Abstract
Intraventricular hemorrhage (IVH) is a cause of significant morbidity and mortality and is an independent predictor of a worse outcome in intracerebral hemorrhage (ICH) and germinal matrix hemorrhage (GMH). IVH may result in both injuries to the brain as well as hydrocephalus. This paper reviews evidence on the mechanisms and potential treatments for IVH-induced hydrocephalus. One frequently cited theory to explain hydrocephalus after IVH involves obliteration of the arachnoid villi by microthrombi with subsequent inflammation and fibrosis causing CSF outflow obstruction. Although there is some evidence to support this theory, there may be other mechanisms involved, which contribute to the development of hydrocephalus. It is also unclear whether the causes of acute and chronic hydrocephalus after hemorrhage occur via different mechanisms; mechanical obstruction by blood in the former, and inflammation and fibrosis in the latter. Management of IVH and strategies for prevention of brain injury and hydrocephalus are areas requiring further study. A better understanding of the pathogenesis of hydrocephalus after IVH, may lead to improved strategies to prevent and treat post-hemorrhagic hydrocephalus.
Introduction
Intraventricular hemorrhage (IVH) is a cause of significant morbidity and mortality and is an independent predictor of worse outcome after germinal matrix hemorrhage (GMH) in neonates 1,2 and intracerebral hemorrhage (ICH) in adults 3,4. IVH is common in neonates and usually occurs as a result of GMH. The incidence of GMH-IVH is higher in very low birth weight (VLBW, birth weight < 1500 grams) infants and, although the rate of IVH in this group has decreased in recent years (Philip, 1989), the overall number of preterm infants who survive is increasing. 22% of VLBW infants display GMH-IVH with one quarter developing ventricular dilation. 34% of those with persistent ventricular dilation will require surgical treatment with a reservoir or a shunt2.
In adults, ICH is common with an incidence of 12–15/100,000 cases annually5 and IVH occurs in 42% to 52% of those presenting with spontaneous, non-traumatic ICH3,4,6,7. Hydrocephalus develops in up to 67% of patients with intraventricular extension of ICH3,4 and is itself associated with a higher mortality8. The presence of IVH in patients with ICH lowered the rate of favorable outcome from 31% to 15% and it was an independent predictor of worse outcome along with hydrocephalus in the STICH trial of surgical intervention for ICH3. The volume of blood contained within the ventricles was also independently associated with a worse Glasgow Coma Scale9.
IVH can lead to both immediate obstructive hydrocephalus and delayed communicating hydrocephalus in addition to direct brain injury10. The mechanisms by which ventricular hemorrhage causes brain injury and hydrocephalus is still not totally clear. Determining those mechanisms may allow identification of additional therapeutic targets to decrease IVH-induced hydrocephalus and potentially mitigate deleterious brain effects. Evaluating the mechanisms of CSF accumulation in pathologic states, such as IVH, requires an understanding of normal absorption of CSF in both animals and humans and there are still some deficiencies in that regard (see below). This review will address hydrocephalus after IVH, both in neonate (which commonly occurs with extension of GMH into the ventricles) and in the adult (which often occurs via extension of ICH) with emphasis on the mechanisms of hydrocephalus development.
Intraventricular hemorrhage: etiology
In neonates, GMH arises from the germinal matrix, an area of rapidly dividing progenitor cells destined to be both neurons and glia which is present until 34 weeks gestation11,12. This area is located between the ventricular wall and caudate nucleus in the thalamostriate groove1. It is highly vascularized and undergoes rapid angiogenesis during development. The vascular network is comprised of immature, fragile vessels that lack connective tissue support. Pericytes, which are important for maintenance of tight junctions that function in the blood-brain barrier (BBB), are decreased in the germinal matrix vasculature13,14. A lack of muscle and type IV collagen around the capillaries15, increased fibrinolysis, presence of immature glial cells around the capillaries11, and a direct communication between the arterial blood supply and deep venous system may all contribute to the propensity of this area to hemorrhage during development. This propensity to hemorrhage is coupled with multiple possible inciting events in the neonate, most of which render the germinal matrix susceptible to rapid changes in blood flow. GMH usually occurs within the first 3 days of life and is thought to be associated with stressors that may include hypertension, hypercarbia-induced increases in cerebral blood flow, increased venous pressure, coagulopathy, and impaired cerebral autoregulation15. As the germinal matrix is no longer present in term infants, IVH in this group is thought to occur as a result of choroid plexus hemorrhage15.
IVH in adults can occur secondary to extension of ICH, aneurysmal SAH, vascular malformations and trauma. ICH with intraventricular extension is more likely if the hemorrhage occurs in periventricular locations such as the thalamus and caudate7. In adults, older age, increased volume of ICH, and significant hypertension are also associated with ICH-associated IVH16.
IVH/Hydrocephalus Grading
In neonates, GMH-IVH is graded based on the extent of hemorrhage and presence of ventricular dilation17. Grade I is defined as hemorrhage confined to the subependymal germinal matrix. Grade II is GMH that extends into the ventricle without ventricular dilation. Grade III is GMH-IVH accompanied by ventricular dilation. Grade IV is GMH-IVH with hemorrhage extension into the brain parenchyma beyond the germinal matrix (figure 1). Grading of post-hemorrhagic ventricular dilation is not uniform and although some groups18 have suggested criteria for ventricular dilation, there is no commonly accepted system in place. In adults, there are multiple grading scales for IVH in the setting of ICH19–21. These scores incorporate measurements of the amount and location of intraventricular blood as well as the degree of ventricular distension. When compared against one another, all were able to predict outcome when used at admission and within 6 days after hemorrhage4. There is no consistent grading score in the adult for hydrocephalus alone, particularly in the presence of a mass lesion, such as in ICH8.
Figure 1.

Cranial ultrasound showing grade IV germinal matrix hemorrhage-intraventricular hemorrhage.
IVH-induced brain injury
Neonatal and adult IVH causes immediate and delayed injury to the brain resulting in ischemia, hypoxia, white matter injury, periventricular leukomalacia, free radical damage, hemorrhagic infarction of the parenchyma, release of inflammatory cytokines and free radicals into the CSF, gliosis and decreased cerebral perfusion pressure15. These effects may culminate in cerebral palsy, seizures, cognitive deficits, hydrocephalus and death. IVH results in damage to precursor cells of the germinal matrix and subventricular zone, and in neonates this may be of even more importance as the brain is actively developing. Decreased Ki67, a marker of proliferation, has been found in the germinal matrix adjacent to the site of the hematoma22. In addition there is suppression of cell proliferation in the ganglionic eminence within 24 hours of hemorrhage in human autopsy specimens 23.
Hydrocephalus itself causes injury to the brain by a number of mechanisms including inflammation, ventricular distension disrupting periventricular fibers, and increased intracranial pressure with resultant decreased cerebral perfusion3,7,24,25. In neonates, hydrocephalus is thought to cause white matter injury through multiple mechanisms including increased ICP, iron-induced free radical damage, and inflammatory cytokines26.
Neonatal Animal Models of IVH and Hydrocephalus
Animal models have attempted to replicate the pathology found in neonatal IVH. Animal models include direct induction of IVH via injection of blood into the ventricles of young or premature animals. Other models attempt to mimic GMH directly by premature delivery in combination with systemic treatments to cause fluctuations in intracranial pressure to precipitate GMH. The former has the advantage of controlling the amount and timing of IVH. The latter more closely mimics the pathology in neonates with GMH.
The neonatal GMH-IVH models have been developed in a number of species. A number of rodent models have been developed as the developmental state of the rodent brain at the time of birth is comparable to 24–26 week gestation human brain27 and newborn rodents do not require supportive care required by other prematurely delivered animals. A current rodent animal model involves bilateral ventricular injection of 80 microliters of blood with 65% of rats injected with citrated blood and 50% of those injected with artificial CSF developing dilated lateral ventricles 28. Another newborn rodent model involves injection of autologous blood into the periventricular region where 3 of 21 rats injected with blood developed hydrocephalus29. Lekic et al, 2011, developed a GMH model by injection of clostridial collagenase into the right germinal matrix, however the presence of IVH and ventricular enlargement was not addressed30. A similar neonatal collagenase model did not see hydrocephalus in any of the animals examined31. A mouse model of IVH with injection of blood into the periventricular region of newborn mice resulted in mild ventricular enlargement at 2 weeks22.
Other animal models have used rabbits, beagles, sheep, and pigs. There is rabbit model of GMH-IVH32, in which GMH is precipitated by intraperitoneal injection of glycerol and premature delivery at 29 days gestation (full term is 32 days) 33,34. Up to 20% of rabbit pups delivered by Cesarian section have IVH and 70% develop GMH/IVH after injection of glycerol, which induces intracranial hypotension. GMH has also been also induced with Lasix 27. Beagle pups, in particular, are a good model as the germinal matrix is still present in the first 1–2 days after birth, which then involutes by postnatal day 10. Sheep, along with beagle pups, have a carotid rete mirables that mitigates systemically-induced increases in intracranial pressure, and so would not mimic human pathology as well27. A neonatal piglet model has also been used, where blood is injected into the ventricle at birth35.
Although there are variable rates of hydrocephalus in IVH models, this offers a chance to compare differences between those animals that ultimately develop hydrocephalus and those that do not. Arachnoid granulations, which have been implicated as a potential site of pathology for the generation of hydrocephalus after IVH, are not present in a number of species, including rodents, complicating studies of the mechanisms of hydrocephalus development36. In humans, however, arachnoid granulations are not present prior to birth, and the lack of arachnoid granulations in certain animal models may not be significant.
Information on hydrocephalus induction can be also be gathered from animal models of congenital hydrocephalus caused by genetic mutations and acquired hydrocephalus secondary to injection of materials which block CSF flow, induce inflammation, alter CSF osmolality or cause infection. The first animal models of hydrocephalus were created by Dandy and Blackfan in 191337, who created obstructive hydrocephalus at the level of the cerebral aqueduct in a dog. Since that time, there have been a number of models of acquired hydrocephalus including Kaolin injection into the cisterna magna or injection of substances, which result inflammation. Injection of hyperosmolar dextran and fibroblast growth factor into the CSF both cause hydrocephalus in rats presumably by increasing the osmolality of the CSF and drawing additional water into the ventricular compartment38 through the choroid plexus or across the ependymal surface.
Genetic models of congenital hydrocephalus in the rat include the HTX and LEW/Jms rats, both of which develop obstructive hydrocephalus prior to reaching adulthood, with the HTX rat first developing transitory communicating hydrocephalus. In the mouse, there are multiple genetic models39, including hy3, aquaporin 4 −/−, TGF-B1 overexpression40, and L1-cell adhesion molecule deficient mutants41.
Adult Animal Models of IVH and Hydrocephalus
There has been a paucity of IVH studies in adult animals. The animal models that have been created have used a stereotactic injection of blood into the ventricles42. Our group has developed an IVH model in the adult rat where intraventricular injection of 200 μl autologous blood results in ventricular dilation, which peaked at 2 days and persisted for 8 weeks43. Pang evaluated ventricular size after the injection of 9 ml of autologous blood into the ventricles of adult dogs44 and Mayfrank et al. examined IVH and ventricular size in adult pigs45.
IVH-mediated brain injury and hydrocephalus have not been systematically evaluated in ICH animal models but rather in animal models of IVH alone 45,46. As IVH is a common complication of ICH in humans, animal models that combine these two pathologies are needed. There are also differences between neonatal and adult IVH and, therefore, appropriate models are needed for both age groups. For example, the neonatal mouse brain is more severely damaged than mature mouse brain after intracerebral injection of blood, thrombin and plasminogen47.
Mechanisms of hydrocephalus after IVH
It is a commonly held view that IVH-induced hydrocephalus is due to alterations in the CSF drainage pathway, particularly related to the cerebral aqueduct, 4th ventricular outlets and the arachnoid villi or granulations. This section discusses the evidence for this along with potential roles of alternate drainage pathways, alterations in the ependyma, BBB and aquaporin expression that may alter fluid flow within the brain. It also examines growing evidence for a role of iron and inflammation in hydrocephalus and IVH-induced brain injury. Expansion of ventricles may also occur secondary to loss of brain tissue from atrophy. Although this may contribute to chronic ventriculomegly after hemorrhage, is not likely to be the case after acute IVH.
CSF dynamics
The most commonly held view of IVH-induced hydrocephalus is that it is due to a blockage in the CSF drainage pathway. Thus, acute obstructive hydrocephalus after IVH occurs from blood blocking the cerebral aqueduct or the 4th ventricular outlets (foramen of Luschka and foraman of Magendie). Tetraventrciular hydrocephalus results from blockage at the level of the cortical subarachnoid space or less commonly blockage at the level of the outlets of the 4th ventricle (formanima of Luschka and Magendie). There are few animal studies that actually investigate either the cortical subarachnoid space or the outlets of the 4th ventricle. One early study attributed post-hemorrhagic hydrocephalus to fibrous thickening of the subtentorial leptomeninges with occlusion of the 4th ventricular outlets48, not the arachnoid granulations.
In contrast to obstructive hydrocephalus, dilation of all ventricles is often referred to as communicating hydrocephalus, even though a blockage may be present, for example, in the subarachnoid space. Communicating hydrocephalus after IVH is commonly thought to occur by blocking CSF outflow at the level of the arachnoid villi and arachnoid granulations by microthrombi49. As communicating hydrocephalus often occurs in a delayed fashion, inflammation causing scarring of the arachnoid granulations has also been implicated as a mechanism. Larroche in 1972 demonstrated that post-hemorrhagic ventricular dilation occurs through obliterative arachnoiditis in the posterior fossa (foramina of Luschka and Magendie) and to a lesser extent by obstruction of CSF at the cerebral aqueduct48,49. The idea of arachnoid villi and granulations being the site of obstruction of CSF outflow after hemorrhage has been widely accepted, however data to support this theory are often lacking. For instance, GMH-IVH induced hydrocephalus is most common in VLBW infants, most of whom do not have arachnoid granulations as they are not usually seen until the time of term gestation. In these individuals, there are thought to be additional routes of CSF drainage, which may be the location of impaired drainage (see below). These routes of absorption have not been adequately addressed particularly as there are no arachnoid granulations in multiple animal models including rats, mice, rabbits and cats36, as well as the human fetus.
Sites of absorption-arachnoid granulations
In order to begin to understand the pathophysiology of IVH-induced hydrocephalus, an appreciation of all of the possible absorptive sites of CSF is necessary. The idea of CSF reabsorption occurring via the arachnoid granulations originated in the early 1900s by Weed50. Microscopic arachnoid villi and macroscopic arachnoid granulations consist of protrusions of arachnoid through the dura into the venous sinuses, and are most prominent adjacent to the superior sagittal sinus. Both the presence and morphology of arachnoid granulations are different between humans and animals51. Rats, a common species for animal models of hydrocephalus, do not have arachnoid granulations and have very few arachnoid villi. The arachnoid villi in rats may only function under conditions of elevated CSF pressure and although they extend into the wall of the sinus, they do not protrude into the lumen of the sinus. Many of the theories supporting arachnoid granulations as the primary mode of CSF absorption stemmed from Dandy’s work on experimental hydrocephalus. However in his work from 1919 Dandy noted that the injection of India-ink into the CSF took 45–75 minutes to reach areas along the longitudinal sinus and that by this time 20–25% of the absorption had already taken place, indicating an alternative site for absorption52.
Alternative CSF absorption sites are particularly important in the developing brain since the arachnoid granulations are not yet fully functioning36. In addition to arachnoid villi along the superior sagittal sinus, ventricular ependyma via the subependymal vein, leptomeninges via the cortical vein, pia-arachnoid capillaries through the venous system, choroid plexus through the deep venous system and the perineural space by way of lymphatic channels were noted as additional sites of CSF absorption.
Studies involving SAH have also implicated the arachnoid villi in pathogenesis of hydrocephalus53–55. Possible mechanisms for interruption of CSF flow through the villi include an inflammatory-mediated or blood product provoking proliferation of arachnoid cells53 or blockage of the arachnoid villi by erythrocytes55. Arachnoid granulations have a collagenous center consisting of trabechulae which may contain macrophages 56. After subarachnoid hemorrhage erythrocytes, but not fibrin are found in arachnoid granulations 56. To enhance blood clearance and decrease that chance for occlusion of the arachnoid granulations there have been multiple trials investigating the effect of intraventricular tissue plasminogen activator (t-PA)44,57. Not all neonates respond to thrombolytic therapy and the presence of plasminogen activator inhibitor-1 (and decreased fibrinolysis) is thought to play a role in this lack of response and may be involved in the pathogenesis of IVH-induced hydrocephalus58,59.
Alternative sites of absorption
As noted above, the traditional theory about CSF reabsorption through arachnoid granulations, may not be entirely complete, as there is evidence of accessory pathways via nasal lymphatics, nerve sheaths, spinal subarachnoid space and across the ependymal and endothelial surfaces in the brain. As neonates do not have arachnoid granulations, these additional pathways may be more active in the developing nervous system and may play a particular role in pathogenesis of hydrocephalus after GMH/IVH in the neonate. Precursors to arachnoid villi appear at 26 weeks gestation as depressions in the venous wall of the dura. Arachnoid cells are contained in the depressions and are found in a subendothelial location. The depressions develop into protrusions up to the time of birth at which time they become arachnoid villi60. Animal studies have shown that nasal lymphatics are an important mechanism of CSF drainage through the cribriform plate into the nasal mucosa and from there the nasal lymphatics 61. In HTX rats, there is decreased CSF outflow to the nasal lymphatics compared with control animals62. CSF also drains along spinal nerve root sheaths and spinal CSF outflow comprises up to 25% of CSF drainage63,64.
Because arachnoid granulations do not exist in rats and mice, there have been multiple attempts to find appropriate models to study CSF drainage. In sheep, CSF drainage assessed with 125I -labeled human serum albumin, occurred through both nasal lymphatics and arachnoid villi65 and there was an equal contribution of both routes to CSF drainage66. CSF drainage via arachnoid granulations in chicken has been shown to mimic that which occurs in humans67. The chicken is microsmatic and has a less well developed lymphatic CSF drainage system, similar to humans. As information regarding the passage of CSF into the dural venous sinuses through arachnoid villi and granulations is lacking, some studies have examined flow in ex-vivo models consisting of human arachnoid granulations from cadaver specimens 68. There have not been any studies evaluating changes to accessory CSF drainage pathways after IVH, and this is an area that warrants further investigation.
Hydrodynamic theory and communicating hydrocephalus
Communicating hydrocephalus frequently develops after IVH. The term communicating hydrocephalus is not precise and different theories exist as to the mechanisms54,69,70. The CSF bulk flow hypothesis explanation is based on CSF absorption resulting from a slight increase in pressure at the site of absorption which is created by pressure generated by CSF at the site of formation at the choroid plexus. The hydrodynamic theory states that decreased intracranial compliance causes increased arterial pulse pressure which mediates an increase in ventricular pressure, which subsequently distends the ventricles and decreases the subarachnoid space71. This theory is able to explain why the subarachnoid space decreases in size in the setting of communicating hydrocephalus, when it might be expected to increase if the point of obstruction was indeed at the arachnoid granulations. This theory includes the idea that capillaries are able to absorb fluid as well70. The hydrodynamic theory suggests a mechanism by which chronic obstructive hydrocephalus can develop from acute obstructive hydrocephalus, where initial ventricular distension is counteracted by venous stasis69. The hydrodynamic theory is intriguing in that it offers an explanation for hydrocephalus other than that of the arachnoid granulations, however it does not offer a consistent mechanism by which intracranial compliance is altered, particularly after IVH.
Ependymal and subependymal damage
Ependymal surface damage occurs after IVH and neonates with IVH and hydrocephalus have more severe damage compared to those with IVH alone72. Ependymal cell loss with formation of subependymal rosettes is evident, possibly due to disruption of the ependyma by the blood itself or increased pressure44. In general, ependymal injury results in atrophy, discontinuity of the surface with enlargement of the ventricle, gliosis, and the formation of subependymal rosettes73. Damage to the integrity of the ependymal surface itself can result in collapse of the walls of the cerebral aqueduct, resulting in obstructive hydrocephalus. Furthermore, it has been suggested that ependymal cells are terminally differentiated and do not regenerate and, therefore, any ependymal injury is expected to have long-lasting effects.
The ependyma is a cuboidal epithelium and contains motile cilia, primary cilia and microvilli74. The multi-ciliated ependymal cells are thought to direct CSF flow75–79 and are regulated by CSF components and neurotransmitters such as serotonin 80. Cilia are also involved in migration of new neurons from the subventricular zone along the rostral migratory stream to the olfactory bulb76. The development of cilia is thought to occur postnatally 81 and the organization of such cilia may be disrupted in GMH-IVH. Ciliary dysfunction has been associated with hydrocephalus 74,78,82,83, as in a case of primary ciliary dyskeinesia and development of neonatal hydrocephalus. Ciliary defects are thought to result in hydrocephalus by multiple mechanisms including aqueductal stenosis and altered CSF flow74,84. In the case of aqueductal stenosis, cilia clump together occluding the aqueduct. Destruction of the ependyma by IVH results in the destruction of motile cilia and may affect CSF flow and production and migration of new neurons from the subventricular zone76.
Primary cilia also project into the ventricle from progenitor cells in the subventricular zone75, neuroepithelial cells and radial glia. They have mechano-sensory function and may regulate activity of the stem cells79. IVH may impact these cilia and, thereby, stem cell function.
With destruction of the ventricular ependyma comes disruption of the underlying subventricular zone (SVZ), which may affect neurogenesis and the migration of new neurons. The germinal matrix is also susceptible to damage after GMH-IVH in neonates as it is located beneath the ependymal lining adjacent to the lateral ventricles 23. It contains neural precursor cells15 and begins to produce astrocytes and oligodendrocytes in the third trimester. The production of new neurons by the subependymal zone continues throughout development and into adulthood 75. Frank destruction of the ependymal surface and possibly the underlying SVZ is demonstrated by the ability of markers of progenitor cells to be cultured from CSF of preterm infants with post-hemorrhagic hydrocephalus85. Furthermore stem cell dysfunction may play a role in ventricular enlargement, as conditional ablation of PTEN, which is important in neural stem cell proliferation, results in hydrocephalus86.
Bone morphogenetic protein (BMP) signaling controls the fate of SVZ progenitor cells and its expression can induce astrocyte proliferation. Noggin, a BMP inhibitor, increases oligodendrogliosis and, when used to suppress BMP signaling in a neonatal IVH model, resulted in improved neurologic recovery87. BMP-4 is also increased in the CSF of preterm infants with IVH and may be involved in changes in signaling within the SVZ87. This change in signaling is one example of how differential regulation of the SVZ, which may be disrupted after IVH, can result in changes in cell lineage. This may be important not only for the development of hydrocephalus, but also for pathogenesis of white matter injury following GMH-IVH. Finally, the ependyma itself, continues to mature into the post-natal period and may regulate radial glial cells neuroblast migration through expression of S-10088. Disruption of the ependyma may alter such migration.
Alterations to the blood-brain and blood-CSF barriers
The blood brain barrier (BBB) is composed of cerebral endothelial cells and their linking tight junctions although, astrocytes and pericytes play a role in regulating BBB function12. The blood-CSF barrier is situated primarily at the choroid plexus epithelial cells and their linking tight junctions. Disruption of these barriers may result in increased CSF protein content resulting in osmotic shifts. Following IVH there is abnormal uptake of IgG by epithelial cells lining the choroid plexus as well as by ependymal cells lining the ventricles, which is a phenomena similar to that which is seen with BBB breakdown89. In a SAH model, BBB tight junctions were altered, where ZO-1, a tight junction protein, expression was predominately cytoplasmic rather than intercellular 90. BBB development in the germinal matrix lags behind that of white matter 12 as GFAP+ cells (an astrocytic marker) is not present in the germinal matrix until after 28 weeks. The immature BBB in the germinal matrix may contribute to different pathophysiology in the neonate compared with adults. As a result of breakdown of these barriers, IVH may introduce an atmosphere of increased protein content and contribute to altered osmotic gradients. A number of proteins are elevated in the CSF after IVH including thrombopoietin, ferritin, glial fibrillary protein (GFAP) and S-10091, plasminogen activator inhibitor 159, TGFβ-1, TGFβ-2 and VEGF38 and increased levels of a number of proteins may result in the production of protein-rich vasogenic edema 90. This has been demonstrated in an animal model where intraventricular injection of hyperosmolar dextran resulted in hydrocephalus38 presumably through secretion of water into the ventricular space via the choroid plexus or across the ependymal wall. However it is unclear if increasing osmolarity or protein content would be able to maintain hydrocephalus as the increased osmolarity would be expected to dissipate over time.
Aquaporins
As ventricular enlargement and hydrocephalus most often occur through a net increase in overall brain water content, alterations to water regulation may be a contributory factor. Water is movement across cell membranes can be facilitated by water channels called aquaporins (AQPs). AQP 4 is the most abundant aquaporin in the brain and is present at astrocyte endfeet, glia limitans, and basolateral surface of ependymal cells92,93. In the presence of cerebral ischemia and cytotoxic edema, the lack of AQP 4 appears to be protective as AQP 4−/− mice display less edema after such insults94. In contrast, those mice have increased intracranial pressure and brain water content after insults resulting in vasogenic edema 95 and have greater injury after ICH with increased peri-lesional edema 96.
AQP 4 channels are found as early as 23 weeks of gestation in premature infants 12. AQP 4 channels are upregulated in hydrocephalus 97 and are thought to be involved in compensatory transependymal water flow. Hydrocephalus occurs in 10% of AQP 4−/− mice and occurs either through defective water transport or through breakdown of the ependymal surface and subsequent obstruction of small CSF apertures such as the cerebral aqueduct or 4th ventricular outlets98. Additionally AQP 4−/− mice develop hydrocephalus more rapidly99 as AQP 4 is responsible for transependymal flow of water and is upregulated in hydrocephalus. In a kaolin-induced model of hydrocephalus in adult rats, AQP 4 was found to be upregulated after 2 weeks with co-localization with GFAP in the glial limitans and perivascular astroglia, although there was an initial decrease in levels at 2 days100. AQP 4 has also been implicated in cell adhesion 101 and ependymal integrity 102 with AQP 4−/− mice displaying an altered BBB phenotype103.
Another aquaporin, AQP 1, is also present within the brain at the apical membrane of the choroid plexus epithelium where it is involved in CSF secretion. There is a paucity of studies evaluating AQP 1 and hydrocephalus, with a recent review on the topic only finding 5 studies, of which 1 was a case report and one a small case series104. Not surprisingly, in AQP 1−/− mice CSF production is decreased105 and these mice do not develop hydrocephalus94. There was no difference in AQP-1 levels at 4 weeks and 9 months 97 in a kaolin-induced model of hydrocephalus, whereas there was an initial decrease in choroid plexus AQP 1 expression in the hydrocephalic HTX rat106. AQP 1 function may be regulated by intracellular localization rendering it nonfunctional, while preserving overall protein levels93.
Inflammation
Inflammation is thought to be involved in the pathogenesis of cerebral palsy in preterm infants107. It has also been investigated as a possible cause of hydrocephalus after GMH-IVH, where there is fibrosing arachnoiditis, meningeal fibrosis and subependymal gliosis44,108. Inflammation and subsequent scarring may prevent the flow of CSF either through the cerebral aqueduct, 4th ventricular outlets, basal cisterns or arachnoid granulations.
Transforming growth factor (TGF)-β1 is involved in regulating extracellular matrix proteins and it causes communicating hydrocephalus when injected into the subarachnoid space in mice109. Interestingly, there was no obstruction to CSF outflow from the 4th ventricle or at the level of the cerebral aqueduct in this model. However, the authors did note destruction of cilia on the ependymal surface. Preterm infants with GMH-IVH and ventricular enlargement have been reported to have elevated inflammatory markers, such as TNF-α, in their CSF110. However, in that particular study, the control group consisted of preterm infants with normal cranial ultrasound and, therefore, did not have GMH-IVH so changes in the inflammatory markers may relate to IVH, hydrocephalus or both. Periventricular TGF-β is present after IVH in neonatal rats, however there was no difference in immunohistochemical staining between animals that went on to develop hydrocephalus and those who did not111. TGF-β1 release following IVH upregulates fibronectin and laminin 15, and an increase in perivascular fibronectin has been reported in rats that develop hydrocephalus after IVH 111. However, two studies have found that TGF-β inhibitors do not attenuate ventricular dilation after IVH in rats112,113.
Activation of NF-κB signaling at the choroid plexus and ependymal lining has been reported in a rat IVH model89, suggesting that disruption of the ependymal lining through an inflammatory mechanism may be involved in the pathogenesis of hydrocephalus. Macrophages are normally found in the ventricular system114. In a 6-aminonicotinamide model of hydrocephalus, there was an increase in intraventricular macrophages and these cells were found to contain erythrocytes, which may be important in IVH-mediated hydrocephalus115. Intraventricular macrophages express transferrin receptors and may also be involved in iron regulation, which is also of possible importance after IVH116 as hemosiderin is seen within macrophages after hemorrhage73. The subarachnoid space is also subject to the effects of inflammation post hemorrhage, as arachnoid cells are able to serve as antigen presenting cells after SAH117. Although inflammatory cells are seen in the subarachnoid space and arachnoid villi post hemorrhage, multiple groups have not demonstrated arachnoid villus fibrosis after SAH53,55.
Complement activation occurs after injury and is involved in ICH-mediated cerebral edema and RBC lysis118,119. Complement activation may also play a role in hydrocephalus as there was complement type 3 receptor upregulation in intraventricular macrophages in a rat model of hydrocephalus115. Complement-mediated erythrocyte lysis may expose the CSF and brain to the damaging effects of iron and we are currently examining the role of complement in hydrocephalus and neuronal death after IVH.
Iron
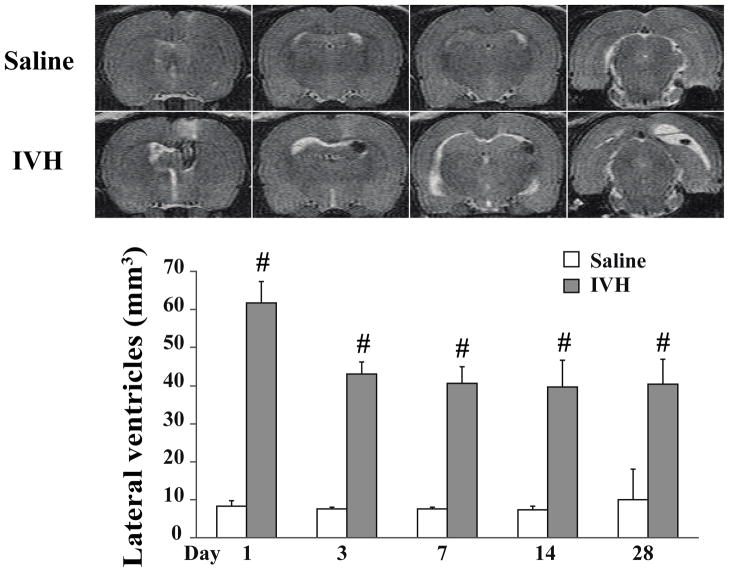
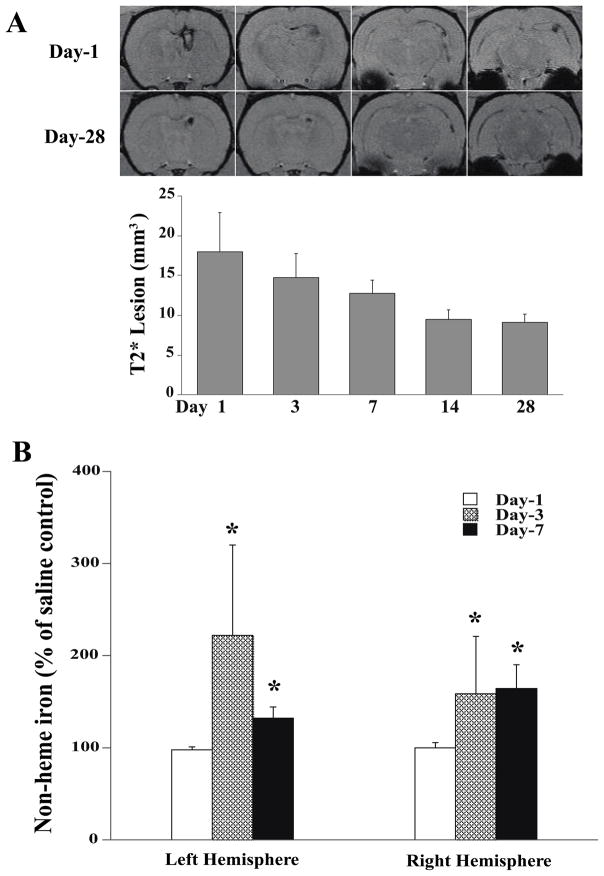
Iron, has long been known to cause neuropathology in neurodegenerative diseases such as Parkinson’s, Alzheimer’s and superficial siderosis. Iron is a degradation product of hemoglobin and has been looked at as a mechanism of injury after ICH, SAH and IVH120–122. Non protein bound iron is elevated in neonates with PHVD compared to neonates without hemorrhage123. After IVH in rats, non-heme iron, and the iron-handling proteins, heme-oxygenase 1 and ferritin, were increased along with ventricular size42. Our group has established an adult rodent IVH model with stereotactic injection of 200 ul autologous blood (or saline control) into the right lateral ventricle. With this model enlargement of the lateral ventricles occurs within 1 day after IVH and persists up to 28 days (figure 2). Iron accumulation was measured using T2* MRI sequences and was highest 1 day after IVH and slowly declined up to 28 days (figure 3a). Non-heme iron was also quantified and was elevated in both hemispheres on day 3 and 7 after IVH, compared with saline controls (figure 3b). Intraventricular injection of lysed erythrocytes and elemental iron causes rapid ventricular enlargement and death in adult rats, compared with injection of packed erythrocytes (Xi et al, unpublished data). Further support for a role of iron comes from studies with iron chelators such as deferoxamine and minocycline. Deferoxamine reduces ventricular enlargement after IVH42 and white matter injury after ICH124, and minocycline reduces edema after ICH125 although it is possible that some of the effects of these drugs are via mechanisms other than iron chelation.
Figure 2.
T2 weighted MRI scans (coronal brain sections) at day 1 after saline injection and IVH, and the time-course of lateral ventricular volume changes. Values are mean ±S.D., n=7, #p<0.01 vs. saline group. Figure reprinted with permission from Chen et al., Stroke 2011;42:465–470.
Figure 3.
(A) T2* weighted MRI scans (coronal sections) at day 1 and day 28 after IVH, and the time-course of the T2* lesion (hypodensity) after IVH. Values are expressed as the means ± S.D., n=7. (B) Time course of non-heme brain tissue iron in the left and right hemisphere after IVH. Values are mean ± S.D., n=6, *p<0.05 vs. saline control (same time point) and day 1. Figure reprinted with permission from Chen et al., Stroke 2011;42:465–470.
Hemosiderin deposits are seen in the in the ventricular wall in hydrocephalus after IVH72. Ependymal cells, are thought to take up iron from the CSF and prevent iron diffusion to the rest of the brain126. Under the condition with iron release into CSF after IVH, there is the potential for iron-induced ependymal damage. Similarly, oligodendrocytes are iron scavengers within the CNS. The white matter-lined ventricles contain numerous oligodendrocytes and exposure of the CSF to blood, may result in iron uptake and possible iron overload in those cells. Recent work from our lab demonstrated an increase in ferritin containing cells both in the periventricular area and hippocampus after IVH compared with saline control (figure 4).
Figure 4.
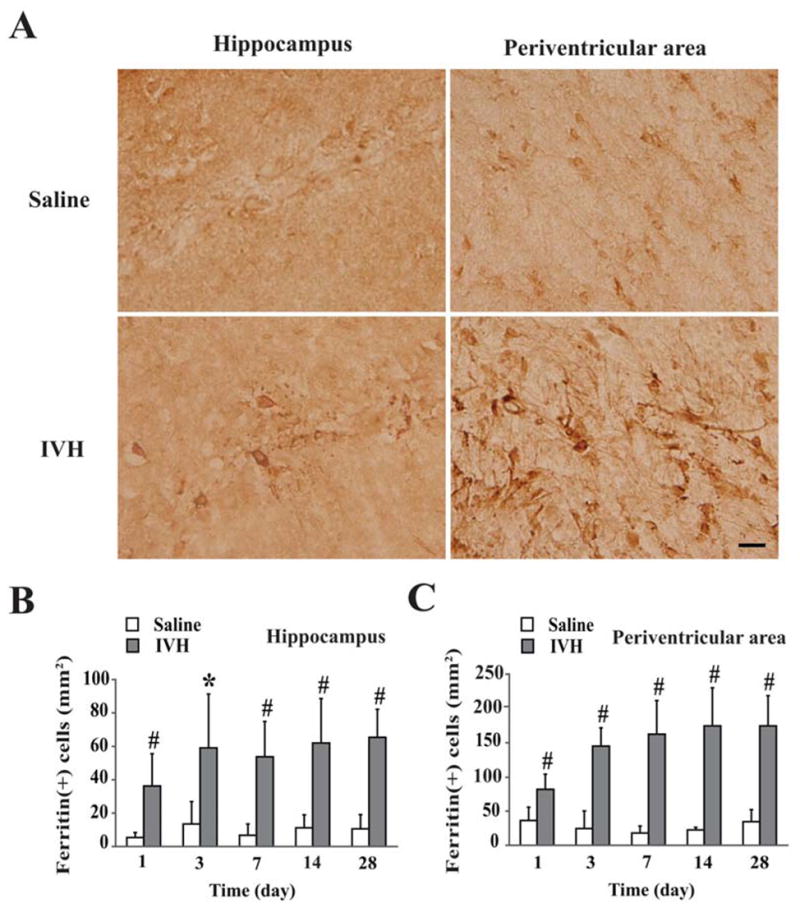
(A) Immunoreactivity of ferritin in the hippocampus and the periventricular area day 28 after IVH or saline control. Scale bar = 20 μm. (B) Time course of ferritin levels in the hippocampus. (C) Time course of ferritin levels in the periventricular area. Values are mean ± SD, n=3~7 in saline group and n=5~7 in IVH group, *p<0.05 and #p<0.01 vs. saline group. Figure reprinted with permission from Chen et al., Stroke 2011;42:465–470.
Iron regulation differs between the adult and the developing brain, which may impact the effects of IVH. After intraventricular administration, iron and transferrin are more widely distributed throughout the brain early in development. In addition, the uptake and iron content in the brain increases after birth126.
Iron and AQP 4 have been implicated in brain injury after ICH. In a rat model of ICH, iron deposition and AQP 4 were present in the perihematomal area and the iron chelator, deferoxamine, reduced both perihematomal edema and AQP 4 upregulation 127. Periventricular iron accumulation after IVH may cause upregulation of AQP 4 and subsequently result in enlargement of the ventricles128 or edema. There have been no studies looking at AQP 4 or AQP 1 and IVH.
Therapeutic interventions
Studies investigating treatment for IVH and post-hemorrhagic hydrocephalus have focused on the neonatal population as GMH-IVH occurs in a specific population at a predicted time point (i.e. in premature infants within 72 hours of birth), offering an opportunity to intervene prior to the time of potential injury18,129. Antenatal attempts to reduce GMH-IVH have included maternal administration of phenobarbital, vitamin K, corticosteroids, indomethacin, and magnesium15. Maternal administration of corticosteroids has been the only antenatal treatment to reduce the incidence of IVH in preterm infants130. Postnatal treatments that have been investigated include phenobarbital, paralytics, indomethacin, ethamsylate and vitamin E. Of these, indomethacin is the only treatment that has shown any possible effect on IVH131.
Shooman et al, in their review of treatment for post-hemorrhagic hydrocephalus, noted only 13 randomized trials addressing this topic129. Of those trials, there was no therapy that reduced the incidence of shunt surgery in preterm infants with GMH-IVH and hydrocephalus. As the amount and duration of blood in the ventricular system is associated with rates of hydrocephalus, there has been some interest in clot removal as a therapeutic intervention. However, removing blood through drainage, lysis with TPA and irrigation was investigated in GMH-IVH132 and there was no difference in rates of shunt surgery compared with reservoir tapping and there was an increase in secondary hemorrhage in those treated with drainage, TPA and irrigation. In addition, early removal of CSF by lumbar puncture or subcutaneous reservoir does not decrease the risk of shunt dependence129. Diuretics to decrease CSF production have shown no benefit and in fact, resulted in worse outcome and increased risk of death133
Adult studies on the treatment of IVH have focused on intraventricular t-PA administration. Some have suggested that intraventricular thrombolysis10 may result in decreased mortality and better functional outcomes compared with untreated controls. The IVH thrombolysis trial was recently published, which showed an acceptable safety profile for intraventricular low-dose recombinant t-PA after ICH with IVH57. Rates of shunt surgery are not a primary outcome in a number of the studies on intraventricular t-PA, however, there appears to be a trend towards reduced incidence of shunt surgery10. There may be an additional effect on inflammation by t-PA, as in one retrospective study individuals who received intraventricular t-PA demonstrated fewer CSF white blood cells 5–6 days after hemorrhage24.
Conclusion
IVH is a cause of significant morbidity and mortality as a complication of neonatal GMH and adult ICH. IVH causes hydrocephalus in 25–50% of these individuals and results in additional damage to the brain. There has been little research on the mechanisms of IVH-induced hydrocephalus in recent years. Arachnoid granulations may be involved initially through obstruction by microthrombi and subsequently by inflammation and fibrosis, however the data supporting this are sparse. Other mechanisms of hydrocephalus may involve accessory CSF drainage pathways, damage to the ependymal surface and cilia, changes in expression of aquaporin channels, and pathways related to iron and inflammation. Investigating the pathogenesis of hydrocephalus after IVH may lead to additional treatment strategies, which are needed as the current mainstay of treatment has not changed in the last 50 years.
Figure 5.
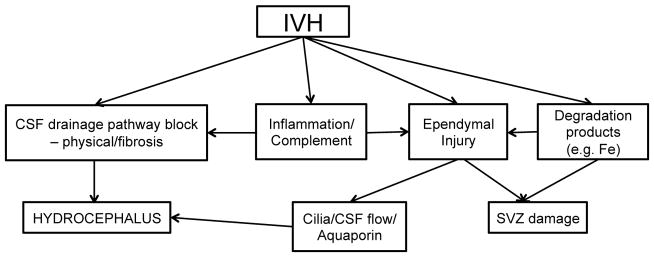
Outline of some of the injury pathways that may be initiated by an intraventricular hemorrhage. SVZ = subventricular zone
Acknowledgments
This work was supported by grants NS-039866 and NS-073595 from the National Institutes of Health (NIH) and 0840016N from American Heart Association (AHA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and AHA.
References
- 1.Bassan H. Intracranial Hemorrhage in the Preterm Infant: Understanding It, Preventing It. Clinics in Perinatology. 2009;36:737–762. doi: 10.1016/j.clp.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, Horwood LJ, Volpe JJ. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed. 2002;87:F37–41. doi: 10.1136/fn.87.1.F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattathiri PS, Gregson B, Prasad KSM, Mendelow AD STICH Investigators. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir Suppl. 2006;96:65–68. doi: 10.1007/3-211-30714-1_16. [DOI] [PubMed] [Google Scholar]
- 4.Hwang BY, Bruce SS, Appelboom G, Piazza MA, Carpenter AM, Gigante PR, Kellner CP, Ducruet AF, Kellner MA, Deb-Sen R, Vaughan KA, Meyers PM, Connolly ES. Evaluation of intraventricular hemorrhage assessment methods for predicting outcome following intracerebral hemorrhage. J Neurosurg. 2012;116:185–192. doi: 10.3171/2011.9.JNS10850. [DOI] [PubMed] [Google Scholar]
- 5.Woo D, Broderick JP. Spontaneous intracerebral hemorrhage: epidemiology and clinical presentation. Neurosurg Clin N Am. 2002;13:265. doi: 10.1016/s1042-3680(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 6.Zahuranec DB, Gonzales NR, Brown DL, Lisabeth LD, Longwell PJ, Eden SV, Smith MA, Garcia NM, Hoff JT, Morgenstern LB. Presentation of intracerebral haemorrhage in a community. J Neurol Neurosurg Psychiatr. 2006;77:340–344. doi: 10.1136/jnnp.2005.077164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallevi H, Albright KC, Aronowski J, Barreto AD, Martin-Schild S, Khaja AM, Gonzales NR, Illoh K, Noser EA, Grotta JC. Intraventricular hemorrhage: Anatomic relationships and clinical implications. Neurology. 2008;70:848–852. doi: 10.1212/01.wnl.0000304930.47751.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: a previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke. 1998;29:1352–1357. doi: 10.1161/01.str.29.7.1352. [DOI] [PubMed] [Google Scholar]
- 9.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Critical Care Medicine. 1999;27:617. doi: 10.1097/00003246-199903000-00045. [DOI] [PubMed] [Google Scholar]
- 10.Staykov D, Bardutzky J, Huttner HB, Schwab S. Intraventricular fibrinolysis for intracerebral hemorrhage with severe ventricular involvement. Neurocrit Care. 2011;15:194–209. doi: 10.1007/s12028-010-9390-x. [DOI] [PubMed] [Google Scholar]
- 11.Gould SJ, Howard S. Glial differentiation in the germinal layer of fetal and preterm infant brain: an immunocytochemical study. Pediatr Pathol. 1988;8:25–36. doi: 10.3109/15513818809022277. [DOI] [PubMed] [Google Scholar]
- 12.Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview. Neurobiology of Disease. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Braun A, Xu H, Hu F, Kocherlakota P, Siegel D, Chander P, Ungvari Z, Csiszar A, Nedergaard M, Ballabh P. Paucity of Pericytes in Germinal Matrix Vasculature of Premature Infants. Journal of Neuroscience. 2007;27:12012–12024. doi: 10.1523/JNEUROSCI.3281-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitelaw A. Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Seminars in Neonatology. 2001;6:135–146. doi: 10.1053/siny.2001.0047. [DOI] [PubMed] [Google Scholar]
- 16.Steiner T, Diringer MN, Schneider D, Mayer SA, Begtrup K, Broderick J, Skolnick BE, Davis SM. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006;59:767–73. doi: 10.1227/01.NEU.0000232837.34992.32. discussion 773–4. [DOI] [PubMed] [Google Scholar]
- 17.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. The Journal of Pediatrics. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 18.Whitelaw A, Aquilina K. Management of posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal Ed. 2011 doi: 10.1136/adc.2010.190173. [DOI] [PubMed] [Google Scholar]
- 19.Hallevi H, Dar NS, Barreto AD, Morales MM, Martin-Schild S, Abraham AT, Walker KC, Gonzales NR, Illoh K, Grotta JC, Savitz SI. The IVH score: a novel tool for estimating intraventricular hemorrhage volume: clinical and research implications. Critical Care Medicine. 2009;37:969–74. e1. doi: 10.1097/CCM.0b013e318198683a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graeb DA, Robertson WD, Lapointe JS, Nugent RA, Harrison PB. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology. 1982;143:91–96. doi: 10.1148/radiology.143.1.6977795. [DOI] [PubMed] [Google Scholar]
- 21.LeRoux PD, Haglund MM, Newell DW, Grady MS, Winn HR. Intraventricular hemorrhage in blunt head trauma: an analysis of 43 cases. Neurosurgery. 1992;31:678–84. doi: 10.1227/00006123-199210000-00010. discussion 684–5. [DOI] [PubMed] [Google Scholar]
- 22.Xue M, Balasubramaniam J, Buist RJ, Peeling J, Del Bigio MR. Periventricular/intraventricular hemorrhage in neonatal mouse cerebrum. J Neuropathol Exp Neurol. 2003;62:1154–1165. doi: 10.1093/jnen/62.11.1154. [DOI] [PubMed] [Google Scholar]
- 23.Del Bigio MR. Cell proliferation in human ganglionic eminence and suppression after prematurity-associated haemorrhage. Brain. 2011;134:1344–1361. doi: 10.1093/brain/awr052. [DOI] [PubMed] [Google Scholar]
- 24.Hallevi H, Walker KC, Kasam M, Bornstein N, Grotta JC, Savitz SI. Inflammatory response to intraventricular hemorrhage: Time course, magnitude and effect of t-PA. J Neurol Sci. 2011 doi: 10.1016/j.jns.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Del Bigio MR. Pathophysiologic consequences of hydrocephalus. Neurosurg Clin N Am. 2001;12:639–49. vii. [PubMed] [Google Scholar]
- 26.Whitelaw A. Posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal Ed. 2002;86:72F–74. doi: 10.1136/fn.86.2.F72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balasubramaniam J, Del Bigio MR. Animal models of germinal matrix hemorrhage. J Child Neurol. 2006;21:365–371. doi: 10.1177/08830738060210050201. [DOI] [PubMed] [Google Scholar]
- 28.Cherian SS, Love S, Silver IA, Porter HJ, Whitelaw AGL, Thoresen M. Posthemorrhagic ventricular dilation in the neonate: development and characterization of a rat model. J Neuropathol Exp Neurol. 2003;62:292–303. doi: 10.1093/jnen/62.3.292. [DOI] [PubMed] [Google Scholar]
- 29.Balasubramaniam J, Xue M, Buist RJ, Ivanco TL, Natuik S, Del Bigio MR. Persistent motor deficit following infusion of autologous blood into the periventricular region of neonatal rats. Experimental Neurology. 2006;197:122–132. doi: 10.1016/j.expneurol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Lekic T, Manaenko A, Rolland W, Tang J, Zhang JH. A novel preclinical model of germinal matrix hemorrhage using neonatal rats. Acta Neurochir Suppl. 2011;111:55–60. doi: 10.1007/978-3-7091-0693-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alles YCJ, Greggio S, Alles RM, Azevedo PN, Xavier LL, DaCosta JC. A novel preclinical rodent model of collagenase-induced germinal matrix/intraventricular hemorrhage. Brain Research. 2010;1356:130–138. doi: 10.1016/j.brainres.2010.07.106. [DOI] [PubMed] [Google Scholar]
- 32.Lorenzo AV, Welch K, Conner S. Spontaneous germinal matrix and intraventricular hemorrhage in prematurely born rabbits. J Neurosurg. 1982;56:404–410. doi: 10.3171/jns.1982.56.3.0404. [DOI] [PubMed] [Google Scholar]
- 33.Chua CO, Chahboune H, Braun A, Dummula K, Chua CE, Yu J, Ungvari Z, Sherbany AA, Hyder F, Ballabh P. Consequences of intraventricular hemorrhage in a rabbit pup model. Stroke. 2009;40:3369–3377. doi: 10.1161/STROKEAHA.109.549212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Georgiadis P, Xu H, Chua C, Hu F, Collins L, Huynh C, Lagamma EF, Ballabh P. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke. 2008;39:3378–3388. doi: 10.1161/STROKEAHA.107.510883. [DOI] [PubMed] [Google Scholar]
- 35.Aquilina K, Hobbs C, Cherian S, Tucker A, Porter H, Whitelaw A, Thoresen M. A neonatal piglet model of intraventricular hemorrhage and posthemorrhagic ventricular dilation. J Neurosurg. 2007;107:126–136. doi: 10.3171/PED-07/08/126. [DOI] [PubMed] [Google Scholar]
- 36.Oi S, Di Rocco C. Proposal of “evolution theory in cerebrospinal fluid dynamics” and minor pathway hydrocephalus in developing immature brain. Childs Nerv Syst. 2006;22:662–669. doi: 10.1007/s00381-005-0020-4. [DOI] [PubMed] [Google Scholar]
- 37.Dandy WE, Blackfan KD. An experimental and clinical study of internal hydrocephalus. JAMA: The Journal of the American Medical Association. 1913;61:2216–2217. [Google Scholar]
- 38.Krishnamurthy S, Li J, Schultz L, McAllister JP. Intraventricular infusion of hyperosmolar dextran induces hydrocephalus: a novel animal model of hydrocephalus. Cerebrospinal Fluid Res. 2009;6:16. doi: 10.1186/1743-8454-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Williams MA, Rigamonti D. Genetics of human hydrocephalus. J Neurol. 2006;253:1255–1266. doi: 10.1007/s00415-006-0245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galbreath E, Kim SJ, Park K, Brenner M, Messing A. Overexpression of TGF-beta 1 in the central nervous system of transgenic mice results in hydrocephalus. J Neuropathol Exp Neurol. 1995;54:339–349. doi: 10.1097/00005072-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 41.McAllister JP., II . Experimental Hydrocephalus. In: Winn HR, editor. Youmans Neurological Surgery. 6. Elsevier; 2011. [Google Scholar]
- 42.Chen Z, Gao C, Hua Y, Keep RF, Muraszko K, Xi G. Role of Iron in Brain Injury After Intraventricular Hemorrhage. Stroke. 2011;42:465–470. doi: 10.1161/STROKEAHA.110.602755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lodhia KR, Shakui P, Keep RF. Hydrocephalus in a rat model of intraventricular hemorrhage. Acta Neurochir Suppl. 2006;96:207–211. doi: 10.1007/3-211-30714-1_45. [DOI] [PubMed] [Google Scholar]
- 44.Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 3. Effects of intraventricular urokinase on clot lysis and posthemorrhagic hydrocephalus. Neurosurgery. 1986;19:553–572. doi: 10.1227/00006123-198610000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Mayfrank L, Kissler J, Raoofi R, Delsing P, Weis J, Küker W, Gilsbach JM. Ventricular dilatation in experimental intraventricular hemorrhage in pigs. Characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke. 1997;28:141–148. doi: 10.1161/01.str.28.1.141. [DOI] [PubMed] [Google Scholar]
- 46.Adeoye O, Clark JF, Khatri P, Wagner KR, Zuccarello M, Pyne-Geithman GJ. Do Current Animal Models of Intracerebral Hemorrhage Mirror the Human Pathology? Transl Stroke Res. 2011;2:17–25. doi: 10.1007/s12975-010-0037-1. [DOI] [PubMed] [Google Scholar]
- 47.Xue M, Del Bigio MR. Injections of blood, thrombin, and plasminogen more severely damage neonatal mouse brain than mature mouse brain. Brain Pathol. 2005;15:273–280. doi: 10.1111/j.1750-3639.2005.tb00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larroche JC. Post-haemorrhagic hydrocephalus in infancy. Anatomical study. Biol Neonate. 1972;20:287–299. doi: 10.1159/000240472. [DOI] [PubMed] [Google Scholar]
- 49.Hill A, Shackelford GD, Volpe JJ. A potential mechanism of pathogenesis for early posthemorrhagic hydrocephalus in the premature newborn. Pediatrics. 1984;73:19–21. [PubMed] [Google Scholar]
- 50.Weed LH. Meninges and Cerebrospinal Fluid. J Anat. 1938;72:181–215. [PMC free article] [PubMed] [Google Scholar]
- 51.Mann JD, Butler AB, Rosenthal JE, Maffeo CJ, Johnson RN, Bass NH. Regulation of intracranial pressure in rat, dog, and man. Ann Neurol. 1978;3:156–165. doi: 10.1002/ana.410030212. [DOI] [PubMed] [Google Scholar]
- 52.Dandy WE. Experimental Hydrocephalus. Annals of Surgery. 1919;70:129–142. doi: 10.1097/00000658-191908000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Massicotte EM, Del Bigio MR. Human arachnoid villi response to subarachnoid hemorrhage: possible relationship to chronic hydrocephalus. J Neurosurg. 1999;91:80–84. doi: 10.3171/jns.1999.91.1.0080. [DOI] [PubMed] [Google Scholar]
- 54.Greitz D. Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev. 2004;27:145–65. doi: 10.1007/s10143-004-0326-9. discussion 166–7. [DOI] [PubMed] [Google Scholar]
- 55.Torvik A, Bhatia R, Murthy VS. Transitory block of the arachnoid granulations following subarachnoid haemorrhage. A postmortem study. Acta Neurochir (Wien) 1978;41:137–146. doi: 10.1007/BF01809144. [DOI] [PubMed] [Google Scholar]
- 56.Upton ML, Weller RO. The morphology of cerebrospinal fluid drainage pathways in human arachnoid granulations. J Neurosurg. 1985;63:867–875. doi: 10.3171/jns.1985.63.6.0867. [DOI] [PubMed] [Google Scholar]
- 57.Naff N, Williams MA, Keyl PM, Tuhrim S, Bullock MR, Mayer SA, Coplin W, Narayan R, Haines S, Cruz-Flores S, Zuccarello M, Brock D, Awad I, Ziai WC, Marmarou A, Rhoney D, McBee N, Lane K, Hanley DF. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial. Stroke. 2011;42:3009–3016. doi: 10.1161/STROKEAHA.110.610949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitelaw A, Mowinckel MC, Abildgaard U. Low levels of plasminogen in cerebrospinal fluid after intraventricular haemorrhage: a limiting factor for clot lysis? Acta Paediatr. 1995;84:933–936. doi: 10.1111/j.1651-2227.1995.tb13795.x. [DOI] [PubMed] [Google Scholar]
- 59.Hansen A, Whitelaw A, Lapp C, Brugnara C. Cerebrospinal fluid plasminogen activator inhibitor-1: a prognostic factor in posthaemorrhagic hydrocephalus. Acta Paediatr. 1997;86:995–998. doi: 10.1111/j.1651-2227.1997.tb15186.x. [DOI] [PubMed] [Google Scholar]
- 60.Gomez DG, Ehrmann JE, Gordon Potts D, Pavese AM, Gilanian A. The arachnoid granulations of the newborn human: An ultrastructural study. International Journal of Developmental Neuroscience. 1983;1:139–147. doi: 10.1016/0736-5748(83)90040-0. [DOI] [PubMed] [Google Scholar]
- 61.Papaiconomou C, Bozanovic-Sosic R, Zakharov A, Johnston M. Does neonatal cerebrospinal fluid absorption occur via arachnoid projections or extracranial lymphatics? Am J Physiol Regul Integr Comp Physiol. 2002;283:R869–76. doi: 10.1152/ajpregu.00173.2002. [DOI] [PubMed] [Google Scholar]
- 62.Rammling M, Madan M, Paul L, Behnam B, Pattisapu JV. Evidence for reduced lymphatic CSF absorption in the H-Tx rat hydrocephalus model. Cerebrospinal Fluid Res. 2008;5:15. doi: 10.1186/1743-8454-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edsbagge M, Tisell M, Jacobsson L, Wikkelso C. Spinal CSF absorption in healthy individuals. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1450–5. doi: 10.1152/ajpregu.00215.2004. [DOI] [PubMed] [Google Scholar]
- 64.Bozanovic-Sosic R, Mollanji R, Johnston MG. Spinal and cranial contributions to total cerebrospinal fluid transport. Am J Physiol Regul Integr Comp Physiol. 2001;281:R909–16. doi: 10.1152/ajpregu.2001.281.3.R909. [DOI] [PubMed] [Google Scholar]
- 65.Boulton M, Armstrong D, Flessner M, Hay J, Szalai JP, Johnston M. Raised intracranial pressure increases CSF drainage through arachnoid villi and extracranial lymphatics. Am J Physiol. 1998;275:R889–96. doi: 10.1152/ajpregu.1998.275.3.R889. [DOI] [PubMed] [Google Scholar]
- 66.Boulton M, Flessner M, Armstrong D, Mohamed R, Hay J, Johnston M. Contribution of extracranial lymphatics and arachnoid villi to the clearance of a CSF tracer in the rat. Am J Physiol. 1999;276:R818–23. doi: 10.1152/ajpregu.1999.276.3.R818. [DOI] [PubMed] [Google Scholar]
- 67.Kelkenberg U, von Rautenfeld DB, Brinker T, Hans VH. Chicken arachnoid granulations: a new model for cerebrospinal fluid absorption in man. Neuroreport. 2001;12:553–557. doi: 10.1097/00001756-200103050-00024. [DOI] [PubMed] [Google Scholar]
- 68.Glimcher SA, Holman DW, Lubow M, Grzybowski DM. Ex Vivo Model of Cerebrospinal Fluid Outflow across Human Arachnoid Granulations. Investigative Ophthalmology & Visual Science. 2008;49:4721–4728. doi: 10.1167/iovs.08-2238. [DOI] [PubMed] [Google Scholar]
- 69.Greitz D. The hydrodynamic hypothesis versus the bulk flow hypothesis. Neurosurg Rev. 2004;27:299–300. doi: 10.1007/s10143-004-0349-2. [DOI] [PubMed] [Google Scholar]
- 70.Greitz D. Paradigm shift in hydrocephalus research in legacy of Dandy’s pioneering work: rationale for third ventriculostomy in communicating hydrocephalus. Child’s Nervous System. 2007;23:487–489. doi: 10.1007/s00381-007-0303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Egnor M, Zheng L, Rosiello A, Gutman F, Davis R. A model of pulsations in communicating hydrocephalus. Pediatr Neurosurg. 2002;36:281–303. doi: 10.1159/000063533. [DOI] [PubMed] [Google Scholar]
- 72.Fukumizu M, Takashima S, Becker LE. Neonatal posthemorrhagic hydrocephalus: neuropathologic and immunohistochemical studies. Pediatr Neurol. 1995;13:230–234. doi: 10.1016/0887-8994(95)00183-g. [DOI] [PubMed] [Google Scholar]
- 73.Sarnat HB. Ependymal reactions to injury. A review. J Neuropathol Exp Neurol. 1995;54:1–15. doi: 10.1097/00005072-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 74.Del Bigio MR. Ependymal cells: biology and pathology. Acta Neuropathol. 2010;119:55–73. doi: 10.1007/s00401-009-0624-y. [DOI] [PubMed] [Google Scholar]
- 75.Ihrie RA, Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sawamoto K. New Neurons Follow the Flow of Cerebrospinal Fluid in the Adult Brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 77.Mirzadeh Z, Han Y-G, Soriano-Navarro M, García-Verdugo JM, Alvarez-Buylla A. Cilia organize ependymal planar polarity. Journal of Neuroscience. 2010;30:2600–2610. doi: 10.1523/JNEUROSCI.3744-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, Bell PD, Schwiebert EM, Yoder BK. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005;132:5329–5339. doi: 10.1242/dev.02153. [DOI] [PubMed] [Google Scholar]
- 79.Han Y-G, Alvarez-Buylla A. Role of primary cilia in brain development and cancer. Curr Opin Neurobiol. 2010;20:58–67. doi: 10.1016/j.conb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nguyen T, Chin WC, O’Brien JA, Verdugo P, Berger AJ. Intracellular pathways regulating ciliary beating of rat brain ependymal cells. J Physiol (Lond) 2001;531:131–140. doi: 10.1111/j.1469-7793.2001.0131j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spassky N. Adult Ependymal Cells Are Postmitotic and Are Derived from Radial Glial Cells during Embryogenesis. Journal of Neuroscience. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greenstone MA, Jones RW, Dewar A, Neville BG, Cole PJ. Hydrocephalus and primary ciliary dyskinesia. Arch Dis Child. 1984;59:481–482. doi: 10.1136/adc.59.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Santi MM, Magni A, Valletta EA, Gardi C, Lungarella G. Hydrocephalus, bronchiectasis, and ciliary aplasia. Arch Dis Child. 1990;65:543–544. doi: 10.1136/adc.65.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ibanez-Tallon I. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Human Molecular Genetics. 2004;13:2133–2141. doi: 10.1093/hmg/ddh219. [DOI] [PubMed] [Google Scholar]
- 85.Krueger RC, Wu H, Zandian M, Danielpour M, Kabos P, Yu JS, Sun YE. Neural progenitors populate the cerebrospinal fluid of preterm patients with hydrocephalus. The Journal of Pediatrics. 2006;148:337–340. doi: 10.1016/j.jpeds.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 86.Ohtoshi A. Hydrocephalus caused by conditional ablation of the Pten or beta-catenin gene. Cerebrospinal Fluid Res. 2008;5:16. doi: 10.1186/1743-8454-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dummula K, Vinukonda G, Chu P, Xing Y, Hu F, Mailk S, Csiszar A, Chua C, Mouton P, Kayton RJ, Brumberg JC, Bansal R, Ballabh P. Bone Morphogenetic Protein Inhibition Promotes Neurological Recovery after Intraventricular Hemorrhage. Journal of Neuroscience. 2011;31:12068–12082. doi: 10.1523/JNEUROSCI.0013-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sarnat HB. Role of human fetal ependyma. Pediatr Neurol. 1992;8:163–178. doi: 10.1016/0887-8994(92)90063-5. [DOI] [PubMed] [Google Scholar]
- 89.Simard PF, Tosun C, Melnichenko L, Ivanova S, Gerzanich V, Simard JM. Inflammation of the Choroid Plexus and Ependymal Layer of the Ventricle Following Intraventricular Hemorrhage. Transl Stroke Res. 2011;2:227–231. doi: 10.1007/s12975-011-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Simard JM, Geng Z, Woo SK, Ivanova S, Tosun C, Melnichenko L, Gerzanich V. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2009;29:317–330. doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Whitelaw A, Rosengren L, Blennow M. Brain specific proteins in posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal Ed. 2001;84:F90–1. doi: 10.1136/fn.84.2.F90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Venero JL, Vizuete ML, Machado A, Cano J. Aquaporins in the central nervous system. Prog Neurobiol. 2001;63:321–336. doi: 10.1016/s0301-0082(00)00035-6. [DOI] [PubMed] [Google Scholar]
- 93.Brian OK, Tom P, Wang D. Aquaporins: relevance to cerebrospinal fluid physiology and therapeutic potential in hydrocephalus. 2010. pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Owler BK, Pitham T, Wang D. Aquaporins: relevance to cerebrospinal fluid physiology and therapeutic potential in hydrocephalus. Cerebrospinal Fluid Res. 2010;7:15. doi: 10.1186/1743-8454-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- 96.Tang Y, Wu P, Su J, Xiang J, Cai D, Dong Q. Effects of Aquaporin-4 on edema formation following intracerebral hemorrhage. Experimental Neurology. 2010;223:485–495. doi: 10.1016/j.expneurol.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 97.Mao X, Enno TL, Del Bigio MR. Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur J Neurosci. 2006;23:2929–2936. doi: 10.1111/j.1460-9568.2006.04829.x. [DOI] [PubMed] [Google Scholar]
- 98.Feng X, Papadopoulos MC, Liu J, Li L, Zhang D, Zhang H, Verkman AS, Ma T. Sporadic obstructive hydrocephalus in Aqp4 null mice. J Neurosci Res. 2009;87:1150–1155. doi: 10.1002/jnr.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bloch O, Auguste KI, Manley GT, Verkman AS. Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J Cereb Blood Flow Metab. 2006;26:1527–1537. doi: 10.1038/sj.jcbfm.9600306. [DOI] [PubMed] [Google Scholar]
- 100.Skjolding AD, Rowland IJ, Søgaard LV, Praetorius J, Penkowa M, Juhler M. Hydrocephalus induces dynamic spatiotemporal regulation of aquaporin-4 expression in the rat brain. Cerebrospinal Fluid Res. 2010;7:20. doi: 10.1186/1743-8454-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hiroaki Y, Tani K, Kamegawa A, Gyobu N, Nishikawa K, Suzuki H, Walz T, Sasaki S, Mitsuoka K, Kimura K, Mizoguchi A, Fujiyoshi Y. Implications of the aquaporin-4 structure on array formation and cell adhesion. J Mol Biol. 2006;355:628–639. doi: 10.1016/j.jmb.2005.10.081. [DOI] [PubMed] [Google Scholar]
- 102.Li X, Kong H, Wu W, Xiao M, Sun X, Hu G. Aquaporin-4 maintains ependymal integrity in adult mice. Neuroscience. 2009;162:67–77. doi: 10.1016/j.neuroscience.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 103.Zhou J, Kong H, Hua X, Xiao M, Ding J, Hu G. Altered blood–brain barrier integrity in adult aquaporin-4 knockout mice. Neuroreport. 2008;19:1–5. doi: 10.1097/WNR.0b013e3282f2b4eb. [DOI] [PubMed] [Google Scholar]
- 104.Kalani MYS, Filippidis AS, Rekate HL. Hydrocephalus and aquaporins: the role of aquaporin-1. Acta Neurochir Suppl. 2012;113:51–54. doi: 10.1007/978-3-7091-0923-6_11. [DOI] [PubMed] [Google Scholar]
- 105.Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005;19:76–78. doi: 10.1096/fj.04-1711fje. [DOI] [PubMed] [Google Scholar]
- 106.Paul L, Madan M, Rammling M, Chigurupati S, Chan SL, Pattisapu JV. Expression of aquaporin 1 and 4 in a congenital hydrocephalus rat model. Neurosurgery. 2011;68:462–473. doi: 10.1227/NEU.0b013e3182011860. [DOI] [PubMed] [Google Scholar]
- 107.Carlo WA, McDonald SA, Tyson JE, Stoll BJ, Ehrenkranz RA, Shankaran S, Goldberg RN, Das A, Schendel D, Thorsen P, Skogstrand K, Hougaard DM, Oh W, Laptook AR, Duara S, Fanaroff AA, Donovan EF, Korones SB, Stevenson DK, Papile L-A, Finer NN, O’Shea TM, Poindexter BB, Wright LL, Ambalavanan N, Higgins RD. Cytokines and Neurodevelopmental Outcomes in Extremely Low Birth Weight Infants. The Journal of Pediatrics. 2011;159:919–925.e3. doi: 10.1016/j.jpeds.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cherian S, Whitelaw A, Thoresen M, Love S. The Pathogenesis of Neonatal Post-hemorrhagic Hydrocephalus. Brain Pathology. 2006;14:305–311. doi: 10.1111/j.1750-3639.2004.tb00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tada T, Kanaji M, Shigeaki K. Induction of communicating hydrocephalus in mice by intrathecal injection of human recombinant transforming growth factor-β1. Journal of Neuroimmunology. 1994;50:153–158. doi: 10.1016/0165-5728(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 110.Savman K, Blennow M, Hagberg H, Tarkowski E, Thoresen M, Whitelaw A. Cytokine response in cerebrospinal fluid from preterm infants with posthaemorrhagic ventricular dilatation. Acta Paediatr. 2002;91:1357–1363. doi: 10.1111/j.1651-2227.2002.tb02834.x. [DOI] [PubMed] [Google Scholar]
- 111.Cherian S, Thoresen M, Silver IA, Whitelaw A, Love S. Transforming growth factor-betas in a rat model of neonatal posthaemorrhagic hydrocephalus. Neuropathol Appl Neurobiol. 2004;30:585–600. doi: 10.1111/j.1365-2990.2004.00588.x. [DOI] [PubMed] [Google Scholar]
- 112.Aquilina K, Hobbs C, Tucker A, Whitelaw A, Thoresen M. Do drugs that block transforming growth factor beta reduce posthaemorrhagic ventricular dilatation in a neonatal rat model? Acta Paediatrica. 2008;97:1181–1186. doi: 10.1111/j.1651-2227.2008.00903.x. [DOI] [PubMed] [Google Scholar]
- 113.Hoque N, Thoresen M, Aquilina K, Hogan S, Whitelaw A. Decorin and Colchicine as Potential Treatments for Post-Haemorrhagic Ventricular Dilatation in a Neonatal Rat Model. Neonatology. 2011;100:271–276. doi: 10.1159/000327842. [DOI] [PubMed] [Google Scholar]
- 114.Jordan FL, Thomas WE. Brain macrophages: questions of origin and interrelationship. Brain Research. 1988;472:165–178. doi: 10.1016/0165-0173(88)90019-7. [DOI] [PubMed] [Google Scholar]
- 115.Lu J, Kaur C, Ling EA. An immunohistochemical study of the intraventricular macrophages in induced hydrocephalus in prenatal rats following a maternal injection of 6-aminonicotinamide. J Anat. 1996;188 ( Pt 2):491–495. [PMC free article] [PubMed] [Google Scholar]
- 116.Ling EA, Kaur C, Lu J. Origin, nature, and some functional considerations of intraventricular macrophages, with special reference to the epiplexus cells. Microsc Res Tech. 1998;41:43–56. doi: 10.1002/(SICI)1097-0029(19980401)41:1<43::AID-JEMT5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 117.Xin Z-L, Wu X-K, Xu J-R, Li X. Arachnoid cell involvement in the mechanism of coagulation-initiated inflammation in the subarachnoid space after subarachnoid hemorrhage. J Zhejiang Univ Sci B. 2010;11:516–523. doi: 10.1631/jzus.B1000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hua Y, Xi G, Keep RF, Hoff JT. Complement activation in the brain after experimental intracerebral hemorrhage. J Neurosurg. 2000;92:1016–1022. doi: 10.3171/jns.2000.92.6.1016. [DOI] [PubMed] [Google Scholar]
- 119.Xi G, Hua Y, Keep RF, Younger JG, Hoff JT. Systemic complement depletion diminishes perihematomal brain edema in rats. Stroke. 2001;32:162–167. doi: 10.1161/01.str.32.1.162. [DOI] [PubMed] [Google Scholar]
- 120.Lee J-Y, Keep RF, He Y, Sagher O, Hua Y, Xi G. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J Cereb Blood Flow Metab. 2010;30:1793–1803. doi: 10.1038/jcbfm.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakamura T, Keep RF, Hua Y, Hoff JT, Xi G. Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Research. 2005;1039:30–36. doi: 10.1016/j.brainres.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 122.Iwanowski L, Olszewski J. The effects of subarachnoid injections of iron-containing substances on the central nervous system. J Neuropathol Exp Neurol. 1960;19:433–448. doi: 10.1097/00005072-196007000-00010. [DOI] [PubMed] [Google Scholar]
- 123.Savman K, Nilsson UA, Blennow M, Kjellmer I, Whitelaw A. Non-protein-bound iron is elevated in cerebrospinal fluid from preterm infants with posthemorrhagic ventricular dilatation. Pediatr Res. 2001;49:208–212. doi: 10.1203/00006450-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 124.Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40:2241–2243. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao F, Hua Y, He Y, Keep RF, Xi G. Minocycline-induced attenuation of iron overload and brain injury after experimental intracerebral hemorrhage. Stroke. 2011;42:3587–3593. doi: 10.1161/STROKEAHA.111.623926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moos T. Brain iron homeostasis. Dan Med Bull. 2002;49:279–301. [PubMed] [Google Scholar]
- 127.Qing WG, Dong YQ, Ping TQ, Lai LG, Fang LD, Min HW, Xia L, Heng PY. Brain edema after intracerebral hemorrhage in rats: the role of iron overload and aquaporin 4. J Neurosurg. 2009;110:462–468. doi: 10.3171/2008.4.JNS17512. [DOI] [PubMed] [Google Scholar]
- 128.Takeuchi S, Nawashiro H. Letter by Takeuchi and Nawashiro Regarding Article, “Role of Iron in Brain Injury After Intraventricular Hemorrhage”. Stroke. 2011;42:e378–e378. doi: 10.1161/STROKEAHA.111.613547. [DOI] [PubMed] [Google Scholar]
- 129.Shooman D, Portess H, Sparrow O. A review of the current treatment methods for posthaemorrhagic hydrocephalus of infants. Cerebrospinal Fluid Res. 2009;6:1. doi: 10.1186/1743-8454-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Neilson JP. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Obstet Gynecol. 2007;109:189–190. doi: 10.1097/01.aog.0000251610.51286.b1. [DOI] [PubMed] [Google Scholar]
- 131.Fowlie PW, Davis PG. Prophylactic indomethacin for preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2003;88:F464–6. doi: 10.1136/fn.88.6.F464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Whitelaw A, Jary S, Kmita G, Wroblewska J, Musialik-Swietlinska E, Mandera M, Hunt L, Carter M, Pople I. Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics. 2010;125:e852–8. doi: 10.1542/peds.2009-1960. [DOI] [PubMed] [Google Scholar]
- 133.Whitelaw A, Kennedy CR, Brion LP. Diuretic therapy for newborn infants with posthemorrhagic ventricular dilatation. Cochrane Database Syst Rev. 2001:CD002270. doi: 10.1002/14651858.CD002270. [DOI] [PMC free article] [PubMed] [Google Scholar]