Abstract
Preface
Significant advances in our understanding of the in vivo functions of human cells, tissues and immune systems have resulted from the development of mouse strains that are based on severely immunodeficient mice expressing mutations in the interleukin-2 (IL-2) receptor common γ-chain locus. These mouse strains support the engraftment of a functional human immune system and permit detailed analysis of human immune biology, development and functions. In this Review, we discuss recent advances in the development of humanized mice, the lessons learned, the remaining challenges and the promise of using humanized mice for the in vivo study of human immunology.
Introduction
A fundamental understanding of many biological processes in humans has stemmed from experimental studies in animal models, particularly in rodents. Using these models, key aspects of the development and regulation of the haematopoietic and immune systems have been elucidated at the cellular and molecular levels. However, many aspects of mammalian biological systems, particularly their immune systems, are species specific. Moreover, rodents are refractory to certain human specific infectious agents, and many of the new therapeutic and immunomodulatory reagents that have been developed are human specific.
For many years, investigators have relied on chimpanzees to bridge the final gap between rodent models and humans. However, the use of chimpanzees for biomedical research has been banned in Europe, and a recent NIH directive has stopped all new research on chimpanzees in the United States (http://www.nih.gov/news/health/dec2011/od-15.htm). Based on these restrictions it is unclear whether experimentation in chimpanzees will be a feasible approach in the future for moving discoveries made in rodents through the pre-clinical phase to human trials.
Therefore, to address the limitations of translating discoveries in rodents into clinical applications, sophisticated small animal models that more closely recapitulate human biological systems, termed “humanized” mice, are more acutely required. We define humanized mice in this review as immunodeficient mice that have been engrafted with human primary haematopoietic cells and tissues that generate a functional human immune system. We1 and others2, 3 have reviewed the history of humanized mice. . There has been a rapid growth in use of humanized mice that emanated from breakthroughs following the development of immunodeficient mice with a mutation in the interleukin-2 (IL-2) receptor γ-chain locus (Il2rg; also known as γc and CD132) in the mid 2000's4-6. The Il2rg mutation leads to severe impairments in B and T cell development and function, and completely prevents natural killer (NK) cell development. In addition, this mutation affects the development of lymph node anlagen, resulting in poor lymph node development and organization. The focus of this Review is on the progress in the generation and use of new models of immune engrafted humanized mice that has occurred over the last few years, the limitations present in the earlier models that have recently been overcome, the challenges yet to be conquered and the promise that humanized mice provide for the future of biomedical research.
Model systems and technologies
To understand the progress, promise and remaining challenges in the use of humanized mice, it is important to recognize the variety and complexity of the model systems being used in a particular study to appropriately interpret the results. There are many and quite diverse strains of immunodeficient mice with a mutant Il2rg gene that have been developed, as well as a complex nomenclature surrounding the various strains and models (Text Box 1). Any one specific model will not be optimal for addressing the myriad of questions that might be considered, and it is important to not only choose the appropriate model system for the specific question at hand, but to also be innovative in designing questions and experiments that can provide valid data that can be properly interpreted using the individual models.
Text Box 1. Platforms for Creating Human Immune System Engrafted Mice (reviewed in REF1).
| Platform Stocks of Immunodeficient Mice | Common Abbreviation | Characteristics |
| NOD.Cg-PrkdcscidIl2rgtm1Wjl | NSG | The Il2rg targeted mutation is a complete null, is not expressed and will not bind cytokines |
| NOD.cg-PrkdcscidIl2rgtm1Sug | NOG | The Il2rg chain lacks the intracytoplasmic domain. It Is expressed and will bind cytokines but will not signal |
| C;129S4- Rag2tm1.1Flv | BRG | On a mixed BALB/c x 129 Rag2null strain. Il2rg is complete null, is not expressed and will not bind cytokines |
| Platform Genes to Create Immunodeficient Mice | ||
| protein kinase, DNA activated, catalytic polypeptide | Prkdcscid (often abbreviated as scid) | Double strand DNA repair defect that prevents TCR and BCR recombination |
| recombination activating gene 1 recombination activating gene 2 | Rag1−/−Rag2−/− | T and B cell receptor recombination defects T and B cell receptor recombination defects |
There are three main platforms of mouse strains that are used: NOD.Cg-PrkdcscidIl2rgtm1Wjl (NSG), NOD.cg-PrkdcscidIl2rgtm1Sug (NOG), and C;129S4- Rag2tm1.1Flv (BRG), Text Box 1). Several of the strains of NSG mice and BRG mice are available from The Jackson Laboratory (http://www.jax.org/), whereas NOG mice are available from Taconic (http://www.taconic.com/wmspage.cfm?parm1=16). The derivation and unique characteristics of each of the immunodeficient mouse models derived from immunodeficient Il2rgnull mice have been reviewed in detail1.
Recent evidence shows that these mouse models differ in their ability to support the engraftment of functional human immune systems. For example, NSG and NRG mice support higher levels of human haematopoietic stem cell (HSC) engraftment and T cell development than do BRG mice7 and NSG mice support higher levels of human HSC engraftment in bone marrow than do NOG mice8. Choice of the specific mouse model will impact the engraftment levels of the transplanted human haematopoietic and immune systems, the types of cells that can be successfully engrafted, and the functional capabilities of specific immune cells.
There are four different technological approaches for the engraftment of a functional human immune system in these immunodeficient mouse models, each with distinct advantages and caveats (Text Box 2, Figure 1). Depending on the question, the investigator will need to choose the appropriate human immune system engrafted mouse for their studies.
Text Box 2. Human immune system engraftment models.
| Human Immune System Models | Common Abbreviati on | Establishment of Model | Advantages | Limitations | |
| Human Peripheral Blood Lymphocyte engrafted immunodeficient (SCID) mouse | Hu-PBL-SCID | Injection of peripheral lymphoid cells |
|
|
|
| Human Scid-Repopulating Cell SCID | Hu-SRC-SCID | Injection of newborn or adult mice with hematopoietic stem cells derived from fetal liver, cord blood, bone marrow or from granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood |
|
|
|
| SCID-Human | SCID-Hu | Co-implantation of human fetal liver and thymus under the renal capsule |
|
|
|
| Bone marrow, Liver, Thymus | BLT | Human fetal liver and autologous thymus fragments are co-implanted under the renal capsule of an immunodeficient mouse, and following preconditioning human HSCs isolated from the same fetal liver are then injected intravenously to engraft the bone marrow48 |
|
|
|
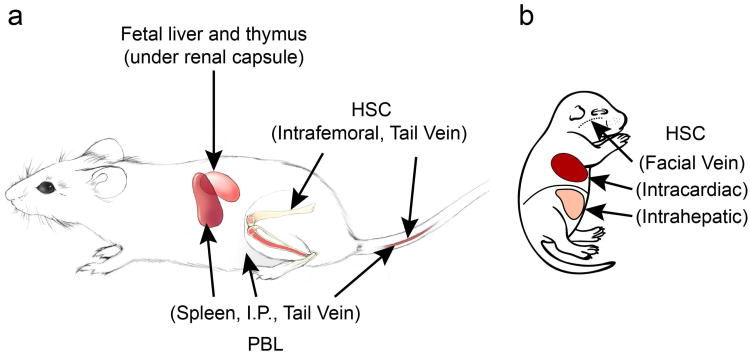
Figure 1. Approaches for engraftment of human immune systems in immunodeficient mice.
Legend to Figure 1: a: To establish Hu-PBL-SCID mice, injection routes include IV, IP, and intrasplenic into adult recipients. To establish Hu-SRC-SCID mice, injection routes of HSC in adult recipients include IV and intrafemoral. b: Injection routes into newborn mice include facial vein, intracardiac, and intrahepatic. IP injection of HSC into newborn or adult recipients results in very low engraftment. To establish SCID-Hu mice, fetal liver and thymus fragments are implanted under the renal capsule into adult mice. To establish BLT mice, fetal liver and thymus fragments are implanted under the renal capsule into irradiated adult mice, and HSC derived from the same fetal liver are injected IV. HSC, haematopoietic stem cell; IP, intraperitoneal; IV, intravenous; PBL, peripheral blood lymphocytes.
Overcoming limitations in humanized mice
There were previously several limitations inherent in humanized mice due, in part, to mouse-versus human-specific differences in the growth factors and cytokines required for human haematopoietic and immune system development (Figure 2), and due to the presence of mouse MHC versus human HLA molecules (reviewed in REF 1). Additional limitations included deficiencies in the development of lymph nodes, poorly organized lymphoid architecture and impaired affinity maturation and class switching of antibodies following immunization in the humanized mice1. In addition, species-specificity of homing molecules may impede the appropriate trafficking of human immune cells. Finally, remaining mouse innate immunity in the humanized mouse models represented a continuing impediment to human cell engraftment and investigation of human immune function1.
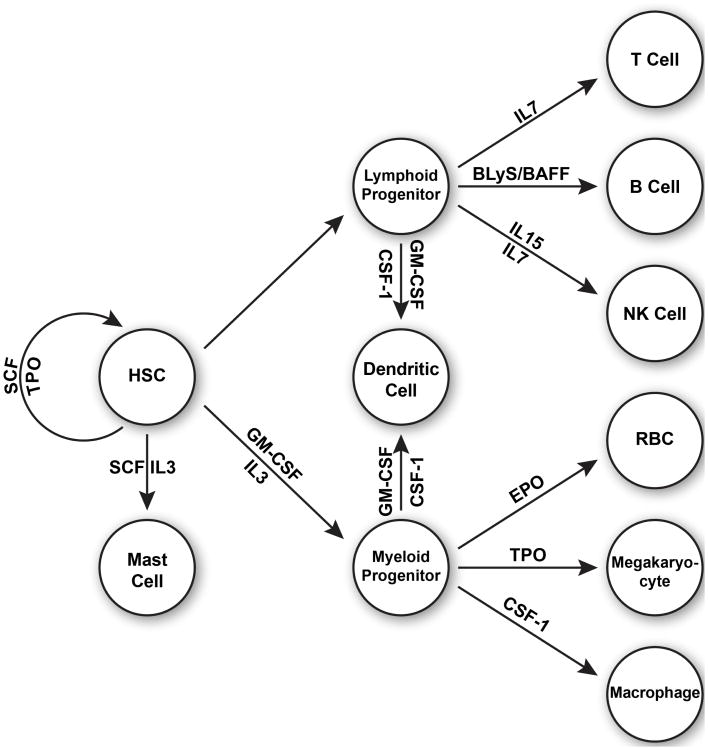
Figure 2. Cytokines expressed transgenically in immunodeficient IL2rγnull strains.
Legend to Figure 2: Human cytokines that have been, or are currently under development, expressed transgenically in immunodeficient IL2rγnull strains to enhance human hematopoietic cell engraftment and immune system development and function. SCF, stem cell factor, TPO, thrombopoietin, HSC, hematopoietic stem cell, GM-CSF, granulocyte macrophage colony stimulating factor, IL3, interleukin 3, EPO, erythropoietin, CSF-1, colony stimulating factor-1, BLyS/BAFF, B lymphocyte stimulator/B cell activating factor, IL7, interleukin 7, IL15, interleukin 15.
Expression of human transgenes and targeted inactivation of mouse genes encoding factors that control the development and function of the innate immune system have recently addressed many of these limitations, resulting in increased human immune system function in the humanized mice. The technological approaches for developing these “next generation” immunodeficient Il2rgnull mice are quite diverse, and differences in the recipient strain and levels of functional engraftment of human immune systems differ depending on the technology used.
Technologies to deliver human species-specific factors to enhance human haematopoiesis and immune system development and function are also diverse. The simplest approach is to provide the needed factors by injection of recombinant proteins (reviewed in REF 9). Other technologies include the transduction of human HSCs with genes encoding factors such as mouse CD47 10, which regulates phagocytic uptake of cells (that is, a do not eat me signal), and delivery of plasmid DNA encoding human cytokines by hydrodynamic tail vein injection 11. In addition, lentiviral vectors have been used to deliver human IL7 into BRG mice, promoting homeostatic proliferation of both adoptively transferred and endogenously generated T cells12.
As described below, three main genetic engineering technologies have been adopted: transgenic expression of cDNA constructs driven by tissue-specific or ubiquitous promoters; transgenic expression of bacterial artificial chromosomes (BACs); and knock-in technology.
New tools
Strains of transgenic immunodeficient Il2rgnull mice have been developed using cDNA constructs expressing human genes under the control of host tissue specific or ubiquitous promoters. However, this approach often leads to transgene expression at non-physiological levels or lacking temporal controls leading to adverse effects on the development and function of the human immune system in humanized mice. A second approach is the use of human bacterial artificial chromosomes (BACs) that often contain critical regulatory elements for gene expression. The use of BACs to create transgenic mice facilitates gene expression at physiological levels, when appropriate during development, and when functionally needed. Using these transgenic approaches, the homologous mouse gene is left intact and therefore both the mouse and human gene of interest are expressed. A third approach is to use knock-in technology in which the mouse gene is replaced by the corresponding human gene, resulting in the expression of the human gene product in the absence of the mouse gene product. This strategy has been successfully used for multiple genes in C;129RG mice due to the availability of embryonic stem (ES) cells of the C;129RG strain3. These ES cells can also be used to develop knockout mice in which a targeted mouse gene is specifically inactivated. The development of knock-in and knockout mice on the NOD strain was previously hindered by the lack of available NOD ES cells, but this limitation has recently been overcome by the generation of ES cells based on the NOD13 and NSG strain (http://research.jax.org/collaboration/escell.html) and will result in the development of knock-in and knockout NSG and NRG mice.
Additional emerging technologies that can alter the genome of mice have been described, including zinc finger technology (ZFN) and the transcription activator like effector nuclease (TALEN) technology. Zinc finger nucleases are engineered DNA-binding proteins that cause double stranded DNA breaks at specific locations and enables targeted editing of the genome14. TALENS utilize transcription activator-like effectors, secreted by plant bacteria that can be designed to bind to specific DNA sequences15. ZFN and TALEN technology can be applied directly in embryos and thus can be used for the development of new strains with increasing genetic complexity that cannot easily be generated using traditional crosses. Although, neither ZFN nor TALEN technology have yet been applied to the field of humanized mice, these technologies will likely make an important impact on future humanized mouse model development.
New models
Many genetically modified strains of immunodeficient Il2rgnull mice are now available for the study of human immunobiology (Table 1) and it is likely that many new stocks will soon be available.
Table 1.
Immunodeficient Il2rgnull mouse strains genetically engineered to enhance immunity in humanized mice.
| Immunodeficient mouse strain | Commonly used strain abbreviation | Human factors/ targeted mutations | Technology used for human factor expression | Expression and effect on host mouse | Effect on engraftment of human HSCs, PBMCs and fetal tissues | Ref. |
|---|---|---|---|---|---|---|
| C;129S4- Rag2tm1.1FlvT hpotm1.1(TPO)FlvIl2rgtm1.1Flv | C;129 Rag2G TPO-KI | Thrombopoietin | Knockin | Thrombocytopenia Decreased HSC numbers | Increased human myelopoiesis; decreased lymphopoiesis; Increased HSC self-renewal capacity | 17 |
| C;129S4- Rag2tm1.1FlvCsf1tm1.1(CSF 1)FlvIl2rgtm1.1Flv | C;129 Rag2G CSF1-KI | Macrophage colony-stimulating factor | Knockin | No pathologic changes Normal hematopoiesis | Increased frequencies of human monocytes/macrophages; augmented macrophage function | 16 |
| C;129S4- Rag2tm1.1FlvCsf2/IL3tm1.1(CSF2,IL3)FlvIl2rgtm1.1Flv | C;129 Rag2G (GM-CSF, IL-3)-KI | IL-3 and GM-CSF | Knockin | (PAP) | Enhanced development of human alveolar macrophages; Partial rescue of PAP | 18 |
| C;129S4- Rag2tm1.1FlvIl2rgtm1.1Flv Tg(SIRPA)1Flv | C;129 Rag2G Tg(Hu-SIRPA) | Signal regulatory protein α | BAC transgenic | No pathological changes Normal hematopoiesis | Enhanced human HSC engraftment and maintenance in BM; Enhanced antigen-specific IgM Ab responses | 27 |
| NOD.Cg-PrkdcscidIl2rgtm1Wjl Tg(CMV-IL3,CSF2,KITLG)1Eav | NSG Tg(Hu-GM-CSF, IL-3, SCF) | IL-3, GM-CSF, SCF | Transgene construct under control of a CMV promoter/enhancer | No pathological changes | Reduced frequencies of human HSC; Increased frequencies of human myeloid cells in BM; Increased frequencies of CD4+ FoxP3+ cells; Decreased B cell frequencies | 19 |
| NOD.Cg-PrkdcscidIl2rgtm1Wjl Tg(PGK1-KITL*220)441Daw | NSG Tg(Hu-bSCF) | Membrane-bound human SCF | Transgenic construct | No pathological changes | Supports neonatal HSC engraftment without host irradiation; Increased human mast cell numbers | 21, 22 |
| NOD.Cg-PrkdcscidIl2rgtm1WjlB2mtm1Unc | NSG B2m−/− | MHC class I KO | Targeted mutation | Prevents MHC class I expression; Hemachromatosis | Decreases severity of GVHD mediated by allo anti-MHC class 1 responses following PBL engraftment | 24 |
| NOD.Cg-Prkdcscidl2rgtm1WjlH2-Ab1tm1Gru | NSG IA−/− | MHC class II KO | Targeted mutation | No pathological changes | Decreases severity of GVHD mediated by allo anti-MHC class II following PBL engraftment | 24 |
| NOD.Cg-Prkd cscidIl2rgtm1WjlTg(HLA- A/H2-D/B2M)1Dvs/SzJ | NSG Tg(HLA-A2, B2M) | HLA-A2, Huβ2M | Transgene construct | No pathological changes | Supports development of human HLA-A2-restricted T cells following human HSC engraftment | 61, 62 |
| NOD.Cg-PrkdcscidIl2rgtm1WjlTg(HLA-A2.1)1Eng | NSG Tg(HLA-A2) | HLA-A2 | Transgene construct | No pathological changes | Supports development of human HLA-A2-restricted T cells following human HSC engraftment | 60 |
| NOD.Cg-PrkdcscidIl2rgtm1WjlH2-Ab1tm1Gru Tg(HLA-DRB1)31Dmz | NSG I-A−/− Tg(HLA-DR4) | HLA-DR4 Mouse Class II KO | Transgene construct | No pathological changes | Target for allo-GVH following PBMC engraftment | 25 |
| NOD-Cg-Rag1tm1MomIL2rgnullTg(HLA-DRB1)31Dmz | NSG Tg(HLA-DR4) | HLA-DR4 | Transgene construct | No pathological changes | Enhanced human immune function | 43 |
KI, knock-in; TPO, thrombopoietin; CSF1, macrophage colony-stimulating factor 1; GM-CSF, granulocyte macrophage colony-stimulating factor; IL-3, interleukin-3; SIRP, signal regulatory protein-α; SCF, stem cell factor; BAC, bacterial chromosomal construct; CMV, cytomegalovirus; BM, bone marrow; PAP, pulmonary alveolar proteinosis; HSC, haematopoietic stem cell; mbSCF, membrane bound human stem cell factor; KO, knockout; GVHD, graft-versus-host disease; Tg, transgenic; PBMCs, peripheral blood mononuclear cells; C;129 Rag2G, C;129S4- Rag2tm1.1Flv; NSG, NOD-scid Il2rgnull; NOG, NOD-scid Il2rgtrunc; B2M, beta 2-microglobulin; HSC, hematopoietic stem cell, PBL, peripheral blood lymphocytes; BLT, bone marrow, liver, thymus; RBC, red blood cells.
Using knock-in technology, three new strains of BRG mice expressing human haematopoietic growth factors that are important in myeloid cell development have been developed3. As expected, each human growth factor enhanced the development of the human cell lineage targeted by that specific factor. For example, transgenic expression of macrophage colony-stimulating factor (CSF1) increased the frequencies of human monocytes and macrophages following human HSC engraftment and enhanced the production of pro-inflammatory cytokines following challenge with the Toll-like receptor 4 (TLR4) agonist lipopolysaccharide16. However, adverse events were also observed. For example, in the thrombopoietin (TPO) knockin strain, increased myelopoiesis and HSC self-renewal was observed, but thrombocytopaenia developed17. Although human TPO can support murine thrombocytopoiesis, the levels of human TPO that were expressed in the mouse was ∼10 fold lower than the normal levels of mouse TPO. This led to decreased numbers of mouse platelets, and because only very low levels of human platelets circulate in the blood of humanized mice, thrombocytopaenia developed. In IL3 and granulocyte macrophage colony-stimulating factor (GM-CSF) knockin mice, human HSC engraftment resulted in enhanced development of human alveolar macrophages, but non-engrafted mice developed pulmonary alveolar proteinosis caused by the absence of mouse GM-CSF18.
Transgenic expression of three hematopoietic growth factors (IL3, GM-CSF and SCF) under the control of a ubiquitous promoter led to increased frequencies of human myeloid cells in the bone marrow of NSG mice engrafted with human HSC19. However, earlier studies with these co-integrated transgenes showed that GM-CSF-mediated HSC mobilization resulted in reduced frequencies of human HSCs in the bone marrow compared with non-transgenic NOD-scid mice20. Increased levels of CD4+Foxp3+ T cells in the peripheral tissues were also apparent following HSC engraftment of triply transgenic NSG mice, suggesting that non-physiological exposure to cytokines during T cell development can alter the cell population generated19. This deficiency might be overcome using the knockin approach in which physiological levels of cytokines would be expected. In contrast, the transgenic expression of membrane-bound human SCF (mbSCF) driven by a ubiquitous promoter results in increased human mast cell levels and enhanced HSC engraftment in NSG mice with no reported pathological changes in the murine host21. Moreover newborn NSG mice expressing mbSCF support high levels of human HSC engraftment without the need for X-irradiation conditioning22. A caveat associated with expressing human factors in immunodeficient mice is that although some murine cytokines do not function on human cells, many of the human factors cross-react with murine cells23. This could cause unexpected phenotypic changes. For example, transgenic expression of a factor used to increase levels of human innate immunity following human HSC engraftment could upregulate mouse innate immunity and reduce levels of human engraftment. In addition, even cross-reactive mouse cytokines that work in vitro may have lower in vivo potency than their corresponding human cytokines.
Additional immunodeficient mouse strains have been developed to minimize reactivity of human immune cells against host tissue and include NSG MHC class I- and MHC class II-deficient mice24, 25. These mice were specifically designed to reduce xenogeneic GVHD, and when combined with transgenic expression of human HLA molecules, they permit the development of HLA-restricted human T cells in the absence of H2-resticted human T cells in Hu-SRC-SCID mice25.
Although the current models are appropriate for addressing many questions of human immune system development and function, recognition of remaining deficiencies in the models continues to evolve. These deficiencies are systematically being addressed using genetic approaches to improve human immune system engraftment and function. Continued development of mice expressing human-specific growth factors and HLA molecules, combined with additional approaches to reduce murine H2 expression and decrease host innate immunity, promise to provide models with increased levels of human immune system engraftment and improved functional activity.
Lessons learned from humanized mice
Haematopoiesis
To generate a functional human immune system that contains multiple cell lineages required for innate and adaptive immunity, immunodeficient Il2rgnull mice are engrafted with human HSCs. For many years, it was known that NOD-scid mice engrafted at higher levels with human HSCs than did immunodeficient CB17, BALB/c and C57BL/6 mice1. Of interest was the report that the signal regulatory protein α(Sirpa) gene expressed in NOD mice has a polymorphism that is very similar to the human gene, and that the SIRPα expressed by C57BL/6 and BALB/c mice have much less homology to human SIRPα26. Appropriate interaction of SIRPα on host macrophages with human CD47 on engrafted haematopoietic cells is important for hematopoietic cell survival and therefore expression of human or NOD (human-like) SIRPα is a major determinant for engraftment and survival of human haematopoietic cells in mice26. BRG mice transgenically expressing a human SIRPα increase engraftment to levels achieved in NSG and NRG mice, indicating that SIRPα is a causal factor in controlling engraftment levels27. A NSG mouse strain transgenically expressing human SIRPα is currently under development to identify additional strain-specific factors important in human HSC engraftment.
Key insights into the biology of human immune system development from HSCs have been gained using humanized mice. HSCs derived from human fetal liver, fetal bone marrow and adult bone marrow were used to engraft immunodeficient mice28. Fetal liver and fetal bone marrow generated higher numbers of CD4+CD25+Foxp3+ regulatory T (Treg) cells in the thymus than did adult HSCs. The data suggest a “layering” of immune system development during ontogeny in humans that is due to intrinsic differences in HSC populations, leading to distinct immune systems in fetal versus adult individuals28. Another report using humanized mice showed that human myeloid and plasmacytoid dendritic cells can arise from a common precursor that is independent of conventional myeloid and lymphoid haematopoietic pathways29. Humanized mice have also been used to analyze the in vivo function of HSCs derived from human induced pluripotent stem (iPS) cells. Although the human iPS cell-derived CD34+ HSC had bipotential ability to generate both hematopoietic and endothelial lineages in vitro, these studies demonstrated preferential differentiation into endothelial cells in vivo30. Investigations into potential mechanisms of human immune system development and function following engraftment of HSCs in humanized mice will continue to provide insights in human immune cell differentiation and function.
Immune system
Approaches for studying human immunity in humanized mice include engraftment of mature lymphoid cells (the Hu-PBL-SCID model) or stem cells (the Hu-SRC-SCID and the BLT models).
The use of Hu-PBL-SCID mice to study effector mechanisms in a xenogeneic GVHD system has focused on the efficacy of drugs and Treg cells in preventing disease24. Recent modifications of the Hu-PBL-SCID model have permitted investigation of the infiltration and rejection of human islet, skin and arterial allografts and the cell populations involved in the immune rejection process31-33. The transfer of human Treg cells that were expanded ex vivo prevented rejection of human skin and arterial grafts in the Hu-PBL-SCID model34, 35. These observations demonstrate that human Tregs can modulate transplantation rejection and that humanized mice provide both a platform for testing the efficacy of Tregs as a cellular therapy as well as a tool with which to identify the mechanisms underlying their in vivo regulatory activity.
Of interest was the demonstration that survival of adoptively transferred antigen-specific central memory T cells is IL-15 dependent, and that this T cell population is superior to effector memory T cells in their ability to survive in adoptive recipients, providing guidance for clinical trials of adoptive T cell therapy36. Although rejection of allografts and the development of xenogeneic GVHD have been readily demonstrated in Hu-PBL-SCID mice, antigen-specific activation of T cells has been much harder to document, perhaps due to the limited engraftment of human APCs in this model24. To address this, donor-matched human dendritic cells have been transferred with PBLs to NSG mice. Following immunization, adenovirus antigen-specific T cells developed37, and this model therefore provides an opportunity to test vaccines and investigate mechanisms underlying antigen-specific T cell immune responses.
One caveat of interpreting results following engraftment of human effector T cells from PBL or other sources in the Hu-PBL-SCID model is the confounding effect of the xenogeneic GVHD response, due primarily to human T cell reactivity against mouse MHC molecules24. To address this, NSG mice lacking MHC class I and class II molecules (NSG B2m−/− mice and NSG I-A−/− mice, respectively) have been developed and these mice have reduced xenogeneic GVHD24. Xenogeneic GVHD is further reduced following engraftment of NSG IA−/− mice with purified human CD4+ T cells25. When human HLA-DR4−CD4+ T cells are engrafted into NSG-HLA-DR4 transgenic mice lacking mouse MHC class II molecules (NSG I-A−/− Tg(HLA-DR4), an allogeneic GVHD response develops in the absence of xenogeneic GVHD, providing for the first time a model system for the study of human allogeneic GVHD25.
Although all lineages of immune cells (including T cells) are generated in Hu-SRC-SCID and BLT mice, the immune system generated is not as robust as desired1. Splenic germinal centers are poorly organized, preventing the development of a robust antigen-specific antibody response23. Antigen-specific human IgM antibody responses and IgM hybridomas are generated following immunization of HSC-engrafted mice, but achievement of affinity maturation and class switching from IgM to IgG isotype has been particularly difficult38. A recent study has suggested that most human B cells that develop in HSC-engrafted humanized mice are immature CD5+ B cells39. However, antibody repertoires in human B cells generated in HSC-engrafted NSG mice are very similar to the repertoire observed in B cells in humans38, 40. Also, mouse B cell activating factor (BAFF; also known as BLyS), which is a critical B cell differentiation and survival factor, is ineffective on human B cells41. Administration of recombinant human BAFF enhances B cell engraftment and survival in the Hu-PBL-SCID model, in which human B cells usually fail to efficiently engraft41. Use of recombinant human BAFF during immunization of humanized mice may support affinity maturation of the immune response and class switching from IgM to IgG isotype and analysis of immunodeficient Il2rgnull mice transgenically expressing BAFF is underway. Furthermore, providing both human BAFF and IL-7 may further enhance the antibody response in humanized mice41, 42.
Another critical factor may be the requirement for HLA expression in the mouse host. Engraftment of HLA-DR4+ HSCs into NSG or NOG Tg(HLA-DR4) mice permitted generation of human IgG antibodies following tetanus toxoid vaccination, although antibody levels were still much lower than observed in immunized individuals43, 44.
In contrast to difficulties in generating antigen-specific B cell responses in Hu-SRC-SCID and BLT humanized mice, they readily generate antigen-specific T cell responses. NSG mice develop a highly diverse T cell repertoire following engraftment with cord blood HSCs45 and human CD4+, CD8+ and Treg cells develop in both the Hu-SRC-SCID and BLT models7, 46. Delayed type hypersensitivity (DTH) responses can be elicited in humanized mice, and IL-17-specific antibody can block the DTH response to collagen V, suggesting a role for IL-17 in human DTH responses47.
Moreover, a human T cell-mediated immune response to the superantigen toxic shock syndrome toxin 1 (TSST1) has been demonstrated in BLT mice, permitting the mechanisms underlying this response to be investigated and effective therapies to be tested48. The development of functional human T cells in humanized mice can be improved by administration of recombinant human IL-75, 42. However, insights into the role of IL-7 in human T cell development and function using humanized mice suggests that the timing of administration of human IL-7 is critical, as exposure to IL-7 prior to the entrance of T cell progenitors into the thymus impedes rather than enhances human T cell development and function49. Of great interest is the use of humanized mice to study the mechanisms underlying human-specific drugs that target T cells. For example, mechanisms underlying the beneficial effects of Teplizumab (a CD3-specific antibody) in type 1 diabetics was shown using humanized mice to induce gut-tropic Treg cells, and this observation was then confirmed in patients treated with Teplizumab50.
Human allograft rejection has been studied in the Hu-SRC-SCID model. Using BRG mice, islet allograft rejection was not observed, likely due to the low levels of human T cells that are generated in this model51. In contrast, using NSG mice that develop higher levels of human T cells7, islet allograft rejection was observed52. A variation combining the Hu-PBL-SCID and Hu-SRC-SCID models has been used to study human allograft rejection.
A Hu-SRC-SCID model generated by engraftment of mobilized peripheral blood stem cells was used to generate B cells and macrophages in the absence of naive T cells. In this study CD34+ enriched circulating mononuclear cells isolated by leukapheresis following G-CSF mobilization were injected along with adoptive transfer of PBMCs autologous to the HSC donor. This treatment led to rejection of human artery allograft endothelial cells33. Additional studies using this model demonstrated that effector memory CD4+ T cells directly recognize and destroy allogeneic endothelial cells in the tissue graft, and that treatment with a neutralizing human IL-6-specific antibody enhances human Treg cells and prevents rejection53.
Humanized mice can also be used to study cells of the innate immune system. NK cells develop in HSC-engrafted mice1. However, their ability to kill targets is not robust. Pre-activation of NK cells or trans-presentation of recombinant human IL-15 enhances human NK cell development in humanized mice, and they begin to acquire cytotoxic activity54, 55. However, complete “licensing” of NK cells will require human HLA transgenic expression by the murine host to permit fully functional human NK cell development. An additional component of the human immune system that has only recently been able to be studied in rodents due to species-specific differences is the CD1 antigen presenting system. Using NSG BLT mice, it was shown that a functional human CD1-dependent cellular compartment can develop in these mice56. This was confirmed by CD1d tetramer staining of human invariant NKT (iNKT) cells, secretion of IFNγ by CD1-restricted iNKT cells, and rapid production of IFNγ and IL-4 following injection of α-galactosylceramide56. This now permits for the first time investigation of the human CD1 system in a small animal model.
Infectious diseases
A major experimental use of humanized mice over the last few years has been for the study of human infectious agents (Table 2). An example of the utility of Hu-SRC-SCID mice for infectious disease experiments was the study of Salmonella enteric serovar Thyphi infection57, 58. S. Typhi is highly adapted to humans and fails to cause progressive infection in mice. Much of the current understanding of Salmonella pathogenesis resulted from studies of S. Typhimurium in mice, but many differences exist between S. Typhi and S. Typhimurium. In a report using NSG Hu-SRC-SCID mice infected with S. Typhi, granulomatous inflammation with mononuclear cell infiltration in the spleen, which mimics human pathology, was observed, and infections induced rapid fatality. Screening of transposon pools in infected NSG Hu-SRC-SCID mice revealed previously unrecognized Salmonella virulence determinants57. In another report using BRG Hu-SRC-SCID mice, prolonged survival following S. Typhi infection was associated with innate and adaptive human immune responses58. These differences in pathogenesis and lethality following S. Typhi infection highlight the differences in the different strains of humanized mice (NSG versus BRG mice), each having its own advantages and constraints.
Table 2.
Pathogenic microorganisms studied in humanized Il2rgnull mice.
| Infectious agent | Mouse strain | Engraftment | Ref. |
|---|---|---|---|
| HIV | Many | Multiple models HSC,PBL,SCID/Hu, BLT | 64, 65 |
| HTLV1 | BRG | HSC | 111 |
| EBV | NSG Tg(HLA-A2, B2M) | HSC | 60, 61 |
| EBV | NOG | HSC | 59, 60, 112 |
| Ebola virus | NSG | PBL | 113 |
| Dengue virus | NSG | HSC | 114, 115 |
| Dengue virus | NSG Tg(HLA-A2, B2M) | HSC, BLT | 62 |
| Dengue virus | BRG | HSC | 116 |
| Hepatitis C virus | BRG FK506 Caspase 8 | HSC and fetal hepatocytes | 108, 117-119 |
| Hepatitis B virus | STOCK Prkdcscid Tg(Alb1-Plau)Bri | Adult hepatocytes | 118-120 |
| Salmonella enteric serovar Thyphi | NSG, C;129RG | HSC | 57, 58 |
| Borrelia hermsii | NSG | HSC | 121 |
| Plasmodium falciparum | NSG | Hu RBC | 122 |
| Cytomegalovirus virus | NSG | HSC | 123 |
HIV, human immunodeficiency virus; HTLV1, human T-lymphotropic virus 1; EBV, Epstein–Barr virus; Alb1-Plau, human urokinase-type plasminogen activator gene under control of the albumin promoter
A NOG HSC-engrafted model of Epstein-Barr virus was described that closely mimicked the pathological conditions observed following infection of humans with EBV, resembling a syndrome termed hemophagocytic lympho-histiocytosis, a rare but devastating disorder in EBV-infected individuals.59 The availability of HLA-A2- transgenic immunodeficient IL2rgnull mice permitted extension of infection models to the study of HLA-A2-restricted human CD8+ T cell responses to infectious agents60-62. Infection of HSC engrafted immunodeficient NSG-HLA-A2 transgenic mice with Epstein–Barr virus (EBV) led to the development of HLA-A2-restricted human CD8+ T cell responses and protective CD4+ and CD8+ T cell-mediated immunity60, 61. A humanized mouse model of EBV infection is particularly important, as pre-symptomatic stages of EBV infection in humans are largely unexplored but can now be investigated in humanized mice. HLA-A2-restricted human T cell responses have also been observed following dengue virus infection of NSG-HLA-A2 transgenic mice engrafted with HSCs or in BLT mice62. Infected humans can develop severe capillary leakage syndrome (dengue hemorrhagic fever) following a second infection with a different dengue virus serotype, although little is known about the immune response or mechanisms by which this occurs63. Humanized mice may become a useful model for studying the role of prior immunity to dengue virus in contributing to this disease63.
One of the greatest impacts of humanized mice has been in the field of HIV research. HIV infection of humanized mice has utilized all four models (Hu-PBL-SCID, Hu-SRC-SCID, SCID-Hu, and BLT) of immune system engraftment, and have been extensively reviewed elsewhere64-67, therefore only key recent advances are discussed here. Numerous studies have tested HIV therapeutic interventions that limited viral replication and CD4+ T cell depletion. These therapeutic interventions include: systemic antiretroviral therapy68, systemic delivery of HIV-neutralizing antibodies69, 70, transplantation of genetically modified HSCs71, 72 and novel approaches for delivering small inhibitory RNA to either cellular or viral targets73-75. In addition, recent studies using antiretrovirals to suppress viremia demonstrated that HIV latency in CD4+ T cells occurs in humanized mice, highlighting the value of these models for evaluating HIV eradication strategies76-78. In a related study, Hu-SRC-SCID mice were used to examine the potential of HSCs to serve as a reservoir of latent HIV79. In the BLT model, robust mucosal human immune systems render these animals susceptible to rectal and vaginal HIV transmission80, 81. The susceptibility of BLT mice to mucosal HIV transmission permits evaluation of HIV prevention strategies using topically or systemically applied viral inhibitors75, 80, 81. Importantly, infection of BLT mice results in rapid systemic dissemination of the virus and peripheral CD4+ T cell depletion including gastrointestinal CD4+ T cells, similar to what is observed in humans80. Together these studies demonstrate the broad applicability of humanized mice in pre-clinical HIV research.
Both the function and HIV-associated dysfunction of the human immune system have been described in HIV infected humanized mice. In BLT mice, the production of low levels of HIV-specific IgG was detected by 8 weeks post-infection82 and by 16 weeks all infected mice were producing human HIV-specific immunoglobulin83. Furthermore, HIV-epitope restricted cytotoxic T-lymphocyte (CTL) responses were detected in HIV-infected BLT mice83. Additional evidence of CTL function against HIV in humanized mice include the finding that depletion of human CD8+ T cells accelerated HIV-induced immunopathology, suggesting a role of these cells in controlling infection84. Extensive sequencing analysis showed that immune selective pressure drove evolution of the HIV env gene in Hu-SRC-SCID mice85, and functional innate immune responses mediated by the antiretroviral cytidine deaminase APOBEC3 toward HIV have been demonstrated86. Immune dysfunction in HIV disease is, in part, characterized by abnormal immune activation87 and a similar activated T cell phenotype has been recently described in BLT mice88. Hu-SRC-SCID mice have been utilized to demonstrate that HIV infection activates and impairs plasmacytoid DC function89. Finally, the influx of human macrophages into the brain of Hu-SRC-SCID mice exhibiting progressive HIV infection was linked with viral neuropathogenesis90, 91. Although immune responses generated in humanized mice are not protective against infection, these studies reveal functional human immune responses in humanized mice and show that known and new HIV-induced immune dysfunctions can be studied in these models.
Autoimmunity
There are only a few reports of successfully recapitulating autoimmune diseases in humanized mice. In one study using the Hu-PBL-SCID model engrafted with PBLs from individuals with systemic lupus erythematosus (SLE), mice developed microthrombi and infiltration of B and T cells into the glomeruli, which are features of SLE92. In another report, HLA-A2-matched PBLs from individuals with type 1 diabetes (T1D) transferred to NSG-HLA-A2 mice allowed recovery of islet-associated T cells, suggesting that this might provide a model for studying diabetogenic T cells93. Using the Hu-SRC-SCID model, bone marrow-derived HSCs from patients with osteoarthritis transferred into anti-CD122-treated NK cell-depleted NOD-scid mice developed synovial inflammation following intra-articular injection of Chlamydia trachomatis to induce arthritis94. To date, studies of the immunological mechanisms of autoimmunity have yet to be performed using these models. However, as the next generation of humanized mice becomes available, modeling of autoimmune disorders and investigation into the immunological mechanisms underlying autoimmune diseases will follow.
Tumour immunology
Humanized mice have permitted new types of human tumours to be grown and studied in vivo1. These new models have been used to characterize cancer stem cells and other facets of tumour biology 95, 96, and reports are now appearing on features of the human anti-tumor immune response. Transplantation of primary lung tumors revealed the existence of tumor infiltrating effector memory T cells that could be activated by administration of human IL-1297. Primary human epithelial ovarian tumors engrafted in NSG mice also contain human T cells and efforts are underway to activate the intra-tumor T cells for anti-ovarian tumour immunity98.
Humanized mice have also been used to study tumour therapies. CD47 is expressed on most solid human tumours, and administration of human CD47-specific antibody to human tumour-bearing NSG mice leads to tumour cell phagocytosis and elimination, demonstrating that tumour-engrafted NSG mice can be used to evaluate antibody-mediated cancer therapies99. Engraftment of BLT mice using HSCs transduced with anti-tumor CD8+ T cell receptors have been shown to control human melanomas100. This model will be important for optimizing human immune responses to melanomas for cellular adoptive therapies. Efficacy of adoptive transfer of human T cells genetically engineered to express melanoma antigen-specific T cell receptors has been evaluated in NOG mice101. In a novel approach, newborn NSG mice were co-engrafted with human HSCs and human breast cancer cells. The tumours grew in the presence of the immune system, and the generation of tumour-specific T cells was observed, as well as NK cell accumulation at the tumour site102. Understanding the resistance of the tumour to the engrafted immune system and how to modulate the immune system to overcome this resistance will provide new approaches for cancer immunotherapy.
Future promise and remaining challenges
The new models that have been described and used to study human immunity are only the beginning of the next generation of humanized mice that will soon be available (Figure 2). Remaining challenges are to generate new strains of mice needed for further enhancement of human immunity with reduced mouse innate immunity (Table 3)1, 2.
Table 3. Remaining challenges in humanized mice and approaches to overcome these challenges.
| Challenges | Opportunities |
|---|---|
| Impaired mature B cell development and function following PBL or HSC engraftment | Provide human cytokines that promote B cell development (BAFF, IL-6) |
| Low IgG antibody responses | Enhance T cell help for class switch Enhance lymph node germinal center development |
| Develop HLA-restricted T cells for multiple HLA class I and II alleles | Develop a panel of HLA transgenic mice |
| Engraftment with mature T cells results in GVHD | Targeting of mouse MHC class I and II genes |
| Infection with human host-specific pathogens | Engraft with human infectable cells |
| Modeling human autoimmune diseases | Engraft with human target cells and with human autoreactive immune system |
| Rapid clearance of human RBCs from circulation | Deplete mouse macrophages and express human SIRPα protein |
| Engraft with human tumor cells that grow poorly in existing models | Provide needed human growth factors, hormones, etc; Engraft tumors in orthotopic site |
| Development of HSC and other functional cell populations from human ES cells or iPS cells | Provide needed microenvironment and growth factors |
| Lack of appropriate trafficking of human immune cells | Generate human adhesion molecule transgenic mice to facilitate appropriate trafficking of human immune cells |
A critical issue remaining is the limited development of lymph nodes in immunodeficient Il2rgnull mice. Although mesenteric lymph nodes develop, other peripheral lymph nodes are small with little to no lymphoid organization103. Current approaches to address this include administration of human IL-7, which is critical in neonatal maturation of lymph nodes104 and provision of human agonist anti-lymphotoxin β receptor during a critical early maturation step in lymph node development105. One of the cell populations deficient in Il2rgnull mice is the lymphoid tissue-inducer cell, which is required for lymph node and gut-associated lymphoid tissue formation. Efforts to identify approaches to enhance development of these cell populations are continuing.
Another limitation being addressed is the generation of immunodeficient Il2rgnull HLA transgenic mice that represent many of the common HLA class I superfamilies106. For the study of autoimmunity, HLA class II transgenic NSG mice are being developed. These new models will facilitate investigations of T1D, multiple sclerosis and rheumatoid arthritis, autoimmune disorders strongly associated with specific HLA class II alleles. For the study of human NK cells, NSG-HLA transgenic mice expressing HLA-B27 or HLA-Cw*0304, which are important in NK cell licensing, are under study. Use of HLA transgenic mice co-expressing IL-7 and/or BAFF may increase T and B cell function, respectively, and enhance immune responses. Overcoming poor adaptive humoral immune responses in humanized mice will result in more effective models for vaccine testing.
Additional modifications are needed for studies of infectious agents that require circulating human RBCs or resident hepatocytes as part of their life cycle, such as Plasmodium falciparum, which has obligate stages in both hepatocytes and RBCs. Mouse macrophages are a significant impediment to the survival of human RBCs in NSG mice and depletion of mouse macrophages allows enhanced development and survival of peripheral human RBCs in BLT mice107. Alternatively, the study of hepatitis B and C virus infections in the presence of an immune system requires the presences of both human hepatocytes and HSCs in the humanized mouse. One approach to achieve this has been to engraft human fetal liver hepatocytes and HSCs into an immunodeficient recipient with additional genetic modifications that induced host liver cell damage. Both human liver and immune systems developed, and the mice supported infection with hepatitis C virus108.
For proper immune function, appropriate trafficking of human immune cells to non-lymphoid tissues is required. Due to species-specific differences in adhesion molecules and potential incompatibilities of receptor-ligand pairs, human lymphocyte homing may not occur properly in immunodeficient mice. For example, there are known species-specific differences in the mouse/human lymphocyte function-associated antigen-1 (LFA-1) and intercellular adhesion molecule-1 (ICAM-1). Mouse LFA-1 can bind to human ICAM-1, but murine LFA-1 does not bind to human ICAM-1109.
One of the final obstacles in establishing a full human chimera is the inability of a number of human hematopoietic cell lineages, including granulocytes, platelets and RBCs, to circulate and traffic appropriately in the murine host. When the murine host is conditioned sufficiently to replace essentially all of the murine bone marrow with human hematopoietic cells, the mice die, likely due to a severe anemia and/or greatly reduced levels of circulating platelets and granulocytes21. Although human RBCs, platelet and granulocyte progenitors are detected in the bone marrow, few of these cell populations are found in the circulation, likely due to their removal in the circulation by murine macrophages110.
Conclusion and perspective
The diversity of humanized model strains, differences in approaches for engraftment of human cells and tissues, and the various technological approaches being used to generate the next generation of immunodeficient Il2rgnull mice leads to the key caveat in the use of humanized mice for the study of human immunology. Experiments using humanized mice, or any animal model system, need to be designed to address a mechanistic question rather than attempting to fully recapitulate the human biological process or pathology. With these caveats in mind, humanized mice are becoming increasing important tools in translational biomedical research, permitting insights into our understanding of human hematopoiesis, innate and adaptive immunity, autoimmunity, infectious disease and cancer immunology.
Acknowledgments
We thank D. Serreze and R. Maser for critical review of the manuscript. The authors are supported by the US National Institutes of Health (grants AI46629, UO1 DK089572, DK092758 and AI073146), an institutional Diabetes Endocrinology Research Center grant (DK32520), a Cancer Core Grant (CA034196), the Juvenile Diabetes Research Foundation International, and the Leona M. and Harry B. Helmsley Charitable Trust. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the US National Institutes of Health.
Glossary terms
- γc
A type I cytokine receptor chain that is shared by the receptors for the interleukins IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21.
- Severe combined immunodeficiency
(SCID). Humans or mice with this rare genetic disorder lack functional B and T cells owing to a mutation in a gene that is involved in B cell and/or T cell development, and consequently they suffer from recurrent infections. Several forms of SCID have been described, including mutations in the IL-2 receptor γ-chain, which is shared by several interleukin receptors, protein kinase, DNA activated, catalytic polypeptide (PRKDC), Janus activated kinase 3 (JAK3) deficiency and adenosine deaminase deficiency.
- Graft-versus-host disease
(GVHD). Tissue damage in a recipient of allogeneic tissue (usually a bone-marrow transplant) that results from the activity of donor cytotoxic T lymphocytes recognizing the tissues of the recipient as foreign. GVHD varies markedly in extent, but it can be life threatening in severe cases. Damage to the liver, skin and gut mucosa are common clinical manifestations.
- Bacterial artificial chromosome
(BAC). A cloning vector derived from a single-copy F-plasmid of Escherichia coli that carries the F replication and partitioning systems that ensure low copy number and faithful segregation of plasmid DNA to daughter cells. Large genomic fragments can be cloned into such vectors and they are faithfully replicated, which makes BACs useful for constructing genomic libraries.
- Knock-in technology
The introduction of a transgene into a precise location in the genome, rather than a random integration site. Knocking-in uses the same technique of homologous recombination as a knockout strategy but the targeting vector is designed to allow expression of the introduced transgene under control of the regulatory elements of the targeted gene.
- thrombocytopaenia
A reduced number of circulating platelets, owing to either the failure of production from bone-marrow megakaryocytes or increased clearance from the circulation, predominantly in the spleen.
- Central memory T cells
Antigen-experienced T cells that lack immediate effector function but can mediate rapid recall responses. They also rapidly develop the phenotype and function of effector memory T cells after restimulation with antigen. Central memory T cells retain the migratory properties of naive T cells and therefore circulate through the secondary lymphoid organs.
- Effector memory T cells
Memory T cells that home to peripheral tissues and plasma cells that home to the bone marrow and secrete antibodies. They are responsible for immediate protection to a re-infection.
- Germinal centre
A lymphoid structure that arises within follicles after immunization with, or exposure to, a T cell-dependent antigen. It is specialized for facilitating the development of high-affinity, long-lived plasma cells and memory B cells.
- Delayed-type hypersensitivity
A cellular immune response to antigen that develops over 24–72 hours with the infiltration of T cells and monocytes, and is dependent on the production of TH1 cell-specific cytokines.
- α-Galactosylceramide
(α-GalCer). A synthetic or marine-sponge-derived glycolipid containing an a-anomeric glycosidic linkage of the galactose residue to the sphingosine base. This lipid, and structurally related ones, potently activates CD1d-restricted natural killer T cells that express the semi-invariant Vα214–Jα18 T cell receptor in mice (and the Vα24–Jα18 equivalent receptor in humans).
- Systemic lupus erythematosus
(SLE). An autoimmune disease in which autoantibodies specific for DNA, RNA or proteins associated with nucleic acids form immune complexes. These complexes damage small blood vessels, especially in the kidneys. Patients with SLE generally have abnormal B and T cell function.
- lymphoid-tissue inducer cell
A cell that is present in developing lymph nodes, Peyer's patches and nasopharynx-associated lymphoid tissue (NALT). Lymphoid-tissue inducer cells are required for the development of these lymphoid organs. The inductive capacity of these cells for the generation of Peyer's patches and NALT has been shown by adoptive transfer, and it is generally assumed that they have a similar function in the formation of lymph nodes.
Footnotes
Competing interest statement: D.L.G. and L.D.S. are consultants for ViaCord Inc and Biogen/IDEC Inc. M.A.B. receives grant support from Biogen/IDEC Inc. J.V.G. is a consultant for Calimmune, GSK,and Boehringer Ingelheim.
References
- 1.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 2.Ito R, Takahashi T, Katano I, Ito M. Current advances in humanized mouse models. Cell Mol Immunol. 2012;9:208–214. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willinger T, Rongvaux A, Strowig T, Manz MG, Flavell RA. Improving human hemato-lymphoid-system mice by cytokine knock-in gene replacement. Trends Immunol. 2011;32:321–327. doi: 10.1016/j.it.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Ito M, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 5.Shultz LD, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2rγnull mice engrafted with mobilized human hematopoietic stem cell. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 6.Traggiai E, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 7.Brehm MA, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010;135:84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott SP, Eppert K, Lechman ER, Doedens M, Dick JE. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood. 2010;116:193–200. doi: 10.1182/blood-2010-02-271841. [DOI] [PubMed] [Google Scholar]
- 9.Drake AC, Chen Q, Chen J. Engineering humanized mice for improved hematopoietic reconstitution. Cell Mol Immunol. 2012;9:215–224. doi: 10.1038/cmi.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legrand N, et al. Functional CD47/signal regulatory protein alpha (SIRP(alpha)) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. Proc Natl Acad Sci U S A. 2011;108:13224–13229. doi: 10.1073/pnas.1101398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci U S A. 2009;106:21783–21788. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Connell RM, et al. Lentiviral vector delivery of human interleukin-7 (hIL-7) to human immune system (HIS) mice expands T lymphocyte populations. PLoS ONE. 2010;5:e12009. doi: 10.1371/journal.pone.0012009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang W, et al. Pluripotin combined with leukemia inhibitory factor greatly promotes the derivation of embryonic stem cell lines from refractory strains. Stem Cells. 2009;27:383–389. doi: 10.1634/stemcells.2008-0974. [DOI] [PubMed] [Google Scholar]
- 14.Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 15.Baker M. Gene-editing nucleases. Nat Methods. 2012;9:23–26. doi: 10.1038/nmeth.1807. [DOI] [PubMed] [Google Scholar]
- 16.Rathinam C, et al. Efficient differentiation and function of human macrophages in humanized CSF-1 mice. Blood. 2011;118:3119–3128. doi: 10.1182/blood-2010-12-326926. [DOI] [PubMed] [Google Scholar]
- 17.Rongvaux A, et al. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc Natl Acad Sci U S A. 2011;108:2378–2383. doi: 10.1073/pnas.1019524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willinger T, et al. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci U S A. 2011;108:2390–2395. doi: 10.1073/pnas.1019682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Billerbeck E, et al. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rgamma(null) humanized mice. Blood. 2011;117:3076–3086. doi: 10.1182/blood-2010-08-301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicolini FE, Cashman JD, Hogge DE, Humphries RK, Eaves CJ. NOD/SCID mice engineered to express human IL-3, GM-CSF and Steel factor constitutively mobilize engrafted human progenitors and compromise human stem cell regeneration. Leukemia. 2004;18:341–347. doi: 10.1038/sj.leu.2403222. [DOI] [PubMed] [Google Scholar]
- 21.Takagi S, et al. Membrane-bound human SCF/KL promotes in vivo human hematopoietic engraftment and myeloid differentiation. Blood. 2012;119:2768–2777. doi: 10.1182/blood-2011-05-353201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brehm MA, et al. Engraftment of human HSC in non-irradiated newborn NOD-scid IL2rgammanull mice is enhanced by transgenic expression of membrane-bound human SCF. Blood. 2012;119:2778–2788. doi: 10.1182/blood-2011-05-353243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manz MG. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity. 2007;26:537–541. doi: 10.1016/j.immuni.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 24.King MA, et al. Hu-PBL-NOD-scid IL2rgnull mouse model of xenogeneic graft-versus-host-like disease and the role of host MHC. Clinical & Experimental Immunology. 2009;157:104–118. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Covassin L, et al. Human peripheral blood CD4 T cell-engrafted non-obese diabetic-scid IL2rgamma(null) H2-Ab1 (tm1Gru) Tg (human leucocyte antigen D-related 4) mice: a mouse model of human allogeneic graft-versus-host disease. Clin Exp Immunol. 2011;166:269–280. doi: 10.1111/j.1365-2249.2011.04462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takenaka K, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 27.Strowig T, et al. Transgenic expression of human signal regulatory protein alpha in Rag2-/-{gamma}c-/- mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci U S A. 2011;108:13218–13223. doi: 10.1073/pnas.1109769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mold JE, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishikawa F, et al. The developmental program of human dendritic cells is operated independently of conventional myeloid and lymphoid pathways. Blood. 2007;110:3591–3660. doi: 10.1182/blood-2007-02-071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian X, et al. Bioluminescent Imaging Demonstrates Transplanted Human Embryonic Stem Cell-derived Cd34(+) Cells Preferentially Develop into Endothelial Cells. Stem Cells. 2009;27:2675–2685. doi: 10.1002/stem.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King M, et al. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol. 2008;126:303–314. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Racki WJ, et al. NOD-scid IL2rgnull (NSG) mouse model of human skin transplantation and allograft rejection. Transplantation. 2010;89:527–536. doi: 10.1097/TP.0b013e3181c90242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirkiles-Smith NC, et al. Development of a humanized mouse model to study the role of macrophages in allograft injury. Transplantation. 2009;87:189–197. doi: 10.1097/TP.0b013e318192e05d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Issa F, et al. Ex vivo-expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model. Transplantation. 2010;90:1321–1327. doi: 10.1097/TP.0b013e3181ff8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nadig SN, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16:809–813. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, et al. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011;117:1888–1898. doi: 10.1182/blood-2010-10-310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harui A, Kiertscher SM, Roth MD. Reconstitution of huPBL-NSG mice with donor-matched dendritic cells enables antigen-specific T-cell activation. J Neuroimmune Pharmacol. 2011;6:148–157. doi: 10.1007/s11481-010-9223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker PD, et al. Generation of human antigen-specific monoclonal IgM antibodies using vaccinated “human immune system” mice. PLoS ONE. 2010;5:e13137. doi: 10.1371/journal.pone.0013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biswas S, et al. Humoral immune responses in humanized BLT mice immunized with West Nile virus and HIV-1 envelope proteins are largely mediated via human CD5+ B cells. Immunol. 2011;134:419–433. doi: 10.1111/j.1365-2567.2011.03501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ippolito GC, et al. Antibody Repertoires in Humanized NOD-scid-IL2Rgamma(null) Mice and Human B Cells Reveals Human-Like Diversification and Tolerance Checkpoints in the Mouse. PLoS ONE. 2012;7:e35497. doi: 10.1371/journal.pone.0035497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt MR, et al. Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PLoS ONE. 2008;3:e3192. doi: 10.1371/journal.pone.0003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unsinger J, McDonough JS, Shultz LD, Ferguson TA, Hotchkiss RS. Sepsis-induced human lymphocyte apoptosis and cytokine production in “humanized” mice. J Leukoc Biol. 2009;86:219–227. doi: 10.1189/jlb.1008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danner R, et al. Expression of HLA class II molecules in humanized NOD. Rag1KO.IL2RgcKO mice is critical for development and function of human T and B cells. PLoS ONE. 2011;6:e19826. doi: 10.1371/journal.pone.0019826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki M, et al. Induction of human humoral immune responses in a novel HLA-DR-expressing transgenic NOD/Shi-scid/gammacnull mouse. Int Immunol. 2012;24:243–252. doi: 10.1093/intimm/dxs045. [DOI] [PubMed] [Google Scholar]
- 45.Marodon G, et al. High diversity of the immune repertoire in humanized NOD.SCID.gamma c-/- mice. Eur J Immunol. 2009;39:2136–2145. doi: 10.1002/eji.200939480. [DOI] [PubMed] [Google Scholar]
- 46.Onoe T, et al. Human natural regulatory T cell development, suppressive function, and postthymic maturation in a humanized mouse model. J Immunol. 2011;187:3895–3903. doi: 10.4049/jimmunol.1100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajesh D, et al. Th1 and Th17 immunocompetence in humanized NOD/SCID/IL2rgammanull mice. Hum Immunol. 2010;71:551–559. doi: 10.1016/j.humimm.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melkus MW, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 49.van Lent AU, et al. IL-7 enhances thymic human T cell development in “human immune system” Rag2-/-IL-2Rgammac-/- mice without affecting peripheral T cell homeostasis. J Immunol. 2009;183:7645–7655. doi: 10.4049/jimmunol.0902019. [DOI] [PubMed] [Google Scholar]
- 50.Waldron-Lynch F, et al. Teplizumab induces human gut-tropic regulatory cells in humanized mice and patients. Sci Transl Med. 2012;4:118ra12. doi: 10.1126/scitranslmed.3003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobson S, et al. Alloreactivity but failure to reject human islet transplants by humanized Balb/c/Rag2gc mice. Scand J Immunol. 2010;71:83–90. doi: 10.1111/j.1365-3083.2009.02356.x. [DOI] [PubMed] [Google Scholar]
- 52.Brehm MA, et al. Human immune system development and rejection of human islet allografts in spontaneously diabetic NOD-Rag1null IL2rgnull Ins2Akita mice. Diabetes. 2010;59:2265–2270. doi: 10.2337/db10-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fogal B, et al. Neutralizing IL-6 Reduces Human Arterial Allograft Rejection by Allowing Emergence of CD161+ CD4+ Regulatory T Cells. J Immunol. 2011;187:6268–6280. doi: 10.4049/jimmunol.1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huntington ND, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strowig T, et al. Human NK cells of mice with reconstituted human immune system components require preactivation to acquire functional competence. Blood. 2010;116:4158–4167. doi: 10.1182/blood-2010-02-270678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lockridge JL, et al. Analysis of the CD1 antigen presenting system in humanized SCID mice. PLoS ONE. 2011;6:e21701. doi: 10.1371/journal.pone.0021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Libby SJ, et al. Humanized nonobese diabetic-scid IL2rgammanull mice are susceptible to lethal Salmonella Typhi infection. Proc Natl Acad Sci U S A. 2010;107:15589–15594. doi: 10.1073/pnas.1005566107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song J, et al. A mouse model for the human pathogen Salmonella typhi. Cell Host Microbe. 2010;8:369–376. doi: 10.1016/j.chom.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato K, et al. A novel animal model of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in humanized mice. Blood. 2011;117:5663–5673. doi: 10.1182/blood-2010-09-305979. [DOI] [PubMed] [Google Scholar]
- 60.Strowig T, et al. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med. 2009;206:1423–1434. doi: 10.1084/jem.20081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shultz LD, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A. 2010;107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaiswal S, et al. Enhanced humoral and HLA-A2-restricted dengue virus-specific T cell responses in humanized BLT NSG mice. Immunology. 2012 doi: 10.1111/j.1365-2567.2012.03585.x. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 64.Denton PW, Garcia JV. Humanized Mouse Models of HIV Infection. AIDS Rev. 2011;13:135–148. [PMC free article] [PubMed] [Google Scholar]
- 65.Berges BK, Rowan MR. The utility of the new generation of humanized mice to study HIV-1 infection: transmission, prevention, pathogenesis, and treatment. Retrovirology. 2011;8:65. doi: 10.1186/1742-4690-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sato K, Koyanagi Y. The mouse is out of the bag: insights and perspectives on HIV-1-infected humanized mouse models. Exp Biol Med (Maywood) 2011;236:977–985. doi: 10.1258/ebm.2011.010294. [DOI] [PubMed] [Google Scholar]
- 67.Duyne RV, et al. Humanized mouse models of HIV-1 latency. Curr HIV Res. 2011;9:595–605. doi: 10.2174/157016211798998781. [DOI] [PubMed] [Google Scholar]
- 68.Choudhary SK, et al. Suppression of human immunodeficiency virus type 1 (HIV-1) viremia with reverse transcriptase and integrase inhibitors, CD4+ T-cell recovery, and viral rebound upon interruption of therapy in a new model for HIV treatment in the humanized Rag2-/-{gamma}c-/- mouse. J Virol. 2009;83:8254–8258. doi: 10.1128/JVI.00580-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balazs AB, et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joseph A, et al. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. J Virol. 2010;84:6645–6653. doi: 10.1128/JVI.02339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holt N, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28:839–847. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimizu S, et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood. 2010;115:1534–1544. doi: 10.1182/blood-2009-04-215855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar P, et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim SS, et al. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol Ther. 2010;18:370–376. doi: 10.1038/mt.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neff CP, et al. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4(+) T cell decline in humanized mice. Sci Transl Med. 2011;3:66ra6. doi: 10.1126/scitranslmed.3001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Denton PW, et al. Generation of HIV latency in humanized BLT mice. J Virol. 2012;86:630–634. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choudhary SK, et al. Latent HIV-1 infection of resting CD4 T cells in the humanized Rag2/gammac/ mouse. J Virol. 2012;86:114–120. doi: 10.1128/JVI.05590-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marsden MD, et al. HIV latency in the humanized BLT mouse. J Virol. 2012;86:339–347. doi: 10.1128/JVI.06366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carter CC, et al. HIV-1 utilizes the CXCR4 chemokine receptor to infect multipotent hematopoietic stem and progenitor cells. Cell Host Microbe. 2011;9:223–234. doi: 10.1016/j.chom.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Denton PW, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol. 2011;85:7582–7593. doi: 10.1128/JVI.00537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Denton PW, et al. Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice. PLoS ONE. 2010;5:e8829. doi: 10.1371/journal.pone.0008829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun Z, et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med. 2007;204:705–714. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brainard DM, et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol. 2009;83:7305–7321. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gorantla S, et al. CD8+ cell depletion accelerates HIV-1 immunopathology in humanized mice. J Immunol. 2010;184:7082–7091. doi: 10.4049/jimmunol.1000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ince WL, et al. Evolution of the HIV-1 env gene in the Rag2-/- gammaC-/-humanized mouse model. J Virol. 2010;84:2740–2752. doi: 10.1128/JVI.02180-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sato K, et al. Dynamics of memory and naive CD8+ T lymphocytes in humanized NOD/SCID/IL-2Rgammanull mice infected with CCR5-tropic HIV-1. Vaccine. 2010;28(2):B32–B37. doi: 10.1016/j.vaccine.2009.10.154. [DOI] [PubMed] [Google Scholar]
- 87.Liu Z, et al. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 88.Long BR, Stoddart CA. Interferon alpha and HIV Infection Cause Activation of Human T Cells in NSG-BLT Mice. J Virol. 2012;86:3327–3336. doi: 10.1128/JVI.06676-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang L, et al. Efficient infection, activation, and impairment of pDCs in the BM and peripheral lymphoid organs during early HIV-1 infection in humanized rag2/gamma C/ mice in vivo. Blood. 2011;117:6184–6192. doi: 10.1182/blood-2011-01-331173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dash PK, et al. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. J Neurosci. 2011;31:3148–3157. doi: 10.1523/JNEUROSCI.5473-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gorantla S, et al. Links between progressive HIV-1 infection of humanized mice and viral neuropathogenesis. Am J Pathol. 2010;177:2938–2949. doi: 10.2353/ajpath.2010.100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Andrade D, et al. Engraftment of peripheral blood mononuclear cells from systemic lupus erythematosus and antiphospholipid syndrome patient donors into BALB-RAG-2-/- IL-2Rgamma-/- mice: a promising model for studying human disease. Arthritis Rheum. 2011;63:2764–2773. doi: 10.1002/art.30424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Whitfield-Larry F, et al. HLA-A2-matched peripheral blood mononuclear cells from type 1 diabetic patients, but not nondiabetic donors, transfer insulitis to NOD-scid/gammac(null)/HLA-A2 transgenic mice concurrent with the expansion of islet-specific CD8+ T cells. Diabetes. 2011;60:1726–1733. doi: 10.2337/db10-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang NH, Inman RD, Dick JE, Wither JE. Bone marrow-derived human hematopoietic stem cells engraft NOD/SCID mice and traffic appropriately to an inflammatory stimulus in the joint. J Rheumatol. 2010;37:496–502. doi: 10.3899/jrheum.090317. [DOI] [PubMed] [Google Scholar]
- 95.Leskov I, et al. Rapid generation of human B-cell lymphomas via combined expression of Myc and Bcl2 and their use as a preclinical model for biological therapies. Oncogene. 2012 doi: 10.1038/onc.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barabe F, Kennedy JA, Hope KJ, Dick JE. Modeling the initiation and progression of human acute leukemia in mice. Science. 2007;316:600–604. doi: 10.1126/science.1139851. [DOI] [PubMed] [Google Scholar]
- 97.Simpson-Abelson MR, et al. Long-term engraftment and expansion of tumor-derived memory T cells following the implantation of non-disrupted pieces of human lung tumor into NOD-scid IL2Rgamma(null) mice. J Immunol. 2008;180:7009–7018. doi: 10.4049/jimmunol.180.10.7009. [DOI] [PubMed] [Google Scholar]
- 98.Bankert RB, et al. Humanized mouse model of ovarian cancer recapitulates patient solid tumor progression, ascites formation, and metastasis. PLoS ONE. 2011;6:e24420. doi: 10.1371/journal.pone.0024420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Willingham SB, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vatakis DN, et al. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc Natl Acad Sci U S A. 2011;108:E1408–E1416. doi: 10.1073/pnas.1115050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shirakura Y, et al. T-cell receptor gene therapy targeting melanoma-associated antigen-A4 inhibits human tumor growth in non-obese diabetic/SCID/gammac(null) mice. Cancer Sci. 2012;103:17–25. doi: 10.1111/j.1349-7006.2011.02111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wege AK, et al. Humanized tumor mice-A new model to study and manipulate the immune response in advanced cancer therapy. Int J Cancer. 2011;129:2194–2206. doi: 10.1002/ijc.26159. [DOI] [PubMed] [Google Scholar]
- 103.Vuyyuru R, Patton J, Manser T. Human immune system mice: current potential and limitations for translational research on human antibody responses. Immunol Res. 2011;51:257–266. doi: 10.1007/s12026-011-8243-9. [DOI] [PubMed] [Google Scholar]
- 104.Chappaz S, Finke D. The IL-7 signaling pathway regulates lymph node development independent of peripheral lymphocytes. J Immunol. 2010;184:3562–3569. doi: 10.4049/jimmunol.0901647. [DOI] [PubMed] [Google Scholar]
- 105.Rennert PD, James D, Mackay F, Browning JL, Hochman PS. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity. 1998;9:71–79. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 106.Greiner DL, et al. Humanized mice for the study of type 1 and type 2 diabetes. Ann N Y Acad Sci. 2011;1245:55–58. doi: 10.1111/j.1749-6632.2011.06318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu Z, Van RN, Yang YG. Macrophages prevent human red blood cell reconstitution in immunodeficient mice. Blood. 2011;118:5938–5946. doi: 10.1182/blood-2010-11-321414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Washburn ML, et al. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 2011;140:1334–1344. doi: 10.1053/j.gastro.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Johnston SC, Dustin ML, Hibbs ML, Springer TA. On the species specificity of the interaction of LFA-1 with intercellular adhesion molecules. J Immunol. 1990;145:1181–1187. [PubMed] [Google Scholar]
- 110.Rozemuller H, et al. Enhanced engraftment of human cells in RAG2/gammac double-knockout mice after treatment with CL2MDP liposomes. Exp Hematol. 2004;32:1118–1125. doi: 10.1016/j.exphem.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 111.Villaudy J, et al. HTLV-1 propels thymic human T cell development in “human immune system” Rag2/ gamma c/ mice. PLoS Pathog. 2011;7:e1002231. doi: 10.1371/journal.ppat.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cocco M, et al. CD34+ cord blood cell-transplanted Rag2-/- gamma(c)-/- mice as a model for Epstein-Barr virus infection. Am J Pathol. 2008;173:1369–1378. doi: 10.2353/ajpath.2008.071186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shultz LD, Brehm MA, Bavari S, Greiner DL. Humanized mice as a preclinical tool for infectious disease and biomedical research. Ann N Y Acad Sci. 2011;1245:50–54. doi: 10.1111/j.1749-6632.2011.06310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Subramanya S, et al. Targeted delivery of siRNA to human dendritic cells to suppress Dengue viral infection and associated proinflammatory cytokine production. J Virol. 2009;84:2490–2501. doi: 10.1128/JVI.02105-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mota J, Rico-Hesse R. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J Virol. 2009;83:8638–8645. doi: 10.1128/JVI.00581-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuruvilla JG, Troyer RM, Devi S, Akkina R. Dengue virus infection and immune response in humanized RAG2(-/-)gamma(c)(-/-) (RAG-hu) mice. Virology. 2007;369:143–152. doi: 10.1016/j.virol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 117.Mercer DF, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- 118.Bissig KD, et al. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest. 2010;120:924–930. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meuleman P, et al. Human hepatocytes secrete soluble CD14, a process not directly influenced by HBV and HCV infection. Clin Chim Acta. 2006;366:156–162. doi: 10.1016/j.cca.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 120.Brezillon N, et al. Antiviral activity of Bay 41-4109 on hepatitis B virus in humanized Alb-uPA/SCID mice. PLoS ONE. 2011;6:e25096. doi: 10.1371/journal.pone.0025096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vuyyuru R, Liu H, Manser T, Alugupalli KR. Characteristics of Borrelia hermsii infection in human hematopoietic stem cell-engrafted mice mirror those of human relapsing fever. Proc Natl Acad Sci U S A. 2011;108:20707–20712. doi: 10.1073/pnas.1108776109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jimenez-Diaz MB, et al. Improved murine model of malaria using Plasmodium falciparum competent strains and non-myelodepleted NOD-scid IL2Rgammanull mice engrafted with human erythrocytes. Antimicrob Agents Chemother. 2009;53:4533–4536. doi: 10.1128/AAC.00519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Smith MS, et al. Granulocyte-colony stimulating factor reactivates human cytomegalovirus in a latently infected humanized mouse model. Cell Host Microbe. 2010;8:284–291. doi: 10.1016/j.chom.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Watanabe Y, et al. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/gammac(null) (NOG) mice (hu-HSC NOG mice) Int Immunol. 2009;21:843–858. doi: 10.1093/intimm/dxp050. [DOI] [PubMed] [Google Scholar]