Abstract
Cryptococcus neoformans is a basidiomycete human fungal pathogen that causes meningoencephalitis in both immunocompromised and immunocompetent individuals. The ability to sense and respond to diverse extracellular signals is essential for the pathogen to infect and cause disease in the host. Four major stress-activated signaling (SAS) pathways have been characterized in C. neoformans, including the HOG (high osmolarity glycerol response), PKC/Mpk1 MAPK (mitogen-activated protein kinase), calcium-dependent calcineurin, and RAS signaling pathways. The HOG pathway in C. neoformans not only controls responses to diverse environmental stresses, including osmotic shock, UV irradiation, oxidative stress, heavy metal stress, antifungal drugs, toxic metabolites, and high temperature, but also regulates ergosterol biosynthesis. The PKC (Protein kinase C)/Mpk1 pathway in C. neoformans is involved in a variety of stress responses, including osmotic, oxidative, and nitrosative stresses and breaches of cell wall integrity. The Ca2+/calmodulin- and Ras-signaling pathways also play critical roles in adaptation to certain environmental stresses, such as high temperature and sexual differentiation. Perturbation of the SAS pathways not only impairs the ability of C. neoformans to resist a variety of environmental stresses during host infection, but also affects production of virulence factors, such as capsule and melanin. A drug(s) capable of targeting signaling components of the SAS pathway will be effective for treatment of cryptococcosis.
Keywords: Cryptococcus neoformans, Human fungal pathogens, SAS pathways, Signaling, Stress-response
Cryptococcus neoformans is a basidiomycete human fungal pathogen that causes meningitis in patients immunocompromised by AIDS, organ-transplantation, or anticancer-chemotherapy and immunocompetent individuals alike. C. neoformans is generally classified into four serotypes (A to D) with different pathogenic characteristics during host infection (Hoang et al., 2004). Serotype A (C. neoformans var. grubii) is the most commonly isolated clade worldwide, and along with serotype D (C. neoformans var. neoformans) causes life-threatening meningitis mainly in immunocompromised populations. In contrast, the serotype B and C clades (C. neoformans var. gattii), recently renamed Cryptococcus gattii, can cause the fatal disease in both healthy individuals and immunocompromised patients.
C. neoformans has both heterothallic sexual and homothallic unisexual life cycles. Supporting the existence of the heterothallic life cycle, C. neoformans is isolated as either the a (MAT a) or α (MAT α) mating type. In nature, the C. neoformans MAT α strain is more prevalent and more virulent (in serotype D but not serotype A) than the MAT a strain (Kwon-Chung et al., 1992; Lin and Heitman, 2006). Even though no difference in virulence was observed between serotype A MAT α (H99) and MAT a (KN99) strains, the MAT α strain was found to be more efficient in disseminating into the central nervous system (CNS) and passing through the blood-brain-barrier (BBB) than the MAT a strain during co-infection of both mating types (Nielsen et al., 2005). When MAT α and MAT a strains co-exist under certain environmental conditions, such as nutrient limitation, each mating-type cell secretes a small peptide, called a pheromone, to trigger cell-cell fusion and filamentous growth (Kwon-Chung, 1976), after which a dikaryon is formed. Subsequently, a basidium is formed at the end of the filamentous structure where karyogamy (nuclear fusion) occurs. As a result of the mating event, four chains of basidiospores are produced from the surface of basidium (Fraser et al., 2003). However, the ratio difference of α and a mating type cells is so big that the sexual differentiation by mating is not common in the environment (Kwon-Chung and Bennett, 1978). C. neoformans also undergoes a homothalic unisexual life-cycle, named monokatyotic fruiting (Lin et al., 2005). This type of differentiation is regarded as a form of self-sex, an occurrence which could increase the ability to survive under harsh conditions. During monkaryotic fruiting, two cells of the same mating type (mostly α type) fuse together and undergo hyphae-like sexual mating. During the process, ploidy changes occur, leading to the production of diploid blastospores (Tscharke et al., 2003; Lin et al., 2005). Generally, MAT α and serotype D strains are more efficient in monokaryotic fruiting than MAT a and serotype A strains, respectively (Wickes et al., 1996).
C. neoformans is a ubiquitous fungus found in diverse environmental niches, including soil, trees and bird guano (Idnurm et al., 2005). Infections propagules have form of either spore or dried yeast cells. The main infection route is the respiratory tract of the human host, which leads to the pulmonary infection. Subsequently, the pathogen disseminates into diverse organs (but mainly to the CNS), resulting in life-threatening meningoencephalitis (Sukroongreung et al., 1998; Liu et al., 2002).
C. neoformans has several virulence factors. Two well known virulence factors are antiphagocytic polysaccharide capsule (Chang et al., 1996) and antioxidant melanin (Nosanchuk et al., 1999). The capsule prevents C. neoformans from being phagocytosed by macrophages and from being dehydrated (Aksenov et al., 1973). Capsule formation is influenced by iron limitation and physiological CO2 levels (Zaragoza et al., 2003). Four genes (CAP10, CAP59, CAP60 and CAP64) are known to be required for capsule formation, but their biochemical properties have not been reported (Chang and Kwon-Chung, 1994, 1998, 1999; Chang et al., 1995, 1996, 1997). Mutants with these genes deleted lack capsule formation and are avirulent (Chang and Kwon-Chung, 1994). The C. neoformans capsule is composed of several carbohydrate components. The major component is glucuronoxylomannan, accounting for more than 80% of capsule components, and the minor components are galactoxylomannan and mannoprotein (Vartivarian et al., 1993). Like capsule, the virulence factor melanin plays an important role in debilitating the host defense mechanism during infection (Kwon-Chung et al., 1982). Melanin is converted from catecholamine by Lac1 or Lac2. Originally, it was thought that Lac1 was the only enzyme capable of producing melanin, but microarray data demonstrated that Lac2 is not only homologous to Lac1, but also contributes to melanin production (Zhu and Williamson 2004; Pukkila-Worley et al., 2005). Cells with the ability to synthesize melanin are more resistant to oxidation, ultraviolet irradiation, and high temperature, than non-melanized cells (Jacobson and Emery, 1991; Wang et al., 1995; Wang and Casadevall, 1994; Rosas and Casadevall, 1997).
The ability to sense and respond to diverse extracellular signals is indispensable for all living organism to survive under a plethora of environmental stresses. Fungal pathogens maintain cellular homeostasis by developing their unique signaling pathways to counteract these environmental cues. Therefore, the stress response has been one of major virulence determinants for C. neoformans. For example, the ability to survive at host physiological temperature, 37~39℃, is considered to be one of the major virulence factors for human fungal pathogens including C. neoformans and C. albicans (Kwon-Chung and Rhodes, 1986; Ernst, 2000). The ability to sense and respond to diverse environmental stresses is mediated by four major signaling pathways in C. neoformans. These pathways are the: i) HOG (high osmolarity glycerol response), ii) PKC/Mpk1 MAPK (mitogen-activated protein kinase), iii) calcium-dependent calcineurin, and iv) RAS signaling pathways. In this review, we focus on the major stress-activated signaling (SAS) pathways controlling stress response and virulence of C. neoformans.
The HOG Pathway
From fungi to mammals, the stress-activated MAPK pathway is evolutionarily conserved and plays a crucial role in responding to a plethora of environmental cues (Bahn, 2008). Depending on the species, however, the MAPK pathway has some unique characteristics. For example, a two-component-like phosphorelay system is found to be a major signaling cascade upstream of the stress-activated MAPK pathway in fungi, but not in mammals (Bahn, 2008). S. cerevisiae contains a stress-activated MAPK, named Hog1, a protein involved in sensing, responding, and adapting to diverse environmental cues including pheromone sensing and initiation of sporulation during mating, filamentous growth under nitrogen starvation, and the response to osmotic and cell wall stresses (Chen and Thorner, 2007).
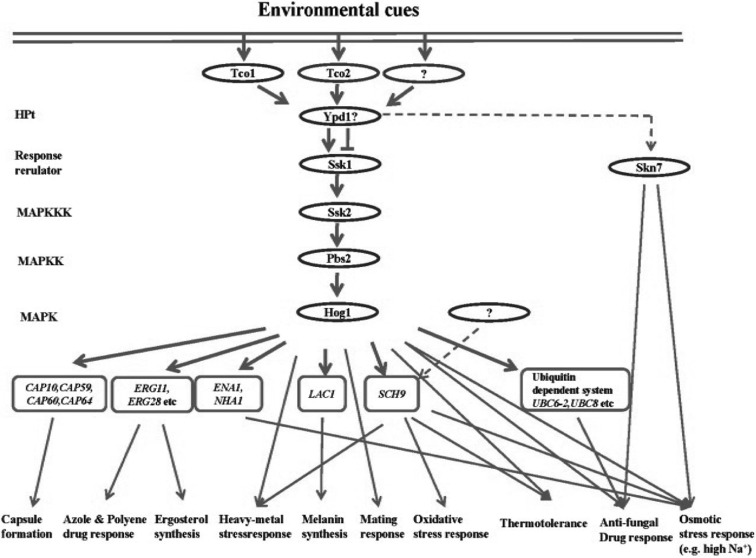
The HOG pathway is one of the key SAS pathways in C. neoformans and consists of multiple signaling components (Fig. 1). The signaling cascade upstream of the Hog1 MAPK module is the multi-component phosphorelay system, comprised of a hybrid sensor histidine kinase (HK), including Tco1 and Tco2 (Bahn et al., 2006), a histidine-containing phosphotransfer protein (HPt) Ypd1, and response regulators (RR), Ssk1 and Skn7. The Hog1 MAPK module consists of Ssk2 MAPK kinase kinase (MAPKKK), Pbs2 MAPK kinase (MAPKK), and Hog1 MAPK. Downstream of the Hog1 MAPK, multiple stress defense and protein kinase genes have been identified recently (Ko et al., 2009).
Fig. 1.
The C. neoformans HOG pathway. The HOG pathway consists of multiple signaling components and is involved in a variety of stress responses, including osmotic, oxidative, and heavy metal stresses, breaches of cell wall integrity, and anti-fungal drug responses. Following exposure to an environmental cue, signals are transmitted to downstream genes of the HOG pathway via a two-component-like phosphorelay system and a HOG MAPK module composed of Ssk2 MAPK kinase kinase, Pbs2 MAPK kinase, and Hog1 MAPK.
The general regulatory mechanism of the phosphorelay system in fungi is as follows. After exposure to certain external cues, a hybrid sensor HK in the phosphorelay system autophosphorylates a His residue in its HK domain and transfers the phosphate group to an Asp residue in the RR domain in the same hybrid sensor HK. Subsequently, the phosphate in the RR domain is delivered to a His residue in the HPt and then transferred to an Asp residue in a response regulator (Bahn, 2008). Next, the activated response regulator interacts with the autoinhibitory domain of the Ssk2-like MAPKKK and induces the autophosphorylation of a Thr residue in the Ser/Thr kinase domain. In turn, the activated MAPKKK phosphorylates the Ser and Thr residues of the Pbs2-like MAPKK, which then dually phosphorylates the Thr and Tyr residues of the Hog1-like MAPK (Bahn, 2008). In C. neoformans, Tco1 and Tco2 HKs act redundantly and independently for either positive or negative regulation of the Hog1 MAPK. However, the Ssk1 response regulator appears to positively regulate the Hog1 MAPK module since phenotypes of the ssk1Δ mutant are mostly identical to those of the hog1Δ mutant (Bahn et al., 2006). Another C. neoformans response regulator, Skn7, is also involved in stress responses to high salt conditions, oxidative damages (e.g. tert-butyl hydroperoxide), and the antifungal drug fludioxonil. Skn7, however, appears to act independently from the Hog1 MAPK module (Fig. 1) (Bahn et al., 2006).
The Hog1 MAPK is found in both C. neoformans serotype A and serotype D. The role of Hog1 in C. neoformans, and its regulatory mechanism, however, varies between different clinical and environmental C. neoformans strains (Hoang et al., 2004). Most notably, the Hog1 MAPKs in a majority of C. neoformans are uniquely regulated in response to environmental stresses. In most other fungi and in mammals, the Hog1 MAPK is unphosphorylated and localizes to the cytosol under unstressed condition, but is rapidly phosphorylated for translocation into the nucleus and activation of target genes. By contrast, in a number of C. neoformans strains, including the serotype A H99 strain, Hog1 is constitutively phosphorylated, found in both the cytosol and nucleus even under unstressed conditions, and rapidly dephosphorylated for activation by stress (Bahn, 2008). This type of Hog1 regulation is not observed in all C. neoformans strains. In a minority of C. neoformans strains, such as the serotype D JEC21 stain, Hog1 is regulated in a conventional manner similar to other fungi (Bahn, 2008). This difference appears to account for the different roles of the HOG pathway between C. neoformans strains. For instance, the hog1Δ mutant generated in the H99 strain background, but not the hog1Δ mutant generated in the JEC21 background, shows an increased sensitivity to high temperature (39~40℃) and hydrogen peroxide. In addition, the mating capability is enhanced in the hog1Δ mutant of the H99 strain, but not of the JEC21 strain. In the H99 strain background, mutation of genes in the HOG pathway induces pheromone production and cell-cell fusion in the early stage of mating (Bahn et al., 2005). The major benefit of having constitutively phosphorylated Hog1 appears to be increased stress-resistance. Notably, the C. neoformans strains with constitutively phosphorylated Hog1 are more stress-resistant than those having unphosphorylated Hog1 under unstressed condition.
The HOG pathway in C. neoformans not only responds to osmotic stress, but also controls diverse environmental stress responses against UV irradiation, oxidative stress, heavy metal stress, antifungal drugs, toxic metabolites, and high temperature (Bahn et al., 2005, 2006, 2007; Kojima et al., 2006; Bahn, 2008). Moreover, the HOG pathway is involved in controlling production of two major C. neoformans virulence factors, capsule and melanin, which are also regulated by the cAMP signaling pathway (Alspaugh et al., 1997, 2002; D'Souza et al., 2001; Bahn et al., 2004). Supporting this finding, DNA microarray analyses have shown that genes required for production of capsule, such as CAP10, CAP59, CAP60 and CAP64, and melanin, such as LAC1, were found to be induced following inactivation of the HOG pathway (Ko et al., 2009).
Recently, a genome-wide transcriptome analysis of the HOG pathway has led to the discovery of a number of HOG downstream target genes. In response to osmotic stress, the ENA1 gene, encoding a putative P-type ATPase sodium pump, was found to be mainly regulated by Ssk1 and Hog1 (Ko et al., 2009). In S. cerevisiae, Ena1 is required for long-term adaptation to osmotic shock and is also activated by Hog1 (Proft and Struhl, 2004). The C. neoformans ena1Δ mutant is hypersensitive to osmotic shock under glucose starvation and low pH conditions (Ko et al., 2009; Idnurm et al., 2009). Interestingly, Idnurm and co-workers reported that Ena1 is required for full virulence of C. neoformans (Idnurm et al., 2009). Following exposure to oxidative shock, a number of genes are regulated in a Hog1-dependent manner (Ko et al., 2009). These include SCH9, encoding a protein kinase, and UBC6-2 and UBC8, two genes that appear to be involved in the ubiquitin proteasome system. In S. cerevisiae, Sch9 kinase regulates transcription factors, such as Sko1, that respond to osmotic shock (Pascual-Ahuir and Proft, 2007). The C. neoformans sch9Δ mutant shows hypersensitivity to osmotic shock and fludioxonil, phenotypes that are distinguished from those of the hog1Δ mutant, indicating that Sch9 appears to be governed by both Hog and other pathways (Ko et al., 2009). The protein degradation pathway, including the ubiquitin proteasome system, is also commonly activated by oxidative stress in other eukaryotic organisms (Vandenbroucke et al., 2008).
The most notable observation of the HOG pathway transcriptome analysis is that it regulates genes involved in ergosterol biosynthesis. Inhibition of the HOG pathway, such as mutation of the SSK1 and HOG1 genes, increases the expression levels of a number of genes, such as ERG11, a gene involved in ergosterol biosynthesis. Supporting this finding, it was observed that cellular ergosterol contents increase by inhibiting the HOG pathway. Confirming this, the hog1Δ mutant was found to be resistant to azole drugs, such as fluconazole, but is hypersusceptible to amphotericin B (Ko et al., 2009). This provides an important combination antifungal therapeutic method, one which will increase efficacy but decrease toxicity of amphotericin B, a drug that is currently the most widely used for treatment of cryptococcosis.
The PKC/Mpk1 Pathway
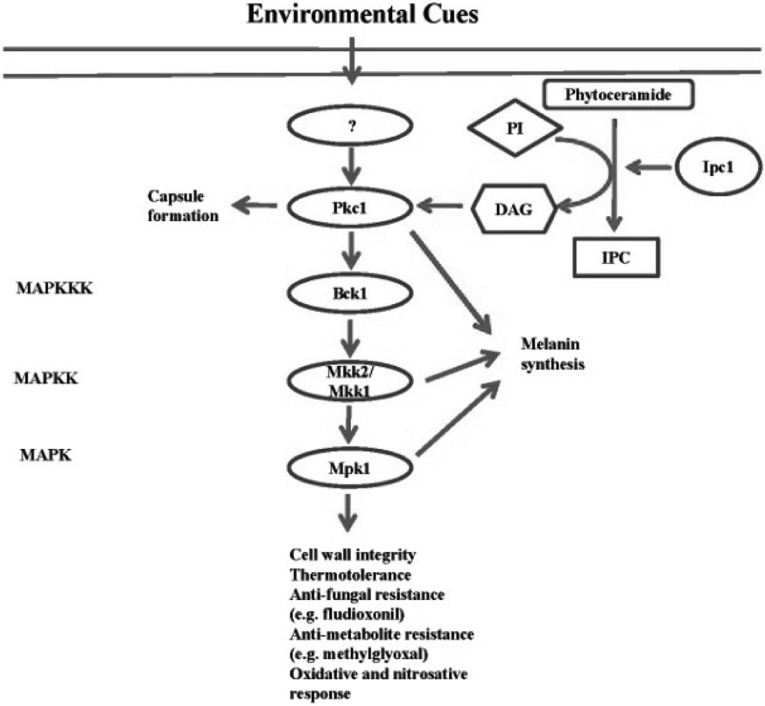
In C. neoformans, the PKC (Protein kinase C)/Mpk1 signaling pathway is involved in responding to a variety of environmental stresses, including osmotic stress, oxidative stress, nitrosative stress, and breaches of cell wall integrity. Moreover, deletion of the PKC1 gene induces an increase in capsule size and reduces the ability to synthesize melanin when compared to the wild-type. This pathway comprises several signaling components, including the small GTP-binding protein Rho1, PKC, Bck1 MAPKKK, Mkk1/Mkk2 MAPKK, and Mpk1 MAPK, a gene which is homologous to Slt2 in S. cerevisiae (Levin, 2005). It has been reported that DAG (diacylglycerol) functions as a second messenger activating mammalian protein kinase C. The production of DAG is regulated by Ipc1 (inositol-phosphorylceramide synthase-1) (Heung et al., 2004). Specifically, DAG influences melanin synthesis in C. neoformans by regulating Pkc1 (Fig. 2).
Fig. 2.
The C. neoformans PKC/Mpk1 pathway. The PKC (Protein kinase C)/Mpk1 pathway governs cell wall integrity and stress responses against nitrosative and oxidative damaging agents and anti-fungal drugs. This pathway is composed of several components including the small GTP-binding protein Rho1, PKC, Ser/Thr MAPKKK Bck1, MAPKK Mkk1/Mkk2, MAPK Mpk1, and Ipc1 (inositol-phosphorylceramide synthase-1), an enzyme which produces DAG (diacylglycerol) for activating PKC.
The PKC/Mpk1 pathway has been well characterized in S. cerevisiae. The PKC/Mpk1 MAPK pathway in S. cerevisiae regulates the organization of the actin cytoskeleton and cell wall integrity (Delley and Hall, 1999). Pkc1 plays a more crucial role in sensing and responding to environmental cues (Levin et al., 1990). In response to an oxidative stress, such as hydrogen peroxide and diamide, Pkc1 is activated by phosphorylation. This activation results in the formation of disulfide bonds in cytoskeletal proteins and induces disruption of the cell wall.
In C. neoformans, the PKC/Mpk1 pathway is also involved in responding to a diversity of stresses, including oxidative stress, and breaches of cell wall integrity (Fig. 2) (Gerik et al., 2008). Following exposure to reactive oxygen or nitrogen species (ROS and RNS, respectively), the Mpk1 MAPK is activated by phosphorylation downstream of the PKC pathway in C. neoformans. Activation of the Mpk1 MAPK is not observed in the pkc1Δ mutant following exposure to nitrosative stress (e.g. NaNO2), oxidative stress (e.g. hydrogen peroxide), or a cell wall damaging agent (e.g. calcoflour white), clearly indicating that phosphorylation of Mpk1 is governed by Pkc1 (Gerik et al., 2008). Recently, it has been shown that the bck1Δ, mkk1Δ and mpk1Δ mutants exhibited hypersensitivity to fludioxonil, methylglyoxal, hydrogen peroxide, and high temperature, but not osmotic stress (Bahn et al., 2007). Furthermore, the pkc1Δ mutant is hypersensitive to cell wall destabilizers such as SDS, calcoflour and congo red (Berridge et al., 2003), indicating that Pkc1 plays an essential role in maintaining cell wall integrity. These characteristics may be related to the hyper-susceptibility of the pkc1Δ mutant to high temperature (Gerik et al., 2008).
The PKC pathway influences capsule formation and melanin synthesis. Deletion of the PKC1 gene induces an increase in capsule size, a phenotype not observed in the bck1Δ, mkk2Δ and mpk1Δ mutants (Gerik et al., 2008). In addition, the pkc1Δ and mkk2Δ mutants show a reduction in melanin synthesis (Gerik et al., 2005, 2008). For regulation of melanin formation, the PKC pathway is activated by the sphingolipid pathway. The role of the PKC pathway in capsule and melanin biosynthesis could be related to the ability to maintain cell wall integrity since the capsule is deposited into the cell wall and a laccase, an essential enzyme for the production of melanin, is located in the cell wall (Zhu et al., 2001; Reese and Doering, 2003; Heung et al., 2004).
The Ca2+/Calcineurin Pathway
Ca2+ is an important secondary messenger governing a variety of stress responses in eukaryotic organisms. In C. neoformans, the Ca2+-mediated signaling pathway also plays a critical role in adaptation to certain environmental stresses, such as high temperature and cell wall stress (Fig. 3) (Fox and Heitman, 2002; Kraus and Heitman, 2003). In response to certain external cues, the concentration of cytosolic Ca2+ is changed, an event which results in the transmission of signals from the membrane to the nucleus. For example, a rise in concentration of cytosolic Ca2+ leads to the activation of PKC, an enzyme which functions in a variety of cellular responses including cellular growth and activation of transcription factors (Berridge et al., 2003).
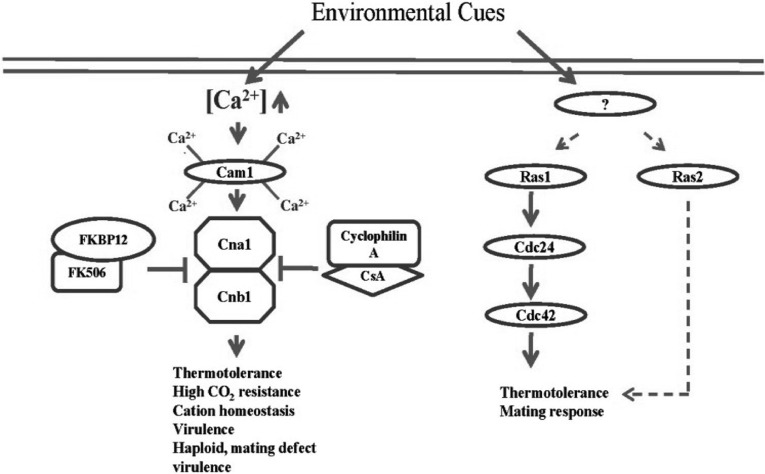
Fig. 3.
The Ca2+/calcineurin pathway and Ras-signaling pathway in C. neoformans. These two pathways are also involved in a variety of stress responses, including thermotolerance and pheromone-responsive mating. Calmodulin (Cam1) binds four Ca2+ molecules via four EF hand motifs. Subsequently, Ca2+-bound Cam1 activates calcineurin, a complex composed of a catalytic subunit A and a regulatory subunit B. Cyclophilin A and FK506-binding protein (FKBP12) bound with CsA and FK506, respectively, inhibit the activation of calcineurin. The Ras-signaling pathway governs thermotolerance in a Cdc24 (a guanine nucleotide exchange factor) and Cdc42 (GTPase)-dependent manner. Ras1 and Ras2 share some functions in both thermotolerance and mating.
The Ca2+ signaling pathway is well conserved from fungi to mammals (Kraus et al., 2005). In this pathway, calmodulin and calcineurin play essential roles in the stress response of fungi (Kraus et al., 2005). Calmodulin is a small cytosolic protein that binds four Ca2+ ions via the EF hand motifs forming a Ca2+/calmodulin complex that acts as a Ca2+ sensor (Kraus and Heitman, 2003). The bound Ca2+ induces a conformational change of the calmodulin, resulting in the release of free energy (Ikura, 1996). In S. cerevisiae, the Ca2+ signaling pathway is also involved in cellular mechanisms, such as mitosis (by regulation of Nuflp/Spc110p, stress responses), and cytoskeleton rearrangement (Chin and Means, 2000; Cyert, 2001).
Calcineurin, a Ser/Thr specific phosphatase, is the target of Ca2+ bound calmodulin and its function is well conserved among eukaryotic cells (Aramburu et al., 2000). The calmodulin-mediated activation of calcineurin is inhibited by chemical compounds such as cyclosporine A (CsA) and tacrolimus (FK506), two well known immunosuppressive drugs (Hemenway and Heitman, 1999). In the cell, CsA and FK506 form complexes with cyclophilin A and FK506-binding protein (FKBP12), respectively, and these complexes inhibit the action of calcineurin (Liu et al., 1991). Calcineurin is a heterodimer composed of catalytic subunit A (Cna1), containing autoinhibitory and calmodulin binding domains, and Ca2+ binding regulatory B subunit (Cnb1) (Watanabe et al., 1996).
The Ca2+/calcineurin pathway is also involved in a variety of stress responses in C. neoformans (Fig. 3). The CsA does not affect the viability of C. neoformans at low temperature, but does at 37℃. This indicates that calcineurin is not essential at low temperature, but indispensable during infection of the host (Odom et al., 1997). Furthermore, the cna1Δ mutant is hypersensitive to high CO2 concentration and pH 7.3, conditions which are essential for the cells to infect the host. As a result, the cna1Δ mutant is avirulent (Odom et al., 1997). Also, calcineurin A in C. neoformans is related to cation homeostasis. The mutant lacking calcineurin is sensitive to Li+ and provides resistance for Mn2+, which is the opposite phenotype observed in S. cerevisiae. This indicates that the function of calcineurin diverged between the species.
Calcineurin B (subunit B) in C. neoformans is also indispensable for growth at 37℃. The cnb1Δ has defects in haploid fruiting and mating response, phenotypes not seen in cna1Δ mutants (Fox et al., 2001). The cnb1Δ mutant is also avirulent like the cna1Δ.
The RAS Pathway
The Ras-signaling pathway is another important SAS pathway that governs thermotolerance of C. neoformans. Ras is a monomeric GTPase activated by replacement of bound GDP with GTP, an event mediated by a guanine nucleotide-exchange factor (GEF), or inactivated by a GTPase-activating protein (GAP) (Bourne et al., 1990; Bourne et al., 1991). The Ras-signaling pathway has been implicated in growth, differentiation, and stress response of eukaryotic organisms. In mammals, Ras regulates cell proliferation via the Erk MAPK and a mutation in the RAS gene is regarded as one of the main causes of human cancers (Barbacid, 1987). In fungi, Ras is also involved in a variety of cellular responses, including cell cycle regulation and cAMP production (Thevelein, 1994; Jiang et al., 1998). Although Ras is conserved from fungi to mammals, the role of Ras in cells varies among species. S. cerevisiae, for example, has two Ras proteins, Ras1 and Ras2 that control a variety of cellular responses, including the production of cAMP, regulation of MAPK signaling, diploid and haploid growth, and polarization of actin cytoskeleton (Toda et al., 1985; Gimeno et al., 1992; Stanhill et al., 1999; Ho and Bretscher, 2001). In Schizosaccharomyces pombe, Ras also controls the MAPK pathway in the pheromone response during mating, but is not involved in the production of cAMP (Nielsen et al., 1992; Hughes, 1995).
The Ras-signaling pathway is involved in cAMP signaling, environmental stress response, and pheromone response during mating in C. neoformans (Fig. 3) (D'Souza et al., 2001; Waugh et al., 2002, 2003). C. neoformans also expresses Ras1 and Ras2, and these two proteins not only have distinct roles in stress response, such as thermotolerance, but also share some functions (Waugh et al., 2002). Although mutating both RAS1 and RAS2 in S. cerevisiae causes lethality, C. neoformans ras1Δ ras2Δ double mutants are viable but with growth defects under normal conditions (Kataoka et al., 1984; Tatchell et al., 1984; Waugh et al., 2002). Ras1, but not Ras2, is required for growth at high temperature, mating response, and virulence (Alspaugh et al., 2000; Waugh et al., 2003). Overexpression of Ras2, however, partly restores the growth of the ras1Δ mutant at high temperature and completely suppresses its mating defect, indicating that these Ras proteins share some functions. Unlike other SAS pathways, however, the Ras-signaling pathway is not involved in production of virulence factors, such as melanin and capsule (Waugh et al., 2002).
Recently, the downstream signaling network of the Ras pathway was partly elucidated. The guanine nucleotide exchange factor Cdc24 was found to be the downstream effector of Ras1 (Nichols et al., 2007). The cdc24Δ mutant shows the same phenotype as the ras1Δ mutant in response to high temperature and actin polarization. The cdc24Δ ras1Δ double mutant shows no synergistic defect of growth at 37℃ compared to the cdc24Δ and ras1Δ, clearly indicating that Ras1 and Cdc24 share a common signal transduction pathway. Recently, Cdc42 was found to be the target of the Ras1-Cdc24 signaling complex in C. neoformans (Nichols et al., 2007).
Conclusion and Future Insights
C. neoformans is armed with various stress-activated signaling (SAS) pathways that promote survival of the pathogen in the harsh host environment. The SAS pathways of C. neoformans include the HOG, Ca2+/calcineurin, PKC/Mpk1 MAPK, and Ras-signaling pathways. Perturbation of the SAS pathways not only impairs the capability of C. neoformans to resist a variety of environmental stresses during host infection, but also affects production of virulence factors, such as capsule and melanin. A drug(s) targeted to signaling components of the SAS pathway will be effective for treatment of cryptococcosis. This therapeutic strategy, however, has the following problems. First, most of the central signaling components of the SAS pathways are evolutionarily conserved and therefore their inhibitors could have side-toxicity. Exceptions include components of the two-component system, which are fungal-specific. Further identification and characterization of the downstream targets of the SAS pathways by DNA microarray or proteomics approaches will likely provide more ideal targets for development of antifungal therapies. Secondly, drugs targeting the SAS pathway will not be fungicidal. This problem could only be overcome by using them in combination with other drugs. For example, fluconazole and fludioxonil are known to have synergistic antifungal activity with the calcineurin inhibitor FK506 (Del Poeta et al., 2000; Kojima et al., 2006). Furthermore, it has been shown that inhibition of the HOG pathway renders cells hypersensitive to amphotericin B (Ko et al., 2009). In conclusion, complete understanding of SAS pathways will provide unprecedented opportunities to develop novel antifungal therapy against C. neoformans.
Acknowledgements
This work was supported by the Korea Research Foundation grant funded by the Korean Government (MOEHRD; Basic Research Promotion Fund) (KRF-2008-331-C00245) and in part by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (R11-2008-062-02001-0).
References
- 1.Aksenov SI, Babyeva IP, Golubev VI. On the mechanism of adaptation of micro-organisms to conditions of extreme low humidity. Life Sci Space Res. 1973;11:55–61. [PubMed] [Google Scholar]
- 2.Alspaugh JA, Cavallo LM, Perfect JR, Heitman J. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol Microbiol. 2000;36:352–365. doi: 10.1046/j.1365-2958.2000.01852.x. [DOI] [PubMed] [Google Scholar]
- 3.Alspaugh JA, Perfect JR, Heitman J. Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev. 1997;11:3206–3217. doi: 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alspaugh JA, Pukkila-Worley R, Harashima T, Cavallo LM, Funnell D, Cox GM, Perfect JR, Kronstad JW, Heitman J. Adenylyl cyclase functions downstream of the Galpha protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot Cell. 2002;1:75–84. doi: 10.1128/EC.1.1.75-84.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aramburu J, Rao A, Klee CB. Calcineurin: from structure to function. Curr Top Cell Regul. 2000;36:237–295. doi: 10.1016/s0070-2137(01)80011-x. [DOI] [PubMed] [Google Scholar]
- 6.Bahn YS. Master and commander in fungal pathogens: the two-component system and the HOG signaling pathway. Eukaryot Cell. 2008;7:2017–2036. doi: 10.1128/EC.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahn YS, Geunes-Boyer S, Heitman J. Ssk2 mitogen-activated protein kinase kinase kinase governs divergent patterns of the stress-activated Hog1 signaling pathway in Cryptococcus neoformans. Eukaryot Cell. 2007;6:2278–2289. doi: 10.1128/EC.00349-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahn YS, Hicks JK, Giles SS, Cox GM, Heitman J. Adenylyl cyclase associated protein Aca1 regulates virulence and differentiation of Cryptococcus neoformans via the cyclic AMP-protein kinase A cascade. Eukaryot Cell. 2004;3:1476–1491. doi: 10.1128/EC.3.6.1476-1491.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahn YS, Kojima K, Cox GM, Heitman J. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol Biol Cell. 2005;16:2285–2300. doi: 10.1091/mbc.E04-11-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahn YS, Kojima K, Cox GM, Heitman J. A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans. Mol Biol Cell. 2006;17:3122–3135. doi: 10.1091/mbc.E06-02-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 12.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 13.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348:125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- 14.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 15.Chang YC, Cherniak R, Kozel TR, Granger DL, Morris LC, Weinhold LC, Kwon-Chung KJ. Structure and biological activities of acapsular Cryptococcus neoformans 602 complemented with the CAP64 gene. Infect Immun. 1997;65:1584–1592. doi: 10.1128/iai.65.5.1584-1592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang YC, Kwon-Chung KJ. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994;14:4912–4919. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang YC, Kwon-Chung KJ. Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect Immun. 1998;66:2230–2236. doi: 10.1128/iai.66.5.2230-2236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang YC, Kwon-Chung KJ. Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J Bacteriol. 1999;181:5636–5643. doi: 10.1128/jb.181.18.5636-5643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang YC, Penoyer LA, Kwon-Chung KJ. The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect Immun. 1996;64:1977–1983. doi: 10.1128/iai.64.6.1977-1983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang YC, Wickes BL, Kwon-Chung KJ. Further analysis of the CAP59 locus of Cryptococcus neoformans: structure defined by forced expression and description of a new ribosomal protein-encoding gene. Gene. 1995;167:179–183. doi: 10.1016/0378-1119(95)00640-0. [DOI] [PubMed] [Google Scholar]
- 21.Chen RE, Thorner J. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2007;1773:1311–1340. doi: 10.1016/j.bbamcr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10:322–328. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 23.Cyert MS. Genetic analysis of calmodulin and its targets in Saccharomyces cerevisiae. Annu Rev Genet. 2001;35:647–672. doi: 10.1146/annurev.genet.35.102401.091302. [DOI] [PubMed] [Google Scholar]
- 24.D'Souza CA, Alspaugh JA, Yue C, Harashima T, Cox GM, Perfect JR, Heitman J. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol Cell Biol. 2001;21:3179–3191. doi: 10.1128/MCB.21.9.3179-3191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Poeta M, Cruz MC, Cardenas ME, Perfect JR, Heitman J. Synergistic antifungal activities of bafilomycin A(1), fluconazole, and the pneumocandin MK 0991/caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685,818 against Cryptococcus neoformans. Antimicrob Agents Chemother. 2000;44:739–746. doi: 10.1128/aac.44.3.739-746.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delley PA, Hall MN. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J Cell Biol. 1999;147:163–174. doi: 10.1083/jcb.147.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernst JF. Transcription factors in Candida albicans - environmental control of morphogenesis. Microbiology. 2000;146(Pt 8):1763–1774. doi: 10.1099/00221287-146-8-1763. [DOI] [PubMed] [Google Scholar]
- 28.Fox DS, Heitman J. Good fungi gone bad: the corruption of calcineurin. Bioessays. 2002;24:894–903. doi: 10.1002/bies.10157. [DOI] [PubMed] [Google Scholar]
- 29.Fox DS, Cruz MC, Sia RA, Ke H, Cox GM, Cardenas ME, Heitman J. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Mol Microbiol. 2001;39:835–849. doi: 10.1046/j.1365-2958.2001.02295.x. [DOI] [PubMed] [Google Scholar]
- 30.Fraser JA, Subaran RL, Nichols CB, Heitman J. Recapitulation of the sexual cycle of the primary fungal pathogen Cryptococcus neoformans var. gattii: implications for an outbreak on Vancouver Island, Canada. Eukaryot Cell. 2003;2:1036–1045. doi: 10.1128/EC.2.5.1036-1045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerik KJ, Bhimireddy SR, Ryerse JS, Specht CA, Lodge JK. PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans. Eukaryot Cell. 2008;7:1685–1698. doi: 10.1128/EC.00146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerik KJ, Donlin MJ, Soto CE, Banks AM, Banks IR, Maligie MA, Selitrennikoff CP, Lodge JK. Cell wall integrity is dependent on the PKC1 signal transduction pathway in Cryptococcus neoformans. Mol Microbiol. 2005;58:393–408. doi: 10.1111/j.1365-2958.2005.04843.x. [DOI] [PubMed] [Google Scholar]
- 33.Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 34.Hemenway CS, Heitman J. Calcineurin. Structure, function, and inhibition. Cell Biochem Biophys. 1999;30:115–151. doi: 10.1007/BF02737887. [DOI] [PubMed] [Google Scholar]
- 35.Heung LJ, Luberto C, Plowden A, Hannun YA, Del Poeta M. The sphingolipid pathway regulates Pkc1 through the formation of diacylglycerol in Cryptococcus neoformans. J Biol Chem. 2004;279:21144–21153. doi: 10.1074/jbc.M312995200. [DOI] [PubMed] [Google Scholar]
- 36.Ho J, Bretscher A. Ras regulates the polarity of the yeast actin cytoskeleton through the stress response pathway. Mol Biol Cell. 2001;12:1541–1555. doi: 10.1091/mbc.12.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoang LM, Maguire JA, Doyle P, Fyfe M, Roscoe DL. Cryptococcus neoformans infections at Vancouver Hospital and Health Sciences Centre (1997~2002): epidemiology, microbiology and histopathology. J Med Microbiol. 2004;53:935–940. doi: 10.1099/jmm.0.05427-0. [DOI] [PubMed] [Google Scholar]
- 38.Hughes DA. Control of signal transduction and morphogenesis by Ras. Semin Cell Biol. 1995;6:89–94. doi: 10.1016/1043-4682(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 39.Idnurm A, Bahn YS, Nielsen K, Lin X, Fraser JA, Heitman J. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat Rev Microbiol. 2005;3:753–764. doi: 10.1038/nrmicro1245. [DOI] [PubMed] [Google Scholar]
- 40.Idnurm A, Walton FJ, Floyd A, Reedy JL, Heitman J. Identification of ENA1 as a virulence gene of the human pathogenic fungus Cryptococcus neoformans through signature-tagged insertional mutagenesis. Eukaryot Cell. 2009;8:315–326. doi: 10.1128/EC.00375-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikura M. Calcium binding and conformational response in EF-hand proteins. Trends Biochem Sci. 1996;21:14–17. [PubMed] [Google Scholar]
- 42.Jacobson ES, Emery HS. Catecholamine uptake, melanization, and oxygen toxicity in Cryptococcus neoformans. J Bacteriol. 1991;173:401–403. doi: 10.1128/jb.173.1.401-403.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang Y, Davis C, Broach JR. Efficient transition to growth on fermentable carbon sources in Saccharomyces cerevisiae requires signaling through the Ras pathway. EMBO J. 1998;17:6942–6951. doi: 10.1093/emboj/17.23.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kataoka T, Powers S, McGill C, Fasano O, Strathern J, Broach J, Wigler M. Genetic analysis of yeast RAS1 and RAS2 genes. Cell. 1984;37:437–445. doi: 10.1016/0092-8674(84)90374-x. [DOI] [PubMed] [Google Scholar]
- 45.Ko YJ, Yu YM, Kim GB, Lee GW, Maeng PJ, Kim S, Floyd A, Heitman J, Bahn YS. Remodeling of global transcription patterns of Cryptococcus neoformans genes mediated by the stress-activated HOG signaling pathways. Eukaryot Cell. 2009;8:1197–1217. doi: 10.1128/EC.00120-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kojima K, Bahn YS, Heitman J. Calcineurin, Mpk1 and Hog1 MAPK pathways independently control fludioxonil antifungal sensitivity in Cryptococcus neoformans. Microbiology. 2006;152:591–604. doi: 10.1099/mic.0.28571-0. [DOI] [PubMed] [Google Scholar]
- 47.Kraus PR, Heitman J. Coping with stress: calmodulin and calcineurin in model and pathogenic fungi. Biochem Biophys Res Commun. 2003;311:1151–1157. doi: 10.1016/s0006-291x(03)01528-6. [DOI] [PubMed] [Google Scholar]
- 48.Kraus PR, Nichols CB, Heitman J. Calcium- and calcineurin-independent roles for calmodulin in Cryptococcus neoformans morphogenesis and high-temperature growth. Eukaryot Cell. 2005;4:1079–1087. doi: 10.1128/EC.4.6.1079-1087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon-Chung KJ. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia. 1976;68:821–833. [PubMed] [Google Scholar]
- 50.Kwon-Chung KJ, Bennett JE. Distribution of alpha and alpha mating types of Cryptococcus neoformans among natural and clinical isolates. Am J Epidemiol. 1978;108:337–340. doi: 10.1093/oxfordjournals.aje.a112628. [DOI] [PubMed] [Google Scholar]
- 51.Kwon-Chung KJ, Polacheck I, Popkin TJ. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J Bacteriol. 1982;150:1414–1421. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwon-Chung KJ, Rhodes JC. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986;51:218–223. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwon-Chung KJ, Wickes BL, Stockman L, Roberts GD, Ellis D, Howard DH. Virulence, serotype, and molecular characteristics of environmental strains of Cryptococcus neoformans var. gattii. Infect Immun. 1992;60:1869–1874. doi: 10.1128/iai.60.5.1869-1874.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levin DE, Fields FO, Kunisawa R, Bishop JM, Thorner J. A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell. 1990;62:213–224. doi: 10.1016/0092-8674(90)90360-q. [DOI] [PubMed] [Google Scholar]
- 56.Lin X, Heitman J. The biology of the Cryptococcus neoformans species complex. Annu Rev Microbiol. 2006;60:69–105. doi: 10.1146/annurev.micro.60.080805.142102. [DOI] [PubMed] [Google Scholar]
- 57.Lin X, Hull CM, Heitman J. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature. 2005;434:1017–1021. doi: 10.1038/nature03448. [DOI] [PubMed] [Google Scholar]
- 58.Liu H, Cottrell TR, Pierini LM, Goldman WE, Doering TL. RNA interference in the pathogenic fungus Cryptococcus neoformans. Genetics. 2002;160:463–470. doi: 10.1093/genetics/160.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 60.Nichols CB, Perfect ZH, Alspaugh JA. A Ras1-Cdc24 signal transduction pathway mediates thermotolerance in the fungal pathogen Cryptococcus neoformans. Mol Microbiol. 2007;63:1118–1130. doi: 10.1111/j.1365-2958.2006.05566.x. [DOI] [PubMed] [Google Scholar]
- 61.Nielsen K, Cox GM, Litvintseva AP, Mylonakis E, Malliaris SD, Benjamin DK, Jr, Giles SS, Mitchell TG, Casadevall A, Perfect JR, Heitman J. Cryptococcus neoformans a strains preferentially disseminate to the central nervous system during coinfection. Infect Immun. 2005;73:4922–4933. doi: 10.1128/IAI.73.8.4922-4933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nielsen O, Davey J, Egel R. The ras1 function of Schizosaccharomyces pombe mediates pheromone-induced transcription. EMBO J. 1992;11:1391–1395. doi: 10.1002/j.1460-2075.1992.tb05184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nosanchuk JD, Rudolph J, Rosas AL, Casadevall A. Evidence that Cryptococcus neoformans is melanized in pigeon excreta: implications for pathogenesis. Infect Immun. 1999;67:5477–5479. doi: 10.1128/iai.67.10.5477-5479.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16:2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pascual-Ahuir A, Proft M. The Sch9 kinase is a chromatin-associated transcriptional activator of osmostress-responsive genes. EMBO J. 2007;26:3098–3108. doi: 10.1038/sj.emboj.7601756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Proft M, Struhl K. MAP kinase-mediated stress relief that precedes and regulates the timing of transcriptional induction. Cell. 2004;118:351–361. doi: 10.1016/j.cell.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 67.Pukkila-Worley R, Gerrald QD, Kraus PR, Boily MJ, Davis MJ, Giles SS, Cox GM, Heitman J, Alspaugh JA. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot Cell. 2005;4:190–201. doi: 10.1128/EC.4.1.190-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reese AJ, Doering TL. Cell wall alpha-1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol Microbiol. 2003;50:1401–1409. doi: 10.1046/j.1365-2958.2003.03780.x. [DOI] [PubMed] [Google Scholar]
- 69.Rosas AL, Casadevall A. Melanization affects susceptibility of Cryptococcus neoformans to heat and cold. FEMS Microbiol Lett. 1997;153:265–272. doi: 10.1111/j.1574-6968.1997.tb12584.x. [DOI] [PubMed] [Google Scholar]
- 70.Stanhill A, Schick N, Engelberg D. The yeast Ras/cyclic AMP pathway induces invasive growth by suppressing the cellular stress response. Mol Cell Biol. 1999;19:7529–7538. doi: 10.1128/mcb.19.11.7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sukroongreung S, Kitiniyom K, Nilakul C, Tantimavanich S. Pathogenicity of basidiospores of Filobasidiella neoformans var. neoformans. Med Mycol. 1998;36:419–424. [PubMed] [Google Scholar]
- 72.Tatchell K, Chaleff DT, DeFeo-Jones D, Scolnick EM. Requirement of either of a pair of ras-related genes of Saccharomyces cerevisiae for spore viability. Nature. 1984;309:523–527. doi: 10.1038/309523a0. [DOI] [PubMed] [Google Scholar]
- 73.Thevelein JM. Signal transduction in yeast. Yeast. 1994;10:1753–1790. doi: 10.1002/yea.320101308. [DOI] [PubMed] [Google Scholar]
- 74.Toda T, Uno I, Ishikawa T, Powers S, Kataoka T, Broek D, Cameron S, Broach J, Matsumoto K, Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985;40:27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- 75.Tscharke RL, Lazera M, Chang YC, Wickes BL, Kwon-Chung KJ. Haploid fruiting in Cryptococcus neoformans is not mating type alpha-specific. Fungal Genet Biol. 2003;39:230–237. doi: 10.1016/s1087-1845(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 76.Vandenbroucke K, Robbens S, Vandepoele K, Inze D, Van de Peer Y, Van Breusegem F. Hydrogen peroxide-induced gene expression across kingdoms: a comparative analysis. Mol Biol Evol. 2008;25:507–516. doi: 10.1093/molbev/msm276. [DOI] [PubMed] [Google Scholar]
- 77.Vartivarian SE, Anaissie EJ, Cowart RE, Sprigg HA, Tingler MJ, Jacobson ES. Regulation of cryptococcal capsular polysaccharide by iron. J Infect Dis. 1993;167:186–190. doi: 10.1093/infdis/167.1.186. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y, Aisen P, Casadevall A. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect Immun. 1995;63:3131–3136. doi: 10.1128/iai.63.8.3131-3136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Casadevall A. Decreased susceptibility of melanized Cryptococcus neoformans to UV light. Appl Environ Microbiol. 1994;60:3864–3866. doi: 10.1128/aem.60.10.3864-3866.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Watanabe Y, Perrino BA, Soderling TR. Activation of calcineurin A subunit phosphatase activity by its calcium-binding B subunit. Biochemistry. 1996;35:562–566. doi: 10.1021/bi951703+. [DOI] [PubMed] [Google Scholar]
- 81.Waugh MS, Nichols CB, DeCesare CM, Cox GM, Heitman J, Alspaugh JA. Ras1 and Ras2 contribute shared and unique roles in physiology and virulence of Cryptococcus neoformans. Microbiology. 2002;148:191–201. doi: 10.1099/00221287-148-1-191. [DOI] [PubMed] [Google Scholar]
- 82.Waugh MS, Vallim MA, Heitman J, Alspaugh JA. Ras1 controls pheromone expression and response during mating in Cryptococcus neoformans. Fungal Genet Biol. 2003;38:110–121. doi: 10.1016/s1087-1845(02)00518-2. [DOI] [PubMed] [Google Scholar]
- 83.Wickes BL, Mayorga ME, Edman U, Edman JC. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the alpha-mating type. Proc Natl Acad Sci USA. 1996;93:7327–7331. doi: 10.1073/pnas.93.14.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zaragoza O, Fries BC, Casadevall A. Induction of capsule growth in Cryptococcus neoformans by mammalian serum and CO2. Infect Immun. 2003;71:6155–6164. doi: 10.1128/IAI.71.11.6155-6164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu X, Gibbons J, Garcia-Rivera J, Casadevall A, Williamson PR. Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infect Immun. 2001;69:5589–5596. doi: 10.1128/IAI.69.9.5589-5596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu X, Williamson PR. Role of laccase in the biology and virulence of Cryptococcus neoformans. FEMS Yeast Res. 2004;5:1–10. doi: 10.1016/j.femsyr.2004.04.004. [DOI] [PubMed] [Google Scholar]