Abstract
Dendritic cells (DC) are crucial for the induction of immune responses and thus an inviting target for modulation by pathogens. We have previously shown that Plasmodium falciparum-infected erythrocytes inhibit the maturation of DCs. Intact P. falciparum-infected erythrocytes can bind directly to CD36 and indirectly to CD51. It is striking that these receptors, at least in part, also mediate the phagocytosis of apoptotic cells. Here we show that antibodies against CD36 or CD51, as well as exposure to early apoptotic cells, profoundly modulate DC maturation and function in response to inflammatory signals. Although modulated DCs still secrete tumor necrosis factor-α, they fail to activate T cells and now secrete IL-10. We therefore propose that intact P. falciparum-infected erythrocytes and apoptotic cells engage similar pathways regulating DC function. These findings may have important consequences for the treatment of malaria and may suggest strategies for modulating pathological immune responses in autoimmune diseases.
Adaptive immune responses are initiated by antigen-presenting cells, among which dendritic cells (DC) are crucial because they are most efficient in activating naïve T cells. Immature DCs reside in almost all tissues and continually sample antigens. Proinflammatory cytokines as well as bacterial products provide strong stimuli provoking their maturation and migration into the T-cell areas of draining lymph nodes and spleen. During maturation, the DCs cease phagocytosis and up-regulate the surface expression of costimulatory molecules, adhesion molecules, and stable HLA/peptide complexes that allow them to prime naïve and boost memory T cells (reviewed in ref. 1). Depending on the local cytokine environment and progression in their maturation, DCs can induce both Th1 and Th2 T-cell responses (2, 3). However, DCs also play an important role in the induction of CD8 + T-cell and B-cell function (4, 5).
Recent studies have shown that tissue injury provides an endogenous maturation signal for DCs. Necrotic cells derived from primary fibroblasts in mice, or from established cell lines in humans, stimulated DC maturation, whereas apoptotic cells did not (6, 7). On the basis of these and other studies, it has been hypothesized that DCs that have ingested apoptotic bodies in the absence of maturation stimuli might induce T-cell tolerance directly or transfer antigens to other bystander DCs (8, 9). If, however, DCs receive inflammatory signals while ingesting apoptotic cells, they might cross-present apoptotic cell-derived peptides within MHC class I molecules and so activate CD8 + T cells, promoting immune responses to the activating insult (10). Thus, depending on the local environment and the signals they receive, DCs seem to be pivotal not only for the induction of immune responses to invading pathogens but also for the regulation of harmful immune responses directed against environmental or self-antigens. However, the precise signals and pathways determining whether DCs activate or dampen immune responses remain elusive.
One route to understanding the regulation of DCs in the normal immune system may be through the mechanisms by which pathogens subvert their function. Plasmodium falciparum, in its intraerythrocytic stages, causes a wide spectrum of clinical disease. Clinically protective immune responses require repeated infections despite large amounts of circulating antigen during the acute phases of the disease (11, 12). P. falciparum-infected erythrocytes (iRBC) express a clonally variant protein (PfEMP-1) in the erythrocyte membrane that mediates binding to host cells (reviewed in ref. 13). Almost all variants of PfEMP1 analyzed so far bind to CD36 and/or thrombospondin (TSP), and individual variants may bind additionally to a variety of other host receptors such as CD31 [platelet-endothelial cell adhesion molecule (PECAM-1)], CD35 (complement receptor 1), CD51 (αv integrin chain), or CD54 [intercellular adhesion molecule-1 (ICAM-1)] (14–18). We have recently shown that intact iRBC that adhere to CD36 and/or TSP profoundly inhibit the maturation of DCs into fully costimulatory antigen-presenting cells (19). We were intrigued by this observation because CD36, and indirectly TSP, by bridging the binding of apoptotic cells to a receptor complex of CD36 and αvβ3, are involved in the recognition and uptake of apoptotic cells (reviewed in ref. 20). Together, these data suggested that iRBC mimic apoptotic cells. We now show that not only mAbs to CD36 and CD51 but also exposure to apoptotic cells can modulate DC maturation in response to inflammatory signals in a manner similar to iRBC. We therefore hypothesize that the engagement of CD36 or CD51 by apoptotic cells regulates DC maturation and function in the normal immune system.
Materials and Methods
Cell Culture.
Immature DCs were derived from peripheral blood cells by using standard procedures. Briefly, monocytes were cultivated either in RPMI medium 1640 supplemented with 2 mM glutamine/50 μg/ml of kanamycin/1% nonessential amino acids (GIBCO/BRL)/1% pooled human AB serum (National Blood Service)/50 ng/ml each of IL-4 (specific activity > 2 × 106 units/mg, PeproTech, Rocky Hill, NJ) and granulocyte macrophage–colony-stimulating factor (specific activity > 1 × 107 units/mg, Schering-Plough) for 6 days or in serum-free XVIVO-15 medium (BioWhittaker) supplemented with 50 μg/ml kanamycin and the cytokines listed above. At day six of culture, nonadherent immature DCs were harvested and depleted of contaminating lymphocytes with the aid of magnetic beads (Dynal, Great Neck, NY) and anti-CD3 and anti-CD19 mAb (Dako). P. falciparum-infected erythrocytes (cytoadherent line ITO/C24 and Malayan Camp) were maintained in group O RBC (National Blood Service) at a parasitemia of 2–10% in RPMI supplemented with 10% pooled human serum/2 mM glutamine/20 mM Hepes/2 mM hypoxanthine/20 mM glucose/10 μg/ml of gentamicin at 37°C under 95% N2, 1% O2, 4% CO2 (21). All cell cultures were tested at regular intervals for contamination with Mycoplasma spp. by using a PCR-based mycoplasma-detection kit (American Type Culture Collection) (22).
Induction of Apoptosis.
Apoptosis was induced by irradiation of autologous DCs, neutrophils, or monocytes with a calibrated UV lamp at a dose of 2,500 mJ/cm2 at a density of 1 × 106/ml in six-well plates in RPMI or X-VIVO15 medium supplemented as described above. In pilot studies, we monitored apoptosis serially after UV irradiation by staining with FITC-annexinV (AV) and propidium iodide (PI) according to the manufacturer's recommendations (Roche Diagnostics). Apoptotic cells binding FITC-AV but excluding PI were detectable 3 h after and secondary necrotic cells (AV + PI+) 6 h after UV irradiation. Absolute values varied slightly with the cell type used [for apoptotic DCs (mean % ±SD): 3 h: 17 ± 5 AV+, 0 ± 1 PI+; 6 h: 31 ± 7 AV+, 8 ± 5 PI+; 12 h: 82 ± 26 AV+, 13 ± 7 PI+]. After 24 h, all cells had undergone secondary necrosis and were AV+/PI+. Necrosis was induced by at least three cycles of rapid freezing at −70°C and thawing at 37°C. Thereafter, more than 90% of cells were permeable to trypan blue. Whereas these cells were cocultured with live autologous dendritic cells immediately, apoptotic cells were first cultured alone for 3 h after UV irradiation.
Maturation of DCs.
For maturation assays in the presence of modulating agents, 1 × 106 purified DCs were incubated in duplicate wells as described above with or without 25 μg/ml of isotype-control or test mAb or with apoptotic or necrotic cells or iRBC for at least 3 h at 37°C. Thereafter, DCs were matured as indicated with either 100 ng/ml of lipopolysaccharide (LPS) (Salmonella typhimurium, Sigma), 50 ng/ml of tumor necrosis factor (TNF)-α (PeproTech), 1 μg/ml of soluble chimeric CD40L (Alexis, San Diego, CA), or 50% monocyte-conditioned medium (MCM) (23) for 48 h or left untreated as a control. All maturation assays in the presence of apoptotic cells or iRBC were performed in parallel in RPMI (with serum) and X-VIVO15 medium (without serum), both supplemented with granulocyte macrophage–colony-stimulated factor and IL-4, but we found no differences in the results (data not shown). In some experiments, we included either 10 μg/ml of blocking anti-IL-10 or isotype control mAbs.
mAbs and Flow Cytometry.
The following mAbs directed against the respective human surface markers were used: CD3 clone OKT3, HLA A,B,C clone W6/32, CD14 clone Tük4, CD54 clone 6.5B5, CD19 clone HD37 (Dako); CD36 clone 89 (IgG1) or clone SMQ (IgM), CD80 clone BB1, CD40 clone LOB7/6, CD86 clone BU63, HLA-DR clone BF-1 (Serotec), CD83 clone HB15e (PharMingen). Staining of DCs was performed and analyzed by using a flow cytometer (Becton Dickinson), as described (19). Dead cells were excluded from analysis by using PI. We assayed maturation in the presence of the following azide-free mAbs: CD36 clone SMQ (IgM, Immunocontact, Frankfurt, Germany) and clone 89 (IgG1, Serotec, now discontinued), both of which inhibit binding of iRBC to purified CD36 and uptake of apoptotic cells by DC (ref. 24 and unpublished observations), CD51 clone 13C2 (IgG1, Immunocontact), which inhibits binding and uptake of apoptotic cells by DCs (ref. 25 and unpublished observations), HLA A,B,C, clone G46–2.6 (IgG1, PharMingen) binding to a monomorphic epitope, CD54 clone HA58 (IgG1, PharMingen), which blocks allogeneic mixed leukocyte reaction (MLR) (26), IgM isotype control clone MOPC 104E, IgG1 isotype control clone MOPC 21 (Sigma), IL-10 clone 23738.111 (IgG2b, R & D Systems).
T-Cell Proliferation Assays.
For allogeneic MLRs, total adult T cells were purified by using Cellect columns (TCS Medical Products, Southampton, PA). DCs were added in increasing numbers (156–10,000) to 1 × 105 T cells in triplicate and incubated for 5 days. We added 0.5 μCi 3H-thymidine/well for the last 18 h of the culture. For clonal T-cell responses, 1 × 106 DCs were pulsed for 6 h with 0.025 μM acetylcholine receptor (AChR) α:3–181 polypeptide before or 1 μM AChR α:144–163 peptide after maturation (27). In these experiments, increasing numbers of MHC class II-sharing DCs were then incubated with 3 × 104 T cells for 72 h. Proliferation was measured as above.
ELISA.
Supernatants from DC cultures under the conditions described were collected 24 h after addition of LPS, when TNF-α, IL-12, and IL-10 were at plateau levels (data not shown). Supernatants from proliferation assays of the T-cell clone TB-2 were collected after 60 h of culture (27). The concentrations of IL-4, IFN-γ, TNF-α, IL-12p70, IL-10, and TGF-β1 were measured according to the manufacturer's specifications (R & D Systems).
Statistical Analysis.
The relative increase in surface marker expression was calculated by dividing the mean fluorescence intensity (MFI) of LPS-exposed DCs by the MFI of immature DCs after subtracting values obtained with isotype-control antibody. Likewise, the cytokine concentrations in the supernatants of LPS-matured DCs were divided by those of immature DCs after subtracting background values with medium alone. In either case, the increases from at least three independent experiments were compared by using a paired-sample Student's t test. To compare the effects on MLR, responses were normalized as the percentage of the proliferation achieved with LPS-matured control DCs at a DC/T-cell ratio of 1:20 after subtraction of background. Statistical analysis was performed by using spss Ver. 9 (SPSS, Chicago).
Results
mAbs to CD36 and the α-v-Integrin CD51 Inhibit DC Maturation.
We wished to establish whether ligation of CD36 on the surface of DCs could account for their modulation by intact iRBC. We therefore exposed immature DCs to IgM and IgG1 mAbs against CD36 or to control Igs and analyzed their maturation in response to inflammatory signals. Immature DCs exposed to medium alone, to isotype-matched irrelevant Igs, or to mAbs against CD54 or MHC class I molecules, and then matured with LPS increased their surface expression of HLA class II molecules, costimulatory molecules, and CD83 (Fig. 1). By contrast, DCs exposed to anti-CD36 mAbs (whether IgM or IgG) consistently failed to mature despite stimulation with LPS, showing no significant increase in any of the surface markers analyzed; phenotypically, they closely resembled immature DCs (Fig. 1). The differences in MFI (mean of at least three independent experiments) compared with LPS-matured DCs alone were statistically significant for all the markers (P < 0.05; Table 1). Surface expression of some markers sometimes appeared to be even below that of immature DCs, but these differences were not statistically significant. Inhibition of phenotypic DC maturation by anti-CD36 mAbs was observed whether we used LPS, TNF-α, CD40L, or MCM as maturation stimulus (data not shown). The qualitative and quantitative effects of anti-CD36 mAb on DC maturation were very similar to those observed with iRBC (Table 1).
Figure 1.
Antibodies against CD36 and CD51 inhibit the LPS-induced maturation of DCs. Immature DCs (DC) were exposed to control mouse IgM, anti-CD36 (IgM), or anti-CD51 (IgG1) mAbs, control anti-CD54, or anti-MHC class I mAbs, as indicated, and left untreated or matured with LPS (+LPS). Subsequently, DCs were stained for the indicated surface molecules and analyzed by FACScan. Dead cells were excluded with PI. The MFI is indicated in each histogram. Shown is one representative experiment of six.
Table 1.
Different ligands of CD36 and/or CD51 induce a similar phenotype in dendritic cells exposed to LPS
| Modulation of dendritic cell with
|
||||||
|---|---|---|---|---|---|---|
| None | Anti-CD36 | Anti-CD51 | Anti-CD36/Anti-CD51 | Apoptotic DC | iRBC | |
| Phenotypic maturation† | ||||||
| Fold increase of CD83 ± SD | 24 ± 6 | 0.6 ± 0.16** | 1 ± 0.1** | 1.4 ± 0.5** | 1 ± 0.47** | 0.5 ± 0.1** |
| Fold increase of CD86 ± SD | 3.9 ± 0.3 | 0.6 ± 0.2* | 0.7 ± 0.1* | 0.6 ± 0.1* | 1.3 ± 0.5* | 0.8 ± 0.2* |
| n = 16 | n = 6 | n = 6 | n = 6 | n = 4 | n = 10 | |
| MLR (percent proliferation ± SD)‡ | 100 ± 0 | 11 ± 5.2** | 19 ± 6.9** | 11 ± 1** | 24 ± 12** | 11 ± 2.3** |
| n = 9 | n = 3 | n = 3 | n = 3 | n = 3 | n = 6 | |
| Fold increase in secretion of§ | ||||||
| TNF-α ± SD | 5 ± 1 | 4 ± 0.5 | 5 ± 0.3 | ND | 4 ± 0.9 | 7 ± 3 |
| IL-12 ± SD | 7 ± 1.4 | 0.4 ± 0.1** | 0.7 ± 0.2** | ND | 0.9 ± 0.7** | 0.8 ± 0.3** |
| IL-10 ± SD | 0.7 ± 0.3 | 7 ± 0.6** | 6 ± 1.4** | ND | 6 ± 1.8** | 6 ± 0.5** |
| n = 13 | n = 13 | n = 13 | n = 7 | n = 4 | ||
Shown are mean and standard deviation for n experiments. Control DCs and modulated DCs were significantly different with
, P < 0.05 and
, P < 0.01 (Student's t test).
Fold increase in surface expression calculated from MFI on matured DCs over that of immature DCs.
Proliferation of allogeneic T cells relative to mature DCs.
Fold increase in secretion of cytokines relative to immature dendritic cells.
The integrins αvβ5 and αvβ3 are involved in the ingestion of apoptotic cells by DCs (25, 28); moreover iRBC of at least one laboratory P. falciparum line adhere to CD51 (αv-integrin chain) (17). When tested, mAbs to CD51 also significantly inhibited phenotypic DC maturation (Fig. 1) again regardless of the maturation stimulus used (data not shown). The observed effect was maximal with either anti-CD36 or anti-CD51 mAb alone (Table 1).
Apoptotic Cells Inhibit the Maturation and Function of DCs.
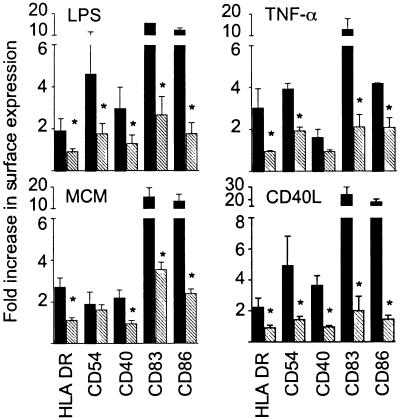
Both CD36 and CD51 mediate, at least in part, the recognition and uptake of apoptotic cells by immature DCs (25, 28). We therefore investigated whether apoptotic cells would inhibit phenotypic DC maturation in a manner similar to these mAbs. Three hours after UV-irradiation, apoptosing monocytes, neutrophils or DCs were cocultured with autologous DCs at a ratio of 2:1 for a minimum of a further 3 h before addition of a maturation stimulus. During this period, the percentage of early apoptotic cells (binding AV) increased from ≈15 to 30%. However, after 24 h, all UV-irradiated cells had progressed to secondary necrosis and were permeable to PI, which, plus their scatter profile, clearly distinguished them from live DCs (Fig. 2A) when their LPS-induced maturation was assessed by FACScan analysis (Fig. 2B). After coculture with apoptosing cells, DCs consistently failed to mature phenotypically in response to LPS (Fig. 2B). The surface expression of all markers was statistically significantly lower than on control DCs (mean of three independent experiments: P < 0.05, Table 1). Similar results were obtained not only when we used TNF-α, CD40L, or MCM as a maturation stimulus (Fig. 3) but also with apoptotic monocytes and neutrophils instead of the apoptotic DC shown here. As has been observed by Sauter et al. (7), necrotic cells derived from primary cell isolates did not mature DCs (data not shown) but permitted LPS-induced maturation (Fig. 2B).
Figure 2.
Apoptotic but not necrotic cells inhibit the maturation of viable DCs. (A) When cells are harvested for FACS analysis, live DCs can be distinguished from apoptotic DCs by forward scatter (FSC) and exclusion of PI. (B) Immature DCs (DC) were left untreated (Med), matured with LPS (LPS), or exposed to autologous apoptotic (apoptotic DC + LPS) or necrotic DCs (necrotic DC + LPS) before maturation. Subsequently, DCs were analyzed by FACScan as for Fig. 1. Shown is one representative experiment of four.
Figure 3.
Apoptotic cells inhibit DC maturation in response to different stimuli. Immature DCs were exposed to medium (black bars) or to apoptotic cells (hatched bars) with or without the indicated maturation stimulus and analyzed by FACScan as for Fig. 1. The fold increase in surface marker expression was calculated from the MFI as in Table 1. All data represent the mean of three independent experiments (*, P < 0.05, paired Student's t test).
Modulated DCs Fail to Activate T Cells.
Because mature DCs are potent activators of T cells, we next examined whether this phenotypic modulation by anti-CD36, anti-CD51 mAbs, or apoptotic cells was reflected in their capacity to activate T cells.
First, in a MLR, DCs exposed to each of these agents before maturation evoked minimal allogeneic T-cell responses (Fig. 4 and Table 1). Interestingly, the allogeneic T-cell responses induced by these modulated DCs before and after maturation with LPS were even lower than those induced by immature DCs, possibly because the latter begin to mature through CD40/CD40–ligand interactions with T cells during the MLR (29). This suggested a profound functional defect in the dendritic cells rather than a mere lack of phenotypic maturation.
Figure 4.
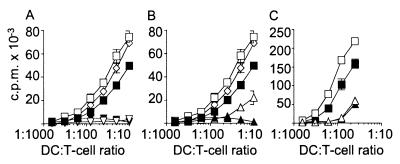
Modulated DCs fail to induce allogeneic T-cell responses. Proliferative allogeneic T-cell responses to DCs modulated by anti-CD36 mAb (A), by anti-CD51 mAb (B), or by apoptotic cells (C). The stimulator DCs were: immature DC alone (filled squares) or matured with LPS (open squares); DCs exposed to isotype-control Igs and then matured with LPS (open diamond); DCs exposed to anti-CD36 in (A), anti-CD51 mAb in (B) or apoptotic cells in (C) alone (filled triangle) or matured with LPS (open triangle). Shown is one representative experiment of at least three. Stimulation of T cells by modulated DCs was significantly reduced (P < 0.01) compared with mature DCs.
We studied also the proliferation of the CD4+ T-cell clone TB-2 to its acetylcholine receptor epitope (α:145–163) presented by MHC class II-sharing DCs (27). Established T-cell clones have less stringent requirements for costimulation than primary T cells and—if the specific peptide is known—their responses can be assessed independently of antigen uptake and processing. When DCs were exposed to anti-CD36 and/or anti-CD51 mAbs before maturation, the T-cell responses were almost abolished (Fig. 5). This effect was observed irrespective of whether DCs were pulsed with the 178-aa polypeptide before maturation or were loaded exogenously with the specific 19-aa peptide afterwards (Fig. 5 A and B); in either case, it was highly significant (P < 0.01). The inhibition of T-cell proliferation was equally strong when DCs were exposed to apoptotic cells instead (Fig. 5C). Moreover, secretion of IL-4 and IFN-γ was reduced to a similar extent (P < 0.01) (Fig. 5 D and E). These results suggest that modulated DCs have a defect in costimulation and/or antigen presentation by MHC class II molecules rather than in antigen processing.
Figure 5.
Modulated DCs fail to induce antigen-specific T-cell responses. Proliferation of the T-cell clone TB-2 to DCs modulated by anti-CD36 or anti-CD51 mAb (A, B) and pulsed with the specific polypeptide α:3–181 before maturation (A) or the specific peptide α:145–163 after maturation (B) or to DCs modulated by apoptotic cells (C) and pulsed with the specific polypeptide α:3–181 before maturation with LPS. (D, E) Secretion of IFN-γ and IL-4 by the T-cell clone TB-2 in response to DCs in medium alone (med) or modulated with isotype control Ig (iso), anti-CD36 mAb (anti-CD36), or apoptotic DCs (apoDC) before maturation with LPS with or without the specific peptide α:145–163 (pep). Shown are the mean and SD of IFN-γ and IL-4 secretion by T cells (*, P < 0.01). Symbols for A–C: open or filled symbols indicate DCs pulsed with or without antigen, respectively. DCs were exposed to isotype-control Igs (circle), anti-CD36 mAb (inverse triangle), anti-CD51 mAb (triangle), or apoptotic cells (square) and then matured with LPS. Shown is one representative experiment of at least three.
Modulated DCs Secrete IL-10 but Not IL-12.
After establishing these phenotypic and functional effects, we investigated whether the modulating agents, including intact iRBC, affected the secretion of cytokines that could influence either DCs or the responses of the interacting T cells.
DCs were first exposed to medium alone, anti-CD36 mAbs, apoptotic cells, or iRBC and then matured with LPS or left untreated. The pattern of cytokines they secreted over the next 24 h was similar with all three modulating agents but strikingly different from that of control DCs (Fig. 6). Secretion of TNF-α was comparable in normal and modulated DCs, whereas IL-12 p70 secretion was almost abolished by modulation (P < 0.01). In stark contrast, secretion of IL-10 became appreciable only after modulation of DCs and was almost undetectable in control cultures (P < 0.01). The absolute levels of IL-10 varied considerably with the modulating agent (Fig. 6) but consistently increased 6- to 10-fold with each of them (Table 1). TGF-β1 was not detected in any of the supernatants (data not shown). IL-10 can inhibit the maturation of DCs in response to TNF-α (30, 31). However, blocking anti-IL-10 mAbs failed to prevent the modulation of DCs by anti-CD36 mAb or apoptotic cells in three independent experiments (data not shown); evidently, it is largely IL-10 independent.
Figure 6.
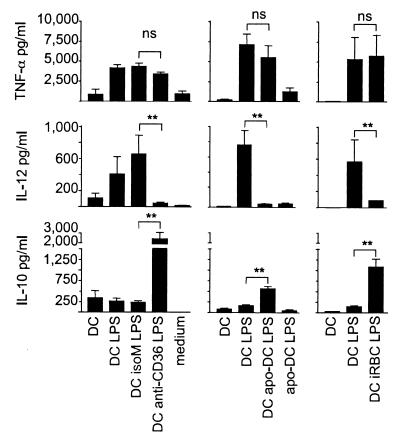
Effect of modulating agents on LPS-induced secretion of TNF-α, IL-12p70, and IL -10 by DCs. Cytokine secretion over 24 h by immature DCs (DC) and LPS-matured DCs alone (DC LPS) or exposed to anti-CD36 (DC anti-CD36 LPS) or isotype control (DC isoM LPS) mAb, apoptotic cells (DC apo-DC LPS), or iRBC (DC iRBC LPS). The cytokine levels in culture medium alone (medium) or supernatants from apoptotic cells alone (apo-DC) are also indicated. Shown are the mean and SD of cytokine secretion by DCs and controls exposed to mAbs (n = 13), to apoptotic cells (n = 7), or to iRBC (n = 4). ns, not significant. **, P < 0.01.
Discussion
We have recently reported that intact iRBC, but not lysates thereof, inhibit the maturation and function of DCs in response to inflammatory stimuli (19). This modulating effect was observed with cytoadherent parasite lines that bound to CD36 and to the soluble serum protein TSP, which in turn can bind to CD36 and the integrin-chain αv (CD51) complexed with β3. Therefore, CD36 and CD51 were prime candidates in our quest for the host receptor(s) mediating the inhibition of dendritic cell maturation and function by intact iRBC. Here, we report that single mAbs against either CD36 or CD51 mimic these effects. Intriguingly, CD36 and CD51 are also involved in the recognition and ingestion of apoptotic cells, which themselves modulate DC function in response to inflammatory stimuli in a similar manner (Table 1). Because only apoptotic cells occur naturally in the human body, our results suggest that their binding to CD36 and/or CD51 is part of a pathway regulating DC function in the normal immune system. Thus, intact iRBC may functionally mimic apoptotic cells and so take advantage of an Achilles' heel in the human immune system: the need to compromise between maintenance of responsiveness to pathogens and protection from autoaggression.
The recognition and ingestion of apoptotic cells by monocytes and macrophages comprise an apparently redundant system of receptor complexes that include the scavenger receptor CD36, αvβ3 and αvβ5, CD14, the recently described phosphatidyl serine receptor, other integrins such as β1 and β2, as well as the serum proteins C1q, β2 glycoprotein I, and TSP (reviewed in refs. 20 and 32). Whereas the function of some of these proteins in the clearance of apoptotic cells is well understood, the effects of others and their interactions remain largely unknown. On DCs, recognition and uptake of apoptotic cells seem to be mediated, at least in part, by αvβ5 and CD36 (26); expression of other candidate receptors is low (CD14) or not yet established (phosphatidyl serine receptor) (33). However, it seems likely that the interaction of apoptotic cells with DCs is more complex; as further receptors are described, analyzing their effects on DC function will be of considerable interest.
In our experiments, DCs that had ingested apoptotic cells appeared phenotypically and functionally immature despite exposure to such diverse inflammatory stimuli as LPS, TNF-α, MCM, and CD40L. However, they were not totally unresponsive to inflammatory signals because they still secreted TNF-α and now produced IL-10. This cytokine profile, in combination with the functional modulation, clearly defines a distinctive DC phenotype (Table 1); further studies are now needed to address its physiological significance.
The response of DCs to apoptotic cells is in stark contrast with that of monocytes and macrophages, which down-regulate proinflammatory cytokines and instead secrete anti-inflammatory cytokines such as TGF-β and IL-10, and so contribute to the resolution of inflammation (34, 35). Consistent with a role as initiators rather than effectors of immune responses, DCs that have ingested apoptotic cells might amplify local danger signals through secretion of TNF-α even though they do not activate T cells themselves. TNF-α may not only confine the spread of intracellular infections but also recruit further immune effector cells to sites of inflammation (36). Although evidently not the prime mediator of the modulation of DCs by apoptotic cells, IL-10 may raise the threshold for maturation and antigen presentation of bystander DCs at sites of inflammation and tissue injury (30, 31). If so, then the integration of several, sometimes even opposing, signals may determine which phenotype any given DC acquires. The presence of modulated as well as matured DCs may be necessary to ensure the induction of effective immune responses against invading pathogens while maintaining peripheral tolerance to self antigens.
The shift from the secretion of IL-12, which is normally observed in mature DCs, to IL-10 after modulation might have profound consequences for any interacting T cells. IL-12 drives Th1 T-cell responses by inducing IFN-γ secretion in T cells and natural killer cells (37). By contrast, IL-10 is involved in the deviation of T-cell subsets to a Th2-phenotype (38, 39) and in the induction of anergy (40). While our preliminary evidence suggests that blocking of IL-10 does not reverse the profound failure of modulated DCs to induce T-cell proliferation (B.U., unpublished observations), repeated stimulation of T cells with modulated dendritic cells might induce regulatory T cells (41).
Our observations on DC modulation may illuminate some puzzling observations on field isolates of iRBC. Although almost all field and laboratory isolates bind to CD36 and/or TSP (42–44), those with higher affinity for CD36 are more frequently isolated from children with mild than with severe malaria (43, 45). Furthermore, a nonsense mutation in CD36 is common in African populations. Although one study reported that the frequency of this mutation is increased in patients with cerebral malaria, our data suggest that it is reduced in patients suffering from respiratory distress, severe malarial anemia, or hypoglycemia (46, 47). Without further functional studies, the consequences of this mutation for the immune response to malaria cannot be deduced.
However, modulation of DCs by iRBC is a contact-dependent process; if it occurred in vivo, it most likely would affect DCs in the circulation as well as in the liver and in the marginal zone of the spleen. Modulation should depend on a critical level of iRBC and on their affinity for CD36. Cumulative modulation of DCs might result in a progressive polarization or inhibition of T-cell priming and so may dampen antiparasite immune responses; at the same time, it might reduce associated immunopathology. Indeed, some studies suggest that induction of primary immune responses is impaired during acute phases of malarial disease (48–50). Nevertheless, T-cell-dependent humoral immune responses are clearly induced during acute malaria, and adults living in endemic areas often show high levels of antimalarial IgG (51), although antibodies against individual parasite antigens frequently seem to be short-lived (52, 53). Possibly the secretion of IL-10, at least in part by iRBC-modulated DCs, may promote the induction of Th2 responses, whereas the progressive impairment of DC function could interfere with the induction of memory in T and B cells.
Clearly, the immunological consequences of parasite-mediated modulation of DCs are not yet fully understood, but they may contribute significantly to mechanisms of immune evasion by the asexual blood stages of P. falciparum. Unraveling these mechanisms may provide therapeutic clues for the treatment of malaria. Conversely, investigating iRBC-mediated modulation of DCs may lead us to new approaches for regulating the pathological immune responses in autoimmune diseases and transplantation.
Acknowledgments
We thank Dr. Jonathan M. Austyn for critical discussion of the manuscript and Drs. Barry Elford and Chris I. Newbold for their overall encouragement and support. This study was funded by the Sir E. P. Abraham Research Trust, University of Oxford, Oxford, U.K.
Abbreviations
- DC
dendritic cells
- LPS
lipopolysaccharide
- iRBC
P. falciparum-infected erythrocytes
- TNF
tumor necrosis factor
- TSP
thrombospondin
- MCM
monocyte-conditioned medium
- MLR
mixed leukocyte reaction
- PI
propidium iodide
- AV
annexin V
- MFI
mean fluorescence intensity
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebeque S, Liu Y-J, Paulendaran B, Palucka K. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka H, Demeure C E, Rubio M, Delespesse G, Sarfati M. J Exp Med. 2000;19:405–412. doi: 10.1084/jem.192.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 4.Ridge J P, Di Rosa F, Matzinger P. Nature (London) 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 5.Dubois B, Vanbervliet B, Fayette J, Massacrier C, Van Kooten C, Briere F, Banchereau J, Caux C. J Exp Med. 1997;185:941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallucci S, Lolkema M, Matzinger P. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 7.Sauter B, Albert M L, Francisco L, Larsson M, Somersan S, Bhardwaj N. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurts C, Kosaka H, Carbone F R, Miller J F, Heath W R. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman R M, Turley S, Mellman I, Inaba K. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert M L, Sauter B, Bhardwaj N. Nature (London) 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 11.Miller L H, Good M F, Milon G. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 12.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, Pasvol G, Snow R. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 13.Newbold C I. Curr Opin Microbiol. 1999;2:420–425. doi: 10.1016/S1369-5274(99)80074-5. [DOI] [PubMed] [Google Scholar]
- 14.Barnwell J W, Asch A S, Nachman R L, Yamaya M, Aikawa M, Ingravallo P. J Clin Invest. 1989;84:765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baruch D I, Gormely J A, Ma C, Howard R J, Pasloske B L. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowe J A, Moulds J M, Newbold C I, Miller L H. Nature (London) 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- 17.Siano J P, Grady K K, Millet P, Wick T M. Am J Trop Med Hyg. 1998;59:77–79. doi: 10.4269/ajtmh.1998.59.77. [DOI] [PubMed] [Google Scholar]
- 18.Treutiger C J, Heddini A, Fernandez V, Muller W A, Wahlgren M. J Exp Med. 1999;182:15–20. [Google Scholar]
- 19.Urban BC, Ferguson D J P, Pain A, Willcox N, Plebanski M, Austyn J, Roberts D J. Nature (London) 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 20.Savill J. Br Med Bull. 1997;53:491–508. doi: 10.1093/oxfordjournals.bmb.a011626. [DOI] [PubMed] [Google Scholar]
- 21.Trager W, Jensen J B. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 22.Salio M, Cerundolo V, Lanzavecchia A. Eur J Immunol. 2000;30:705–708. doi: 10.1002/1521-4141(200002)30:2<705::AID-IMMU705>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 24.Pain A, Ferguson D J P, Kai O, Urban B C, Lowe B, Marsh K, Roberts D J. Proc Natl Acad Sci USA. 2001;98:1805–1810. doi: 10.1073/pnas.98.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albert M L, Pearce SF, Francisco L M, Sauter B, Roy P, Silverstein R L, Bhardwaj N. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlossman S, Bloumsell L, Gilks W, editors. Leucocyte Typing. New York: Oxford Univ. Press; 1995. [Google Scholar]
- 27.Nagvekar N, Moody A M, Moss P, Roxanis I, Curnow J, Beeson D, Pantic N, Newsom-Davis J, Vincent A, Willcox N. J Clin Invest. 1998;101:2268–2277. doi: 10.1172/JCI2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubartelli A, Poggi A, Zocchi M R. Eur J Immunol. 1997;27:1893–1900. doi: 10.1002/eji.1830270812. [DOI] [PubMed] [Google Scholar]
- 29.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk A H. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 31.Sato K, Nagayama H, Tadokoro K, Juji T, Takahashi T A. J Immunol. 1999;162:3865–3872. [PubMed] [Google Scholar]
- 32.Savill J, Fadok V. Nature (London) 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 33.Fadok V A, Bratton D L, Rose D M, Pearson A, Ezekewitz R A, Henson P M. Nature (London) 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 34.Voll R E, Herrmann M, Roth E A, Stach C, Kalden J R, Girkontaite I. Nature (London) 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 35.Fadok V A, Bratton D L, Konowal A, Freed P W, Westcott J Y, Henson P M. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schluter D, Deckert M. Microbes Infect. 2000;2:1285–1292. doi: 10.1016/s1286-4579(00)01282-x. [DOI] [PubMed] [Google Scholar]
- 37.Gately M K, Renzetti L M, Magram J, Stern A S, Adorini L, Gubler U, Presky D H. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 38.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Eur J Immunol. 1997;27:1229–1235. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Rich B E, Inobe J, Chen W, Weiner H L. Int Immunol. 1998;10:1017–1026. doi: 10.1093/intimm/10.8.1017. [DOI] [PubMed] [Google Scholar]
- 40.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries J E, Roncarolo M G. Nature (London) 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 41.Joss A, Akdis M, Faith A, Blaser K, Akdis C A. Eur J Immunol. 2000;30:1683–1690. doi: 10.1002/1521-4141(200006)30:6<1683::AID-IMMU1683>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 42.Baruch D I, Ma X C, Singh H B, Bi X, Pasloske B L, Howard R J. Blood. 1997;90:3766–3775. [PubMed] [Google Scholar]
- 43.Newbold C, Warn P, Black G, Berendt A, Craig A, Snow B, Msobo M, Peshu N, Marsh K. Am J Trop Med Hyg. 1997;57:389–398. doi: 10.4269/ajtmh.1997.57.389. [DOI] [PubMed] [Google Scholar]
- 44.Rogerson S J, Tembenu R, Dobano C, Plitt S, Taylor T E, Molyneux M E. Am J Trop Med Hyg. 1999;61:467–472. doi: 10.4269/ajtmh.1999.61.467. [DOI] [PubMed] [Google Scholar]
- 45.Traore B, Muanza K, Looareesuwan S, Supavej S, Khusmith S, Danis M, Viriyavejakul P, Gay F. Am J Trop Med Hyg. 2000;62:38–44. doi: 10.4269/ajtmh.2000.62.38. [DOI] [PubMed] [Google Scholar]
- 46.Aitman T J, Cooper L D, Norsworthy P J, Wahid F N, Gray J K, Curtis B R, McKeigue P M, Kwiatkowski D, Greenwood B M, Snow R W, Hill A V, Scott J. Nature (London) 2000;405:1015–1016. doi: 10.1038/35016636. [DOI] [PubMed] [Google Scholar]
- 47.Pain A, Urban B C, Kai O, Casals-Pascuals C, Marsh K, Roberts D J. Lancet. 2001;357:1502–1503. doi: 10.1016/S0140-6736(00)04662-6. [DOI] [PubMed] [Google Scholar]
- 48.McGregor I A, Barr M. Trans R Soc Trop Med Hyg. 1962;56:364–367. doi: 10.1016/0035-9203(62)90006-8. [DOI] [PubMed] [Google Scholar]
- 49.Williamson W A, Greenwood B M. Lancet. 1978;1:1328–1329. doi: 10.1016/s0140-6736(78)92403-0. [DOI] [PubMed] [Google Scholar]
- 50.Greenwood B M, Bradley A K, Blakebrough I S, Whittle H C, Marshall T F, Gilles H M. Trans R Soc Trop Med Hyg. 1980;74:340–346. doi: 10.1016/0035-9203(80)90095-4. [DOI] [PubMed] [Google Scholar]
- 51.Cohen S, McGregor I A, Carrington S. Nature (London) 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 52.Giha H A, Staalsoe T, Dodoo D, Elhassan I M, Roper C, Satti G M, Arnot D E, Theander T G, Hviid L. Infect Immun. 1999;67:4092–4098. doi: 10.1128/iai.67.8.4092-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavanagh D R, Elhassan I M, Roper C, Robinson V J, Giha H, Holder A A, Hviid L, Theander T G, Arnot D E, McBride J S. J Immunol. 1998;161:347–359. [PubMed] [Google Scholar]