Abstract
Asthma, a chronic airway inflammatory disease is typically associated with high levels of exhaled nitric oxide (NO). Over the past decades, extensive research has revealed that NO participates in a number of metabolic pathways that contribute to animal models of asthma and human asthma. In asthmatic airway, high levels of NO lead to greater formation of reactive nitrogen species (RNS), which modify proteins adversely affecting functional activities. In contrast, high levels of NO are associated with lower than normal levels of S-nitrosothiols, which serve a bronchodilator function in the airway. Detailed mechanistic studies have enabled the development of compounds that target NO metabolic pathways, and provide opportunities for novel asthma therapy. This review discusses the role of NO in asthma with the primary focus on therapeutic opportunities developed in recent years.
Introduction
Asthma, a chronic airway inflammatory disease is characterized by high levels of exhaled NO. Classically, NO binds to soluble Guanylate Cyclase, which generates production of intracellular messenger cGMP, leading to smooth muscle relaxation that results in vasodilation or bronchodilation [1,2]. However, considerable evidence supports that the high levels of airway-derived NO mainly participate in asthma pathogenesis by formation of detrimental reactive nitrogen species (RNS) that mediate inflammation and injury. Increased generation of RNS along with reactive oxygen species (ROS) are well documented in asthma [3,4]. Infiltration of eosinophils and neutrophils in the asthmatic airway introduced increased release peroxidases, e.g. eosinophil-peroxidase (EPO) and myeloperoxidase (MPO) [5]. MPO uses nitrite (an oxidation product of NO) as a substrate to generate the nitrogen dioxide radical [5–7] that can nitrate protein tyrosine and form 3-nitrotyrosine [7–10]. The detection of nitrotyrosine in the asthmatic lung along with identification of modified proteins by mass spectroscopy definitively establishes several biochemical targets of NO [5,7,11]. In contrast, levels of S-nitrosothiols are lower in asthmatic airways as compared to healthy controls [5,12]. Recent studies identify increased expression of S-nitroso glutathione (GSNO) reductase is responsible for the loss of S-nitroso-thiols [13]. NO levels may also be affected by arginase enzymes, which metabolize L-Arginine, the substrate for NO synthases. The discovery of the importance of arginine/NO metabolic pathways in asthma offers the opportunity for novel therapeutic strategies to treat asthma.
Enzymes in Asthma Pathophysiology
Nitric Oxide Synthases
In the respiratory tract, resident and inflammatory cells are capable of NO production. NO is generated by oxidation of L-arginine (Fig 1); this reaction is typically catalyzed by the NO synthases (NOS) [14]. NOS exists in three distinct isoforms: neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS), of which nNOS and eNOS are constitutively expressed, typically in neuronal and endothelial cells [15]. NO derived from the constitutive NOS along with other NO-adduct molecules, in particular nitrosothiols, are able to release NO at levels that modulate airway as well as pulmonary vascular tone [15,16]. On the other hand NO derived from iNOS, seems to play a primary role as pro-inflammatory mediator. All isoforms are present in the lung. Although eNOS is present in epithelium, iNOS is the predominant enzyme in airway epithelium [17].
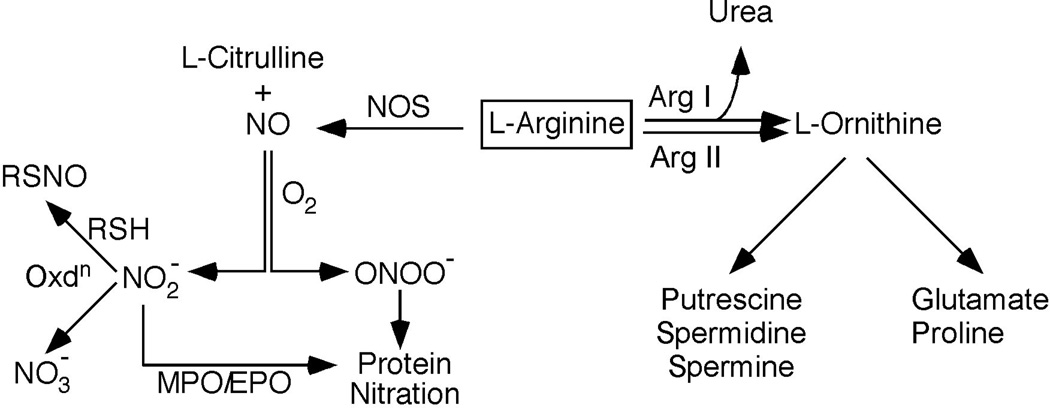
Fig.1.
Metablism of NO. Nitric oxide synthases use L-Arginine as substrate to produce NO and L-Citrulline. L-Arginine is also consumed by Arginases I and II to generate urea and L-ornithine. L-ornithine serves as a precursor for Glutamine, Proline, Putrescine, Spermidine and Spermine, which are important to cell proliferation and extracellular matrix formation. NO can be rapidly oxidized to nitrite (NO2-), which can be further oxidized to nitrate (NO3-). Under disease conditions with superoxide generation, NO can rapidly oxidize to peroxynitrite (ONOO-), which can readily nitrate tyrosine residues of proteins. Myeloperoxidase (MPO) and eosinophil peroxidase (EPO) can also use nitrite as a substrate to nitrate protein tyrosines. In the presence of thiols (RSH), nitrite undergoes nitrosylation reaction to generate S-nitrosothiols (RSNO).
The iNOS protein expression levels are increased in asthmatic airway epithelial cells [18,19]. The iNOS expression is mostly regulated at the level of transcription [20–24], and Janus kinase (JAK)/signal transducer, activator of transcription 1 (STAT-1), nuclear factor NF-κβ and AP-1 are essential for iNOS gene expression [15,22,23]. Early studies in mice, with targeted deletions of the three isoforms of NOS, identified a role for iNOS in the disease progression. Ovalbumin (OVA) sensitized and challenged wild-type (WT) mice lung showed significant up regulation of iNOS, whereas iNOS expression was undetectable in similarly treated iNOS knock out (KO) mice. However airway hyperresponsiveness between these two groups was similar. Airway hyperresponsiveness in nNOS-deficient and n/eNOS-deficient mice was significantly less than that observed in WT mice [25]. On the other hand, iNOS KO mice sensitized and challenged with OVA had less inflammatory cell infiltration of the lung, particularly eosinophils, as compared to WT animals [26]. Similarly, pharmacologic inhibition of iNOS-derived NO abrogated bronchoconstriction, inflammation, and remodeling processes in a guinea pig model of allergic asthma [27]. In humans, of the presence of higher than normal exhaled NO is strongly associated with transcriptional activation of the iNOS gene [19,28]. In support of transcriptional control of iNOS and exhaled NO levels, Salam et al showed an interaction of particulate matter exposure, iNOS promoter haplotypes and methylation on exhaled NO levels in children [29]. Recent study also confirms the importance of iNOS in virus-induced asthma which involves Th1 and Th17 responses [30]. These data suggest that iNOS inhibitors may be of utility in asthma. However, although iNOS inhibitors L-NAME [L-NG-Nitroarginine Methyl Ester] and L-NIL [N6-(1-iminoethyl)-L-lysine hydrochloride] cause significant reduction of exhaled NO, neither improve lung function in the asthmatics [31], despite the fact that these inhibitors are protective in animal models. Clearly there are differences between the animal models and human disease, which present challenges to development of potent iNOS inhibitors for the treatment of human asthma.
Arginase
Studies over the past decade indicate that Arginases contribute to the pathophysiology of asthma. Arginases exist in two isoforms: Arginase I and Arginase II. Arginase I is a cytosolic enzyme highly expressed in the liver, where it subserves the urea cycle. Arginase II is localized in the mitochondria; present in most tissues, including airway epithelium, the function of arginase II in cell metabolism is unclear [32–35]. Arginases use L-arginine to produce L-ornithine and urea (Fig 1). In cells without a complete urea cycle, arginase II may be responsible for ornthine as a final product, which is used as a precursor of polyamines, glutamate, and proline [32](Fig 1). Arginases may also regulate NO biosynthesis by modulating arginine availability for NOS. Up regulation of Arginase genes expression in lung has been linked to asthma in clinical studies of human patients and in mouse models of OVA-induced airway inflammation [36,37]. Arginase II and I are prominent among the asthma signature genes in the murine asthma model [37]. Similarly, asthmatic patients have higher levels of serum arginase activity and lower levels of serum L-arginine (suggesting greater consumption by arginases) as compared with healthy controls [34]. This led to the concept that Arginase may limit L-arginine availability to iNOS, which may result in superoxide anion production by iNOS, generating peroxynitrite, and consequent protein nitration. Greater synthesis of L-ornithine by arginase may also promote airway remodelling in asthma via greater cell proliferation and collagen production [38]. In support of this concept, arginine bioavailability is correlated with airway obstruction in severe asthma [39]. Breton et al performed a detailed study on DNA methylation of NOS-Arginase pathway and its association with exhaled NO in asthmatic children. Increase in DNA methylation of Arginase II was significantly associated with decrease in exhaled NO in asthmatic children. Differences in exhaled NO were also observed for Arginase I in asthmatics. However, DNA methylation in NOS genes was not associated with exhaled NO [40]. Recently, Yamamoto et al studied the relationship of iNOS and Arginase II expression with asthma severity using bronchial brushing and sputum from healthy and asthmatic subjects. The iNOS protein, exhaled NO and nitrotyrosine (reactive nitrogen species), all were correlated with sputum eosinophils, with the strongest relationships in severe asthma [41].
London’s group performed a detailed study with 433 case-parent triads to investigate the association between gene polymorphisms in Arginase I and II and childhood asthma. Arginase I single nucleotide polymorphisms (SNPs) and haplotypes were associated with atopy as determined by positive skin test, and with relative risk of asthma. The association was stronger among children with smoking parents. Altogether the data suggest that genetic polymorphisms in Arginase genes may contribute to origins of asthma and atopy [42].
NO, Nitration and S-nitrosylation
NO and its byproducts nitrite (NO2- ), nitrate (NO3-) and reactive nitrogen species (RNS), such as peroxynitrite (ONOO-) all can execute biologic functions and influence cellular metabolism [43,44]. Hence, all NO products may contribute to asthma pathophysiology. Typically, asthmatics have high levels of exhaled NO and high levels of NO3-, and nitrotyrosine in the airway lining fluid. NO reacts rapidly with superoxide (O2•-), generated during asthma exacerbations, to produce ONOO-. ONOO- may decay to NO3- or nitrate tyrosine residues of proteins, leading to structural change and alterations in biological function [5,45]. Hemeperoxidases such as MPO and EPO, use NO2- as a substrate to generate 3-nitrotyrosine (Fig 1). Immunohistochemical studies suggest that airway epithelium and eosinophils are major cellular sources for nitrotyrosine in asthmatic airways [5]. Some murine model studies suggest that asthmatic responses can occur independent of iNOS and RNS production [25,46], while others indicate a significant role of iNOS in inflammation [26]. Nitration generally represents a pathophysiological condition, but recent work suggests that enzyme denitrase(s) may reverse nitration events [47]. A role for denitrase enzyme(s) in asthma is currently unknown.
Proteomic survey of nitrated proteins in the OVA mouse model of asthma has revealed several critical proteins in different cellular compartments are modified. Antioxidant proteins (catalase, MnSOD, glutathione S-transferase, antioxidant protein 2) are found to have nitrative modification in asthma [4]. Studies confirm that along with nitration, oxidation also plays an important role in the proteins modifications and inactivation [4,48]. NO and ONOO- are capable of modifying thiol residues of cysteines in proteins to produce S-nitrosothiols (SNO), which can alter protein expression and/or function [49,50]. GSNO and other nitrosothiols are commonly found in airway lining fluid where they contribute to host defense and bronchodilation [51]. A number of studies now link GSNO deficiency to asthma pathophysiology [12,13,52]. RSNO are present at lower than normal levels in the tracheal aspirates of asthmatic children [51,53]. Increased catabolism of GSNO results in the overall lower levels of GSNO in asthmatic airway [51], which is due to greater GSNO-reductase (GSNOR) expression and activity in asthmatic airways [13].
Importantly, NO also attenuates NF-κB DNA binding [54]. Several different NF-kB proteins e.g. IKK, p65 and p50 are regulated by S-nitrosylation, whih is the molecular mechanism by which NO alters NF-κB signaling [49,54,55]. The presence of s-nitrosylated p65 and p50 have inverse relationship with the presence of NF-κB p50-p65 DNA binding and NF-κB-dependent transcription [55]. Thus, the alterations of GSNO/NO metabolism in asthma likely contribute to the pathologic consequences in asthma.
Asthma and Recent Therapeutic Opportunities
NF κb and other signaling molecules to target the arginine/NO pathway
Inhibition of the NF-kB pathway has been an active area of study for the discovery of new agents to treat inflammatory disease [41]. Recently, Licochalcone B and Licochalcone D, along with Licochalcone A, were found to inhibit phosphorylation of NF-κB p65 in LPS mediated signaling pathway. Licochalcone B and Licochalcone D consequently were also reported to reduce LPS mediated NO production [56]. Andrographolide, an active component of the medicinal plant Andrographis paniculata may also have therapeutic value in asthma. Andrographolide blocked p65 nuclear translocation and DNA-binding activity in the nuclear extracts from lung tissues of OVA-challenged mice [57], and attenuated OVA-induced eosinophilia and IL-4, IL-5, and IL-13 levels in bronchoalveolar lavage fluid. In parallel, tissue eosinophilia, airway hyper responsiveness and mucus production, and iNOS expression in lung were all reduced by this compound. Fisetin, a bioactive flavonol with a similar mode of action on p65 as andrographolide, also reduces eosinophils, IL-4, IL-5, and IL-13 levels in bronchoalveolar lavage fluid in a murine asthma model [58]. Fisetin also attenuated airway hyper responsiveness to methacoline. These bioactive compounds that reduce iNOS and/or NO and attenuate asthmatic responses all execute their therapeutic value via down regulation of NF-κB pathway, but other pathways that exert control over iNOS may also be effective. For example, epimagnolin and fargesin, which are components of the magnolia flower also inhibit iNOS expression and reduces NO production but via effects on the Extracellular signal-regulated kinases (ERK) pathway [59]. Further work is needed to establish efficacy of signal transduction targeted therapies.
Enzymatic inhibition of NOS
Inhibitors of NOS have been studied for their effect on asthma in animal models as well as humans with varying results. For example, L-NIL effectively caused iNOS inhibition, and reduced exhaled NO in asthmatics within 15 min of oral treatment [60]. The NOS inhibitor 1400W effectively reduced eosinophilia and airway resistance in animal models of asthma, but L-NAME failed to improve airway hyperresponsiveness or airway collagen deposition [27,61]]. Other highly selective iNOS inhibitors (GW274150 and BYK402750) have been shown to be promising in decreasing inflammation in a cigarette-smoke exposure mouse model [31]. This may indicate that specific iNOS inhibition is important to achieve therapeutic utility in airway inflammation. On the other hand, potentiation of downstream NO effects via the phosphodiesterase inhibitor sildenafil effectively relaxes carbachol-induced contractions in isolated tracheal rings prepared from a rodent model of allergic asthma, which indicates a cautious approach to NOS inhibitors that may paradoxically also cause loss of bronchodilator effects [62].
Arginase blockade as a strategy for asthma
Morris CR et al has suggested an effective line of treatment might aim at arginase inhibition or oral arginine supplementation to balance arginine utilization in asthma [63]. The pharmacologic inhibitor of arginase, nor-NOHA [L-2-Amino-(4-(2'-hydroxyguanidino)butyric acid], increases NO production as measured by NO2- and NO3-[64]. Treatment with nor-NOHA attenuates airway hyperreactivity and eosinophilia in the intranasal mite-induced NC/Nga mouse model. Although nitrite and nitrate in the lung remained elevated with nor-NOHA, mRNA for IL-4, IL-5, and IL-13, eotaxin-1 and -2, were reduced. Consistent with this, the numbers of bronchial goblet cells were decreased by nor-NOHA [65]. On the other hand, BEC [(S)-(2- Boronoethyl)-L-cysteine], a highly potent arginase inhibitor, increased protein s-nitrosylation and nitration in a mouse asthma model, suggesting that specificity and potency of inhibitors may be important in altering effects on outcomes in asthma [66].
Augmentation of S-nitrosothiol through blockade of GSNOR
Studies have tested the therapeutic role of S-nitrosothiols in the murine model of allergic inflammation and found that instillation of GSNO suppressed NF-kB activation and bronchial hyperreactivity, but did not significantly alter airway inflammation [67,68]. Since blockade of GSNOR is protective in murine asthma model [13], Foster et al recently explored the possibility of using GSNOR inhibitors, GSNORi, to treat asthma [67]. GSNORi raised levels of S-nitrosylated proteins in cytokine stimulated murine macrophage cells and interestingly lowered levels of other immunomodulators, e.g. osteopontin, cyclooxygenase-2, and iNOS [69]. Sun X et al have identified a pyrrole based potent and novel GSNORi, N6022, that lowers GSNOR activity, and leads to bronchodilation in the OVA induced asthmatic mouse model [70].
Summary
Alterations of NO and its end products are well delineated in asthma. A multitude of studies have revealed detrimental effects of RNS and beneficial functions of S-nitrosothiols in asthma. Thus, design of compounds to target the arginine/NO pathway in asthma will optimally preserve, or augment, the production of S-nitrosothiols and reduce the formation of toxic RNS. Further detailed studies of NO metabolism will enable innovative treatments that reverse pathologic metabolism in the airway, and recover beneficial products of NO.
Table 1.
Compounds that Target the Arginine/NO Pathway
| Molecular Target |
Compounds | Biological Effect | References |
|---|---|---|---|
| NF-kB p65 | Licochalcone, Andrographolide, Fisetin |
Reduce NO production, attenuate airway hyperresponsiveness, mucus production, eosinophilia |
[56–58] |
| ERK pathway |
Epimagnolin, fargesin |
Reduce NO production | [59] |
| NOS | L-NIL, 1400W | Reduce exhaled NO, eosinophilia and airway resistance |
[27,60] |
| Arginase | Nor-NOHA | Attenuate airway hyperreactivity, eosinophilia and mucus hyperplasia |
[65] |
| NO-cGMP- K+channel |
Sildenafil | Reduce tracheal hyperresponsiveness | [62] |
| GSNOR | GSNO instillation, GSNORi, N6022 |
Increase S-nitrosylated proteins; reduce iNOS levels, attenuate bronchoconstriction |
[67,68,70] |
Acknowledgements
The authors acknowledge grant support from HL081064, HL691701, HL109250, and HL103453. SCE is a Senior Fellow of the American Asthma Foundation Sandler Program for Asthma Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The author(s) have no conflict of interest to declare.
References
- 1.Munzel T, et al. Physiology and pathophysiology of vascular signaling controlled by guanosine 3',5'-cyclic monophosphate-dependent protein kinase [corrected] Circulation. 2003;108(18):2172–2183. doi: 10.1161/01.CIR.0000094403.78467.C3. [DOI] [PubMed] [Google Scholar]
- 2.Ricciardolo FL, et al. Randomised double-blind placebo-controlled study of the effect of inhibition of nitric oxide synthesis in bradykinin-induced asthma. Lancet. 1996;348(9024):374–377. doi: 10.1016/s0140-6736(96)04450-9. [DOI] [PubMed] [Google Scholar]
- 3.Gerard C. Biomedicine. Asthmatics breathe easier when it's SNO-ing. Science. 2005;308(5728):1560–1561. doi: 10.1126/science.1114163. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh S, et al. Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation. J Immunol. 2006;176(9):5587–5597. doi: 10.4049/jimmunol.176.9.5587. [DOI] [PubMed] [Google Scholar]
- 5.Dweik RA, et al. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci U S A. 2001;98(5):2622–2627. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan ML, et al. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem. 2002;277(20):17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 7.MacPherson JC, et al. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol. 2001;166(9):5763–5772. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- 8.Eiserich JP, et al. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391(6665):393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 9.van der Vliet A, et al. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272(12):7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 10.Wu W, et al. Eosinophil peroxidase nitrates protein tyrosyl residues. Implications for oxidative damage by nitrating intermediates in eosinophilic inflammatory disorders. J Biol Chem. 1999;274(36):25933–25944. doi: 10.1074/jbc.274.36.25933. [DOI] [PubMed] [Google Scholar]
- 11.Duguet A, et al. Eosinophil peroxidase mediates protein nitration in allergic airway inflammation in mice. Am J Respir Crit Care Med. 2001;164(7):1119–1126. doi: 10.1164/ajrccm.164.7.2010085. [DOI] [PubMed] [Google Scholar]
- 12.Gaston B, et al. Bronchodilator S-nitrosothiol deficiency in asthmatic respiratory failure. Lancet. 1998;351(9112):1317–1319. doi: 10.1016/S0140-6736(97)07485-0. [DOI] [PubMed] [Google Scholar]
- 13.Que LG, et al. S-nitrosoglutathione reductase: an important regulator in human asthma. Am J Respir Crit Care Med. 2009;180(3):226–231. doi: 10.1164/rccm.200901-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 15.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411(2–3):217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 16.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 17.Kobzik L, et al. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993;9(4):371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- 18.Donnelly LE, Barnes PJ. Expression and regulation of inducible nitric oxide synthase from human primary airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26(1):144–151. doi: 10.1165/ajrcmb.26.1.4477. [DOI] [PubMed] [Google Scholar]
- 19.Guo FH, et al. Molecular mechanisms of increased nitric oxide (NO) in asthma: evidence for transcriptional and post-translational regulation of NO synthesis. J Immunol. 2000;164(11):5970–5980. doi: 10.4049/jimmunol.164.11.5970. [DOI] [PubMed] [Google Scholar]
- 20.Marks-Konczalik J, et al. Cytokine-mediated transcriptional induction of the human inducible nitric oxide synthase gene requires both activator protein 1 and nuclear factor kappaBbinding sites. J Biol Chem. 1998;273(35):22201–22208. doi: 10.1074/jbc.273.35.22201. [DOI] [PubMed] [Google Scholar]
- 21.Uetani K, et al. Central role of double-stranded RNA-activated protein kinase in microbial induction of nitric oxide synthase. J Immunol. 2000;165(2):988–996. doi: 10.4049/jimmunol.165.2.988. [DOI] [PubMed] [Google Scholar]
- 22.Xie QW, et al. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993;177(6):1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu W, et al. STAT-1 and c-Fos interaction in nitric oxide synthase-2 gene activation. Am J Physiol Lung Cell Mol Physiol. 2003;285(1):L137–L148. doi: 10.1152/ajplung.00441.2002. [DOI] [PubMed] [Google Scholar]
- 24.Zheng S, et al. Impaired innate host defense causes susceptibility to respiratory virus infections in cystic fibrosis. Immunity. 2003;18(5):619–630. doi: 10.1016/s1074-7613(03)00114-6. [DOI] [PubMed] [Google Scholar]
- 25.De Sanctis GT, et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med. 1999;189(10):1621–1630. doi: 10.1084/jem.189.10.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong Y, et al. Inhibition of allergic airway inflammation in mice lacking nitric oxide synthase 2. J Immunol. 1999;162(1):445–452. [PubMed] [Google Scholar]
- 27.Prado CM, et al. Effects of nitric oxide synthases in chronic allergic airway inflammation and remodeling. Am J Respir Cell Mol Biol. 2006;35(4):457–465. doi: 10.1165/rcmb.2005-0391OC. [DOI] [PubMed] [Google Scholar]
- 28.Grasemann H, et al. A neuronal NO synthase (NOS1) gene polymorphism is associated with asthma. Biochem Biophys Res Commun. 2000;272(2):391–394. doi: 10.1006/bbrc.2000.2794. [DOI] [PubMed] [Google Scholar]
- 29.Salam MT, et al. Genetic and epigenetic variations in inducible nitric oxide synthase promoter, particulate pollution, and exhaled nitric oxide levels in children. J Allergy Clin Immunol. 129(1):232–239. e231–e237. doi: 10.1016/j.jaci.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin TS, et al. Role of inducible nitric oxide synthase on the development of virus-associated asthma exacerbation which is dependent on Th1 and Th17 cell responses. Exp Mol Med. 42(10):721–730. doi: 10.3858/emm.2010.42.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hesslinger C, et al. Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem Soc Trans. 2009;37(Pt 4):886–891. doi: 10.1042/BST0370886. [DOI] [PubMed] [Google Scholar]
- 32.Colleluori DM, et al. Expression, purification, and characterization of human type II arginase. Arch Biochem Biophys. 2001;389(1):135–143. doi: 10.1006/abbi.2001.2324. [DOI] [PubMed] [Google Scholar]
- 33.King NE, et al. Arginine in asthma and lung inflammation. J Nutr. 2004;134(10 Suppl):2830S–2836S. doi: 10.1093/jn/134.10.2830S. discussion 2853S. [DOI] [PubMed] [Google Scholar]
- 34.Morris CR, et al. Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med. 2004;170(2):148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- 35.Salimuddin, et al. Regulation of the genes for arginase isoforms and related enzymes in mouse macrophages by lipopolysaccharide. Am J Physiol. 1999;277((1 Pt 1)):E110–E117. doi: 10.1152/ajpendo.1999.277.1.E110. [DOI] [PubMed] [Google Scholar]
- 36.Cho WS, et al. Increased expression of arginase I and II in allergic nasal mucosa. Laryngoscope. 121(2):236–240. doi: 10.1002/lary.21288. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann N, et al. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest. 2003;111(12):1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maarsingh H, et al. Arginase and pulmonary diseases. Naunyn Schmiedebergs Arch Pharmacol. 2008;378(2):171–184. doi: 10.1007/s00210-008-0286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lara AR, Erzurum SC. A urinary test for pulmonary arterial hypertension? Am J Respir Crit Care Med. 2005;172(3):262–263. doi: 10.1164/rccm.2505009. [DOI] [PubMed] [Google Scholar]
- 40.Breton CV, et al. DNA methylation in the arginase-nitric oxide synthase pathway is associated with exhaled nitric oxide in children with asthma. Am J Respir Crit Care Med. 184(2):191–197. doi: 10.1164/rccm.201012-2029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto M, et al. Nitric oxide and related enzymes in asthma: relation to severity, enzyme function and inflammation. Clin Exp Allergy. 2001;42(5):760–768. doi: 10.1111/j.1365-2222.2011.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, et al. Genetic polymorphisms in arginase I and II and childhood asthma and atopy. J Allergy Clin Immunol. 2006;117(1):119–126. doi: 10.1016/j.jaci.2005.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghosh S, Erzurum SC. Nitric oxide metabolism in asthma pathophysiology. Biochim Biophys Acta. 2011;1810(11):1008–1016. doi: 10.1016/j.bbagen.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lundberg JO, et al. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 45.Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 12(1):93–124. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koarai A, et al. iNOS depletion completely diminishes reactive nitrogen-species formation after an allergic response. Eur Respir J. 2002;20(3):609–616. doi: 10.1183/09031936.02.00274902. [DOI] [PubMed] [Google Scholar]
- 47.Kamisaki Y, et al. An activity in rat tissues that modifies nitrotyrosine-containing proteins. Proc Natl Acad Sci U S A. 1998;95(20):11584–11589. doi: 10.1073/pnas.95.20.11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Comhair SA, et al. Superoxide dismutase inactivation in pathophysiology of asthmatic airway remodeling and reactivity. Am J Pathol. 2005;166(3):663–674. doi: 10.1016/S0002-9440(10)62288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reynaert NL, et al. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci U S A. 2004;101(24):8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whalen EJ, et al. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129(3):511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 51.Gaston B, et al. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci U S A. 1993;90(23):10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Que LG, et al. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308(5728):1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaston B, et al. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med. 1994;149((2 Pt 1)):538–551. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- 54.Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry. 2001;40(6):1688–1693. doi: 10.1021/bi002239y. [DOI] [PubMed] [Google Scholar]
- 55.Kelleher ZT, et al. NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J Biol Chem. 2007;282(42):30667–30672. doi: 10.1074/jbc.M705929200. [DOI] [PubMed] [Google Scholar]
- 56.Furusawa J, et al. Glycyrrhiza inflata-derived chalcones, Licochalcone A, Licochalcone B and Licochalcone D, inhibit phosphorylation of NF-kappaB p65 in LPS signaling pathway. Int Immunopharmacol. 2009;9(4):499–507. doi: 10.1016/j.intimp.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 57.Bao Z, et al. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-kappaB pathway. Am J Respir Crit Care Med. 2009;179(8):657–665. doi: 10.1164/rccm.200809-1516OC. [DOI] [PubMed] [Google Scholar]
- 58.Goh FY, et al. Fisetin, a bioactive flavonol, attenuates allergic airway inflammation through negative regulation of NF-kappaB. Eur J Pharmacol. 679(1–3):109–116. doi: 10.1016/j.ejphar.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Baek JA, et al. Extracts of Magnoliae flos inhibit inducible nitric oxide synthase via ERK in human respiratory epithelial cells. Nitric Oxide. 2009;20(2):122–128. doi: 10.1016/j.niox.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Hansel TT, et al. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. Faseb J. 2003;17(10):1298–1300. doi: 10.1096/fj.02-0633fje. [DOI] [PubMed] [Google Scholar]
- 61.De Boer J, et al. Effects of endogenous superoxide anion and nitric oxide on cholinergic constriction of normal and hyperreactive guinea pig airways. Am J Respir Crit Care Med. 1998;158(6):1784–1789. doi: 10.1164/ajrccm.158.6.9711005. [DOI] [PubMed] [Google Scholar]
- 62.Sousa CT, et al. Sildenafil decreases rat tracheal hyperresponsiveness to carbachol and changes canonical transient receptor potential gene expression after antigen challenge. Braz J Med Biol Res. 44(6):562–572. doi: 10.1590/s0100-879x2011007500056. [DOI] [PubMed] [Google Scholar]
- 63.Morris CR, et al. Nitric oxide and arginine dysregulation: a novel pathway to pulmonary hypertension in hemolytic disorders. Curr Mol Med. 2008;8(7):620–632. doi: 10.2174/156652408786241447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kenyon NJ, et al. Arginases I and II in lungs of ovalbumin-sensitized mice exposed to ovalbumin: sources and consequences. Toxicol Appl Pharmacol. 2008;230(3):269–275. doi: 10.1016/j.taap.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takahashi N, et al. Direct inhibition of arginase attenuated airway allergic reactions and inflammation in a Dermatophagoides farinae-induced NC/Nga mouse model. Am J Physiol Lung Cell Mol Physiol. 299(1):L17–L24. doi: 10.1152/ajplung.00216.2009. [DOI] [PubMed] [Google Scholar]
- 66.Ckless K, et al. Inhibition of arginase activity enhances inflammation in mice with allergic airway disease, in association with increases in protein S-nitrosylation and tyrosine nitration. J Immunol. 2008;181(6):4255–4264. doi: 10.4049/jimmunol.181.6.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foster MW, et al. S-nitrosoglutathione supplementation to ovalbumin-sensitized and - challenged mice ameliorates methacholine-induced bronchoconstriction. Am J Physiol Lung Cell Mol Physiol. 2011;301(5):L739–L744. doi: 10.1152/ajplung.00134.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Olson N, et al. Modulation of NF-{kappa}B and HIF-1 by S-Nitrosoglutathione Does Not Alter Allergic Airway Inflammation in Mice. Am J Respir Cell Mol Biol. 2011;44(6):813–823. doi: 10.1165/rcmb.2010-0035OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foster MW, et al. Proteomic characterization of the cellular response to nitrosative stress mediated by s-nitrosoglutathione reductase inhibition. J Proteome Res. 2012;11(4):2480–2491. doi: 10.1021/pr201180m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun X, et al. Structure-activity relationship of pyrrole based S-nitrosoglutathione reductase inhibitors: carboxamide modification. Bioorg Med Chem Lett. 22(6):2338–2342. doi: 10.1016/j.bmcl.2012.01.047. [DOI] [PubMed] [Google Scholar]