Our current study results suggest that a nonlinear relationship exists between exposure to heading during the previous year, as measured by the Einstein Heading Questionnaire, and both abnormal white matter microstructure and poorer neurocognitive performance on a memory test.
Abstract
Purpose:
To investigate the association of soccer heading with subclinical evidence of traumatic brain injury.
Materials and Methods:
With institutional review board approval and compliance with HIPAA guidelines, 37 amateur soccer players (mean age, 30.9 years; 78% [29] men, 22% [eight] women) gave written informed consent and completed a questionnaire to quantify heading in the prior 12 months and lifetime concussions. Diffusion-tensor magnetic resonance (MR) imaging at 3.0 T was performed (32 directions; b value, 800 sec/mm2; 2 × 2 × 2-mm voxels). Cognitive function was measured by using a computerized battery of tests. Voxelwise linear regression (heading vs fractional anisotropy [FA]) was applied to identify significant regional associations. FA at each location and cognition were tested for a nonlinear relationship to heading by using an inverse logit model that incorporated demographic covariates and history of concussion.
Results:
Participants had headed 32–5400 times (median, 432 times) over the previous year. Heading was associated with lower FA at three locations in temporo-occipital white matter with a threshold that varied according to location (885–1550 headings per year) (P < .00001). Lower levels of FA were also associated with poorer memory scores (P < .00001), with a threshold of 1800 headings per year. Lifetime concussion history and demographic features were not significantly associated with either FA or cognitive performance.
Conclusion:
Heading is associated with abnormal white matter microstructure and with poorer neurocognitive performance. This relationship is not explained by a history of concussion.
© RSNA, 2013
Introduction
The adverse consequences of repeated sports-related head trauma have been known since the description of dementia pugilistica (1), now known as chronic traumatic encephalopathy. Head injury is recognized in players of many sports (2) including soccer, as in the famous case of British player Jeffrey Astle (3). Although the media, as well as scientific studies, are largely focused on the effects of multiple concussions, the role of “subconcussive” impacts in contact sports is garnering interest as an additional mechanism of cumulative brain injury (4,5).
Soccer play commonly entails “heading,” an offensive or defensive move whereby the player’s unprotected head is used to deliberately impact the ball and direct it during play. Players head the ball, on average, 6–12 times during competitive games, where balls travel at high velocities (up to 80 km per hour or more in nonprofessional matches [6]). During practice sessions, however, heading drills, where the ball, often tossed at low velocity, is headed repeatedly (up to 30 or more closely spaced repetitions), are commonplace (6). Because soccer represents the world’s most popular sport, with more than 265 million active players worldwide, and heading is a common practice, soccer is thus a very common cause of repetitive minor head injury. While heading may be a cause of concussion and postconcussion symptoms, studies indicate that this is not common. The clinical significance of subconcussive heading as a cause of brain injury, however, remains controversial and largely unexplored.
An Institute of Medicine report (7) and medical guidelines (8) note the absence of definitive evidence that soccer heading causes traumatic brain injury (TBI) but acknowledge concern for potential risks. Soccer organizations acknowledge concussion risks but assert heading is not a significant cause of concussion. A recent review noted that “it is impossible to isolate the long-term effects of heading alone [vs. concussion]” (6). Neuropsychological impairment, reported in amateur (9), professional (10), and high school players (11,12), has engendered debate on the role of concussion versus subconcussive heading. A recent research letter attributes brain microstructural differences between a small group of elite soccer players and swimmers to heading (13), but heading has not been directly studied as a predictor of TBI, independent of recognized concussion. Notwithstanding the importance of overt head injuries, heading itself entails repeated impact, acceleration-deceleration of the brain, and, perhaps depending on technique, rotation of the brain. Furthermore, cumulative effects of repetitive minor injury may not manifest for many years, as in chronic traumatic encephalopathy (6). Thus, pathologic evidence of TBI, if detectable, is likely present prior to onset of overt symptoms or disability.
Heading entails repetitive low-level injury; effects may depend on the rate of exposures, the time between exposures, and the vulnerability of individual players. We measured subclinical TBI with diffusion-tensor imaging, a magnetic resonance (MR) imaging measurement that reflects traumatic axonal injury, such as in the review by Niogi and Mukherjee (14), and we measured functional manifestations with neurocognitive tests. We hypothesized that a threshold for heading exposure would be detectable, above which its association with imaging and neurocognitive abnormalities would increase. If this hypothesis were confirmed, defining the heading threshold above which TBI risk increases would have considerable public health importance. Thus, the purpose of this study was to investigate the association of soccer heading with subclinical evidence of TBI.
Materials and Methods
Subjects
The study was reviewed and approved by the local institutional review board and complied with the Health Insurance Portability and Accountability Act. Written informed consent was obtained. Thirty-nine amateur soccer players were recruited from adult amateur leagues in New York City between June and September 2010. Recruitment was accomplished by distributing informational flyers through soccer leagues and at amateur soccer competitions. Recruitment publicity information did not indicate that the study was designed to assess brain injury or cognitive impairment. The first 39 subjects who agreed to participate and met inclusion criteria were enrolled in the study. Subjects completed a medical history and underwent the Mini-International Neuropsychiatric Interview (15) to exclude subjects with psychiatric disorders, including substance use disorders. Smoking was assessed by asking subjects the following question: Have you smoked at least 100 cigarettes in your lifetime and, if yes, do you currently smoke? Handedness was determined by using the Edinburgh Handedness Inventory (16). No subjects were excluded from enrollment because of medical, psychiatric or other criteria. Two subjects were excluded after enrollment, one because of claustrophobia and one because of severe image artifacts. The final study group included 37 subjects (median age, 30 years; 75% [28] men, 25% [nine] women). Subject characteristics were compared by using the Fisher exact test or one-way analysis of variance across subgroups of subjects defined by heading exposure (low, medium, and high).
Exposure Variables
Thirty-seven subjects meeting inclusion and exclusion criteria (Table 1) completed the Einstein Heading Questionnaire (EHQ) (Table 2). The EHQ is a structured questionnaire from which we estimated heading during the prior 12 months, which served as the primary exposure variable of interest. The EHQ also ascertains demographics and lifetime all-cause concussion history. To ascertain concussion history, subjects were asked a series of questions regarding any prior head trauma for which they sought, received, or were recommended to seek medical evaluation or care. A random subsample of 13 subjects participated in a test-retest reliability study of the EHQ. These subjects completed the exposure assessment twice, with a 2-week interval between assessments. Reliability was assessed with the intraclass correlation coefficient.
Table 1.
Enrollment Criteria
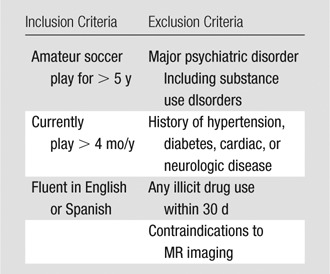
Table 2.
Heading Exposure Assessment Questionnaire
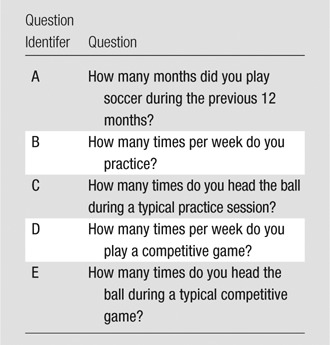
Note.—The prior 12 months of exposure was computed as follows: A ⋅ 4[(B ⋅ C) + (D ⋅ E)], where A, B, C, D, and E are the corresponding question identifiers, respectively.
Cognitive Outcome Variables
Neuropsychological assessment of the primary cognitive outcomes was obtained with a cognitive function test (CogState; CogState, New Haven, Conn), a valid and reliable comprehensive computer-administered battery of tests (17,18). Variables of interest for this study were scores on tests of psychomotor speed, attention, executive function, and memory. Cognitive assessments were configured and supervised by a licensed neuropsychologist (M.E.Z., with 16 years of neuropsychological testing experience).
Imaging Outcome Variables
Whole-brain MR imaging was performed (M.L.L., with 21 years of MR imaging experience) with a 32-channel 3.0-T MR unit (Achieva TX; Philips Medical Systems, Best, the Netherlands) and a 32-channel head coil (Philips Medical Systems). T1-weighted magnetization-prepared rapid acquisition gradient-echo imaging (repetition time msec/echo time msec/inversion time msec, 9.8/4.6/1431; flip angle, 8°; section thickness, 1 mm; matrix, 240 × 187; field of view, 240 × 187; number of signals acquired, one), T2-weighted turbo spin-echo imaging (repetition time msec/[effective] echo time msec, 4000/100; section thickness, 2 mm; matrix, 392 × 384; field of view, 244 × 244; number of signals acquired, one), T2-weighted fluid-attenuated inversion-recovery imaging (11 000/120 [effective]/2800; section thickness, 2 mm; matrix, 352 × 206; field of view, 240 × 240; number of signals acquired, one), and susceptibility-weighted imaging (16/23; flip angle, 15°; section thickness, 1 mm; matrix, 268 × 210; field of view, 240 × 190; number of signals acquired, one) sequences were performed. A board-certified neuroradiologist (M.L.L., with a Certificate of Added Qualification in neuroradiology and 21 years of clinical neuroradiology experience) reviewed these images to detect structural abnormalities and evidence of prior trauma, including microhemorrhage. Diffusion-tensor imaging with single-shot echo-planar imaging (echo time, 51 msec; flip angle, 90°; section thickness, 2 mm; matrix, 128 × 128; field of view, 256 × 256; 32 diffusion directions; b value, 800 sec/mm2) was performed.
Image Processing
After correction for head motion and eddy current effects, fractional anisotropy (FA), the primary imaging outcome, was derived from a tensor model at each voxel, by using software (Oxford Center for Functional Magnetic Resonance Imaging of the Brain Diffusion Toolbox, Oxford, United Kingdom) (19). FA images thus derived were coregistered to the Montreal Neurological Institute brain template by using a multistep procedure that incorporates distortion correction and both linear within-subject and nonlinear across-subject transformations, as described previously (20). Image processing was supervised by one author (M.L.L.), who had 21 years of image processing experience.
Identification of Regions of Interest
We sought to identify a nonlinear association of heading exposure and FA. Specifically, we investigated whether an inverse S shape (ie, after an initial baseline, values decline after some level of exposure) would explain the data. Our approach was also not to restrict the analysis to a priori regions of interest (ROIs). Because the nonlinear regression model is computationally intensive, it would be impractical to implement on a voxelwise basis across the approximately 6 × 106 voxels that comprise the brain volume. Therefore, on the basis of our expectation that at least a weak linear association between heading exposure and FA would be present at locations where the nonlinear association is significant, we performed an initial whole-brain voxelwise linear regression analysis with statistical parametric mapping (SPM 8, Functional Imaging Laboratory, University College London, London, England; Matlab, MathWorks, Natick, Mass) (21) to identify these locations, to which the subsequent nonlinear analysis was restricted. Thus, significant voxels identified during the initial whole-brain linear regression analysis formed the ROIs on which the final nonlinear analysis was subsequently performed. Two subjects were excluded for the purposes of this initial linear analysis, as their exposures (minimum, 32; maximum, 5400) resulted in extreme skewness that could not be resolved through transformation. We used somewhat liberal thresholds for the significance of each individual voxel in the linear analysis but applied a stricter threshold for cluster size to constrain false discoveries (voxelwise, 2.5% [two tailed, uncorrected]; clusterwise, 5% [corrected]). Correction for multiple comparisons was made by using familywise error rate correction, as implemented in SPM 8.
Data Analysis
Two biostatisticians (N.K. and M.K., with 13 and 22 years of experience, respectively) performed statistical analyses. To address our hypothesis, we fit a nonlinear curve (S or inverse S curve) to FA at each ROI (defined as in the previous section, above) and separately to each cognitive performance score as a function of heading, while controlling for demographic variables and concussion history (reported as the number of lifetime concussions). The inverse logit function in the Equation below has been applied to describe curves with an S or inverse S shape (22–24). The inverse logit function was fitted separately for each outcome measure (FA from each ROI and each cognitive score). Regression parameters included y-intercept (β0), scale and direction (β1), the drop-off point (β2), and the slope of the exponential curve (β3), along with linear terms for demographic variables (age [A], sex [G], years of education [E]) and history of concussion, denoted C (α1–α4). Only significant covariates among three demographic variables and history of concussion were included in the final model for each outcome measure, as shown in the Equation. The Y represents outcome measure (FA or heading), i is subject identity, β2 represents the threshold for heading exposure (H), and ERR is error. The nonlinear model was implemented by using the Statistics Toolbox (Matlab, version 2010a; MathWorks). To reduce the influence of outliers (extreme exposure), we used a robust fit algorithm (25), iteratively fitting the nonlinear regression with adjusted weights for outliers. The Equation is rendered thus:
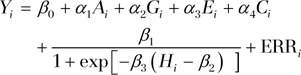 |
Results
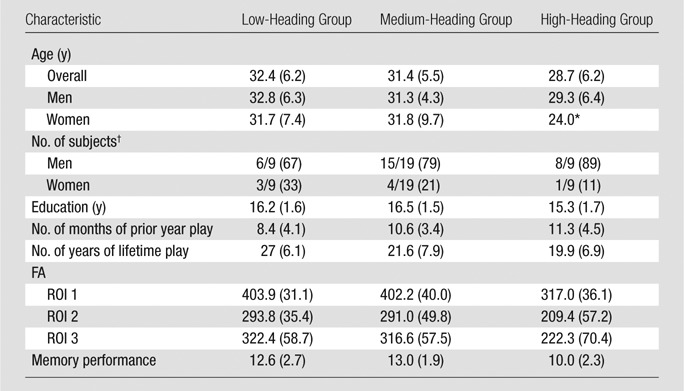
Players were mostly male (n = 29 [78%] vs n = 8 [22%] for female players). Mean age for male subjects was 30.9 years (range, 21–40 years), and the mean age for female players was 30.8 years (range, 24–44 years). The mean age of the entire sample was 30.9 years (range, 21–44 years). The age distributions of male and female subgroups were not significantly different (two-group t test, P = .932). Participants reported having played soccer for an average of 22.4 years and reported having played an average of 10.2 months (range, 4–12 months) during the past year. Demographic details are presented in Table 3.
Table 3.
Subject and ROI Characteristics
Note.—Data are means, and numbers in parentheses are standard deviations except where otherwise indicated.
Only one subject was in this subgroup and no mean was available.
Numbers in parentheses are percentages, and percentages were rounded.
Thirteen subjects participated in the test-retest reliability substudy of the EHQ during 2 weeks. The intraclass correlation coefficient for the EHQ was greater than 0.8. With the use of the EHQ, we estimated the number of heading events per participant during the previous year and found values that ranged from 32 to 5400 (median, 432; interquartile range, 276–1095). We classified the sample in low (≤276 headings per year), medium (277–1095 headings per year), and high (≥1096 headings per year) exposure groups, with nine, 19, and nine subjects, respectively, in each group, to assess the relationship of heading and subject characteristics. These groups did not differ with respect to age (one-way analysis of variance, P = .368), sex (Fisher exact test, P = .675), years of education (one-way analysis of variance, P = .18), handedness (Fisher exact test, P = .314), current or lifetime smoking (Fisher exact test, P = .281 [current] or P = .566 [lifetime]), or concussion (Fisher exact test, P = .38). Higher levels of heading were associated with more months of play during the previous year.
An American Board of Radiology–certified neuroradiologist (M.L.L.) reviewed all brain images. No structural abnormalities or hemorrhage were identified. The linear regression of heading in the prior 12 months and covariates (age, sex, education, history of concussion) on FA identified three ROIs (Fig 1), with significantly lower FA as a function of greater heading exposure, but not explained by the covariates (Table 3).
Figure 1a:
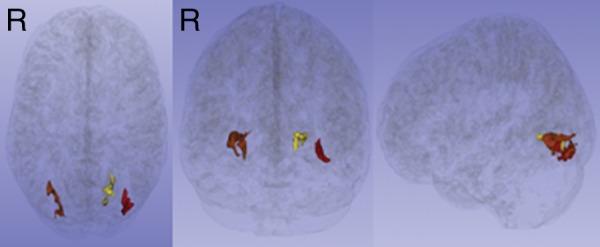
(a, b) Three ROIs in the temporo-occipital white matter detected by the initial voxelwise linear regression of estimated prior 12 months of heading on FA, shown as color regions rendered in three-dimensional images (a) and superimposed on T1-weighted axial (left), coronal (middle), and sagittal (right) images from the Montreal Neurological Institute template (b). FA at each ROI was significantly lower as a function of greater heading exposure. R = right.
Figure 1b:
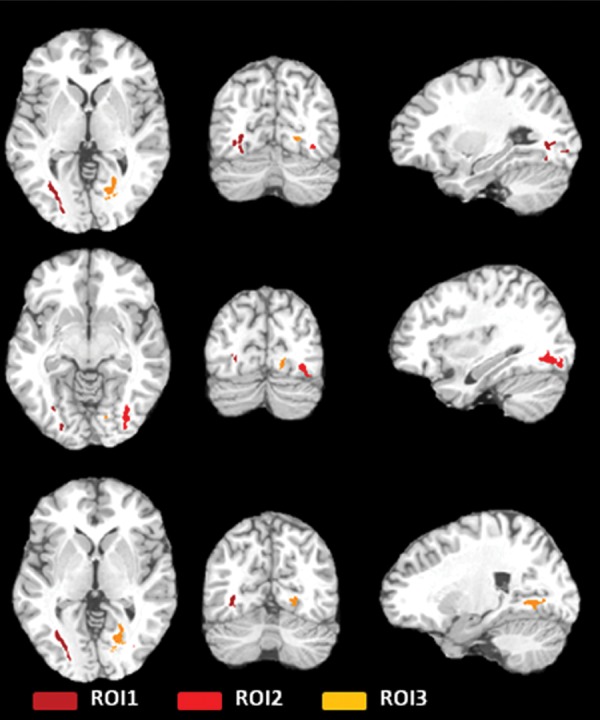
(a, b) Three ROIs in the temporo-occipital white matter detected by the initial voxelwise linear regression of estimated prior 12 months of heading on FA, shown as color regions rendered in three-dimensional images (a) and superimposed on T1-weighted axial (left), coronal (middle), and sagittal (right) images from the Montreal Neurological Institute template (b). FA at each ROI was significantly lower as a function of greater heading exposure. R = right.
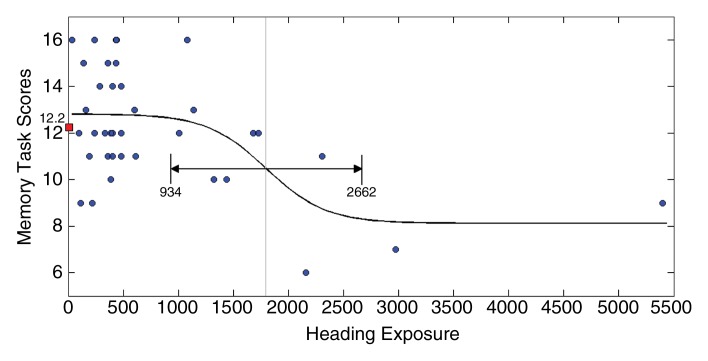
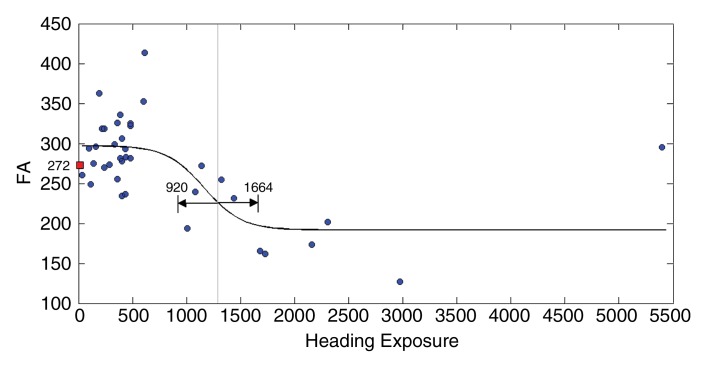
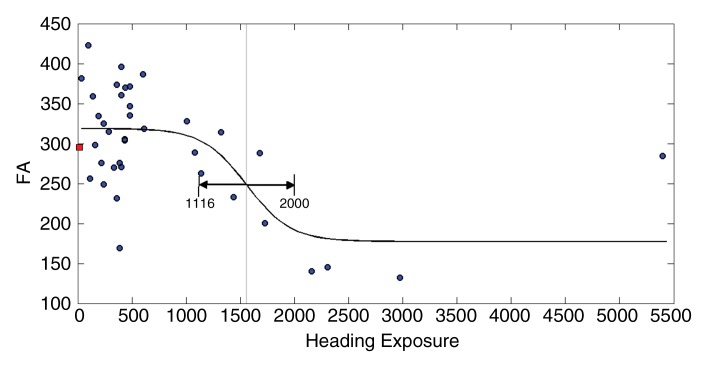
Fits to the nonlinear inverse logit model were significant (P < .00001) and revealed an inverse S relationship between heading and FA at each ROI (Fig 2), with a threshold value (β2) that varied from 885 to 1550, depending on the ROI location. Similarly, the relationship between heading and memory function fit the inverse logit model significantly (P < .00001). The threshold, however, was nearly 1800 headings per year for memory, higher than all thresholds for FA (Fig 2d). Other cognitive measures were not significantly associated with heading exposure. No significant association of any covariate (age, sex, education, or history of concussion) was found in relationship to either FA or the cognitive measures. Therefore, the nonlinear inverse logit model for each of the outcome measures (Equation) includes only four parameters (β0–β3). These parameters are shown in Table 4.
Figure 2a:
(a–c) Inverse logit fits for mean FA values from each of the three ROIs (Fig 1) and (d) for memory versus heading exposure. Observed values and the fitted inverse logit function are expressed in blue circles and black curves, respectively. Light-gray line = estimated threshold (β2) and its 95% confidence interval = black line with double-sided arrows, red box = mean FA and memory performance values.
Figure 2d:
(a–c) Inverse logit fits for mean FA values from each of the three ROIs (Fig 1) and (d) for memory versus heading exposure. Observed values and the fitted inverse logit function are expressed in blue circles and black curves, respectively. Light-gray line = estimated threshold (β2) and its 95% confidence interval = black line with double-sided arrows, red box = mean FA and memory performance values.
Table 4.
Results of the Nonlinear Analysis of FA, Memory, and Heading
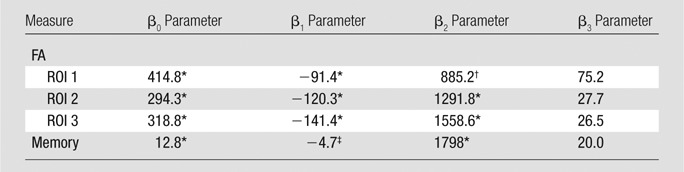
Note.—No significant effect of any covariate (age, sex, years of education, prior concussion) was found for either the cognitive measure or FA.
P < .001.
P < .01.
P < .05.
Figure 2b:
(a–c) Inverse logit fits for mean FA values from each of the three ROIs (Fig 1) and (d) for memory versus heading exposure. Observed values and the fitted inverse logit function are expressed in blue circles and black curves, respectively. Light-gray line = estimated threshold (β2) and its 95% confidence interval = black line with double-sided arrows, red box = mean FA and memory performance values.
Figure 2c:
(a–c) Inverse logit fits for mean FA values from each of the three ROIs (Fig 1) and (d) for memory versus heading exposure. Observed values and the fitted inverse logit function are expressed in blue circles and black curves, respectively. Light-gray line = estimated threshold (β2) and its 95% confidence interval = black line with double-sided arrows, red box = mean FA and memory performance values.
Discussion
Our current study results suggest that a nonlinear relationship exists between exposure to heading during the previous year, as measured by the EHQ, and both abnormal white matter microstructure and poorer neurocognitive performance on a test of memory. Notably, these findings were not significantly associated with prior concussion, yet they are consistent with findings seen in patients with TBI, as indicated in a study by Palacios et al (26). The relationships detected in this current study between heading and both imaging and cognitive outcomes provide some intriguing clues to pathophysiologic mechanisms. It is well established that most patients with mild TBI recover fully over time, indicating that intrinsic mechanisms are generally able to effect repair of low-level injury, as reviewed by Povlishok and Katz (27). Increasing heading exposure in this study, up to a threshold (β2), without associated imaging or cognitive changes, may indicate a range of exposure within which these intrinsic injury repair mechanisms are effective. The appearance of significant associations of imaging and cognitive changes with heading above the threshold, on the other hand, suggests that these repair mechanisms may be unable to keep pace with the cumulative injury that occurs beyond this degree of exposure.
One unexpected feature of our current results is the location of the brain abnormalities opposite the presumed point of heading impact on the forehead. We interpret this as analogous to the phenomenon of contrecoup injury, which occurs opposite the site of impact in contusional brain injury. Of note, the spatial extent and severity of contrecoup contusion injury is typically greater than that at the site of impact (coup injury). Thus, the chance of detecting a subtle degree of injury may in fact be greater opposite the impact location. Another consideration is that heading technique is not necessarily uniform, and variability in impact site is probably common. It is also possible that the imaging abnormalities we have detected index significant injury in general, though it is injury at other locations, too subtle to detect in this small sample, that is the specific cause of the memory changes.
We detected a lower heading threshold for imaging measures compared with the threshold for worse memory function. The imaging measures reveal pathologic changes in tissue (low FA) that would be expected to precede functional manifestations (cognition) related to tissue changes. These disparate thresholds could indicate differential lead time to the evolution of pathologic and clinical manifestations following injury, a widely recognized epidemiologic phenomenon. Virtually all subjects with exposures exceeding the threshold (β2) levels had low FA, suggesting that heading above some defined level may be generally unsafe. The important obverse of this finding is that exposure below the threshold level may be generally safe. Last, although subjects with exposure below the threshold (β2) identified in this study generally had FA and cognitive performance above the group mean, we did detect a subset of individuals with FA and cognitive function below the group mean, despite exposure well below the threshold (β2). This pattern suggests that, although exposure below a threshold may be generally safe, some individuals may be particularly sensitive to the effect of subconcussive heading and at higher risk for brain injury and adverse cognitive outcomes after even modest exposure.
The current study findings add to our understanding of the effects of heading on brain structure and function beyond the recognized role of heading as a relatively uncommon cause of concussion. Researchers in previous studies who have reported on concussion and/or cognitive impairment in soccer players at various skill levels have only addressed heading insofar as it is one of many potential causes of concussion in soccer. Additional limitations of prior studies of concussion in soccer are that they have generally not quantified subconcussive heading exposure and have not used quantitative neuroimaging. On the basis of these published studies, safety guidelines have focused on mitigating concussion caused by many potential types of impact, such as those detailed by Koutures and Gregory (8). Nonetheless, these guidelines have noted concern in regard to the independent risk of heading and note the lack of data addressing heading itself. In one recent study (13), researchers used diffusion-tensor imaging to compare a small number of elite soccer players with no history of concussion with a control group of competitive swimmers, finding low anisotropy in the soccer group and attributing the finding to heading. However, this study did not quantify exposure to heading or assess functional outcomes. To our knowledge, our current study is thus the first to quantify subconcussive heading as an exposure and to assess the association of heading with imaging evidence of brain pathologic findings and neurocognitive function.
An important limitation of this study is its cross-sectional nature, which does not allow us to specifically infer a causal relationship between heading and imaging or functional changes. Although the fits of our data to the nonlinear model used herein were highly significant, such models are most robust with large samples. Further confirmation of these findings in a larger sample is thus important. Factors that contribute heterogeneity to our sample, such as the inclusion of men and women, could be considered a limitation but also reflect the expected prevalence of soccer play among these demographics and, if at all, would be likely to dampen the strength of our associations rather than create a systematic bias. Assessing heading exposure is a complex undertaking, particularly in light of the consideration that cumulative lifetime exposure is likely most important, as suggested by our emerging understanding of long-term effects of concussive brain injury such as chronic traumatic encephalopathy. We validated and used a prior 12-month self-reporting measure for this study, considering recall over this time frame, in the context of total years of play, as a proxy for lifetime exposure. A limitation of any self-reporting measure is that the subject’s ability to recall the number of heading events over the long term is potentially subject to bias. Although heading could be quantified prospectively, such an approach would be only a partial assessment of lifetime exposure and would thus not address the role of lifetime (or even relatively recent) cumulative exposure, as players typically begin heading as children. Another limitation is that we were not able to characterize features of individual heading events, such as site of impact, velocity, rotation, and other features. It is of course likely that heading events vary greatly, but these features could only be captured with prospective use of biomechanical instrumentation. Such an approach, which was beyond the scope of this study, would, however, also be affected by the limitation on assessment of cumulative lifetime exposure described above. Our findings are based on analysis of FA and not of other diffusion parameters. We chose this approach because FA is the parameter that has been most widely studied in TBI and, particularly, in association with cognitive outcomes in TBI patients. Investigation of other diffusion measures may shed additional light on the underlying pathologic mechanisms of the effects we detect and is an area of opportunity for future study. We do not, however, expect that the basic associations we reported here would be altered.
Further research is required to confirm and characterize threshold effects and possible modifiers that are applicable to specific settings and populations. Characterizing the dose-response curve linking heading and TBI toward understanding threshold effects might facilitate safety guidelines that could help minimize the risk of adverse effects on the brain. Prospective monitoring of exposure at the team level, perhaps to be termed head counts, could identify a point at which a player’s heading should be curtailed for a specific recovery period, also to be defined through further research. Such an approach mirrors pitch counts that have mitigated upper extremity injury due to excess exposure in youth baseball (28). Further study is also warranted to identify risk factors, which could become the foundation for public health interventions designed to prevent exposure in vulnerable individuals. Were a genetic variant such as the apolipoprotein ε4 allele, or ApoE4, for example, shown to confer greater risk for heading-related TBI, individuals could be screened and those with apolipoprotein ε4 allele advised not to head. Further research is thus essential to develop evidence-based protective strategies that can ensure the future of safe soccer play.
Advances in Knowledge.
• High frequency of soccer heading (>885–1800 headings per year) in otherwise healthy adult amateur players is associated with lower white matter fractional anisotropy (FA) (P < .00001) and worse memory performance (P < .00001) than in players who performed less heading.
• Heading alone is associated with both low FA and worse memory; whether players do or do not have history of one or more concussions does not explain differences in FA and memory among players (P = .38).
• The associations between heading and diminished FA, as well as between heading and memory, are nonlinear, with evidence of a threshold dose-response relationship.
Implication for Patient Care.
• Repetitive subconcussive head trauma in the setting of heading during soccer may be associated with white matter microstructural and neurocognitive changes similar to those seen in patients with traumatic brain injury.
Disclosures of Conflicts of Interest: M.L.L. No relevant conflicts of interest to disclose. N.K. No relevant conflicts of interest to disclose. M.E.Z. No relevant conflicts of interest to disclose. M.K. No relevant conflicts of interest to disclose. W.F.S. No relevant conflicts of interest to disclose. C.A.B. No relevant conflicts of interesst to disclose. R.B.L. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: receives research support from the National Headache Foundation, and the Migraine Research Fund; serves on the editorial boards of Neurology and Cephalalgia and as senior advisor to Headache; has reviewed for the National Institute on Aging and National Institute of Neurological Disorders and Stroke; holds stock options in eNeura Therapeutics (a company without commercial products); serves as consultant, advisory board member, or has received honoraria from Allergan, American Headache Society, Autonomic Technologies, Boehringer-Ingelheim Pharmaceuticals, Boston Scientific, Bristol Myers Squibb, Cognimed, Colucid, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, MAP, Merck, Nautilus Neuroscience, Novartis, NuPathe, Vedanta, and Zogenix. Other relationships: none to disclose.
Acknowledgments
The authors acknowledge the essential contributions of Nina Ackerman, BA, Albert Einstein College of Medicine of Yeshiva University, Bronx, NY, to subject recruitment and assessment.
Received March 2, 2013; revision requested April 22; final revision received May 7; accepted May 10; final version accepted May 14.
From the 2011 RSNA Annual Meeting.
R.B.L. supported by National Institutes of Health grants PO1 AG03949, PO1AG027734, RO1AG025119, RO1AG022374-06A2, RO1AG034119, RO1AG12101, K23AG030857, K23NS05140901A1, and K23NS47256. Supported by a grant from the Dana Foundation David Mahoney Neuroimaging Program.
Funding: This research was supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (grant R01 NS082432).
Abbreviations:
- EHQ
- Einstein Heading Questionnaire
- FA
- fractional anisotropy
- ROI
- region of interest
- TBI
- traumatic brain injury
References
- 1.Martland HS. Punch drunk. JAMA 1928;91(15):1103–1107 [Google Scholar]
- 2.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009;68(7):709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutherford A, Stephens R, Potter D. The neuropsychology of heading and head trauma in Association Football (soccer): a review. Neuropsychol Rev 2003;13(3):153–179 [DOI] [PubMed] [Google Scholar]
- 4.Spiotta AM, Shin JH, Bartsch AJ, Benzel EC. Subconcussive impact in sports: a new era of awareness. World Neurosurg 2011;75(2):175–178 [DOI] [PubMed] [Google Scholar]
- 5.Gysland SM, Mihalik JP, Register-Mihalik JK, Trulock SC, Shields EW, Guskiewicz KM. The relationship between subconcussive impacts and concussion history on clinical measures of neurologic function in collegiate football players. Ann Biomed Eng 2012;40(1):14–22 [DOI] [PubMed] [Google Scholar]
- 6.Spiotta AM, Bartsch AJ, Benzel EC. Heading in soccer: dangerous play? Neurosurgery 2012;70(1):1–11; discussion 11 [DOI] [PubMed] [Google Scholar]
- 7.Patlak M, Joy JE, Board on Neuroscience and Behavioral Health. Is soccer bad for children’s heads? summary of the IOM Workshop on Neuropsychological Consequences of Head Impact in Youth Soccer. Washington, DC: National Academies Press, 2002 [PubMed] [Google Scholar]
- 8.Koutures CG, Gregory AJ; American Academy of Pediatrics. Council on Sports Medicine and Fitness. Injuries in youth soccer. Pediatrics 2010;125(2):410–414 [DOI] [PubMed] [Google Scholar]
- 9.Matser EJT, Kessels AG, Lezak MD, Jordan BD, Troost J. Neuropsychological impairment in amateur soccer players. JAMA 1999;282(10):971–973 [DOI] [PubMed] [Google Scholar]
- 10.Matser JT, Kessels AG, Jordan BD, Lezak MD, Troost J. Chronic traumatic brain injury in professional soccer players. Neurology 1998;51(3):791–796 [DOI] [PubMed] [Google Scholar]
- 11.Stephens R, Rutherford A, Potter D, Fernie G. Neuropsychological impairment as a consequence of football (soccer) play and football heading: a preliminary analysis and report on school students (13-16 years). Child Neuropsychol 2005;11(6):513–526 [DOI] [PubMed] [Google Scholar]
- 12.Powell JW, Barber-Foss KD. Traumatic brain injury in high school athletes. JAMA 1999;282(10):958–963 [DOI] [PubMed] [Google Scholar]
- 13.Koerte IK, Ertl-Wagner B, Reiser M, Zafonte R, Shenton ME. White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA 2012;308(18):1859–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niogi SN, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil 2010;25(4):241–255 [DOI] [PubMed] [Google Scholar]
- 15.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(suppl 20):22–33; quiz 34–57 [PubMed] [Google Scholar]
- 16.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9(1):97–113 [DOI] [PubMed] [Google Scholar]
- 17.Collie A, Maruff P, Darby DG, McStephen M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. J Int Neuropsychol Soc 2003;9(3):419–428 [DOI] [PubMed] [Google Scholar]
- 18.Maruff P, Thomas E, Cysique L, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol 2009;24(2):165–178 [DOI] [PubMed] [Google Scholar]
- 19.Smith SM, Johansen-Berg H, Jenkinson M, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc 2007;2(3):499–503 [DOI] [PubMed] [Google Scholar]
- 20.Lipton ML, Gulko E, Zimmerman ME, et al. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology 2009;252(3):816–824 [DOI] [PubMed] [Google Scholar]
- 21.Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1994;2(4):189–210 [Google Scholar]
- 22.Fox J, Weisberg S. An R companion to applied regression. 2nd ed. Los Angeles, Calif: Sage, 2011 [Google Scholar]
- 23.Pinheiro J, Bates D. Mixed-effects models in S and S-PLUS. New York, NY: Springer, 2000 [Google Scholar]
- 24.Lindstrom ML, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics 1990;46(3):673–687 [PubMed] [Google Scholar]
- 25.Holland P, Welsch R. Robust regression using interactively reweighted least-squares. Commun Stat Theory Methods 1977;6(9):813–827 [Google Scholar]
- 26.Palacios EM, Sala-Llonch R, Junque C, et al. Long-term declarative memory deficits in diffuse TBI: correlations with cortical thickness, white matter integrity and hippocampal volume. Cortex 2013;49(3):646–657 [DOI] [PubMed] [Google Scholar]
- 27.Povlishok JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehab 2005;20(1):76–94 [DOI] [PubMed] [Google Scholar]
- 28.Kerut EK, Kerut DG, Fleisig GS, Andrews JR. Prevention of arm injury in youth baseball pitchers. J La State Med Soc 2008;160(2):95–98 [PubMed] [Google Scholar]