Abstract
The goal of this study was to characterize the effects of CYP1A2•CYP2B4 complex formation on the rates and efficiency of toluene metabolism by comparing the results from simple reconstituted systems containing P450 reductase (CPR) and a single P450 to those using a mixed system containing CPR and both P450s. In the mixed system, the rates of formation of CYP2B4-specific benzyl alcohol and p-cresol were inhibited, whereas that of CYP1A2-specific o-cresol was increased, results consistent with the formation of a CYP1A2•CYP2B4 complex where the CYP1A2 moiety has higher affinity for CPR binding. Comparison of the rates of NADPH oxidation and production of hydrogen peroxide and excess water by the simple and mixed systems indicated that excess water formed at a much lower rate in the mixed system. The commensurate increase in the rate of CYP1A2-specific product formation suggested the P450•P450 interaction increased the putative rate-limiting step of CYP1A2 catalysis, abstraction of a hydrogen radical from the substrate. Cumene hydroperoxide-supported metabolism was measured to determine whether the effects of the P450•P450 interaction required the presence of CPR. Peroxidative metabolism was not affected by the interaction of the two P450s, even with CPR present. However, CPR did stimulate peroxidative metabolism by the simple system containing CYP1A2. These results suggest the major functional effects of the P450•P450 interaction are mediated by changes in the relative abilities of the P450s to receive electrons from CPR. Furthermore, CPR may play an effector role by causing a conformation change in CYP1A2 that makes its metabolism more efficient.
The cytochromes P450 (P450) represent a superfamily of enzymes that catalyze the metabolism of a wide array of endogenous and xenobiotic substrates (1). The broad substrate specificity of this enzyme superfamily is due to (a) active sites that are capable of binding a wide variety of different compounds, often in multiple orientations, and (b) the multiplicity of P450 enzymes, with each form having its own characteristic substrate selectivity (2).
These heme-containing enzymes use the binding and reduction of molecular oxygen to perform a mixed function oxidation in which the oxygen-oxygen bond is cleaved so that one atom of molecular oxygen is directly inserted into a substrate, and the other atom is reduced to form water (3). The electrons required for this process are obtained by the physical interaction of the P450 enzymes with a redox partner (either NADPH-cytochrome P450 reductase (CPR) or cytochrome b5) (4). P450-mediated metabolism is very inefficient (5). Frequently, the reactive oxygen-bound heme intermediates in the active site of the P450 break down to release reactive oxygen species, such as superoxide and hydrogen peroxide. In addition, a high valent, iron-oxene species that is formed after heterolytic cleavage of a peroxy-iron intermediate can be reduced with extra electrons from CPR resulting in the production of “excess” water (6).
Most of the eukaryotic P450 enzymes and their redox partners are embedded in the endoplasmic reticulum, with the membrane providing a scaffold to allow for the proper alignment and functional interaction of these enzymes (7;8). The interaction between CPR and P450 is necessary for NADPH-dependent electron transfer to the heme protein. The ratio of P450 enzymes to CPR in the liver endoplasmic reticulum is generally in excess of 5:1 (9;10), raising the question, “How are these excess P450s organized to effectively receive electrons from the limiting amounts of CPR?”. One mechanism by which P450-mediated metabolism could be influenced by the limiting amount of CPR involves a simple competition between different P450 enzymes for the reducing equivalents provided by CPR, and has been documented for several P450-mediated reactions (11–13). However, other types of interactions between the components of the P450 monooxygenase system allow for more complex means of altering metabolism by the P450 enzymes – through the formation of P450•P450 complexes (14).
Evidence indicates that the P450 enzymes and CPR may exist as a mixture of various complexes in the membrane (15), where both homomeric (16–19) and heteromeric (20–26) associations of P450 enzymes are likely. In many of these reports, complex formation was shown to alter the enzyme activity for a particular substrate. This phenomenon was originally identified in the study of the metabolism of 7-alkoxyresorufins by reconstituted systems comprised of a mixture of CYP1A2 and CYP2B4 (20). In these studies, the metabolism of the CYP2B4-specific substrate, 7-pentoxyresorufin (27), was inhibited when both P450 enzymes were reconstituted with lipid and subsaturating concentrations of CPR, relative to that observed in a “simple” reconstituted system containing CYP2B4 alone. Conversely, the metabolism of 7-ethoxyresorufin, a CYP1A2-specific substrate (27) was stimulated in the “mixed” reconstituted system containing both CYP1A2 and CYP2B4 (20;21). Overall, the mixed reconstituted systems were shown to exhibit more CYP1A2 character, manifesting increased CYP1A2-selective activity and concomitantly decreased CYP2B4-selective catalysis. These data could not be explained by models involving the simple competition of the P450 enzymes for limiting amounts of CPR and were consistent with a heteromeric CYP1A2•CYP2B4 complex that was associated with an increase, relative to the individual P450 system, in the binding affinity between CPR and the CYP1A2 moiety of the mixed P450 complex (28).
The goal of the present study was to examine the interactions between CYP1A2 and CYP2B4 using toluene, a substrate that is metabolized by both P450 enzymes. CYP2B4 is a more effective catalyst of toluene metabolism, generating primarily benzyl alcohol and smaller amounts of p-cresol. Although CYP1A2 does not generate product as rapidly as CYP2B4, it is capable of producing benzyl alcohol, and unique in being able to produce o-cresol. Therefore, toluene was used not only to analyze changes in substrate selectivity of the mixed reconstituted systems, but also to examine how the CYP1A2•CYP2B4 interaction affects the efficiency of substrate metabolism (reaction coupling) by determining the rates of NADPH oxidation, hydrogen peroxide production, and excess water formation associated with toluene oxidation. Our findings demonstrate that the CYP1A2•CYP2B4 interaction inhibits CYP2B4-mediated metabolism but stimulates CYP1A2-mediated activities. The interaction between CYP1A2 and CYP2B4 increases the degree of coupling of toluene metabolism by causing a decrease in the rate of formation of excess water. Furthermore, the functional effects of the CYP1A2•CYP2B4 interaction require electrons to enter the heme proteins through CPR, as peroxidative metabolism does not exhibit functional changes resulting from the CYP1A2•CYP2B4 complex. These data not only support our previous conclusion that CPR interacts differently with CYP1A2 and CYP2B4 when the two P450s are in a mixed complex (20–22;24), but also are consistent with the CYP1A2•CYP2B4 interaction modifying the CYP1A2 active site to result in an increase in the rate of the putative limiting step of CYP1A2 catalysis.
Experimental Procedures
Unless otherwise noted, all reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO). C41 Cells were purchased through Avidis SA (Biopole Clermont-Limagne, France). Protein extraction reagent BPER came from Pierce-Thermo Chemical Co. (Rockford, IL).
Enzymes
Full-length cytochrome P450 2B4 (CYP2B4) was expressed in Escherichia coli C41 and purified according to published procedures (21). Recombinant rabbit NADPH cytochrome P450 CPR (plasmid: pSC-CPR, provided by Dr. Lucy Waskell (Univ. Michigan)) was expressed in E. coli C41, solubilized, and purified following a modification of methods described previously (29;30). Cytochrome P450 1A2 (CYP1A2) was isolated from βNF-treated rabbit liver microsomes as previously described (31).
Preparation of reconstituted systems of P450 enzymes, CPR, and lipid
An 8 mM solution of the lipid, dilauroylphosphatidylcholine (DLPC), in 50 mM potassium phosphate buffer (pH 7.25) containing 20% glycerol, 0.1 M NaCl, and 5 mM EDTA was clarified by sonication at RT in a bath sonicator for 30 min. The clarified DLPC suspension, the P450 enzymes, and the CPR were mixed at enzyme concentrations greater than 10 μM and preincubated for 2 hours at room temp. These concentrated stock solutions of enzyme and DLPC were then diluted to the final assay concentrations as described below.
Assays to measure the metabolism of toluene, NADPH consumption, and the production of hydrogen peroxide
A saturated solution of the substrate, toluene, was made by vigorously shaking excess toluene in 100 mM potassium phosphate buffer pH 7.25. This saturated buffer is stored in a Brinkman Bottle-top Dispenser and was added to the reaction solution containing the reconstituted system. This allows for the addition of toluene without the need for adding organic solvent to the reaction. The toluene concentration was calculated by measuring its UV absorbance at 262 nm, using and extinction coefficient of 0.237 mM−1cm−1 as described (32).
The enzyme reconstituted systems described above were made up in 7 mL glass vials. The experiments used a DLPC:P450 enzyme ratio of 160:1 and a CPR:P450 enzyme ratio of 1:2. The concentration of each P450 included in the reactions was 0.2 μM. After incubation of the enzymes and lipid for two hours at room temperature, other assay components were added (MgCl2, D,L-isocitric acid, and isocitrate dehydrogenase to final concentrations of 10 mM, 6.2 mM, and 0.3 U/ml respectively), and the volume was brought up to 0.5 ml with toluene saturated potassium phosphate buffer (the final toluene concentration in the buffer ranged from 4.5 mM to 5 mM) (32). The vials were immediately capped with rubber septa and placed into a shaking water bath set at 37°C for 5 minutes. The reactions were initiated by injecting 5 μL of a 50 mM solution of NADPH through the septa with a Hamilton syringe (Reno, NV) at one minute intervals, and the vials were placed back in the water bath. After incubation for 10 min, the vials were removed from the bath, again at one minute intervals, and the vial contents were distributed to measure NADPH consumption, hydrogen peroxide production, and toluene metabolism as indicated below.
Determination of CYP1A2 and CYP2B4 kinetic constants for toluene metabolism
Reactions to measure the rates of metabolism as a function of toluene concentration were carried out as described above except for the following modifications. Higher concentrations of CYP1A2 were needed to measure the rates of toluene metabolism at low substrate concentrations, so 0.4 μM of the P450 was added to the incubations. The Km for toluene metabolism by either CYP1A2 or CYP2B4 was determined as a function of toluene concentration at saturating CPR (2:1 CPR:P450). After the 2 hr preincubation period, the reconstituted systems were mixed with increasing volumes of the saturated toluene solution and brought to their final volume (0.495 ml) with 100 mM potassium phosphate. Reactions were then initiated as described above (5 μl of 50 mM NADPH). The absorbance of the toluene-saturated buffer at 261 nm was determined immediately before dispensing to the reaction vials in order to precisely determine the toluene concentrations in the individual reactions. The rates of the reactions were plotted as a function of the toluene concentrations and the kinetic constants were determined from a nonlinear regression of the data using the Michaelis-Menton equation in Prism 5.0 software (GraphPad Software, San Diego, CA).
NADPH consumption
A volume of 100 μL of the incubation mixture was added to a microfuge tube containing 20 μL of 70% trichloroacetic acid, and the tube was then vortexed and placed on ice. This aliquot was used to determine the depletion of NADPH, as described (5), by adding 100 μL of a 1 mM solution of 2,4-dinitrophenylhydrazine (in 1.0 N HCl). The tube was vortexed and allowed to stand at room temperature (23°C) for 20 minutes. Aqueous NaOH (0.5 mL of a 10% solution) was then added, and the samples were vortexed and incubated at room temperature for 10 min. The tube was then centrifuged at 9,500 × g for 5 minutes, and the absorbance of the supernatant was read at 440 nm. The amount of NADPH consumed in the reaction was determined from the amount of α-ketoglutarate formed by the isocitrate dehydrogenase NADPH regenerating system, and this was calculated by reference to an α-ketoglutarate standard curve.
Hydrogen peroxide generation
Portions of the reaction mixtures (200 μL) also were added to microfuge tubes containing 400 μL of ice-cold, 4% trichloroacetic acid. These aliquots were used for the determination of hydrogen peroxide production (33). The tubes were then vortexed and placed on ice for 10 min before centrifuging at 14,000 rpm for 10 min. An aliquot of the supernatant (0.35 mL) was placed in a 13×100 glass tube with 350 μl of water. An aqueous solution of ferroammonium sulfate (0.15 mL of a 10 mM solution) and immediately thereafter, 0.056 mL of a 2.5 M aqueous solution of KSCN were added. The tube was vortexed, left at room temperature for 10 minutes, and the absorbance at 480 nm was read. Hydrogen peroxide standards (0–50 nmol) were processed and read with the samples.
Product formation
Finally, another aliquot of the reaction mixture (150 μL) was added to a microfuge tube that contained 25 μL of ice-cold, 25% zinc sulfate. Saturated Ba(OH)2 (25 μL) was then added immediately before the tube was vortexed and placed on ice. After the sample was centrifuged at 9500 × g for 5 min, the supernatant was transferred to a clean microfuge tube and was stored at −20°C until 100 μL was used for HPLC. Toluene metabolites were analyzed by HPLC using the method of Nakajima et al. (34). The metabolites were separated using a Phenomenex Primasphere C18-HCl column (250×4.6 mm, 5 micron). The mobile phase was 35% acetonitrile in water running in isocratic mode at 1.0 ml/min. Absorbance was measured at 200 nm. The retention times for the metabolites are as follows: benzyl alcohol at 6–7 minutes, p-cresol at 13–14 minutes, and o-cresol at 14–15 minutes. Standards of these metabolites were made up in the toluene reaction media and processed along with the samples.
Peroxidative metabolism of toluene
When the peroxidative metabolism of toluene by the P450s was measured, all incubation conditions were the same except that the total P450 enzyme concentrations were 1 μM and 2 μM for simple and mixed reconstituted systems, respectively. To determine the effect of CPR, reactions were run in both the presence and absence of CPR at the same relative concentrations used in the NADPH-supported reactions. In addition, the isocitrate dehydrogenase system was not added, and the reactions were initiated with 5 μL of an acetonitrile stock solution of cumene hydroperoxide (200 mM) instead of NADPH. Finally, the reactions were run for only one minute and were quenched by adding 210 μL of the reaction mixture to a microfuge tube containing 75 μL of ice-cold, 25% zinc sulfate. A saturated solution of barium sulfate (75 μL) was then added immediately, and the samples were vortexed and put on ice for 10 min. Attempts to measure the production of both o-cresol and p-cresol from the P450-mediated, peroxidative metabolism of toluene were unsuccessful because these products were not stable in the presence of the cumene hydroperoxide (data not shown). The CYP2B4-specific production of benzyl alcohol was quantified by HPLC as described above.
Peroxidative metabolism of 7-ethoxyresorufin
Because the o-cresol and p-cresol were unstable in the presence of the peroxide, the peroxidative metabolism by CYP1A2 was assessed by using the CYP1A2-specific substrate, 7-ethoxyresorufin (27). The simple and mixed P450 assays contained 0.5 μM and 1.0 μM of P450, respectively. Reactions were performed in the presence and absence of CPR at the same relative concentrations used in the NADPH-supported reactions. The 0.1 mL reactions were run in individual wells of a 96-well plate and were initiated with 1 μL of 200 mM cumene hydroperoxide. The P450-mediated formation of the fluorescent product, 7-hydroxyresorufin, was measured in real time (Ex: 535 nm; Em: 585), and the reactions were typically linear for about a minute.
Results
Metabolism of toluene by different reconstituted systems of CPR, DLPC, and P450
Both CYP2B4 and CYP1A2 are capable of metabolizing toluene. CYP2B4 is the most active of these enzymes primarily generating benzyl alcohol, and to a lesser extent, p-cresol. Although significantly less active, CYP1A2 also metabolizes toluene with a different regioselectivity, generating roughly equal amounts of o-cresol and benzyl alcohol. The goal of this study was to take advantage of the differences in regioselectivity of these P450 enzymes to determine if P450 function is affected by their co-reconstitution. This was accomplished by comparing the rates of formation of each toluene metabolite by “simple” reconstituted systems containing a single P450 enzyme and those formed by “mixed” reconstituted systems containing both CYP1A2 and CYP2B4 (20;35). Furthermore, the effects of the CYP1A2•CYP2B4 interaction on the efficiency of substrate metabolism were assessed by measuring the rates of NADPH oxidation and hydrogen peroxide production by the simple and mixed reconstituted systems.
The simple, reconstituted systems of DLPC and each individual P450 were prepared at a subsaturating CPR:P450 ratio (1:2), and the mixed reconstituted system contained both P450 enzymes at the same concentrations (twice the amount of total P450) and CPR at twice the concentration used in the simple systems. Under these conditions, if the CPR and the P450 enzymes are organized in the same manner in both the simple and mixed reconstituted systems, then a simple additive effect would be expected (35). In other words, the rates of formation by the mixed reconstituted system would be equal to the sum of the rates by the two simple systems.
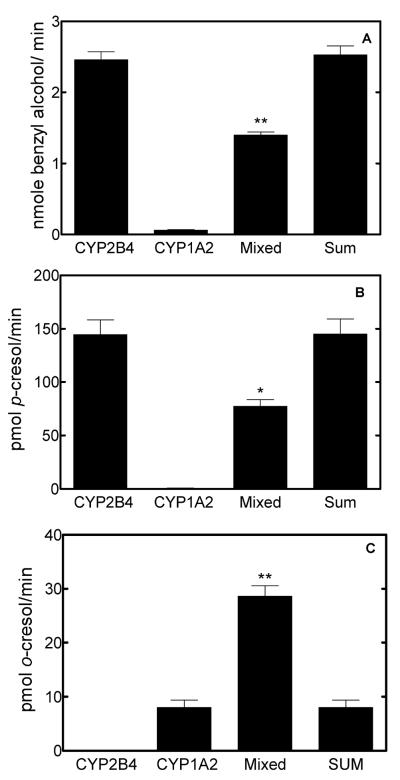
The rates of formation of benzyl alcohol by the various reconstituted systems are shown in Figure 1A. These results demonstrate that benzyl alcohol was generated primarily by CYP2B4 with only minor quantities produced in the simple CYP1A2 reconstituted system. However, production of this CYP2B4-selective metabolite was inhibited by about 45% in the mixed reconstituted system containing both P450 enzymes. Similar results were found with p-cresol formation, the other CYP2B4-specific product (Figure 1B), as the generation of this product also was inhibited by roughly 50% in the mixed reconstituted system.
Figure 1.
Rates of production of toluene metabolites by simple (CPR/CYP2B4 and CPR/CYP1A2) and mixed (CPR/CYP1A2/CYP2B4) systems reconstituted with DLPC. The rates of production of toluene metabolites by simple and mixed reconstituted system in addition to the sum of the rates by the simple systems containing either CYP2B4 or CYP1A2 are shown. The bar labeled “sum” represents the sum of the rates of the simple systems, and would be expected if the combination of P450 proteins in the mixed reconstituted system does not lead to a significant reorganization of the proteins. (A) Benzyl alcohol production. (B) Formation of p-cresol. (C) Formation of o-cresol. Significant differences in the means of the rates obtained with the mixed reconstituted system (n=5 for each condition) when compared to the sum of the rates from the simple reconstituted systems are indicated (*, p < 0.01; **, p < 0.001).
A different result was observed with the CYP1A2 selective product, o-cresol. This product actually showed a 3.6-fold stimulation in the mixed reconstituted system as compared to the sum of the rates of the simple reconstituted systems. These results demonstrate that mixing of CYP1A2 and CYP2B4 into a single reconstituted system enhanced the “CYP1A2 character” of the reconstituted system and simultaneously diminished its “CYP2B4 character”.
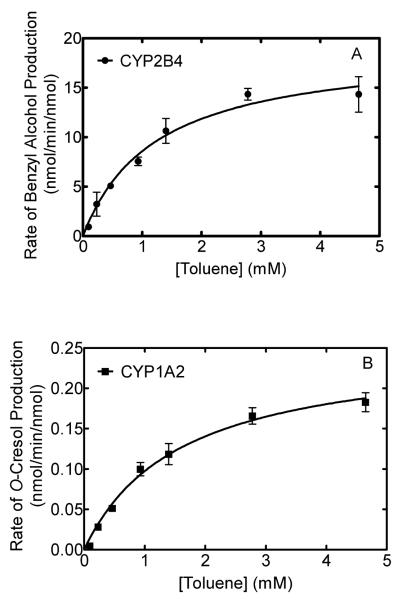
Determination of the Km for toluene with CYP1A2 and CYP2B4
As mentioned above, one of the objectives of this study was to determine how the CYP1A2•CYP2B4 interaction influenced the efficiency of toluene metabolism by the two enzymes. Because P450 enzymes can use reducing equivalents from CPR to generate hydrogen peroxide and excess water when the enzymes are not bound to substrate, it was important to confirm that the substrate, toluene, was at or near saturating concentrations under the experimental conditions. Otherwise, the changes in NADPH consumption and peroxide formation associated with the interaction of the two P450s could have been due to the enzymes that did not contain substrate. Therefore, we determined the kinetic constants for toluene metabolism by CYP2B4 and CYP1A2 (Fig. 2). This information allowed for an estimation of the fraction of P450 enzyme bound by substrate in the saturated toluene solution (approximately 5mM). The analysis generated similar Km values of 1.23 ± 0.29 mM and 1.60 ± 0.274 mM (average ± standard error; n = 4 separate determinations) for CYP2B4 and CYP1A2, respectively, whereas the corresponding Vmax values (in units of nmol/min/nmol) were 19.12 ± 1.74 and 0.252 ± 0.02 for CYP2B4-mediated production of benzyl alcohol and CYP1A2-mediated production of o-cresol, respectively. Using the Km values to approximate the degree of enzyme saturation under the conditions used to measure metabolic efficiencies, it was determined that CYP2B4 and CYP1A2 were approximately 80% and 75% saturated with toluene, respectively. Thus, the high levels of substrate binding by both enzymes provided support for the assumption that the differences in the rates of side product formation by the simple and mixed reconstituted systems were attributable to changes in the efficiencies of substrate metabolism and not in the activities of P450s not bound to substrate.
Figure 2.
The rates of metabolism of toluene by CYP2B4 (A) and CYP1A2 (B) as a function of toluene concentration. Reconstituted systems of DLPC, CPR, and either CYP2B4 or CYP1A2 were prepared and reactions were performed to measure the rates of metabolism of different concentrations of toluene as described in Materials and Methods. Each data point represents the average and standard error of four separate reconstituted systems measured on two different days.
Effect of P450•P450 interaction on the efficiency of toluene metabolism
Metabolism by P450 is mediated by a multi-step reaction cycle, and two of these steps require an interaction with cytochrome b5 and/or CPR to transfer electrons to P450 (6). These electron-transfer steps provide two of three potential branch points in the catalytic cycle at which reactive iron-oxo intermediates can either proceed through the reaction cycle to ultimately oxidize the substrate or degrade to produce either hydrogen peroxide or excess water as side-products. After the first electron-transfer step, the resulting oxyferrous P450 can break down to release superoxide. After the second electron-transfer step, the peroxy-heme species can degrade to form hydrogen peroxide. However, because superoxide rapidly dismutates into hydrogen peroxide and molecular oxygen (36), the cumulative rate of oxyferrous and peroxy-heme degradation also can be measured by the total rate of hydrogen peroxide production alone. The third branch of the catalytic cycle where reducing equivalents can be lost to non-productive metabolism follows the breakage of the oxygen-oxygen bond of the peroxy-heme species, which results in the formation of a high valent, iron-oxene species. This reactive form of the enzyme can either: (1) abstract a hydrogen atom from substrate and rebound to generate product, or (2) be reduced with an extra two electrons from CPR to generate “excess water” (37). The rate of excess water production can be determined by subtraction of the sum of the rates of hydrogen peroxide production and substrate oxidation from that of NADPH oxidation.
Each of these three side reactions creates a futile cycle, which results in returning the P450 catalytic cycle to the starting point and as a result, decreases the efficiency (or “coupling”) of electron flow to product formation. Thus, by simultaneously measuring the rates of NADPH oxidation, substrate metabolism, and hydrogen peroxide production, we were able to assess the effects of the P450•P450 interaction on the coupling of P450-mediated metabolism.
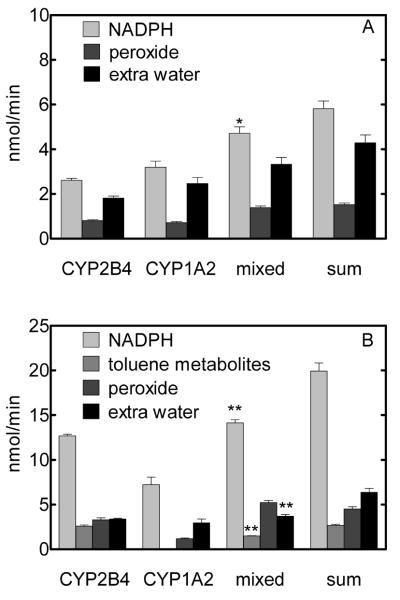
We first examined the rates of hydrogen peroxide and excess water production in the absence of substrate (Fig. 3A). Under these conditions, CPR still oxidized NADPH and transferred electrons to the P450s to generate the side products. These activities were very similar for the two simple reconstituted systems of P450 both in terms of the magnitudes of the rates and the relative proportions of hydrogen peroxide and excess water formed. CYP1A2 formed excess water at a slightly greater rate than that formed by CYP2B4. The interaction of CYP1A2 and CYP2B4 resulted in a slight but significant decrease (as compared to the sum of the rates measured with the two simple systems of P450) in the rate of NADPH oxidation that was associated with a minor decrease in the rate of excess water production (although the latter was not statistically significant). Because the differences in rates observed with the mixed system were minimal in the absence of substrate, it can be assumed that any dramatic changes in the rates of side product formation in the presence of toluene were attributable to a change in the efficiency of substrate metabolism and not to an effect on hydrogen peroxide and/or excess water formation by unbound P450.
Figure 3.
Effect of CYP1A2•CYP2B4 interactions on P450 reaction coupling. Reconstituted systems of DLPC, CPR, and the P450 enzyme(s) were prepared as described in Materials and Methods. The averages and standard errors (n ≥ 5) of the rates of NADPH consumption and the formation of the products are expressed as nmol/min. The significance of the differences in the rates by the mixed reconstituted system and the sum of those by the simple systems are indicated for each reaction product (*, p < 0.05; **, p < 0.001). Panel A shows the rates of NADPH consumption and the production of hydrogen peroxide and excess water in the absence of substrate. Hydrogen peroxide represents the total amount of hydrogen peroxide and superoxide (which dismutates to hydrogen peroxide and oxygen) formed by decay of the peroxyferrous and oxyferrous intermediates, respectively, of the P450 catalytic cycle (3). Thus, a mole of NADPH is consumed for every mole of hydrogen peroxide formed. Excess water was calculated based on the NADPH consumed and the sum of the various products of these P450-dependent reactions. Extra water is formed by two-electron reduction of the putative, high valent iron-oxo intermediate (iron-oxene species) at the third branch point of the catalytic cycle (6). The iron-oxene species, in turn, is formed following the two-electron reduction of molecular oxygen bound to the heme group of the P450. Thus, the formation of a single molecule of excess water requires the reducing equivalents from two molecules of NADPH. In panel B, toluene metabolites include the sum of the rates of formation of o-cresol, p-cresol, and benzyl alcohol.
The P450-mediated metabolism of toluene was associated with higher rates of NADPH consumption and the production of both hydrogen peroxide and excess water (Figure 3B). The metabolism of toluene by both CYP1A2 and CYP2B4 was poorly coupled to the oxidation of NADPH. The metabolism by CYP1A2 was extremely unproductive as only one percent of the oxidized NADPH was used to form toluene metabolites. Although metabolism by CYP2B4 was more efficient, only 12% of the oxidized NADPH was used to form toluene metabolites (Fig. 3B). Most of the NADPH oxidation by both of the simple reconstituted systems containing either CYP2B4 or CYP1A2 was used in the production of excess water. Because two moles of NADPH are consumed in the production of 1 mole of excess water (6), over 60% of the NADPH oxidized by the enzymes was expended on this pathway of reaction uncoupling (Table 1).
Table 1.
Measured and predicted rates of P450-mediated NADPH oxidation and product formation catalyzed by simple and mixed systems.a
| Product or Substrate | CYP2B4 system | CYP1A2 system | Mixed system (Experimental results) | Predicted Contribution from CYP2B4 to mixed RS b | Predicted Contribution from CYP1A2 to mixed RS c | Mixed system (Theoretical) d |
|---|---|---|---|---|---|---|
| NADPH oxidized | 12.7 ± 0.38 | 7.21 ± 0.23 | 14.14 ± 0.38 | 6.79 | 21.63 | 28.42 |
| Toluene metabolites | 2.61 ± 0.28 | 0.01 ± 0.002 | 1.51 ± 0.10 | 1.40 | 0.03 | 1.43 |
| H2O2 formed | 2.01 ± 0.63 | 1.20 ± 0.10 | 5.23 ± 0.86 | 1.08 | 3.60 | 4.68 |
| Excess H2O formed | 4.04 ± 1.29 | 3.00 ± 0.33 | 3.70 ± 1.34 | 2.16 | 9.00 | 11.16 |
aThe experimentally measured rates of NADPH oxidation and formation of side-products and toluene metabolites by CYP2B4, CYP1A2, and mixed CYP2B4-CYP1A2 reconstituted systems were determined as described in Materials and Methods. The theoretical rates (expressed in nmol/min) of NADPH oxidation and the formation of uncoupled products by the individual enzymes in the mixed system were predicted by using the measured changes in the rates of formation of the P450-specific toluene metabolites by the mixed system as proportion factors for these other parameters. More specifically, Figure 1 indicates that mixing of CYP1A2 and CYP2B4 caused a 47% decrease in CYP2B4-mediated product (p-cresol) formation and a 3.75-fold increase in CYP1A2-mediated product (o-cresol) formation. To calculate the theoretical contribution of each enzyme in the mixed system, the rates of NADPH oxidation and uncoupled product formation by the simple systems were multiplied by 0.47 and 3.75 for CYP2B4b- and CYP1A2c-mediated metabolism, respectively. The theoretical rates of metabolism by the mixed systemd are simply the sum of the “predicted contributions” by each of the enzymes. The theoretical rates are calculated from the averages of the measured rates of metabolism and do not include margins of error.
In addition to changing the relative levels of toluene metabolites formed (Fig. 1), the combination of P450 enzymes in the mixed reconstituted system also resulted in changes in the coupling of the enzymatic reactions (Fig. 3B and Table 1). More specifically, the mixed reconstituted system exhibited significant decreases in the rates of NADPH consumption and the formation of both excess water and total toluene metabolites (i.e. the sum of the rates with the simple systems was greater than the rates measured with the mixed reconstituted system) (Fig. 3B). Interestingly in both of the simple reconstituted systems, the formation of excess water exceeded that of hydrogen peroxide; however, in the mixed reconstituted system the relative rates of formation of these products were reversed. These results suggest that the interaction between CYP1A2 and CYP2B4 fundamentally changed metabolism by one or both of the enzymes such that the relative proportions of the metabolites and the products formed by reaction uncoupling were altered. This assumption is demonstrated clearly by Table 1 in which the rates of NADPH oxidation and the formation of uncoupled products in the mixed system were predicted (theoretical) by using the relative changes in the rates of P450-specific product formation observed with the mixed system as proportion factors for the other rates in the assay. This calculation assumed that even though the rates of substrate metabolism were altered by the P450•P450 interaction, the relative proportions of reaction uncoupling were the same in the mixed and simple reconstituted systems. It could be argued that this approach is flawed because it would be impossible to know the individual contributions of each P450 to the total levels of NADPH oxidation and hydrogen peroxide production in a mixed reconstituted system containing both of the enzymes. However, because each P450 made a specific toluene metabolite, it was possible to determine the relative level of change of metabolism by each enzyme in the mixed system and deduce the expected rates of the nonspecific activities by comparison to the observed rates in the simple reconstituted systems. More specifically, as indicated above, CYP1A2-specific metabolism was stimulated about 3.6-fold in the mixed system. Thus, to calculate theoretical rates of excess water and hydrogen peroxide production by the mixed system, the rates for these reactions (observed in the simple system with CYP1A2) were multiplied by 3.6-fold. This would be the expected outcome if the efficiency of CYP1A2 metabolism was unaltered by the interaction of the two P450s. The observed differences between the experimental data and the theoretical adjusted rates of peroxide and extra water formation, clearly show that coupling of the P450s is influenced by the CYP1A2•CYP2B4 complex.
A comparison of the theoretical and experimental rates catalyzed by the mixed system (Table 1) indicated that the interaction of the two P450s caused a dramatic decrease in the rate of excess water production by CYP1A2. This conclusion can be surmised because the measured rate of excess water production by the mixed system was greater than the theoretical rate of excess water production by CYP2B4 but dramatically less than that by CYP1A2. Thus, product formation by CYP1A2 was much more efficient in the mixed reconstituted system. As would be expected, the lower rate of excess water production was also associated with a pronounced decrease in the rate of NADPH oxidation by the mixed system. In contrast to its effect on excess water production, the interaction of the two P450s had no detectable effect on the rates of hydrogen peroxide production by the two enzymes.
Peroxidative metabolism of toluene by simple and mixed reconstituted systems of CPR, DLPC, and P450
NADPH-supported toluene metabolism by a mixed reconstituted system containing CPR and both CYP1A2 and CYP2B4 showed a substantial inhibition of the CYP2B4-selective activities (benzyl alcohol and p-cresol) and a stimulation of CYP1A2-selective o-cresol production. These results raise the question, “Are the functional changes observed in the mixed system due to a direct alteration in the function of the CYP1A2•CYP2B4 complex, or does the CYP1A2•CYP2B4 complex lead to altered electron flow through the CPR?” To distinguish between these possibilities, toluene metabolism was measured using cumene hydroperoxide, rather than NADPH. In these reactions, the peroxide provides the electrons and the oxygen molecule that are used by the P450 to catalyze substrate oxidation, so CPR is not required for these assays. Thus, the peroxide-stimulated P450 reactions can be used to specifically assess the effects of the P450•P450 interaction on the latter-stages of catalysis after delivery of electrons to the active site. Cumene hydroperoxide-supported metabolism of toluene was measured using reconstituted systems containing (1) CYP1A2, (2) CYP2B4 and (3) both CYP1A2 and CYP2B4 (Figure 4). The results show that the same substrate specificity was observed for peroxide- and NADPH-supported metabolism, as CYP2B4 generated benzyl alcohol from toluene at a greater rate than CYP1A2. In contrast to the results obtained with NADPH-supported metabolism, the rate of benzyl alcohol production by the mixed reconstituted system was approximately equal to the sum of the rates observed with the simple systems, suggesting that benzyl alcohol production was not affected by the combined presence of these P450s. Next, we repeated these experiments using reconstituted systems that also contained CPR. Although CPR was present in each of the reconstituted systems, and potentially capable of forming a complex with the P450s, there was no NADPH, preventing any opportunity for electron flow through the proteins. Similar results were obtained showing that, even in the presence of CPR, the rate of metabolism by the mixed system was the sum of the rates of the simple reconstituted systems. These results suggest that electron flow from CPR to CYP2B4 was affected by the interaction between CYP1A2 and CYP2B4.
Figure 4.
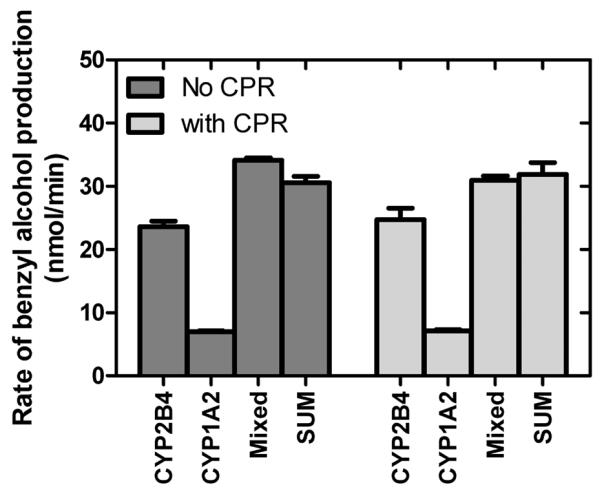
Effect of the interaction between CYP1A2 and CYP2B4 on cumene hydroperoxide-mediated toluene metabolism. The rate of toluene metabolism was examined using cumene hydroperoxide to provide reducing equivalents to support the reactions both in the presence and absence of CPR. Because of instability of the p- and o-cresols in the presence of peroxides, only the major product of toluene metabolism, benzyl alcohol, was measured. Assays to measure the peroxidative metabolism of the substrates were performed by initiating the reactions with 2.5 mM cumene hydroperoxide instead of NADPH as described in Materials and Methods. The results represent the averages and standard error of six determinations from two separate experiments. The P450 concentrations used in the peroxidative assays were 5-fold higher than those used in the NADPH-supported reactions (Figure 1A).
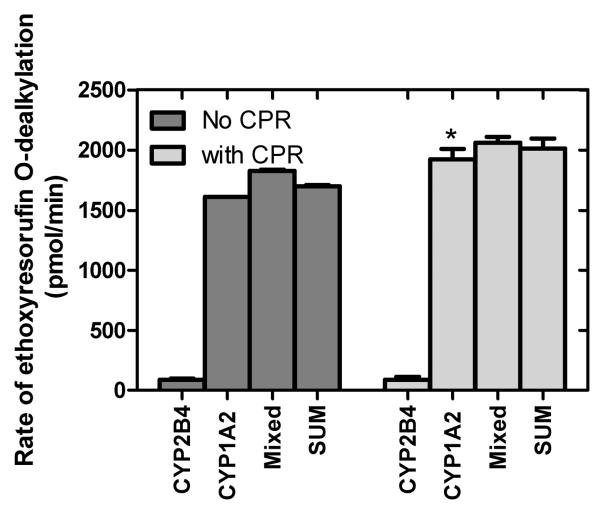
Unfortunately, the peroxide used to stimulate the P450-mediated metabolism reacted with both of the ring-hydroxylation products (o-cresol and p-cresol). Therefore, another CYP1A2-specific substrate, 7-ethoxyresorufin (27), was used to monitor CYP1A2-specific metabolism. Again, in contrast to the NADPH-supported reaction, the rates of metabolism catalyzed by the mixed reconstituted system were not significantly different from the sum of the corresponding rates by the simple systems (Figure 5). The major implication of these findings regarding the peroxidative reactions is that the functional effects of the CYP2B4•CYP1A2 interaction are mediated predominantly by changes in the relative abilities of the two P450s to interact with CPR when they are bound together in a mixed complex. Furthermore, the data indicate the functional changes related to CYP1A2•CYP2B4 complex formation require electrons to be flowing through CPR.
Figure 5.
Effect of the interaction between CYP1A2 and CYP2B4 on cumene hydroperoxide mediated metabolism of 7-ethoxyresorufin. The rate of 7-ethoxyresorufin metabolism was examined using cumene hydroperoxide to provide reducing equivalents to support the reactions both in the presence and absence of CPR. The asterisk (*) indicates the means are significantly different (p < 0.05) from the corresponding experiments in the absence of CPR. The results represent the averages and standard error of six determinations from two separate experiments.
Interestingly, CYP1A2-dependent peroxidative metabolism of 7-ER was slightly stimulated by the presence of CPR (Figure 5). Previous studies have demonstrated that CPR (38) and CYP2B4 (24) would both disrupt the homomeric aggregation of CYP1A2. However, stimulation of CYP1A2 activity was only caused by the interaction with CPR and not CYP2B4. Thus, these data indicate there was a minor positive effect of the physical interaction of CPR and CYP1A2 on catalysis and not just electron delivery to the P450 active site.
Discussion
This study furthers previous work from our laboratory and others demonstrating that CYP1A2 and CYP2B4 physically interact to influence the metabolism of drugs and environmental compounds (14;19;20;23;24;35;39). Consistent with our previous findings using other substrates, the interaction of CYP2B4 and CYP1A2 caused a synergistic stimulation of CYP1A2-mediated toluene metabolism (o-cresol production), and a marked decrease in CYP2B4-mediated production of p-cresol and benzyl alcohol. These results are consistent with our previous assumption that CYP1A2 and CYP2B4 form a heteromeric complex that increases the ability of the CYP1A2 moiety to bind to CPR.
Studies from numerous laboratories have shown that several P450•P450 interactions cause changes in the rates of substrate catalysis by one or both of the enzymes. CYP2E1 and CYP1A2 interacted in a manner that influenced metabolism by both enzymes, and the changes in metabolism were similar to those associated with the interaction of CYP2B4 and CYP1A2 (i.e. CYP2E1 activity was decreased while that of CYP1A2 was increased) (22). Similarly, the interactions between CYP2C9·CYP2C19 (40), CYP2C9·CYP2D6 (25), and CYP2C9·CYP3A4 (26) variably influenced the metabolism by the individual P450s. In the case of CYP2C9, its activity was stimulated by the interaction with CYP2C19 (40) but inhibited by CYP2D6 (25) and CYP3A4 (26). Interestingly, only the full-length CYP3A4 and not the N-truncated derivative affected metabolism by CYP2C9.
In addition to using the regioselectivity of the P450-mediated metabolism of a single substrate to monitor the functional effects of a P450•P450 interaction, we also ascertained the effects of the CYP1A2•CYP2B4 interaction on the efficiency of metabolism by the enzymes. We confirmed that the changes we observed were attributable to the metabolism of toluene by measuring the rates of excess water and hydrogen peroxide production in the absence of substrate and by determining the kinetic constants for toluene metabolism by the two enzymes. By determining the Km of each P450 for toluene metabolism, we confirmed that both enzymes were close to saturation with substrate under the experimental conditions used. Interestingly, our measurement of the Km for toluene metabolism by CYP2B4 was about 15-fold smaller than the value previously reported for this substrate (41). This discrepancy is likely attributable to (1) differences in the methods for protein reconstitution, and (2) the addition of toluene as a methanolic solution in the previous study. Previous studies have shown that the catalytic characteristics of the P450s are extremely sensitive to reconstitution conditions, as the proteins need to be preincubated for at least 1 hr in order to stabilize the catalytic results (42). The duration of enzymes/lipid preincubation is unclear from the previous kinetic analysis of toluene metabolism (41). The second major variant is due to the use of organic solvents as vehicles for hydrophobic substrates. In the literature report on the Km for toluene, the substrate was diluted in methanol at an unreported dilution. In the current study (Figure 3), the kinetics of toluene metabolism were measured in the absence of organic solvent. It is highly likely that the methanol that was used in the older study competed with toluene for binding to the CYP2B4 active site, as reported for other CYP2B enzymes (43). The presence of the substrate (with proportionally larger quantities of the competing solvent at higher substrate concentrations) would be expected to increase the apparent Km for toluene. Thus, our findings indicate that the calculated Km of toluene for rabbit CYP2B4 is in the low mM range.
The theoretical values calculated for hydrogen peroxide production for the mixed reconstituted system provided reasonable estimates for those obtained experimentally (Table 1). However, the actual rates of both NADPH consumption and the production of excess water were much lower than would be expected if the enzymes functioned independently in the mixed reconstituted system. Of course, a decrease in the rate of production of excess water would lead to a significant decrease in NADPH consumption because each molecule of excess water formed requires 2 molecules of NADPH (4 electron total) to be diverted toward this pathway. In terms of the coupling of NADPH oxidation to product formation by the P450 enzymes, the interaction of the two enzymes in the mixed reconstituted system resulted in a dramatic increase in the efficiency of CYP1A2-mediated metabolism (o-cresol formation) and a slight decrease in the efficiency of CYP2B4-mediated metabolism (production of benzyl alcohol and p-cresol). These data suggest that futile cycling of NADPH to form excess water from CYP1A2-related catalysis was greatly diminished when the enzyme was bound in a mixed complex with CYP2B4. Although the rate of excess water production is not measured directly but inferred from the difference in the rate of NADPH oxidation and the sum of the rates of product and hydrogen peroxide formation, it seems unlikely, given the magnitude of the changes associated with the P450•P450 interaction, that the results could be attributable to experimental error.
The possibility that P450•P450 interactions can influence the coupling of reducing equivalents to catalysis has important implications for cellular survival as it is conceivable that a P450•P450 interaction may also influence the generation of reactive oxygen species. The contribution and significance of P450 in the generation of cellular reactive oxygen species has been discussed thoroughly in a previous review of the topic (44). Our findings suggest that the interaction of specific forms of P450s could have a physiologic role in regulating the production of reactive oxygen species by these heme proteins. This possibility has been addressed in more detail in an excellent review on the functional significance of P450 oligomerization (45).
It is important to emphasize that these studies were conducted using DLPC reconstituted systems of P450. Thus, the effects of P450•P450 interactions may be different in the complex lipid bilayer of the endoplasmic reticulum. Although our lab has provided evidence for the effects of the CYP2B4•CYP1A2 interaction on substrate metabolism in both DLPC reconstituted systems and rabbit liver microsomes from animals induced to express both forms of P450 (46), it remains to be determined if the same is true for the effects of the P450•P450 interaction with respect to the efficiency of substrate metabolism. Based on the steps of P450-mediated catalysis (described in the Results), there are two conceivable mechanisms by which the P450•P450 interaction could have the observed effect on excess water production by the P450s. The interaction could either decrease the rate of electron flow from the CPR or accelerate the rate of hydrogen radical abstraction by the reactive iron-oxene species. Interestingly, evidence shows that the rate-limiting step of most CYP1A2-mediated reactions is the abstraction of a hydrogen atom from the substrate by the high valent, iron-oxene species (described above) (47;48). Our findings are fully consistent with this being the rate-limiting step of toluene metabolism by CYP1A2 as the increase in the rate of o-cresol production in the mixed system was matched by a commensurate decrease in the rate of excess water production. Furthermore, if the rate limiting step was one of the electron transfer steps from the CPR, one might expect the rate of hydrogen peroxide production to be altered in concert with an increase in o-cresol production. However, the rate of hydrogen peroxide production was essentially what would be predicted if the P450s did not interact with one another. Thus, consistent with what has been established about catalysis by CYP1A2, our data indicate that the effect of the P450•P450 interaction involves a step at the latter part of the catalytic cycle (after the first two electrons have been delivered to the P450).
This line of reasoning leads to the conclusion that the increase in the rate of o-cresol production observed in the mixed reconstituted system was probably due to an increase in the rate of hydrogen radical abstraction from the substrate by the high valent iron-oxene species of CYP1A2. The increase in the rate of CYP1A2-mediated hydrogen abstraction from toluene would be expected to result in a decrease in the rate of excess water formation because this would limit the possibility for CPR-mediated reduction of the iron-oxene species and the formation of excess water. In addressing the possibility that the decrease in excess water production by the mixed system results from a slower rate of electron delivery from CPR to CYP1A2, the data comparing the rates of excess water production in the absence of substrate are instructive. Although there were slightly lower rates of NADPH oxidation and excess water generation in the mixed reconstituted system in the absence of substrate, the differences were minimal, and the effects were greatly enhanced in the presence of toluene. This suggests that electron flow from CPR to CYP1A2 was not dramatically altered in the mixed system and as a result, the changes were predominantly the result of an increase in the rate of hydrogen radical abstraction from the substrate.
Conversely, the peroxidative metabolism by CYP1A2 that was supported by cumene hydroperoxide was not appreciably stimulated by its interaction with CYP2B4 in the mixed P450 reconstituted system in both the presence and absence of CPR. Because the stimulation of CYP1A2 in the mixed reconstituted system relied on both the presence of CPR and its ability to deliver electrons to the P450 (NADPH was not present in the peroxidative reactions), these results suggested that the mechanism of stimulation involved a change in the functional interaction of CPR and CYP1A2.
Surprisingly, peroxidative metabolism by the simple reconstituted system with CYP1A2 was increased by the interaction with CPR. Thus, it seems possible that the interaction of CYP1A2 with oxidized CPR caused a conformational change in CYP1A2 that facilitated hydrogen radical abstraction from the substrate by the iron-oxene species. Recent studies have shown that the conformations of CPR are dramatically different when its FMN group is oxidized (or partially oxidized in the semiquinone form) and fully reduced (49–51). In the oxidized/partially oxidized state, the FMN domain of CPR interacts with the face of the FAD domain allowing for electron transfer between the flavins. Once NADPH is bound to the FAD domain, electrons are rapidly shuttled from the FAD to the FMN moiety. Continued association of the resulting NADP with CPR causes a conformational change in an intermediate hinge region that turns the FMN domain outward allowing it to interact with its redox partners. Given the dramatic changes in CPR conformation that result from the reduction of its FMN group, it can be concluded that CPR with oxidized/partially oxidized and fully reduced FMN moieties would interact differently with CYP1A2. The fully reduced form of CPR also may affect the rate of substrate hydrogen abstraction by CYP1A2 when it is bound to the P450 although a distinction between the effects of oxidized and reduced CPR on facilitating progression through the P450 catalytic cycle has been noted previously (52).
Although the effect of CPR on cumene hydroperoxide-mediated metabolism by CYP1A2 in the simple reconstituted system) appeared to be minor, the peroxidative reactions required CYP1A2 to first bind and activate the hydroperoxide group of cumene hydroperoxide; next the cumyl alcohol must exit the CYP1A2 active site to allow the substrate to bind; and then the iron-oxo species can abstract the substrate hydrogen. Thus, it also is possible that the additional steps of cumyl hydroperoxide binding, activation, and exit from the active site may be partially rate-limiting and may obscure what otherwise is a major effect of CPR binding on the rate of hydrogen abstraction from the substrate.
Previously, it has been proposed that the mechanism by which P450 metabolism is altered in the mixed CYP2B4•CYP1A2 interaction is by changes in the CPR-binding affinity of the constituent enzymes. The results of this study require that the putative changes in CPR-binding affinity also coincide with a conformation change that causes CYP1A2 metabolism to be more efficient. Otherwise, CPR binding more tightly to CYP1A2 would simply cause electrons to flow more rapidly to CYP1A2, and as a result, the rate of excess water generation would be increased and not decreased by the interaction of CYP1A2 and CYP2B4.
Another mechanism for stimulation of CYP1A2 activity that may act independently or in concert with a CYP2B4-mediated change in the binding affinity of CPR and CYP1A2 relates to the likelihood that CPR is sterically hindered from binding to CYP1A2 when the P450 is in a homomeric complex. P450 complexes are characterized by heterogeneity with respect to the position and spin state equilibria of the constituent enzymes, and this heterogeneity has been proposed to be a means of catalytic regulation (45). In the current instance, our hypothesis proposes that CPR has better access to the proximal side of CYP1A2 when it is bound in a heteromeric complex with CYP2B4. The idea is supported by cross-linking studies showing that CYP1A2 makes larger sized-aggregates than CYP2B4 (24) and by a recent publication showing that the catalytic activity of CYP1A2 is inhibited when the enzyme is in a homomeric complex (19). P450 enzymes have a sidedness that is common to all the members of the P450 superfamily. The heme group is not centrally located in the interior of the enzyme, but instead is much closer to one side of the enzyme than the other. The side that is nearest the heme group is known as the proximal side. This also is the side to which CPR binds in order to transfer electrons to the P450. The opposite, distal side of the P450, contains the substrate access channel to the interior, active site of the enzyme. Most P450 enzymes have been characterized as forming distal to distal contacts (with juxtaposed membrane-binding regions) for adjacent units in crystal structures (53–56). Interestingly, the crystal structure of human CYP1A2 also demonstrated, in addition to distal to distal contacts, an unusual proximal to proximal intermolecular interaction between adjacent CYP1A2 units in the crystal (57). The membrane-spanning domains were also juxtaposed for this type of interaction making the alignment credible as a functional type of complex. If this interaction prevails in simple lipid reconstituted systems with CYP1A2, the ability of CPR to interact with these enzymes may be limited which may explain why the activity of CYP1A2 is limited in its homomeric complex. The inhibition of CYP2B4 and activation of CYP1A2 could be rationalized if some of the CYP1A2 forming a complex where the proximal side is obstructed was replaced by CYP2B4 in the mixed system and was then freed up to bind to CPR.
There are three main conclusions that can be drawn from this study. First, when CYP1A2, CYP2B4 and subsaturating CPR are present in the same membrane system, the relative ratios of toluene metabolites are affected in a manner in which CYP1A2-selective o-cresol production is stimulated and CYP2B4-selective benzyl alcohol and p-cresol are inhibited. These results are consistent with the formation of a CYP1A2•CYP2B4 complex, where the CYP1A2 moiety has a higher affinity for CPR and/or has a greater opportunity to bind to CPR. Second, the interaction between CYP1A2 and CYP2B4 leads to significant changes in coupling of the P450s, which are consistent with a change in the rate limiting step for toluene metabolism by CYP1A2. Third, when the reaction was supported by cumene hydroperoxide, the interaction between CYP1A2 and CYP2B4 did not have the same effects on substrate metabolism – even in the presence of CPR. These results suggest that the interaction between CYP1A2 and CYP2B4 leads to a conformational or positional change that not only alters the ability of CPR to bind, but also fundamentally changes the efficiency by which CYP1A2 metabolizes substrates. Taken together with other findings, the data in this study describe an enzyme system where the P450s in the membrane cannot simply be considered as monomeric proteins that interact with CPR, but function as components of a larger system where P450•P450 complexes as well as interactions with other endoplasmic reticulum constituents will influence the disposition of foreign compounds.
Acknowledgments
Funding Source: These studies were supported by US Public Health Service Research Grants from the National Institute of Environmental Health Sciences ES004344 (WLB) & ES013648 (WLB & JRR).
Abbreviations
- (CYP; P450)
cytochrome P450
- (EROD)
7-ethoxyresorufin-O-dealkylation
- (7-ER)
7-ethoxyresorufin
- (7-EFC)
7-ethoxy-4-trifluromethylcoumarin
- (7-HFC)
7-hydroxy-4-trifluoromethylcoumarin
- (BZP)
benzphetamine
- (NADPH)
reduced nicotinamide adenine dinucleotide phosphate
- (CPR)
NADPH – cytochrome P450 reductase
- (DLPC)
L-α-dilauroyl-sn-glycero-3-phosphocholine
References
- 1.Porter TD, Coon MJ. Cytochrome P-450. Multiplicity of isoforms, substrates, and catalytic and regulatory mechanisms. J Biol. Chem. 1991;266:13469–13472. [PubMed] [Google Scholar]
- 2.Anzenbacher P, Anzenbacherová E. Cytochromes P450 and metabolism of xenobiotics. Cell. Mol. Life. Sci. 2001;58:737–747. doi: 10.1007/PL00000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White RE, Coon MJ. Oxygen activation by cytochrome P-450. Annu. Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]
- 4.Tamburini PP, MacFarquhar S, Schenkman JB. Evidence of binary complex formations between cytochrome P-450, cytochrome b5, and NADPH-cytochrome P-450 reductase of hepatic microsomes. Biochem. Biophys. Res. Commun. 1986;134:519–526. doi: 10.1016/s0006-291x(86)80451-x. [DOI] [PubMed] [Google Scholar]
- 5.Gruenke LD, Konopka K, Cadieu M, Waskell L. The Stoichiometry of the Cytochrome P-450-catalyzed Metabolism of Methoxyflurane and Benzphetamine in the Presence and Absence of Cytochrome b(5) J. Biol. Chem. 1995;270:24707. doi: 10.1074/jbc.270.42.24707. [DOI] [PubMed] [Google Scholar]
- 6.Loida PJ, Sligar SG. Molecular recognition in cytochrome P-450: mechanism for the control of uncoupling reactions. Biochem. 1993;32:11530–11538. doi: 10.1021/bi00094a009. [DOI] [PubMed] [Google Scholar]
- 7.Lu AY, Strobel HW, Coon MJ. Hydroxylation of benzphetamine and other drugs by a solubilized form of cytochrome P-450 from liver microsomes: lipid requirement for drug demethylation. Biochem. Biophys. Res. Commun. 1969;36:545–551. doi: 10.1016/0006-291x(69)90339-8. [DOI] [PubMed] [Google Scholar]
- 8.French JS, Guengerich FP, Coon MJ. Interactions of cytochrome P-450, NADPH-cytochrome P-450 reductase, phospholipid, and substrate in the reconstituted liver microsomal enzyme system. J. Biol. Chem. 1980;255:4112–4119. [PubMed] [Google Scholar]
- 9.Peterson JA, Ebel RE, O'Keeffe DH, Matsubara T, Estabrook RW. Temperature dependence of cytochrome P-450 reduction. A model for NADPH-cytochrome P-450 reductase:cytochrome P-450 interaction. J. Biol. Chem. 1976;251:4010–4016. [PubMed] [Google Scholar]
- 10.Reed JR, Cawley GF, Backes WL. Inhibition of cytochrome P450 1A2-mediated metabolism and production of reactive oxygen species by heme oxygenase-1 in rat liver microsomes. Drug Metab Lett. 2011;5:6–16. doi: 10.2174/187231211794455253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West SB, Lu AYH. Reconstituted liver microsomal enzyme system that hydroxylates drugs, other foreign compounds and endogenous substrates. V. Competition between cytochromes P-450 and P-448 for reductase in 3,4-benzpyrene hydroxylation. Arch. Biochem. Biophys. 1972;153:298–303. doi: 10.1016/0003-9861(72)90448-1. [DOI] [PubMed] [Google Scholar]
- 12.Tan YZ, Patten CJ, Smith P, Yang CS. Competitive interactions between cytochromes P450 2A6 and 2E1 for NADPH-cytochrome P450 oxidoreductase in the microsomal membranes produced by a baculovirus expression system. Arch. Biochem. Biophys. 1997;342:82–91. doi: 10.1006/abbi.1997.9995. [DOI] [PubMed] [Google Scholar]
- 13.Li DN, Pritchard MP, Hanlon SP, Burchell B, Wolf CR, Friedberg T. Competition between cytochrome P-450 isozymes for NADPH-cytochrome P-450 oxidoreductase affects drug metabolism. J. Pharmacol. Exp. Ther. 1999;289:661–667. [PubMed] [Google Scholar]
- 14.Reed JR, Backes WL. Formation of P450.P450 complexes and their effect on P450 function. Pharmacol. Ther. 2012;133:299–310. doi: 10.1016/j.pharmthera.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanck J, Smettan G, Ristau O, Ingelman-Sundberg M, Ruckpaul K. Mechanism of rate control of the NADPH-dependent reduction of cytochrome P-450 by lipids in reconstituted phospholipid vesicles. Eur. J. Biochem. 1984;144:509–513. doi: 10.1111/j.1432-1033.1984.tb08495.x. [DOI] [PubMed] [Google Scholar]
- 16.Davydov DR, Deprez E, Hoa GH, Knyushko TV, Kuznetsova GP, Koen YM, Archakov AI. High-pressure-induced transitions in microsomal cytochrome P450 2B4 in solution: evidence for conformational inhomogeneity in the oligomers. Arch. Biochem. Biophys. 1995;320:330–344. doi: 10.1016/0003-9861(95)90017-9. [DOI] [PubMed] [Google Scholar]
- 17.Davydov DR, Fernando H, Baas BJ, Sligar SG, Halpert JR. Kinetics of dithionite-dependent reduction of cytochrome P450 3A4: heterogeneity of the enzyme caused by its oligomerization. Biochem. 2005;44:13902–13913. doi: 10.1021/bi0509346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davydov DR, Sineva EV, Sistla S, Davydova NY, Frank DJ, Sligar SG, Halpert JR. Electron transfer in the complex of membrane-bound human cytochrome P450 3A4 with the flavin domain of P450BM-3: the effect of oligomerization of the heme protein and intermittent modulation of the spin equilibrium. Biochim. Biophys. Acta. 2010;1797:378–390. doi: 10.1016/j.bbabio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed JR, Connick JP, Cheng D, Cawley GF, Backes WL. Effect of Homomeric P450•P450 Complexes on P450 Function. Biochem. J. 2012;446:489–497. doi: 10.1042/BJ20120636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backes WL, Batie CJ, Cawley GF. Interactions among P450 enzymes when combined in reconstituted systems: formation of a 2B4-1A2 complex with a high affinity for NADPH-cytochrome P450 reductase. Biochem. 1998;37:12852–12859. doi: 10.1021/bi980674a. [DOI] [PubMed] [Google Scholar]
- 21.Kelley RW, Reed JR, Backes WL. Effects of ionic strength on the functional interactions between CYP2B4 and CYP1A2. Biochem. 2005;44:2632–2641. doi: 10.1021/bi0477900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley RW, Cheng D, Backes WL. Heteromeric Complex Formation between CYP2E1 and CYP1A2: Evidence for the Involvement of Electrostatic Interactions. Biochem. 2006;45:15807–15816. doi: 10.1021/bi061803n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davydov DR, Petushkova NA, Archakov AI, Hoa GH. Stabilization of P450 2B4 by its association with P450 1A2 revealed by high-pressure spectroscopy. Biochem. Biophys. Res. Commun. 2000;276:1005–1012. doi: 10.1006/bbrc.2000.3596. [DOI] [PubMed] [Google Scholar]
- 24.Reed JR, Eyer M, Backes WL. Functional interactions between cytochromes P450 1A2 and 2B4 require both enzymes to reside in the same phospholipid vesicle: evidence for physical complex formation. J. Biol. Chem. 2010;285:8942–8952. doi: 10.1074/jbc.M109.076885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanian M, Low M, Locuson CW, Tracy TS. CYP2D6-CYP2C9 protein-protein interactions and isoform-selective effects on substrate binding and catalysis. Drug Metab Dispos. 2009;37:1682–1689. doi: 10.1124/dmd.109.026500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian M, Zhang H, Tracy TS. CYP2C9-CYP3A4 protein-protein interactions in a reconstituted expressed enzyme system. Drug Metab. Dispos. 2010;38:1003–1009. doi: 10.1124/dmd.109.030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burke MD, Thompson S, Elcombe JR, Halpert J, Haaparanta T, Mayer RT. Ethoxy-. Pentoxy-and Benzyloxyphenoxazones and Homologues: A Series of Substrates to Distinguish Between Different Induced Cytochromes P-450. Biochem. Pharmacol. 1985;34:3337–3345. doi: 10.1016/0006-2952(85)90355-7. [DOI] [PubMed] [Google Scholar]
- 28.Backes WL, Kelley RW. Organization of multiple cytochrome P450s with NADPH-cytochrome P450 reductase in membranes. Pharmacology & Therapeutics. 2003;98:221–233. doi: 10.1016/s0163-7258(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 29.Yasukochi Y, Masters BS. Some properties of a detergent-solubilized NADPH-cytochrome c(cytochrome P-450) reductase purified by biospecific affinity chromatography. J. Biol. Chem. 1976;251:5337–5344. [PubMed] [Google Scholar]
- 30.Shen AL, Porter TD, Wilson TE, Kasper CB. Structural analysis of the FMN binding domain of NADPH-cytochrome P-450 oxidoreductase by site-directed mutagenesis. J Biol. Chem. 1989;264:7584–7589. [PubMed] [Google Scholar]
- 31.Koop DR, Coon MJ. Purification of liver microsomal cytochrome P-450 isozymes 3a and 6 from imidazole-treated rabbits. Evidence for the identity of isozyme 3a with the form obtained by ethanol treatment. Mol. Pharmacol. 1984;25:494–501. [PubMed] [Google Scholar]
- 32.Sequeira DJ, Eyer CS, Cawley GF, Nick TG, Backes WL. Ethylbenzene-mediated induction of cytochrome P450 isozymes in male and female rats. Biochem. Pharmacol. 1992;44:1171–1182. doi: 10.1016/0006-2952(92)90382-s. [DOI] [PubMed] [Google Scholar]
- 33.Hildebrandt AG, Roots I, Tjoe M, Heinemeyer G. Hydrogen peroxide in hepatic microsomes. Methods Enzymol. 1978;52:342–350. doi: 10.1016/s0076-6879(78)52037-5. [DOI] [PubMed] [Google Scholar]
- 34.Nakajima T, Wang RS, Elovaara E, Park SS, Gelboin HV, Hietanen E, Vainio H. Monoclonal antibody-directed characterization of cytochrome P450 isozymes responsible for toluene metabolism in rat liver. Biochem. Pharmacol. 1991;41:395–404. doi: 10.1016/0006-2952(91)90536-e. [DOI] [PubMed] [Google Scholar]
- 35.Cawley GF, Batie CJ, Backes WL. Substrate-dependent competition of different P450 isozymes for limiting NADPH-cytochrome P450 reductase. Biochem. 1995;34:1244–1247. doi: 10.1021/bi00004a018. [DOI] [PubMed] [Google Scholar]
- 36.Kuthan H, Ullrich V, Estabrook RW. A quantitative test for superoxide radicals produced in biological systems. Biochem. J. 1982;203:551–558. doi: 10.1042/bj2030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorsky LD, Koop DR, Coon MJ. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. Products of oxygen reduction. J. Biol. Chem. 1984;259:6812–6817. [PubMed] [Google Scholar]
- 38.Gut J, Richter C, Cherry RJ, Winterhalter KH, Kawato S. Rotation of cytochrome P-450. Complex formation of cytochrome P-450 with NADPH-cytochrome P-450 reductase in liposomes demonstrated by combining protein rotation with antibody-induced cross-linking. J. Biol. Chem. 1983;258:8588–8594. [PubMed] [Google Scholar]
- 39.Davydov DR, Petushkova NA, Bobrovnikova EV, Knyushko TV, Dansette P. Association of cytochromes P450 1A2 and 2B4: are the interactions between different P450 species involved in the control of the monooxygenase activity and coupling? Adv. Exp. Med. Biol. 2001;500:335–338. doi: 10.1007/978-1-4615-0667-6_53. [DOI] [PubMed] [Google Scholar]
- 40.Hazai E, Kupfer D. Interactions between CYP2C9 and CYP2C19 in reconstituted binary systems influence their catalytic activity: possible rationale for the inability of CYP2C19 to catalyze methoxychlor demethylation in human liver microsomes. Drug Metab Dispos. 2005;33:157–164. doi: 10.1124/dmd.104.001578. [DOI] [PubMed] [Google Scholar]
- 41.White RE, McCarthy MB. Active site mechanics of liver microsomal cytochrome P-450. Arch. Biochem. Biophys. 1986;246:19–32. doi: 10.1016/0003-9861(86)90445-5. [DOI] [PubMed] [Google Scholar]
- 42.Causey KM, Eyer CS, Backes WL. Dual role of phospholipid in the reconstitution of cytochrome P- 450 LM2-dependent activities. Mol. Pharmacol. 1990;38:134–142. [PubMed] [Google Scholar]
- 43.Backes WL, Canady WJ. Methods for the evaluation of hydrophobic substrate binding to cytochrome P-450. Pharmacol. Ther. 1981;12:133–158. doi: 10.1016/0163-7258(81)90078-4. [DOI] [PubMed] [Google Scholar]
- 44.Zangar RC, Davydov DR, Verma S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol. Appl. Pharmacol. 2004;199:316–331. doi: 10.1016/j.taap.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 45.Davydov DR. Microsomal monooxygenase as a multienzyme system: the role of P450-P450 interactions. Expert Opin. Drug Metab. Toxicol. 2011;7:543–558. doi: 10.1517/17425255.2011.562194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cawley GF, Zhang S, Kelley RW, Backes WL. Evidence supporting the interaction of CYP2B4 and CYP1A2 in microsomal preparations. Drug Metab Dispos. 2001;29:1529–1534. [PubMed] [Google Scholar]
- 47.Yun CH, Miller GP, Guengerich FP. Rate-determining steps in phenacetin oxidations by human cytochrome P450 1A2 and selected mutants. Biochem. 2000;39:11319–11329. doi: 10.1021/bi000869u. [DOI] [PubMed] [Google Scholar]
- 48.Guengerich FP, Krauser JA, Johnson WW. Rate-limiting steps in oxidations catalyzed by rabbit cytochrome P450 1A2. Biochem. 2004;43:10775–10788. doi: 10.1021/bi0491393. [DOI] [PubMed] [Google Scholar]
- 49.Hall DA, Vander Kooi CW, Stasik CN, Stevens SY, Zuiderweg ER, Matthews RG. Mapping the interactions between flavodoxin and its physiological partners flavodoxin reductase and cobalamin-dependent methionine synthase. Proc. Natl. Acad. Sci. U. S A. 2001;98:9521–9526. doi: 10.1073/pnas.171168898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellis J, Gutierrez A, Barsukov IL, Huang WC, Grossmann JG, Roberts GC. Domain motion in cytochrome P450 reductase: conformational equilibria revealed by NMR and small-angle x-ray scattering. J. Biol. Chem. 2009;284:36628–36637. doi: 10.1074/jbc.M109.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xia C, Hamdane D, Shen AL, Choi V, Kasper CB, Pearl NM, Zhang H, Im SC, Waskell L, Kim JJ. Conformational changes of NADPH-cytochrome P450 oxidoreductase are essential for catalysis and cofactor binding. J. Biol. Chem. 2011;286:16246–16260. doi: 10.1074/jbc.M111.230532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reed JR, Hollenberg PF. Examining the mechanism of stimulation of cytochrome P450 by cytochrome b5: the effect of cytochrome b5 on the interaction between cytochrome P450 2B4 and P450 reductase. J Inorg. Biochem. 2003;97:265–275. doi: 10.1016/s0162-0134(03)00275-7. [DOI] [PubMed] [Google Scholar]
- 53.Scott EE, He YA, Wester MR, White MA, Chin CC, Halpert JR, Johnson EF, Stout CD. An open conformation of mammalian cytochrome P450 2B4 at 1.6-A resolution. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13196–13201. doi: 10.1073/pnas.2133986100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cosme J, Johnson EF. Engineering microsomal cytochrome P450 2C5 to be a soluble, monomeric enzyme. Mutations that alter aggregation, phospholipid dependence of catalysis, and membrane binding. J. Biol. Chem. 2000;275:2545–2553. doi: 10.1074/jbc.275.4.2545. [DOI] [PubMed] [Google Scholar]
- 55.Schoch GA, Yano JK, Wester MR, Griffin KJ, Stout D, Johnson EF. Structure of Human Microsomal Cytochrome P450 2C8: evidence for a peripheral fatty acid binding site. J. Biol. Chem. 2004;279:9497–9503. doi: 10.1074/jbc.M312516200. [DOI] [PubMed] [Google Scholar]
- 56.Hu G, Johnson EF, Kemper B. CYP2C8 exists as a dimer in natural membranes. Drug Metab. Dispos. 2010;38:1976–1983. doi: 10.1124/dmd.110.034942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sansen S, Yano JK, Reynald RL, Schoch GA, Griffin KJ, Stout CD, Johnson EF. Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. J. Biol. Chem. 2007;282:14348–14355. doi: 10.1074/jbc.M611692200. [DOI] [PubMed] [Google Scholar]