Abstract
Background
This open-label single-arm exploratory study evaluated the accuracy of the Ingestible Sensor System (ISS), a novel technology for directly assessing the ingestion of oral medications and treatment adherence.
Methods
ISS consists of an ingestible event marker (IEM), a microsensor that becomes activated in gastric fluid, and an adhesive personal monitor (APM) that detects IEM activation. In this study, the IEM was combined to enteric-coated mycophenolate sodium (ECMPS). Twenty stable adult kidney transplants received IEM-ECMPS for a mean of 9.2 weeks totaling 1227 cumulative days.
Results
Eight patients prematurely discontinued treatment due to ECMPS gastrointestinal symptoms (n=2), skin intolerance to APM (n=2), and insufficient system usability (n=4). Rash or erythema due to APM was reported in 7 (37%) patients, all during the first month of use. No serious or severe adverse events and no rejection episode were reported. IEM detection accuracy was 100% over 34 directly observed ingestions; Taking Adherence was 99.4% over a total of 2824 prescribed IEM-ECMPS ingestions. ISS could detect accurately the ingestion of two IEM-ECMPS capsules taken at the same time (detection rate of 99.3%, n=2376).
Conclusions
ISS is a promising new technology that provides highly reliable measurements of intake and timing of intake of drugs that are combined with the IEM.
Keywords: Treatment adherence, Kidney transplantation, Ingestible sensor system, Telemedicine, Enteric-coated mycophenolate sodium
Nonadherence to prescribed medications is an important issue in the clinical management of kidney transplant patients (1–3). It is common in the first months after kidney transplantation and increases by duration of follow-up (4). Accurate detection of nonadherence is a challenging but essential first step that enables further interventions aimed at improving adherence (5, 6). All adherence measurement methods used currently in transplant patients are indirect; each has advantages and disadvantages and all lack accuracy (7). Therefore, more reliable methods that could provide direct confirmation of medication use and identify nonadherence are highly desirable to support transplant patients and healthcare professionals who care for them.
The Ingestible Sensor System (ISS) is a new technology that enables direct and accurate measurement of medication adherence and the capture of medication intake dynamics via the ingestion of microsensors that can accompany or be incorporated into oral dose forms of active pharmaceuticals. This device has been approved for use in European Union (CE-mark) and in the United States (Food and Drug Administration clearance). The ISS technology is composed of the ingestible event marker (IEM), a microsensor that becomes activated after ingestion, and the adhesive personal monitor (APM) affixed to the torso that detects the IEM once activated (Fig. 1). A wireless Bluetooth-based interface within the APM allows the APM to transmit its stored data to a smartphone, which in turn sends the data to a secured, centralized data storage and processing location via the mobile telephony network. The objective of this pilot study was to evaluate the detection accuracy, usability, and safety of ISS combined with enteric-coated mycophenolate sodium (ECMPS) in kidney transplants.
FIGURE 1.
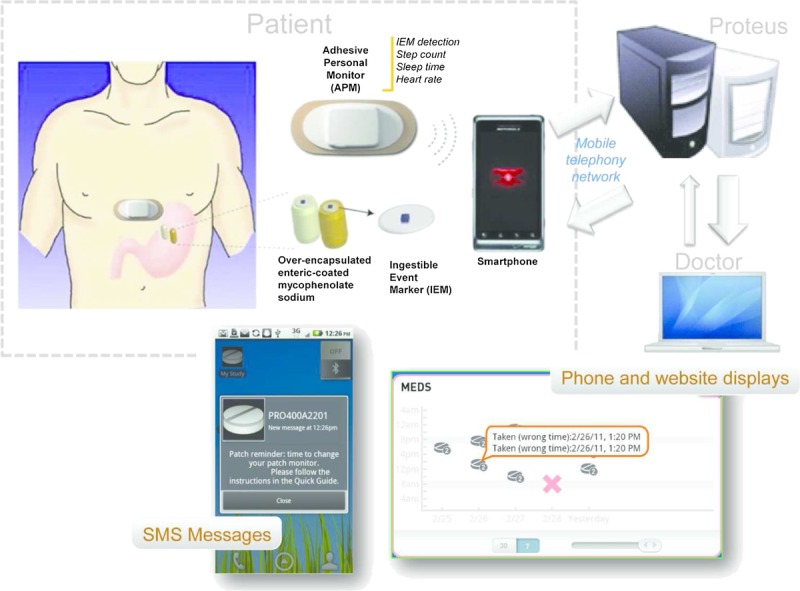
Elements of the ingestible sensor system.
RESULTS
Twenty patients participated in this 12-week uncontrolled pilot study. Patients were in majority Caucasians (19 of 20), males (15 of 20), and 51.7±8.8 years old (mean±SD; range 35–68) and had a body weight of 81.8±12.7 kg (range, 54.5–105.4). They were in stable condition after an average of 6.0±5.0 years after renal transplantation (range, 1–19 years) and taking ECMPS or mycophenolate mofetil in combination with cyclosporine (7 patients), tacrolimus (11 patients), sirolimus (1 patient), or everolimus (1 patient). Three (15%) patients had a second transplant.
Patients were exposed to ISS for an average of 9.2 weeks and a total of 1227 cumulative days. Eight (40%) patients discontinued treatment prematurely: 2 due to skin intolerance to the APM, 2 due to gastrointestinal symptoms shortly after introduction of ECMPS, and 4 because of insufficient system usability that included inadequate mobile telephone service from the network that was used for the study.
Adherence
Positive detection accuracy (PDA) was 100% (95% confidence interval [CI], 89.7–100) in the 34 directly observed ingestions (DOI) performed at clinic visits and in which the contact of the APM to the skin was satisfactory (as measured by APM impedance <4000 Ω) (Table 1). Of the 4136 IEM-ECMPS prescribed ingestions that took place without direct observation, 2824 (68%) occurred, whereas APM impedance was documented to be satisfactory (<4000 Ω for a 24-hr period). The difference between prescribed and detected ingestions (n=1312) is due mostly to instances when the APM was not worn by the patient, either because patients waited for several hours between APM weekly replacements or discontinued APM use a few days before they discontinued the study or even decided not to wear the APM during vacation periods while taking IEM-ECMPS. When the APM was in place, abnormal impedance was detected in only 16 days (1.4%) out of a total of 1181 days of use, often after the recommended period of APM wear (>8 days).
TABLE 1.
Summary of ISS PDA and patient’s adherence
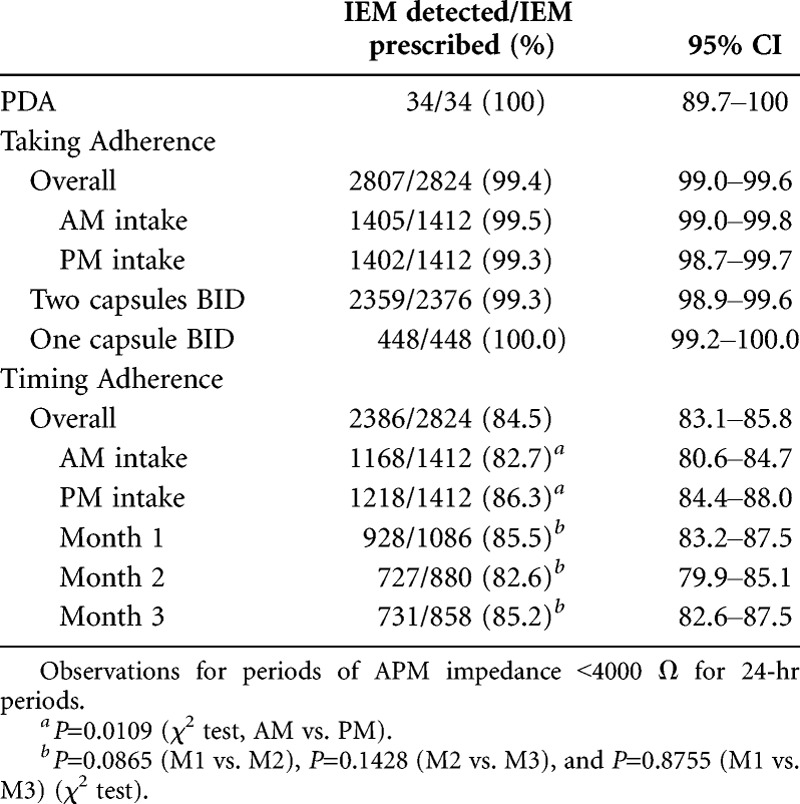
Overall adherence to the number of pills ingested every day relative to the number of pills prescribed (Taking Adherence) was 99.4% (95% CI, 99.0–99.6). In several of the 17 events of missed Taking Adherence, the cause may have been related to a missed IEM detection, as patients reported having taken the drug during the period where no detection occurred. The detection rate of daily medication ingestion was 99.3% (95% CI, 98.9–99.6; n=2376) in patients who took two IEM-ECMPS capsules BID and 100% (95% CI, 99.2–100; n=448) in those who took one IEM-ECMPS capsule BID, demonstrating the system’s ability to detect two IEMs taken at the same time. Adherence to the time of ingestion (Timing Adherence) was 84.5% (95% CI, 83.1–85.8) with some day-to-day variations (Fig. 2) but no significant change over time: Timing Adherence was 85.5%, 82.6%, and 85.2% over the first, second, and third months, respectively (Table 1). Timing Adherence was lower during mornings than evenings (82.7% vs. 86.3%; P=0.0109). Mean deviation from the time window preset for drug intake was 42±50 min (median, 20 min; range, 0.1–180).
FIGURE 2.
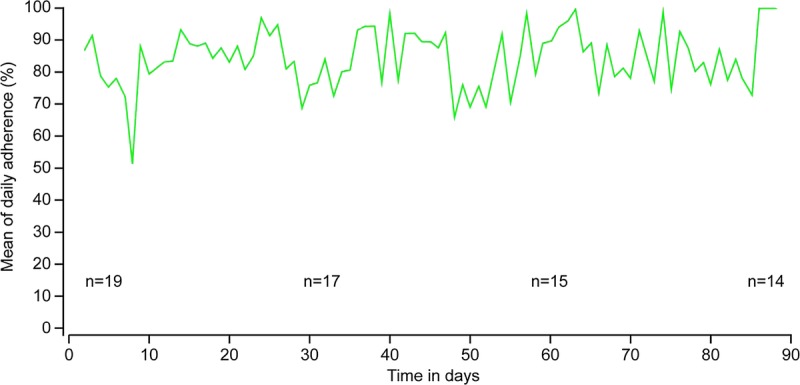
Mean timing adherence over time (periods of APM impedance <4000 Ω).
Individual adherence behavior is illustrated in Figure 3, which shows day-to-day variability in Timing Adherence, while Taking Adherence remains high. In this case, the daily APM-to-phone connectivity interval was frequently longer than 30 min, indicating that this patient was not carrying the smartphone permanently near the APM. This caused no detrimental effect on ISS performance, as data that are collected by the APM remain stored on the APM until the APM returns within Bluetooth range of the smartphone. From the data that were logged by the ISS, it appears that the patient took an “APM holiday” between study days 42 and 49.
FIGURE 3.
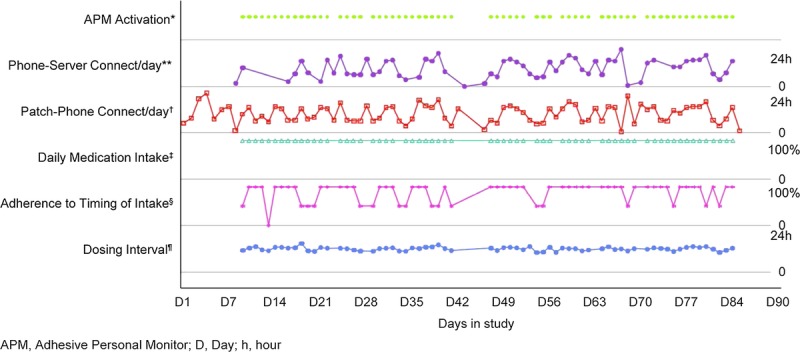
Representative example of an individual patient adherence profile and system functionality records. D, day; h, hour. *For APM activation, each dot represents an APM continuously active for 24 hr. The absence of a dot between days 42 and 49 indicates that the patient was not wearing the patch during this period. **Phone–server connection reports the longest time interval between two connections of the smartphone with the server on each day. For this patient, this time varied between 30 min and 24 hr, indicating difficulties with mobile network access. †Patch–phone connection reports the longest time interval between two connections of the APM with the smartphone on each day. This time interval varied also between 30 min and 24 hr, indicating that the smartphone was not carried permanently by the patient in proximity of the APM. However, because the data were stored on the APM, the APM data were still communicated to the smartphone and then to the server at least once a day. ‡Detection of daily medication ingestion was 100% during the entire study period. §Adherence to the prescribed time of intake fluctuated between 100%, 50% (one of the two daily intakes was not on time), and 0% (time missed for both daily intakes). ¶Dosing interval was the time between morning and evening intakes and oscillated around 12 hr, indicating the absence of major time deviation from the prescribed schedule for medication taking.
All patients received at least one automated short message service (SMS) reminder message in case APM had not been replaced after 7 days and 15 patients received 10 reminders or more during the entire study period. SMS reminders for low adherence to timing of ingestion were sent to 8 of the 13 patients who entered the weeks 8 to 12 study period, 2 of whom received more than five messages.
Safety
No serious or severe adverse events and no rejection episode were reported. Two patients discontinued medication because of ECMPS-related gastrointestinal symptoms (diarrhea) shortly after having started ECMPS, one of which occurred after ECMPS during the run-in period but before using IEM-ECMPS. Seven patients experienced skin reactions to the APM, but in only 2 (10%) did skin intolerance (rash) lead to discontinuation of the system use, respectively, after 2 and 36 days. The other five cases of skin reaction were mild and did not preclude the continuous use of the APM for the 12-week study duration and successful study completion.
DISCUSSION
This is the first study of the use of ISS, a novel technology that uses the fixed combination of the IEM with an immunosuppressant to assess directly daily medication ingestion in transplantation. The system demonstrated very high drug intake detection accuracy during DOIs and the ability to detect two IEMs taken at the same time, with a consistent performance over 12 weeks of continuous use. The uniqueness of this technology is in the physical combination of the IEM with the drug, allowing direct confirmation not only of the time of ingestion but also the type, dose, and number of medications being ingested.
The need for a sensor applied to the skin (the APM) may be considered as a limitation of the technology. However, the combination of the IEM and the APM provides benefits such as allowing for an ultra-low-power highly miniaturized ingestible sensor. Moreover, it ensures security in the data transmission, because the IEM’s modulated current can only be detected for a short period of time and only with the attachment of the APM sensor to the skin. The continuous APM contact to the skin can be monitored in real time by patients and caregivers and, if unsatisfactory, can be acted upon. Issues with system usability led to discontinuation in 20% of patients and were related to the use of U.S. mobile phones using the CDMA technology that led to inconsistent mobile network access in Switzerland. This issue will be resolved by using cell phones that are set to the mobile network of the patient’s country and checking the quality of mobile network access at the patient’s home before prescribing the system. Skin tolerability was a significant limitation to the use of the system in about 10% of the patients but seems to be related to individual sensitivity to adhesives because cases did not increase over time. Work is in progress to use alternative APM adhesives with improved skin tolerability. Patient motivation is also a factor, because the intent of the ISS is to facilitate a dialogue between the patient and the medical team regarding treatment adherence based on an objective assessment of medication intake.
The study was not designed to address the acceptance of the ISS technology across different transplant patient populations and subgroups, particularly in relation to their familiarity with electronic technologies. Although the study tested the technical feasibility of automated SMS reminders as a means of providing feedback on medication use, the study was not designed to modify patient behavior based on these reminders. No systematic intervention by transplant clinicians was planned; therefore, no behavioral change was anticipated.
The ISS technology creates new potential for continual assessment of medication taking in general and to immunosuppressants in transplant patients in particular. However, further studies will be needed to evaluate patient acceptance for the technology, its suitability to support interventions to improve medication taking, and the best situations in which this technology will provide optimal value, such as treatment adherence training early after transplantation, or supporting patients who have nonadherent behavior.
MATERIALS AND METHODS
Patients
Twenty kidney transplant recipients were screened and enrolled in this pilot study between May and August 2011 at five study sites in Switzerland (ClinicalTrials.gov identifier NCT01320358). To be eligible, patients aged 18 years or older had to be in stable condition at least 6 months after transplantation and be taking a stable prescription of immunosuppressive therapy that included ECMPS or mycophenolate mofetil. Before enrollment, patients had to be able to read and to understand the instructions for use of ISS and to sign a written informed consent form.
Ingestible Sensor System
The ISS developed by Proteus Digital Health (Redwood City, CA) is a device that directly confirms medication ingestion events and monitors treatment adherence as well as several physiologic parameters of wellness such as heart rate, body position, and physical activity (Fig. 1).
The IEM is a tiny device (1.0×1.0×0.45 mm) that is composed of an integrated circuit coated with thin layers of minerals and metals that rests in the center of an edible, cellulose-based 5 mm disc. Within a few minutes after ingestion, the sensor uses the electrolytes in the stomach (pH independent) to power a biogalvanic battery formed by thin layers (∼10 μm) of magnesium and copper deposited on the IEM’s surface. The ensuing current is modulated by the IEM’s microprocessor and creates an electric field that propagates through the body tissues to the skin surface. The modulated current is detected, decoded, recorded, and date and time stamped by the APM, which acts as a receiver. The IEM communicates its identifier for a finite period of time of approximately 5 to 10 min and can be detected by an APM only when the APM is adherent to the subject’s skin, thus providing personal privacy for data recording. This mimics the process whereby bioelectric signals from cardiomyocytes reach the electrodes of an electrocardiogram on the surface of the body. The IEM’s current is less than that of a human heartbeat, and each sensor’s identifier is unique. This makes it possible to differentiate multiple IEMs from one another when they are ingested at the same time. The IEM becomes inactive subsequently and is excreted in the feces. The extractable amount of copper and magnesium that can be absorbed by the intestine from a single sensor is very small compared with their daily allowable amounts for human consumption of 0.3% (7.7 ng) and 0.003% (9.8 ng), respectively. In this study, the IEM was combined with ECMPS 360 mg tablets by means of an overencapsulation carrier. The IEM-ECMPS combination product was manufactured according to Good Manufacturing Practices by Novartis Pharma (Basel, Switzerland).
The APM is a wearable, unmedicated, battery-powered, adhesive-backed device measuring 11.4×5.4×1.3 cm containing electronic sensors capable of detecting the ingestion of the IEM and of measuring physiologic parameters. Contact of the APM to the skin is made by three conductive gel electrodes. Adequate quality of the APM skin contact, measured every 20 min, is defined by impedance values lower than 4000 Ω. The APM may be worn continuously for 7 days during all activities, including exercising, swimming, and bathing, and is then replaced. APM replacement, including activation, and Bluetooth pairing of the new one to its dedicated smartphone takes approximately 5 min. Each APM carries a unique serial number that is logged by the system so that APM replacements can be monitored. Every 30 min, the wireless Bluetooth-based interface within the APM is activated to transmit its stored encrypted data in a secured manner to its designated smartphone. The data transmitted by the APM include the IEM information, the APM serial number, impedance, and battery level and the physiologic parameters. In turn, the smartphone sends the data to a secured, centralized data storage and processing location via the mobile telephony network using industry-standard encryption such as the one used by banks. Processed data are then relayed to the patient and a physician designated by the patient via SMS messages, smartphone displays, and/or Web site pages. In case of communication difficulties, the data remain stored in the APM and can be downloaded directly from the APM at a later time whenever necessary. To maintain privacy, data collection, and all data transmissions and displays, occur in a secure manner. No personally identifiable information is stored on the APM or otherwise transmitted between the APM and phone or the phone and the data center. The use of the ISS has been shown to be safe in patients wearing implantable devices such as pacemakers or defibrillators.
Study Design
This is an open-label single-arm exploratory study of 12 weeks’ duration. Main study endpoints were IEM detection accuracy when compared to DOI, Taking Adherence, and Timing Adherence, the latter being defined by IEM detection within the time±1 hr window preset by the patient for morning and evening drug intakes.
At the first visit, patients were trained to use the ISS technology and the mobile phone that was provided for the study and to replace the APM every week. Their treatment was then converted from regular ECMPS to the equivalent number of IEM-ECMPS 360 mg capsules. Patients who were receiving mycophenolate mofetil had their therapy converted and stabilized to the equivalent dose of ECMPS during a 2-week run-in period before using IEM-ECMPS.
DOI took place during clinic visits on day 1 and weeks 1, 4, 8, and 12, and each ingestion was observed by the study nurse who recorded the date and time of ingestion. In the event that a patient had already taken the regular morning dose of IEM-ECMPS, an IEM-placebo combination capsule of equal size was substituted to perform the DOI. Of the 34 DOI, 8 consisted of IEM-placebo ingestions; detection accuracy was 100% for both the IEM-ECMPS and IEM-placebo ingestions. Between visits, the intake of IEM-ECMPS was assessed continuously by the ISS for the duration of the study. During the entire study duration, patients received automated SMS messages to remind them of changing their APM if it had been worn for more than 7 days or when APM battery depletion was detected. In addition, from weeks 8 to 12, patients received automated adherence SMS reminders if their average timing adherence had been less than 84%, or one missed intake, over the previous 72 hr. During the weeks 8 to 12 period, patients had access also to graphical displays of their adherence records on the smartphone. Study site nurses had access to their patients’ full adherence data on the secure study Web site and received also a weekly E-mail report of individual patient’s adherence records over the elapsed week, which they could share with the patient.
Definition of Endpoints and Statistical Methods
All statistical analyses were descriptive. All analyses of adherence were conducted for periods when APMs had satisfactory skin contact (impedance <4000 Ω) because APM can detect IEM ingestion only when it is properly adherent to the body. For DOI, an impedance less than 4000 Ω had to be confirmed before and after the time of DOI recorded by the nurse. For daily adherence assessments, all daily impedance measurements by the APM had to be less than 4000 Ω. Endpoints were defined as follows:
PDA=proportion of IEM detected relative to the number of DOI.
Taking Adherence=number of IEM detected divided by the number of IEM-ECMPS prescribed during the study period.
Timing Adherence=number of IEM detected within the time window preset by the patient divided by the number of IEM-ECMPS capsules prescribed. A 2-hr time window for drug ingestion was defined at time of inclusion by each patient for morning and evening intakes; this time window could not be modified thereafter. The magnitude of time deviations from the target window was also reported for the subset of ingestions occurring outside that window.
The safety analysis included all patients who received at least one dose of IEM-ECMPS.
Study Oversight
This study was conducted in accordance with the International Conference on Harmonisation Tripartite Guidelines for Good Clinical Practice, with applicable local regulations, and with the ethical principles laid down in the Declaration of Helsinki. The institutional review boards of participating centers approved the study protocol (EKBB 285/10). All patients provided written informed consent. Novartis, the study sponsor, was responsible for the study conduct, data collection and statistical analysis; Proteus was responsible for managing the ISS data flow. The data stored on the Proteus server was secure and anonymized and patients were identified only by their study number. Data was then transferred to Novartis and matched with the study database before the statistical analyses that were also conducted by Novartis. The study was cofunded by Novartis and Proteus. The authors had full access to the data, wrote this article, and take responsibility for the accuracy of the reported analysis.
ACKNOWLEDGMENTS
The authors thank the study coinvestigators and coordinators for their support in particular for training patients and providing assistance in the use of the Proteus system: M. Blum and M. Strucker (Zurich), U. Sager (Bern), B. Paul, V. Schurter, and M. Tufail (Aarau), R. Winzeler (Zurich), and L. Berben, S. Mueller, N. Thannberger S. Schaub, and G. Stirnimann (Basle); the Novartis study monitors (O. Meub and C. Boller); the Proteus Digital Health experts and support team (T. Robertson, P. Beaulieu, Y. Behzadi, and T. Schoenberger); and the Novartis team experts (C. Rizzuto, J, Cuine, T. Boggiano, and W. Beiss).
Footnotes
S.K., T.M., and G.F. are employees of Novartis, D.G. is an employee of Quintiles, and A.I. and L.D. are employees of Proteus Digital Health.
E-mail: ute.eisenberger@uk-essen.de
U.E., R.P.W., A.B., P.A., J.S., A.I., S.K., and T.M. participated in the performance of the research. U.E., R.P.W., S.K., D.G., and G.F. participated in the data analysis. U.E., R.P.W., L.D., G.F., and S.D.G. participated in the writing of the article. R.P.W., A.I., S.K., T.M., D.G., L.D., G.F., and S.D.G. participated in the research design.
Received 7 February 2013. Revision requested 27 February 2013.
Accepted 10 May 2013.
REFERENCES
- 1. Butler JA, Roderick P, Mullee M, et al. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation 2004; 77: 769. [DOI] [PubMed] [Google Scholar]
- 2. Dew MA, DiMartini AF, De Vito Dabbs A, et al. Rates and risk factors for non-adherence to the medical regimen after adult solid organ transplantation. Transplantation 2007; 83: 858. [DOI] [PubMed] [Google Scholar]
- 3. Pinsky BW, Takemoto SK, Lentine KL, et al. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant 2009; 9: 2597. [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group Clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9: S1. [DOI] [PubMed] [Google Scholar]
- 5.Sabaté E, ed. Adherence to Long-term Therapies. Geneva, Switzerland: The World Health Organization; 2003 [Google Scholar]
- 6. Fine RN, Becker Y, De Geest S, et al. Nonadherence consensus conference summary report. Am J Transplant 2009; 9: 35. [DOI] [PubMed] [Google Scholar]
- 7. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 35: 487. [DOI] [PubMed] [Google Scholar]


