observed between fecal markers and activity (Table 3). Similarly, sensitivity and specificity for active mucosal inflammation is likely to be lower for CRP compared with fecal markers. For example, Solem et al. observed that the CRP test had 54% sensitivity and 75% specificity for CD in 105 patients 62. In a study of 43 patients with UC, 19 of 37 (51%) with active disease, based on colonoscopy analysis, had increased levels of CRP whereas 0 of 6 patients without endoscopic evidence of disease activity had increased levels of CRP62. Perhaps most importantly from the perspective of a clinician, 37 of 43 patients (86%) with any clinical symptoms of CD and with increased levels of CRP had evidence of mucosal inflammation, based on colonoscopy analysis 62. So, for patients with CD, the combination of increased levels of CRP and clinical symptoms is likely to be sufficient to identify active mucosal disease. Some patients have persistent, normal levels of CRP despite active disease63. For these patients, CRP will not be useful to differentiate quiescent from active disease. This may be a population where fecal biomarkers are particularly useful.
ESR has also been studied as a biomarker for IBD disease activity. Like CRP, some but not all studies have found it to be increased more frequently among patients with active CD than UC63-65. However, tests for ESR are less widely used than tests for CRP because ESR levels do not change as quickly with disease activity63.
Using biomarkers to establish mucosal healing
In patients with UC or CD, mucosal healing in response to medical therapy correlates with a less severe future course of disease 66, 67. Similarly, following ileal resection, the appearance of the neoterminal ileum predicts short term outcomes68. Thus, there is potential to use biomarkers to assess mucosal healing following medical therapy or surgery and to predict the likelihood of relapse.
Roseth et al. demonstrated that patients with CD or UC who had remission following medical therapy had large reductions in levels of fecal calprotectin, (to below 50 μg/g)49. Several additional studies have shown similar results in response to therapy. Sipponen et al. performed one study of patients treated with anti-tumor necrosis factor (TNF) agents and another study of patients treated with other therapies. Among 5 patients that had mucosal healing after treatment with reagents other than anti-TNF agents, 4 (80%) also had normalized levels of fecal calprotectin and lactoferrin. Among 9 patients with no mucosal improvement after therapy, 8 (89%) had increased levels of calprotectin and 6 (67%) had increased levels of lactoferrin 61. Eleven patients that responded to anti-TNF therapy (based on endoscopic appearance), had significant decreases in levels of fecal calprotectin and lactoferrin, whereas 3 non-responders did not have decreased levels of these markers69. Despite the consistency of these results, the studies were limited by small sample sizes and an inability to define an optimal cut point for predicting mucosal healing. However, within the range of cut points tested, there does not appear to be a difference between tests for calprotectin and lactoferrin in determining treatment response.
There are limited data regarding the use of biomarkers to assess CD recurrence following ileocolonic resection; and the results for fecal biomarkers demonstrated only modest sensitivity and specificity70-72. A possible explanation for these observations is that the initial, asymptomatic recurrence of CD results in limited mucosal injury. This small amount of injury, particularly to the ileum, is not likely to increase biomarkers to levels that can be detected in fecal samples.
Biomarkers to predict disease course
One of the major goals of treatment for CD is to prevent complications such as perforation and formation of abscesses, fistulas and strictures. Biomarkers might be used to identify patients who are at high risk for a complicated disease course. At the time of diagnosis, 5%–15% of patients already have strictures, 5%–15% have had a penetrating complication, and the remaining 70%–80% have pure inflammatory disease73, 74. A stricture or penetrating complication occurs in approximately 50% of patients within the first 20 years of disease; most of these patients require surgery within 6 months 74. Similarly, estimates of the cumulative risk of surgery range from 28% to 61% at 10 years for children and adults, respectively75, 76. Approximately 50% of the patients with CD would be expected to have a relatively uncomplicated course during a period of 10–20 years and might be candidates for less aggressive therapy, whereas the remaining 50% would be candidates for more aggressive therapy. The challenge is to identify these populations before the complications have occurred and to find therapies that can effectively prevent these complications.
Results from tests for ASCA and pANCA have been shown to predict complicated disease courses in some, but not all, studies37. Other studies have evaluated the ability of other combinations of seromarkers, quantitative assessment of these markers, and combinations of markers and genetic data to predict disease course. Mow et al. studied 303 patients with CD; 77 ASCA, OmpC and I2 were each associated with various features of complicated disease (Table 4). Furthermore, the number of tests for antibodies that were positive and the concentration of the antibodies, based on sums of quartiles for each marker, were associated with complications that included stenosis, internal penetrating disease, and the need for small bowel surgery; although 40% of patients in the lowest quartiles for concentration of I2, ASCA, and OmpC levels had undergone small bowel surgery. In a follow-up study, Targan et al. associated antibodies to CBir1 with small bowel disease, an internal penetrating phenotype, and fibrostenosis78. Anti-CBir1 antibodies were not associated with small bowel surgery. Similarly, ACCA, ALCA, AMCA, and gASCA have been associated with penetrating or stricturing complications and surgery.44
Table 4. Associations of serologies and NOD2 mutations with disease phenotype.
| Small bowel disease | Fibrostenosis | Internal Perforation | Small bowel surgery | Perianal disease | |
|---|---|---|---|---|---|
| ASCA | + | + | + | + | Not associated |
| pANCA | - | - | Not associated | - | Not associated |
| I2 | + | + | Not associated | + | Not associated |
| OmpC | Not associated | + | + | + | Not associated |
Adapted from Mow WS, Vasiliauskas EA, Lin Y-C, Fleshner PR, Papadakis KA, Taylor KD, Landers CJ, Abreu-Martin MT, Rotter JI, Yang H, Targan SR. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology 2004;126:414-24.
Many studies of the ability of serologic tests to predict disease course have relied on a cross-sectional design in which the disease phenotype was determined at the same time that the serologic factors were measured. However, phenotype and serologic status may change over time.44
To overcome this limitation, studies by Amre et al. and Dubinsky et al. 79, 80 evaluated children with CD and measured these antibodies close to the time of diagnosis or early in the course of therapy. Amre et al. associated a positive result in the ASCA test with a shorter time to fistula or abscess development. The cohort studied by Dubinsky et al. included 167 patients without internal perforations or strictures at the time of serologic analysis. Of patients with 1 or more positive result for ASCA, I2, or OmpC, 8.2% developed complications, compared with 2.7% of those with no positive result. A subsequent study by Dubinsky et al. of a larger cohort of 536 pediatric patients with CD yielded nearly identical results81. Similar to the findings of Mow et al.,77 the number of positive test results and the sum of quartiles for individual results were associated with time to complications or surgery.
While these tests may be useful in carefully selected patients, the following needs to be considered. The serologic profiles associated with high risk for complications identified many patients that did not have internal perforating or stricture complications or require surgery within 5 to 10 years81. Additionally, the available studies did not determine how much the serologic data adds to the predictive ability of other clinical data. Finally, and as importantly, we do not know if early intervention in patients with the worst prognosis, based on positive serology test results, will change long-term outcomes.
The role of biomarkers in predicting disease relapse
Biomarkers might also be developed to identify patients that are likely to experience disease recurrence after treatment. Several studies have shown that in patients with quiescent disease, increased concentrations of fecal calprotectin predict disease relapse within 12 months, particularly in patients with UC (Table 5)82-87. Early studies reported that increased concentrations of fecal calprotectin identified patients that underwent relapse within 12 months with approximately 90% sensitivity and 82% specificity82, 83. Costa et al. reported that increased levels of fecal calprotectin had a positive-predictive value of 81% and a negative-predictive value of 90% for relapse of UC; in patients with CD, the positive predictive value was 87% and the negative-predictive value was 43% 82.
Table 5.
Studies associating increased concentrations of fecal calprotectin with relapse in patients with exacerbations in ulcerative colitis.
| Author | Patient population | Duration of remission at entry | Calprotectin Concentration to Define Elevated Level | Relapse Rate with Low Calprotectin | Relapse Rate with High Calprotectin |
|---|---|---|---|---|---|
| Gisbert84 | UC | >6 mos. | >150μg/g | 9% | 31% |
| Tibble83 | UC | 1-4 mos. | >50μg/g | 10%* | 85%* |
| Tibble83 | CD | 1-4 mos. | >50μg/g | 15% | 85% |
| Costa82 | UC | 1-12 mos. | >150μg/g | 10% | 81% |
| Costa82 | CD | 1-12 mos. | >150μg/g | 57% | 87% |
| D'Inca85 | UC | 3-36 mos. | >130μg/g | 30% | 79% |
| Sipponen86 | UC+CD | > 3mos (51% >12 months) | >100μg/g | 25% | 39% |
| Walkiewicz87# | CD | Not stated | >400μg/g | 11% | 56% |
estimated from Kaplan Meier curves
A study of calprotectin and lactoferrin levels in patients with quiescent IBD84 found that increased levels of either biomarker identified patients who would relapse in 12 months (calprotectin sensitivity 69%, specificity 69%; lactoferrin sensitivity 62%, specificity 65%), although these values were lower than those reported in previous studies. Sipponen and Kolho reported that the relapse rate among patients with increased levels of fecal calprotectin was low, although in this study, more than 50% of the patients had been in remission for more than 1 year86. Increased levels of calprotectin (and likely lactoferrin) shortly after remission might be better predictors of relapse than increased levels of calprotectin 6 months or more after remission.
The abilities of CRP and ESR to identify patients that are most likely to undergo disease relapse have also been examined. In patients with CD, several studies have associated increased levels of CRP and/or ESR with relapse88-93; there are less data available to associate marker levels with relapse of UC. Bitton et al. examined markers of relapse of 74 patients with clinically and endoscopically quiescent UC (27 relapsed during the follow-up period). Although the identification of basal plasmacytosis in rectal biopsy samples was associated with relapse, increased levels of CRP or ESR were not94.
The role of biomarkers in predicting response to therapy
In addition to predicting disease relapse, biomarkers might be used to predict response to therapy. For example, it would be useful to know which patients are most likely to respond to intravenous corticosteroid therapy for UC95-98. Travis et al. demonstrated that the combination of stool frequency and CRP levels on the third day of therapy predicted failure to respond to intravenous steroids96; this finding was validated in a study of children with severe UC. However, the Pediatric Ulcerative Colitis Index (PUCAI), which is based only on symptoms, was found to more accurately identify patients that do not respond to intravenous corticosteroids than the Travis index or levels of calprotectin, CRP, M2-pyruvate kinase, or S100A1297, 98. In analyzing data from these types of studies, it is important to consider that the symptoms that were measured by the PUCAI may have also been used by the treating physician to make therapeutic decisions. If that is the case, it is difficult for biomarkers to outperform symptoms in predicting therapeutic decisions, such as the need for surgery.
ASCA, pANCA and other antibodies have also been tested for their association with responses to specific therapies. Taylor et al. demonstrated a lower response rate among patients with CD treated with infliximab who had positive results from a test for pANCA99. A subsequent study failed to confirm this association, although there was trend toward lower response rates in patients with positive results from pANCA and negative results from ASCA tests100. This same pattern was associated with a reduced response to infliximab therapy in patients with UC (55% of these patients responded to the drug) 101. Most recently, in a study of children with either CD or UC, presence of a positive test for pANCA was again associated with a lower likelihood of responding to infliximab102. Results of tests for anti-I2, but not ASCA, pANCA, or OmpC, were associated with response to fecal diversion (94% response among patients with anti-I2 antibodies vs. 18% response among those without anti-I2 antibodies)103.
Drug levels, metabolites and anti-drug antibodies as biomarkers
The major metabolites of thiopurines (azathioprine and 6-mercaptopurine) are 6 thioguanine nucleotides (6TGN) and 6 methylmercaptopurine (6MMP). A meta-analysis showed that patients in clinical remission were more likely to have levels of 6TGN greater than 230–260 pmol/8 × 108 red blood cells than patients with persistently active disease (62% vs. 36%)104. However, the meta-analysis included only cross-sectional studies, so drug levels were not measured before the outcomes of therapy were known. A small randomized trial reported that dose adjustment based on metabolite levels was not more effective than weight-based dose determination although the study was not completed because of slow enrollment105. A recent study demonstrated that data on thiopurine metabolites helped clinicians make therapeutic decisions for symptomatic patients with IBD (Table 6).106 Less expensive alternatives to directly measure thiopurine metabolite levels, such as measuring the mean corpuscular volume, lymphocyte counts, WBC count and machine learning algorithms have been proposed107. However, these alternatives need to be studied prospectively to determine whether therapeutic adjustment based on these tests will improve clinical outcomes.
Table 6. Proposed algorithms in response to measured drug levels in the setting of symptoms of active disease.
| 6TGN concentration | 6MMP concentration | Interpretation | Strategy |
|---|---|---|---|
| In therapeutic window* | Normal or high^ | Refractory to thiopurines | Change therapy – can discontinue thiopurine or continue at same dose in conjunction with the new therapy |
| Low | Low or normal | Too low of dose or noncompliant | Increase dose or educate regarding compliance |
| High | Normal or high | Refractory to thiopurines | Change therapy and discontinue thiopurine or continue at same dose or lower dose |
| Low | High | Preferential shunting to 6MMP† | Change therapy or reduce dose and add allopurinol |
| Anti-Infliximab antibody | Infliximab concentration | Additional testing | Strategy |
|---|---|---|---|
| Positive | Change to another anti-TNF therapy. If persistent disease activity after changing agents, change to agent with different mechanism | ||
| In therapeutic window | Active disease on endoscopy / radiology | Change to agent with different mechanism | |
| In therapeutic window | Inactive disease on endoscopy / radiology | Investigate for alternative etiology of symptoms | |
| Sub-therapeutic# | Increase dose or frequency. If persistent disease activity, switch to a different anti-TNF agent. | ||
| Sub-therapeutic# | Alternative strategy - Switch to a different anti-TNF agent. If persistent disease activity, change to agent with different mechanism. |
Therapeutic window for 6TGN defined as 235–450 pmol/8 _ 108 red blood cells
Therapeutic level of 6MMP is less than 5700 pmol/8 _ 108 RBC
A ratio of 6MMP:6TGN greater than 11
Sub-therapeutic infliximab concentration defined as <12 mcg/ml at 4 weeks or undetectable trough level
The effectiveness of biologic therapies, such as the anti-TNF drugs for CD and UC, depends on sufficient drug levels 108-112. Beart et al. observed longer duration of response to therapy in those with infliximab concentrations 4 weeks post infusion of greater than 12μg/ml (median 82 days) than in those with lower drug levels (median 69 days)108. Among patients with CD, detectable trough serum infliximab level is associated with maintenance of remission for the entire duration between infusions109. In a recent randomized trial comparing infliximab monotherapy, azathioprine monotherapy, and combination therapy, corticosteroid free remission at week 26 was more common among patients with detectable trough levels at week 30. However, it is noteworthy that 59% of the patients with undetectable infliximab levels had achieved steroid free remission, which is only modestly lower than the approximately 73% steroid free remission rate in those with detectable infliximab levels.110 Thus, detectable trough levels are not essential to achieve clinical remission. Similar studies have demonstrated the predictive value of adalimumab drug levels and antibodies in the management of CD111 and infliximab drug levels for moderate to severely active ulcerative colitis112.
Factors other than the administered dose also contribute to anti-TNF drug levels. Biologic therapies can induce production of anti-drug antibodies, which are associated with shorter duration of response and a higher incidence of adverse events108.
There is uncertainty regarding in what clinical setting measurement of infliximab drug levels and anti-infliximab antibodies is useful, but they are most commonly measured when patients lose response to therapy. Afif and Sandborn found that only 17% of patients with antibodies to infliximab responded to an increased dose of this drug113. However, switching patients to another anti-TNF agent resulted in a clinical response in 92% of these patients. These data led Afif et al. to propose an algorithm (Table 6) in which detection of anti-drug antibodies in serum samples indicates that patients should be given an alternative drug in the same class. Furthermore, undetectable trough levels or low levels of the drugs by 4-weeks after administration indicates that the dose or frequency should be increased (or that an alternate anti-TNF agent should be given); patients with therapeutic drug levels in serum should be evaluated for active disease and switched to a therapeutic with a different mechanism of action.
Conclusions
Biomarkers could have a role at nearly every point in the disease management. When patients present with symptoms suggestive of IBD, combinations of fecal and serologic markers might be used to identify patients that should undergo invasive testing and to help distinguish CD from UC. Tests for serologic biomarkers have been developed to identify patients that are most likely to have a severe course of disease progression, but require further evaluation in prospective studies that also assess clinical predictors (e.g., disease location, nutritional status, routine blood tests. Failure to achieve mucosal healing with therapy has been associated with worse disease course. Biomarkers such as calprotectin and lactoferrin can be used to assess mucosal healing, without the need for invasive testing or radiation. Tests for levels of drug metabolites or anti-drug antibodies can be used to determine why certain patients don't respond to specific therapies and identify alternative treatment strategies.
Acknowledgments
This work was supported in part by NIH grant K24-DK078228.
Footnotes
Adapted from the following manuscripts:
Haines ML, Ajlouni Y, Irving PM, Sparrow MP, Rose R, Gearry RB, Gibson PR. Clinical usefulness of therapeutic drug monitoring of thiopurines in patients with inadequately controlled inflammatory bowel disease. Inflammatory Bowel Diseases 2010 epub ahead of print.
Afif W, Loftus EV, Jr., Faubion WA, Kane SV, Bruining DH, Hanson KA, Sandborn WJ. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. American Journal of Gastroenterology 2010;105:1133-9.
References
- 1.Langhorst J, Elsenbruch S, Mueller T, Rueffer A, Spahn G, Michalsen A, Dobos GJ. Comparison of 4 neutrophil-derived proteins in feces as indicators of disease activity in ulcerative colitis. Inflamm Bowel Dis. 2005;11:1085–91. doi: 10.1097/01.mib.0000187980.08686.18. [DOI] [PubMed] [Google Scholar]
- 2.Saitoh O, Kojima K, Sugi K, Matsuse R, Uchida K, Tabata K, Nakagawa K, Kayazawa M, Hirata I, Katsu K. Fecal eosinophil granule-derived proteins reflect disease activity in inflammatory bowel disease. Am J Gastroenterol. 1999;94:3513–20. doi: 10.1111/j.1572-0241.1999.01640.x. see comment. [DOI] [PubMed] [Google Scholar]
- 3.Sugi K, Saitoh O, Hirata I, Katsu K. Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil-derived proteins. Am J Gastroenterol. 1996;91:927–34. [PubMed] [Google Scholar]
- 4.Poullis A, Foster R, Northfield TC, Mendall MA. Review article: faecal markers in the assessment of activity in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:675–81. doi: 10.1046/j.1365-2036.2002.01196.x. [DOI] [PubMed] [Google Scholar]
- 5.Angriman I, Scarpa M, D'Incx00E Renata, Basso D, Ruffolo C, Polese L, Sturniolo GC, D'Amico DF, Plebani M. Enzymes in feces: useful markers of chronic inflammatory bowel disease. Clin Chim Acta. 2007;381:63–8. doi: 10.1016/j.cca.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Becker K, Niederau C, Frieling T. Fecal excretion of alpha 2-macroglobulin: a novel marker for disease activity in patients with inflammatory bowel disease. Z Gastroenterol. 1999;37:597–605. [PubMed] [Google Scholar]
- 7.Casellas F, Antolx000Edn M, Varela E, Garcx000Fa-Lafuente A, Guarner F, Borruel N, Armengol Mx00E Jos Ram, Malagelada JR. Fecal excretion of human deoxyribonucleic acid as an index of inflammatory activity in ulcerative colitis. Clinical Gastroenterology & Hepatology. 2004:683–9. doi: 10.1016/s1542-3565(04)00291-5. [DOI] [PubMed] [Google Scholar]
- 8.Chung-Faye G, Hayee Bh, Maestranzi S, Donaldson N, Forgacs I, Sherwood R. Fecal M2-pyruvate kinase (M2-PK): a novel marker of intestinal inflammation. Inflamm Bowel Dis. 2007;13:1374–8. doi: 10.1002/ibd.20214. [DOI] [PubMed] [Google Scholar]
- 9.Czub E, Herzig KH, Szaflarska-Popawska A, Kiehne K, Socha P, Wox0015B Halina, Kaminska B, Blaszczynski M, Cichy W, Bala G, Brodzicki J, Grzybowska-Chlebowczyk U, Walkowiak J. Fecal pyruvate kinase: a potential new marker for intestinal inflammation in children with inflammatory bowel disease. Scand J Gastroenterol. 2007;42:1147–50. doi: 10.1080/00365520701320513. [DOI] [PubMed] [Google Scholar]
- 10.de Jong NSH, Leach ST, Day AS. Fecal S100A12: a novel noninvasive marker in children with Crohn's disease. Inflamm Bowel Dis. 2006;12:566–72. doi: 10.1097/01.ibd.0000227626.72271.91. [DOI] [PubMed] [Google Scholar]
- 11.Hocke M, Richter L, Bosseckert H, Eitner K. Platelet activating factor in stool from patients with ulcerative colitis and Crohn's disease. Hepatogastroenterology. 1999;46:2333–7. [PubMed] [Google Scholar]
- 12.Jansson J, Willing B, Lucio M, Fekete A, Dicksved J, Halfvarson J, Tysk C, Schmitt-Kopplin P. Metabolomics reveals metabolic biomarkers of Crohn's disease. PLoS ONE. 2009;4:e6386. doi: 10.1371/journal.pone.0006386. Electronic Resource. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeffery J, Lewis SJ, Ayling RM. Fecal dimeric M2-pyruvate kinase (tumor M2-PK) in the differential diagnosis of functional and organic bowel disorders. Inflamm Bowel Dis. 2009;15:1630–4. doi: 10.1002/ibd.20946. [DOI] [PubMed] [Google Scholar]
- 14.Lundberg JO, Hellstrx000Fm PM, Fagerhol MK, Weitzberg E, Roseth AG. Technology insight: calprotectin, lactoferrin and nitric oxide as novel markers of inflammatory bowel disease. Nature Clinical Practice Gastroenterology & Hepatology. 2005;2:96–102. doi: 10.1038/ncpgasthep0094. [DOI] [PubMed] [Google Scholar]
- 15.Masoodi I, Kochhar R, Dutta U, Vaishnavi C, Prasad KK, Vaiphei K, Kaur S, Singh K. Fecal lactoferrin, myeloperoxidase and serum C-reactive are effective biomarkers in the assessment of disease activity and severity in patients with idiopathic ulcerative colitis. J Gastroenterol Hepatol. 2009;24:1768–74. doi: 10.1111/j.1440-1746.2009.06048.x. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen OH, Gionchetti P, Ainsworth M, Vainer B, Campieri M, Borregaard N, Kjeldsen L. Rectal dialysate and fecal concentrations of neutrophil gelatinase-associated lipocalin, interleukin-8, and tumor necrosis factor-alpha in ulcerative colitis. Am J Gastroenterol. 1999;94:2923–8. doi: 10.1111/j.1572-0241.1999.01439.x. [DOI] [PubMed] [Google Scholar]
- 17.Peterson CGB, Sangfelt P, Wagner M, Hansson T, Lettesjx000F H, Carlson M. Fecal levels of leukocyte markers reflect disease activity in patients with ulcerative colitis. Scand J Clin LabInvest. 2007;67:810–20. doi: 10.1080/00365510701452838. [DOI] [PubMed] [Google Scholar]
- 18.Wagner M, Peterson CGB, Ridefelt P, Sangfelt P, Carlson M. Fecal markers of inflammation used as surrogate markers for treatment outcome in relapsing inflammatory bowel disease. World Journal of Gastroenterology. 2008;14:5584–9. doi: 10.3748/wjg.14.5584. discussion 5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys. Gut. 2006;55:436–31. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roseth AG, Fagerhol MK, Aadland E, Schjonsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27:793–8. doi: 10.3109/00365529209011186. [DOI] [PubMed] [Google Scholar]
- 21.Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, Bridger S, Foster R, Sherwood R, Fagerhol M, Bjarnason I. A simple method for assessing intestinal inflammation in Crohn's disease. Gut. 2000;47:506–13. doi: 10.1136/gut.47.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Roon AC, Karamountzos L, Purkayastha S, Reese GE, Darzi AW, Teare JP, Paraskeva P, Tekkis PP. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803–13. doi: 10.1111/j.1572-0241.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 23.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 341:c3369. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kane SV, Sandborn WJ, Rufo PA, Zholudev A, Boone J, Lyerly D, Camilleri M, Hanauer SB. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol. 2003;98:1309–14. doi: 10.1111/j.1572-0241.2003.07458.x. [DOI] [PubMed] [Google Scholar]
- 25.Gisbert JP, McNicholl AG, Gomollon F. Questions and answers on the role of fecal lactoferrin as a biological marker in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1746–54. doi: 10.1002/ibd.20920. [DOI] [PubMed] [Google Scholar]
- 26.Joishy M, Davies I, Ahmed M, Wassel J, Davies K, Sayers A, Jenkins H. Fecal calprotectin and lactoferrin as noninvasive markers of pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2009;48:48–54. doi: 10.1097/MPG.0b013e31816533d3. [DOI] [PubMed] [Google Scholar]
- 27.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–9. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 28.Silberer H, Kuppers B, Mickisch O, Baniewicz W, Drescher M, Traber L, Kempf A, Schmidt-Gayk H. Fecal leukocyte proteins in inflammatory bowel disease and irritable bowel syndrome. Clin Lab. 2005;51:117–26. [PubMed] [Google Scholar]
- 29.de Jong NS, Leach ST, Day AS. Fecal S100A12: a novel noninvasive marker in children with Crohn's disease. Inflamm Bowel Dis. 2006;12:566–72. doi: 10.1097/01.ibd.0000227626.72271.91. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser T, Langhorst J, Wittkowski H, Becker K, Friedrich AW, Rueffer A, Dobos GJ, Roth J, Foell D. Faecal S100A12 as a non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2007;56:1706–13. doi: 10.1136/gut.2006.113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manolakis AC, Kapsoritakis AN, Georgoulias P, Tzavara C, Valotassiou V, Kapsoritaki A, Potamianos SP. Moderate performance of serum S100A12, in distinguishing inflammatory bowel disease from irritable bowel syndrome. BMC Gastroenterol. 2010;10:118. doi: 10.1186/1471-230X-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beattie RM, Walker-Smith JA, Murch SH. Indications for investigation of chronic gastrointestinal symptoms. Arch Dis Child. 1995;73:354–5. doi: 10.1136/adc.73.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poullis AP, Zar S, Sundaram KK, Moodie SJ, Risley P, Theodossi A, Mendall MA. A new, highly sensitive assay for C-reactive protein can aid the differentiation of inflammatory bowel disorders from constipation- and diarrhoea-predominant functional bowel disorders. Eur J Gastroenterol Hepatol. 2002;14:409–12. doi: 10.1097/00042737-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Shine B, Berghouse L, Jones JE, Landon J. C-reactive protein as an aid in the differentiation of functional and inflammatory bowel disorders. Clin Chim Acta. 1985;148:105–9. doi: 10.1016/0009-8981(85)90219-0. [DOI] [PubMed] [Google Scholar]
- 35.Lewis JD. C-reactive protein: anti-placebo or predictor of response. Gastroenterology. 2005;129:1114–6. doi: 10.1053/j.gastro.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 36.Ruemmele FM, Targan SR, Levy G, Dubinsky M, Braun J, Seidman EG. Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology. 1998;115:822–9. doi: 10.1016/s0016-5085(98)70252-5. [DOI] [PubMed] [Google Scholar]
- 37.Reese GE, Constantinides VA, Simillis C, Darzi AW, Orchard TR, Fazio VW, Tekkis PP. Diagnostic precision of anti-Saccharomyces cerevisiae antibodies and perinuclear antineutrophil cytoplasmic antibodies in inflammatory bowel disease. Am J Gastroenterol. 2006;101:2410–22. doi: 10.1111/j.1572-0241.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 38.Benor S, Russell GH, Silver M, Israel EJ, Yuan Q, Winter HS. Shortcomings of the inflammatory bowel disease Serology 7 panel. Pediatrics. 2010;125:1230–6. doi: 10.1542/peds.2009-1936. [DOI] [PubMed] [Google Scholar]
- 39.Sabery N, Bass D. Use of serologic markers as a screening tool in inflammatory bowel disease compared with elevated erythrocyte sedimentation rate and anemia. Pediatrics. 2007;119:e193–9. doi: 10.1542/peds.2006-1361. [DOI] [PubMed] [Google Scholar]
- 40.McKenzie H, Main J, Pennington CR, Parratt D. Antibody to selected strains of Saccharomyces cerevisiae (baker's and brewer's yeast) and Candida albicans in Crohn's disease. Gut. 1990;31:536–8. doi: 10.1136/gut.31.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Standaert-Vitse A, Jouault T, Vandewalle P, Mille C, Seddik M, Sendid B, Mallet JM, Colombel JF, Poulain D. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn's disease. Gastroenterology. 2006;130:1764–75. doi: 10.1053/j.gastro.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Dotan I, Fishman S, Dgani Y, Schwartz M, Karban A, Lerner A, Weishauss O, Spector L, Shtevi A, Altstock RT, Dotan N, Halpern Z. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn's disease. Gastroenterology. 2006;131:366–78. doi: 10.1053/j.gastro.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 43.Simondi D, Mengozzi G, Betteto S, Bonardi R, Ghignone RP, Fagoonee S, Pellicano R, Sguazzini C, Pagni R, Rizzetto M, Astegiano M. Antiglycan antibodies as serological markers in the differential diagnosis of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:645–51. doi: 10.1002/ibd.20368. [DOI] [PubMed] [Google Scholar]
- 44.Ferrante M, Henckaerts L, Joossens M, Pierik M, Joossens S, Dotan N, Norman GL, Altstock RT, Van Steen K, Rutgeerts P, Van Assche G, Vermeire S. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut. 2007;56:1394–403. doi: 10.1136/gut.2006.108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joossens S, Reinisch W, Vermeire S, Sendid B, Poulain D, Peeters M, Geboes K, Bossuyt X, Vandewalle P, Oberhuber G, Vogelsang H, Rutgeerts P, Colombel JF. The value of serologic markers in indeterminate colitis: a prospective follow-up study. Gastroenterology. 2002;122:1242–7. doi: 10.1053/gast.2002.32980. [DOI] [PubMed] [Google Scholar]
- 46.Joossens S, Colombel JF, Landers C, Poulain D, Geboes K, Bossuyt X, Targan S, Rutgeerts P, Reinisch W. Anti-outer membrane of porin C and anti-I2 antibodies in indeterminate colitis. Gut. 2006;55:1667–9. doi: 10.1136/gut.2005.089623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vandewalle-El Khoury P, Colombel JF, Joossens S, Standaert-Vitse A, Collot M, Halfvarson J, Ayadi A, Landers CJ, Vermeire S, Rutgeerts P, Targan SR, Chamaillard M, Mallet JM, Sendid B, Poulain D. Detection of antisynthetic mannoside antibodies (ASigmaMA) reveals heterogeneity in the ASCA response of Crohn's disease patients and contributes to differential diagnosis, stratification, and prediction. Am J Gastroenterol. 2008;103:949–57. doi: 10.1111/j.1572-0241.2007.01648.x. [DOI] [PubMed] [Google Scholar]
- 48.Roseth AG, Aadland E, Jahnsen J, Raknerud N. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion. 1997;58:176–80. doi: 10.1159/000201441. [DOI] [PubMed] [Google Scholar]
- 49.Roseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol. 2004;39:1017–20. doi: 10.1080/00365520410007971. [DOI] [PubMed] [Google Scholar]
- 50.Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Farkkila M. Crohn's disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn's disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–6. doi: 10.1002/ibd.20312. [DOI] [PubMed] [Google Scholar]
- 51.D'Inca R, Dal Pont E, Di Leo V, Ferronato A, Fries W, Vettorato MG, Martines D, Sturniolo GC. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007;22:429–37. doi: 10.1007/s00384-006-0159-9. [DOI] [PubMed] [Google Scholar]
- 52.Hanai H, Takeuchi K, Iida T, Kashiwagi N, Saniabadi AR, Matsushita I, Sato Y, Kasuga N, Nakamura T. Relationship between fecal calprotectin, intestinal inflammation, and peripheral blood neutrophils in patients with active ulcerative colitis. Digestive Diseases & Sciences. 2004;49:1438–43. doi: 10.1023/b:ddas.0000042243.47279.87. [DOI] [PubMed] [Google Scholar]
- 53.Sipponen T, Karkkainen P, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Farkkila M. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn's disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221–9. doi: 10.1111/j.1365-2036.2008.03835.x. [DOI] [PubMed] [Google Scholar]
- 54.Fagerberg UL, Loof L, Lindholm J, Hansson LO, Finkel Y. Fecal calprotectin: a quantitative marker of colonic inflammation in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;45:414–20. doi: 10.1097/MPG.0b013e31810e75a9. [DOI] [PubMed] [Google Scholar]
- 55.Jones J, Loftus EV, Jr, Panaccione R, Chen LS, Peterson S, McConnell J, Baudhuin L, Hanson K, Feagan BG, Harmsen SW, Zinsmeister AR, Helou E, Sandborn WJ. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn's disease. Clinical Gastroenterology & Hepatology. 2008;6:1218–24. doi: 10.1016/j.cgh.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 56.Sipponen T, Savilahti E, Karkkainen P, Kolho KL, Nuutinen H, Turunen U, Farkkila M. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn's disease. Inflamm Bowel Dis. 2008;14:1392–8. doi: 10.1002/ibd.20490. [DOI] [PubMed] [Google Scholar]
- 57.Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, Seibold F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–9. doi: 10.1038/ajg.2009.545. [DOI] [PubMed] [Google Scholar]
- 58.Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: Correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15:1851–1858. doi: 10.1002/ibd.20986. [DOI] [PubMed] [Google Scholar]
- 59.Sipponen T, Savilahti E, Karkkainen P, Kolho KL, Nuutinen H, Turunen U, Farkkila M. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn's disease. Inflamm Bowel Dis. 2008;14:1392–8. doi: 10.1002/ibd.20490. [DOI] [PubMed] [Google Scholar]
- 60.Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Farkkila M. Crohn's disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn's disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–6. doi: 10.1002/ibd.20312. [DOI] [PubMed] [Google Scholar]
- 61.Sipponen T, Bjorkesten CGAF, Farkkila M, Nuutinen H, Savilahti E, Kolho KL. Faecal calprotectin and lactoferrin are reliable surrogate markers of endoscopic response during Crohn's disease treatment. Scand J Gastroenterol. 2010;45:325–31. doi: 10.3109/00365520903483650. [DOI] [PubMed] [Google Scholar]
- 62.Solem CA, Loftus EV, Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–12. doi: 10.1097/01.mib.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 63.Fagan EA, Dyck RF, Maton PN, Hodgson HJ, Chadwick VS, Petrie A, Pepys MB. Serum levels of C-reactive protein in Crohn's disease and ulcerative colitis. Eur J Clin Invest. 1982;12:351–9. doi: 10.1111/j.1365-2362.1982.tb02244.x. [DOI] [PubMed] [Google Scholar]
- 64.Cakal B, Akoz AG, Ustundag Y, Yalinkilic M, Ulker A, Ankarali H. Red cell distribution width for assessment of activity of inflammatory bowel disease. Dig Dis Sci. 2009;54:842–7. doi: 10.1007/s10620-008-0436-2. [DOI] [PubMed] [Google Scholar]
- 65.Rodgers AD, Cummins AG. CRP correlates with clinical score in ulcerative colitis but not in Crohn's disease. Dig Dis Sci. 2007;52:2063–8. doi: 10.1007/s10620-006-9691-2. [DOI] [PubMed] [Google Scholar]
- 66.Froslie KF, Jahnsen J, Moum BA, Vatn MH, Group I. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–22. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 67.Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, Stokkers P, Hommes D, Rutgeerts P, Vermeire S, D'Haens G. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology. 2010;138:463–8. doi: 10.1053/j.gastro.2009.09.056. quiz e10-1. [DOI] [PubMed] [Google Scholar]
- 68.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99:956–63. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 69.Sipponen T, Savilahti E, Karkkainen P, Kolho KL, Nuutinen H, Turunen U, Farkkila M. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn's disease. Inflamm Bowel Dis. 2008;14:1392–8. doi: 10.1002/ibd.20490. [DOI] [PubMed] [Google Scholar]
- 70.Scarpa M, D'Inca R, Basso D, Ruffolo C, Polese L, Bertin E, Luise A, Frego M, Plebani M, Sturniolo GC, D'Amico DF, Angriman I. Fecal lactoferrin and calprotectin after ileocolonic resection for Crohn's disease. Dis Colon Rectum. 2007;50:861–9. doi: 10.1007/s10350-007-0225-6. [DOI] [PubMed] [Google Scholar]
- 71.Lamb CA, Mohiuddin MK, Gicquel J, Neely D, Bergin FG, Hanson JM, Mansfield JC. Faecal calprotectin or lactoferrin can identify postoperative recurrence in Crohn's disease. Br J Surg. 2009;96:663–74. doi: 10.1002/bjs.6593. [DOI] [PubMed] [Google Scholar]
- 72.Orlando A, Modesto I, Castiglione F, Scala L, Scimeca D, Rispo A, Teresi S, Mocciaro F, Criscuoli V, Marrone C, Platania P, De Falco T, Maisano S, Nicoli N, Cottone M. The role of calprotectin in predicting endoscopic post-surgical recurrence in asymptomatic Crohn's disease: a comparison with ultrasound. Eur Rev Med Pharmacol Sci. 2006;10:17–22. [PubMed] [Google Scholar]
- 73.Romberg-Camps MJ, Dagnelie PC, Kester AD, Hesselink-van de Kruijs MA, Cilissen M, Engels LG, Van Deursen C, Hameeteman WH, Wolters FL, Russel MG, Stockbrugger RW. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol. 2009;104:371–83. doi: 10.1038/ajg.2008.38. [DOI] [PubMed] [Google Scholar]
- 74.Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV., Jr Risk Factors Associated With Progression to Intestinal Complications of Crohn's Disease in a Population-Based Cohort. Gastroenterology. doi: 10.1053/j.gastro.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gupta N, Cohen SA, Bostrom AG, Kirschner BS, Baldassano RN, Winter HS, Ferry GD, Smith T, Abramson O, Gold BD, Heyman MB. Risk factors for initial surgery in pediatric patients with Crohn's disease. Gastroenterology. 2006;130:1069–77. doi: 10.1053/j.gastro.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Munkholm P, Langholz E, Davidsen M, Binder V. Intestinal cancer risk and mortality in patients with Crohn's disease. Gastroenterology. 1993;105:1716–23. doi: 10.1016/0016-5085(93)91068-s. [DOI] [PubMed] [Google Scholar]
- 77.Mow WS, Vasiliauskas EA, Lin YC, Fleshner PR, Papadakis KA, Taylor KD, Landers CJ, Abreu-Martin MT, Rotter JI, Yang H, Targan SR. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology. 2004;126:414–24. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 78.Targan SR, Landers CJ, Yang H, Lodes MJ, Cong Y, Papadakis KA, Vasiliauskas E, Elson CO, Hershberg RM. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn's disease. Gastroenterology. 2005;128:2020–8. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 79.Amre DK, Lu SE, Costea F, Seidman EG. Utility of serological markers in predicting the early occurrence of complications and surgery in pediatric Crohn's disease patients. Am J Gastroenterol. 2006;101:645–52. doi: 10.1111/j.1572-0241.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 80.Dubinsky MC, Lin YC, Dutridge D, Picornell Y, Landers CJ, Farrior S, Wrobel I, Quiros A, Vasiliauskas EA, Grill B, Israel D, Bahar R, Christie D, Wahbeh G, Silber G, Dallazadeh S, Shah P, Thomas D, Kelts D, Hershberg RM, Elson CO, Targan SR, Taylor KD, Rotter JI, Yang H. Serum immune responses predict rapid disease progression among children with Crohn's disease: immune responses predict disease progression. Am J Gastroenterol. 2006;101:360–7. doi: 10.1111/j.1572-0241.2006.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dubinsky MC, Kugathasan S, Mei L, Picornell Y, Nebel J, Wrobel I, Quiros A, Silber G, Wahbeh G, Katzir L, Vasiliauskas E, Bahar R, Otley A, Mack D, Evans J, Rosh J, Hemker MO, Leleiko N, Crandall W, Langton C, Landers C, Taylor KD, Targan SR, Rotter JI, Markowitz J, Hyams J. Increased immune reactivity predicts aggressive complicating Crohn's disease in children. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2008;6:1105–11. doi: 10.1016/j.cgh.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, Ricchiuti A, Marchi S, Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn's disease. Gut. 2005;54:364–8. doi: 10.1136/gut.2004.043406. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. doi: 10.1053/gast.2000.8523. [see comment] [DOI] [PubMed] [Google Scholar]
- 84.Gisbert JP, Bermejo F, Perez-Calle JL, Taxonera C, Vera I, McNicholl AG, Algaba A, Lopez P, Lopez-Palacios N, Calvo M, Gonzalez-Lama Y, Carneros JA, Velasco M, Mate J. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009;15:1190–8. doi: 10.1002/ibd.20933. [DOI] [PubMed] [Google Scholar]
- 85.D'Inca R, Dal Pont E, Di Leo V, Benazzato L, Martinato M, Lamboglia F, Oliva L, Sturniolo GC. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008;103:2007–14. doi: 10.1111/j.1572-0241.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 86.Sipponen T, Kolho KL. Faecal calprotectin in children with clinically quiescent inflammatory bowel disease. Scand J Gastroenterol. 2010;45:872–7. doi: 10.3109/00365521003782389. [DOI] [PubMed] [Google Scholar]
- 87.Walkiewicz D, Werlin SL, Fish D, Scanlon M, Hanaway P, Kugathasan S. Fecal calprotectin is useful in predicting disease relapse in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:669–73. doi: 10.1002/ibd.20376. [DOI] [PubMed] [Google Scholar]
- 88.Consigny Y, Modigliani R, Colombel JF, Dupas JL, Lemann M, Mary JY. A simple biological score for predicting low risk of short-term relapse in Crohn's disease. Inflamm Bowel Dis. 2006;12:551–7. doi: 10.1097/01.ibd.0000225334.60990.5b. [DOI] [PubMed] [Google Scholar]
- 89.Boirivant M, Leoni M, Tariciotti D, Fais S, Squarcia O, Pallone F. The clinical significance of serum C reactive protein levels in Crohn's disease. Results of a prospective longitudinal study. J Clin Gastroenterol. 1988;10:401–5. doi: 10.1097/00004836-198808000-00011. [DOI] [PubMed] [Google Scholar]
- 90.Brignola C, Campieri M, Bazzocchi G, Farruggia P, Tragnone A, Lanfranchi GA. A laboratory index for predicting relapse in asymptomatic patients with Crohn's disease. Gastroenterology. 1986;91:1490–4. doi: 10.1016/0016-5085(86)90206-4. [DOI] [PubMed] [Google Scholar]
- 91.Oussalah A, Chevaux JB, Fay R, Sandborn WJ, Bigard MA, Peyrin-Biroulet L. Predictors of infliximab failure after azathioprine withdrawal in Crohn's disease treated with combination therapy. Am J Gastroenterol. 105:1142–9. doi: 10.1038/ajg.2010.158. [DOI] [PubMed] [Google Scholar]
- 92.Bitton A, Dobkin PL, Edwardes MD, Sewitch MJ, Meddings JB, Rawal S, Cohen A, Vermeire S, Dufresne L, Franchimont D, Wild GE. Predicting relapse in Crohn's disease: a biopsychosocial model. Gut. 2008;57:1386–92. doi: 10.1136/gut.2007.134817. [DOI] [PubMed] [Google Scholar]
- 93.Papi C, Festa V, Leandro G, Moretti A, Tanga M, Koch M, Capurso L. Long-term outcome of Crohn's disease following corticosteroid-induced remission. Am J Gastroenterol. 2007;102:814–9. doi: 10.1111/j.1572-0241.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 94.Bitton A, Peppercorn MA, Antonioli DA, Niles JL, Shah S, Bousvaros A, Ransil B, Wild G, Cohen A, Edwardes MD, Stevens AC. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology. 2001;120:13–20. doi: 10.1053/gast.2001.20912. [DOI] [PubMed] [Google Scholar]
- 95.Ho GT, Lee HM, Brydon G, Ting T, Hare N, Drummond H, Shand AG, Bartolo DC, Wilson RG, Dunlop MG, Arnott ID, Satsangi J. Fecal calprotectin predicts the clinical course of acute severe ulcerative colitis. Am J Gastroenterol. 2009;104:673–8. doi: 10.1038/ajg.2008.119. [DOI] [PubMed] [Google Scholar]
- 96.Travis SP, Farrant JM, Ricketts C, Nolan DJ, Mortensen NM, Kettlewell MG, Jewell DP. Predicting outcome in severe ulcerative colitis. Gut. 1996;38:905–10. doi: 10.1136/gut.38.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turner D, Mack D, Leleiko N, Walters TD, Uusoue K, Leach ST, Day AS, Crandall W, Silverberg MS, Markowitz J, Otley AR, Keljo D, Mamula P, Kugathasan S, Hyams J, Griffiths AM. Severe pediatric ulcerative colitis: a prospective multicenter study of outcomes and predictors of response. Gastroenterology. 2010;138:2282–91. doi: 10.1053/j.gastro.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 98.Turner D, Leach ST, Mack D, Uusoue K, McLernon R, Hyams J, Leleiko N, Walters TD, Crandall W, Markowitz J, Otley AR, Griffiths AM, Day AS. Faecal calprotectin, lactoferrin, M2-pyruvate kinase and S100A12 in severe ulcerative colitis: a prospective multicentre comparison of predicting outcomes and monitoring response. Gut. 2010;59:1207–12. doi: 10.1136/gut.2010.211755. [DOI] [PubMed] [Google Scholar]
- 99.Taylor KD, Plevy SE, Yang H, Landers CJ, Barry MJ, Rotter JI, Targan SR. ANCA pattern and LTA haplotype relationship to clinical responses to anti-TNF antibody treatment in Crohn's disease. Gastroenterology. 2001;120:1347–55. doi: 10.1053/gast.2001.23966. [DOI] [PubMed] [Google Scholar]
- 100.Esters N, Vermeire S, Joossens S, Noman M, Louis E, Belaiche J, De Vos M, Van Gossum A, Pescatore P, Fiasse R, Pelckmans P, Reynaert H, Poulain D, Bossuyt X, Rutgeerts P. Serological markers for prediction of response to anti-tumor necrosis factor treatment in Crohn's disease. Am J Gastroenterol. 2002;97:1458–62. doi: 10.1111/j.1572-0241.2002.05689.x. [DOI] [PubMed] [Google Scholar]
- 101.Ferrante M, Vermeire S, Katsanos KH, Noman M, Van Assche G, Schnitzler F, Arijs I, De Hertogh G, Hoffman I, Geboes JK, Rutgeerts P. Predictors of early response to infliximab in patients with ulcerative colitis. Inflamm Bowel Dis. 2007;13:123–8. doi: 10.1002/ibd.20054. [DOI] [PubMed] [Google Scholar]
- 102.Dubinsky MC, Mei L, Friedman M, Dhere T, Haritunians T, Hakonarson H, Kim C, Glessner J, Targan SR, McGovern DP, Taylor KD, Rotter JI. Genome wide association (GWA) predictors of anti-TNFalpha therapeutic responsiveness in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1357–66. doi: 10.1002/ibd.21174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spivak J, Landers CJ, Vasiliauskas EA, Abreu MT, Dubinsky MC, Papadakis KA, Ippoliti A, Targan SR, Fleshner PR. Antibodies to I2 predict clinical response to fecal diversion in Crohn's disease. Inflamm Bowel Dis. 2006;12:1122–30. doi: 10.1097/01.mib.0000235833.47423.d7. [DOI] [PubMed] [Google Scholar]
- 104.Osterman M, Kundu R, Lichtenstein G, Lewis J. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology. 2006;130:1047–53. doi: 10.1053/j.gastro.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 105.Dassopoulos T, Martin C, Galanko J, Wolf D, Dubinsky M, Sandler R, Hanauer S. A Randomized Trial of Metabolite-Adjusted Versus Weight-Based Dosing of Azathioprine (AZA) in Crohn's Disease (CD) Gastronterology. 2009;136:A–519. [Google Scholar]
- 106.Haines ML, Ajlouni Y, Irving PM, Sparrow MP, Rose R, Gearry RB, Gibson PR. Clinical usefulness of therapeutic drug monitoring of thiopurines in patients with inadequately controlled inflammatory bowel disease. Inflamm Bowel Dis. 2010 doi: 10.1002/ibd.21458. [DOI] [PubMed] [Google Scholar]
- 107.Waljee AK, Joyce JC, Wang S, Saxena A, Hart M, Zhu J, Higgins PD. Algorithms outperform metabolite tests in predicting response of patients with inflammatory bowel disease to thiopurines. Clin Gastroenterol Hepatol. 2010;8:143–50. doi: 10.1016/j.cgh.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 108.Baert F, Noman M, Vermeire S, Van Assche G, DH G, Carbonez A, Rutgeerts P, Baert F, Noman M, Vermeire S, Van Assche G, D' Haens G, Carbonez A, Rutgeerts P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601–8. doi: 10.1056/NEJMoa020888. [see comment] [DOI] [PubMed] [Google Scholar]
- 109.Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:1248–54. doi: 10.1016/j.cgh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 110.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 111.Karmiris K, Paintaud G, Noman M, Magdelaine-Beuzelin C, Ferrante M, Degenne D, Claes K, Coopman T, Van Schuerbeek N, Van Assche G, Vermeire S, Rutgeerts P. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn's disease. Gastroenterology. 2009;137:1628–40. doi: 10.1053/j.gastro.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 112.Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. doi: 10.1136/gut.2009.183095. [DOI] [PubMed] [Google Scholar]
- 113.Afif W, Loftus EV, Jr, Faubion WA, Kane SV, Bruining DH, Hanson KA, Sandborn WJ. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:1133–9. doi: 10.1038/ajg.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saverymuttu SH, Camilleri M, Rees H, Lavender JP, Hodgson HJ, Chadwick VS. Indium 111-granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111-granulocyte excretion. Gastroenterology. 1986;90:1121–8. doi: 10.1016/0016-5085(86)90376-8. [DOI] [PubMed] [Google Scholar]
- 115.Farup PG, Hovde O, Halvorsen FA, Raknerud N, Brodin U. Mesalazine suppositories versus hydrocortisone foam in patients with distal ulcerative colitis. A comparison of the efficacy and practicality of two topical treatment regimens. Scand J Gastroenterol. 1995;30:164–70. doi: 10.3109/00365529509093256. [DOI] [PubMed] [Google Scholar]


