Abstract
The decline of the immune system appears to be an intractable consequence of aging, leading to increased susceptibility to infections, reduced effectiveness of vaccination and higher incidences of many diseases including osteoporosis and cancer in the elderly. These outcomes can be attributed, at least in part, to a phenomenon known as T cell replicative senescence, a terminal state characterized by dysregulated immune function, loss of the CD28 costimulatory molecule, shortened telomeres and elevated production of pro-inflammatory cytokines. Senescent CD8 T cells, which accumulate in the elderly, have been shown to frequently bear antigen specificity against cytomegalovirus (CMV), suggesting that this common and persistent infection may drive immune senescence and result in functional and phenotypic changes to the T cell repertoire. Senescent T cells have also been identified in patients with certain cancers, autoimmune diseases and chronic infections, such as HIV. This review discusses the in vivo and in vitro evidence for the contribution of CD8 T cell replicative senescence to a plethora of age-related pathologies and a few possible therapeutic avenues to delay or prevent this differentiative end-state in T cells. The age-associated remodeling of the immune system, through accumulation of senescent T cells has far-reaching consequences on the individual and society alike, for the current healthcare system needs to meet the urgent demands of the increasing proportions of the elderly in the US and abroad.
Keywords: telomere, telomerase, T cells, immune, human, latent infection, aging, HIV/AIDS, immunosenescence, replicative senescence
Introduction
For the first time in history, older adults constitute the fastest growing population in the world. Projections indicate that by 2025 the cohort over age 65 will be increasing 3.5 times as rapidly as the total population [1] and the proportion of individuals age 60 years and older, which accounted for 10% of the world population in 2000, will increase to approximately 22% of the world population by 2050. Consequently, scientific research on age-related maladies needs to rapidly progress to meet the medical and health-related needs of the progressively increasing elderly population. Moreover, in order to develop appropriate preventative and therapeutic strategies, it is essential that we understand the underlying biological mechanisms that contribute to age-related diseases
Aging is a highly complex and continuous process that affects almost all organ systems, causing molecular and physiological changes, both qualitatively and quantitatively. With age, the immune systems undergoes an extensive but gradual process of remodeling dictated by both genetic and intrinsic events, such as the shrinking of the thymus, and environmental factors, primarily mediated by the lifetime encounters of the body to foreign antigens. These factors synergize with age and result in major alterations in immune function that have been implicated in multiple deleterious outcomes.
It is generally recognized that the function of the immune system declines with age, a process characterized by distinct and dramatic changes on both a cellular and systemic level. This so-called immunosenescence is associated with increased incidence of age-related pathologies, such as osteoporosis, Alzheimer’s disease, cancer and autoimmune disorders [2–5]. Older persons also have an impaired response to vaccinations, which have an efficacy of only 30–40% in this population [6]. In addition to the blunted immune response to vaccination intended to prevent infections, older persons show reduced ability to fight actual infections. Indeed, according to the United States Centers of Disease Control influenza and pneumonia rank as the fifth leading cause of death in persons over the age of 65 [7]. There is also evidence that aging affects the hematopoietic stem cell population; bone marrow from older donors fails to fully reconstitute the immune system of transplant recipients [8]. Morbidity and the prolonged duration of illness are also substantially increased with age. Interestingly, more than 50 years ago, the late Dr. Roy Walford, a pioneer of aging research and caloric restriction studies, proposed that chronological aging is directly related to dysfunctions in immunological processes and thus leads to age-related pathologies [9, 10]. This “immunologic theory of aging” has been confirmed more recently, in longitudinal studies of elderly people, which document significant correlations between specific immune features and all-cause mortality [11].
Immunosenescence describes a multifaceted and dynamic pattern of changes within the immune system during aging, leading to defects in both adaptive and innate immunity. One of the most important age-related immune changes is the impaired generation of primary CD8 T cell responses against infection and reduced vaccine efficacy, documented in mice [10, 12–15], rhesus macaques [16], and humans (reviewed in [17]). Other major alterations that characterize immunosenescence include the reduced number and impaired function of hematopoietic stem cells, diminished amount of circulating naïve T cells, inverted CD4/CD8 ratio and increased levels of proinflammatory cytokines, such as IL-6 and TNFα [18]. There is also a shift from Th1 to Th2 cytokine profiles following T cell activation, which may shift the balance between humoral and cell-mediated immune responses. This altered cytokine profile is likely due to an increased ratio of memory to naive T cells [19, 20]. Another dramatic change observed in the elderly compared to the young is the dramatically reduced T cell production of IL-2 [21], an observation consistent with data from senescent T cell cultures. Although the number of T cell receptors (TCRs) at the individual cell level does not to decline with age, the TCR signal transduction pathways are significantly altered, resulting in impaired transcription of key effector T cell genes [22]. Phosphorylation of critical STAT proteins involved in T cell maintenance is also reduced or delayed in T cells from 90 year-old individuals, a further indication of age-associated T cells defects [23]. Age-related changes within the immune system, as with other complicated diseases and disorders, does not occur at the same pace in all components, or among different individuals, and is strongly influenced by environmental, genetic and epigenetic factors that ultimately shape the severity and magnitude of the dysfunction.
Arguably, the most significant age-related change in the human immune system is the quality and phenotype of the CD8 or so-called cytotoxic T lymphocyte (CTL) subset. With age and in chronic infections such as human immunodeficiency virus (HIV), the majority of CD8 T cells become antigen-experienced and acquires a dysregulated, pro-inflammatory phenotype. These late-differentiated CD8 T cells possess many features of cellular replicative senescence that have been characterized in long-term in vitro cultures [24].
Replicative senescence refers to the process by which normal somatic cells reach an irreversible stage of cell cycle arrest following multiple rounds of replication; this end stage is associated with marked changes in gene expression and function [25]. The parallel phenotypic and functional changes documented in T cells from aged individuals and those observed in T cells driven to replicative senescence in vitro suggests that the in vitro replicative senescence experimental system can be exploited further to elucidate the various factors that contribute to and that may modulate human immunosenescence. Currently, it is known that among the prominent causal agents of T cell replicative senescence in vivo are persistent viruses and tumor antigens.
Several excellent discussions of inflammation and its role in immunosenescence and aging have been covered by other reviews [18, 26, 27]. Here, we will first briefly summarize the main features of the immune system, then discuss the process of T cell replicative senescence and telomerase/telomere dynamics. We will follow with a summary of the current research bridging senescent T cells to several age-related pathologies. Finally the review will conclude with a few lingering, but significant, questions and suggested avenues for future research.
Immunology basics
The primary purpose of the immune system is to maintain and protect our health, by fighting off the glut of pathogens we encounter throughout our lifetime. There are two components of immunity. The innate system comprised of natural killer (NK) cells, macrophages, dendritic cells (DCs), and complement factors, functions relatively non-specifically, but rapidly and efficiently. This immune compartment serves as the first line of defense against environmental pathogens. By contrast, the adaptive component, comprised of T and B cells, requires more time to mount a biochemical response, but utilizes extremely specific targeting to eliminate foreign invaders. Importantly, adaptive immunity allows for the development of immunological memory that is a crucial in both preventing recurring infection by the same strain of pathogen and for the prophylactic effects of vaccination. The innate and adaptive immune cells respond in concert through extensive crosstalk between the two systems. For example, cytokines secreted by different immune cells modulate the activity of innate and adaptive immune cells. Furthermore, the adaptive immune response begins its assault only after it has received signals from the innate component, and cells of the innate system are instructed by the adaptive immune compartment to eliminate weakened or injured pathogens and to clear cell debris. These evolution-driven, complementary components of the human immune system normally provide adequate protection against most bacteria, viruses, and parasites present in the environment.
The key mediators of the adaptive immune response are lymphocytes. T cells, along with B cells, derive from hematopoietic stem cells found in the bone marrow. Through a series of recombination events of variable and constant gene segments encoding different V, D, and J regions, a receptor molecule is formed that is unique to that cell [28]. In this way, a hundred different gene segments can create thousands of distinct receptor chains. Moreover, greater diversity is achieved by pairing two different chains encoded by different genes—in T cells, the chains are the α and β chain—to form a functional antigen receptor. As a result, an astonishing 108 different specificities may be generated to recognize the diverse epitopes of foreign antigens, enabling the immune system to respond to the myriad of different epitopes characterizing unique pathogens [29, 30]. After the cells undergo these intricate gene recombination events and passing through stringent selection tests within the thymic microenvironment, to eliminate inadequate or self-recognizing thymocytes, mature T cells then emigrate out of the thymus into peripheral tissue.
Activation and Clonal Expansion of lymphocytes
Induction of a primary adaptive immune response usually begins when antigen-presenting cells (APC) such as dendritic cells (DC) of the innate immune system engulf pathogens and present the pathogen antigens to naive T cells. TCRs on the T cell surface recognize the antigen bound to major histocompatibility complex (MHC) molecules on the surface of APCs [31]. T cells that possess a TCR specific for the antigen-MHC complex are activated to proliferate (a process known as clonal expansion) and differentiate into effector T cells through induction of many pathways downstream of the TCR and CD28, a co-stimulatory molecule essential for optimal T cell activation. By this process, relevant T cells give rise to many clones with the ability to secrete pro-inflammatory, antiviral cytokines and cytotoxic enzymes to eliminate the pathogens during the first 1–2 weeks of infection [32, 33]. Proliferation of helper CD4 T cells instruct other immune cells such as B cells to clonally expand and differentiate into antibody-producing plasma cells. The antibodies neutralize, or block the pathogen’s ability to access human cells by coating the pathogen and thereby targeting it for phagocytosis by APCs (a process known as opsonization), or by activating the complement system, which promotes pathogen destruction. Pathogens that are not eliminated by the above mechanisms go on to infect cells and then become targeted by CTL. After the peak of immune cell expansion and clearance of the pathogen, 90–95% of antigen specific-T cells undergo cell-mediated apoptosis in a process termed ‘contraction.’ The remaining 5–10% of T cells differentiates into a pool of long-lived memory CD8 T cells that persist at low frequencies but retain cytotoxic effector functions and high proliferative potential, allowing them be on constant surveillance and prevent re-infection of the host by the same virus [33, 34].
Because of the enormous antigenic receptor repertoire, only a very small number of cells recognize and respond to any single antigen. Therefore, the ability to clonally expand in order to generate a sufficient and effective immune response is a paramount aspect of adaptive immunity and the clearance of infection. However, normal somatic cells cannot infinitely divide because of a cell-intrinsic phenomenon known as the Hayflick limit, which is thought to have evolved to protect against the development of cancers [35]. This strict limitation of proliferative potential, referred to as replicative senescence, although possibly beneficial as a tumor suppressor mechanism, has the potential to negatively impact the function of the immune system, as will be discussed in detail below.
CD8 T cell replicative senescence: in vitro studies
In 1961, Drs. Hayflick and Moorhead first described the phenomenon of replicative senescence in cultures established from human lung fibroblasts. Thereafter, many researchers went on to investigate this process in other somatic cell types, including epithelial cells, hepatocytes, endothelial cells and keratinocytes. In vitro senescence is defined by a limited proliferative capacity of somatic cells, resulting in arrested cell cycling and a population of non-dividing, but fully viable senescent cells [36]. However, only recently has this process been examined in the context of the immune system. At first glance, it may seem counterintuitive that there is a limited proliferative capacity of immune cells, since the ability to undergo rapid, extensive, and often repeated rounds of clonal expansion is absolutely essential to their protective functions. However, the observed decline of T cell immune function in elderly persons suggests that T cells may undergo replicative senescence as human beings age chronologically. Indeed, in vitro studies on senescence in T cells have delineated well-characterized and invariable changes in cell surface markers, signal transduction pathways, and functional traits that are similar to T cells from elderly people, as well as to T cells from younger persons chronically infected with HIV. Importantly the presence of various types of senescent cells, including those found in the immune system, have been shown to have some negative health effects, due, at least in part, to their production of pro-inflammatory molecules and cytotoxic enzymes [24, 37].
Following multiple rounds of stimulation with allogeneic or peptide-pulsed autologous antigen-presenting cells (APCs), or with antibody-coated microbeads that mimic APCs, CD8 T cells are driven to senescence in a fairly predictable manner in vitro. Cultured senescent T cells share similar functional changes with other senescent somatic cells including the expression of cell cycle inhibitors, inability to undergo apoptosis (a type of programmed cell death), shortened telomeres, and a pro-inflammatory secretion pattern [38–41]. This arrest is caused by the erosion of telomeres, the repeating hexameric sequences of nucleotides at the ends of linear chromosomes, as cells undergo replication. If telomerase, a holoenzyme that elongates telomeres, declines in activity, the chromosome is recognized as a double strand break and can no longer proliferate [42]. However, senescent T cells still retain some normal effector function, as described in earlier reviews [43, 44]. For instance, senescent CD8 T cells are capable of upregulation of CD25, the alpha chain of the IL-2 receptor, when exposed to antigen [45] and maintain MHC-unrestricted cytotoxicity [46]. With adequate activation and constant exposure to IL-2, which is necessary for survival in cell culture, normal human T cells can undergo between 25 and 40 population doublings, depending on the cell donor, before reaching senescence [47], with an average culture lifespan of approximately 33 population doublings [48].
A critical phenotypic and genetic change as T cells become late-differentiated and progress to senescence is the loss of gene and surface expression of CD28, an essential co-stimulatory receptor that activates T cell pathways such as the Akt and NFκB pathway [49, 50], and modulates important functions such as lipid raft formation, IL-2 gene transcription, apoptosis, stabilization of cytokine mRNA, glucose metabolism and cell adhesion [51]. Therefore, downregulation of CD28 would fundamentally alter normal T cell function, survival, and proliferation. Indeed, senescent T cell cultures were observed to be >95% CD28-negative within the CD8 T cell subset, as compared to over 90% positive in the starting population from healthy, young donors [52]. Although CD28 expression is dynamic and is regulated, in part, by protein turnover and transient transcriptional repression, the loss of CD28 on the cell’s surface and transcription is eventually permanent when senescence is reached. This loss of CD28 parallels the low percentage of CD28+ CD8 T cells found in aged individuals and those afflicted with HIV infections [53, 54].
Because CD28 is necessary to mount effective T cell immune responses, we hypothesized that sustained transcription of CD28 in long-term cultures of CD8 T cells in the previously described in vitro model may prevent the phenotypic and proliferative changes characteristic of replicative senescence. Successfully transduced cells did, in fact, exhibit higher initial mRNA levels and surface expression of CD28, as well as prolonged telomerase activity and significantly increased proliferative potential. Moreover, the senescence-associated proinflammatory cytokine production profile was also prevented. However, proliferation was not indefinite, and the cells eventually stopped dividing and lost CD28 surface, but not gene, expression. At that point, CTLA-4, an inhibitory receptor on T cells that can compete with the CD28 ligands on APC, was upregulated. Fundamental qualities of replicative senescence, including cell cycle arrest, were eventually observed in these cultures, suggesting that sustained transcription of CD28, while significantly delaying senescence, ultimately failed to permanently prevent this process [55].
Another significant feature of senescent CD8 T cells in cell culture is the resistance to apoptosis, consistent with the original findings in fibroblasts [56]. Senescent and late passage T cells exhibited reduced ability to undergo apoptosis as compared to quiescent early passage cultures from the same donor when exposed to six different apoptotic stimuli [39]. Furthermore, culture-driven senescent CD8 T cells expressed less Hsp70, a heat shock protein that promotes survival of cells under stress [57], consistent with previous observations in various cell types such as fibroblasts [58]. This feature parallels in vivo findings, which also documented an apparent decrease in Hsp70 with increasing age [59].
Although replicative senescence is manifested by decline in immune function, in vitro senescent CD8 T cells actually retain some, albeit dysregulated, effector functions in response to antigenic stimulation. As CD8 T cells are driven to senescence in cell culture, they produce more proinflammatory cytokines, mainly IL-6 and TNFα, than their more “youthful” early-passage counterparts [24]. Interestingly, these same inflammatory cytokines are strongly associated with frailty in the elderly [60]. Treatment of T cells with exogenous TNFα in long-term cultures has been shown to accelerate acquisition of senescent characteristics, including downregulation of CD28 gene expression, telomerase activity and IL-2 message [61]. Thus, it is possible that senescent T cells present in vivo may negatively affect other non-senescent T cells by secreting an inflammatory cytokine that promotes loss of CD28 and accelerated senescence. Unlike IL-6 and TNFα, the anti-viral cytokine IFNγ decreases with culture age and antigenic stimulation, diminishing the ability of senescent CD8 T cells to kill virally infected cells [38, 62, 63]. Together, the data suggest that senescent T cells not only fail in their effector function against viral infection, but may also impact the anti-viral function of other T cells, by promoting their premature senescence.
It is important to note, however, that although chronological aging and infections such as HIV, which constitute accelerated aging, are correlated with the accumulation of T cells with senescence markers, there is no clear correlation between in vitro lifespan of T cell cultures and chronological age of the donor [45]. Nevertheless, the accumulated data from multiple lines of research reveals that senescent T cells in long-term cell culture studies do mimic many facets of T cell dysfunction observed in elderly persons, cancer patients, and individuals harboring chronic/persistent viral infections.
The role of telomeres and telomerase in aging
Telomeres are tandem sequences of (TTAGGG)n nucleotide repeats that serve as protective ends of chromosome. Functional telomeres are essential for continued cell proliferation. Because of the incomplete replication of the lagging strand during DNA synthesis, termed the “end replication problem” [64], telomeres can lose up to 200 base pairs in somatic cells with each cell division [65–68]. Progressive telomere erosion eventually leads to the chromosome reaching a critical point at which the exposed DNA end is recognized as a double strand break and cannot pass cell cycle check points [42]. This mechanism—by inducing either senescence or apoptosis-- prevents potentially genetically unstable cells from proliferating indefinitely and developing into cancer. Telomere sequences can be restored by a ribonucleoprotein called telomerase by adding TTAGGG repeats onto the telomeres using its intrinsic RNA, TERC, as a template for reverse transcription [69]. Telomere attrition is not likely to be a linear process, since studies of T cells isolated ex vivo have demonstrated that only after the age of 40 do human cells enter a phase of accelerated telomeric shortening [70].
Telomerase is repressed in most human somatic cells after birth. However, lymphocytes possess the ability to upregulate telomerase upon activation, which allows the extensive proliferation required to combat infection, while maintaining telomere lengths [71]. Similar to this in vivo demonstration of telomerase activation during acute viral infection, ex vivo human CD8 T cells activated with antibodies against CD3 and CD28 have high initial telomerase activity and are able to maintain their initial 10–11kb telomere length through multiple rounds of cell division. Nevertheless, with repeated rounds of stimulation, their telomere lengths progressively shorten to approximately about 5–7kb [40, 48] and their telomerase activity becomes undetectable [72]. Interestingly, in contrast to the CD8 subset, human CD4 T cells do not undergo the same dramatic decline in telomerase activity in vitro [72]. Shortened telomeres and reduced telomerase activity are hallmark features of T cell senescence in vitro and has been associated with many pathological ailments in vivo, which will be discussed in detail later on in this review.
This loss of telomerase activity in vitro parallels loss of CD28 surface expression [72, 73]. Blocking the binding of CD28 to its receptors, B7-1 and B7-2 ligand, on APCs during stimulation significantly reduces telomerase activity, suggesting a role of CD28 in telomerase regulation. Consistent with this observation, sorted CD28+ T cells were found to have significantly greater telomerase activity than the unseparated T cell population after activation in vitro [72]. The outcome of telomerase activity differences based on CD28 expression is that CD28− T cells have shorter telomeres than their respective CD28+ counterparts from the same donor, findings reported by several groups [53, 74, 75]. A recent study that utilized retroviral vectors to constitutively express CD28 in long-term cultures demonstrated enhanced and prolonged telomerase activity as compared to the vector-control transduced T cells [76]. Together, these reports strongly implicate CD28 signaling in the modulation of telomerase and thus maintenance of telomere length.
The central role of telomerase in linking senescence at the cellular level with physiological aging is strikingly illustrated by the multiple abnormalities in persons with the autosomal dominant form of dyskeratosis congenital, a disease caused by mutation in the gene encoding the RNA component of telomerase, TERC [77]. The defects in these patients are most dramatic in tissues in which cells divide rapidly and often, such as hair follicles, skin, lungs, gut and bone marrow. In addition, these patients exhibit numerous age-related pathologies including higher rates of malignancies. Another clinical example is the premature aging syndrome known as childhood progeria, in which individuals have mutated or shortened telomeres. Children with progeria appear old and die of age-related diseases such as heart disease. Genetically engineered mice, which lack telomerase, have been used to analyze progeria in humans. These animals age far more rapidly than normal mice, suffering from multiple age-related conditions, such as osteoporosis, diabetes and neurodegeneration. However, when the inactivated telomerase was switched back on by feeding the mice a chemical called 4-OHT, there was a dramatic reversal of the age-related phenotype: the animals regained their fertility, and other organs such as the brain, spleen, liver and intestines showed correction of their degenerated state [78].
Studies performed on in vitro cultured cells clearly demonstrate that ectopic expression catalytic subunit of telomerase (hTERT) is sufficient to prevent the senescence-induced end-stage and allow proliferation. In addition to fibroblasts and endothelial cells, T cells have also been shown to be amenable to telomerase manipulation [79, 80]. Stable hTERT expression in long-term cultures of HIV-specific CD8 T cells resulted in enhanced proliferative potential and maintenance of telomere length compared to control transduced cultures. Importantly, the telomerized HIV-specific T cells also demonstrated greater inhibition of HIV replication and delayed loss of IFNγ production in the presence of HIV peptides, suggesting that telomerase is absolute essential not only for proliferation, but also for effective immune function [38].
Taken together, these studies suggest that inducing telomerase activity might have positive consequences in T cells that are reactive to viruses such as HIV. As proof of this principle, CD8 T cells from HIV-infected persons were exposed in cell culture to a plant-derived small molecule telomerase activator (“TAT2”). These cells showed upregulation of telomerase activity, retardation of telomere shortening, greater proliferative potential, as well as increase in functional cytokine and chemokine production [81]. When a specific telomerase inhibitor was included along with TAT2 in the culture, all these effects were totally abrogated. These in vitro studies underscore the potential beneficial therapeutic applications of maintaining telomerase activity as an approach to retarding the loss of immune control over infection.
Aging and replicative senescence in vivo
Current animal models of aging are somewhat limited in terms of elucidating certain mechanisms underlying human aging. For instance, mitogen-stimulated murine T cells do become senescent in terms of reduced proliferative potential [82], but cell cycle arrest occurs only after 10–15 doublings, long before significant telomere erosion occurs [83, 84]. Furthermore, humans encounter a plethora of pathogens throughout their lifetime, a process that continually molds the T cell specificity repertoire and affects the quality of their immune system, particularly during old age. It is obvious that this situation cannot be mimicked in short-lived laboratory animals that are protected from the hugely diverse spectrum of bacteria, viruses and parasites to which humans are exposed over many decades.
The reduced adaptive immune function observed with biological aging is, in large part, caused by thymic involution, the shrinking of the thymus, and a decline in naïve T and B cell output from the bone marrow. This leads to a shift in the lymphocyte population composition towards the accumulation of memory T cells. Furthermore, within the total memory cell population, there are changes in the proportional representation of these antigen-experienced cells. Specifically, memory T cells from older persons are often late differentiated or senescent, and are clonally restricted. This phenotype likely results, in part, from the lifetime exposure to prevalent chronic viral infections such as cytomegalovirus (CMV) and Epstein-Barr virus (EBV). These herpes viruses infect the majority of the population early in life, suggesting that they may be the primary drivers of replicative senescence in vivo. Although CMV and EBV are not life threatening, and often remain in a latent state, they persist over many decades and are never eliminated by the immune system. In fact, adaptive immune cells may be working over-time to suppress these viruses and prevent their reemergence. In this way, the constant interaction with herpes virus viral peptides and suppression of viral replication leads to chronic, low levels of T cell activation, proliferation and ultimately senescence and the resultant inflammatory milieu.
Within the total memory pool, arguably, the most dramatic functional changes occur in the CD8+ T cell subset. Similar to what is observed in long term in vitro cultures, there is a progressive loss of CD28 expression, an overall telomere shortening, and a decline in IL-2 production by T cells isolated from the elderly. Studies have shown that older persons have higher proportions of CD28-negative, late-differentiated cells within the CD8 T cell subset (>60%) compared to <10% in younger adults [52]. Importantly, telomere studies have confirmed that the CD28-negative cells had undergone extensive cell division compared to CD28+ T cells from the same individual. Furthermore, within the elderly population, individuals infected with herpes viruses have higher percentage of CD8+CD28− T cells than uninfected persons [85].
In addition to aging itself, autoimmune diseases, chronic infections, and cancer are also thought to drive immune cells towards late-differentiation and replicative senescence. For example, persons with rheumatoid arthritis, an autoimmune disease that leads to excess inflammation of the synovium, have elevated levels of CD28-negative T cells in affected joints, and these cells secrete higher levels of TNFα, one of the cytokines associated with senescence. Interestingly, TNFα has been shown to suppress CD28 gene transcription, suggesting that secretion of this cytokine by senescent cells may function to drive other T cells in the joint to replicative senescence [86]. As noted previously, another chronic infection associated with senescence is HIV, where there is accumulation of CD28-negative cells within the CD8 T cell subset as compared to unaffected age-matched controls [87]. Moreover, telomere length of CD8+CD28− T cells from a group of ~40-year-old HIV+ persons are in the same range as those of lymphocytes isolated from uninfected centenarians [74]. Thus, HIV/AIDS has been proposed as a model of accelerated or premature immunosenescence [88]. Finally, there is accumulating evidence that replicative senescence may play a role in tumor biology as well as cancer immunotherapy, as will be discussed later on.
In addition to the loss of CD28 expression, in vitro replicative senescence is associated with the upregulation of a number of proteins including the cyclin dependent kinase (Cdk) inhibitors p21CIP1/WAF1 (p21) and p16INK4a (p16) [89–92]. More specifically, p21 has been reported to be mostly involved in telomere damage induced replicative senescence, while p16 is mostly involved in stress induced (premature) senescence [93, 94]. Oxidative stress can also enhance telomere dysfunction and promote acquisition of a senescent phenotype [95]. However, there are still some questions regarding how to most accurately define senescent T cells in vivo via their cell markers. The loss of CD28 is commonly used as a biomarker for late-differentiation, as well as senescence, but this observation is an incomplete definition. Researchers also cite the absence of another stimulatory surface molecule, CD27, coupled with upregulation of inhibitory molecules, CD57, a surface marker found on terminally differentiated T cells as a more definitive description of truly senescent T cells. CD28−CD57+ T cells are commonly found in individuals with chronic immune activation, and they increase in frequency with age [96]. In HIV/AIDS, as well as in persons with other chronic infections [97], a large proportion of virus-specific CD8 T cells with CD57 expression that can produce cytokines such as TNFα in response to cognate antigen are unable to proliferate during in vitro culture. Furthermore, CD57 expression on T cells has been associated with shorter telomeres and these cells have undergone more cell divisions compared with other memory cells of other phenotypes [98]. Additionally, the surface receptor killer cell lectin-like receptor G1 (KLRG1) has been shown to be prominently upregulated on T cells in lymphocytic choriomeningitis virus (LCMV)-infected mice, having inhibitory properties by blocking co-stimulatory activities mediated by the AKT pathway such as proliferation [99, 100]. The increased expression of both CD57 and KLRG1 on CD8 T cells and NK cells has been associated with clonal exhaustion and autoimmunity [101]. Finally, cytotoxic T lymphocyte antigen 4 (CTLA-4), which can compete with CD28 for costimulatory ligands [102], has been shown to increase during HIV infection, decrease with the onset of HAART therapy [103] and may serve as a marker for chronically stimulated T cells in vivo. CTLA-4 expression also increases progressively as T cells are driven by repeated stimulation to the end stage of senescence in vitro [76].
Senescent CD8 T cells may acquire new functional properties, such as suppression of other types of immune cells [24]. This will be discussed in the context of cancer later in this review. Interestingly, the only context in which senescent CD8 T cells are associated with a positive effect on health is in organ transplant patients, where their abundance seems to correlate with reduced rejection episodes, suggesting that they may function to suppress immune reactivity to the donor organ. However, in bone marrow transplants, marrow donated by older individuals has been shown to have diminished capacity to reconstitute the immune system of the recipient [8].
Whereas the cell culture model has been highly informative in documenting and characterizing the process of T cell replicative senescence, this model cannot predict all the changes that may occur during in vivo aging. For example, investigative efforts have shown expression of both CD28 and CD57 on some CD8 T cells [104, 105], and the observation of enhanced antigen-induced apoptosis of CD57+ CD8 T cells, in contradiction with in vitro data [98]. Furthermore, it has been reported that T cells with varying levels of expression of CD57, KLRG1 and/or loss of expression of CD28 still have some proliferative potential, depending on the presence of specific cytokine combinations [106–109]. There are also reports of persistent proliferation of some CD8+CD28− cells from older individuals [107]. In addition, a comprehensive analysis did not show reduced proliferation capacity of cells explanted from elderly humans [110], but this study does not eliminate the possibility that during aging, non-dividing, putatively senescent cells accumulate and pack the immunological space. As previously mentioned, many analyses in elderly persons have shown that CMV seropositivity is strongly correlated with the expression of these putative senescent biomarkers, thereby raising the possibility that senescence is simply the negative by-product of the successful long term defense against these latent herpes viruses that infect 85–100% of the population [111–116].
It is generally recognized that senescent-like T cells are present in vivo, and likely contribute to immunosenescence in general, and to the specific associated pathologies observed in aging, as we have discussed so far. A major difficulty in studying cellular aging in vivo is the lack of accurate models, since even the changes observed in mice and other rodents do not precisely mirror those observed in humans. Even in human studies, the divergence between some of the in vitro and in vivo findings needs to be further investigated in order to more fully characterize the molecular changes that define replicative senescence, and how the process affects immune function and health of the elderly population.
Exhaustion versus senescence
The current body of research on T cell dysfunction often categorizes senescence and exhaustion as the same phenomenon because they share similar characteristics, such as the loss of proliferative potential and CD28 surface expression, and changes in cytokine secretion. Research in mice and ex vivo humans cells have shown that both senescence and exhaustion of CD8 T cells result from chronic antigenic stimulation by viruses and are thought to have evolved as a way of preventing excessive and damaging T cell responses to infection [117], but the precise molecular changes that lead to each end-state are still unclear. What is currently understood as a major distinghishing difference is that severe exhaustion results in eventual apoptosis of the exhausted cell, while senescent cells persist ( due their apoptosis resistance). In contrast to senescence, CD8 T cells become exhausted in environments of high antigenic load and lose their function in a specific chronological manner, beginning with the loss of IL-2 production and decline in proliferative potential, and followed by a reduction in TNF production. Finally, IFNγ production and cytotoxic activity are abolished and the cell is marked for degradation [118, 119]. Low levels of CD4 T cell also correlate with greater levels of exhaustion. Compared to naïve or effector CD8 T cells, exhausted murine T cells have altered gene expression profiles in important cellular processes such as chemotaxis, adhesion, co-receptors, migration, metabolism and energy [120].
To date, the most prominent identifying feature of T cell exhaustion is the increased surface expression of the CD28 family member, programmed cell death 1 (PD-1). Senescent T cells, defined primarily by high CD57 and loss of CD28 and CD27 molecules, do not necessarily have PD-1 expression, though they may be present concomitantly, as seen in some HIV-1 infected persons [117, 121, 122]. PD-1 surface expression was first observed in mice infected with lymphocytic choriomeningitis virus (LCMV), where its expression was elevated on CTL that had lost effector functions, but not on functional LCMV-specific CD8 T cells [123]. In humans, virus-specific CD8 T cells during chronic infection with such viruses as human T lymphotrophic virus (HTLV) and hepatitis B, have high PD-1 expression and altered cytokine expression patterns that impact their ability to clear infection. Other important features characterizing exhausted CD8 T cells include the expression of inhibitory receptor CTLA-4, Lymphocyte-activation gene 3 (LAG-3), T cell immunoglobulin mucin-3 (TIM-3) and B lymphocyte-induced maturation protein (Blimp-1) [120]. Significantly, blockade of the PD-1 signaling pathway with antibodies can restore antigen-specific T cell cytokine secretion and proliferation in LCMV-infected mice [124] and in humans with chronic HIV [121, 125, 126]. The mechanisms driving CD8 T cell exhaustion are not completely understood, but almost certainly include intrinsic regulatory pathways (such as signals mediated by the inhibitory receptor PD-1) and extrinsic regulatory pathways (including immunoregulatory cytokines and regulatory T cells) [127].
Downregulation of telomerase activity and shortened telomeres, defining features of senescence, have not been studied extensively in exhausted cells. However, a recent study by Lichterfeld et al. analyzed telomere length and telomerase activity in HIV-1-specific CD8 T cells and found the blocking the PD-1/PD ligand 1 (PD-L1) pathway increased both telomere length and telomerase activity in antigenic peptide-stimulated cells from HIV progressors [128], suggesting that PD-1 may play a role in the decline in telomerase seen in senescent and exhausted T cells. Still, the role of telomerase in the development of exhaustion is largely unknown, and more research will necessary to parse out the exact mechanisms that drive human T cells to reach senescence versus exhaustion. A more critical and thorough review by Akbar and Henson (2011) discusses this topic in greater detail [118].
Inflammation, the immune system and age-related pathologies
Infectious diseases have been a pervasive threat to survival throughout evolution. This suggests that stronger immune responses and inflammation during early life have been central in the survival of the species in the treacherous environments throughout human history. Accordingly, it is likely that certain genes and gene variants associated with robust inflammatory immune responses have been evolutionarily selected for, since they allowed survival to reproductive age. [26, 129]. Furthermore, until recently, most humans did not live to very old age so that there was no evolutionary pressure to prevent or reduce pathologies that arise with age. Thus, there are many age-related diseases and disorders, and in many cases, the immune system may be responsible for the cause, or at least the exacerbation of these conditions. This suggests that judicious manipulation of the immune system may provide novel immune-based therapeutic approaches to treat, prevent or limit the severity and progression of various pathologies.
The primary mode of defense and protection against pathogens by immune cells is through the secretion of inflammatory mediators such as cytokines and chemokines. However, chronic levels of elevated inflammation have negative effects on tissues, organs and overall health. Several studies report that increased levels of circulating proinflammatory cytokines, such as IL-6 and TNFα and their receptors, serve as significant independent risk factors for mortality and morbidity in the elderly [130]. High levels of proinflammatory cytokines in the elderly were also correlated with 2–4 fold increase in production of several acute-phase chemokines such as vascular cell adhesion molecule 1 (VCAM-1), and C-reactive protein (CRP), both soluble molecules that are associated with several age-related pathologies including atherosclerosis and insulin resistance [88, 131–134].
Studies in mice have helped elucidate the relationship between chronic inflammation, disease and lifespan. For example, mice fed a restricted low-calorie diet, which had been previously shown to extend maximum life span, have lower levels of TNFα and IL-6 production by T cells, suggesting that lower cytokine levels may function in increased survival rates [135]. This state of chronically elevated inflammation associated with biological age, termed “inflamm-aging,” [136, 137] underscores the prominent role of inflammation in the age-associated dysfunctional immune changes. It has been proposed that inflamm-aging may represent the common denominator underlying a wide range of age-related diseases.
Inflammation was one of the parameters documented in one of the most thorough longitudinal studies in humans, the Swedish OCTO/NONA studies, which followed free-living persons >85 years of age. The purpose of the study was to determine the biomarkers that predicted morbidity and mortality in this already old, genetically homogeneous population. Interestingly, one striking finding was that low-grade inflammation might predict mortality late in life, often through damage to non-immune organs [11]. The study also identified a so-called “immune risk phenotype” (IRP) representing the cluster of immune parameters associating with 2-, 4- and 6-year survival of the very elderly [138, 139]. A characterization of IRP is as follows: low levels of B cells, increased levels of CD8+CD28−CD57+ T cells, poor T cell proliferative response, CD4/CD8 ratio of <1, and CMV seropositivity [140, 141]. A large population of clonally expanded CMV-specific CD8 cells has been described in elderly individuals and analysis of these cells indicate that they are dysfunctional, more resistant to apoptosis, take up immunologic space, and may be harmful to the immune system [141]. Current findings support the hypothesis that a large proportion of senescent CD8 T cells are driven predominantly by CMV (along with EBV) since between 50–85% of people will be infected with CMV by age 40 and all individuals in the IRP group were CMV-seropositive, compared to around 85% in the non-IRP group.
Additional follow-up studies to the original IRP work have further underscored the negative effect of chronic inflammation in the elderly, with a focus on TNFα and IL-6 as predictors of immune status [142]. Specifically, circulating TNFα levels were found to be a direct predictor of mortality in elderly populations, while IL-6 was the most significant risk biomarker. Another study, which followed 129 elderly nursing home residents documented that a detectable TNFα serum level was associated with death within 13 months [143]. The IRP and studies associating specific cytokine profiles with morbidity and mortality represent prognostic advances that may be further expanded in the clinical setting
Specific age-related pathologies with immune components
Osteoimmunology
The emerging body of work suggesting a role of the immune system in bone-related pathologies had been originally based on the observations relating bone loss in the form of osteoporosis with systemic and local inflammation [144–147]. Reports also showed that increased inflammation was associated with a higher incidence of osteoporosis in postmenopausal women. Furthermore, there is an increased risk of developing osteoporosis in various inflammatory conditions and diseases associated with chronic immune activation, including HIV infection [148–151]. An association between circulating levels of CRP, a strong marker of oxidative stress and inflammation, and bone mineral density has been documented in several immune and inflammatory diseases, as well as in healthy individuals, suggesting a relationship between systemic, low-grade inflammation and osteoporosis [152]. Furthermore, rheumatoid arthritis (RA), characterized by cartilage and bone destruction in the joints, is often cited as a link between inflammation and bone loss, resulting from the release of proteinases (metalloproteinases) and proinflammatory cytokines including TNFα [153]. The immune phenotype of osteoporotic patients also underscores a substantial correlation between T cell senescence and bone remodeling. Analysis of peripheral blood lymphocyte subsets indicates that osteoporotic patients have an increased CD4:CD8 ratio, similar to the IRP findings, and greater numbers of CD45RO effector memory T cells, whereas the naïve subset is markedly decreased [154].
The previously described correlational studies have led to development of an emerging interdisciplinary field known as osteoimmunology, which focuses on the intimate interactions between the skeletal and immune cells primarily within the bone marrow [155]. Immune cells, especially T cells, can influence the balance of bone mineralization, carried out by osteoblasts, and resorption, accomplished by osteoclasts [156]. Cytokines and other protein molecules that regulate osteoclastogenesis, such as receptor activator of NFkB ligand (RANKL), are also key mediators in several immunological responses that are observed to increase with age and/or are associated T cell replicative senescence. For example, in vivo studies in mice and in vitro experiments on human T cells have shown that activated T cells produce cytokines, such as IL-6 or RANKL, that leads to activation and maturation of osteoclasts upon binding on the RANK receptor [157, 158]. Numerous studies have shown that many age-related proinflammatory cytokines, including IL-6, TNFα and IL-1 may play direct roles in the pathogenesis of osteoporosis as well [142, 159]. IL-6 can also directly enhance osteoclast activity by RANKL-independent mechanisms by extending the lifespan of the osteoclasts by inhibiting their apoptosis. TNFα has been shown to both promote osteoclast generation and to stimulate mature osteoclasts to undergo a greater number of resorption cycles through modulation of RANKL activity. Furthermore, TNFα can induce downregulation of collagen synthesis in osteoblasts and enhanced degradation of the extracellular matrix [160]. In inflammatory states and autoimmune diseases, activated T cells produce RANKL and pro-inflammatory cytokines, all of which can induce RANK expression in osteoblasts [161]. In this way, the constant activity of senescent or chronically activated T cells can cause extensive bone loss, as seen in diseases such as rheumatoid arthritis. Thus, immunosenescence may render older adults susceptible to bone diseases. Indeed, a recent study found that elderly patients with osteoporotic fractures had significantly more CD8 T cells with a senescent phenotype, including CD57 surface expression, in their peripheral blood [162].
Reduced bone mass within the oral cavity is also observed in the elderly, and has been linked to other age-related pathologies including atherosclerosis. Both activated and matured CD4 and CD8 T cells are found in the periodontal lesions [163, 164]. In addition, CD8 T cells lacking CD28 expression were found in gingival tissue [165], suggesting that replicative senescence may play a role in oral bone loss. There have been reports of increased production of prostaglandin E2 (PGE2) in people with periodontal diseases as well [166]. Our own recent studies have shown that PGE2, which is an important and ubiquitous mediator of inflammation, causes cultured CD8 T cells to progress more quickly to senescence, with a more rapid loss of CD28 protein and gene expression, reduced proliferative potential and suppressed telomerase activity (manuscript in preparation). Moreover, exposure of human T cells to PGE2 in concert with activation increases their RANKL production; if a similar process occurs in vivo, this would function to enhance osteoclast activity and bone breakdown. In support of this hypothesis, in patients with periodontitis, high levels of PGE2 were related to the severity of periodontal disease and the increase in alveolar bone loss [167, 168]. In addition, there is a greater production of PGE2 by old periodontal ligament cells [169], and this might promote recruited inflammatory T cells to acquire a senescent phenotype, enhancing the rate of alveolar bone resorption in elderly patients.
As a means to reduce address this clinical problem, our group has demonstrated a role of vitamin D in ameliorating the T-cell mediated destruction of bone. Vitamin D had long been known as a key modulator of bone integrity by promoting calcium absorption in the gut and maintaining adequate serum calcium and phosphate concentrations [170], but only when the vitamin D receptor (VDR) was detected on T cells did research on its role in immune/skeletal interactions become active. Initial studies suggested an immunosuppressive role of this metabolite, since it decreased the secretion of Th cell-associated cytokines and increased the population of T regulatory cells [171, 172]. We found that when we treated CD8 T cells from both HIV-negative and HIV-positive persons with low concentrations of 1,25-dihydroxyvitamin D3 (1–10nM), T cells transcribed less RANKL, TACE, which is the enzyme that cleaves RANKL from the surface of the cell into its soluble form, and conferred a less senescent T cell phenotype over time, including greater CD28 and CD27 surface expression that their untreated counterparts. Thus, vitamin D, in addition to its beneficial functions on innate immunity, specifically through the induction of cathelicidin production by macrophages [173], may play a critical role in modulating CD8 T cell function and may, modulate certain facets of the replicative senescence program.
T cell replicative senescence and cancer
Aging is the single highest risk factor for developing cancer. CD8 T cells, the immune system component that is central to the process of cancer immune surveillance, can recognize and kill cells that express tumor-specific antigens in the context of MHC. However, the combined effect of the accumulation of senescent T cells, which occupy “immunological space” and age-related thymic involution, which limits the naïve T cell repertoire, results in diminished immune surveillance capacity by old age. These changes synergize with a decline in innate immune function, all of which may contribute to the increased cancer incidence in the elderly population,
A second aspect of T cell-tumor biology relates to the possible role of chronic exposure to tumor-associated antigens, which may be also driving certain T cells to senescence. Although there is no direct causal data, there have been many correlational studies that support this hypothesis. For example, tumors associated with latent viral infections are increased with age, and are also associated with reduced immune function, as in immunosuppressed organ transplant patients and persons with HIV/AIDS. In AIDS patients, Kaposi’s sarcoma (KS, a rare vascular neoplasm) is uniformly associated with the latent herpes virus HHV8. Interestingly, co-culture of CD8+CD28− T cells from HIV-infected persons to normal vascular endothelial cells causes the latter to develop morphologic features of KS. Additionally, the rate of cervical cancer, which is associated with high-risk strains of human papillomavirus, increases during immune suppression [174].
Non-viral associated tumor antigens may also have the potential to drive antigen-specific T cells to become senescent. A striking example is the strong reactivity and immune response of CD8 T cells from prostate cancer patients to prostate-specific antigen (PSA), which is elevated in these individuals [175, 176]. Patients with certain cancers often times have increased proportions of senescent T cells, and this has been associated with poor clinical outcomes. For example, CD8+CD28− T cells can be purified from various types of human tumors, including lung [177], colorectal [178], ovarian [179], lymphoma [180] and breast gruber [181, 182], as well as from metastatic satellite lymph nodes and peripheral blood of cancer patients [177, 183]. Expanded clonal populations of cytotoxic T cells with other features of replicative senescence (e.g., CD57 expression) are also present in patients with melanoma and multiple myeloma [184, 185], and the abundance of senescent CD8 T cells correlates with tumor mass in head and neck cancer [186]. Moreover, in renal cancer, the proportion of CD8 T cells with features of replicative senescence is actually predictive of patient survival [187].
Interestingly, human T cells from healthy donors incubated with tumor cells for as little as 6 h at a low tumor: T cell ratio acquire a senescence-like phenotype, including the loss of surface expression of costimulatory receptors CD27 and CD28, telomere shortening, and induction of the cell cycle arrest proteins, p53, p21 and p16. Initiation of senescence is likely caused by the secretion of soluble factors by the cancer cells. Moreover, these T cells may play an active role, based on the observation that they can suppress proliferation of responder T cells after being in physical contact with them [188]. Highlighting the role of telomere length in carcinogenesis, a recent meta-analysis of 27 studies found a significant inverse correlation between telomere length of blood cells and cancer incidence [189]. However, these studies do not necessarily prove that these naturally occurring senescent T cells stimulate the progression of tumors in vivo in humans. In order to define the role of senescent T cells on the progression of age-related cancers in humans, it would be necessary to eliminate senescent cells and/or their soluble mediators from cancer-prone tissues and determine the consequences on tumor pathology. Conversely, the chronic activation by tumor antigens, like antigens of latent viruses, may indeed contribute to driving T cells to senescence and resulting in their failure to detect other tumor cells.
Another important issue regarding senescent T cells and cancer emerges from a recent study, in which adoptive immunotherapy with autologous anti-tumor tumor-infiltrating lymphocytes (TILs) following chemotherapy led to tumor regression in half of the patients with metastatic melanoma. Significantly, tumor regression was strongly correlated with proliferative potential and telomerase activity of the transferred T cells [190]. Complementing this finding is study in which the investigators observed that patients with objective clinical responses had TILs with a mean telomere length of 6.3 kb, whereas telomeres of TILs that were not associated with significant clinical responses were markedly shorter. Expression of the costimulatory molecule CD28 also corresponded to longer telomeres and T cell persistence [191]. These studies suggest that in the transferred cells, telomere length and proliferative potential, both prominent features that are reduced in senescent T cells, may play a considerable role in mediating response to adoptive immunotherapy in the treatment of cancer. The results underscore the importance of senescent T cells in the clinical setting, since they can be used in predicting effectiveness of adoptive immunotherapy and the status of the cancer.
In practice, most primary tumors can be removed surgically, followed by radiation, chemotherapy or adjuvant therapy. However, these therapies are largely ineffective against metastases. Recent advances have shown some promising results of cancer vaccines against metastases. However, it seems likely that immunosenescence would significantly impact the efficacy of prophylactic vaccination, based on a large body of evidence that the elderly respond significantly less to vaccination than the young [192, 193]. Several vaccine studies in preclinical cancer models at old age demonstrated that in most cases T cell responses were hardly detectable [194]. Impaired immune responses in elderly can, in part, be attributed to the decline in CD28. Loss of this important costimulatory molecule would cause decreased T cell responses in aged humans.
The previous illustrations are likely reasons that immunological and chronological age is associated with decreased responsiveness to cancer vaccination. Therefore, in order to develop potential effective therapies against cancer and improve immune function, it is important first that we distinguish changes caused by persistent infection from those caused by cancer itself.
As an important catalyst of carcinogenesis, the hormone-like substance, PGE2, is overproduced in many tumors. It accelerates cancer progression by promoting angiogenesis and metastasis, and by influencing the immune response [195, 196]. Studies have also found that the enzyme that produces PGE2, cyclooxygenase (COX)-2, increases its activity in macrophages from old mice [197]. Our own recent studies addressed the potential effect of PGE2 on human T cells, since lymphocyte functional decline is a significant defect observed in persons with cancer. Our preliminary findings indicate that in the presence of physiologically relevant concentrations of PGE2, T cells exhibit reduced proliferation and telomerase activity, lower production levels of antiviral cytokines including IFNγ, and reduced CD28 protein and message. The effects of PGE2 would engender a more senescent-like phenotype to T cells and reducing their ability to detect and eliminate tumor cells.
Role of the immune system in age-related neurological diseases
Brain-infiltrating lymphocytes can directly modulate brain homeostasis under both physiological conditions and in the inflammatory state caused by disease or injury, during which proinflammatory cytokines such as IL-6 and TNFα are found to be elevated [198, 199]. Because senescent CD8 T cells are increased with age and produce significantly higher concentrations of inflammatory mediators, there is a possibility that they are among the cells recruited to the brain during disease or injury, and may contribute to the inflammation. This increase in inflammation seen in neuropathologies correlates with deterioration of cognitive function, including the reduced speed of information processing, reduced working memory capacity and impaired spatial memory often observed in dementia, Parkinson’s disease (PD), multiple sclerosis (MS), progressive multifocal leukoencephalopathy (PML) and Alzheimer’s disease (AD). Indeed, a correlational study implicated senescent T cells in influencing the progression or development of AD, as telomere length in T lymphocytes of AD patients was negatively associated with worse disease status [200, 201].
CD8 T cells are increasingly being recognized as potential drivers of neuronal damage, based on studies showing that they can directly target neurons or the myelin sheath and oligodendrocytes (ODCs) in both the CNS gray and white matter in vitro [202] and in vivo [203]. Importantly, neuropathologic studies have shown that CD8 T cells are the most numerous cell types in the inflammatory infiltrate in acute and chronic MS lesions gay [204]. Axonal damage in MS lesions correlates with the number of CD8 T cells [205, 206], and neurons and all other CNS cell types show increased MHC I expression [207]. Moreover, in patients suffering from MS with depressive symptoms, there are elevated levels of proinflammatory cytokines such as IL-6, IL-1β and TNFα, which correlate to greater negative behavioral consequences [208]. Collectively, these findings suggest that dysregulated CD8 T cells, including senescent cells, that accumulate in the elderly, could contribute as effector cells to the damage of glial and neuronal cells by direct recognition of MHC class I, as well as through the secretion of excessive inflammatory cytokines. However, to date, no group has explicitly investigated whether these infiltrating T cells in persons with MS have features of replicative senescence.
In contrast to the potential deleterious effects of CD8 T cells described above, there is a growing body of evidence documenting a beneficial, neuroprotective role of T cells after CNS damage and in certain neurodegenerative conditions. These T cells can help maintain homeostasis via their interactions with resident microglia, and they may also support neurogenesis. Mice deficient in CNS-specific T cells have reduced rates of hippocampal granular cell progenitor cell proliferation [209], and reduced spatial memory [199]. Regulation of a key molecule necessary for maintenance of brain plasticity, brain-derived neurotrophic factor (BDNF), has been reported to be modulated by T cells, which can either produce it endogenously, or induce its production by glial cells [210–212]. Levels of BDNF production by hippocampal neurons were associated with CNS-specific T cell activity and, in situations of mental stress and depression, T cells were able to restore the reduced BDNF levels [209, 213]. Furthermore, during acute injury and neurodegenerative diseases, T cells can recuit monoctyes and act in tandem with resident microglia to remove excess toxins and debris, such as plaques seen in AD [214–216]. However, as noted previously, senescent T cells, which are show multiple alterations compared to non-senescent T cells, exhibit less ability to proliferate and secrete large quantities of inflammatory cytokines, features associated with negative outcomes in brain disorders. The emerging picture emphasizes the delicate balance between the normal physiological activity of T cells that supports brain health and the possibility that dysregulation of T cells in old age may negatively impact cognitive well-being.
T cell senescence and cardiovascular disease
As the most prevalent killer globally, cardiovascular diseases (CVD) involve complex sets of interactions between vascular endothelial cells and the immune system that lead to the development of cardiac and arterial pathologies. The principal cause of heart disease, myocardial infarction, and stroke is atherosclerosis, a chronic inflammatory disease characterized by lipid and cholesterol accumulation within the walls of large and medium arteries [217]. The immune system plays a major role in this process through the development of monocytes into foam cells upon exposure of oxidized lipids [218]. In addition, T cells have been documented to be present in atherosclerotic lesions [218–221] and vascular endothelial cells, macrophages and smooth muscle cells may communicate with T cells through CD40 [222] and secreted cytokines and chemokines [218].
By taking up a large proportion of the T cell antigen-receptor repertoire and interacting with vascular tissues, senescent T cells may modulate development of atherosclerosis and coronary heart disease. Indeed, epidemiological correlation studies show accelerated telomere shortening seen in patients with cardiovascular disease [223], atherosclerosis [224], and myocardial infarction [225]. Furthermore, patients with degenerative calcific aortic valve stenosis, an archetypal age-related cardiovascular disorder, were shown to have shorter PBMC telomeres than matched controls [226]. Shorter telomeres have also been associated with vascular dementia [95]. Moreover, a longitudinal study of 143 individuals also found that shorter PBMC telomere length at age 60 was associated with a 3.2-fold higher subsequent mortality rate from CVD [227]. Moreover, in HIV-infected persons, the presence of dysfunctional senescent T cells and their associated inflammatory and immunologic factors increases the risk of developing cardiovascular diseases. In one study, among the HIV-infected group, increased levels of T cell activation and a high proportion of senescent CD8 T cells were associated with an increased prevalence of carotid artery lesions. These effects persisted after adjustment for age, antiretroviral medication class, HIV RNA levels, and other cardiovascular disease risk factors [228].
Because these are associative studies, it is not yet known whether telomere shortening of T cells is increasing susceptibility to cardiovascular risk factors or, alternatively, if shorter telomeres are the byproduct of chronic systemic inflammation associated with cardiovascular diseases. However, the former possibility seems more likely, since the same cytokines secreted by senescent T cells (i.e., TNFα and IL-6) are also seen within atherosclerotic lesions. Furthermore, TNF-α and IL-6 facilitate a variety of functions that impact disease progression, including the enhanced recruitment of macrophages, and, therefore, foam cell formation [229]. Recent work in our lab suggests that exposure of CD8 T cells in long term culture to the same oxidized lipids that cause foam cell formation in vivo results in the enhancement of a number of senescence-like features, including reduced telomerase activity. Taken together, inflammatory mediators such as oxidized lipids and inflammatory cytokines may exert negative effects on T cells, enabling them to facilitate the development of vascular pathologies.
Interestingly, senescent T cells have been implicated in playing a role in diabetes mellitus, which in itself is associated to increase risk of having cardiovascular events. One recent study found that CD4+CD28− T cells, a rare long-lived subset of senescent T cells with proatherogenic and plaque-destabilizing properties, are expanded and are associated with poor glycemic control. The presence of these cells also correlates with the occurrence of a first cardiovascular event and with a worse outcome thereafter [230].
Stress and aging
An emerging body of work demonstrates the influential role of psychological health and the immune system. In this interdisciplinary field, termed psychoneuroimmunology, recent research has showed that many age-associated immune changes discussed in this review can be exacerbated by emotional stress such as in MS. Conversely, because T cells can cross the blood-brain barrier, their ability to function normally and regulate inflammation is key to neuronal and glial health. Dysregulated, senescent T cells that may be able to access and interact with brain cells can cause excessive secretion of inflammatory cytokines, which is often a symptom of age-related neurological diseases including Alzheimer’s disease.
It appears that the effects of both chronological and immunological aging, leading to the accumulation of senescent T cells in the T cell repertoire, can be exacerbated by psychological stress. Reduced antibody responsiveness to influenza vaccination has been documented in older caregivers of relatives with dementia. These caregivers also showed reduced telomerase activity versus matched controls [231]. A more recent study found that psychological stress, due to caring for a chronically ill child, is significantly correlated with higher oxidative stress, lower telomerase activity, and shorter telomere length in peripheral blood mononuclear cells from healthy women [232, 233]. In fact, the mothers with the highest levels of perceived stress had, on average, telomere lengths of women 10 years older compared to low stress women. These findings suggest that, at the cellular level, stress may promote earlier onset of senescence, at least in PBMC, and possibly also age-related diseases.
Clinical depression is an important mental condition affecting millions of people, including the elderly, and associated with high levels of psychological stress. Inflammatory and oxidative stress markers, CRP, IL-1, and IL-6, are positively associated with clinical depression in a dose-dependent manner, indicating possible bidirectional crosstalk between senescent T cells and emotional response [234]. Indeed, it has been shown that psychological stress can boost inflammation [235] as well as oxidative stress [232], and both can accelerate telomere attrition [236]. Researchers compared persons with chronic depression versus a control group, and found that the depressed group, as a whole, did not differ from the controls in telomere length of their leukocytes, but individuals with more chronic courses of major depression had significantly shorter telomeres. The mean telomere length in the chronically depressed subjects was 281 base pairs shorter than that in controls, a difference that corresponded to approximately seven years of accelerated cell aging. [237]. Significantly, in HIV infected patients, depressive symptoms were significantly associated with higher viral load levels and altered CD8 T cell function [238]. Indeed, even among the HIV-negative population, psychological stress has been associated in a dose-response manner with an increased risk of acute infectious respiratory illness, specifically the common cold [239]. One explanation for these observations relates to cortisol, a major stress hormone that is elevated in depressed individuals [240]. Exposure of human T cells to cortisol in concert with activation results in a significant reduction in telomerase activity, a change that, over the long term, would be predicted to accelerate the progression to replicative senescence and, in turn, might lead to suppression of immune responses to vaccines and other antigenic interactions [241].
A decline in immune function can also arise in situations of extreme stress through the re-emergence of latent viruses including CMV and EBV, which we discussed as contributing drivers of replicative senescence. For example, physically healthy and fit astronauts have shown reactivation and viral shedding of CMV during spaceflight [242]. Some astronauts also show subclinical reactivation of the varicella zoster virus (VZV) and Epstein-Barr virus (EBV) while levels of cortisol and catecholamines, both hormones involved in ‘fight-or-flight’ responses, were also elevated on the day of landing [243]. Other purportedly high-stressed individuals, such as medical students, showed increased expression of latent EBV during examination periods. Higher antibody titers to EBV IgG were identified at the time of examination, in contrast to 1 month prior to the exam [244, 245].
CD4 T cell senescence and autoimmunity
The primary focus of the discussion on replicative senescence has been on CD8 T cells, since this T cell subset undergoes the most dramatic phenotypic and functional changes during aging. However, recent efforts have begun to investigate the role of senescent CD4 T cells, characterized according to the same criteria described in CD8 T cell senescence, namely, loss of CD28 expression and telomerase activity, as well as shortened telomeres. When CD4 T cells, which normally function to orchestrate multiple signals between T cells, B cells and APCs, reach senescence, they acquire potent cytolytic abilities, a quality reserved primary to CD8 T subsets. Senescent CD4 T cells have been shown to kill endothelial cells [246], which may promote instability in atherosclerotic plaques. The purported senescent CD4 T cells are less common than their CD8 counterparts, but emerging reports suggest that the end-stage CD4 T cells may play an equally critical role in the development of age-related pathologies, particularly in the context of autoimmune diseases such as multiple sclerosis, rheumatoid arthritis and Wengener’s granulomatosis, a rare type of small artery and vein inflammatory disease.
Elderly individuals suffer from autoimmunity more frequently [247] and autoimmune diseases are thought to lead to persistent immune stimulation, much like the situation of latent infections and cancers discussed earlier. The disease-causing autoantigens, though, are often unknown and are apparently not eliminated by the immune system, thus leading to repeated T cell activation. In the prototypic autoimmune disease, rheumatoid arthritis, which often afflicts the elderly, there is an increase frequency in CD28-negative CD4 T cells, which, like CD28-negative CD8 T cells, have been shown to have impaired telomerase activity [70].
The increased frequency of CD28-negative T cells in the elderly, discussed earlier, is often attributed to CMV infection, which is a major component of the IRP. Interestingly, the presence of CD4+CD28− T cells in several autoimmune diseases has been found to be accompanied by CMV infection. For example the expansion of CD28-negative CD4 T cells in Wegener’s granulomatosis and in RA has been strongly associated with CMV infection [248, 249]. Additionally, the T cell infiltrates in the muscle lesions of patients with Dermatomyositis and Polymyositis, both autoimmune connective tissue diseases, primarily consist of cells lacking CD28, and are CMV reactive [250]. However, it is still unclear the mechanism and extent to which CMV infection contributes to the expansion of CD28− CD4 T cells in persons with autoimmune diseases.
Additional information on CD4 T cell senescence is provided in studies on HIV-infected persons who are being treated with highly active anti-retroviral therapy (HAART). In those patients with low CD4 counts, there was greater expression of another senescence biomarker, CD57, on CD4 T cells compared to those with increased, reconstituted of CD4 T cell levels. The cohort with low CD4 T cell counts also showed reduced thymic activity, leading to a lower, more senescent-like CD4 T cell in the peripheral blood [251]. Together, these observations suggest that there are several different related mechanisms contributing to premature immune senescence in HIV-infected patients with low-level CD4 repopulation on HAART.
Future directions
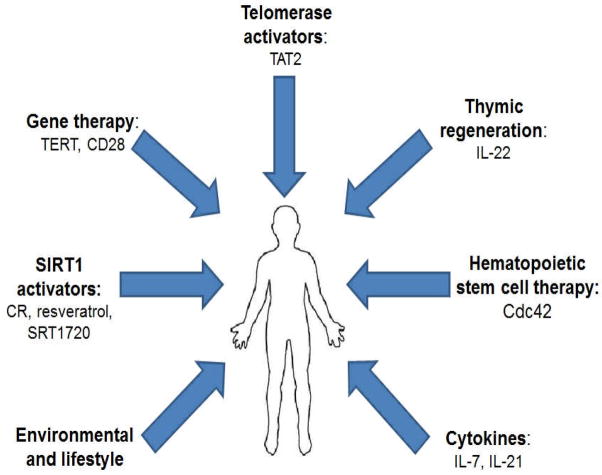
In this review, we have focused on the well-documented observation that senescent T cells, resulting, in part, from environmental factors such as exposure to chronic infections, constitute a significant proportion of the T cell compartment in elderly persons. There are several promising approaches to combating the decline of the immune system during aging or chronic infection, as summarized in fig. 1. Firstly, in considering strategies to prevent the generation of these cells, prophylactic childhood vaccination against CMV is an attractive option. In that regard, a preliminary report of a vaccine used to prevent primary CMV infection in pregnant CMV-seronegative mothers is quite encouraging [252]. It is also possible that development of a therapeutic vaccine, targeting both humoral and cellular immunity, might function to blunt the development of senescent CMV-specific T cells. However the challenge would be to elicit immunity that is more robust than the immunity resulting from natural infection, which we know does not achieve clearance of CMV from infected individuals. It is encouraging that a therapeutic vaccine against a different herpes virus, Varicella Zoster Virus (VZV), which is associated with shingles, has been moderately successful. Specifically, a 50–60% reduction of Zoster incidence was documented in the vaccinated vs. a matched control treated group [253], raising hopes that a similar vaccination strategy might be achievable for CMV. With the development of an effective CMV vaccine, it will be possible to (a) define the extent to which CMV infection contributes to immunological aging; and (b) possibly prevent or decrease the proportion of age-associated senescent T cells, and thus, their contribution to inflamm-aging.
Figure 1. Possible strategies for rejuvenating the immune system and preventing replicative senescence.
Several different therapeutic approaches may be able to reinvigorate T cell function, such as activation of SIRT1, telomerase upregulators, and gene therapy using viral vectors containing the TERT or CD28 genes. Promising methods to promote naïve T cell production and development include stem cell therapy, the use of homeostatic cytokines and thymic regeneration.
An additional primary goal of ongoing research is develop strategies to maintain telomere length and prolong telomerase activity in specific populations of T cells, particularly those that are involved in combating viral infections. Many age-related pathologies, as well as overall mortality and morbidity, are significantly associated with telomere length in T cells. Gene therapy using catalytic human telomerase as well as chemotherapeutic agents, such as TAT2, activating the catalytic subunit of human telomerase, hTERT, could prevent telomere attrition and thus avert the loss of immune control over microbial infections [81].
It should be emphasized that upregulation of telomerase as a treatment strategy must avoid possible oncogenic effects of this reverse transcriptase enzyme. If pharmaceutical small molecules, such as TAT2, can be directed to selected populations of immune cells, telomerase upregulators can promote the maintenance of healthy, functionally competent T cells. Alternatively, targeting regulatory pathways that stabilize telomere caps, maintain CD28 on the cell surface, or attenuate excess chronic inflammation may represent equally effective therapies to treat a number of age-related maladies.
In contrast to trying to re-invigorate expanded populations of late-differentiated T cells, another promising approach focuses on naïve T cell populations, for example, through the treatment of cytoprotective cytokines such as IL-7 and IL-21. Studies have documented that administration of recombinant IL-7 to normal or lymphopenic mice, non-human primates, and humans result in increased T cell proliferation and T cell numbers by increasing thymic output and inducing maintenance of long-lived KLRG1hiCD127lo memory T cells [254, 255]. In addition, ongoing research demonstrated that exogenous IL-7 added to T cells co-cultured with tumor cells inhibited loss of CD27 and CD28, two features of replicative senescence, and allowed normal proliferative capacity and IL-2 production [256]. IL-7 can also induce telomerase activity [257]. Interestingly, an ongoing Phase I clinical trial using the non-glycosylated form of human IL-7 shows promise, in that it seems to cause the expansion of naïve and memory CD4 and CD8 T cell populations.
IL-21, a pleiotropic cytokine produced by CD4 T cells, also has profound effects on the adaptive immune system, including enhancing resting T cell proliferation in vitro by synergizing with IL-7 and promoting antigen-specific CD8 T cell expansion in vivo [234]. In HIV studies, IL-21 levels positively correlate with CD4+ T cell count and CD8 cytotoxic and antiviral activity [258]. Liu et al. found that IL-21 is capable of sustaining CD28 expression in CD8 naive T cells [259], and therefore prevents the accumulation of the CD28−CD8+ T cells that characterize senescence during aging and in HIV disease. Together, these potential immunotherapic approaches may ultimately lead to rejuvenation of the immune responses of older adults, thereby reducing morbidity and increasing overall health span.
Another novel method to revitalize the immune system, independent of preventing progression to replicative senescence, is through regeneration of the thymus. Because the thymus is necessary to produce naïve T cells [260] and thymic involution is a hallmark consequence of chronological aging, leading to the accumulation of late-differentiated, senescent T cells, targeted therapies to rejuvenate the thymus may serve to delay the impairment of the adaptive arm of the immune system. Recently Dudakov et al. demonstrated a role for a subset of innate lymphoid cells in regenerating the thymus. After damage is done to thymic epithelial tissue by chemical or infectious stimuli, lymphoid tissue inducer (LTi)-like cells produce IL-22 and IL-17 [261]. The researchers had discovered that IL-22 may enhance proliferation and survival of thymic epithelial cells, which are essential for T cell development, and administration of exogenous IL-22 accelerated thymic reconstitution after irradiation in mice [262]. However, currently there is no data directly supporting utility in preventing or reversing thymic involution during aging. The results by Dudakov et al. suggest a key role for intrathymic LTi-like cells in working to restore tissue homeostasis during periods of insult (e.g. chemotherapy and radiation insults) and perhaps during various conditions such as chronic stress and inflammation, both known to enhance senescence. Future research in aged animal models or ex vivo in older individuals would need to address the role of IL-22 in mitigating thymic involution during aging.
The life-extending benefits of calorie restriction (CR) originally document by Clive McCay in the 1930s opened up an entire field of gerontological investigation. Compelling research on (CR)-mediated lifespan extension has been documented in model organisms such as yeast [263], flies [264], worms [265] and mammals [135, 266, 267]. The collective body of research strongly suggests that CR may decrease the incidence of age-related diseases in mammals and reduce biomarkers of accelerated aging in humans [268–270]. Currently, it is the only intervention that is known to retard aging in most organisms. Several genetic modulators of CR have been identified, most notably, SIR2 (silent information regulator 2) in yeast [263] and SIR homologs in mammals [271, 272]. These are NAD+-dependent histone deacetylases and belong to the conserved 7-member sirtuin family. Sirtuins have important functions in the regulation of metabolism, growth, differentiation, inflammation, cellular survival, as well as in senescence and lifespan extension [273–276]. Of note is the effect of activating sirtuin-1 (SIRT1), which has been cited to have many beneficial consequences on cellular homeostasis. SIRT1 is known to be involved in deacetylation of non-histone targets to regulate metabolism and metabolic diseases. One way that SIRT1 modulates metabolism is through interactions with Forkhead transcriptional factors (FOXOs), which function as sensors of the insulin signaling pathway and cell longevity [277]. SIRT1 is downregulated in insulin-resistant cells and increased expression of SIRT1 improves insulin sensitivity, especially under insulin-resistant conditions [278]. Furthermore, Westerheide et al have demonstrated that SIRT1 can modulate stress responses by deacetylating heat shock factors (HSF1), which serve as molecular chaperones that facilitate nascent protein folding and refolding or degradation of abnormally folded proteins. Deacetylation would support adequate expression levels of protective genes, such as HSP70, in response to external stress factors such as free radical assault, which is commonly associated accelerated immune aging and its related degenerative processes [279]. Increased stress resistance also correlates with longevity in model organisms [280]. In addition, SIRT1 can induce autophagy, a highly conserved cellular housekeeping process that removes aberrant and dysfunctional molecules and organelles from interfering with cellular homeostasis and functions. Autophagic degradation has been observed to decline during aging and purportedly contributes to cell death or dysfunction [281]. Therefore, the ability of SIRT1 to modulate stress resistance, metabolism, and autophagy, as well as its reported roles in modulating circadian rhythms [282, 283], improving genome stability [284], suppressing tumors [285], decreasing chronic inflammation such as [286], and protecting against neurodegenerative diseases [287, 288] in mammalian cells suggests that SIRT1 is a highly promising target for pharmacological therapies.
Since it is unlikely that long-term CR (i.e., reducing daily caloric intake by ~30%) is a feasible strategy for the majority of humans, discovering other modulators of the sirtuin family would be the ideal goal. A popular putative SIRT1 activator, resveratrol, a small polyphenol found in red wine, has been suggested as a CR mimetic [289, 290]. Resveratrol mimics the transcriptional profiles associated with caloric restriction [291, 292] and prevents early mortality in obese mice [293]. However, resveratrol has many targets in mammalian cells, and the complete signaling pathways on which it operates to activate sirtuins is not yet fully understood, though recently Park et al have found that resveratrol may be inhibiting cAMP phosphodiesterases [294]. However, controversy about the lifespan-extending effects of resveratrol still continues as other reports have suggested that it failed to impart positive benefits [295, 296]. In addition to resveratrol, several new small-molecule SIRT1 activators have been developed, such as SRT1720, which is produced by a subsidiary of GlaxoSmithKline. Although some studies have demonstrated possible benefits of these SIRT1 activations [297–301], SRT1720 and other similar molecules may also exhibit substrate-specific effects on SIRT1 activity in vitro, functioning effectively only when conjugated to a fluorophore [302, 303]. Currently there is still much debate regarding the effectiveness and efficacy of resveratrol and other SIRT1 activators in extending life and revitalizing declining health; however, the research on lifespan extension and the role of sirtuins is still in its nascent stages, and it appears that there is much therapeutic potential in exploiting sirtuins to benefit human health.
Another exciting alternative to combat the apparent decline of the immune system is the application of progenitor stem cells to revive thymic output of functional, naïve T cells. Current research has found that hematopoietic stem cells (HSCs) from old animals have reduced expression of genes involved with immune-cell development compared with HSCs from young animals [304]. With increasing age, HSCs become more numerous but less effective at regenerating blood cells and immune cells, leading to increased susceptibility to infections and disease, such as pneumonia. Recently, Florian et al. have discovered that elevated levels of Cdc42, a small RhoGTPase involved in the cell cycle, in aged HSCs is directly linked to HSC aging. Inhibition of Cdc42 signaling reversed HSC aging and reinstated function and intracellular polarity similar to that of younger stem cells. Inducing premature aging of HSCs in mice by genetically increasing Cdc42 activity led to poor structural organization and polarity, and this disorganization contributed to the cells’ decreased functional efficiency. Finally, the group reported that old HSCs rejuvenated by targeting Cdc42 do function similarly to young stem cells when engrafted into recipient mice [305].
A final strategy for extension of both health and lifespan via gene therapy was recently reported. Bernardes de Jesus et al. tested the effects of a telomerase gene therapy in adult (1 year of age) and old (2 years of age) mice and found that treatment of 1- and 2-year old mice with an adeno-associated virus (AAV) expressing mouse TERT showed beneficial health effects, including increased insulin sensitivity, reduced rates of osteoporosis, neuromuscular coordination and decreases in several molecular biomarkers of aging, as well as increased median life span of 13–24%. There was also no increase incidence of cancer in the treated group, suggesting that the tumorigenic activity of telomerase may not be a major concern in adult animals using AAV vectors. Although mice and human telomere dynamics differ In several ways, this research demonstrates a role of TERT in delaying physiological aging and extending longevity in normal mice through gene therapy [306].
Concluding remarks
The effects of immunosenescence are widespread, and impact multiple aspects of the quality of life of the aging population; these, in turn, have huge societal implications, since the cost of caring for the elderly has a significant impact on the overall healthcare system. Our ever-increasing life expectancy, coupled with the goal of maintaining good health throughout life, mandates that biomedical research be expanded to address the multiple factors affecting aging, at both the basic science level and in translational studies. Importantly, research on aging must be extended to address the influences of early development, including in utero events, on subsequent gene expression and disease processes that occur decades later. Finally, animal model systems are critical in identifying potential pathways involved in basic aging, but ultimately, a full understanding of human aging, particularly the immune system, requires expanded investigation of actual human populations.
Acknowledgments
The research described in this review has been supported by the following NIH grants to RBE: AG032422 and AI060362.
References
- 1.Oeppen J, Vaupel JW. Broken Limits to Life Expectancy. Science. 2002 May 10;296(5570):1029–31. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- 2.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007 Sep;70(3):179–89. doi: 10.1111/j.1399-0039.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 3.Aspinall R, Del Giudice G, Effros RB, Grubeck-Loebenstein B, Sambhara S. Challenges for vaccination in the elderly. Immun Ageing. 2007;4:9. doi: 10.1186/1742-4933-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostan R, Bucci L, Capri M, Salvioli S, Scurti M, Pini E, et al. Immunosenescence and immunogenetics of human longevity. Neuroimmunomodulation. 2008;15(4–6):224–40. doi: 10.1159/000156466. [DOI] [PubMed] [Google Scholar]
- 5.Candore G, Colonna-Romano G, Balistreri CR, Di Carlo D, Grimaldi MP, Listi F, et al. Biology of longevity: role of the innate immune system. Rejuvenation Res. 2006 Spring;9(1):143–8. doi: 10.1089/rej.2006.9.143. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006 Feb 20;24(8):1159–69. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 7.Murphy SL, Xu Jiaquan, Kochanek Kenneth D. Deaths: Preliminary Data for 2010. National Vital Statistics Reports. 2010 Jan 11;60(4) [PubMed] [Google Scholar]
- 8.Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001 Oct 1;98(7):2043–51. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 9.Walford RL. The Immunologic Theory of Aging. Gerontologist. 1964 Dec;57:195–7. doi: 10.1093/geront/4.4.195. [DOI] [PubMed] [Google Scholar]
- 10.Effros RB, Walford RL. T-Cell Cultures and the Hayflick Limit. Human Immunology. 1984;9(1):49–65. doi: 10.1016/0198-8859(84)90006-5. [DOI] [PubMed] [Google Scholar]
- 11.Wikby A, Nilsson BO, Forsey R, Thompson J, Strindhall J, Lofgren S, et al. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006 Aug;127(8):695–704. doi: 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004 Nov 15;200(10):1347–58. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008 Mar 17;205(3):711–23. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murasko DM, Jiang J. Response of aged mice to primary virus infections. Immunol Rev. 2005 Jun;205:285–96. doi: 10.1111/j.0105-2896.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 15.Brien JD, Uhrlaub JL, Hirsch A, Wiley CA, Nikolich-Zugich J. Key role of T cell defects in age-related vulnerability to West Nile virus. J Exp Med. 2009 Nov 23;206(12):2735–45. doi: 10.1084/jem.20090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cicin-Sain L, Smyk-Pearson S, Currier N, Byrd L, Koudelka C, Robinson T, et al. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol. 2010 Jun 15;184(12):6739–45. doi: 10.4049/jimmunol.0904193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McElhaney JE. Prevention of infectious diseases in older adults through immunization: the challenge of the senescent immune response. Expert Rev Vaccines. 2009 May;8(5):593–606. doi: 10.1586/erv.09.12. [DOI] [PubMed] [Google Scholar]
- 18.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stacy S, Krolick KA, Infante AJ, Kraig E. Immunological memory and late onset autoimmunity. Mechanisms of Ageing and Development. 2002;123(8):975–85. doi: 10.1016/s0047-6374(02)00035-0. [DOI] [PubMed] [Google Scholar]
- 20.Hsu H-C, Mountz JD. Origin of late-onset autoimmune disease. Immunology and Allergy Clinics of North America. 2003;23(1):65–82. doi: 10.1016/s0889-8561(02)00074-7. [DOI] [PubMed] [Google Scholar]
- 21.Douziech N, Seres I, Larbi A, Szikszay E, Roy PM, Arcand M, et al. Modulation of human lymphocyte proliferative response with aging. Exp Gerontol. 2002 Jan-Mar;37(2–3):369–87. doi: 10.1016/s0531-5565(01)00204-2. [DOI] [PubMed] [Google Scholar]
- 22.Fulop T, Jr, Gagne D, Goulet AC, Desgeorges S, Lacombe G, Arcand M, et al. Age-related impairment of p56lck and ZAP–70 activities in human T lymphocytes activated through the TcR/CD3 complex. Exp Gerontol. 1999 Apr;34(2):197–216. doi: 10.1016/s0531-5565(98)00061-8. [DOI] [PubMed] [Google Scholar]
- 23.Varin A, Larbi A, Dedoussis GV, Kanoni S, Jajte J, Rink L, et al. In vitro and in vivo effects of zinc on cytokine signalling in human T cells. Experimental Gerontology. 2008;43(5):472–82. doi: 10.1016/j.exger.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005 Jun;205:147–57. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 25.Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2007 Jan 8;25(4):599–604. doi: 10.1016/j.vaccine.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 26.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007 Jan;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Brunner S, Herndler-Brandstetter D, Weinberger B, Grubeck-Loebenstein B. Persistent viral infections and immune aging. Ageing Res Rev. 2011 Jul;10(3):362–9. doi: 10.1016/j.arr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Oltz EM. Regulation of antigen receptor gene assembly in lymphocytes. Immunol Res. 2001;23(2–3):121–33. doi: 10.1385/IR:23:2-3:121. [DOI] [PubMed] [Google Scholar]
- 29.Saito TGR. The generation and selection of the T cell repertoire: insights from studies of the molecular basis of T cell recognition. Immunol Res. 1998 Jan;101:81–113. doi: 10.1111/j.1600-065x.1988.tb00733.x. [DOI] [PubMed] [Google Scholar]
- 30.Janeway CA, Jr, Travers P, Walpert MSM. The immune system in health and disease. Princeton, NJ: Garland Publishing Inc; 2001. [Google Scholar]
- 31.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999 Nov 18;402(6759):255–62. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 32.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 33.Wherry EJ, Ahmed R. Memory CD8 T-Cell Differentiation during Viral Infection. Journal of Virology. 2004 Jun 1;78(11):5535–45. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachmann MF, Barner M, Viola A, Kopf M. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. European Journal of Immunology. 1999;29(1):291–9. doi: 10.1002/(SICI)1521-4141(199901)29:01<291::AID-IMMU291>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Hayflick L. Recent advances in the cell biology of aging. Mech Ageing Dev. 1980 Sep-Oct;14(1–2):59–79. doi: 10.1016/0047-6374(80)90106-2. [DOI] [PubMed] [Google Scholar]
- 36.Hayflick L, Moorhead P. The serial cultivation of human diploid cell strains. Experimental Cell Research. 1961;25(3):585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 37.Rodier F, Campisi J. Four faces of cellular senescence. The Journal of Cell Biology. 2011 Feb 21;192(4):547–56. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dagarag M, Evazyan T, Rao N, Effros RB. Genetic Manipulation of Telomerase in HIV-Specific CD8+ T Cells: Enhanced Antiviral Functions Accompany the Increased Proliferative Potential and Telomere Length Stabilization. The Journal of Immunology. 2004 Nov 15;173(10):6303–11. doi: 10.4049/jimmunol.173.10.6303. [DOI] [PubMed] [Google Scholar]
- 39.Spaulding C, Guo W, Effros RB. Resistance to apoptosis in human CD8+ T cells that reach replicative senescence after multiple rounds of antigen-specific proliferation. Experimental Gerontology. 1999;34(5):633–44. doi: 10.1016/s0531-5565(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 40.Vaziri H, Schachter F, Uchida I, Wei L, Zhu X, Effros R, et al. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet. 1993 Apr;52(4):661–7. [PMC free article] [PubMed] [Google Scholar]
- 41.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010 May;16(5):238–46. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Letters. 2005;579(4):859–62. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 43.Globerson A, Effros RB. Ageing of lymphocytes and lymphocytes in the aged. Immunol Today. 2000 Oct;21(10):515–21. doi: 10.1016/s0167-5699(00)01714-x. [DOI] [PubMed] [Google Scholar]
- 44.Effros RB. Long-term immunological memory against viruses. Mech Ageing Dev. 2000 Dec 20;121(1–3):161–71. doi: 10.1016/s0047-6374(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 45.Perillo NL, Naeim F, Walford RL, Effros RB. The in vitro senescence of human T lymphocytes: failure to divide is not associated with a loss of cytolytic activity or memory T cell phenotype. Mech Ageing Dev. 1993 Feb;67(1–2):173–85. doi: 10.1016/0047-6374(93)90121-7. [DOI] [PubMed] [Google Scholar]
- 46.Pawelec G, Schneider EM, Wernet P. Acquisition of suppressive activity and natural killer-like cytotoxicity by human alloproliferative “helper” T cell clones. J Immunol. 1986 Jan;136(2):402–11. [PubMed] [Google Scholar]
- 47.Effros RB. Replicative senescence in the immune system: impact of the Hayflick limit on T-cell function in the elderly. Am J Hum Genet. 1998 May;62(5):1003–7. doi: 10.1086/301845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Effros RB, Pawelec G. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion? Immunol Today. 1997 Sep;18(9):450–4. doi: 10.1016/s0167-5699(97)01079-7. [DOI] [PubMed] [Google Scholar]
- 49.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunological Reviews. 2009;229(1):12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.SCHMITZ ML, BACHER S, DIENZ O. NF-κB activation pathways induced by T cell costimulation. The FASEB Journal. 2003 Dec 1;17(15):2187–93. doi: 10.1096/fj.02-1100rev. [DOI] [PubMed] [Google Scholar]
- 51.Thompson CB, Lindsten T, Ledbetter JA, Kunkel SL, Young HA, Emerson SG, et al. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1333–7. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Effros RB, Boucher N, Porter V, Zhu X, Spaulding C, Walford RL, et al. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994 Nov-Dec;29(6):601–9. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 53.Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009 Jul;30(7):306–12. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vallejo AN, Weyand CM, Goronzy JJ. Functional disruption of the CD28 gene transcriptional initiator in senescent T cells. J Biol Chem. 2001 Jan 26;276(4):2565–70. doi: 10.1074/jbc.M005503200. [DOI] [PubMed] [Google Scholar]
- 55.Parish ST, Wu JE, Effros RB. Sustained CD28 expression delays multiple features of replicative senescence in human CD8 T lymphocytes. J Clin Immunol. 2010 Nov;30(6):798–805. doi: 10.1007/s10875-010-9449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang E, Lee MJ, Pandey S. Control of fibroblast senescence and activation of programmed cell death. J Cell Biochem. 1994 Apr;54(4):432–9. doi: 10.1002/jcb.240540410. [DOI] [PubMed] [Google Scholar]
- 57.Effros RB, Zhu X, Walford RL. Stress response of senescent T lymphocytes: reduced hsp70 is independent of the proliferative block. J Gerontol. 1994 Mar;49(2):B65–70. doi: 10.1093/geronj/49.2.b65. [DOI] [PubMed] [Google Scholar]
- 58.Bonelli MA, Alfieri RR, Petronini PG, Brigotti M, Campanini C, Borghetti AF. Attenuated expression of 70-kDa heat shock protein in WI-38 human fibroblasts during aging in vitro. Exp Cell Res. 1999 Oct 10;252(1):20–32. doi: 10.1006/excr.1999.4614. [DOI] [PubMed] [Google Scholar]
- 59.Rea IM, McNerlan S, Pockley AG. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontol. 2001 Feb;36(2):341–52. doi: 10.1016/s0531-5565(00)00215-1. [DOI] [PubMed] [Google Scholar]
- 60.Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009 Sep;13(9B):3103–9. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parish ST, Wu JE, Effros RB. Modulation of T lymphocyte replicative senescence via TNF-{alpha} inhibition: role of caspase-3. J Immunol. 2009 Apr 1;182(7):4237–43. doi: 10.4049/jimmunol.0803449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dagarag M, Ng H, Lubong R, Effros RB, Yang OO. Differential impairment of lytic and cytokine functions in senescent human immunodeficiency virus type 1-specific cytotoxic T lymphocytes. J Virol. 2003 Mar;77(5):3077–83. doi: 10.1128/JVI.77.5.3077-3083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang OO, Lin H, Dagarag M, Ng HL, Effros RB, Uittenbogaart CH. Decreased perforin and granzyme B expression in senescent HIV-1-specific cytotoxic T lymphocytes. Virology. 2005 Feb 5;332(1):16–9. doi: 10.1016/j.virol.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 64.Watson JD. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 65.Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973 Sep 14;41(1):181–90. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 66.de Lange t DR. Unlimited mileage from telomerase? Scinece. 1999 Feb 12;283(5404):947–9. doi: 10.1126/science.283.5404.947. [DOI] [PubMed] [Google Scholar]
- 67.Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346(6287):866–8. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 68.Lindsey J, McGill NI, Lindsey LA, Green DK, Cooke HJ. In vivo loss of telomeric repeats with age in humans. Mutat Res. 1991 Jan;256(1):45–8. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- 69.Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, et al. The RNA component of human telomerase. Science. 1995 Sep 1;269(5228):1236–41. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 70.Koetz K, Bryl E, Spickschen K, O’Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):9203–8. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maini MK, Soares MV, Zilch CF, Akbar AN, Beverley PC. Virus-induced CD8+ T cell clonal expansion is associated with telomerase up-regulation and telomere length preservation: a mechanism for rescue from replicative senescence. J Immunol. 1999 Apr 15;162(8):4521–6. [PubMed] [Google Scholar]
- 72.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002 Nov;105(2):117–25. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 73.Weng N, Levine BL, June CH, Hodes RJ. Regulation of telomerase RNA template expression in human T lymphocyte development and activation. J Immunol. 1997 Apr 1;158(7):3215–20. [PubMed] [Google Scholar]
- 74.Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang L, et al. Shortened telomeres in the expanded CD28−CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996 Jul;10(8):F17–22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 75.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28−CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol. 1996 May 15;156(10):3587–90. [PubMed] [Google Scholar]
- 76.Parish S, Wu J, Effros R. Sustained CD28 Expression Delays Multiple Features of Replicative Senescence in Human CD8 T Lymphocytes. Journal of Clinical Immunology. 2010;30(6):798–805. doi: 10.1007/s10875-010-9449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Knudson M, Kulkarni S, Ballas ZK, Bessler M, Goldman F. Association of immune abnormalities with telomere shortening in autosomal-dominant dyskeratosis congenita. Blood. 2005 Jan 15;105(2):682–8. doi: 10.1182/blood-2004-04-1673. [DOI] [PubMed] [Google Scholar]
- 78.Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011 Jan 6;469(7328):102–6. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998 Jan 16;279(5349):349–52. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 80.Yang J, Chang E, Cherry AM, Bangs CD, Oei Y, Bodnar A, et al. Human endothelial cell life extension by telomerase expression. J Biol Chem. 1999 Sep 10;274(37):26141–8. doi: 10.1074/jbc.274.37.26141. [DOI] [PubMed] [Google Scholar]
- 81.Fauce SR, Jamieson BD, Chin AC, Mitsuyasu RT, Parish ST, Ng HL, et al. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. J Immunol. 2008 Nov 15;181(10):7400–6. doi: 10.4049/jimmunol.181.10.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aspinall R. Does the immune system of a mouse age faster than the immune system of a human? Bioessays. 1999 Jun;21(6):519–24. doi: 10.1002/(SICI)1521-1878(199906)21:6<519::AID-BIES8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 83.Sherr CJ, DePinho RA. Cellular senescence: mitotic clock or culture shock? Cell. 2000 Aug 18;102(4):407–10. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 84.Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000 Oct;1(1):72–6. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- 85.Klatt T, Ouyang Q, Flad T, Koetter I, Bühring H, Jr, Kalbacher H, et al. Expansion of peripheral CD8+ CD28- T cells in response to Epstein-Barr virus in patients with rheumatoid arthritis. The Journal of Rheumatology. 2005 Feb 1;32(2):239–51. [PubMed] [Google Scholar]
- 86.Lewis DE, Merched-Sauvage M, Goronzy JJ, Weyand CM, Vallejo AN. Tumor necrosis factor-alpha and CD80 modulate CD28 expression through a similar mechanism of T-cell receptor-independent inhibition of transcription. J Biol Chem. 2004 Jul 9;279(28):29130–8. doi: 10.1074/jbc.M402194200. [DOI] [PubMed] [Google Scholar]
- 87.Borthwick NJ, Bofill M, Gombert WM, Akbar AN, Medina E, Sagawa K, et al. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28- T cells. AIDS. 1994 Apr;8(4):431–41. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 88.Appay V, Rowland-Jones SL. Premature ageing of the immune system: the cause of AIDS? Trends in Immunology. 2002;23(12):580–5. doi: 10.1016/s1471-4906(02)02338-4. [DOI] [PubMed] [Google Scholar]
- 89.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005 Feb 25;120(4):513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 90.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 91.Sharpless NE. Ink4a/Arf links senescence and aging. Experimental Gerontology. 2004;39(11–12):1751–9. doi: 10.1016/j.exger.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 92.Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009 Aug;8(4):439–48. doi: 10.1111/j.1474-9726.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ben-Porath I, Weinberg RA. When cells get stressed: an integrative view of cellular senescence. J Clin Invest. 2004 Jan;113(1):8–13. doi: 10.1172/JCI200420663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shawi M, Autexier C. Telomerase, senescence and ageing. Mech Ageing Dev. 2008 Jan-Feb;129(1–2):3–10. doi: 10.1016/j.mad.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 95.von Zglinicki T. Oxidative stress shortens telomeres. Trends in Biochemical Sciences. 2002;27(7):339–44. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 96.Tarazona R, DelaRosa O, Alonso C, Ostos B, Espejo J, Pena J, et al. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000 Dec 20;121(1–3):77–88. doi: 10.1016/s0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 97.Le Priol Y, Puthier D, Lecureuil C, Combadiere C, Debre P, Nguyen C, et al. High cytotoxic and specific migratory potencies of senescent CD8+ CD57+ cells in HIV-infected and uninfected individuals. J Immunol. 2006 Oct 15;177(8):5145–54. doi: 10.4049/jimmunol.177.8.5145. [DOI] [PubMed] [Google Scholar]
- 98.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003 Apr 1;101(7):2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 99.Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral Infections Induce Abundant Numbers of Senescent CD8 T Cells. The Journal of Immunology. 2001 Nov 1;167(9):4838–43. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 100.Henson SM, Franzese O, Macaulay R, Libri V, Azevedo RI, Kiani-Alikhan S, et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009 Jun 25;113(26):6619–28. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 101.Wood KL, Twigg HL, 3rd, Doseff AI. Dysregulation of CD8+ lymphocyte apoptosis, chronic disease, and immune regulation. Front Biosci. 2009;14:3771–81. doi: 10.2741/3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 Selectively Recruit CTLA–4 and CD28 to the Immunological Synapse. Immunity. 2004;21(3):401–13. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 103.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8(11):1246–54. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 104.Ibegbu CC, Xu YX, Harris W, Maggio D, Miller JD, Kourtis AP. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J Immunol. 2005 May 15;174(10):6088–94. doi: 10.4049/jimmunol.174.10.6088. [DOI] [PubMed] [Google Scholar]
- 105.Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beyersdorf NB, Ding X, Karp K, Hanke T. Expression of inhibitory “killer cell lectin-like receptor G1” identifies unique subpopulations of effector and memory CD8 T cells. Eur J Immunol. 2001 Dec;31(12):3443–52. doi: 10.1002/1521-4141(200112)31:12<3443::aid-immu3443>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 107.Brzezinska A, Magalska A, Szybinska A, Sikora E. Proliferation and apoptosis of human CD8(+)CD28(+) and CD8(+)CD28(−) lymphocytes during aging. Exp Gerontol. 2004 Apr;39(4):539–44. doi: 10.1016/j.exger.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 108.Chiu WK, Fann M, Weng NP. Generation and growth of CD28nullCD8+ memory T cells mediated by IL-15 and its induced cytokines. J Immunol. 2006 Dec 1;177(11):7802–10. doi: 10.4049/jimmunol.177.11.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chong LK, Aicheler RJ, Llewellyn-Lacey S, Tomasec P, Brennan P, Wang EC. Proliferation and interleukin 5 production by CD8hi CD57+ T cells. Eur J Immunol. 2008 Apr;38(4):995–1000. doi: 10.1002/eji.200737687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith CR, Jaramillo A, Poindexter NJ, Steward NS, Lu KC, Brennan DC, et al. In vitro T cell proliferation from kidney allograft biopsies with unremarkable pathology: new strategies for an old problem. Transplantation. 2002 Jan 15;73(1):142–5. doi: 10.1097/00007890-200201150-00026. [DOI] [PubMed] [Google Scholar]
- 111.Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000 Dec 20;121(1–3):187–201. doi: 10.1016/s0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 112.Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002 Jan-Mar;37(2–3):445–53. doi: 10.1016/s0531-5565(01)00212-1. [DOI] [PubMed] [Google Scholar]
- 113.Koch S, Larbi A, Ozcelik D, Solana R, Gouttefangeas C, Attig S, et al. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann N Y Acad Sci. 2007 Oct;1114:23–35. doi: 10.1196/annals.1396.043. [DOI] [PubMed] [Google Scholar]
- 114.Vasto S, Colonna-Romano G, Larbi A, Wikby A, Caruso C, Pawelec G. Role of persistent CMV infection in configuring T cell immunity in the elderly. Immun Ageing. 2007;4:2. doi: 10.1186/1742-4933-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Derhovanessian E, Larbi A, Pawelec G. Biomarkers of human immunosenescence: impact of Cytomegalovirus infection. Curr Opin Immunol. 2009 Aug;21(4):440–5. doi: 10.1016/j.coi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 116.Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009 Jan;19(1):47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- 117.Hodes RJ, Hathcock KS, Weng NP. Telomeres in T and B cells. Nat Rev Immunol. 2002 Sep;2(9):699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- 118.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011 Apr;11(4):289–95. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 119.Wherry EJ. T cell exhaustion. Nat Immunol. 2011 Jun;12(6):492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 120.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003 Apr;77(8):4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Petrovas C, Chaon B, Ambrozak DR, Price DA, Melenhorst JJ, Hill BJ, et al. Differential association of programmed death-1 and CD57 with ex vivo survival of CD8+ T cells in HIV infection. J Immunol. 2009 Jul 15;183(2):1120–32. doi: 10.4049/jimmunol.0900182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004 Feb;2(2):E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984 Aug 1;160(2):521–40. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006 Feb 9;439(7077):682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 125.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006 Sep 21;443(7109):350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 126.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006 Oct;12(10):1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 127.Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008 Jun;134(7):1927–37. 37 e1–2. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lichterfeld M, Mou D, Cung TD, Williams KL, Waring MT, Huang J, et al. Telomerase activity of HIV-1-specific CD8+ T cells: constitutive up-regulation in controllers and selective increase by blockade of PD ligand 1 in progressors. Blood. 2008 Nov 1;112(9):3679–87. doi: 10.1182/blood-2008-01-135442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ottaviani E, Franceschi C. The invertebrate phagocytic immunocyte: Clues to a common evolution of immune and neuroendocrine systems. Immunology Today [Article] 1997 Apr;18(4):169–74. doi: 10.1016/s0167-5699(97)84663-4. [DOI] [PubMed] [Google Scholar]
- 130.Pedersen M, Bruunsgaard H, Weis N, Hendel HW, Andreassen BU, Eldrup E, et al. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mechanisms of Ageing and Development. 2003;124(4):495–502. doi: 10.1016/s0047-6374(03)00027-7. [DOI] [PubMed] [Google Scholar]
- 131.Bo S, Gambino R, Pagani A, Guidi S, Gentile L, Cassader M, et al. Relationships between human serum resistin, inflammatory markers and insulin resistance. Int J Obes Relat Metab Disord. 2005;29(11):1315–20. doi: 10.1038/sj.ijo.0803037. [DOI] [PubMed] [Google Scholar]
- 132.Mariani E, Meneghetti A, Neri S, Ravaglia G, Forti P, Cattini L, et al. Chemokine production by natural killer cells from nonagenarians. Eur J Immunol. 2002 Jun;32(6):1524–9. doi: 10.1002/1521-4141(200206)32:6<1524::AID-IMMU1524>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 133.Najib E, Puranik R, Duflou J, Xia Q, Bao S. Age related inflammatory characteristics of coronary artery disease. International Journal of Cardiology. 2012;154(1):65–70. doi: 10.1016/j.ijcard.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 134.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011 Feb;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 135.Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997 Feb;93(1–3):87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- 136.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000 Jun;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 137.Ginaldi L, De Martinis M, Monti D, Franceschi C. Chronic antigenic load and apoptosis in immunosenescence. Trends Immunol. 2005 Feb;26(2):79–84. doi: 10.1016/j.it.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 138.Wikby A, Johansson B, Ferguson F, Olsson J. Age-related changes in immune parameters in a very old population of Swedish people: a longitudinal study. Exp Gerontol. 1994 Sep-Oct;29(5):531–41. doi: 10.1016/0531-5565(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 139.Ferguson FG, Wikby A, Maxson P, Olsson J, Johansson B. Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison between survivors and nonsurvivors. J Gerontol A Biol Sci Med Sci. 1995 Nov;50(6):B378–82. doi: 10.1093/gerona/50a.6.b378. [DOI] [PubMed] [Google Scholar]
- 140.Pawelec G, Ferguson FG, Wikby A. The SENIEUR protocol after 16 years. Mechanisms of Ageing and Development. 2001;122(2):132–4. doi: 10.1016/s0047-6374(00)00240-2. [DOI] [PubMed] [Google Scholar]
- 141.Pawelec G, Ouyang Q, Wagner W, Wikby A. Pathways to a robust immune response in the elderly. Immunology and Allergy Clinics of North America. 2003;23(1):1–13. doi: 10.1016/s0889-8561(02)00075-9. [DOI] [PubMed] [Google Scholar]
- 142.Bruunsgaard H. Effects of tumor necrosis factor-alpha and interleukin-6 in elderly populations. Eur Cytokine Netw. 2002 Oct-Dec;13(4):389–91. [PubMed] [Google Scholar]
- 143.Mooradian AD, Reed RL, Osterweil D, Scuderi P. Detectable serum levels of tumor necrosis factor alpha may predict early mortality in elderly institutionalized patients. J Am Geriatr Soc. 1991 Sep;39(9):891–4. doi: 10.1111/j.1532-5415.1991.tb04456.x. [DOI] [PubMed] [Google Scholar]
- 144.Goldring SR. Inflammatory mediators as essential elements in bone remodeling. Calcif Tissue Int. 2003 Aug;73(2):97–100. doi: 10.1007/s00223-002-1049-y. [DOI] [PubMed] [Google Scholar]
- 145.Yun AJ, Bazar KA, Lee PY. Pineal attrition, loss of cognitive plasticity, and onset of puberty during the teen years: is it a modern maladaptation exposed by evolutionary displacement? Med Hypotheses. 2004;63(6):939–50. doi: 10.1016/j.mehy.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 146.Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immun Ageing. 2005 Nov 4;2:14. doi: 10.1186/1742-4933-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.De Martinis M, Di Benedetto MC, Mengoli LP, Ginaldi L. Senile osteoporosis: is it an immune-mediated disease? Inflamm Res. 2006 Oct;55(10):399–404. doi: 10.1007/s00011-006-6034-x. [DOI] [PubMed] [Google Scholar]
- 148.Haugeberg G, Lodder MC, Lems WF, Uhlig T, Orstavik RE, Dijkmans BA, et al. Hand cortical bone mass and its associations with radiographic joint damage and fractures in 50–70 year old female patients with rheumatoid arthritis: cross sectional Oslo-Truro-Amsterdam (OSTRA) collaborative study. Ann Rheum Dis. 2004 Oct;63(10):1331–4. doi: 10.1136/ard.2003.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bultink IE, Lems WF, Kostense PJ, Dijkmans BA, Voskuyl AE. Prevalence of and risk factors for low bone mineral density and vertebral fractures in patients with systemic lupus erythematosus. Arthritis Rheum. 2005 Jul;52(7):2044–50. doi: 10.1002/art.21110. [DOI] [PubMed] [Google Scholar]
- 150.Mikuls TR, Saag KG, Curtis J, Bridges SL, Jr, Alarcon GS, Westfall AO, et al. Prevalence of osteoporosis and osteopenia among African Americans with early rheumatoid arthritis: the impact of ethnic-specific normative data. J Natl Med Assoc. 2005 Aug;97(8):1155–60. [PMC free article] [PubMed] [Google Scholar]
- 151.Annapoorna N, Rao GV, Reddy NS, Rambabu P, Rao KR. An Increased Risk of Osteoporosis during Acquired Immunodeficiency Syndrome. Int J Med Sci. 2004;1(3):152–64. doi: 10.7150/ijms.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Koh J-M, Khang Y-H, Jung C-H, Bae S, Kim D, Chung Y-E, et al. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre-and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporosis International. 2005;16(10):1263–71. doi: 10.1007/s00198-005-1840-5. [DOI] [PubMed] [Google Scholar]
- 153.Saidenberg-Kermanac’h N, Cohen-Solal M, Bessis N, De Vernejoul MC, Boissier MC. Role for osteoprotegerin in rheumatoid inflammation. Joint Bone Spine. 2004 Jan;71(1):9–13. doi: 10.1016/S1297-319X(03)00131-3. [DOI] [PubMed] [Google Scholar]
- 154.Effros RB. Replicative senescence of CD8 T cells: effect on human ageing. Experimental Gerontology. 2004;39(4):517–24. doi: 10.1016/j.exger.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 155.Arron JR, Choi Y. Bone versus immune system. Nature. 2000 Nov 30;408(6812):535–6. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- 156.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000 Sep 1;289(5484):1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 157.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-[gamma] Nature. 2000;408(6812):600–5. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 158.Theill LE, Boyle WJ, Penninger JM. RANK-L AND RANK: T Cells, Bone Loss, and Mammalian Evolution. Annual Review of Immunology. 2002;20(1):795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 159.Nanes MS. Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene. 2003 Dec 4;321:1–15. doi: 10.1016/s0378-1119(03)00841-2. [DOI] [PubMed] [Google Scholar]
- 160.Muller B. Cytokine imbalance in non-immunological chronic disease. Cytokine. 2002 Jun 21;18(6):334–9. doi: 10.1006/cyto.2002.0882. [DOI] [PubMed] [Google Scholar]
- 161.Hofbauer LC. Osteoprotegerin ligand and osteoprotegerin: novel implications for osteoclast biology and bone metabolism. Eur J Endocrinol. 1999 Sep;141(3):195–210. doi: 10.1530/eje.0.1410195. [DOI] [PubMed] [Google Scholar]
- 162.Pietschmann P, Grisar J, Thien R, Willheim M, Kerschan-Schindl K, Preisinger E, et al. Immune phenotype and intracellular cytokine production of peripheral blood mononuclear cells from postmenopausal patients with osteoporotic fractures. Experimental Gerontology. 2001;36(10):1749–59. doi: 10.1016/s0531-5565(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 163.Lorenzo J. Interactions between immune and bone cells: new insights with many remaining questions. J Clin Invest. 2000 Sep;106(6):749–52. doi: 10.1172/JCI11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Seguier S, Godeau G, Leborgne M, Pivert G, Brousse N. Immunohistologic and morphometric analysis of cytotoxic T lymphocytes in gingivitis. J Periodontol. 1999 Nov;70(11):1383–91. doi: 10.1902/jop.1999.70.11.1383. [DOI] [PubMed] [Google Scholar]
- 165.Wassenaar A, Reinhardus C, Abraham-Inpijn L, Kievitz F. Type-1 and type-2 CD8+ T-cell subsets isolated from chronic adult periodontitis tissue differ in surface phenotype and biological functions. Immunology. 1996 Jan;87(1):113–8. [PMC free article] [PubMed] [Google Scholar]
- 166.Fracon RN, Teofilo JM, Satin RB, Lamano T. Prostaglandins and bone: potential risks and benefits related to the use of nonsteroidal anti-inflammatory drugs in clinical dentistry. J Oral Sci. 2008 Sep;50(3):247–52. doi: 10.2334/josnusd.50.247. [DOI] [PubMed] [Google Scholar]
- 167.Preshaw PM, Heasman PA. Prostaglandin E2 concentrations in gingival crevicular fluid: observations in untreated chronic periodontitis. J Clin Periodontol. 2002 Jan;29(1):15–20. doi: 10.1034/j.1600-051x.2002.290103.x. [DOI] [PubMed] [Google Scholar]
- 168.Alpagot T, Remien J, Bhattacharyya M, Konopka K, Lundergan W, Duzgunes N. Longitudinal evaluation of prostaglandin E2 (PGE2) and periodontal status in HIV+ patients. Arch Oral Biol. 2007 Nov;52(11):1102–8. doi: 10.1016/j.archoralbio.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mayahara K, Kobayashi Y, Takimoto K, Suzuki N, Mitsui N, Shimizu N. Aging stimulates cyclooxygenase-2 expression and prostaglandin E2 production in human periodontal ligament cells after the application of compressive force. J Periodontal Res. 2007 Feb;42(1):8–14. doi: 10.1111/j.1600-0765.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 170.Holick MF. Vitamin D and bone health. J Nutr. 1996 Apr;126(4 Suppl):1159S–64S. doi: 10.1093/jn/126.suppl_4.1159S. [DOI] [PubMed] [Google Scholar]
- 171.Mattner F, Smiroldo S, Galbiati F, Muller M, Di Lucia P, Poliani PL, et al. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D3. European Journal of Immunology. 2000;30(2):498–508. doi: 10.1002/1521-4141(200002)30:2<498::AID-IMMU498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 172.Bhalla A, Amento E, Serog B, Glimcher L. 1,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. The Journal of Immunology. 1984 Oct 1;133(4):1748–54. [PubMed] [Google Scholar]
- 173.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005 Jul;19(9):1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 174.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of Human Papillomavirus-Associated Cancers Among Persons With AIDS. Journal of the National Cancer Institute. 2009 Aug 19;101(16):1120–30. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chakraborty NG, Stevens RL, Mehrotra S, Laska E, Taxel P, Sporn JR, et al. Recognition of PSA-derived peptide antigens by T cells from prostate cancer patients without any prior stimulation. Cancer Immunol Immunother. 2003 Aug;52(8):497–505. doi: 10.1007/s00262-003-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kennedy-Smith AG, McKenzie JL, Owen MC, Davidson PJ, Vuckovic S, Hart DN. Prostate specific antigen inhibits immune responses in vitro: a potential role in prostate cancer. J Urol. 2002 Aug;168(2):741–7. [PubMed] [Google Scholar]
- 177.Meloni F, Morosini M, Solari N, Passadore I, Nascimbene C, Novo M, et al. Foxp3 expressing CD4+ CD25+ and CD8+CD28− T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Human Immunology. 2006 Jan-Feb;67(1–2):1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 178.Ye SW, Wang Y, Valmori D, Ayyoub M, Han Y, Xu XL, et al. Ex-vivo analysis of CD8+ T cells infiltrating colorectal tumors identifies a major effector-memory subset with low perforin content. J Clin Immunol. 2006 Sep;26(5):447–56. doi: 10.1007/s10875-006-9040-4. [DOI] [PubMed] [Google Scholar]
- 179.Webb JR, Wick DA, Nielsen JS, Tran E, Milne K, McMurtrie E, et al. Profound elevation of CD8+ T cells expressing the intraepithelial lymphocyte marker CD103 (alphaE/beta7 Integrin) in high-grade serous ovarian cancer. Gynecol Oncol. 2010 Sep;118(3):228–36. doi: 10.1016/j.ygyno.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 180.Urbaniak-Kujda D, Kapelko-Slowik K, Wolowiec D, Dybko J, Halon A, Jazwiec B, et al. Increased percentage of CD8+CD28− suppressor lymphocytes in peripheral blood and skin infiltrates correlates with advanced disease in patients with cutaneous T-cell lymphomas. Postepy Hig Med Dosw (Online) 2009;63:355–9. [PubMed] [Google Scholar]
- 181.Gruber IV, El Yousfi S, Durr-Storzer S, Wallwiener D, Solomayer EF, Fehm T. Down-regulation of CD28, TCR-zeta (zeta) and up-regulation of FAS in peripheral cytotoxic T-cells of primary breast cancer patients. Anticancer Res. 2008 Mar-Apr;28(2A):779–84. [PubMed] [Google Scholar]
- 182.Schule JM, Bergkvist L, Hakansson L, Gustafsson B, Hakansson A. CD28 expression in sentinel node biopsies from breast cancer patients in comparison with CD3-zeta chain expression. J Transl Med. 2004 Dec 21;2(1):45. doi: 10.1186/1479-5876-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, et al. CD8+ CD28− T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007 Oct 1;179(7):4323–34. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 184.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009 Aug 20;114(8):1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sze DM, Giesajtis G, Brown RD, Raitakari M, Gibson J, Ho J, et al. Clonal cytotoxic T cells are expanded in myeloma and reside in the CD8(+)CD57(+)CD28(−) compartment. Blood. 2001 Nov 1;98(9):2817–27. doi: 10.1182/blood.v98.9.2817. [DOI] [PubMed] [Google Scholar]
- 186.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(−) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003 Oct;52(10):599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Characiejus D, Pasukoniene V, Kazlauskaite N, Valuckas KP, Petraitis T, Mauricas M, et al. Predictive value of CD8highCD57+ lymphocyte subset in interferon therapy of patients with renal cell carcinoma. Anticancer Res. 2002 Nov-Dec;22(6B):3679–83. [PubMed] [Google Scholar]
- 188.Montes CL, Chapoval AI, Nelson J, Orhue V, Zhang X, Schulze DH, et al. Tumor-induced senescent T cells with suppressor function: a potential form of tumor immune evasion. Cancer Res. 2008 Feb 1;68(3):870–9. doi: 10.1158/0008-5472.CAN-07-2282. [DOI] [PubMed] [Google Scholar]
- 189.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011 Jun;20(6):1238–50. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shen X, Zhou J, Hathcock KS, Robbins P, Powell DJ, Jr, Rosenberg SA, et al. Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length. J Immunother. 2007 Jan;30(1):123–9. doi: 10.1097/01.cji.0000211321.07654.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere Length of Transferred Lymphocytes Correlates with In Vivo Persistence and Tumor Regression in Melanoma Patients Receiving Cell Transfer Therapy. The Journal of Immunology. 2005 Nov 15;175(10):7046–52. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.McElhaney JE. Influenza vaccination in the elderly: seeking new correlates of protection and improved vaccines. Aging health. 2008 Dec 1;4(6):603–13. doi: 10.2217/1745509X.4.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Grubeck-Loebenstein B, Della Bella S, Iorio AM, Michel JP, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res. 2009 Jun;21(3):201–9. doi: 10.1007/BF03324904. [DOI] [PubMed] [Google Scholar]
- 194.Gravekamp C. The importance of the age factor in cancer vaccination at older age. Cancer Immunology, Immunotherapy. 2009;58(12):1969–77. doi: 10.1007/s00262-009-0683-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):3336–40. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Singh B, Berry JA, Shoher A, Ramakrishnan V, Lucci A. COX-2 overexpression increases motility and invasion of breast cancer cells. Int J Oncol. 2005 May;26(5):1393–9. [PubMed] [Google Scholar]
- 197.Wu D, Marko M, Claycombe K, Paulson KE, Meydani SN. Ceramide-induced and age-associated increase in macrophage COX-2 expression is mediated through up-regulation of NF-kappa B activity. J Biol Chem. 2003 Mar 28;278(13):10983–92. doi: 10.1074/jbc.M207470200. [DOI] [PubMed] [Google Scholar]
- 198.Pedemonte E, Mancardi G, Giunti D, Corcione A, Benvenuto F, Pistoia V, et al. Mechanisms of the adaptive immune response inside the central nervous system during inflammatory and autoimmune diseases. Pharmacology & Therapeutics. 2006;111(3):555–66. doi: 10.1016/j.pharmthera.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 199.Ron-Harel N, Segev Y, Lewitus GM, Cardon M, Ziv Y, Netanely D, et al. Age-dependent spatial memory loss can be partially restored by immune activation. Rejuvenation Res. 2008 Oct;11(5):903–13. doi: 10.1089/rej.2008.0755. [DOI] [PubMed] [Google Scholar]
- 200.Franco S, Blasco MA, Siedlak SL, Harris PLR, Moreira PI, Perry G, et al. Telomeres and telomerase in Alzheimer’s disease: Epiphenomena or a new focus for therapeutic strategy? Alzheimer’s and Dementia. 2006;2(3):164–8. doi: 10.1016/j.jalz.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 201.Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, et al. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol Aging. 2003 Jan-Feb;24(1):77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 202.Medana IM, Day NP, Hien TT, Mai NT, Bethell D, Phu NH, et al. Axonal injury in cerebral malaria. Am J Pathol. 2002 Feb;160(2):655–66. doi: 10.1016/S0002-9440(10)64885-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bien CG, Bauer J, Deckwerth TL, Wiendl H, Deckert M, Wiestler OD, et al. Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen’s encephalitis. Ann Neurol. 2002 Mar;51(3):311–8. doi: 10.1002/ana.10100. [DOI] [PubMed] [Google Scholar]
- 204.Gay FW, Drye TJ, Dick GW, Esiri MM. The application of multifactorial cluster analysis in the staging of plaques in early multiple sclerosis. Identification and characterization of the primary demyelinating lesion. Brain. 1997 Aug;120( Pt 8):1461–83. doi: 10.1093/brain/120.8.1461. [DOI] [PubMed] [Google Scholar]
- 205.Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Bruck W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002 Oct;125(Pt 10):2202–12. doi: 10.1093/brain/awf235. [DOI] [PubMed] [Google Scholar]
- 206.Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997 Mar;120( Pt 3):393–9. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- 207.Hoftberger R, Aboul-Enein F, Brueck W, Lucchinetti C, Rodriguez M, Schmidbauer M, et al. Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol. 2004 Jan;14(1):43–50. doi: 10.1111/j.1750-3639.2004.tb00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mikova O, Yakimova R, Bosmans E, Kenis G, Maes M. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur Neuropsychopharmacol. 2001 Jun;11(3):203–8. doi: 10.1016/s0924-977x(01)00081-5. [DOI] [PubMed] [Google Scholar]
- 209.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006 Feb;9(2):268–75. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 210.Moalem G, Gdalyahu A, Shani Y, Otten U, Lazarovici P, Cohen IR, et al. Production of neurotrophins by activated T cells: implications for neuroprotective autoimmunity. J Autoimmun. 2000 Nov;15(3):331–45. doi: 10.1006/jaut.2000.0441. [DOI] [PubMed] [Google Scholar]
- 211.Hohlfeld R, Kerschensteiner M, Stadelmann C, Lassmann H, Wekerle H. The neuroprotective effect of inflammation: implications for the therapy of multiple sclerosis. Neurol Sci. 2006 Mar;27( Suppl 1):S1–7. doi: 10.1007/s10072-006-0537-7. [DOI] [PubMed] [Google Scholar]
- 212.Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007 Feb;55(3):233–8. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- 213.Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, et al. Vaccination as a novel approach for treating depressive behavior. Biol Psychiatry. 2009 Feb 15;65(4):283–8. doi: 10.1016/j.biopsych.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 214.Butovsky E, Juknat A, Goncharov I, Elbaz J, Eilam R, Zangen A, et al. In vivo up-regulation of brain-derived neurotrophic factor in specific brain areas by chronic exposure to Delta-tetrahydrocannabinol. J Neurochem. 2005 May;93(4):802–11. doi: 10.1111/j.1471-4159.2005.03074.x. [DOI] [PubMed] [Google Scholar]
- 215.Shaked I, Tchoresh D, Gersner R, Meiri G, Mordechai S, Xiao X, et al. Protective autoimmunity: interferon-gamma enables microglia to remove glutamate without evoking inflammatory mediators. J Neurochem. 2005 Mar;92(5):997–1009. doi: 10.1111/j.1471-4159.2004.02954.x. [DOI] [PubMed] [Google Scholar]
- 216.Butovsky O, Bukshpan S, Kunis G, Jung S, Schwartz M. Microglia can be induced by IFN-gamma or IL-4 to express neural or dendritic-like markers. Mol Cell Neurosci. 2007 Jul;35(3):490–500. doi: 10.1016/j.mcn.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 217.Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–41. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Libby P, Sukhova G, Lee RT, Galis ZS. Cytokines regulate vascular functions related to stability of the atherosclerotic plaque. J Cardiovasc Pharmacol. 1995;25( Suppl 2):S9–12. doi: 10.1097/00005344-199500252-00003. [DOI] [PubMed] [Google Scholar]
- 219.Schmitz G, Herr AS, Rothe G. T-lymphocytes and monocytes in atherogenesis. Herz. 1998 May;23(3):168–77. doi: 10.1007/BF03044602. [DOI] [PubMed] [Google Scholar]
- 220.Seko Y, Sato O, Takagi A, Tada Y, Matsuo H, Yagita H, et al. Perforin-secreting killer cell infiltration in the aortic tissue of patients with atherosclerotic aortic aneurysm. Jpn Circ J. 1997 Dec;61(12):965–70. doi: 10.1253/jcj.61.965. [DOI] [PubMed] [Google Scholar]
- 221.Zhdanov VS, Chumachenko PV, Cherpachenko NM, Beskrovnova NN, Lavrova NF. Subpopulations of lymphocytes and monocytes/macrophages at the early stages of human aorta atherosclerosis. Arkh Patol. 1995 Jul-Aug;57(4):67–71. [PubMed] [Google Scholar]
- 222.Mach F, Schonbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, et al. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):1931–6. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Spyridopoulos I, Hoffmann J, Aicher A, Brümmendorf TH, Doerr HW, Zeiher AM, et al. Accelerated Telomere Shortening in Leukocyte Subpopulations of Patients With Coronary Heart Disease. Circulation. 2009 Oct 6;120(14):1364–72. doi: 10.1161/CIRCULATIONAHA.109.854299. [DOI] [PubMed] [Google Scholar]
- 224.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001 Aug 11;358(9280):472–3. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 225.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003 May 1;23(5):842–6. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 226.Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004 May 1;117(Pt 11):2417–26. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 227.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003 Feb 1;361(9355):393–5. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 228.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011 Feb 15;203(4):452–63. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–97. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Giubilato S, Liuzzo G, Brugaletta S, Pitocco D, Graziani F, Smaldone C, et al. Expansion of CD4+CD28null T-lymphocytes in diabetic patients: exploring new pathogenetic mechanisms of increased cardiovascular risk in diabetes mellitus. European Heart Journal. 2011 May 1;32(10):1214–26. doi: 10.1093/eurheartj/ehq499. [DOI] [PubMed] [Google Scholar]
- 231.Vedhara K, Cox NK, Wilcock GK, Perks P, Hunt M, Anderson S, et al. Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination. Lancet. 1999 Feb 20;353(9153):627–31. doi: 10.1016/S0140-6736(98)06098-X. [DOI] [PubMed] [Google Scholar]
- 232.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004 Dec 7;101(49):17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009 Feb;18(2):551–60. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005 Jan 3;201(1):139–48. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003 Jul 22;100(15):9090–5. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Aviv A. Telomeres and human aging: facts and fibs. Sci Aging Knowledge Environ. 2004 Dec 22;(51):pe43. doi: 10.1126/sageke.2004.51.pe43. [DOI] [PubMed] [Google Scholar]
- 237.Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, et al. Leukocyte Telomere Length in Major Depression: Correlations with Chronicity, Inflammation and Oxidative Stress - Preliminary Findings. PLoS ONE. 2011;6(3):e17837. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Evans DL, Ten Have TR, Douglas SD, Gettes DR, Morrison M, Chiappini MS, et al. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am J Psychiatry. 2002 Oct;159(10):1752–9. doi: 10.1176/appi.ajp.159.10.1752. [DOI] [PubMed] [Google Scholar]
- 239.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991 Aug 29;325(9):606–12. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 240.Sachar EJ, Asnis G, Nathan RS, Halbreich U, Tabrizi MA, Halpern FS. Dextroamphetamine and Cortisol in Depression: Morning Plasma Cortisol Levels Suppressed. Arch Gen Psychiatry. 1980 Jul 1;37(7):755–7. doi: 10.1001/archpsyc.1980.01780200033003. [DOI] [PubMed] [Google Scholar]
- 241.Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun. 2008 May;22(4):600–5. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis. 2000 Dec;182(6):1761–4. doi: 10.1086/317624. [DOI] [PubMed] [Google Scholar]
- 243.Pierson DL, Stowe RP, Phillips TM, Lugg DJ, Mehta SK. Epstein-Barr virus shedding by astronauts during space flight. Brain Behav Immun. 2005 May;19(3):235–42. doi: 10.1016/j.bbi.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 244.Glaser R, Pearl DK, Kiecolt-Glaser JK, Malarkey WB. Plasma cortisol levels and reactivation of latent Epstein-Barr virus in response to examination stress. Psychoneuroendocrinology. 1994;19(8):765–72. doi: 10.1016/0306-4530(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 245.Glaser R, Pearson GR, Jones JF, Hillhouse J, Kennedy S, Mao HY, et al. Stress-related activation of Epstein-Barr virus. Brain Behav Immun. 1991 Jun;5(2):219–32. doi: 10.1016/0889-1591(91)90018-6. [DOI] [PubMed] [Google Scholar]
- 246.Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, et al. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002 Feb 5;105(5):570–5. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- 247.Yung RL, Julius A. Epigenetics, aging, and autoimmunity. Autoimmunity. 2008 May;41(4):329–35. doi: 10.1080/08916930802024889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Morgan MD, Pachnio A, Begum J, Roberts D, Rasmussen N, Neil DA, et al. CD4+CD28− T cell expansion in granulomatosis with polyangiitis (Wegener’s) is driven by latent cytomegalovirus infection and is associated with an increased risk of infection and mortality. Arthritis Rheum. 2011 Jul;63(7):2127–37. doi: 10.1002/art.30366. [DOI] [PubMed] [Google Scholar]
- 249.Hooper M, Kallas EG, Coffin D, Campbell D, Evans TG, Looney RJ. Cytomegalovirus seropositivity is associated with the expansion of CD4+CD28− and CD8+CD28− T cells in rheumatoid arthritis. J Rheumatol. 1999 Jul;26(7):1452–7. [PubMed] [Google Scholar]
- 250.Fasth AE, Dastmalchi M, Rahbar A, Salomonsson S, Pandya JM, Lindroos E, et al. T cell infiltrates in the muscles of patients with dermatomyositis and polymyositis are dominated by CD28null T cells. J Immunol. 2009 Oct 1;183(7):4792–9. doi: 10.4049/jimmunol.0803688. [DOI] [PubMed] [Google Scholar]
- 251.Fernandez S, French MA, Price P. Immunosenescent CD57+CD4+ T-cells accumulate and contribute to interferon-gamma responses in HIV patients responding stably to ART. Dis Markers. 2011 Jan 1;31(6):337–42. doi: 10.3233/DMA-2011-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Schleiss MR. Cytomegalovirus vaccines and methods of production (WO20009049138): the emerging recognition of the importance of virus neutralization at the epithelial/endothelial interface. Expert Opin Ther Pat. 2010 Apr;20(4):597–602. doi: 10.1517/13543770903584882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hata A, Asanuma H, Rinki M, Sharp M, Wong RM, Blume K, et al. Use of an Inactivated Varicella Vaccine in Recipients of Hematopoietic-Cell Transplants. New England Journal of Medicine. 2002;347(1):26–34. doi: 10.1056/NEJMoa013441. [DOI] [PubMed] [Google Scholar]
- 254.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003 Dec;4(12):1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 255.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011 May;11(5):330–42. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhang Y, Pfannenstiel LW, Bolesta E, Montes CL, Zhang X, Chapoval AI, et al. Interleukin-7 inhibits tumor-induced CD27−CD28− suppressor T cells: implications for cancer immunotherapy. Clin Cancer Res. 2011 Aug 1;17(15):4975–86. doi: 10.1158/1078-0432.CCR-10-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001 Dec 15;167(12):6869–76. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 258.Parmigiani A, Pallin MF, Schmidtmayerova H, Lichtenheld MG, Pahwa S. Interleukin-21 and cellular activation concurrently induce potent cytotoxic function and promote antiviral activity in human CD8 T cells. Hum Immunol. 2011 Feb;72(2):115–23. doi: 10.1016/j.humimm.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Liu S, Lizee G, Lou Y, Liu C, Overwijk WW, Wang G, et al. IL-21 synergizes with IL-7 to augment expansion and anti-tumor function of cytotoxic T cells. Int Immunol. 2007 Oct;19(10):1213–21. doi: 10.1093/intimm/dxm093. [DOI] [PubMed] [Google Scholar]
- 260.Boehm T, Bleul CC. The evolutionary history of lymphoid organs. Nat Immunol. 2007 Feb;8(2):131–5. doi: 10.1038/ni1435. [DOI] [PubMed] [Google Scholar]
- 261.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009 Jan 16;206(1):35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, et al. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012 Apr 6;336(6077):91–5. doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004 Jan 1;18(1):12–6. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004 Nov 9;101(45):15998–6003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001 Mar 8;410(6825):227–30. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 266.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986 Apr;116(4):641–54. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 267.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005 Sep;126(9):913–22. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 268.Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010 Mar;21(3):134–41. doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009 Jul 10;325(5937):201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003 Feb 1;17(3):313–21. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- 271.Chen D, Guarente L. SIR2: a potential target for calorie restriction mimetics. Trends Mol Med. 2007 Feb;13(2):64–71. doi: 10.1016/j.molmed.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 272.Qiu X, Brown KV, Moran Y, Chen D. Sirtuin regulation in calorie restriction. Biochim Biophys Acta. 2010 Aug;1804(8):1576–83. doi: 10.1016/j.bbapap.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 273.Salminen A, Kaarniranta K. SIRT1: regulation of longevity via autophagy. Cell Signal. 2009 Sep;21(9):1356–60. doi: 10.1016/j.cellsig.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 274.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007 May 15;404(1):1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006 Dec 14;444(7121):868–74. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- 276.Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006 Jul 28;126(2):257–68. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 277.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004 Mar 26;303(5666):2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 278.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007 Oct;6(4):307–19. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 279.Westerheide SD, Anckar J, Stevens SM, Jr, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009 Feb 20;323(5917):1063–6. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Harshman LG, Haberer BA. Oxidative stress resistance: a robust correlated response to selection in extended longevity lines of Drosophila melanogaster? J Gerontol A Biol Sci Med Sci. 2000 Sep;55(9):B415–7. doi: 10.1093/gerona/55.9.b415. [DOI] [PubMed] [Google Scholar]
- 281.Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000 Oct 6;275(40):31505–13. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- 282.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008 Jul 25;134(2):329–40. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008 Jul 25;134(2):317–28. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 284.Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008 Nov 28;135(5):907–18. doi: 10.1016/j.cell.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3(4):e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, et al. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009 Mar;29(5):1363–74. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Jeong H, Cohen DE, Cui L, Supinski A, Savas JN, Mazzulli JR, et al. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med. 2012 Jan;18(1):159–65. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007 Jul 11;26(13):3169–79. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003 Sep 11;425(6954):191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 290.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004 Aug 5;430(7000):686–9. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 291.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3(6):e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008 Aug;8(2):157–68. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006 Nov 16;444(7117):337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012 Feb 3;148(3):421–33. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011 Feb;66(2):191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hector KL, Lagisz M, Nakagawa S. The effect of resveratrol on longevity across species: a meta-analysis. Biol Lett. 2012 Jun 20; doi: 10.1098/rsbl.2012.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Yamazaki Y, Usui I, Kanatani Y, Matsuya Y, Tsuneyama K, Fujisaka S, et al. Treatment with SRT1720, a SIRT1 activator, ameliorates fatty liver with reduced expression of lipogenic enzymes in MSG mice. Am J Physiol Endocrinol Metab. 2009 Nov;297(5):E1179–86. doi: 10.1152/ajpendo.90997.2008. [DOI] [PubMed] [Google Scholar]
- 298.Knutson MD, Leeuwenburgh C. Resveratrol and novel potent activators of SIRT1: effects on aging and age-related diseases. Nutr Rev. 2008 Oct;66(10):591–6. doi: 10.1111/j.1753-4887.2008.00109.x. [DOI] [PubMed] [Google Scholar]
- 299.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007 Nov 29;450(7170):712–6. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Funk JA, Odejinmi S, Schnellmann RG. SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther. 2010 May;333(2):593–601. doi: 10.1124/jpet.109.161992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Donmez G, Guarente L. Aging and disease: connections to sirtuins. Aging Cell. 2010 Apr;9(2):285–90. doi: 10.1111/j.1474-9726.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- 302.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010 Mar 12;285(11):8340–51. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, et al. SIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem. 2010 Oct 22;285(43):32695–703. doi: 10.1074/jbc.M110.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005 Jun 28;102(26):9194–9. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Florian MC, Dorr K, Niebel A, Daria D, Schrezenmeier H, Rojewski M, et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 2012 May 4;10(5):520–30. doi: 10.1016/j.stem.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Bernardes de Jesus B, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, et al. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med. 2012 May 15; doi: 10.1002/emmm.201200245. [DOI] [PMC free article] [PubMed] [Google Scholar]