Abstract
Objective
Statins inhibit cholesterol synthesis but can upregulate cholesterol absorption, with higher doses producing larger effects. Ezetimibe inhibits cholesterol absorption but also upregulates synthesis. We tested whether ezetimibe added to ongoing statin therapy would be most effective in lowering LDL-cholesterol (LDL-C) in subjects on high-potency statins and whether these effects would be related to alterations in cholesterol absorption (β-sitosterol) and synthesis (lathosterol) markers.
Methods
Hypercholesterolemic subjects (n=874) on statins received ezetimibe 10 mg/day. Plasma lipids, lathosterol, and β-sitosterol were measured at baseline and on treatment. Subjects were divided into low- (n=133), medium- (n=582), and high- (n=159) statin potency groups defined by predicted LDL-C–lowering effects of each ongoing statin type and dose (reductions of ~20-30%, ~31-45%, or ~46-55%, respectively).
Results
The high-potency group had significantly lower baseline lathosterol (1.93 vs. 2.58 vs. 3.17 μmol/l; p <0.001) and higher baseline β-sitosterol values (6.21 vs. 4.58 vs. 4.51 μmol/l, p <0.001) than medium-/low-potency groups. Ezetimibe treatment in the high-potency group produced significantly greater reductions from baseline in LDL-C than medium-/low-potency groups (−29.1% vs. −25.0% vs. −22.7%; p <0.001) when evaluating unadjusted data. These effects and group differences were significantly (p <0.05) related to greater β-sitosterol reductions and smaller lathosterol increases. However, LDL-C reduction differences between groups were no longer significant after controlling for placebo effects, due mainly to modest LDL-C lowering by placebo in the high-potency group.
Conclusion
Patients on high-potency statins have the lowest levels of cholesterol synthesis markers and the highest levels of cholesterol absorption markers at baseline, and the greatest reduction in absorption markers and the smallest increases in synthesis markers with ezetimibe addition. Therefore, such patients may be good candidates for ezetimibe therapy if additional LDL-C lowering is needed.
Keywords: non-cholesterol sterol, lathosterol, β-sitosterol, statin potency, dyslipidemia
1. Introduction
Statins play a central role in the treatment of atherogenic dyslipidemia and reduction of cardiovascular disease (CVD) risk. The cholesterol-lowering response to statin therapy, however, can vary widely between individuals [1, 2] and contributes to the substantial number of patients with LDL-C levels above guideline-recommended targets [3-6]. Recent studies suggest that statin efficacy may be determined not only by their direct inhibitory effect on cholesterol synthesis but also by compensatory downstream changes in cholesterol metabolism. Statins reduce markers of cholesterol synthesis (e.g., lathosterol, desmosterol), which can elicit subsequent increases in markers of cholesterol absorption (e.g., campesterol, β-sitosterol) [7-11]. The magnitude of change in these sterol markers has been reported to vary by statin dose, with lower doses having smaller effects [8, 10]. Differences between statins have also been observed. Atorvastatin was found to reduce serum lathosterol/cholesterol ratios more than simvastatin, while it increased plant sterol/cholesterol ratios more than simvastatin in patients with coronary heart disease [12]. We previously reported that atorvastatin 80 mg/day and rosuvastatin 40 mg/day caused similar reduction in markers of cholesterol synthesis, but rosuvastatin increased markers of cholesterol absorption significantly less than atorvastatin [13]. This study also suggested that the combined effect of statins on cholesterol synthesis and absorption may influence treatment efficacy, since the greatest reduction in total cholesterol and LDL-C was seen in subjects with the largest reduction in lathosterol and no compensatory increase in campesterol while treatment efficacy was the lowest in subjects where the converse was true.
Ezetimibe is a selective cholesterol absorption inhibitor that blocks the transport of cholesterol and phytosterols across the intestinal wall and significantly reduces LDL-C levels by 15-20% [14, 15]. Ezetimibe decreases markers of cholesterol absorption but also produces a compensatory increase in markers of cholesterol synthesis [16]. Co-administration of ezetimibe with a statin has been shown to inhibit cholesterol absorption as well as synthesis [17] and these complementary effects produce significantly greater reductions in LDL-C than either drug alone [15, 18, 19].
The effects of ezetimibe added to different statins on cholesterol lowering and cholesterol homeostasis has not been well studied, especially not in a large head-to-head comparison study. The goals of this post hoc analysis of the EASE (Ezetimibe Add-on to Statin for Effectiveness) study were to compare the effects of adding ezetimibe 10 mg to different statins and doses on plasma lipid-lowering effects and non-cholesterol sterol levels. Subjects enrolled in the EASE study had LDL-C levels above National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) recommended targets while on statin therapy, and were randomized to receive either placebo or ezetimibe in addition to their ongoing statin [20]. We used lipid and non-cholesterol sterol data from the ezetimibe arm of this study to test the hypothesis that ezetimibe, when added to statin therapy, would be most effective in LDL-C lowering in subjects on high-potency statins and that these effects would be related to alterations in markers of cholesterol absorption (β-sitosterol, β-sitosterol/cholesterol) and synthesis (lathosterol, lathosterol/cholesterol).
2. Methods
2.1 Subjects and study design
This study included subjects from the ezetimibe add-on to statin arm of the EASE study (http://clinicaltrials.gov identifier NCT00092586; Study Protocol 040). Details of the study design and outcomes have been published previously [20, 21]. Briefly, the EASE study was a multicenter, randomized, double-blind, placebo-controlled, 6-week parallel-group study. Participants with hypercholesterolemia were recruited from community based practices across the United States. Inclusion criteria were: 1) age ≥18 years, 2) on a stable, approved dose of any statin, 3) following a cholesterol-lowering diet for ≥ 6 weeks before study entry, and 4) LDL-C levels above risk-based NCEP ATP III targets. Subjects receiving lipid-altering agents other than statins during the 6 weeks before screening were excluded. Patients were randomized to receive ezetimibe 10 mg/day or placebo plus their current statin therapy and dose for 6 weeks. Statin type and dose were maintained throughout the study. The research protocol was approved by the investigational review boards at each site, and all participants provided written informed consent prior to study start.
For this current post hoc analysis, we assessed subjects who were randomized into the ezetimibe 10 mg plus statin arm of the EASE study (n=1940). Of the 1124 subjects with samples available for measurement, only those who had complete sterol and lipid data at baseline and at the end of the 6-week study were included in this analysis (n=874). The mean age and other baseline characteristics of those included and excluded from this analysis were similar.
Comparison of subjects was based on statin type or potency (low, medium, high) subgroups. The low-potency statin group (predicted LDL-C reduction of ~ 20-30%) included subjects receiving simvastatin ≤ 10 mg/day, lovastatin ≤ 20 mg/day, pravastatin ≤ 20 mg/day, and fluvastatin ≤ 40 mg/day. The medium-potency statin group (predicted LDL-C reduction of ~ 31-45%) included subjects receiving simvastatin > 10 to ≤ 40 mg/day, atorvastatin ≤ 20 mg/day, lovastatin > 20 to 80 mg/day, pravastatin > 20 to 80 mg/day, and fluvastatin > 40 to 80 mg/day. The high-potency statin group (predicted LDL-C reduction of ~ 46-55%) included subjects receiving simvastatin > 40 to 80 mg/day, and atorvastatin > 20 to 80 mg/day.
2.2. Measurement of lipoproteins and non-cholesterol sterols
Plasma total cholesterol (total C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were analyzed using standardized methods at the central laboratory of the trial (PPD Global Central Labs, Highland Heights, Kentucky, USA). LDL-C was calculated using the Friedewald formula [22]. Non-HDL-C was calculated by subtracting HDL-C from total C. Apolipoprotein (Apo) A-I, Apo B, and high-sensitivity C-reactive protein (hs-CRP) were measured by automated immunoassays at the central laboratory. Within and between coefficients for all assays were <10%. Plasma lathosterol and β-sitosterol were quantified by gas chromatography mass spectrometry after lipid extraction as previously described [16]. Since these plasma sterols are mainly carried in the LDL fraction [23], it is common practice to adjust them for total plasma cholesterol by expressing them as a ratio to cholesterol. An alternative is to express them as a ratio representing both absorption and synthesis (i.e., sitosterol/lathosterol). Plasma sterols were therefore expressed either in absolute terms, as a ratio to cholesterol, or as a ratio of β-sitosterol to lathosterol.
2.3 Statistical analysis
All continuous variables were expressed as means ± standard deviation (SD) for normally distributed data, or medians ± robust SD if non-normally distributed. Subjects receiving lovastatin and fluvastatin were grouped together as “other statins” when evaluating statin types due to the small number of subjects. For samples with sterol levels below the limit of detection (0.5 μg/ml) either at the time of randomization or at study end, a value of 0.25 μg/ml was assigned to prevent any bias of excluding subjects with very low sterol levels. All subjects were receiving statin therapy at study entry and baseline values represent levels while on treatment. Baseline cholesterol synthesis and absorption markers among the different statins were compared using an ANOVA model with terms for statin type and statin dose within statin type. Changes from baseline in plasma lipids, apolipoproteins, and hs-CRP after ezetimibe treatment were assessed using an ANOVA model with terms for statin type or statin potency. Data were presented as mean and 95% confidence interval (CI) or median and 95% CI for non-normally distributed data. Since baseline LDL-C levels can influence the lipid-lowering effect of hypolipidemic agents, data were also calculated as percent changes from baseline. Lipid data were also calculated by adjustment for lipid values from the placebo arm of the original EASE study. ANOVA with term for statin type or statin potency were used to compare changes from baseline of plasma lipid values among groups as well as changes in lathosterol and β-sitosterol. Correlation between changes from baseline of these sterols with changes of plasma lipids values were assessed using Pearson’s correlation. Multivariate analysis was performed to assess factors associated with changes in plasma lathosterol and β-sitosterol levels with an add-on ezetimibe treatment.
3. Results
3.1. Effect of statin potency, type, and dose on baseline lipids and sterols
Baseline characteristics, lipid values, and non-cholesterol sterol levels for the overall population are presented in Table 1. The mean age was 61.3 years old, 54.1% were male, and the majority of subjects were Caucasian (82.6%, 7.7% were African American and 9.7% were other ethnic groups). Mean lipid levels were 210.7 mg/dl for total C, 129.5 mg/dl for LDL-C, and 48.1 mg/dL for HDL-C, while the median TG level was 151.0 mg/dl. Of the 874 subjects studied, 133 (15.2%) were receiving low-potency statins, 582 (66.6%) were receiving medium-potency statins, and 159 (18.2%) were receiving high-potency statins (Table 1A). Subjects in the low- and high-potency statin groups had similar baseline lipid values, while subjects in the medium-potency statin group had lower baseline total C, LDL-C, non-HDL-C, total C/HDL-C ratio, and Apo B levels based on post hoc Tukey analysis. Subjects receiving high-potency statins also had lower Apo A-1 levels than those in the medium-potency statin group. The distribution of subjects by statin type were 345 (39.5%) for atorvastatin, 233 (26.7%) for simvastatin, 209 (23.9%) for pravastatin, and 87 (9.9%) for lovastatin or fluvastatin (Table 1B). Subjects on simvastatin had significantly lower baseline total C, LDL-C, non-HDL-C, and Apo B levels than those receiving other statin types.
Table 1.
Baseline characteristics, lipids, and sterols
|
A. Overall and subgroups by statin potency
| ||||
|---|---|---|---|---|
| Characteristic | Overall | Statin potency | ||
| (n=874) | Low (N=133) |
Medium (N=582) |
High (N=159) |
|
| Mean age (yr) | 61.3 ±11.2 | 62.0 ±11.2 | 61.4 ±11.2 | 60.5 ±11.0 |
| Male (%) | 473 (54.1) | 68 (51.1) | 312 (53.6) | 93 (58.5) |
| Race | ||||
| Caucasian, n (%) | 722 (82.6) | 97 (72.9) | 485 (83.3) | 140 (88.1) |
| African American, n (%) | 67 (7.7) | 10 (7.5) | 45 (7.7) | 12 (7.5) |
| Others, n (%) | 85 (9.7) | 26 (19.5) | 52 (8.9) | 7 (4.4) |
| DM, n (%) | 361 (41.3) | 61 (45.9) | 253 (43.5) | 47 (29.6) |
| Body mass index (kg/m2) | 30.8 ±6.6 | 30.1 ±7.5 | 31.0 ±6.5 | 30.7 ±6.1 |
| Total C (mg/dl) * | 210.7 ±34.2 | 215.8 ±35.2 | 208.7 ±32.4 | 213.6 ±39.1 |
| LDL-C (mg/dl) ** | 129.5 ±29.0 | 135.1 ±30.1 | 127.0 ±26.4 | 134.1 ±35.2 |
| Triglyceride (mg/dl) a | 151.0 ±83.7 | 152.0 ±75.3 | 151.0 ±81.9 | 150.0 ±91.2 |
| HDL-C (mg/dl) | 48.1 ±11.3 | 47.9 ±11.9 | 48.6 ±11.3 | 46.5 ±10.5 |
| Total C/HDL-C ratio ** | 4.56 ±1.12 | 4.74 ±1.29 | 4.46 ±1.06 | 4.76 ±1.15 |
| Non-HDL-C (mg/dl) ** | 162.5 ±33.1 | 167.8 ±35.8 | 160.1 ±31.0 | 167.1 ±37.0 |
| Apo B (mg/dl) ** | 129.2 ±24.8 | 132.1 ±27.1 | 127.3 ±23.6 | 133.8 ±26.7 |
| Apo A1 (mg/dl) * | 158.1 ±26.9 | 157.8 ±27.2 | 159.7 ±27.1 | 152.4 ±25.2 |
| hs-CRP (mg/L) a | 2.50 ±3.72 | 2.80 ±3.91 | 2.50 ±3.53 | 2.40 ±4.37 |
| Lathosterol (μmol/L) *** | 2.55 ±1.70 | 3.17 ±1.70 | 2.58 ±1.70 | 1.93 ±1.63 |
| β-sitosterol (μmol/L) *** | 4.86 ±2.62 | 4.51 ±2.03 | 4.58 ±2.46 | 6.21 ±3.14 |
| Lathosterol/Total C (μmol/mmol) *** | 0.46 ±0.27 | 0.56 ±0.27 | 0.47 ±0.27 | 0.34 ±0.24 |
| β-sitosterol/Total C (μmol/mmol) *** | 0.91 ±0.50 | 0.83 ±0.39 | 0.86 ±0.47 | 1.15 ±0.60 |
| β-sitosterol/Lathosterol *** | 3.16 ±3.53 | 2.03 ±2.22 | 2.77 ±3.08 | 5.50 ±4.78 |
|
B. Subgroups by statin type
| ||||
|---|---|---|---|---|
| Characteristic | Atorvastatin | Simvastatin | Pravastatin | Other statinsa |
| (n=345) | (n=233) | (n=209) | (n=87) | |
| Mean age (yr) | 60.0±11.7 | 61.6±10.3 | 61.6±11.4 | 63.2±10.2 |
| Male (%) | 182 (52.8%) | 143 (61.4%) | 104 (49.8%) | 44 (50.6%) |
| Race | ||||
| Caucasian, n (%) | 286 (82.9) | 195 (83.7) | 167 (79.9) | 74 (85.1) |
| African American, n (%) | 29 (8.4) | 19 (8.2) | 14 (6.7) | 5 (5.7) |
| Other, n (%) | 30 (8.7) | 19 (8.2) | 28 (13.4) | 8 (9.2) |
| DM, n (%) | 136 (39.4) | 98 (42.1) | 88 (42.1) | 39 (44.8) |
| Body mass index (kg/m2) | 31.1 ±6.5 | 30.8 ±6.5 | 30.2 ±5.9 | 30.9 ±8.8 |
| Total C (mg/dl) ** | 209.7 ±35.4 | 205.5 ±31.4 | 215.5 ±34.9 | 216.4 ±33.2 |
| LDL-C (mg/dl) ** | 130.0 ±30.9 | 124.3 ±26.1 | 133.1 ±28.3 | 133.4 ±28.1 |
| Triglyceride (mg/dl) b | 149.0± 85.6 | 149.0 ±88.4 | 155.0 ±80.0 | 157.0 ±69.8 |
| HDL-C (mg/dl) | 47.8 ±10.7 | 48.2 ±11.8 | 48.6 ±12.0 | 48.4 ±10.3 |
| Total C/HDL-C ratio ** | 4.55 ±1.05 | 4.46 ± 1.10 | 4.65 ±1.25 | 4.64 ±1.09 |
| Non-HDL-C (mg/dl) ** | 162.0 ±33.6 | 157.4 ±30.2 | 166.9 ±34.5 | 168.0 ±33.1 |
| Apo B (mg/dl) * | 129.2 ±25.4 | 125.8 ±23.2 | 131.3 ±26.0 | 133.4 ±23.0 |
| Apo A1 (mg/dl) | 156.3 ±26.1 | 157.7 ±26.3 | 160.5 ±28.2 | 160.0 ±28.0 |
| hs-CRP (mg/l) b | 2.40 ±3.53 | 2.50 ± 3.81 | 2.70 ±4.09 | 2.50 ± 3.53 |
| Lathosterol (μmol/L) *** | 2.20 ±1.70 | 2.47 ±1.68 | 3.08 ±1.69 | 2.91 ±1.42 |
| β-sitosterol (μmol/L) *** | 5.40 ±2.93 | 4.33 ±2.17 | 4.72 ±2.51 | 4.48 ±2.25 |
| Lathosterol/Total C (μmol/mmol) *** | 0.39 ±0.26 | 0.46 ±0.28 | 0.55 ±0.26 | 0.52 ±0.24 |
| β-sitosterol/Total C (μmol/mmol) *** | 1.01 ±0.57 | 0.82 ±0.40 | 0.86 ±0.48 | 0.81 ±0.42 |
| β-sitosterol/Lathosterol *** | 4.22 ±4.39 | 2.86 ±2.96 | 2.11 ±2.10 | 2.23 ±2.62 |
Values are expressed as mean ± standard deviation (SD) or median ± robust SD
p <0.05;
p <0.01;
p <0.001 indicate significant differences between statin potency groups by ANOVA
Apo; apolipoprotein; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; Total C, total cholesterol
other statins include lovastatin and fluvastatin
Values are expressed as mean ± standard deviation (SD) or median ± robust SD
p <0.05;
p <0.01;
p <0.001 indicate significant differences between statin potency groups by ANOVA
Abbreviations as in Table 1A
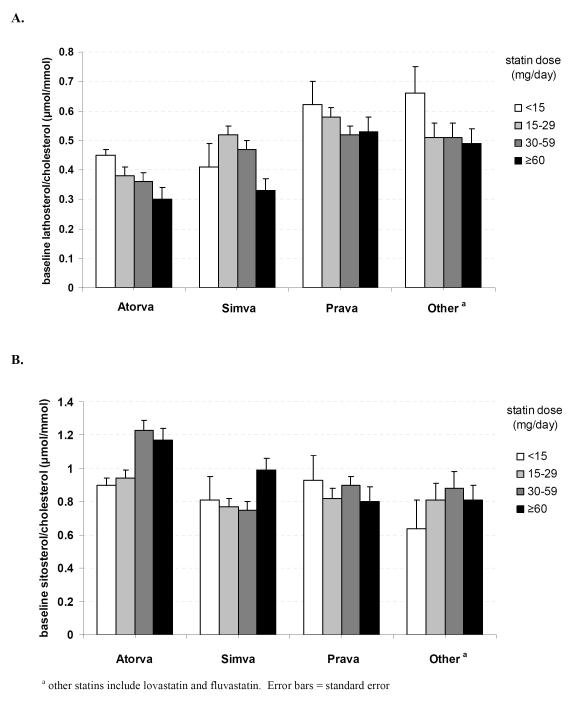
Significant differences in baseline cholesterol synthesis and absorption marker levels were seen when evaluating statin potency, type, and dose. Subjects in the high-potency statin group had significantly lower baseline lathosterol and lathosterol/cholesterol levels (p <0.001) and significantly higher β-sitosterol, β-sitosterol/cholesterol, and sitosterol/lathosterol levels (p <0.001) than subjects in the low-potency statin group (Table 1A). Mean values for high- vs. medium- vs. low-potency statin groups were 0.34 vs. 0.47 vs. 0.56 μmol/mmol for baseline lathosterol/cholesterol levels, 1.15 vs. 0.86 vs. 0.83 μmol/mmol for baseline β-sitosterol/cholesterol levels, and 5.50 vs. 2.77 vs. 2.03 for baseline β-sitosterol/lathosterol, respectively. In addition, mean lathosterol/cholesterol ratios were significantly lower (p <0.001) while mean β-sitosterol/cholesterol and β-sitosterol/lathosterol ratios were significantly higher (p <0.001) in those who used atorvastatin than those who used other statins (Table 1B). In an ANOVA model, statin type and statin dose used within statin type were significantly associated with the ratios of lathosterol/cholesterol and β-sitosterol/cholesterol (p <0.001). These findings were also confirmed when these markers were assessed in absolute terms. Baseline lathosterol/cholesterol and β-sitosterol/cholesterol ratios classified by the different statins and their dosage further demonstrate that statin type and dose are significantly associated with levels of cholesterol synthesis and absorption markers (Figure 1).
Figure 1.
- baseline lathosterol/cholesterol
- baseline β-sitosterol/cholesterol
3.2 Effects of ezetimibe add-on therapy: plasma lipids, apolipoproteins, and hs-CRP
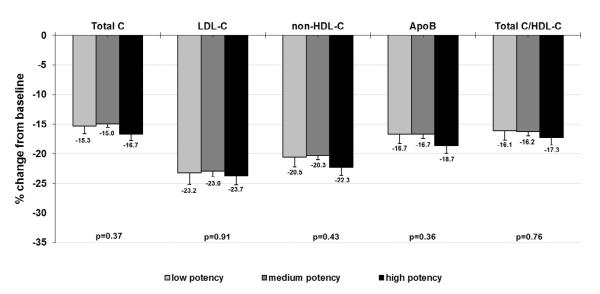
Evaluation of data from the ezetimibe add-on to ongoing statin treatment arm of the EASE study showed significant reductions in total C, LDL-C, TG, non-HDL-C, total C/HDL-C, and Apo B from baseline across all statin potency groups (Supplementary Table 1). The high-potency statin group, however, showed significantly greater reductions in total C, LDL-C, non HDL-C, Apo B, and total C/HDL-C than observed for the other groups. Since baseline LDL-C levels are known to affect the LDL-C–lowering response of hypolipidemic agents, percent reduction of these lipid values were also evaluated and found to be significantly greater in the high potency group (Supplementary Figure 1). The mean percent LDL-C reduction in the high-, medium-, and low-potency statin groups were 29.1%, 25.0%, and 22.7%, respectively (p = 0.002). When results were adjusted for lipid values from the placebo arm of the EASE study, the high-potency statin group had the greatest reductions in total C, LDL-C, non-HDL-C, Apo B, and total C/HDL-C; however, these differences were no longer statistically significant from the other groups (Figure 2). This result may be due in part to modest reductions from baseline in total C, LDL-C, non-HDL-C, Apo B and total C/HDL-C that were observed with placebo treatment, with the greatest effect seen in the high-potency statin group (Supplementary Table 2).
Figure 2.
Percent change in lipid values from statin-treated baseline after ezetimibe add-on therapy (adjusted for values from the placebo arm of EASE)
The addition of ezetimibe to all statin types resulted in significant reductions from baseline in total C, LDL-C, non-HDL-C, Apo B, and total C/HDL-C, with no between-type differences (Supplementary Table 1). The percent LDL-C reductions from baseline for ezetimibe combinations with atorvastatin, pravastatin, simvastatin, and lovastatin or fluvastatin were 26.4%, 23.1%, 26.4%, and 24.1% respectively, p = 0.08 (data not shown).
Response to ezetimibe add-on therapy was also evaluated in subgroups of subjects with statin-treated baseline absorption marker levels above the median (potentially higher absorbers) or ≤ the median (potentially lower absorbers) in order to determine if absorption marker status was associated with treatment efficacy. No significant between-group differences were seen for any of the baseline absorption marker groups (sitosterol, sitosterol/cholesterol, sitosterol/lathosterol) when evaluating absolute change from baseline in total C, LDL-C, triglycerides, non-HDL-C, Apo B, and total C/HDL-C (Supplementary Table 4). Assessment of percent change from baseline, however, showed significantly greater reductions in LDL-C (−26.7% vs. −24.1%, p=0.020), non-HDL-C (−24.2% vs. −21.6%, p=0.011) and total C/HDL-C (−19.0% vs. −17.0%, p=0.030) for subjects with baseline sitosterol/lathosterol levels above the median, while no significant differences were seen when evaluating subgroups based on baseline sitosterol or sitosterol/cholesterol marker levels.
3.3. Effects of ezetimibe add-on therapy: markers of cholesterol absorption and synthesis
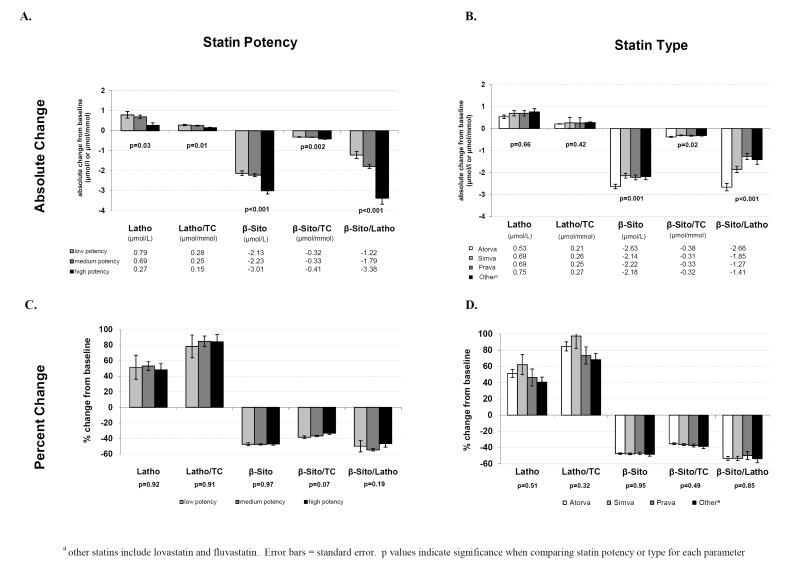
Add-on ezetimibe therapy resulted in significant increases in cholesterol synthesis markers and significant reductions in cholesterol absorption markers from baseline for each statin type and statin potency level. Mean lathosterol levels in the overall study population increased by 52% (95% CI; 42.9, 61.0) and the mean lathosterol/cholesterol ratio increased by 83.8% (95% CI; 73.3, 94.2). Mean β-sitosterol levels decreased by 47.4% (95% CI; −48.7, −46.2) and the mean β-sitosterol/cholesterol ratio decreased by 36.3% (95% CI; −37.7, −34.9). Overall percent change in the mean β-sitosterol/lathosterol ratio was −52.4% (95% CI; −55.8, −49.0). All sterol changes were highly significant (p <0.001). Addition of ezetimibe to high-potency statins produced significantly lower increases in cholesterol synthesis markers than seen with medium- and low-potency statins (Figure 3A). In addition, combination therapy with high-potency statins resulted in significantly greater reductions in cholesterol absorption markers and β-sitosterol/lathosterol than medium- and low-potency statins. When changes in plasma non-cholesterol levels from baseline classified by statin type were compared, there were no significant differences between lathosterol and lathosterol/cholesterol among the four groups (Figure 3B). Adding ezetimibe to atorvastatin decreased β-sitosterol, β-sitosterol/cholesterol, and β-sitosterol/lathosterol levels more than when added to the other statins, but there were no significant differences among other stain types. Evaluation of percent change from baseline in plasma non-cholesterol levels showed no significant difference between statin potencies or statin types (Figure 3C and 3D).
Figure 3.
Effect of 6-week ezetimibe add-on treatment on plasma non-cholesterol sterol levels – absolute and percent change by statin potency and type
3.4. Correlation between lipid-lowering efficacy and changes in cholesterol synthesis and absorption markers
Table 2 shows the correlations between changes in plasma lipids and sterols overall and classified by statin potency groups after 6 weeks of ezetimibe-add on therapy. Changes in lathosterol and β-sitosterol levels were significantly correlated with changes in total C, LDL-C, non-HDL-C, total C/HDL-C, and Apo B for the overall population (all p <0.001). Correlations with changes in TG were significant but considerably weaker. Correlations of changes in lathosterol with these plasma lipid values were strongest in the high-potency statin group, while correlations of changes in β-sitosterol with plasma lipid values were strongest in the low potency statin group. Correlations of changes in lathosterol/cholesterol were also significantly associated with changes in total C, LDL-C, non-HDL-C, total C/HDL-C, and Apo B overall, but were weaker than the absolute values and remained significant only for high- and medium- (Apo B only) potency statin groups (Table 2) and ezetimibe-atorvastatin group (Supplementary Table 3). Changes in β-sitosterol/cholesterol were negatively and weakly correlated with changes in total C, non-HDL-C, and Apo B overall. Again, only the high- and medium- (Apo B only) potency statin and ezetimibe-atorvastatin groups were significant.
Table 2.
Correlation between changes of cholesterol synthesis and absorption markers with changes of plasma lipids and apolipoprotein B levels after 6 weeks of ezetimibe add-on therapy
| Parameter | Changes in lathosterol | Changes in β-sitosterol | ||||||
| All | Low potency |
Medium potency |
High potency |
All | Low potency |
Medium potency |
High potency |
|
|
| ||||||||
| changes in Total C | 0.339 ** | 0.203 * | 0.291 ** | 0.646 ** | 0.260 ** | 0.364 ** | 0.229 ** | 0.217 * |
| changes in LDL-C | 0.288 ** | 0.146 | 0.240 ** | 0.601 ** | 0.245 ** | 0.346 ** | 0.207 ** | 0.208 * |
| changes in Triglycerides | 0.164 ** | 0.149 | 0.161 ** | 0.220 * | 0.073 * | 0.043 | 0.057 | 0.144 |
| changes in HDL-C | 0.022 | −0.068 | 0.039 | 0.016 | 0.061 | 0.003 | 0.103 * | −0.033 |
| changes in Total C/HDL-C | 0.241 ** | 0.204 * | 0.186 ** | 0.485 ** | 0.170 ** | 0.207 * | 0.133 * | 0.177 * |
| changes in Non-HDL-C | 0.338 ** | 0.216 * | 0.288 ** | 0.641 ** | 0.250 ** | 0.359 ** | 0.210 ** | 0.222 * |
| changes in Apo B | 0.311 ** | 0.131 | 0.274 ** | 0.610 ** | 0.218 ** | 0.258 * | 0.189 ** | 0.191 * |
|
| ||||||||
| Parameter | Changes in lathosterol/cholesterol | Changes in β-sitosterol/cholesterol | ||||||
| All | Low potency |
Medium potency |
High potency |
All | Low potency |
Medium potency |
High potency |
|
|
| ||||||||
| changes in Total C | 0.103 * | −0.066 | 0.076 | 0.382 ** | −0.079 * | 0.077 | −0.071 | −0.229 * |
| changes in LDL-C | 0.086 * | −0.099 | 0.065 | 0.354 ** | −0.064 | 0.085 | −0.052 | −0.229 * |
| changes in Triglycerides | 0.068 * | 0.126 | 0.058 | 0.111 | −0.043 | −0.026 | −0.067 | 0.016 |
| changes in HDL-C | −0.024 | −0.139 | −0.012 | −0.005 | 0.029 | 0.018 | 0.059 | −0.063 |
| changes in Total C/HDL-C | 0.076 * | 0.069 | 0.033 | 0.290 ** | −0.061 | 0.001 | −0.075 | −0.107 |
| changes in Non-HDL-C | 0.109 * | −0.036 | 0.080 | 0.381 ** | −0.086 * | 0.072 | −0.086 | −0.217 * |
| changes in Apo B | 0.106 ** | −0.090 | 0.088 * | 0.374 ** | −0.093 * | 0.002 | −0.085 * | −0.231 * |
Values are expressed as correlation coefficients
p ≤0.05;
p <0.001
3.5. Factors related to changes in cholesterol synthesis and absorption markers
Multivariate analysis was used to assess factors associated with changes in lathosterol and β-sitosterol after 6 weeks of ezetimibe add-on therapy (Table 3). Factors significantly associated with changes in lathosterol were baseline total cholesterol, changes in total cholesterol, baseline lathosterol, and statin potency. Factors significantly associated with changes in β-sitosterol were baseline LDL-C, changes in total cholesterol, baseline β-sitosterol, and statin potency. For changes in lathosterol and β-sitosterol, the baseline values of lathosterol and β-sitosterol, respectively, were the strongest predictors during ezetimibe add-on treatment. Changes in LDL-C and stain type were not significantly associated with any sterol change.
Table 3.
Multivariate analysis for variables associated with changes in lathosterol and β-sitosterol after 6 weeks of ezetimibe add-on therapy
| Factor | Change in lathosterol | Change in β-sitosterol | ||
|---|---|---|---|---|
| Beta | P value | Beta | P value | |
| - baseline total cholesterol | 0.009 | 0.0350 | 0.002 | 0.1936 |
| - baseline LDL-C | 0.004 | 0.4538 | 0.005 | 0.0210 |
| - changes in total cholesterol | 0.026 | <0.0001 | 0.012 | <0.0001 |
| - changes in LDL-C | 0.001 | 0.8297 | 0.005 | 0.1015 |
| - baseline lathosterol | −0.409 | <0.0001 | ||
| - baseline β-sitosterol | −0.540 | <0.0001 | ||
| - statin type | 0.5976 | 0.7147 | ||
| - statin potency | ||||
| high-potency | −0.491 | 0.0065 | 0.201 | 0.0215 |
| medium-potency | 0.000 | 0.000 | ||
| low-potency | 0.230 | 0.004 | ||
4. Discussion
This post hoc analysis of the EASE study evaluated the effects of ezetimibe on lipid and sterol markers when added to ongoing statin treatment. To our knowledge, this is the first head-to-head comparison evaluating the effect of ezetimibe add-on to low-, medium- and high-potency statins on changes in cholesterol synthesis and absorption markers and LDL-C. Although it is the second study comparing the addition of ezetimibe to a variety of statins on markers of cholesterol synthesis and absorption, the previous study evaluated the effects of statin, ezetimibe, or both in subjects with primary hypercholesterolemia [24] while the current study assessed the effects of ezetimibe add-on therapy in subjects already on statins and above NCEP ATP III recommended LDL-C targets.
As expected, our study found that addition of ezetimibe to ongoing atorvastatin, simvastatin, pravastatin, and lovastatin or fluvastatin resulted in significant LDL-C lowering from treated baseline and no significant differences between statins were observed, which was consistent with the primary study results [20]. A pooled analysis from four different statin trials in patients with primary hypercholesterolemia also found that ezetimibe 10 mg/day co-administered with lovastatin, simvastatin, pravastatin, or atorvastatin resulted in similar percentage LDL-C reduction from baseline across statin types and statin doses [25]. We hypothesized, however, that the addition of ezetimibe to high-potency statins would result in greater LDL-C reductions than seen with medium- or low-potency statins, and that these effects should be related to alterations in markers of cholesterol synthesis and absorption. Statins inhibit cholesterol synthesis, but effects on lowering LDL-C may be moderated by a compensatory up-regulation of cholesterol absorption, with higher-potency statins having a greater effect [8, 10]. Ezetimibe add-on therapy might therefore be expected to have the greatest LDL-C lowering effect in subjects with higher levels of cholesterol absorption induced by high-potency statins.
In the current study, subjects receiving high-potency statins had significantly higher levels of cholesterol absorption markers (β-sitosterol and β-sitosterol/cholesterol) and lower levels of cholesterol synthesis markers (lathosterol, lathosterol/cholesterol) compared with lower-potency statin groups. These results are consistent with studies comparing different doses of atorvastatin and rosuvastatin which found that higher doses of statin resulted in greater reductions in cholesterol synthesis marker levels and greater increases in cholesterol absorption marker levels than lower doses of the same statin [8, 10]. In addition, the Scandinavian Simvastatin Survival Study (4S) also showed that the higher the dose, the longer the treatment period, and the higher the baseline absorption sterol ratios, the higher was the relative increase of absorption sterol ratios in subjects treated with simvastatin [26]. We found that addition of ezetimibe to high-potency statins resulted in greater reductions in cholesterol absorption markers and smaller increases in cholesterol synthesis markers than observed with medium- and low-potency statins. Greater reductions in LDL-C levels were also observed for this high-potency statin group compared with medium- and low-potency groups. However, these results did not reach statistical significance when adjusted for LDL-C changes observed in the placebo arm of the study. The differential effect of placebo on change from baseline LDL-C in the low- (+0.1%), medium- (−2.0%), and high- (−3.3%) potency statin groups may have contributed to the lack of significance after adjustment. Substantial variability in response to both statin and ezetimibe therapy is well known, and future assessment of individual changes rather than group comparisons may provide additional insight into the effects of ezetimibe when administered with statins of differing potencies.
Controversy exists whether baseline non-cholesterol sterol levels predict LDL-C–lowering response to statin and/or ezetimibe treatment. Miettinen and colleagues have proposed that subjects with high cholesterol absorption and low synthesis respond poorly to statins and may need combination therapy to lower serum cholesterol more effectively and prevent an increase in the levels of plant sterols [26, 27]. Some studies do not support the benefit of using baseline cholesterol absorption marker levels in predicting response to statin and ezetimibe/simvastatin treatment [28, 29]. However, two recent studies suggest that statins were most effective in subjects who did not upregulate cholesterol absorption during statin therapy [13, 24]. Subjects enrolled in the EASE study were above NCEP ATP III recommended LDL-C levels while on statins, and therefore may represent less responsive patients with increased levels of cholesterol absorption, but other factors cannot be ruled out. Within this population, comparison of subjects with baseline cholesterol absorption marker levels either above or ≤ the median found no significant differences in the LDL-C–lowering response to ezetimibe add-on therapy when evaluating absolute change from statin-treated baseline and minimally significant differences when assessing percent change (only seen for sitosterol/lathosterol subgroups). Our post hoc evaluation of EASE was not designed to evaluate the ability of baseline non-cholesterol levels to predict the therapeutic efficacy of statins or ezetimibe because we did not measure baseline values of cholesterol synthesis and absorption markers before statin therapy was initiated, and further studies are needed to address this issue.
We did find that statin type affected levels of cholesterol synthesis and absorption markers. Subjects who were on atorvastatin had lower lathosterol/cholesterol and higher β-sitosterol/cholesterol levels than subjects who were on other statins. In concurrence with our findings, previous studies have shown that atorvastatin reduced precursor sterols more than simvastatin (e.g., −50% vs. −42% when comparing sterol/cholesterol ratios), and plant sterols increased more with atorvastatin than with simvastatin (e.g. 82% vs. 39% when comparing sterol/cholesterol ratios) [12, 24]. We also found that addition of ezetimibe to atorvastatin produced significantly greater reduction of β-sitosterol, β-sitosterol/cholesterol, and β-sitosterol/lathosterol levels than when added to other statins, while increases in markers of cholesterol synthesis were similar across all statins. Despite these differences, addition of ezetimibe to any statin lowered LDL-C by 23-26% and no significant differences between statins were observed. Few other studies have evaluated the influence of statin type on cholesterol synthesis and absorption marker levels during combination therapy with ezetimibe, and none have assessed concomitant changes in LDL-C. In one post hoc evaluation of subjects with primary hypercholesterolemia, ezetimibe plus atorvastatin reduced lathosterol by 62.4% and β-sitosterol by 49.4% while co-administration of ezetimibe and simvastatin reduced lathosterol by 47.6% and β-sitosterol by 52.1%, supporting the current finding that different statins may differentially influence markers of cholesterol synthesis and absorption [24].
Common factors that were significantly associated with changes in lathosterol and β-sitosterol included baseline values of those sterols, changes in total cholesterol, and statin potency. Baseline sterol levels were the strongest predictors of sterol changes during an ezetimibe add-on treatment, indicating that baseline lathosterol may be related to changes in cholesterol synthesis and baseline β-sitosterol to reduction of cholesterol absorption. In addition, changes in sterol levels significantly correlated with changes in LDL-C following ezetimibe add-on therapy. A previous study also reported correlations of baseline non-cholesterol sterol levels with changes in these sterols [28] and sterol changes with those of LDL-C during treatment with ezetimibe and simvastatin [28, 29]. It should be noted that the baseline sterol levels in this study were obtained during statin treatment prior to the addition of ezetimibe, while the baseline values in other studies were obtained before initiation of any therapy.
While this is one of the few studies to assess the effects of ezetimibe add-on therapy on both lipids and sterol markers, certain limitations were present. This study was a post hoc analysis of the EASE trial. Because subjects enrolled in this study had LDL-C levels above NCEP ATP III recommended targets while on statin therapy, results may not be generalizable to other patient populations and may potentially include some bias. Non-cholesterol sterol data was not available for the entire study cohort which could potentially bias the results; however no significant differences in baseline characteristics were seen between those included and excluded from the analysis. In addition, non-cholesterol sterols were not measured in the placebo arm; therefore, we were unable to determine placebo-adjusted values for non-cholesterol sterols after the addition of ezetimibe. Further prospective randomized studies are needed to clarify this issue. The treatment duration of ezetimibe in this study was 6 weeks, and thus longer-term changes in these sterol markers were not assessed. However, a previous study has reported similar changes of cholesterol synthesis and absorption markers levels between short-term therapy and two-year treatment in subjects with familial hypercholesterolemia [28]. Finally, we did not have baseline values for cholesterol synthesis and absorption markers prior to the initiation of statin therapy and therefore were not able to determine if baseline levels predict LDL-C– lowering response to overall treatment. We did, however, have the values of the sterol markers during on-going statin therapy thus providing responses of the subjects to ezetimibe treatment.
5. Conclusions
Results from this study indicate that statin potency and type can significantly affect cholesterol synthesis and cholesterol absorption marker levels. Patients on high-potency statins had the lowest levels of cholesterol synthesis markers and the highest levels of cholesterol absorption markers at baseline, and the greatest reduction in absorption markers and the smallest increases in synthesis markers with ezetimibe addition. Compared with baseline values, ezetimibe was most effective in reducing LDL-C when added to high-potency statin therapy; however, this finding was no longer significant after adjusting for placebo effects. Nevertheless, these results highlight the complementary effects that statins and ezetimibe have on modulating markers of cholesterol synthesis and absorption, and suggest that patients on high-potency statins may be good candidates for ezetimibe therapy if additional LDL-C lowering is required to reach LDL-C goals.
Supplementary Material
Highlights.
“Effects of ezetimibe added to statin therapy on markers of cholesterol absorption and synthesis and LDL-C lowering in hyperlipidemic patients”
To our knowledge, this study is the first head-to-head comparison evaluating the effect of ezetimibe combined with low-, medium-, and high-potency statins on cholesterol synthesis and absorption markers and LDL-C lowering. Furthermore, our study included a relatively large number of subjects as compared with the earlier non-cholesterol sterol studies. We found that statin type and dosage significantly affect cholesterol synthesis and cholesterol absorption markers levels, and that statin potency influenced changes in cholesterol synthesis and absorption marker levels when adding ezetimibe to statin therapy.
Acknowledgments
The study was designed by scientists at Merck Sharp & Dohme Corp., including R.S.L., J.E.T, and A.M.T. as an ancillary substudy to the EASE (Ezetimibe Add-on to Statin for Effectiveness) study. The overall study, the gas chromatography analysis, and other aspects of the study were supported by Merck Sharp & Dohme Corp. The data analysis plan was formulated by E.J.S., N.T., J.L., A.K.S, R.S.L., J.E.T., and A.M.T., and the statistical analysis was performed by J.L. and A.K.S. The manuscript was written by N.T. with input and assistance from all authors, as well as editorial support by Martha Carroll Vollmer, MA of Merck Sharp & Dohme Corp. The content is solely the responsibility of the authors and does not necessarily represent the official views of any organization including the National Institutes of Health, the U.S. Department of Agriculture, or Merck Sharp & Dohme Corp.
Funding This study was supported by Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA. N.T. received a post-doctoral fellowship from the Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand. E.J.S. was supported by grants R01 HL-60935, HL 74753, and PO50HL083813 from the National Institutes of Health and contract 53-3K– 06 from the United Department of Agriculture Research Service.
Footnotes
Conflicts of interest N.T. reports no conflicts of interest. E.J.S. is an employee of Tufts University, and has served as a consultant for the following companies: Amarin, Amgen, AstraZeneca, Boston Heart Diagnostics, Bristol Myers Squibb, DuPont, Genentech/Roche, Merck Sharp & Dohme, and Unilever. J.L., R.S.L., J.E.T., A.K.S., and A.M.T. are employees of Merck Sharp & Dohme Corp. and may own stock and/or hold stock options in the company.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR Trial) Am J Cardiol. 2003;92:152–160. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- [2].Weng TC, Yang YH, Lin SJ, Tai SH. A systematic review and meta-analysis on the therapeutic equivalence of statins. J Clin Pharm Ther. 2010;35:139–151. doi: 10.1111/j.1365-2710.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- [3].Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- [4].Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- [5].Catapano AL, Reiner Z, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Atherosclerosis. 2011;217:3–46. doi: 10.1016/j.atherosclerosis.2011.06.028. [DOI] [PubMed] [Google Scholar]
- [6].Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- [7].Reihnér E, Rudling M, Stáhlberg D, et al. Influence of pravastatin, a specific inhibitor of HMG-CoA reductase, on hepatic metabolism of cholesterol. N Engl J Med. 1990;323:224–228. doi: 10.1056/NEJM199007263230403. [DOI] [PubMed] [Google Scholar]
- [8].Lamon-Fava S, Diffenderfer MR, Barrett PH, et al. Effects of different doses of atorvastatin on human apolipoprotein B-100, B-48, and A-I metabolism. J Lipid Res. 2007;48:1746–1753. doi: 10.1194/jlr.M700067-JLR200. [DOI] [PubMed] [Google Scholar]
- [9].Vanhanen H, Miettinen TA. Pravastatin and lovastatin similarly reduce serum cholesterol and its precursor levels in familial hypercholesterolaemia. Eur J Clin Pharmacol. 1992;42:127–130. doi: 10.1007/BF00278470. [DOI] [PubMed] [Google Scholar]
- [10].Ooi EM, Barrett PH, Chan DC, Nestel PJ, Watts GF. Dose-dependent effect of rosuvastatin on apolipoprotein B-100 kinetics in the metabolic syndrome. Atherosclerosis. 2008;197:139–146. doi: 10.1016/j.atherosclerosis.2007.03.004. [DOI] [PubMed] [Google Scholar]
- [11].De Cuyper I, Wolthers BG, van Doormaal JJ, Wijnandts PN. Determination of changes in serum lathosterol during treatment with simvastatin to evaluate the role of lathosterol as a parameter for whole body cholesterol synthesis. Clin Chim Acta. 1993;219:123–130. doi: 10.1016/0009-8981(93)90203-g. [DOI] [PubMed] [Google Scholar]
- [12].Miettinen TA, Gylling H, Lindbohm N, Miettinen TE, Rajaratnam RA, Relas H. Serum noncholesterol sterols during inhibition of cholesterol synthesis by statins. J Lab Clin Med. 2003;141:131–137. doi: 10.1067/mlc.2003.9. [DOI] [PubMed] [Google Scholar]
- [13].van Himbergen TM, Matthan NR, Resteghini NA, et al. Comparison of the effects of maximal dose atorvastatin and rosuvastatin therapy on cholesterol synthesis and absorption markers. J Lipid Res. 2009;50:730–739. doi: 10.1194/jlr.P800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Davidson MH, McGarry T, Bettis R, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40:2125–2134. doi: 10.1016/s0735-1097(02)02610-4. [DOI] [PubMed] [Google Scholar]
- [15].Gagne C, Gaudet D, Bruckert E. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation. 2002;105:2469–2475. doi: 10.1161/01.cir.0000018744.58460.62. [DOI] [PubMed] [Google Scholar]
- [16].Sudhop T, Lutjohann D, Kodal A, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- [17].Gouni-Berthold I, Berthold HK, Gylling H, et al. Effects of ezetimibe and/or simvastatin on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase gene expression: a randomized trial in healthy men. Atherosclerosis. 2008;198:198–207. doi: 10.1016/j.atherosclerosis.2007.09.034. [DOI] [PubMed] [Google Scholar]
- [18].Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107:2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- [19].Morrone D, Weintraub WS, Toth PP, et al. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: A pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis. 2012;223:251–261. doi: 10.1016/j.atherosclerosis.2012.02.016. [DOI] [PubMed] [Google Scholar]
- [20].Pearson TA, Denke MA, McBride PE, Battisti WP, Brady WE, Palmisano J. A community-based, randomized trial of ezetimibe added to statin therapy to attain NCEP ATP III goals for LDL cholesterol in hypercholesterolemic patients: the ezetimibe add-on to statin for effectiveness (EASE) trial. Mayo Clin Proc. 2005;80:587–595. doi: 10.4065/80.5.587. [DOI] [PubMed] [Google Scholar]
- [21].Pearson TA, Denke MA, McBride PE, et al. Effectiveness of ezetimibe added to ongoing statin therapy in modifying lipid profiles and low-density lipoprotein cholesterol goal attainment in patients of different races and ethnicities: a substudy of the Ezetimibe add-on to statin for effectiveness trial. Mayo Clin Proc. 2006;81:1177–1185. doi: 10.4065/81.9.1177. [DOI] [PubMed] [Google Scholar]
- [22].Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- [23].Miettinen TA, Tilvis RS, Kesaniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol. 1990;131:20–31. doi: 10.1093/oxfordjournals.aje.a115479. [DOI] [PubMed] [Google Scholar]
- [24].Assmann G, Kannenberg F, Ramey DR, Musliner TA, Gutkin SW, Veltri EP. Effects of ezetimibe, simvastatin, atorvastatin, and ezetimibe-statin therapies on non-cholesterol sterols in patients with primary hypercholesterolemia. Curr Med Res Opin. 2008;24:249–259. doi: 10.1185/030079908x253663. [DOI] [PubMed] [Google Scholar]
- [25].Davidson MH, Ballantyne CM, Kerzner B, et al. Efficacy and safety of ezetimibe coadministered with statins: randomised, placebo-controlled, blinded experience in 2382 patients with primary hypercholesterolemia. Int J Clin Pract. 2004;58:746–755. doi: 10.1111/j.1368-5031.2004.00289.x. [DOI] [PubMed] [Google Scholar]
- [26].Miettinen TA, Strandberg TE, Gylling H. Noncholesterol sterols and cholesterol lowering by long-term simvastatin treatment in coronary patients: relation to basal serum cholestanol. Arterioscler Thromb Vasc Biol. 2000;20:1340–1346. doi: 10.1161/01.atv.20.5.1340. [DOI] [PubMed] [Google Scholar]
- [27].Nissinen MJ, Miettinen TE, Gylling H, Miettinen TA. Applicability of non-cholesterol sterols in predicting response in cholesterol metabolism to simvastatin and fluvastatin treatment among hypercholesterolemic men. Nutr Metab Cardiovasc Dis. 2010;20:308–316. doi: 10.1016/j.numecd.2009.04.014. [DOI] [PubMed] [Google Scholar]
- [28].Jakulj L, Vissers MN, Groen AK, et al. Baseline cholesterol absorption and the response to ezetimibe/simvastatin therapy: a post-hoc analysis of the ENHANCE trial. J Lipid Res. 2010;51:755–762. doi: 10.1194/jlr.M001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lakoski SG, Xu F, Vega GL, et al. Indices of Cholesterol Metabolism and Relative Responsiveness to Ezetimibe and Simvastatin. J Clin Endocrinol Metab. 2010;95:800–809. doi: 10.1210/jc.2009-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.