Abstract
In type 1 diabetes, the insulin-producing β-cells are destroyed by the immune system. One way of restoring glucose control is to transplant β-cells from a donor. Although this procedure may restore endogenous insulin production, immunosuppressive treatment is needed to prevent the recipient from rejecting the donor-derived islets. We investigated the possibilities of transient expression of the immunosuppressive cytokine transforming growth factor (TGF)-β within islets to achieve long-term graft tolerance. We found that brief expression of TGF-β prevented rejection of syngeneic islets, that there was reduction of dendritic cell (DC) activation in the graft, and that there was reduced reactivation of T cells in the graft-draining lymph nodes. In vitro exposure of bone marrow–derived DCs to TGF-β reduced expression of costimulatory molecules CD80 and CD86, as well as production of proinflammatory cytokines such as interleukin-12 p70 in DCs, but did not alter levels of major histocompatibility complex classes I and II. Furthermore, the capacity of TGF-β–treated bone marrow–derived DCs to activate both CD4+ and CD8+ T cells was reduced. Adding TGF-β–conditioned tolerogenic DCs to the grafted islets led to long-term survival of the graft, demonstrating that TGF-β–induced tolerogenic DCs can provide an effective means to restore immune tolerance in an already established autoimmune disease.
Type 1 diabetes is caused by immune-mediated destruction of the β-cells. The immune cell infiltrate present in the inflamed islets of patients with recent-onset diabetes includes B cells, macrophages, and NK cells, but it is typically dominated by CD8+ T cells (1,2). Cytolytic CD8+ killer T cells are present in the circulation of patients with type 1 diabetes and can kill human β-cells (3). Although insulin injections can provide good glycemic control, many patients with type 1 diabetes still have complications of hyperglycemia, including cardiovascular disease, retinopathy, and neuropathy. The incidence of type 1 diabetes is increasing (4), and prevention or cure of the disease is of major importance.
The currently favored method of restoring endogenous insulin production is islet transplantation (5) and, since the introduction of the Edmonton protocol by Shapiro et al. (6), >750 islet transplantations have been performed with an outcome of >50% insulin independence 5 years after transplantation (7). A drawback of using any transplant from a genetically different donor is that the recipient must have life-long immunosuppression to preserve the graft. Immunosuppression is associated with long-term susceptibility to infection and certain malignancies (8). Currently, only patients with severe episodic hypoglycemia and those receiving a second graft, such as a kidney, are considered for islet transplantation (7). Any short-term treatment that could establish long-term tolerance of the graft without continuous immunosuppression would increase the number of type 1 diabetic patients for whom an islet transplantation could improve quality of life.
Transforming growth factor (TGF)-β was first described 30 years ago (9) and has been found to modulate many biological processes, such as wound healing (10), fibrosis (11,12), autoimmune disease (13–15), and cancer (16–18). TGF-β contributes to the maintenance of peripheral tolerance by promoting the survival of naturally occurring CD4+Foxp3+ T-regulatory (Treg) cells (15,19) and by inducing differentiation of induced CD4+Foxp3+ Treg cells (20–23). Ablation of the cytokine or its signaling receptors results in multiorgan inflammatory disease and subsequent death a few weeks after birth (13–15,19). The potent immunosuppressive effects of TGF-β make it a good candidate for manipulation in autoimmune and alloimmune reactions.
Prevention of diabetes through constitutive expression of TGF-β in the islets of nonobese diabetic (NOD) mice was complicated by the severe side effects of pancreatic fibrosis (24). Likewise, studies of the effects of TGF-β on islet graft tolerance to date have been unsuccessful because of the deleterious effects of constitutive TGF-β expression on islet morphology and fibrosis, with poor efficacy of transplants being reported (25–27). Recently, we used a unique transgenic NOD mouse in which transient expression of TGF-β in islets under control of the doxycycline gene transcription system (28) led to a significant delay in diabetes progression after a brief production of TGF-β specifically during the diabetes phase when anti-islet cytotoxic T lymphocytes are active. We showed that delay in disease development was not linked to increased Foxp3+ Treg cell activity. Instead, we found that TGF-β decreased effector and memory CD8+ T-cell responses and reduced CD8+ T-cell killing of islets, and this was responsible for the delayed development of diabetes. The fact that a brief pulse of TGF-β expression in islets could protect them from immune cell–mediated destruction offered a major opportunity for establishing islet graft tolerance with only brief exposure to an immunosuppressive agent.
In this study, we have investigated the effect of brief expression of TGF-β in islets transplanted into diabetic recipients. We found that TGF-β expression can prevent rejection of syngeneic islet grafts, and that this effect is caused by reduced activation of dendritic cells (DCs) after they are exposed to TGF-β in the grafted tissue, leading to reduced activation and infiltration of islet-specific T cells. Cotransplantation of syngeneic islets with in vitro generated bone marrow–derived DCs (BMDCs) exposed to TGF-β led to long-lasting graft survival and function.
RESEARCH DESIGN AND METHODS
Mice and diabetes detection.
C57Bl/6 mice, rat insulin promoter (RIP)–tumor necrosis factor (TNF) NOD (29), Tet-TGF NOD (28), G9 T-cell receptor (TCR) transgenic NOD mice (30,31), OT-II TCR transgenic mice with OVA-specific CD4+ T cells (32), OT-I TCR transgenic mice with OVA-specific CD8+ T cells (33), NOD-scid mice, and Tet-TGF-β–NOD-scid mice (Tet-TGF NOD bred to a NOD scid background) were bred and maintained under specific pathogen-free barrier conditions. In the Tet-TGF NOD mice, islet-specific TGF-β expression is controlled by the doxycycline-controlled on/off switch (34). Littermates of these mice that were exposed to the same levels of doxycycline but did not express the Tet-TGF-β transgene were used to isolate islets for control transplants. Diabetes was detected using Diastix reagent strips (Bayer Diagnostics, Basingstoke, U.K.) and confirmed by a blood glucose measurement of >13.3 mmol/L, using a Breeze2 blood glucose meter (Bayer). All animal work was conducted according to U.K. Home Office project license regulations after approval by the Ethical Review Committee of the University of Cambridge.
Flow cytometry.
CD4 allophycocyanin (APC; clone L3T4), CD8 APC or eFluor 450 (clone M1/70), CD44 phycoerythrin (PE) or eFluor 450 (clone IM7), CD80 fluorescein isothiocyanate (FITC) (clone 16–10A1), CD86 PE (clone GL-1), CD103 FITC (clone 2E7), CD40 PE (clone 1C10), interferon (IFN)-γ PE or APC (cloneXMG1.2), interleukin (IL)-10 PE (JES5–16E3), Foxp3 PE (clone FJK-16s), and H2Kb APC (clone AF6–88.5.5.3) antibodies (eBioscience); H2Kd APC (clone SF/111) and CD45 PerCp-Cy5.5 (clone 30-F11) antibodies (BD Bioscience); and major histocompatibility complex (MHC) II PE or FITC (clone 7FT) antibody (Serotec) were used. Intracellular staining was performed using the fixation/permeabilization buffer kit from eBioscience. Flow cytometry was performed on a CyAn ADP (Dako), and data were analyzed using FlowJo software (Tree Star).
Islet isolation and transplantation.
Pancreatic islets were isolated through inflation of the pancreas via the bile duct (35), and islet transplantation was performed according to standard protocols (36). Confirmed diabetic recipient mice received between 300 and 500 islets, resulting in ∼15 islets per gram of body weight. For cotransplantation with DCs, 5 × 105 in vitro generated BMDCs were incubated overnight with or without 5 ng/mL TGF-β (Peprotech) and then were washed twice and mixed with the islets.
Cytokine detection.
For intracellular cytokine detection using flow cytometry, cells were resuspended in Dulbecco's modified Eagle's medium supplemented with 10% FCS, 50 μmol/L β-mercaptoethanol, L-glutamine, and sodium pyruvate and 50 units/mL penicillin and streptomycin (GIBCO). Cells were seeded into the wells of a 96-well plate and stimulated with phorbol myristic acid (12.5 ng/mL) and ionomycin (125 ng/mL) (both Sigma-Aldrich) for 5 h at 37°C with 5% CO2. Monensin (BD Bioscience) was added during the last 4 h of culture at a concentration of 0.13 µL/200 µL of culture. Cytokines in BMDC culture supernatants were detected by electrochemical luminescence immunoassay using a mouse seven-plex proinflammatory cytokine kit (MesoScale Discovery).
BMDCs.
Bone marrow was harvested from the femurs of NOD or C57Bl/6 mice and cultured in 50 mL Dulbecco's modified Eagle's medium supplemented as described plus 10 ng/mL granulocyte–macrophage colony-stimulating factor (Peprotech) for 10 days.
For bead uptake assays and analysis of surface markers, the BMDCs were conditioned for 24 h with or without 5 ng/mL TGF-β (Peprotech), activated overnight with 100 ng/mL lipopolysaccharide (LPS), and then washed twice. Fluorescent microbeads (Bangs Laboratories) were coated with insulin β−chain amino acids 15-23 peptide (insB15-23) according to the manufacturer’s instructions. The beads were added to the BMDCs at a concentration of 25 μg beads/106 cells and incubated for 2 h at 37°C; afterwards, bead uptake was assessed using flow cytometry.
Proliferation assays.
For proliferation assays, OVA-specific CD4+ T cells from OT-II TCR transgenic mice, OVA-specific CD8+ T cells from OT-I TCR transgenic mice, or insB15-23–specific CD8+ T cells from G9 TCR transgenic mice were sorted using negative-selection magnetic bead kits from MACS Miltenyi. The purity was always >95%. For OVA-specific cultures, BMDCs were pulsed with 10 μg/mL OVA for 24 h with or without 5 ng/mL TGF-β (Peprotech) and then were washed and incubated with 100 ng/mL LPS overnight. The next day, the BMDCs were washed again, and 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled (Invitrogen) T cells were added at a concentration of 2 × 105 cells per well. For insulin β-chain–specific cultures, BMDCs were treated as described, but 1 μg/mL insB15-23 peptide was added after activation with or without TGF-β treatment and was present in the cultures during incubation.
After 72 h, the cells were stained for surface markers and 7-amino-actinomycin D (7AAD) to exclude dead cells (BD Bioscience) and were assessed by flow cytometry.
Immunofluorescence.
Graft-bearing kidneys were snap-frozen in liquid nitrogen. Guinea pig anti-insulin antibody (DAKO) was detected with anti-guinea pig Alexa 546 (Molecular Probes). Antibodies against Foxp3 (eBioscience), CD4, CD8, B220, and CD11b (BD Bioscience) were detected with anti-rat Alexa 488 (Molecular Probes). Nuclei were visualized with DAPI (Molecular Probes). The sections were viewed with the Axioskop 2 plus fluorescence microscope (Zeiss), and three fields of view were counted for each graft.
PCR.
Grafts were removed 5 or 7 days after transplantation as indicated in the text, and mRNA was prepared using the Qiagen miniprep kit according to the manufacturer’s instructions. Real-time PCR was performed in technical duplicates using an Applied Biosystems 7500 fast thermocycler and QuantiFAST fast cycling SYBRGreen master mix (Qiagen). Real-time PCR primers for genes of interest were supplied by Qiagen. Relative gene expression was normalized to the reference gene glyceraldehyde 3-phosphate dehydrogenase using the Δ cycle threshold (ΔCT) method.
RESULTS
TGF-β decreases T-cell infiltration of graft islets and prolongs graft survival.
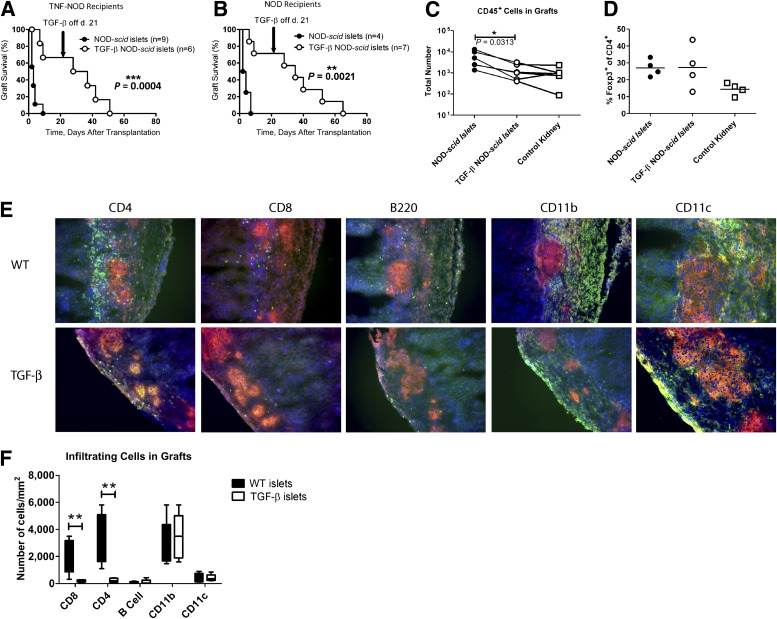
As transplant recipients we used either wild-type (WT) NOD mice or RIP-TNF NOD mice that had been genetically modified to express TNF under the influence of RIP, resulting in a 100% incidence of diabetes (29) on the NOD mouse genetic background. Diabetes develops in RIP-TNF NOD mice in a manner similar to that of NOD mice, but disease progression is more rapid and synchronized (29). Diabetic NOD mice (Fig. 1A) or RIP-TNF NOD mice (Fig. 1B) received an islet graft from TGF-β–NOD-scid mice on day 0. After 21 days, TGF-β expression was switched off by administration of doxycycline-supplemented water. Diabetic mice receiving control NOD-scid islets (normal NOD islets that are not affected by preexisting immune infiltration) rapidly rejected these, and the mice were all diabetic again within 7 days. In contrast, mice receiving the TGF-β–expressing islets maintained normal blood glucose for significantly longer. Although recipients eventually rejected the graft and became diabetic after TGF-β expression was switched off, graft failure in all recipients was not apparent until ∼40 days after TGF-β silencing. There was a possibility that the transgenic expression of TNF in any remaining β-cells in the RIP-TNF NOD mouse recipients led to overturning of tolerance to the graft; therefore, we also investigated graft survival in diabetic WT NOD recipients. There was no statistically significant difference in graft rejection between WT NOD mouse recipients and RIP-TNF NOD mouse recipients (Fig. 1A and B), indicating that the preexisting immune response to islet antigens is sufficient to precipitate rejection after TGF-β expression is silenced, even when there is no additional immunogenicity provided by residual TNF-expressing β-cells. Because the expression of TNF in the endogenous recipient islets before progression to diabetes made no difference for graft survival, the subsequent experiments all have been performed on RIP-TNF NOD mouse recipients. The TGF-β–expressing grafts displayed less infiltration of immune cells than control grafts as measured by flow cytometry of whole grafts and gating on CD45+ cells (Fig. 1C). There were no significant differences in the graft CD4+Foxp3+ Treg cell populations between the two recipient groups (Fig. 1D). Immunofluorescence microscopy of sections prepared from the grafts 5 days after transplantation also showed reduced infiltration of T cells in the TGF-β–expressing grafts (bottom, Fig. 1E) compared with WT grafts (top, Fig. 1E), but infiltration with CD11b+ and CD11c+ antigen-presenting cells was similar between the two groups. Few B cells could be detected in the grafts at this time point.
FIG. 1.
Induced expression of TGF-β for the first 21 days after transplantation protects islet grafts from destruction. RIP-TNF NOD mice (A) or WT NOD mice (B) were confirmed to be diabetic and transplanted with an islet graft from either NOD-scid (●) or TGF-β–NOD-scid mice (○). The difference between the groups was plotted using a Kaplan-Meier plot, and differences between groups were determined using the log-rank test. Absolute numbers of cells infiltrating the grafts of RIP-TNF NOD mice were assessed by flow cytometry 5 days after transplantation (C). Lines connect grafts performed on the same occasion, and the Wilcoxon signed rank test was used to assess differences between groups. The percentage of Foxp3+ cells in the CD4+ T-cell gate in these cells was assessed (D). Sections of the grafts from RIP-TNF NOD mice were stained for insulin (red) and the indicated immune cell (green). DAPI was used as a nuclear stain (blue) (E). Three fields of vision were assessed for at least five mice from each group, and the numbers of infiltrating cells of the indicated subtypes were determined (F). Differences between groups were determined using the Mann-Whitney test (P = 0.0079 for CD8+ T cells and P = 0.0079 for CD4+ T cells). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Statistical analysis of several grafts demonstrated that TGF-β–expressing grafts had less infiltration of T cells but a similar presence of antigen-presenting cells expressing CD11b and CD11c (Fig. 1F).
Mice with TGF-β–expressing grafts have fewer T cells expressing cytokines in the draining lymph nodes.
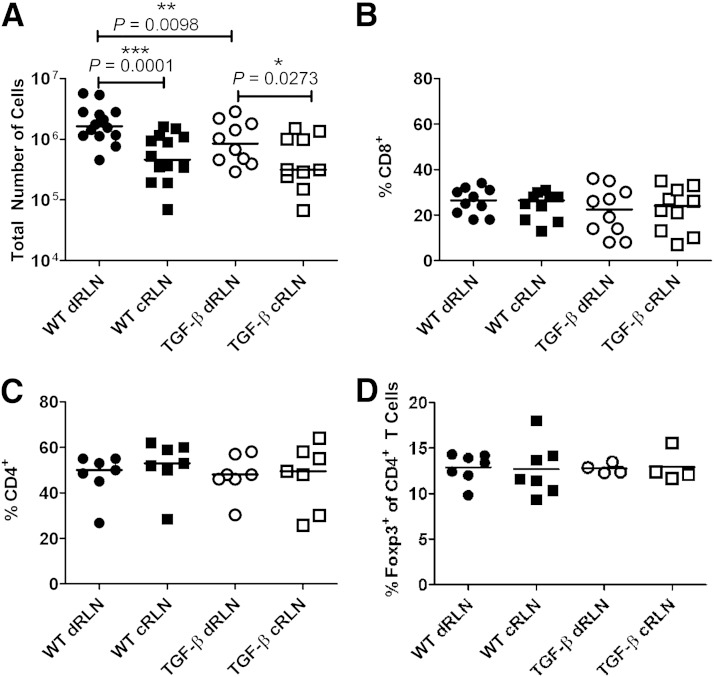
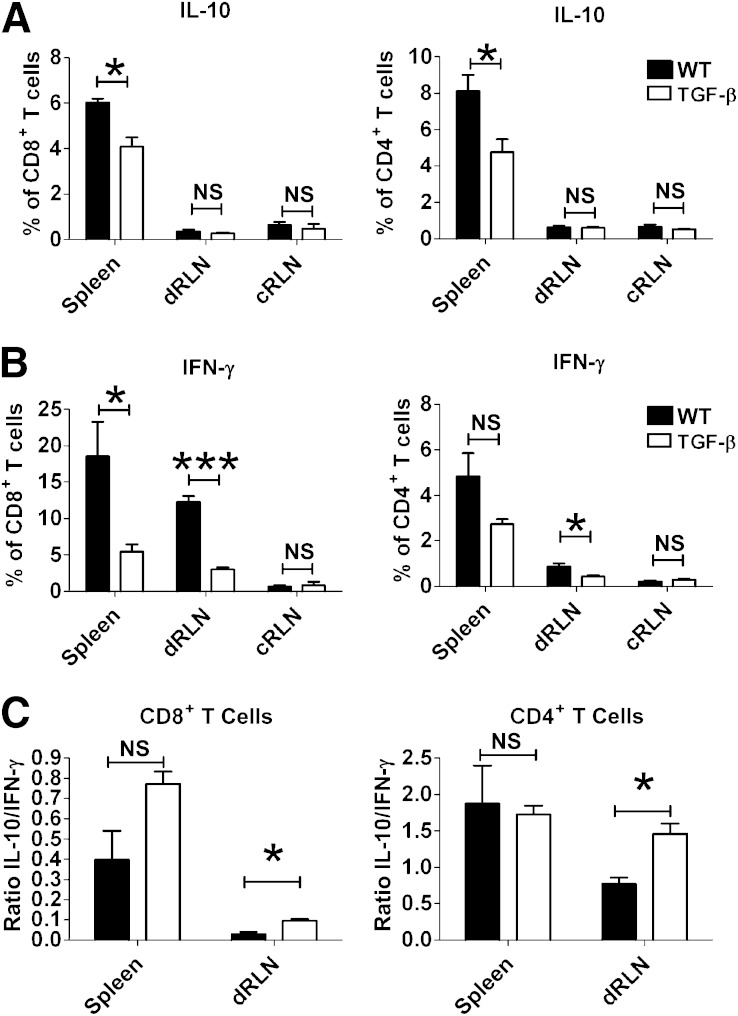
The lower infiltration of T cells into the TGF-β–expressing grafts could be caused either by reduced migration to the graft or by decreased activation in the draining lymph node. To test this, we assessed responses in the graft-draining lymph nodes. Total cell numbers in the draining renal lymph nodes (RLNs) of TGF-β–expressing grafts were lower than in those draining control grafts (Fig. 2A), whereas the cell numbers in draining RLNs from both groups were higher than in control RLNs (Fig. 2A). Within these populations, the percentages of CD4+ and CD8+ T cells were similar (Fig. 2B and C), and the percentages of Foxp3+ Treg cells also were similar within the CD4+ population (Fig. 2D). In accordance with these results, the absolute numbers of both CD4+ and CD8+ T cells were significantly lower in draining RLNs of TGF-β–expressing grafts than in those draining control grafts, whereas there was no significant difference detected in the absolute numbers of Foxp3+ Treg cells (Supplementary Fig. 1). The activation status of the T cells in the draining RLNs of TGF-β–expressing grafts also was decreased, as demonstrated by the lower production of proinflammatory cytokine IFN-γ as well as immunosuppressive cytokine IL-10 (Fig. 3A and B) after ex vivo restimulation with phorbol myristic acid and ionomycin. Examining the ratios of IL-10 produced compared with IFN-γ, it appeared that there was a skewing toward a more IL-10–dominant and less inflammatory profile in the draining RLNs of TGF-β–expressing grafts (Fig. 3C).
FIG. 2.
Lower absolute cell numbers in the draining lymph nodes of TGF-β–expressing grafts. Total numbers of cells retrieved from the graft-draining RLNs (draining RLNs [dRLNs]) of TGF-β–expressing grafts were lower than those draining WT grafts. The nondraining RLNs (control RLN [cRLNs]) had lower total numbers of cells than the graft-draining lymph nodes (A). The composition of the cell populations in the lymph nodes was similar with regard to CD8+ T (B) and CD4+ T cells (C). Within the CD4+ T-cell population, percentages of Foxp3+ Treg cells also were similar between groups (D). Each symbol (●, ○, ■, and □) represents the result from one mouse, and differences between groups were assessed using the Wilcoxon signed rank test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
FIG. 3.
Fewer cytokine-producing cells in the draining lymph nodes of TGF-β–expressing grafts. Spleen, draining RLNs (dRLNs), and control RLNs (cRLNs) were harvested 5 days after transplantation and restimulated for 5 h with phorbol myristic acid and ionomycin. IL-10 (A) and IFN-γ (B) production was assessed by intracellular staining for flow cytometry and was lower in the CD4+ and CD8+ T-cell populations in mice receiving TGF-β–expressing grafts than in those receiving WT grafts. Differences between groups (technical triplicates) were assessed using the Student t test, and the results are representative of four independent experiments, each comparing one recipient of WT islets and one recipient of TGF-β–expressing islets. The ratio of IL-10 to IFN-γ–positive cells in the spleen and dRLNs was assessed in CD8+ T cells (left) and CD4+ T cells (right) (C). NS, not significant. *P ≤ 0.05, ***P ≤ 0.001.
TGF-β–exposed DCs are less capable of inducing antigen-specific proliferation.
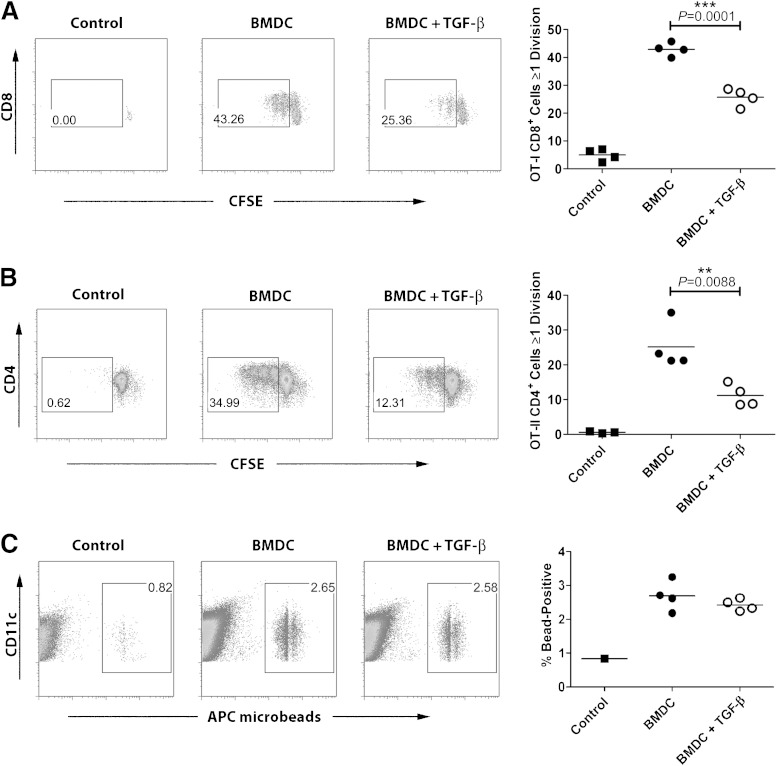
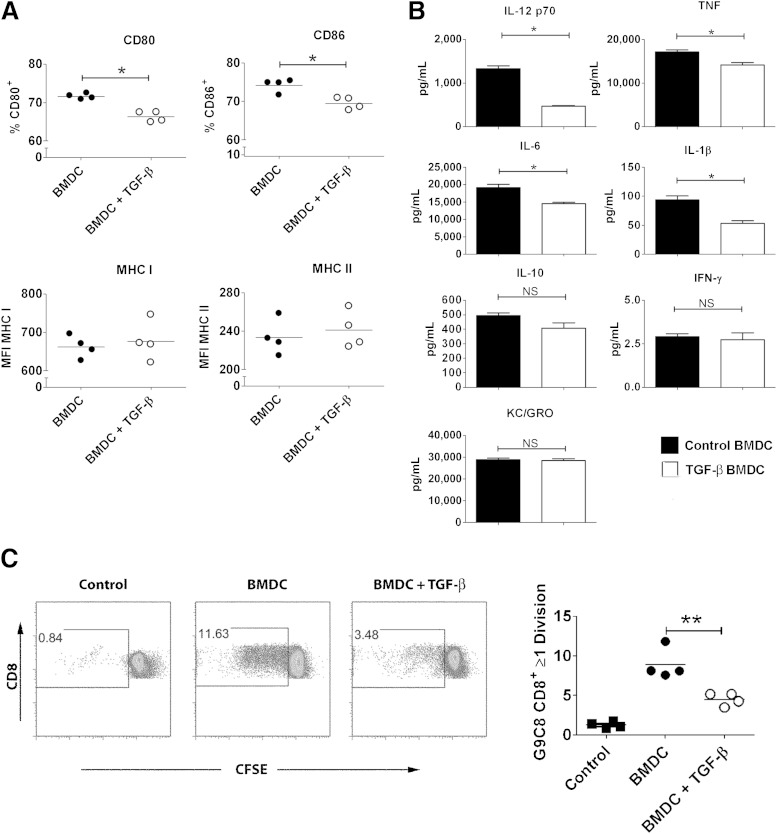
The results depicted in Fig. 1E and F showed that although T-cell infiltration into grafts was reduced in the presence of TGF-β, the presence of antigen-presenting cells expressing CD11c and CD11b was not affected. We hypothesized that exposure to TGF-β would change the capacity of these antigen-presenting cells to efficiently present antigen to islet-specific cells and thus prevent recurring islet destruction. To test this, we used the well-described OVA-TCR system (32,33) and we performed in vitro experiments using BMDCs that we exposed to TGF-β. Exposure to TGF-β during pulsing with whole OVA made BMDC less efficient at activating both CD8+ OVA–specific OT-I T cells (Fig. 4A) and CD4+ OVA–specific OT-II T cells (Fig. 4B). This decrease in activation was not caused by decreased antigen uptake because TGF-β–exposed BMDCs took up fluorescent beads as well as control BMDCs (Fig. 4C). To find out why the TGF-β–exposed BMDCs induced weaker proliferation, we assessed expression of costimulatory molecules on their surface. We found that after LPS stimulation, TGF-β–exposed BMDCs displayed a small but significant decrease of surface expression of costimulatory molecules CD80 and CD86 (Fig. 5A, top), which are crucial for the second activating signal needed in addition to TCR-MHC interaction, mediated through CD28 on the T cell (37). The TGF-β–exposed BMDCs did not show any decrease in surface expression of MHC molecules (Fig. 5A, bottom). TGF-β–exposed BMDCs also produced lower levels of inflammatory cytokines after LPS stimulation, showing three-fold reduced production of IL-12 p70 as well as reduced TNF, IL-6, and IL-1β. In contrast to the reduction observed in these proinflammatory cytokines, production of the anti-inflammatory cytokine IL-10 was preserved in TGF-β–treated DCs. KC/GRO levels (also known as CXCL1) were not different between TGF-β–treated DCs and controls. Levels of IFN-γ were very low in both (Fig. 5B). To further investigate whether the effect of TGF-β was primarily on antigen uptake or processing or on surface costimulatory molecule expression, we tested whether the suppressive effect of TGF-β was still apparent when bypassing antigen uptake through use of a short peptide for T-cell stimulation. We used the diabetes-relevant insulin peptide–specific TCR transgenic system G9 TCR transgenic NOD mice (30,31), which were created using an islet-specific CD8+ T-cell clone isolated from infiltrated NOD mouse islets. BMDCs were exposed to TGF-β and then pulsed with the insB15-23. The pulsed BMDCs were used as APC in culture with the insB15-23–specific G9C8 TCR transgenic CD8+ T cells; although no antigen uptake or processing is required for T cell stimulation in this culture, there was a marked decrease in T-cell proliferation in cultures in which BMDCs had been exposed to TGF-β (Fig. 5C).
FIG. 4.
Exposure to TGF-β during antigen uptake reduces the capacity of BMDCs to present antigen efficiently to T cells. C57BL/6 BMDCs were pulsed with whole OVA in the presence or absence of 5 ng/mL recombinant TGF-β. After washing, they were cultured together with CFSE-labeled OT-II OVA-specific CD4+ T cells (A) or OT-I OVA-specific CD8+ T cells (B) and proliferation was assessed. Filled squares, proliferation without DC; filled circles, proliferation with control BMDC; open circles, proliferation with TGF-β–exposed BMDC. To determine antigen uptake, APC-labeled microbeads were added to the BMDCs in the presence or absence of 5 ng/mL recombinant TGF-β at 37°C. Control BMDCs were incubated with beads at 4°C. Filled squares, bead uptake at 4°C; filled circles, bead uptake in control BMDC; open circles, bead uptake in TGF-β–exposed BMDC (C). Differences between groups were assessed using the Mann-Whitney test. Results show four cultures from each condition with DCs and T cells originating from the same source and are representative of at least three independent experiments. **P ≤ 0.01, ***P ≤ 0.001.
FIG. 5.
Exposure to TGF-β reduced expression of costimulatory molecules on BMDCs and reduced their capacity to stimulate T cells. NOD BMDCs were activated with 100 ng/mL LPS after preconditioning overnight with 5 ng/mL TGF-β. Expressions of costimulatory molecules CD80 and CD86 as well as MHC class I and class II were assessed using flow cytometry (A), and production of cytokines in supernatants was determined using seven-plex analysis (B). NOD BMDCs were activated with LPS after preconditioning overnight with 5 ng/mL TGF-β, pulsed with insulin β-chain peptide 15-23 (ins15-23), and cultured for 72 h with CFSE-labeled insB15-23–specific CD8+ T cells from the G9 TCR transgenic mouse. Proliferation was assessed by flow cytometry. Filled squares, proliferation without DC; filled circles, proliferation with control BMDC; open circles, proliferation with TGF-β–exposed BMDC (C). Differences in surface markers and cytokine expression between groups were determined using the Mann-Whitney test, whereas differences in proliferation were assessed using the Student t test. Results show technical replicates and are representative of at least three independent experiments. NS, not significant. *P ≤ 0.05, **P ≤ 0.01.
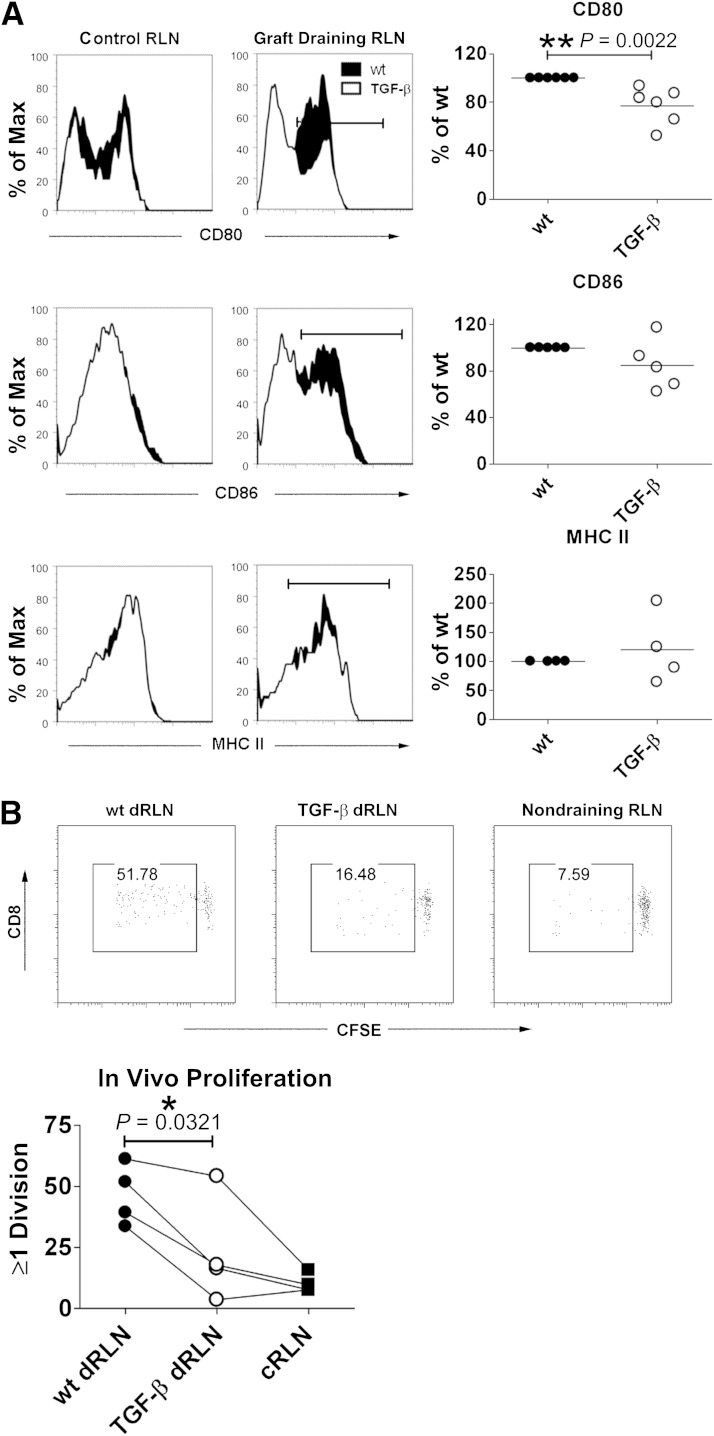
Islet graft expression of TGF-β leads to reduced activation of DCs and less islet antigen–specific proliferation in the graft-draining RLNs.
To investigate whether islet graft expression of TGF-β affected DCs in vivo as seen in the in vitro experiments, we isolated CD11c+ cells from the draining RLNs of graft recipients and assessed surface expression of CD80, CD86, and MHC II. We found that CD80 expression was significantly reduced in DCs from draining RLNs of TGF-β–expressing grafts (Fig. 6A, top). There was a trend toward lower CD86 expression in DCs from draining RLNs of TGF-β–expressing grafts (Fig. 6A, middle), but this was not statistically significant. Just as in the in vitro experiments, MHC II expression was similar between groups (Fig. 6A, bottom panels). To further assess expression of immunosuppressive molecules in TGF-β–expressing grafts, we performed PCR analysis of grafts removed from the recipients 5 or 7 days after transplantation (Supplementary Fig. 2). We investigated expression of proinflammatory cytokines TNF and IFN-γ, anti-inflammatory TGF-β and IL-10, and also markers of either alternative or classical activation of APC such as arginase 1 and inducible nitric oxide synthase (38,39). Of all of these markers, only TGF-β was consistently elevated as expected in all TGF-β–expressing grafts. There was a trend toward higher expression of arginase 1, which was higher in three out of four TGF-β–expressing grafts, but the mixed nature of the sampled tissue and variability between samples make it difficult to draw any conclusions about the gene expression of DCs and macrophages within the grafts. To investigate whether islet-specific T-cell proliferation was affected by the reduced DC activation in graft-draining RLNs, we performed adoptive transfer of CFSE-labeled insB15-23–specific G9 transgenic T cells (30,31) into graft recipients and determined their proliferation. These cells proliferated less well in the RLN-draining TGF-β–expressing grafts (Fig. 6B), indicating that DCs that have been exposed to TGF-β are less effective in activating the β-cell–specific T-cell response necessary for islet destruction and recurrence of type 1 diabetes.
FIG. 6.
Decreased activation of DCs and lower proliferation of islet antigen–specific T cells in draining lymph nodes of TGF-β–expressing grafts. Graft-draining RLNs (draining RLNs [dRLNs]) and control RLNs (cRLNs) were isolated from mice that had received a TGF-β–expressing or WT islet graft 5 days previously. DCs were CD11c+CD3−7AAD− and assessed for expression of CD80 (top), CD86 (middle), and MHC II (bottom) (A). To the left are representative flow cytometry histogram overlays comparing WT grafts (black histograms) with TGF-β–expressing grafts (white histograms) in cRLNs and dRLNs. To the right are graphs showing the relative decrease in expression in the repeat experiments (separate biological replicates; each dot represents the data obtained from one mouse), with the value from the WT graft set as 100% (12 mice for CD80, 10 mice for CD86, and 8 mice for MHC II). Differences between groups were assessed using the Mann-Whitney test (A). Filled circles, control grafts; open circles, TGF-β–producing grafts. CFSE-labeled insB15-23–specific CD8+ T cells were transferred into CD45 congenic RIP-TNF NOD mice having received either a TGF-β–expressing graft or a WT graft 2 days previously. Seventy-two hours later, the draining and nondraining lymph nodes were harvested and proliferation was assessed as dilution of CFSE signal in the CD45.1+CD45.2−CD8+7AAD− gate (B). The experiment was repeated four times, with a compilation of all four experiments (eight mice) at the bottom and representative fluorescence-activated cell sorter plots depicted above. Differences in proliferation between the draining RLNs of WT and TGF-β–expressing grafts were determined using a paired Student t test. *P ≤ 0.05, **P ≤ 0.01.
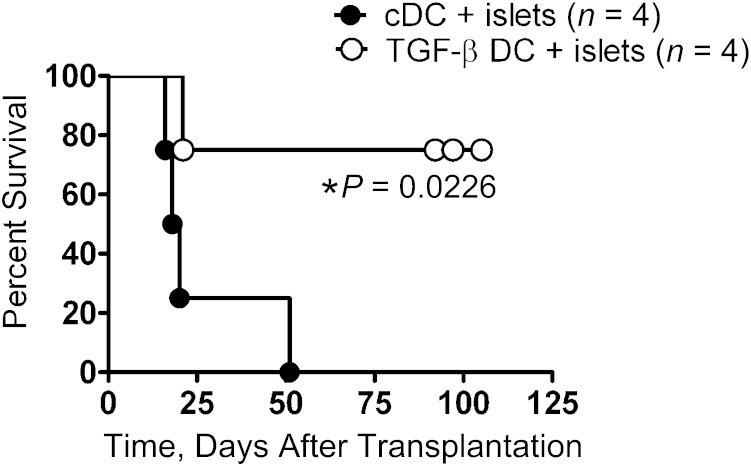
TGF-β–exposed BMDCs prolong the survival of syngeneic islet grafts.
Finally, we wished to determine whether in vitro generated BMDCs exposed to TGF-β, impaired in their expression of costimulatory molecules and their production of proinflammatory cytokines, could actually prevent or delay rejection of syngeneic islets transplanted into a diabetic NOD mouse with an already established anti-islet antigen immune memory. We mixed 5 × 105 control BMDCs or BMDCs that had been exposed to TGF-β for 24 h with the islets for grafting. Recipients of islets mixed with any type of BMDC retained their grafts for longer than recipients that received syngeneic islets without any BMDCs. The diabetic recipients that received the TGF-β–exposed BMDCs along with the islet graft retained graft function significantly longer than recipients of grafts with control BMDCs, demonstrating that TGF-β–generated BMDCs can induce long-lasting graft acceptance (Fig. 7). Recipients of WT syngeneic NOD islets without any BMDCs mixed in rejected the grafts within 10 days, usually sooner (Fig. 1A). Although mice that received control BMDCs mixed with the islet graft eventually rejected their grafts, they survived more than twice as long (≥20 days) as WT NOD islet grafts, indicating that even BMDCs without any TGF-β conditioning can affect graft survival. Mice that received islet grafts mixed with TGF-β–exposed DCs displayed considerable infiltration of CD8+ T cells 10 days after grafting, at levels similar to those receiving islets mixed with control DCs (Supplementary Fig. 3A). In mice that received TGF-β–exposed DCs and control DCs mixed with their islet grafts, there was a considerable infiltration of Foxp3+ cells around the grafted islets, indicating no added recruitment of such cells by the TGF-β–exposed DCs (Supplementary Fig. 3A). At later time points ∼3 weeks after transplantation, the recipients of control DCs with mixed islet grafts developed recurrent diabetes, and at that time point there was, as expected, a large infiltration of CD8+ T cells (Supplementary Fig. 3B). Perhaps less expected was the considerable presence of Foxp3+ regulatory cells that, despite evidently homing to the graft, failed to prevent rejection (Supplementary Fig. 3B, top right). The long-term protected graft in a recipient of islets mixed with TGF-β–exposed DCs, still euglycemic 4 months after transplantation, displayed considerable infiltration of both CD8+ cells (Supplementary Fig. 3B, bottom left) and Foxp3+ cells (Supplementary Fig. 3B, bottom right).
FIG. 7.
TGF-β–preconditioned BMDCs prolong islet graft survival. RIP-TNF NOD mice were confirmed to be diabetic and transplanted with an islet graft mixed with 5 × 105 in vitro generated control BMDCs (●) or BMDCs incubated overnight with 5 ng/mL TGF-β (○). The difference between the groups is depicted using a Kaplan-Meier plot, and the difference was determined using the log-rank test.
DISCUSSION
The immunosuppressive qualities of TGF-β make it an attractive candidate for achieving suppression of autoimmune responses. However, the cytokine has effects on many pathophysiological processes including cancer and fibrosis (40,41). For TGF-β to be used therapeutically, it is crucial to control the site and duration of expression to harness the power of TGF-β for immunotherapy while avoiding undesired side effects.
In our present study, we found that expression of TGF-β–protected islet grafts from memory islet–specific responses in autoimmune diabetic recipients, and that the resulting reduced infiltration of T cells into the grafts corresponded with reduced activation of T cells in the graft-draining lymph nodes. The fact that the TGF-β–expressing grafts displayed similar infiltration of APCs indicated that the APCs were not inhibited in homing to the grafts but that they were affected by the local TGF-β expression in ways that reduced their antigen-presenting abilities. In vitro experiments demonstrated that TGF-β–exposed BMDCs displayed lower levels of costimulatory molecules on their surface; produced less IL-12 p70, TNF, and IL-6; and were impaired in activating antigen-specific T cells. This was also true in vivo because adoptively transferred islet antigen–specific T cells proliferated less well in the RLNs draining TGF-β–expressing grafts, and DCs isolated from these lymph nodes displayed lower levels of costimulatory molecules. Cotransplantation of islets with in vitro generated BMDCs conditioned with TGF-β resulted in prolonged graft survival.
Tolerogenic DCs are immature, maturation-resistant, or, alternatively, activated DCs that have a low ratio of costimulatory to inhibitory signals and produce low levels of IL-12p70. Tolerogenic DCs can be generated in vitro by coculture with various immunosuppressive and anti-inflammatory agents such as IL-10, prostaglandin E2, and vitamin D3. Immunosuppressive drugs such as cyclosporine, tacrolimus, rapamycin, mycophenolate mofetil, and corticosteroids also have been used to promote tolerogenic DC differentiation in vitro, because all these molecules prevent maturation of DCs and impair their capacity to produce IL-12 (42). Tolerogenic DCs generated through culture with dexamethasone and vitamin D3 can inhibit experimental collagen-induced arthritis severity and progression in an antigen-dependent manner (43). Injection of alternatively activated macrophages can reduce and prevent experimental autoimmune encephalomyelitis (44,45). Prevention and significant delay of development of diabetes in NOD mice have been achieved by administration of tolerogenic DCs differentiated in the presence of IL-10 (46), IL-4 (47,48), or flt-3L (49) and also through administration of alternatively activated macrophages (50).
The mechanisms through which these tolerogenic DCs suppress immune responses vary depending on the protocol. Because the tolerogenic DCs express low levels of IL-12 p70, they are less effective at promoting Th1 T-cell responses, and their lower levels of costimulatory molecules reduce their capacity to induce T-cell activation. Many groups also report that tolerogenic DCs induce preferential proliferation and differentiation of Treg (51–53) or Th2 cells (47,48), whereas others report that tolerogenic DCs prevent activation of naïve CD8+ T cells and induce depletion of memory CD8+ T cells (54). A compelling explanation has been offered by Cobbold et al., suggesting that the capacity to deplete essential amino acids or ATP makes certain DCs tolerogenic. Treatment of DC with vitamin D3, IL-10, or TGF-β leads to upregulation of essential amino acid–depleting enzymes (55), and such enzymes are upregulated in tolerated skin grafts (38). In this study, we observed a trend toward higher expression of the amino acid–depleting enzyme arginase 1 in TGF-β–expressing grafts, but this was not consistent in all grafts assessed. This could be caused by technical limitations attributable to the presence of many cell populations other than DCs in the analyzed whole grafts. Studies of protein content in vitamin D3–induced tolerogenic DCs demonstrated changes in metabolic pathways involving lipids, glucose, and oxidative phosphorylation, which may affect the production of reactive oxygen species as well as response to stimulation by the modulated DCs (56).
In our study, the BMDCs we added to the islet grafts were not pulsed with any antigen. In several studies, antigen pulsing has been necessary for transferred tolerogenic DCs to have an effect (43,57). This may be because in our experiments the DCs are directly mixed with the islets, allowing them to take up antigen during the process rather than injected systemically. Additionally, in our experiments the BMDCs were not matured with LPS in the in vivo experiments, which allows greater antigen uptake capacity, so they can take up antigen during and after transplantation. Other studies using immature, non-LPS-pulsed, tolerogenic DCs to alleviate experimental collagen-induced arthritis found no need for antigen pulsing to achieve protection (50,58). A surprising finding was that addition of immature BMDCs could delay rejection of islet grafts even when not exposed to TGF-β. Our findings complement previous studies showing that immature autologous DCs prolong heart graft survival in Lewis rats (59), even when not further differentiated toward a tolerogenic phenotype, indicating that a state of immaturity can be enough to at least delay immunity. We identified the induction of tolerogenic DCs as a potential mechanism for the protection seen with expression of TGF-β, but, interestingly, TGF-β–exposed BMDCs were more efficient at inducing long-term protection of islet grafts than the direct exposure to TGF-β within the graft. Because the protection afforded by cotransplantation with BMDCs was long-lived, it could be interpreted as active immunological tolerance; however, the short-lived effects of direct expression of TGF-β in the graft show the hallmarks of reversible immunosuppression. We found no evidence of increased presence of Foxp3+ regulatory T cells in the grafts cotransplanted with TGF-β–exposed BMDCs (Supplementary Fig. 3) and no decrease in CD8+ T cells even in long-term accepted grafts at 125 days after transplantation, indicating that despite a heavy infiltrate of autoreactive T cells, these cells retain the pattern of respectful insulitis and do not damage the islets. This pattern is different from the reduced infiltration of T cells seen in TGF-β–expressing islet grafts (Fig. 1) and indicates that the mechanisms for suppression between direct expression of TGF-β and coadministration of TGF-β–exposed BMDCs are qualitatively different.
DC-based cell therapy has demonstrated considerable promise for the promotion of transplant tolerance in animal models, and now considerable efforts are being made to investigate whether it can be safely adapted for use in humans. Repetitive stimulation with immature DCs pulsed with an HLA-A2–derived allopeptide can expand alloantigen-specific Treg cells ex vivo (60), and subcutaneous injection of immature DCs pulsed with influenza-derived peptide induced a severely impaired cytolytic response (61). Human DCs, conditioned by exposure to vitamin D3 and dexamethasone, induce anergy in alloreactive CD4+ T cells (62). A clinical study evaluating safety for treatment of rheumatoid arthritis with autologous DCs generated with dexamethasone and vitamin D3 and loaded with synovial fluid (http://www.clinicaltrials.gov/ct2/show/NCT01352858) is ongoing in Newcastle, U.K. At the University of Pittsburgh (Pittsburgh, PA), the first trial for the treatment of type 1 diabetic patients with autologous DCs treated ex vivo with antisense phosphorothioate-modified oligonucleotides targeting the primary transcripts of the CD40, CD80, and CD86 has passed an initial phase I trial for safety (63). The results from these studies will be of great interest to the scientific community and will instruct further adaptation of tolerogenic DC–based therapies for use in human disease and transplantation tolerance.
In summary, our results demonstrate that short-term local exposure to TGF-β in vivo or exposure of DCs to TGF-β ex vivo can prolong the life of islet grafts. In the case of tolerogenic DC transfer, this protection appears to be long-lasting and sustained. The use of cell-mediated immunotherapy is a rapidly expanding field (64), and we propose that conditioned APC administered at the time of transplantation may become a useful and invaluable tool in promoting graft tolerance along with conventional immunosuppressive agents.
ACKNOWLEDGMENTS
D.C.T. is a National Institute for Health Research Clinical Lecturer funded by Cambridge Institute for Medical Research/Wellcome Trust Next Generation Fellowship. The authors acknowledge funding from the Medical Research Council (to F.S.W.). M.W. is an RD Lawrence Fellow funded by Diabetes U.K. The Cambridge Institute for Medical Research is supported by a Wellcome Trust Strategic Award (079895).
No potential conflicts of interest relevant to this article were reported.
D.C.T. researched data and wrote the manuscript. F.S.W. contributed the G9 mice, contributed to discussion of the manuscript, and reviewed and edited the manuscript. P.Z. researched and discussed data. E.A.G. contributed to discussion of the manuscript and reviewed and edited the manuscript. M.W. researched data and wrote the manuscript. M.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Professor Anne Cooke and her laboratory (University of Cambridge) and Dr. Sarah Howlett (University of Cambridge) for generous sharing of reagents and constructive discussions. The authors also thank Christien Lien (University of Cambridge), Orval Lyle (University of Cambridge), Charlotte Ferguson (University of Cambridge), and Martin Rice (University of Cambridge) for excellent technical assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-1740/-/DC1.
M.W. is currently affiliated with the Department of Pathology, University of Cambridge, Cambridge, U.K.
REFERENCES
- 1.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 2009;155:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coppieters KT, von Herrath MG. Histopathology of type 1 diabetes: old paradigms and new insights. Rev Diabet Stud 2009;6:85–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skowera A, Ellis RJ, Varela-Calviño R, et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope [corrected in J Clin Invest 2009;119:2844]. J Clin Invest 2008;118:3390–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes 2002;51:3353–3361 [DOI] [PubMed] [Google Scholar]
- 5.Robertson RP, Davis C, Larsen J, Stratta R, Sutherland DE, American Diabetes Association . Pancreas and islet transplantation in type 1 diabetes. Diabetes Care 2006;29:935. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343:230–238 [DOI] [PubMed] [Google Scholar]
- 7.Shapiro AM. State of the art of clinical islet transplantation and novel protocols of immunosuppression. Curr Diab Rep 2011;11:345–354 [DOI] [PubMed] [Google Scholar]
- 8.Ravindra KV, Wu S, McKinney M, Xu H, Ildstad ST. Composite tissue allotransplantation: current challenges. Transplant Proc 2009;41:3519–3528 [DOI] [PubMed] [Google Scholar]
- 9.Roberts AB, Lamb LC, Newton DL, Sporn MB, De Larco JE, Todaro GJ. Transforming growth factors: isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci USA 1980;77:3494–3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA 1986;83:4167–4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margetts PJ, Bonniaud P, Liu L, et al. Transient overexpression of TGF-beta1 induces epithelial mesenchymal transition in the rodent peritoneum. J Am Soc Nephrol 2005;16:425–436 [DOI] [PubMed] [Google Scholar]
- 12.Reynolds LE, Conti FJ, Silva R, et al. alpha3beta1 integrin-controlled Smad7 regulates reepithelialization during wound healing in mice. J Clin Invest 2008;118:965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 1992;359:693–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulkarni AB, Huh CG, Becker D, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 1993;90:770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 2006;25:441–454 [DOI] [PubMed] [Google Scholar]
- 16.Glick AB, Kulkarni AB, Tennenbaum T, et al. Loss of expression of transforming growth factor beta in skin and skin tumors is associated with hyperproliferation and a high risk for malignant conversion. Proc Natl Acad Sci USA 1993;90:6076–6080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol 2004;172:7335–7340 [DOI] [PubMed] [Google Scholar]
- 18.Terabe M, Ambrosino E, Takaku S, et al. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody. Clin Cancer Res 2009;15:6560–6569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 2006;25:455–471 [DOI] [PubMed] [Google Scholar]
- 20.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol 2004;172:5149–5153 [DOI] [PubMed] [Google Scholar]
- 21.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol 2007;178:4022–4026 [DOI] [PubMed] [Google Scholar]
- 22.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 2007;178:2018–2027 [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003;198:1875–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grewal IS, Grewal KD, Wong FS, et al. Expression of transgene encoded TGF-beta in islets prevents autoimmune diabetes in NOD mice by a local mechanism. J Autoimmun 2002;19:9–22 [DOI] [PubMed] [Google Scholar]
- 25.Yamasaki S, Kurita N, Hata J, Moritani M, Itakura M, Shimada M. The effect of transgenic expression of TGF-beta1 on transplanted islet graft survival. Hepatogastroenterology 2007;54:1617–1621 [PubMed] [Google Scholar]
- 26.Suarez-Pinzon WL, Marcoux Y, Ghahary A, Rabinovitch A. Gene transfection and expression of transforming growth factor-beta1 in nonobese diabetic mouse islets protects beta-cells in syngeneic islet grafts from autoimmune destruction. Cell Transplant 2002;11:519–528 [PubMed] [Google Scholar]
- 27.King C, Davies J, Mueller R, et al. TGF-beta1 alters APC preference, polarizing islet antigen responses toward a Th2 phenotype. Immunity 1998;8:601–613 [DOI] [PubMed] [Google Scholar]
- 28.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci USA 2004;101:4572–4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green EA, Eynon EE, Flavell RA. Local expression of TNFalpha in neonatal NOD mice promotes diabetes by enhancing presentation of islet antigens. Immunity 1998;9:733–743 [DOI] [PubMed] [Google Scholar]
- 30.Wong FS, Karttunen J, Dumont C, et al. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat Med 1999;5:1026–1031 [DOI] [PubMed] [Google Scholar]
- 31.Wong FS, Siew LK, Scott G, et al. Activation of insulin-reactive CD8 T-cells for development of autoimmune diabetes. Diabetes 2009;58:1156–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol 1998;76:34–40 [DOI] [PubMed] [Google Scholar]
- 33.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell 1994;76:17–27 [DOI] [PubMed] [Google Scholar]
- 34.Shockett P, Difilippantonio M, Hellman N, Schatz DG. A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc Natl Acad Sci USA 1995;92:6522–6526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szot GL, Koudria P, Bluestone JA. Murine pancreatic islet isolation (Abstract). J Vis Exp 2007;7:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szot GL, Koudria P, Bluestone JA. Transplantation of pancreatic islets into the kidney capsule of diabetic mice (Abstract). J Vis Exp 2007;9:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev 2009;229:41–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cobbold SP, Adams E, Farquhar CA, et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci USA 2009;106:12055–12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010;32:593–604 [DOI] [PubMed] [Google Scholar]
- 40.Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 2003;3:807–821 [DOI] [PubMed] [Google Scholar]
- 41.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors 2011;29:196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol 2004;4:24–34 [DOI] [PubMed] [Google Scholar]
- 43.Stoop JN, Harry RA, von Delwig A, Isaacs JD, Robinson JH, Hilkens CM. Therapeutic effect of tolerogenic dendritic cells in established collagen-induced arthritis is associated with a reduction in Th17 responses. Arthritis Rheum 2010;62:3656–3665 [DOI] [PubMed] [Google Scholar]
- 44.Vaknin I, Kunis G, Miller O, et al. Excess circulating alternatively activated myeloid (M2) cells accelerate ALS progression while inhibiting experimental autoimmune encephalomyelitis. PLoS ONE 2011;6:e26921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wållberg M, Harris RA. Co-infection with Trypanosoma brucei brucei prevents experimental autoimmune encephalomyelitis in DBA/1 mice through induction of suppressor APCs. Int Immunol 2005;17:721–728 [DOI] [PubMed] [Google Scholar]
- 46.Tai N, Yasuda H, Xiang Y, et al. IL-10-conditioned dendritic cells prevent autoimmune diabetes in NOD and humanized HLA-DQ8/RIP-B7.1 mice. Clin Immunol 2011;139:336–349 [DOI] [PubMed] [Google Scholar]
- 47.Morel PA, Srinivas M, Turner MS, et al. Gene expression analysis of dendritic cells that prevent diabetes in NOD mice: analysis of chemokines and costimulatory molecules. J Leukoc Biol 2011;90:539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feili-Hariri M, Dong X, Alber SM, Watkins SC, Salter RD, Morel PA. Immunotherapy of NOD mice with bone marrow-derived dendritic cells. Diabetes 1999;48:2300–2308 [DOI] [PubMed] [Google Scholar]
- 49.Morin J, Faideau B, Gagnerault MC, Lepault F, Boitard C, Boudaly S. Passive transfer of flt-3L-derived dendritic cells delays diabetes development in NOD mice and associates with early production of interleukin (IL)-4 and IL-10 in the spleen of recipient mice. Clin Exp Immunol 2003;134:388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parsa R, Andresen P, Gillett A, et al. Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes 2012;61:2881–2892 [DOI] [PMC free article] [PubMed]
- 51.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol 2007;178:7018–7031 [DOI] [PubMed] [Google Scholar]
- 52.Min WP, Zhou D, Ichim TE, et al. Inhibitory feedback loop between tolerogenic dendritic cells and regulatory T cells in transplant tolerance. J Immunol 2003;170:1304–1312 [DOI] [PubMed] [Google Scholar]
- 53.Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol 2009;39:3147–3159 [DOI] [PubMed] [Google Scholar]
- 54.Kleijwegt FS, Jansen DT, Teeler J, et al. Tolerogenic dendritic cells impede priming of naive CD8(+) T cells and deplete memory CD8(+) T cells. Eur J Immunol 2013;43:85–92 [DOI] [PubMed]
- 55.Cobbold SP, Adams E, Nolan KF, Regateiro FS, Waldmann H. Connecting the mechanisms of T-cell regulation: dendritic cells as the missing link. Immunol Rev 2010;236:203–218 [DOI] [PubMed] [Google Scholar]
- 56.Ferreira GB, Kleijwegt FS, Waelkens E, et al. Differential protein pathways in 1,25-dihydroxyvitamin d(3) and dexamethasone modulated tolerogenic human dendritic cells. J Proteome Res 2012;11:941–971 [DOI] [PubMed] [Google Scholar]
- 57.Salazar L, Aravena O, Abello P, et al. Modulation of established murine collagen-induced arthritis by a single inoculation of short-term lipopolysaccharide-stimulated dendritic cells. Ann Rheum Dis 2008;67:1235–1241 [DOI] [PubMed] [Google Scholar]
- 58.Kim SH, Kim S, Evans CH, Ghivizzani SC, Oligino T, Robbins PD. Effective treatment of established murine collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express IL-4. J Immunol 2001;166:3499–3505 [DOI] [PubMed] [Google Scholar]
- 59.Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce ag-specific T cell regulation and prolong graft survival. Am J Transplant 2005;5:228–236 [DOI] [PubMed] [Google Scholar]
- 60.Jiang S, Camara N, Lombardi G, Lechler RI. Induction of allopeptide-specific human CD4+CD25+ regulatory T cells ex vivo. Blood 2003;102:2180–2186 [DOI] [PubMed] [Google Scholar]
- 61.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med 2001;193:233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson AE, Sayers BL, Haniffa MA, et al. Differential regulation of naïve and memory CD4+ T cells by alternatively activated dendritic cells. J Leukoc Biol 2008;84:124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care 2011;34:2026–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kantoff PW, Higano CS, Shore ND, et al. IMPACT Study Investigators . Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411–422 [DOI] [PubMed] [Google Scholar]