Abstract
Marked improvement in glycemic control occurs in patients with type 2 diabetes mellitus shortly after Roux-en-Y gastric bypass surgery (RYGB) and before there is major weight loss. The objective of this study was to determine whether the magnitude of this change is primarily due to caloric restriction or is unique to the surgical procedure. We studied eleven subjects who underwent RYGB and fourteen subjects mean-matched for BMI, HbA1c, and diabetes duration who were admitted to our inpatient research unit and given a very low–calorie diet (VLCD) of 500 kcal/day with a macronutrient content similar to that consumed by patients after RYGB. Frequently sampled intravenous glucose tolerance tests were performed before and after interventions. Both groups lost an equivalent amount of weight over a mean study period of 21 days. Insulin sensitivity, acute insulin secretion after intravenous glucose administration, and β-cell function as determined by disposition index improved to a similar extent in both groups. Likewise, changes in fasting glucose and fructosamine levels were similar. Based on these data, VLCD improves insulin sensitivity and β-cell function just as well as RYGB in the short term.
The prevalence of obesity and the associated health consequences, including type 2 diabetes mellitus (T2DM), continues to rise (1). The typical progression of T2DM is one of deteriorating β-cell function that requires an increasing amount of oral medical therapy and finally insulin treatment to achieve adequate glycemic control (2). Ultimately, there is pancreatic β-cell failure (3). Calorie restriction and subsequent weight loss have been shown to be effective treatment modalities of T2DM (4). Caloric restriction can improve hyperglycemia through regulation of hepatic glucose production (5). In addition to cumulative weight loss, the rapidity with which the weight loss is achieved also exerts an effect on glycemic control (6). Unfortunately, most individuals are unable to maintain a reduced body weight through diet alone (7). In contrast, weight loss achieved by bariatric surgery has been shown to result in a lesser degree of recidivism than nonsurgical treatments and is associated with marked improvement of glycemic control (8).
While Roux-en-Y gastric bypass surgery (RYGB) produces profound weight loss, glycemic control improves within the first 2–3 weeks after the procedure and before much of the weight loss occurs, leading to the hypothesis that factors in addition to weight loss are involved (9,10). In an animal study of nonobese Goto-Kakizaki rats, bypass of the duodenum and jejunum has been shown to control hyperglycemia unrelated to weight reduction (11), leading to the hypothesis that routing nutrients away from the proximal small intestine produces an antidiabetes effect. Rapid delivery of nutrients to the distal small intestine also enhances postprandial secretion of the incretin glucagon-like peptide-1 (GLP-1). Substantial changes in GLP-1 levels have been observed after RYGB but not after gastric banding or equivalent weight loss achieved by diet (12–14), thus providing another mechanism for improvement in glucose homeostasis after RYGB.
We have previously demonstrated that there was less improvement in β-cell function of subjects with T2DM on an 800 kcal/day low-calorie diet compared with a matched cohort of RYGB subjects when assessed with an intravenous glucose challenge after equivalent weight reduction (15). A limitation of the study was that the low-calorie diet group took longer than the surgery group to achieve equivalent weight loss, indicating that the degree of caloric restriction was not equivalent to the RYGB group. The difference in caloric intake confounds interpretation of the results because the degree of caloric restriction in addition to the amount of weight loss affects glucostatic parameters. For example, Wing et al. (16) demonstrated that an 11% reduction in body weight with a 400 kcal/day diet resulted in significantly greater insulin sensitivity compared with the same weight reduction achieved over a longer period of time on a 1,100 kcal/day diet. Henry et al. (4) demonstrated that on 330 kcal/day, the majority of improvement in fasting plasma glucose occurred within the first 10 days of caloric restriction preceding most of the weight loss. Minimal further improvement followed after continued weight loss, thus demonstrating that most of the improvement in glucose occurs within the first few days of caloric restriction.
Based on our previous study, we were unable to rule out the possibility that the greater improvement in β-cell function after RYGB was due to the surgical procedure itself as opposed to greater caloric restriction. Therefore, in this study, we sought to confirm our previous finding by changing the diet to a very low–calorie diet (VLCD) of 500 kcal/day, which is similar to the typical intake in the early post-RYGB period. This VLCD resulted in the same amount of weight loss over the same period of time as the RYGB group. β-Cell function was assessed by frequently sampled intravenous glucose tolerance tests (fsIVGTTs) before and at a mean of 21 days after the interventions.
RESEARCH DESIGN AND METHODS
Two groups of subjects with a self-reported history of T2DM were recruited, consisting of individuals who were scheduled to undergo RYGB (n = 11) or willing to participate in a nonsurgical inpatient VLCD program (n = 14). The decision to undergo surgery was made between patient and physician, independent of this research protocol. Subjects for the VLCD were recruited by word of mouth and flyers placed throughout the Medical Center. Main inclusion criteria were HbA1c 6.5–12% (48–108 mmol/mol), age 18–65 years, and BMI >35 kg/m2. Major exclusion criteria were the use of thiazolidinedione or insulin at a dose of >60 units/day, use of dipeptidyl peptidase-4 inhibitor or GLP-1 receptor agonist for >12 months, fasting triglycerides >400 mg/dL, weight change >5% in the previous 3 months, or significant illness. Of study participants, 16% were Caucasian, 44% African American, and 40% Hispanic. The study was approved by the Columbia University Institutional Review Board, and written informed consent was obtained from all subjects.
After an overnight fast, volunteers in the VLCD group were admitted as inpatients for the duration of the study to the Clinical Research Resource in the Irving Institute of Clinical and Translational Research and were placed on a clear diet equivalent to the inpatient postoperative RYGB diet of 360 kcal for the day. On day 2 and the next 14–24 days (duration depended upon subjects’ personal schedules), a diet similar in amount and macronutrient content that is recommended by the bariatric dietitians during this early postoperative period was provided. The diet consisted of 500 kcal/day (50% protein, 35% carbohydrate, and 15% fat) consumed as six mini-meals prepared with low-fat milk, pureed fruit and vegetables, Crystal Light, and Jell-O pudding mixes (Kraft Foods, Northfield, IL). Protein intake was met with the incorporation of UNJURY whey protein isolate (ProSynthesis Laboratories, Sterling, VA). Appropriate vitamin and mineral supplementation was provided as well as noncaloric noncarbonated caffeine-free beverages, sugar-free chewing gum, and sodium-free flavored bouillon. After the first 4 days, subjects were allowed day passes to leave the unit when needed to run personal errands, and if necessary, were provided a cooler containing a meal to ensure adherence to diet and schedule. RYGB was performed as previously described (13). Although patients were encouraged to lose weight prior to surgery, none of our participants were on a reduced-calorie diet at the time of the first fsIVGTT or experienced weight change between the time of testing and the day of surgery.
fsIVGTT procedure and calculations.
fsIVGTT was performed prior to and postintervention. Subjects were instructed to avoid strenuous exercise for 2 days prior to the procedure. Oral diabetes medications were held 2–3 days prior to testing and injectable medications held 1 day prior. After a 10-h fast, blood samples were collected for hormone and metabolic analyses. Glucose (0.3g/kg body wt as dextrose 50 g/dL) was administered intravenously within 2 min at t = 0, and subsequent samples were obtained at 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 24, 25, 27, 30, 40, 50, 60, 70, 90, 100, 120, 140, 160, and 180 min. At 20 min, an intravenous injection of regular insulin (0.05 units/kg body wt) was administered to increase the accuracy of measuring insulin sensitivity in diabetic subjects (17). Insulin sensitivity was assessed using the Bergman minimal model analysis (MINMOD Millennium 6.02 software) of fsIVGTT (18,19). The equations of this model provide measures of glucose-dependent glucose elimination, the sensitivity of glucose elimination to insulin (Si), and the acute insulin response to glucose (AIR). Disposition index (DI) is derived from the product of Si and AIR and is a measure of insulin secretion in relation to insulin sensitivity. Acute C-peptide response (ACPR) is the relative mean increase (in percent) from fasting in C-peptide levels 3–5 min after glucose administration. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as reported by Matthews et al. (20).
Analytic assays.
Serum insulin, C-peptide, and high-sensitivity C-reactive protein (CRP) were measured with the Immulite Analyzer (Siemens, Los Angeles, CA). Leptin, total ghrelin, and glucose were measured as previously described (21). Total PYY was measured by ELISA (Millipore, St. Charles, MO) with a sensitivity of 10 pg/mL and 2.3% intra-assay and 7.2% interassay coefficients of variation. Total GLP-1 was measured by radioimmunoassay after alcohol extraction according to the manufacturer’s protocol (Millipore). Sensitivity of the assay is 3 pmol/L, and recovery in each assay was tested by parallel extraction of standards. High–molecular weight adiponectin was quantified by ELISA (Millipore) with an assay sensitivity of 0.5 ng/mL and 2.4% intra-assay and 5.5% interassay coefficients of variation. All samples analyzed by radioimmunoassay or ELISA were run in duplicate.
Statistical analysis.
Based on our previous work, we estimated that ΔDI for VLCD versus RYGB would equal 160 with an estimated SD equal to 120. Nine subjects in each group would provide 80% probability of detecting this estimated difference with a P α < 0.05% (15). SAS version 9.2 software (Cary, NC) was used for statistical analysis. Group differences in the distribution of continuous variables at baseline were tested with a Student independent t test, as were group differences at end point. Within-group differences between pre- and posttreatment were tested with dependent t tests. The between-group differences in the change from pre- to posttreatment were tested with independent t tests. All tests were two tailed, with P values <0.05 considered statistically significant. No adjustment of the critical value of the test statistic was made for the separate tests of different peptides or for HOMA-IR. Model estimated means and SEs are presented.
RESULTS
Clinical characteristics.
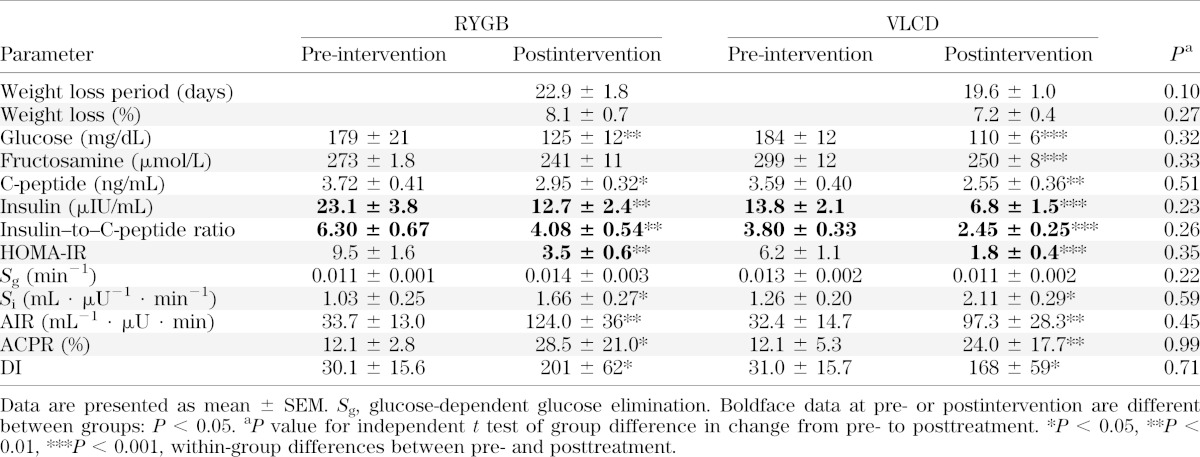
Baseline clinical characteristics of study subjects were similar between groups (Table 1). The study cohort had a mean BMI of 41.2 kg/m2. The mean duration of diabetes was 5.7 ± 0.9 years (range 0.5–15), and the mean HbA1c was 8.4 ± 0.3% (6.2–11.1) (68 ± 3 mmol/mol [range 44–98]). All subjects except one person in the VLCD were taking antihyperglycemic medications, with two subjects in each group on additional insulin therapy. Table 2 shows that there was no difference in the time period (21 ± 1 days) needed to achieve a similar loss of body weight (7.6 ± 0.4%) between treatment groups.
TABLE 1.
Subject characteristics at baseline
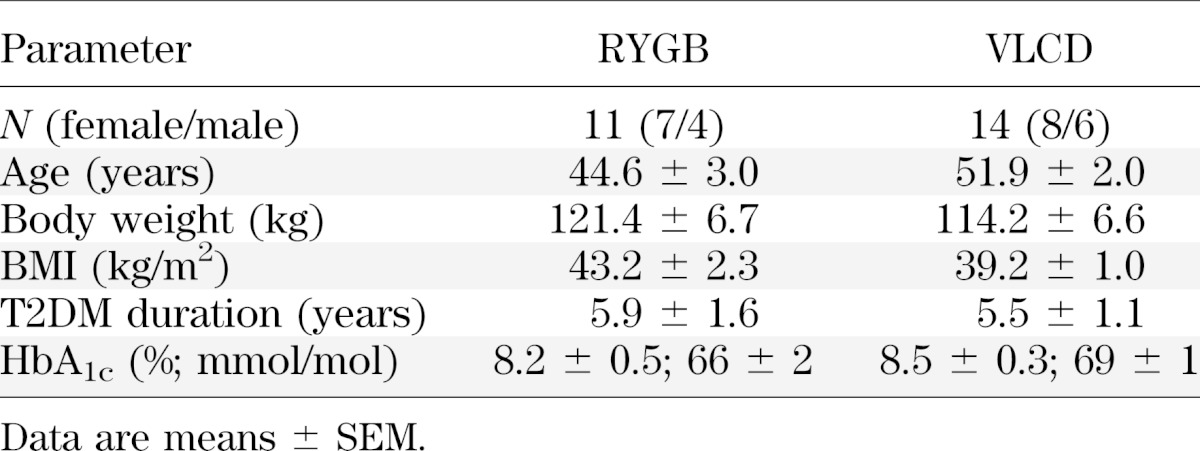
TABLE 2.
Weight loss and glucostatic parameters before and after interventions
Glucostatic parameters before and after interventions.
Baseline glucostatic parameters, with the exception of fasting insulin concentrations, were similar in both groups and are presented in Table 2. Decreases in fasting glucose and C-peptide concentrations and increases in Si, AIR, ACPR, and DI occurred in both groups to a similar extent (Table 2 and Figs. 1 and 2). While the insulin–to–C-peptide ratio was different between groups, the decrease in these values was not different (P = 0.26). Within-group change in fructosamine was significant in VLCD group but did not reach significance in RYGB group (P = 0.057). However, the change in fructosamine when adjusted for baseline values was not significant between groups (P = 0.33). After interventions, three RYGB and four VLCD subjects remained on antihyperglycemic medication.
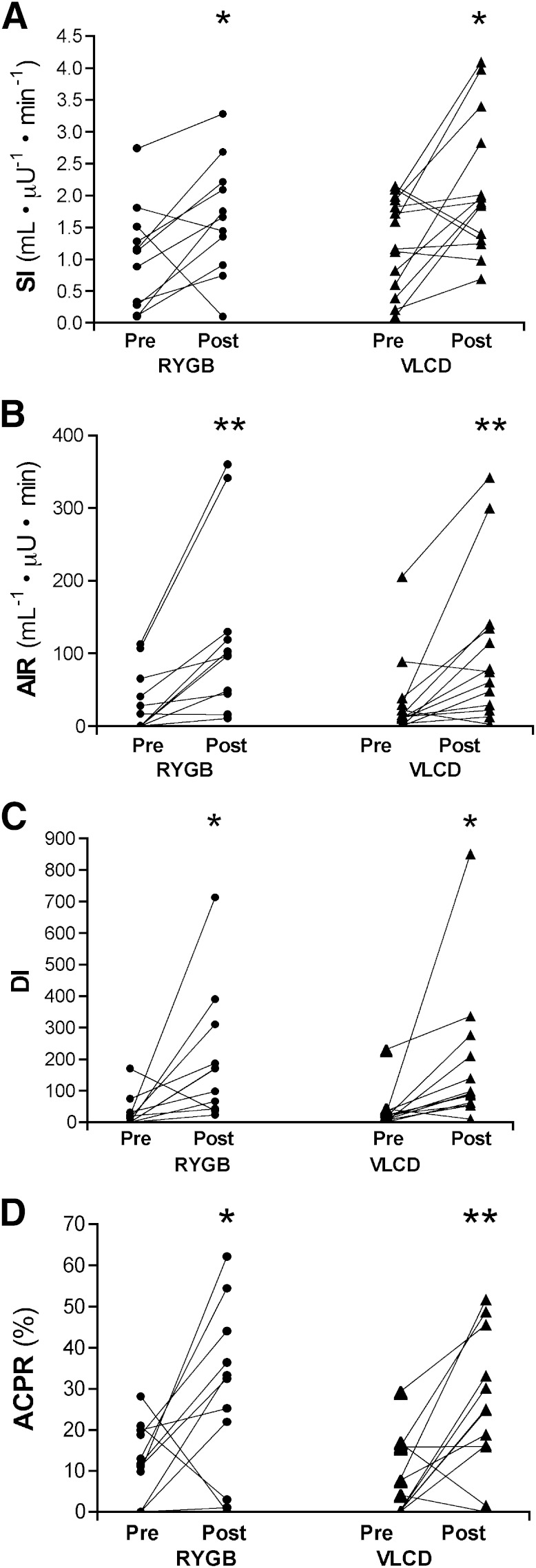
FIG. 1.
Changes in Si (A), AIR (B), DI (C), and ACPR (D) for all subjects shown individually. *P < 0.05, **P < 0.01, ***P < 0.001 vs. baseline.
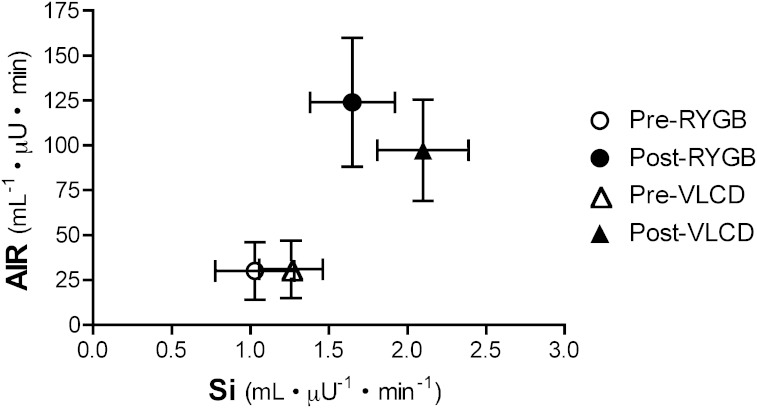
FIG. 2.
Graphic representation of the relationship between insulin sensitivity and insulin secretion before and after interventions.
Linear regression was used to determine whether baseline characteristics correlated with changes in DI. Duration of diabetes was not predictive of change in DI in RYGB (β = −1.66; P = 0.29) or VLCD (β = −1.56; P = 0.33), and the initial value of HbA1c was not predictive either (β = −4.8, P = 0.29, and β = 7.6, P = 0.26, in RYGB and VLCD, respectively). Baseline DI was not associated with change in DI (β = −1.9, P = 0.19, and β = −0.2, P = 0.86). Baseline Si and AIR were not predictive of the change in DI in either group (data not shown). The amount of weight loss was not correlated with change in DI in either the RYGB (P = 0.92) or VLCD (P = 0.99) group.
Plasma hormone and lipid levels.
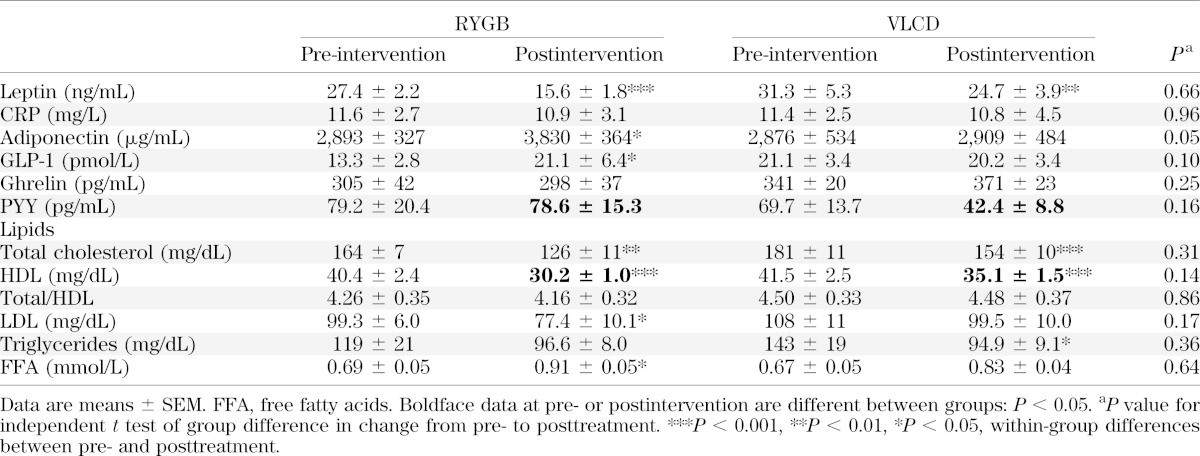
There were significant increases in adiponectin and GLP-1 in the RYGB group, and the difference in change from baseline between groups approached significance (Table 3) (P = 0.05 and 0.10 for change in adiponectin and GLP-1, respectively). There were no significant changes in fasting plasma levels of ghrelin and PYY. As expected, with both methods of weight loss plasma leptin levels decreased. CRP levels did not change in either group.
TABLE 3.
Plasma hormone levels and lipids before and after interventions
Baseline and posttreatment measurements of lipids were similar between groups, although there was some variation in the pattern of changes (Table 3). Three subjects in the RYGB group and four in the VLCD were taking cholesterol-lowering medication. Free fatty acid levels increased after RYGB and VLCD (P = 0.029 and 0.062, respectively).
DISCUSSION
Contrary to our expectations, this study demonstrates that RYGB in subjects with T2DM does not result in greater improvement in β-cell function compared with equivalent weight loss achieved over the same time period by VLCD. As evaluated by fsIVGTT, both groups demonstrated significant and similar increases in acute insulin secretion and insulin sensitivity. We previously demonstrated a greater improvement in DI in subjects with T2DM after RYGB compared with individuals who achieved equivalent weight loss on an outpatient low-calorie diet (15). However, the degree to which calories were restricted was not equivalent to the RYGB, and therefore, the rate of weight loss for the RYGB was greater. Based on studies of caloric restriction, the degree of caloric restriction is a major factor that exerts a glucose-lowering effect independent from the amount of weight loss (4,16). This is the first study of individuals with T2DM that compares RYGB to a diet group matched for both the amount and rate of weight loss. Others have evaluated β-cell function after surgery, but there has not been a simulation of a gastric bypass diet without the surgery in patients with T2DM. Isbell et al. (22), for example, evaluated RYGB with matched obese subjects on a VLCD 2–7 days after intervention. There was in both groups a 25% improvement in insulin sensitivity as quantified by HOMA-IR, but a significant decrease in fasting glucose levels was demonstrated in the diet group only. These results are somewhat difficult to interpret given that the groups were a mix of subjects with and without T2DM, and there may be a confounding effect of residual inflammation in this very early postoperative state.
Differences in the methods used to assess β-cell function, characteristics of the patient population, and differences in operative procedures among surgeons also make it difficult to compare studies with sometimes seemingly conflicting outcomes. For example, with use of the hyperglycemic clamp it was found that 4 weeks after RYGB there was an increase in insulin sensitivity, but DI remained unchanged (23). An explanation for this finding in comparison with our study is that metabolic testing was carried out when subjects were consuming an 800-calorie liquid diet prior to surgery. It is also unclear whether diabetes medications, including metformin and thiazolidinediones, were held prior to testing. Similar to our results, Lin et al. (24) showed that DI improved 23-fold (from 23 to 403) 1 month after RYGB. Nannipieri et al. (25) also showed that β-cell glucose sensitivity improved (but did not normalize) 45 days after RYGB in patients with T2DM. Interestingly, insulin sensitivity but not β-cell glucose sensitivity improved in proportion to weight loss. Certainly, duration of diabetes also influences β-cell function (26). Lim et al. (27) studied patients with T2DM and restricted caloric intake to 600 kcal/day for 8 weeks and demonstrated not just improvement but normalization of both β-cell function and hepatic insulin sensitivity. The duration of diabetes, however, was <4 years, which limits comparison with our study cohort with a longer duration of diabetes. In the absence of a gold standard for assessing β-cell function and the inclusion of a diverse population, comparison of different studies is indeed problematic (28).
Similar to dietary interventions (4,6), there is considerable variability in the glycemic response after RYGB. From a clinical perspective, it would be helpful to predict the glycemic response to surgery from known or easily measureable baseline characteristics. In retrospective studies, it appears that patients with a longer history of diabetes or on insulin therapy were less likely to achieve euglycemia off antihyperglycemic medications (29,30). Magnitude of weight loss has been associated with better glycemic outcome (29–31). From our data, it appears that duration of T2DM, baseline β-cell function, or HbA1c and the amount of weight loss did not correlate with changes in DI. However, in this short-term study we are unable to assess the maximal improvement in glycemic parameters, as it has been shown that Si continues to improve with further weight loss between 6 and 24 months after surgery (24). Likewise, we cannot predict the durability of these changes, as weight tends to increase with time, and deterioration of glycemic control, even with maintenance of weight loss, has been observed (30,32–34).
It has been well documented that RYGB produces profound postprandial stimulation of GLP-1 secretion and a greater incretin effect than observed with diet-induced weight loss (14). RYGB also alters glucose absorption, causing a marked increase in the early rate of appearance of ingested glucose into the systemic circulation that would be expected to alter the pattern of insulin secretion (35). For these reasons, we evaluated β-cell function in the absence of enteral nutrient passage and without the confounding effect of altered glucose kinetics. Although gut hormones were not measured during the fsIVGTT, others have demonstrated that there is negligible change in GLP-1 concentrations upon intravenous glucose infusion (14,36). Thus, the findings in our study suggest that there are changes in β-cell function, independent of glucose absorption from the gut and the incretin effect, that occur to a similar extent after RYGB and VLCD. Because of the incretin effect, however, we did expect that overall glycemic control would improve to a greater extent after RYGB. Contrary to our expectations, decreases in fructosamine levels from baseline were of similar magnitude between RYGB and VLCD. It is likely that the greater incretin effect noted after larger meal challenges in RYGB patients may not have provided much advantage in the setting of very small meals and less demand for prandial insulin secretion. In this vein, it has been noted that β-cell function (15) and clinical outcome after RYGB (14) are better than equivalent weight loss achieved by LCD; however, the caloric intake and duration of the weight loss periods were greater in the diet groups of both studies.
Another component of insulin and glucose metabolism is insulin clearance, which has been demonstrated to increase after 11% weight loss on a 500–600 kcal/day diet (37). We did not directly measure insulin clearance, but given that fasting insulin was greater in the RYGB group at baseline while C-peptide levels were nearly identical it appears that insulin clearance may have been greater in the VLCD group. It is unclear why there was a difference, since groups were fairly well matched for some of the factors that are associated with clearance such as Si, AIR, fasting glucose, and BMI (38,39). Unfortunately, we do not have measurements of waist circumference or visceral adipose tissue that could conceivably be different in this relatively small sample size and might affect insulin clearance. Nevertheless, the change in the insulin–to–C-peptide ratio was nearly identical between groups, suggesting that the interventions do not differentially affect clearance.
Limitations of this study are the nonrandomized intervention scheme, as well as the relatively small sample size. The study was designed to detect a 1.3-SD difference in change in DI between groups. Smaller differences may not have been detected, but the clinical significance of smaller differences is somewhat questionable. It is possible that with more subjects changes in some of the secondary end points would reach statistical significance, such as the increase in adiponectin in the VLCD group and decrease in fructosamine after RYGB. Although the groups were matched for duration of diabetes, this was a self-reported time of diagnosis that likely varies from the actual onset of the disease. Another factor to consider is that acute inflammation, even with a minimally invasive laparoscopic procedure, could have increased insulin resistance blunting the response of the RYGB patients. We did not perform detailed assessment of residual inflammation; however, CRP levels were not statistically different between both groups. Physical activity levels were not monitored, although given the inpatient setting we were able to limit the activity in the VLCD group to that expected of postsurgical patients. The RYGB group was not studied under inpatient observation because the surgical procedure itself imposes limitation of caloric intake to ~500 kcal/day in the first 2–3 weeks after surgery.
These data indicate that the changes in glucose homeostasis that occur within 2–3 weeks after RYGB are primarily due to very low energy intake as opposed to specific surgically-induced hormonal effects. Clearly, this does not mean that RYGB is not more beneficial in the long term, since the degree of caloric restriction required to mimic surgical results cannot be maintained in most individuals. As in our prior study, even when the diet was 800 kcal/day instead of 500 kcal/day less improvement in β-cell function was noted. It would be expected that in the longer term, the average blood glucose would begin to increase in the VLCD group as caloric intake was liberalized even if the weight loss were maintained (4,5). The RYGB group may maintain or further their improvements by virtue of continued weight loss. Furthermore, even if it were possible to match caloric intake with RYGB for a much longer period, there may be changes in nutrient absorption (i.e., amino acids, fatty acids) and bile acid secretion specific to the bypass procedure that could affect clinical outcome independent of weight loss and calorie restriction.
In summary, our data suggest that RYGB is not superior to VLCD with regard to early changes in β-cell function in obese subjects with T2DM when tested in the absence of an enteral nutrient stimulus. Observing glucostatic parameters in the longer term would be useful to investigate the durability of these changes. Certainly, incretins play a role in improving glucose homeostasis after RYGB, but there are likely nonenteral mechanisms associated with glycemic control in the short and longer term that result from calorie restriction. Further study is required in order to define preoperative characteristics that could better predict an individual’s response to surgical interventions for the treatment of type 2 diabetes.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) Grant DK072011 (to J.K.), National Center for Research Resources (NCRR) Grant UL1 RR024156, and a pilot grant from the Columbia Diabetes Research Center (NIH Grant DK63608). C.J. was supported by NIH T32 Training Grant DK07271.
J.K. has received research grant support from Covidien and payment for serving on the Scientific Advisory Board of Nutrisystem. No other potential conflicts of interest related to this article were reported.
C.J. was responsible for patient recruitment and care and data management and participated in manuscript preparation. W.K. designed food menus and supervised the bionutrition staff. G.F. was responsible for patient recruitment and performance of glucose tolerance tests. I.M.C. performed all hormone assays and assisted with sample preparation and data entry. L.A. and M.B. assisted with patient recruitment. D.J.M. performed data analysis. J.K. conceived and designed the study, supervised the experiments and collection of data, and prepared the manuscript. J.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 30th Annual Scientific Meeting of the Obesity Society, San Antonio, Texas, 20–24 September 2012.
The bionutrition staff of the NCRR were invaluable for the performance of this study. The authors also thank the participants in this study; Nancy Restuccia, MS, RD, CDN (Columbia University Medical Center, New York, New York), for guidance with bariatric nutrition guidelines; and Dr. Leona Plum (Profil Institut für Stoffwechselforschung, Neuss, Germany) for critical review of the manuscript.
Footnotes
Clinical trial reg. no. NCT00627484, clinicaltrials.gov.
See accompanying commentary, p. 3017.
REFERENCES
- 1.Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med 2012;42:563–570 [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care 1992;15:318–368 [DOI] [PubMed] [Google Scholar]
- 3.Leahy JL, Hirsch IB, Peterson KA, Schneider D. Targeting beta-cell function early in the course of therapy for type 2 diabetes mellitus. J Clin Endocrinol Metab 2010;95:4206–4216 [DOI] [PubMed] [Google Scholar]
- 4.Henry RR, Scheaffer L, Olefsky JM. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1985;61:917–925 [DOI] [PubMed] [Google Scholar]
- 5.Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1993;77:1287–1293 [DOI] [PubMed] [Google Scholar]
- 6.Watts NB, Spanheimer RG, DiGirolamo M, et al. Prediction of glucose response to weight loss in patients with non-insulin-dependent diabetes mellitus. Arch Intern Med 1990;150:803–806 [PubMed] [Google Scholar]
- 7.Wadden TA. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med 1993;119:688–693 [DOI] [PubMed] [Google Scholar]
- 8.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moo TA, Rubino F. Gastrointestinal surgery as treatment for type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 2008;15:153–158 [DOI] [PubMed] [Google Scholar]
- 10.Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 2009;33(Suppl. 1):S33–S40 [DOI] [PubMed] [Google Scholar]
- 11.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 2004;239:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 2007;3:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plum L, Ahmed L, Febres G, et al. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity (Silver Spring) 2011;19:2149–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994;17:30–36 [DOI] [PubMed] [Google Scholar]
- 17.Welch S, Gebhart SS, Bergman RN, Phillips LS. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab 1990;71:1508–1518 [DOI] [PubMed] [Google Scholar]
- 18.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667–E677 [DOI] [PubMed] [Google Scholar]
- 19.Saad MF, Anderson RL, Laws A, et al. A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose tolerance. Insulin Resistance Atherosclerosis Study. Diabetes 1994;43:1114–1121 [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 21.Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 2005;90:359–365 [DOI] [PubMed] [Google Scholar]
- 22.Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 2010;33:1438–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashyap SR, Daud S, Kelly KR, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2010;34:462–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin E, Liang Z, Frediani J, et al. Improvement in ß-cell function in patients with normal and hyperglycemia following Roux-en-Y gastric bypass surgery. Am J Physiol Endocrinol Metab 2010;299:E706–E712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nannipieri M, Mari A, Anselmino M, et al. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab 2011;96:E1372–E1379 [DOI] [PubMed] [Google Scholar]
- 26.Clauson P, Linnarsson R, Gottsäter A, Sundkvist G, Grill V. Relationships between diabetes duration, metabolic control and beta-cell function in a representative population of type 2 diabetic patients in Sweden. Diabet Med 1994;11:794–801 [DOI] [PubMed] [Google Scholar]
- 27.Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011;54:2506–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrannini E, Mingrone G. Impact of different bariatric surgical procedures on insulin action and beta-cell function in type 2 diabetes. Diabetes Care 2009;32:514–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003;238:467–484; discussion 84–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg 2013;23:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003;237:751–756; discussion 757–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sjöström L, Lindroos AK, Peltonen M, et al. Swedish Obese Subjects Study Scientific Group . Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 33.DiGiorgi M, Rosen DJ, Choi JJ, et al. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis 2010;6:249–253 [DOI] [PubMed] [Google Scholar]
- 34.Chikunguwo SM, Wolfe LG, Dodson P, et al. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2010;6:254–259 [DOI] [PubMed] [Google Scholar]
- 35.Bradley D, Conte C, Mittendorfer B, et al. Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest 2012;122:4667–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haltia LT, Savontaus E, Vahlberg T, Rinne JO, Kaasinen V. Acute hormonal changes following intravenous glucose challenge in lean and obese human subjects. Scand J Clin Lab Invest 2010;70:275–280 [DOI] [PubMed] [Google Scholar]
- 37.Svendsen PF, Jensen FK, Holst JJ, Haugaard SB, Nilas L, Madsbad S. The effect of a very low calorie diet on insulin sensitivity, beta cell function, insulin clearance, incretin hormone secretion, androgen levels and body composition in obese young women. Scand J Clin Lab Invest 2012;72:410–419 [DOI] [PubMed] [Google Scholar]
- 38.Lee CC, Haffner SM, Wagenknecht LE, et al. Insulin Clearance and the Incidence of Type 2 Diabetes in Hispanics and African Americans: the IRAS Family Study. Diabetes Care 2013;36:901–907 [DOI] [PMC free article] [PubMed]
- 39.Goodarzi MO, Cui J, Chen YD, Hsueh WA, Guo X, Rotter JI. Fasting insulin reflects heterogeneous physiological processes: role of insulin clearance. Am J Physiol Endocrinol Metab 2011;301:E402–E408 [DOI] [PMC free article] [PubMed] [Google Scholar]