Abstract
Fusarium is a fungal pathogen of immunosuppressed lung transplant patients associated with a high mortality in those with severe and persistent neutropenia. The principle portal of entry for Fusarium species is the airways, and lung involvement almost always occurs among lung transplant patients with disseminated infection. In these patients, the immunoprotective mechanisms of the transplanted lungs are impaired, and they are, therefore, more vulnerable to Fusarium infection. As a result, fusariosis occurs in up to 32% of lung transplant patients. We studied fusariosis in 6 patients following lung transplantation who were treated at Massachusetts General Hospital during an 8-year period and reviewed 3 published cases in the literature. Cases were identified by the microbiology laboratory and through discharge summaries. Patients presented with dyspnea, fever, nonproductive cough, hemoptysis, and headache. Blood tests showed elevated white blood cell counts with granulocytosis and elevated inflammatory markers. Cultures of Fusarium were isolated from bronchoalveolar lavage, blood, and sputum specimens.
Treatments included amphotericin B, liposomal amphotericin B, caspofungin, voriconazole, and posaconazole, either alone or in combination. Lung involvement occurred in all patients with disseminated disease and it was associated with a poor outcome. The mortality rate in this group of patients was high (67%), and of those who survived, 1 patient was treated with a combination of amphotericin B and voriconazole, 1 patient with amphotericin B, and 1 patient with posaconazole. Recommended empirical treatment includes voriconazole, amphotericin B or liposomal amphotericin B first-line, and posaconazole for refractory disease. High-dose amphotericin B is recommended for treatment of most cases of fusariosis. The echinocandins (for example, caspofungin, micafungin, anidulafungin) are generally avoided because Fusarium species have intrinsic resistance to them. Treatment should ideally be based on the Fusarium isolate, susceptibility testing, and host-specific factors. Prognosis of fusariosis in the immunocompromised is directly related to a patient’s immune status. Prevention of Fusarium infection is recommended with aerosolized amphotericin B deoxycholate, which also has activity against other important fungi.
INTRODUCTION
Lung transplantation is now a viable and widely accepted treatment of end-stage lung disease. The first lung transplant was carried out in 1963 as a single left lung transplant from a cadaver where the recipient survived just 18 days, eventually succumbing to renal failure and malnutrition.36,37 From that time until the early 1980s, the main problems facing lung transplantation were organ rejection and healing of airway anastamoses. Advances during the early 1980s saw the advent of cyclosporine A, a potent immunosuppressive drug, and improved surgical techniques. This led to the first successful heart-lung transplant for idiopathic pulmonary artery hypertension in 1981, single lung transplant for idiopathic pulmonary fibrosis in 1983, and double lung transplant for emphysema in 1986.21,96,119 From January 1, 1988, to September 30, 2009, 471,304 single lung transplantations have been carried out the United States alone.120 Some diseases that cause respiratory failure and may ultimately benefit from lung transplantation include suppurative diseases like chronic obstructive airways disease, bronchiectasis, chronic bronchitis, and cystic fibrosis, and nonsuppurative diseases like emphysema, idiopathic pulmonary fibrosis, primary and secondary pulmonary hypertension, alpha-1 antitrypsin disease, Good-pasture disease, sarcoidosis, rheumatoid disease, hemosiderosis, occupational lung disease, and inhalation burns.83,117 Lung transplantation is considered when life expectancy is less than 24–36 months and patients have New York Heart Association (NYHA) class III and IV symptoms.69
Lung transplantation is often complicated by bacterial and fungal infections.45 Invasive fungal infections are serious and potentially life-threatening complications of lung transplantation, occurring in 14%–32% of recipients.15,45,51 Fungal pneumonias are a significant contributor to the 35% of pneumonia deaths in the first year following lung transplantation.1,17 In this group of patients the epidemiology of fungal infections has changed with the use of fluconazole prophylaxis; there has been a significant decrease in the incidence of yeast infections, namely Candida species, and an increase in mold infections.4,15,64,112,124 Among these mold infections the incidence of invasive Fusarium species infections is rising, now second only to Aspergillus species.15,73,77,106,112 The diagnosis of invasive fusariosis currently has a very poor prognosis.50 Early diagnosis and treatment are essential to improve the chances of survival. Given the magnitude and severity of the problem, surprisingly little is known about the pathogenesis, clinical characteristics, laboratory diagnosis, and management of fusariosis.15
The main factors contributing to the acquisition of invasive fusarial infections in lung transplant patients are the following: 1) the protective physical barriers to infection in the lungs are compromised during lung transplantation; 2) the protective mucociliary clearance and cough reflexes are reduced due to denervation of the transplanted lung; 3) the transplanted lung is continuously exposed to the open environment and its microorganisms; 4) patients are immunosuppressed with potent pharmacologic agents; and 5) the incidence of infections caused by drug-resistant and aggressive strains of fungi is increasing.17,53,59,64,65 Note that Fusarium species can be acquired from the environment by the recipient following transplantation, or alternatively Fusarium species can be present as a pretransplant colonizing organism in the donor lung that can manifest following transplantation, causing clinical disease in the recipient.17 The main entry site for Fusarium species is the airways via inhalation of airborne conidia, and the lungs are involved in 31%–41% of all fusariosis cases.33,68,127 In support of this is the development of sinusitis and pneumonia in the absence of fungemia.75 Lung involvement can also occur following hematongenous spread of the fungus, which almost always occurs in the immunocompromised.75
To better understand the emerging role of this pathogen in lung transplant patients, we here examine the clinical characteristics of Fusarium infections in this patient population and evaluate possible risk factors, clinical findings, laboratory diagnosis, response to therapy, and outcomes from clinical cases and the literature.
METHODS
Clinical Data Collection
Six cases of Fusarium infection in lung transplant patients were identified in our institution (Massachusetts General Hospital) by the microbiology laboratory and from discharge summaries. The review period was from January 2001 to November 2008 inclusive; over this time, a total of 73 lung transplants were carried out at Massachusetts General Hospital.120 Data for these cases were obtained from a detailed study of computerized patient medical records. In addition, we reviewed the English-language literature to identity similar cases of fusariosis in lung transplant patients, searching the MEDLINE database (National Library of Medicine, Bethesda, MD) using the key words “transplant” and “Fusarium.” We did not constrain the search to any specific time period due to the limited number of published case reports.
Identification and Determination of MIC of Fusarium Species
The Fusarium isolate from Patient 6 (Tables 1 and 2) was obtained and the species was identified by amplifying and sequencing a portion of the translation elongation factor (TEF) 1α coding region as described by O’Donnell et al.80 The minimum inhibitory concentration (MIC) of the isolate was determined spectrophotometrically using RPMI 1640 media (Mediatech, Inc., Manassas, VA) following the Clinical and Laboratory Standards Institute microbroth dilution method. All antifungal compounds tested were obtained from Sigma-Aldrich (St. Louis, MO), with the exception of caspofungin (Merck, Whitehouse Station, NJ).
TABLE 1.
Clinical Features of 6 Cases of Fusarium Infection in Lung Transplant Patients at Massachusetts General Hospital
| Patient (Age in yr/Sex) | Reason for Lung Transplant |
Positive Culture Specimen |
Immunosuppression at Diagnosis |
Signs and Symptoms |
Fusarium Treatment |
Outcome | Reason for Death | Acute Rejection 6 mo Before Infection |
Antifungal Prophylaxis or Previous Therapy |
1Y3 B-D Glucan | Galactomannan Antigen |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (20/F) | Cystic fibrosis | BAL | Tacrolimus, prednisone | SOB, fever, abdominal distention, PE, postural hypotension, crackles in left chest (WBC 19.2, %PMN 89, ANC 17,100) | Liposomal amphotericin B, caspofungin | Died | Invasive aspergillosis | Yes | No | ND | ND |
| 2 (55/M) | Alpha-1 antitrypsin deficiency | BAL | Cyclosporine, azathioprine, antithymocyte globulin (ATG), prednisone | SOB, fever (97.9-F), pneumonia, hemoptysis, fine crackles on auscultation, diminished BS at base (WBC 7.1, %PMN 75, ANC 6270) | None | Died | Sepsis, ARF, scedosporium infection | No | No | ND | ND |
| 3 (59/F) | Interstitial pulmonary fibrosis | Blood, BAL | Tacrolimus, prednisone | SOB, fever, nonproductive cough, ARF, intubated, hypoxia, fine crackles (WBC 3.9, %PMN 94, ANC 3660) | Liposomal amphotericin B, voriconazole | Died | Sepsis, Fusarium infection, CMV pneumonitis | No | Voriconazole, caspofungin | 53 | 0.09 |
| 4 (66/F) | Chronic obstructive airways disease | BAL | Antithymocyte globulin (ATG), prednisone | Fever (98.1-F), hypoxia, dimished BS, otherwise asymptomatic (WBC 14.5, %PMN ND, ANC ND) | Amphotericin B, voriconazole | Survived | — | No | Fluconazole | ND | ND |
| 5 (19/F) | Cystic fibrosis | BAL | Cyclosporine, prednisone | SOB, fever (100.3-F), hypoxia, diminished BS on affected side, no cervical lymphadenopathy, fatigue, headache, dry heaves (WBC 4.9, %PMN 78, ANC 3850) | Voriconazole | Died | Unknown | Yes | No | 31 | 0.206 |
| 6 (36/F) | Interstitial pulmonary fibrosis | Sputum | Cyclosporine, prednisone | SOB, fever (99.5-F), chills, hypoxia, nonproductive cough, headache, coarse crackles on affected side, expiratory wheezing (WBC 8, %PMN 86, ANC 7610) | Liposomal amphotericin B, voriconazole | Died | Respiratory distress, sepsis | No | Itraconazole | ND | 0.59 |
Abbrevations: ANC = absolute neutrophil count, ARF = acute respiratory failure, BS = breath sounds, CMV = cytomegalovirus, ND = no data, PE = pulmonary embolism, PMN = polymorphonuclear cells (granulocytes), SOB = shortness of breath, WBC = white blood cell count, 106cells/mL.
TABLE 2.
Laboratory Features of 6 Cases of Fusarium Infection in Lung Transplant Patients at Massachusetts General Hospital
| Patient | Reason for Lung Transplant | Positive Culture Specimen | Previous Fungal Infections | Cultured Fusarium | Fungal Stain and Microscopy | Concomitant Infection at Diagnosis |
|---|---|---|---|---|---|---|
| 1 | Cystic fibrosis | BAL | Candida albicans, C. glabrata, Aspergillus fumigatus | Fusarium spp ND | Positive (septated hyphae) | Aspergillus fumigatus, Pseudomonas aeruginosa |
| 2 | Alpha-1 antitrypsin deficiency | BAL | None | Fusarium spp ND | Negative | C. albicans, Acinetobacter baumanni |
| 3 | Interstitial pulmonary fibrosis | Blood, BAL | None | Fusarium spp ND | Positive (septated hyphae) | CMV infection |
| 4 | Chronic obstructive airways disease | BAL | C. albicans, Alternaria spp, Non-albicans yeast, Penicillium spp | Fusarium spp 1 colony | Negative | Chryseobacterium (Flavobacterium) meningosepticum, Nonenteric gram-negative rods, Stenotrophomonas maltophilia, Alcaligenes xylosoxidans, Corynebacterium spp, C. albicans, Alternaria spp |
| 5 | Cystic fibrosis | BAL | Aspergillus versicolor | Fusarium spp ND | Negative | Pseudomonas aeruginosa (2 types) |
| 6 | Interstitial pulmonary fibrosis | Sputum | Mycobacterium avian intracellulare, Stenotrophomonas | F. proliferatum 2 colonies | NA | Nocardia asteroides, Aspergillus spp |
Abbrevations: ND = not determined, NA = not available.
RESULTS
Case Series
We identified 6 lung transplant patients with fusariosis (mean age, 42.6 yr; range, 19–66 yr) (Table 1). The reasons for lung transplant were cystic fibrosis (2 patients), interstitial pulmonary fibrosis (2 patients), alpha-1 antitrypsin deficiency (1 patient), and chronic obstructive pulmonary disease (1 patient). The patients with cystic fibrosis had bilateral lung transplants, while the others had single lung transplants (2 patients with left lung transplants and 2 patients with right lung transplants). All 4 patients with single lung transplants had clinical and radiologic evidence of infection in the transplanted lung. Only 1 of 6 patients had acute rejection of their transplants before Fusarium infection. Importantly, 4 of the 6 patients had fungal infections with other organisms before infection with Fusarium. All patients were on a combination of 2 or more immunosuppressive drugs, and 3 of the 6 patients were on antifungal prophylaxis.
Clinical findings included shortness of breath in 4 of the 6 patients, fever in 5 of the 6 patients (range, 36.6°C to 37.9°C), nonproductive cough in 2 of the 6 patients, hemoptysis in 1 of the 6 patients, and headache in 2 of the 6 patients. Clinical examination findings included crackles in 4 of the 6 patients, expiratory wheeze in 1 of the 6 patients, and reduced breath sounds on the affected side in 2 of the 6 patients. Interestingly, there was no sinus involvement in any of the patients. All patients had evidence of infection on chest radiographs and computed tomography (CT) scans.
Laboratory findings included an elevated white blood cell count in 2 of the 6 patients (white cell count range, 4.9–19.2 × 109 L−1) and granulocytosis in 5 of the 6 patients (polymorphonuclear cells range, 75%–94%). Fusarium was identified in bronchoalveolar lavage (BAL) cultures in 5 of 6 patients, blood cultures in 1 of 6 patients, and sputum cultures in 1 of 6 patients; 2 of 6 patients had septated fungal hyphae recognized by microscopy. Infections were within a year of lung transplantation in 4 of 6 patients, a little over 2 years in 1 patient, and over 15 years in the remaining patient. As noted above, all patients had concomitant infections with various organisms (Table 2); 2 of 6 patients had positive 1→3 β-D-Glucan (BDG) and galactomannan tests, while 1 of 6 patients had a lone positive galactomannan test (Table 1).
All 6 patients had radiologic investigations including chest radiographs and CT scans. In each case, there was evidence of lung involvement (Figure 1). Of the radiologic investigations that were formally reported by a radiologist, the following features were listed: consolidation (1 patient), atelectasis (1 patient), nodular opacities (2 patients), mediastinal lymphadenopathy (1 patient), pleural effusions (2 patients), ground glass opacities (1 patient), and bronchiectasis (2 patients). Note that each patient had some, but not all, of these features.
FIGURE 1.
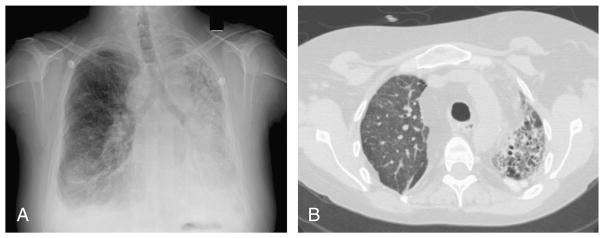
Radiologic images of Patient 6 from this case study, with Fusarium proliferatum, Aspergillus species, and Nocardia asteroides isolated from sputum samples: A, Anteroposterior chest radiograph showing focal consolidation in the mid- to lower right lung likely representing pneumonia following a right thoracotomy and right lung transplantation. There are small bilateral pleural effusions, and left hemithoracic volume loss and interstitial fibrosis or traction bronchiectasis of native left lung. B, Axial CT scan without contrast showing fibrosis in the native left lung as evidenced by the honeycombing architectural distortion. The lower lobes demonstrate ground glass opacity and consolidation. The right transplant lung demonstrates diffuse ground glass opacity and focal consolidation with associated centrilobular nodularity.
Liposomal amphotericin B and caspofungin were used to treat 1 of 6 patients, liposomal amphotericin B and voriconazole in 2 of 6 patients, amphotericin B and voriconazole in 1 of 6 patients, and voriconazole alone in 1 of 6 patients. One patient did not receive treatment for fusariosis. The patient treated with amphotericin B and voriconazole was the sole survivor and was alive more than a year after infection; the other 5 patients all succumbed to the infection.
Literature Review
We identified 3 patients with Fusarium infection following a lung transplant reported in the English-language literature for which adequate clinical data were available.9,35,41 The mean age of infection was 44.3 years (range, 18–62 yr) (Table 3). The reasons for lung transplantation included advanced bullous emphysema in 1 patient, emphysema due to alpha-1 antitrypsin deficiency and tobacco use in 1 patient, and cystic fibrosis in the remaining patient. The patient with cystic fibrosis had a double lung transplant, while the other 2 patients had a single right lung transplant. All 3 patients were on a combination of 3 immuno-suppressive agents when they developed Fusarium infections: prednisolone, azathioprine, and cyclosporine.
TABLE 3.
Features of 3 Cases of Fusarium Infection in Lung Transplant Patients, Previous Reports
| Reference (Age in yr/Sex) | Reason for Lung Transplant | Positive Culture Specimen | Immunosuppression at Diagnosis | Signs and Symptoms | Fusarium Treatment | Outcome | Reason for Death | Antifungal Prophylaxis or Previous Therapy | Fusarium Isolate |
|---|---|---|---|---|---|---|---|---|---|
| 9 (53/M) | Advanced bullous emphysema | BAL | Prednisone, azathioprine, cyclosporine | Productive cough, pleuritic chest pain, low-grade fever (98.6 -F) (WBC 7.4) | Amphotericin B | Alive after 1 yr | NA | Co-trimazole | F. solani |
| 35 (18/F) | Cystic fibrosis | Central venous catheter blood cultures, BAL | Prednisone, azathioprine, cyclosporine | Apyrexial, vegetation on tricupsid valve (WBC 7.7, 75% neutrophils) | Liposomal amphotericin B | Died | Convulsion crisis, septic shock | No | F. solani |
| 41 (63/M) | Emphysema (>1-antitrypsin deficiency and tobacco use) | Sputum | Prednisone, azathioprine (changed to mycophenlate mofetil), cyclosporine | Mild fever, asthenia, weight loss, productive cough, SOB (WBC 3.8, ANC 2430) | Pozaconazole | Survived | NA | Itraconazole | F. proliferatum |
Abbrevations: See previous tables.
Clinical findings included shortness of breath in 1 of 3 patients, fever in 2 of 3 patients, productive cough in 2 of 3 patients, pleuritic chest pain in 1 of 3 patients, asthenia in 1 of 3 patients, and weight loss in 1 of 3 patients. There were no signs of sinus involvement in these patients. All 3 patients had evidence of infection on chest radiographs and CT scans.
Laboratory findings included normal white cell counts in all 3 patients. Fusarium was identified on BAL cultures in 2 of 3 patients, sputum cultures in 1 of 3 patients, and blood cultures in 1 of 3 patients. All 3 patients developed fusariosis soon after lung transplantation. Two of the 3 patients were infected with F. solani, while the other patient was infected with F. proliferatum. MIC data were available for 2 of 3 patients (Table 4). Both of these isolates were resistant to fluconazole and susceptible to amphotericin B.
TABLE 4.
Antifungal Susceptibilities of Fusarium Species, Previous and Present Reports
| MIC (Kg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Species | Reference | Amphotericin B | Flucytosine | Itraconazole | Fluconazole | Ketoconazole | Posaconazole | Caspofungin |
| F. solani | 9 | 2 | >64 | >64 | ND | ND | ND | ND |
| F. prolifertarum | 41 | 2 | ND | >8 | >64 | ND | 4 | ND |
| F. prolifertarum | PR | 4 | >128 | >128 | >128 | 32 | ND | 128 |
Abbreviations: See Table 1. PR = present report.
Amphotericin B was used to treat 1 of the 3 patients, liposomal amphotericin B in 1 of the 3 patients, and posaconazole in 1 of the 3 patients. The patient treated with liposomal amphotericin B died, while the others survived.
DISCUSSION
Fusarium organisms are filamentous fungi found in the soil and air worldwide, especially in tropical and temperate regions.71,130 They are mainly plant pathogens but occasionally cause severe infection in farm animals.28,71,75 Fusarium causes a wide range of disease in humans including superficial, locally invasive, and disseminated infections and allergic disease; fusarial mycotoxins have been linked to food poisoning, pancytopenia, and esophageal cancer.71,74,75,107,129 Although many species of Fusarium exist, most of the clinical isolates belong to 3 groups: the Fusarium solani species complex (FSSC), the Fusarium oxysporum species complex, and the Gibberella fujikuroi species complex. Isolates from the FSSC are responsible for a majority of the Fusarium mycoses, and at least 20 phylogenetically distinct species of the FSSC have been isolated from clinical specimens.81 Clinical isolates of FSSC members are phylogenetically similar to isolates from other environmental sources, suggesting that human infections are the result of a susceptible host contacting the FSSC isolate in the environment.131 F. oxysporum isolates are the second most common cause of Fusarium infections, and a single clonal widespread lineage is responsible for most F. oxysporum infections.82 Several members from the G. fujikuroi species complex are able to infect humans, most notably F. verticillioides (F. moniliforme) and F. proliferatum.79
Disseminated infections usually occur only in patients with severe and persistent neutropenia.75 Clinicians are increasingly aware of Fusarium as an important pathogen of immunocompromised patients associated with high mortality.15,29,74 Of note, Fusarium species and Aspergillus species are often confused because they have similar histologic appearances with septate branching hyphae and they can cause similar clinical syndromes.38,115 Some patients are consequently started on amphotericin B, an agent used to treat aspergillosis but with little activity against some Fusarium species.5,6,49,81,97,122 It is, therefore, important to make the correct diagnosis to optimize treatment and improve prognosis.29
Epidemiology and Clinical Spectrum
The clinical manifestation of fusariosis depends on the patient’s immune status and the source of the infection. In immunocompetent hosts, there is a broad spectrum of infection ranging from keratitis with contact lens use, peritonitis in patients with intraabdominal catheters, to sinusitis, pneumonia, thromboplebhitis, endophthamitis, fungemia, septic arthritis, and osteomyelitis.14,26,30,46,47,52,58,70,74,75,92,99,105,114 Immunocompromised patients with prolonged and extreme neutropenia and severe T-cell immunodeficiency, such as lung transplant patients, are those most at risk of developing fusariosis.15 They develop similar infections to those in immunocompetent patients but generally have more locally invasive or disseminated fusarial infections.74,75
The main site of entry for Fusarium species in lung transplant patients is the airways by inhalation of fusarial conidia followed by areas of skin and mucosal membrane breakdown.75 Fusarium is ubiquitous in the environment and fusarial conidia have been detected in outdoor air samples.7,15,66 One study18 found Fusarium to be more prevalent in air samples than Aspergillus. Fusarium species have also been isolated from indoor water storage systems and outdoor water bodies. Water-related activities, such as showering and outdoor water sports, can aerosolize fusarial conidia, thereby making them airborne and facilitating transmission through the airways.75,82
The lungs are involved in the majority of cases of disseminated fusariosis in lung transplant patients.75 Even after controlling for immune status, lung involvement is associated with a higher mortality.76 Lung lesions in fusarial pneumonia include nodular and cavitary lesions and nonspecific alveolar or interstitial infiltrates.76 The clinical picture is usually non-specific, with shortness of breath, nonproductive cough, and pleuritic chest pain.75,76
Our results conform with those reported above in the literature. In the current case series, 2 patients had disseminated fusariosis with positive blood cultures, and both these patients had lung involvement with positive BAL samples. Furthermore, all 9 lung transplant patients studied had proven lung involvement with positive BAL samples or sputum cultures and radiologic evidence of infection. There was a high mortality rate (67%) for this group of patients.
Lung transplant recipients are at high risk for other molds that are clinically, histopathologically, and radiologically very similar to fusariosis. This group of patients is susceptible to infection with Aspergillus species and other molds such as Zygomycetes, Scedosporium, and dematiaceous molds.67,108,109 We will briefly touch on 2 of the most common mold infections, Aspergillus and Zygomycetes, but these have been covered elsewhere in more detail.22,43,72,85,87,100 The incidence of invasive Aspergillus infections in lung transplant patients is between 6% and 16% with a high mortality rate.22,108,109 The risk factors for developing aspergillosis are largely the same as those listed above for developing fusariosis, and the clinical presentation of aspergillosis is similar to that of fusariosis with the exception of skin lesions and myalgia occurring more commonly in fusariosis.22 Furthermore, the radiologic features of aspergillosis are similar to those of fusariosis, with subpeural nodular opacities and cavitation.22 Lung transplant patients can develop Aspergillus infections anytime from months to years after the lung transplant, but the majority of cases are diagnosed within 6 months of the transplantation.67,109
The most common non-Aspergillus molds that colonize airways in lung transplant recipients include Cladosporium, Phialemonium, and Zygomycetes.108 Zygomycetes are ubiquitous filamentous fungi that can also cause life-threatening infection in lung transplant patients.22,100 Risk factors for zygomycosis includes immunosuppression, malignancy, diabetes mellitus, injection drug use, prematurity, receiving deferoxamine therapy, and long-term prophylaxis with voriconazole.22,100,108 Pulmonary disease is most common in immunocompromised patients, and the most common causative organisms include, from most common to least common, Rhizopus species, Mucor species, and Cunninghamella bertholletiae, among others.22,100 Common symptoms include fever, dyspnea, chest pain, rhinocerebral infection, and multi-organ failure.113 Like fusariosis, radiologic features include subpleural lung nodules.113
Diagnosis
Diagnosis of fusariosis usually depends on both the clinical and laboratory findings. The following criteria can be used to discern true infection in lung transplant patients:
isolation of several colonies of Fusarium from the same specimen or isolation of the same fungus from different specimens;
positive identification of the fungus on direct visualization of laboratory samples; and
isolation of Fusarium from a site that is more likely to indicate infection than not.75 (For example, Fusarium identified in a sputum sample from a healthy immunocompetent patient may be the result of recent inhalation of conidia; however, Fusarium isolated from a BAL culture from an immunosuppressed transplant patient should be considered diagnostic of infection, and antifungal therapy initiated.)
Blood cultures are frequently positive in fusariosis, unlike aspergillosis, because Fusarium species sporulate, which facilitates bloodborne dissemination and growth.56,75 Confirmation of fusariosis may require histopathology, polymerase chain reaction (PCR), or mass spectrometry. A significant problem with histopathologic diagnosis is that biopsy specimens are sometimes hard to obtain, especially in patients who are critically ill. Furthermore, morphologic discrimination between Fusarium and Aspergillus is highly subjective and difficult because their hyphae appear very similar on direct visualization. Finding hyphae and spores in tissue samples from lung transplant patients is highly suggestive of fusariosis. Distinguishing Fusarium from other fungi may also require in situ hybridization in paraffin-embedded tissue specimens.38,75 In the current study, only 2 of the 6 patients had positive fungal stains on microscopy with septate hyphae.
Culture methods typically have a low sensitivity for identifying Fusarium, although the fungus can be identified by its microscopic appearance.48 Species identification may be difficult and may require the use of molecular techniques, and fortunately, PCR-based methods are now available to identify Fusarium species from culture media, blood, and tissue.39,44 The detection of Fusarium from blood cultures, however, has poor sensitivity, and species identification is complex and requires a great deal of time and expertise.40,75,128 Furthermore, using the TEF1α gene as a target for species identification remains questionable in some Fusarium species.62 Some traditional PCR assays use the internal transcribed spacer (ITS) region between the 18S rDNA and 28S rDNA, which has high specificity for certain Fusarium strains such as F. proliferatum, and a combination of several other conserved sequenced regions as the current best molecular method to identify Fusarium isolates to the species level.107
Molecular markers of fungal infection, such as BDG and galactomannan, can also be used to help diagnose infection. BDG is a nonspecific marker of fungal infection that is usually positive in invasive fusariosis. However, this assay cannot distinguish between Fusarium and other fungi.78,84 A positive BDG test and a negative galactomannan test in a lung transplant patient with a mold infection is highly suggestive of Fusarium infection.75 In the current study, 2 patients had a positive BDG test and a negative galactomannan test, while another patient had a lone positive galactomannan test (defined as a test result of > 0.5).10,88
Khan et al48 evaluated the diagnostic usefulness of a BDG test and DNA detection using a highly sensitive and specific nested PCR method on serum and BAL specimens from mice infected with F. oxysporum. They found that the BDG and PCR sensitivity in BAL and serum samples were 15 and 98%, and 92 and 75%, respectively. When combining the 2 tests for serum samples the sensitivity rose to 98% with a negative predictive value of 92%. The specificity and positive predictive value were 100% for both serum and BAL specimens. Of note, however, the BDG test remained positive throughout the infection period, while a positive PCR result declined over time. Nonetheless, combining the BDG and PCR tests on serum promises to be a highly sensitive and specific diagnostic method for invasive Fusarium infection. There is, however, a lack of published data in human subjects to validate this hypothesis.
The use of mass spectrometry to identify clinical isolates of Fusarium has recently gained popularity. Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) is a tool that has been used to characterize fungi.19,55,121,126 At the moment, though, there is no standard protocol or validated database to use as a benchmark.62 A major advantage of mass spectrometry is that fungal identification can be completed in 1 hour compared to culture-based methods, which require serial subcultures and microscopic examination of morphologic characteristics that usually take over a week to yield results. PCR-based methods take a minimum of 2 days to yield results. Other advantages to using MALDI-TOF analysis are that each run can include many species and strains of fungi, the results are very consistent, and it is cost efficient.62,107
Treatment
The increase in the incidence of severe fusarial infections and the rapid progression of disease demands early and aggressive treatment of patients with disseminated and invasive infections.115 The management of fusariosis includes conservative medical and surgical measures. Conservative measures include removal of indwelling lines and central venous catheters in patients with confirmed catheter-related fungemia, while surgical measures include debulking of necrotic and infected tissue in locally invasive disease.15,57,75,123 In addition, adjunctive therapies exist and can be used to optimize management of these patients.60,75,93,101
There are currently inconsistent and conflicting recommendations in the literature with regards to the treatment of fusariosis. In the current study, monotherapy with voriconazole, amphotericin B, liposomal amphotericin B, and posaconazole, or dual combination therapy with liposomal amphotericin B and caspofungin, liposomal amphotericin B and voriconazole, and amphotericin B and voriconazole were used for treatment. Mortality was high in this group of patients (67%), and of those who survived, 1 patient was treated with a combination of amphotericin B and voriconazole, 1 patient with amphotericin B, and 1 patient with posaconazole. In general, however, voriconazole is the recommended first-line treatment, with amphotericin B, its lipid formulation, and posaconazole as possible alternatives.2,60,75,102 The echinocandins (for example, caspofungin, micafungin, anidulafungin) are avoided because Fusarium species have intrinsic resistance to the echinocandins.8,25 In a study of 273 patients with refractory invasive fungal infections caused by Candida species, Aspergillus species, Fusarium species, Scedosporium species, Cryptococcus neoformans, and Penicillium species, and 28 patients requiring primary treatment of their fungal infections, treatment with voriconazole was associated with a good response in 50% of patients and 45.5% (5 of 11 patients) of patients with fusariosis.8,91 Voriconazole was well tolerated as <10% of the patients required a reduction in dose or removal from voriconazole therapy. Voriconazole is approved by the United States Food and Drug Administration for salvage therapy, and posaconazole can also be used as salvage therapy for refractory invasive fusarial infections.8,60,95,111 Should either of these newer generation triazoles be chosen for Fusarium treatment, they require therapeutic drug monitoring. Variability in plasma drug levels are associated with drug interactions, patient age, nonlinear pharmacokinetics, polymorphisms of the cytochrome P450 CYP2C19 gene, and variable enteral absorption of drugs.60 A plasma concentration of voriconazole below 1 mg/L is likely to be subtherapeutic, and above 5 mg/L there is neurologic toxicity.86 The therapeutic levels of voriconazole are not, however, well defined, and a clinical response should be taken into account. The MIC of voriconazole for Fusarium species varies widely and is between 0.25–8 mg/L.8 Therefore, the initial target level for voriconazole should be between 1 and 5 mg/L watching for a clinical response. If there is no clinical response, the levels should be increased up to 8 mg/L while monitoring for side effects. If there are side effects or if there is no clinical response at 8 mg/L, therapy should be switched to a second-line agent.
Isavuconazole, formerly BAL4815, is a new broad-spectrum triazole antifungal in the late stages of clinical testing. Guinea et al34 compared the in vitro activity of isavuconazole to that of voriconazole and fluconazole. The MIC50 for isavuconazole and voriconazole were both 16 mg/L, and the MIC90 were 16 mg/L and 4 mg/L against Fusarium species, respectively. This shows that isavuconazole and voriconazole have similar but limited activity against the Fusarium species tested.
Naturally, the treatment of fusariosis should depend on the type of infection, the Fusarium isolate, its susceptibility to antifungals, and host-specific factors. However, given the difficulties in laboratory identification, the species of Fusarium is not always known, let alone the antifungal susceptibilities. At present, amphotericin B and its lipid formulation appear to be better than the newer triazoles (voriconazole, posaconazole, ravuconazole, albaconazole) against F. oxysporum and F. solani.8,111,125 O’Donnell et al81 tested 19 FSSC isolates with 10 antifungals and terbinafine, a synthetic allylamine antifungal, and amphotericin B showed the lowest MICs. Amphoterin B, though, has clinically poor activity and renal toxicity.103
Ruiz-Cendoya et al103 studied the interaction between the new triazoles (voriconazole and posaconazole) with amphotericin B in mice infected with F. oxysporum. Monotherapies of each antifungal agent showed very poor efficacy in vivo. There was a poor response to the combination of amphotericin B and voriconazole; however, there was a much better response to the combination of amphotericin B and posaconazole.103 The poor efficacy of the amphotericin B and voriconazole combination has been reported in clinical studies, although 2 published cases report that this combination of antifungals led to clinical improvements in patients with F. solani infections before the resolution of neutropenia.42,75,101,102 This combination was tested in mice infected with F. solani and showed only modest treatment efficacy.102 Posaconazole shows high MICs for Fusarium species in general, but it is better against F. oxysporum than F. solani.3,8,116 One case report described poor efficacy using combination amphotericin B and posaconazole, although the level of posaconazole was subtherapeutic due to the patient’s poor diet.54
Azor et al11 studied the susceptibility of F. verticillioides and F. thapsium to antifungals. F. verticillioides commonly causes fusariosis in humans. The results showed that terbinafine was most effective in treating F. verticillioides in vitro. In decreasing order of potency against F. verticillioides, terbinafine was followed by posaconazole, ravuconazole, voriconazole, amphotericin B, ketoconazole, albaconazole, and itraconazole.11 Again, terbinafine was the most effective drug in vitro in treating F. thapsium. Voriconazole and amphotericin B followed with equal potencies. The other antifungals tested showed no activity again F. thapsium.
The treatment of other molds differs from the treatment of fusariosis in some important ways. Amphotericin B deoxycholate has broad-spectrum activity against Aspergillus species and Zygomycetes.22,104 Liposomal amphotericin B has the same spectrum of activity but fewer side effects. Concerning the azoles: fluconazole is not active against molds; itraconazole has good in vitro activity against Aspergillus but not Fusarium or Zygomycetes; voriconazole has good activity against most Aspergillus species but no activity against Zygomycetes; and posaconazole has good in vitro activity against Aspergillus and Zygomycetes.13,20,22,31,94,104 Caspofungin, anidulafungin, and micafungin all have some activity against Aspergillus but are not effective in monotherapy against invasive aspergillosis and have little or no activity against Zygomycetes.13,22,25,27
In treating lung transplant patients with fusariosis, antifungal therapy should be complemented by reversal of as many underlying predisposing factors as possible. Low neutrophil and macrophages counts are 2 such predisposing factors to Fusarium infection, which can be treated with immunotherapy.60 Immunotherapy includes growth factors (granulocyte colony-stimulating factor [G-CSF] and granulocytemacrophage colony-stimulating factor [GM-CSF]) and granulocyte transfusions for neutropenic patients, and gamma interferon and/or GM-CSF for patients with adequate neutrophil counts.75 There is, however, a lack of clinical data on the role of immune reconstitution in Fusarium infection, and the best treatment strategy remains unclear. Because of the poor prognosis of fusariosis, especially in lung transplant patients with profound and prolonged neutropenia, G-CSF and granulocyte transfusions are often given.75,101 Immunotherapy is well tolerated.93 To our knowledge, there is currently only 1 case report in the literature of successful treatment of invasive fusariosis with a combination of pharmacologic treatment and immunotherapy.75,101
The response to treatment in lung transplant patients can be assessed by 1) disappearance of fevers and other clinical signs of infection, 2) resolution of fungemia, and 3) attenuation of radiologic features of the disease.75 Note that radiologic features of infection may persist making, their interpretation difficult. Positron emission tomography (PET) and indium-labeled white blood cell scintigraphy can be used to detect inflammation if necessary.12,61,75
Prognosis
The prognosis of fusariosis in lung transplant patients is directly related to the immune status of the patient, with high mortality rates in patients with prolonged and profound neutropenia. Multivariate predictors of poor outcome include persistent neutropenia, recent corticosteroid therapy, disseminated disease, and lung involvement. Those with no risk factors or whose only risk factor is corticosteroid therapy have statistically significant higher survival rates.75–77 In the current study, all 9 lung transplant patients had some predictors of a poor outcome listed above. The mortality rate in this patient group was high (67%), which was consistent with trends published in the scientific literature.
Prevention
Given the poor prognosis of fusariosis and the limited susceptibility to antifungals, prevention of infection is better than the cure. Precautions should be taken to prevent lung transplant patients from coming in contact with known sources of Fusarium.75 Decreasing the level of immunosuppression should be attempted where possible, especially in patients with a prior history of fusariosis. In addition, active infections, such as skin lesions, should be treated before commencing immuno-suppressive therapy.74,75
Antifungal prophylaxis should be based on local or organism-specific patterns of susceptibility. Prophylactic antifungals reduce mortality and the incidence of fungal infections in lung transplant patients.32,45 Yet there is no standardized approach to antifungal prophylaxis. Husain et al45 surveyed 50 lung transplant centers worldwide, where roughly 63% of all lung transplants are carried out. They found that aerosolized amphotericin B deoxycholate alone or in combination with itraconazole was the most common prophylaxis strategy used with good results. Itraconazole itself has poor in vitro activity against Fusarium species.16,110 Nevertheless, the use of aerosolized amphotericin B deoxycholate with itraconazole might be a more appropriate approach because it has good activity against Aspergillus species, Fusarium species, and Candida species, among others.2,23,60,63,89,90,98 The drug-drug interactions associated with itraconazole should, however, be taken into account. Other prophylactic strategies used with some success to prevent fungal infections in general include single drug dosing with liposomal amphotericin B, fluconazole, voriconazole, posaconazole, and oral nystatin.24,60,98,118 Limitations to antifungal prophylaxis include selection of resistance, drug toxicity and interactions to the patient, high cost, and interference with some diagnostic assays.45
Conclusions
The Fusarium species is an important but relatively uncommon pathogen of immunocompromised lung transplant patients associated with a high mortality rate. Fusarium species that infect lung transplant patients most frequently are F. solani, F. oxysporum, F. verticillioides, and F. proliferatum. The principle portal of entry for Fusarium species is the airways, followed by the sites of skin breakdown and possibly mucosal membranes. The presentation of fusariosis depends on the immune status of the host and the portal of entry. In humans, Fusarium causes a wide range of disease including superficial, locally invasive, and disseminated infections and allergic disease, and its toxins are associated with food poisoning, pancytopenia, and esophageal cancer. In the lungs, the Fusarium organism causes a nonspecific illness with shortness of breath, nonproductive cough, and pleuritic chest pain. Laboratory confirmation of fusariosis is based on fungal cultures, microscopy, BDG test, galactomannan test, and PCR. Laboratory diagnosis would benefit greatly from MALDI-TOF mass spectrometry, which is a versatile, reliable, and cost-effective assay. Treatment should ideally be based on the Fusarium isolate, susceptibility testing, and host-specific factors. The current recommendations for treating fusariosis empirically in lung transplant patients are voriconazole, amphotericin B, or liposomal amphotericin B first-line and posaconazole for refractory disease. The echinocandins are avoided in treatment. The prognosis of fusariosis is directly related to the immune status of the patient, with high mortality rates in patients with severe and persistent neutropenia. Given the poor outcome of fusariosis, preventing infection is one of the cornerstones of management.
Acknowledgments
This work was supported by a R01 award AI075286 from the NIH and a R21 award R21A1070569 to EM.
Abbreviations
- BAL
bronchoalveolar lavage
- BDG
1→3 β-D-Glucan
- CT
computed tomography
- FSSC
Fusarium solani species complex
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- MALDI-TOF
matrix-assisted laser desorption ionization-time of flight
- MIC
minimum inhibitory concentration
- PCR
polymerase chain reaction
- TEF
translation elongation factor
References
- 1.Aguilar-Guisado M, Givalda J, Ussetti P, Ramos A, Morales P, Blanes M, Bou G, de la Torre-Cisneros J, Roman A, Borro JM, Lama R, Cisneros JM. Pneumonia after lung transplantation in the RESITRA cohort: a multicenter prospective study. Am J Transplant. 2007;7:1989–1996. doi: 10.1111/j.1600-6143.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Abdely HM. Management of rare fungal infections. Curr Opin Infect Dis. 2004;17:527–532. doi: 10.1097/00001432-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Alastruey-Izquierdo A, Cuenca-Estrella M, Monzon A, Mellado E, Rodriguez-Tudela JL. Antifungal susceptibility profile of clinical Fusarium spp. isolates identified by molecular methods. J Antimicrob Chemother. 2008;61:805–809. doi: 10.1093/jac/dkn022. [DOI] [PubMed] [Google Scholar]
- 4.Anaissie E, Kantarjian H, Jones P, Barlogie B, Luna M, Lopez-Berestein G, Bodey GP. Fusarium. A newly recognized fungal pathogen in immunosuppressed patients. Cancer. 1986;57:2141–2145. doi: 10.1002/1097-0142(19860601)57:11<2141::aid-cncr2820571110>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Anaissie E, Kantarjian H, Ro J, Hopfer R, Rolston K, Fainstein V, Bodey G. The emerging role of Fusarium infections in patients with cancer. Medicine (Baltimore) 1988;67:77–83. doi: 10.1097/00005792-198803000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Anaissie EJ, Hachem R, Legrand C, Legenne P, Nelson P, Bodey GP. Lack of activity of amphotericin B in systemic murine fusarial infection. J Infect Dis. 1992;165:1155–1157. [PubMed] [Google Scholar]
- 7.Anaissie EJ, Kuchar RT, Rex JH, Francesconi A, Kasai M, Muller FM, Lozano-Chiu M, Summerbell RC, Dignani MC, Chanock SJ, Walsh TJ. Fusariosis associated with pathogenic fusarium species colonization of a hospital water system: a new paradigm for the epidemiology of opportunistic mold infections. Clin Infect Dis. 2001;33:1871–1878. doi: 10.1086/324501. [DOI] [PubMed] [Google Scholar]
- 8.Aperis G, Mylonakis E. Newer triazole antifungal agents: pharmacology, spectrum, clinical efficacy and limitations. Expert Opin Investig Drugs. 2006;15:579–602. doi: 10.1517/13543784.15.6.579. [DOI] [PubMed] [Google Scholar]
- 9.Arney KL, Tiernan R, Judson MA. Primary pulmonary involvement of Fusarium solani in a lung transplant recipient. Chest. 1997;112:1128–1130. doi: 10.1378/chest.112.4.1128. [DOI] [PubMed] [Google Scholar]
- 10.Aspergillus Testing: Antibody, EPH, Galactomannan. [Accessed on April 12, 2010];Department of Pathology, Miller School of Medicine at the University of Miami Web site. [ http://www.pathology.med.miami.edu/x143.xml.
- 11.Azor M, Gene J, Cano J, Sutton DA, Fothergill AW, Rinaldi MG, Guarro J. In vitro antifungal susceptibility and molecular characterization of clinical isolates of Fusarium verticillioides (F. moniliforme) and Fusarium thapsinum. Antimicrob Agents Chemother. 2008;52:2228–2231. doi: 10.1128/AAC.00176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldwin JE, Wraight EP. Indium labelled leucocyte scintigraphy in occult infection: a comparison with ultrasound and computed tomography. Clin Radiol. 1990;42:199–202. doi: 10.1016/s0009-9260(05)81934-8. [DOI] [PubMed] [Google Scholar]
- 13.Boucher HW, Groll AH, Chiou CC, Walsh TJ. Newer systemic antifungal agents: pharmacokinetics, safety and efficacy. Drugs. 2004;64:1997–2020. doi: 10.2165/00003495-200464180-00001. [DOI] [PubMed] [Google Scholar]
- 14.Bourguignon RL, Walsh AF, Flynn JC, Baro C, Spinos E. Fusarium species osteomyelitis. Case report. J Bone Joint Surg Am. 1976;58:722–723. [PubMed] [Google Scholar]
- 15.Boutati EI, Anaissie EJ. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years_ experience at a cancer center and implications for management. Blood. 1997;90:999–1008. [PubMed] [Google Scholar]
- 16.Bueno JG, Martinez C, Zapata B, Sanclemente G, Gallego M, Mesa AC. In vitro activity of fluconazole, itraconazole, voriconazole and terbinafine against fungi causing onychomycosis. Clin Exp Dermatol. 2010;35:658–663. doi: 10.1111/j.1365-2230.2009.03698.x. Epub. 2009 Oct 23. [DOI] [PubMed] [Google Scholar]
- 17.Campos S, Caramori M, Teixeira R, Afonso J, Jr, Carraro R, Strabelli T, Samano M, Pego-Fernandes P, Jatene F. Bacterial and fungal pneumonias after lung transplantation. Transplant Proc. 2008;40:822–824. doi: 10.1016/j.transproceed.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 18.Caplin I, Unger DL. Molds on the Southern California deserts. Ann Allergy. 1983;50:260–263. [PubMed] [Google Scholar]
- 19.Chen HY, Chen YC. Characterization of intact Penicillium spores by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:3564–3568. doi: 10.1002/rcm.2229. [DOI] [PubMed] [Google Scholar]
- 20.Chryssanthou E, Cherif H, Petrini B, Kalin M, Bjorkholm M. Surveillance of triazole susceptibility of colonizing yeasts in patients with haematological malignancies. Scand J Infect Dis. 2004;36:855–859. doi: 10.1080/00365540410021108. [DOI] [PubMed] [Google Scholar]
- 21.Cooper JD, Patterson GA, Grossman R, Maurer J. Double-lung transplant for advanced chronic obstructive lung disease. Am Rev Respir Dis. 1989;139:303–307. doi: 10.1164/ajrccm/139.2.303. [DOI] [PubMed] [Google Scholar]
- 22.Cornely OA. Aspergillus to Zygomycetes: causes, risk factors, prevention, and treatment of invasive fungal infections. Infection. 2008;36:296–313. doi: 10.1007/s15010-008-7357-z. [DOI] [PubMed] [Google Scholar]
- 23.Cornely OA, Bohme A, Buchheidt D, Einsele H, Heinz WJ, Karthaus M, Krause SW, Kruger W, Maschmeyer G, Penack O, Ritter J, Ruhnke M, Sandherr M, Sieniawski M, Vehreschild JJ, Wolf HH, Ullmann AJ. Primary prophylaxis of invasive fungal infections in patients with hematologic malignancies. Recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Haematologica. 2009;94:113–122. doi: 10.3324/haematol.11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 25.Denning DW. Echinocandin antifungal drugs. Lancet. 2003;362:1142–1151. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 26.Doczi I, Gyetvai T, Kredics L, Nagy E. Involvement of Fusarium spp. in fungal keratitis. Clin Microbiol Infect. 2004;10:773–776. doi: 10.1111/j.1469-0691.2004.00909.x. [DOI] [PubMed] [Google Scholar]
- 27.Espinel-Ingroff A. In vitro antifungal activities of anidulafungin and micafungin, licensed agents and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature. Rev Iberoam Micol. 2003;20:121–136. [PubMed] [Google Scholar]
- 28.Evans J, Levesque D, de Lahunta A, Jensen HE. Intracranial fusariosis: a novel cause of fungal meningoencephalitis in a dog. Vet Pathol. 2004;41:510–514. doi: 10.1354/vp.41-5-510. [DOI] [PubMed] [Google Scholar]
- 29.Fleming RV, Walsh TJ, Anaissie EJ. Emerging and less common fungal pathogens. Infect Dis Clin North Am. 2002;16:915–933. doi: 10.1016/s0891-5520(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 30.Gabriele P, Hutchins RK. Fusarium endophthalmitis in an intravenous drug abuser. Am J Ophthalmol. 1996;122:119–121. doi: 10.1016/s0002-9394(14)71976-2. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher JC, MacDougall C, Ashley ES, Perfect JR. Recent advances in antifungal pharmacotherapy for invasive fungal infections. Expert Rev Anti Infect Ther. 2004;2:253–268. doi: 10.1586/14787210.2.2.253. [DOI] [PubMed] [Google Scholar]
- 32.Gordon SM, Avery RK. Aspergillosis in lung transplantation: incidence, risk factors, and prophylactic strategies. Transpl Infect Dis. 2001;3:161–167. doi: 10.1034/j.1399-3062.2001.003003161.x. [DOI] [PubMed] [Google Scholar]
- 33.Guarro J, Gene J. Opportunistic fusarial infections in humans. Eur J Clin Microbiol Infect Dis. 1995;14:741–754. doi: 10.1007/BF01690988. [DOI] [PubMed] [Google Scholar]
- 34.Guinea J, Pelaez T, Recio S, Torres-Narbona M, Bouza E. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob Agents Chemother. 2008;52:1396–1400. doi: 10.1128/AAC.01512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guinvarc_h A, Guilbert L, Marmorat-Khuong A, Lavarde V, Chevalier P, Amrein C, Guillemain R, Berrebi A. Disseminated Fusarium solani infection with endocarditis in a lung transplant recipient. Mycoses. 1998;41:59–61. doi: 10.1111/j.1439-0507.1998.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 36.Hachem RR. [Accessed on April 12, 2010];Lung transplantation: An overview. UpToDate.com Web site. [ http://www.uptodateonline.com/patients/content/topic.do?topicKey=~A6G8pzUei/zJ4x&selectedTitle=2~145&source=search_result.
- 37.Hardy JD, Webb WR, Dalton ML, Jr, Walker GR., Jr Lung homotransplantation in man. JAMA. 1963;186:1065–1074. doi: 10.1001/jama.1963.63710120001010. [DOI] [PubMed] [Google Scholar]
- 38.Hayden RT, Isotalo PA, Parrett T, Wolk DM, Qian X, Roberts GD, Lloyd RV. In situ hybridization for the differentiation of Aspergillus, Fusarium, and Pseudallescheria species in tissue section. Diagn Mol Pathol. 2003;12:21–26. doi: 10.1097/00019606-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Healy M, Reece K, Walton D, Huong J, Frye S, Raad II, Kontoyiannis DP. Use of the Diversi Lab System for species and strain differentiation of Fusarium species isolates. J Clin Microbiol. 2005;43:5278–5280. doi: 10.1128/JCM.43.10.5278-5280.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hennequin C, Abachin E, Symoens F, Lavarde V, Reboux G, Nolard N, Berche P. Identification of Fusarium species involved in human infections by 28S rRNA gene sequencing. J Clin Microbiol. 1999;37:3586–3589. doi: 10.1128/jcm.37.11.3586-3589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herbrecht R, Kessler R, Kravanja C, Meyer MH, Waller J, Letscher-Bru V. Successful treatment of Fusarium proliferatum pneumonia with posaconazole in a lung transplant recipient. J Heart Lung Transplant. 2004;23:1451–1454. doi: 10.1016/j.healun.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 42.Ho DY, Lee JD, Rosso F, Montoya JG. Treating disseminated fusariosis: amphotericin B, voriconazole or both? Mycoses. 2007;50:227–331. doi: 10.1111/j.1439-0507.2006.01346.x. [DOI] [PubMed] [Google Scholar]
- 43.Hosseini-Moghaddam SM, Husain S. Fungi and molds following lung transplantation. Semin Respir Crit Care Med. 31:222–233. doi: 10.1055/s-0030-1249118. [DOI] [PubMed] [Google Scholar]
- 44.Hue FX, Huerre M, Rouffault MA, de Bievre C. Specific detection of fusarium species in blood and tissues by a PCR technique. J Clin Microbiol. 1999;37:2434–2438. doi: 10.1128/jcm.37.8.2434-2438.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Husain S, Zaldonis D, Kusne S, Kwak EJ, Paterson DL, McCurry KR. Variation in antifungal prophylaxis strategies in lung transplantation. Transpl Infect Dis. 2006;8:213–218. doi: 10.1111/j.1399-3062.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 46.Jakle C, Leek JC, Olson DA, Robbins DL. Septic arthritis due to Fusarium solani. J Rheumatol. 1983;10:151–153. [PubMed] [Google Scholar]
- 47.Kerr CM, Perfect JR, Craven PC, Jorgensen JH, Drutz DJ, Shelburne JD, Gallis HA, Gutman RA. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis. Ann Intern Med. 1983;99:334–336. doi: 10.7326/0003-4819-99-3-334. [DOI] [PubMed] [Google Scholar]
- 48.Khan ZU, Ahmad S, Theyyathel AM. Diagnostic value of DNA and (1–3)- beta-D-glucan detection in serum and bronchoalveolar lavage of mice experimentally infected with Fusarium oxysporum. J Med Microbiol. 2008;57:36–42. doi: 10.1099/jmm.0.47301-0. [DOI] [PubMed] [Google Scholar]
- 49.Kiehn TE, Nelson PE, Bernard EM, Edwards FF, Koziner B, Armstrong D. Catheter-associated fungemia caused by Fusarium chlamydosporum in a patient with lymphocytic lymphoma. J Clin Microbiol. 1985;21:501–504. doi: 10.1128/jcm.21.4.501-504.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kontoyiannis DP, Lewis RE. Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect Dis Clin North Am. 2006;20:581–607. doi: 10.1016/j.idc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Kubak BM. Fungal infection in lung transplantation. Transpl Infect Dis. 2002;4(Suppl 3):24–31. doi: 10.1034/j.1399-3062.4.s3.4.x. [DOI] [PubMed] [Google Scholar]
- 52.Kurien M, Anandi V, Raman R, Brahmadathan KN. Maxillary sinus fusariosis in immunocompetent hosts. J Laryngol Otol. 1992;106:733–736. doi: 10.1017/s0022215100120729. [DOI] [PubMed] [Google Scholar]
- 53.Kwon DS, Mylonakis E. Posaconazole: a new broad-spectrum antifungal agent. Expert Opin Pharmacother. 2007;8:1167–1178. doi: 10.1517/14656566.8.8.1167. [DOI] [PubMed] [Google Scholar]
- 54.Lewis R, Hogan H, Howell A, Safdar A. Progressive fusariosis: unpredictable posaconazole bioavailability, and feasibility of recombinant interferon-gamma plus granulocyte macrophage-colony stimulating factor for refractory disseminated infection. Leuk Lymphoma. 2008;49:163–165. doi: 10.1080/10428190701724819. [DOI] [PubMed] [Google Scholar]
- 55.Li TY, Liu BH, Chen YC. Characterization of Aspergillus spores by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2000;14:2393–2400. doi: 10.1002/1097-0231(20001230)14:24<2393::AID-RCM178>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 56.Liu K, Howell DN, Perfect JR, Schell WA. Morphologic criteria for the preliminary identification of Fusarium, Paecilomyces, and Acremonium species by histopathology. Am J Clin Pathol. 1998;109:45–54. doi: 10.1093/ajcp/109.1.45. [DOI] [PubMed] [Google Scholar]
- 57.Lupinetti FM, Giller RH, Trigg ME. Operative treatment of Fusarium fungal infection of the lung. Ann Thorac Surg. 1990;49:991–992. doi: 10.1016/0003-4975(90)90885-a. [DOI] [PubMed] [Google Scholar]
- 58.Madhavan M, Ratnakar C, Veliath AJ, Kanungo R, Smile SR, Bhat S. Primary disseminated fusarial infection. Postgrad Med J. 1992;68:143–144. doi: 10.1136/pgmj.68.796.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maertens J. Evaluating prophylaxis of invasive fungal infections in patients with haematologic malignancies. Eur J Haematol. 2007;78:275–282. doi: 10.1111/j.1600-0609.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- 60.Maertens J, Meersseman W, Van Bleyenbergh P. New therapies for fungal pneumonia. Curr Opin Infect Dis. 2009;22:183–190. doi: 10.1097/QCO.0b013e328328cfff. [DOI] [PubMed] [Google Scholar]
- 61.Mahfouz T, Miceli MH, Saghafifar F, Stroud S, Jones-Jackson L, Walker R, Grazziutti ML, Purnell G, Fassas A, Tricot G, Barlogie B, Anaissie E. 18F-fluorodeoxyglucose positron emission tomography contributes to the diagnosis and management of infections in patients with multiple myeloma: a study of 165 infectious episodes. J Clin Oncol. 2005;23:7857–7863. doi: 10.1200/JCO.2004.00.8581. [DOI] [PubMed] [Google Scholar]
- 62.Marinach-Patrice C, Lethuillier A, Marly A, Brossas JY, Gene J, Symoens F, Datry A, Guarro J, Mazier D, Hennequin C. Use of mass spectrometry to identify clinical Fusarium isolates. Clin Microbiol Infect. 2009;15:634–642. doi: 10.1111/j.1469-0691.2009.02758.x. [DOI] [PubMed] [Google Scholar]
- 63.Marino E, Gallagher JC. Prophylactic antifungal agents used after lung transplantation. Ann Pharmacother. 44:546–556. doi: 10.1345/aph.1M377. [DOI] [PubMed] [Google Scholar]
- 64.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 65.Marty FM, Cosimi LA, Baden LR. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell transplants. N Engl J Med. 2004;350:950–952. doi: 10.1056/NEJM200402263500923. [DOI] [PubMed] [Google Scholar]
- 66.Mattiuzzi GN, Alvarado G, Giles FJ, Ostrosky-Zeichner L, Cortes J, O_Brien S, Verstovsek S, Faderl S, Zhou X, Raad, Bekele BN, Leitz GJ, Lopez-Roman I, Estey EH. Open-label, randomized comparison of itraconazole versus caspofungin for prophylaxis in patients with hematologic malignancies. Antimicrob Agents Chemother. 2006;50:143–147. doi: 10.1128/AAC.50.1.143-147.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehrad B, Paciocco G, Martinez FJ, Ojo TC, Iannettoni MD, Lynch JP., III Spectrum of Aspergillus infection in lung transplant recipients: case series and review of the literature. Chest. 2001;119:169–175. doi: 10.1378/chest.119.1.169. [DOI] [PubMed] [Google Scholar]
- 68.Merz WG, Karp JE, Hoagland M, Jett-Goheen M, Junkins JM, Hood AF. Diagnosis and successful treatment of fusariosis in the compromised host. J Infect Dis. 1988;158:1046–1055. doi: 10.1093/infdis/158.5.1046. [DOI] [PubMed] [Google Scholar]
- 69.Moffatt-Bruce SD. Lung Transplantation. eMedicine.com Web site. [Accessed on April 12, 2010];2009 May 13; [ http://emedicine.medscape.com/article/429499-overview.
- 70.Murray CK, Beckius ML, McAllister K. Fusarium proliferatum superficial suppurative thrombophlebitis. Mil Med. 2003;168:426–427. [PubMed] [Google Scholar]
- 71.Nelson PE, Dignani MC, Anaissie EJ. Taxonomy, biology, and clinical aspects of Fusarium species. Clin Microbiol Rev. 1994;7:479–504. doi: 10.1128/cmr.7.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neofytos D, Fishman JA, Horn D, Anaissie E, Chang CH, Olyaei A, Pfaller M, Steinbach WJ, Webster KM, Marr KA. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis. 2010;12:220–229. doi: 10.1111/j.1399-3062.2010.00492.x. Epub 2010 Jan 25. [DOI] [PubMed] [Google Scholar]
- 73.Nucci M. Emerging moulds: Fusarium, Scedosporium and Zygomycetes in transplant recipients. Curr Opin Infect Dis. 2003;16:607–612. doi: 10.1097/00001432-200312000-00015. [DOI] [PubMed] [Google Scholar]
- 74.Nucci M, Anaissie E. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin Infect Dis. 2002;35:909–920. doi: 10.1086/342328. [DOI] [PubMed] [Google Scholar]
- 75.Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20:695–704. doi: 10.1128/CMR.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nucci M, Anaissie EJ, Queiroz-Telles F, Martins CA, Trabasso P, Solza C, Mangini C, Simoes BP, Colombo AL, Vaz J, Levy CE, Costa S, Moreira VA, Oliveira JS, Paraguay N, Duboc G, Voltarelli JC, Maiolino A, Pasquini R, Souza CA. Outcome predictors of 84 patients with hematologic malignancies and Fusarium infection. Cancer. 2003;98:315–319. doi: 10.1002/cncr.11510. [DOI] [PubMed] [Google Scholar]
- 77.Nucci M, Marr KA, Queiroz-Telles F, Martins CA, Trabasso P, Costa S, Voltarelli JC, Colombo AL, Imhof A, Pasquini R, Maiolino A, Souza CA, Anaissie E. Fusarium infection in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2004;38:1237–1242. doi: 10.1086/383319. [DOI] [PubMed] [Google Scholar]
- 78.Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, Ketchum PA, Finkelman MA, Rex JH, Ostrosky-Zeichner L. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis. 2004;39:199–205. doi: 10.1086/421944. [DOI] [PubMed] [Google Scholar]
- 79.O_Donnell K, Cigelnik E, Casper HH. Molecular phylogenetic, morphological, and mycotoxin data support reidentification of the Quorn mycoprotein fungus as Fusarium venenatum. Fungal Genet Biol. 1998;23:57–67. doi: 10.1006/fgbi.1997.1018. [DOI] [PubMed] [Google Scholar]
- 80.O_Donnell K, Kistler HC, Cigelnik E, Ploetz RC. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc Natl Acad Sci U S A. 1998;95:2044–2049. doi: 10.1073/pnas.95.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O_Donnell K, Sutton DA, Fothergill A, McCarthy D, Rinaldi MG, Brandt ME, Zhang N, Geiser DM. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J Clin Microbiol. 2008;46:2477–2490. doi: 10.1128/JCM.02371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O_Donnell K, Sutton DA, Rinaldi MG, Magnon KC, Cox PA, Revankar SG, Sanche S, Geiser DM, Juba JH, van Burik JA, Padhye A, Anaissie EJ, Francesconi A, Walsh TJ, Robinson JS. Genetic diversity of human pathogenic members of the Fusarium oxysporum complex inferred from multilocus DNA sequence data and amplified fragment length polymorphism analyses: evidence for the recent dispersion of a geographically widespread clonal lineage and nosocomial origin. J Clin Microbiol. 2004;42:5109–5120. doi: 10.1128/JCM.42.11.5109-5120.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.OPTN: Organ Procurement and Transplantation Network. U. S. Department of Health & Human Services Web site; [Accessed on April 12, 2010]. [ http://optn.transplant.hrsa.gov/organDatasource/about.asp?display=Lung. [Google Scholar]
- 84.Ostrosky-Zeichner L, Alexander BD, Kett DH, Vazquez J, Pappas PG, Saeki F, Ketchum PA, Wingard J, Schiff R, Tamura H, Finkelman MA, Rex JH. Multicenter clinical evaluation of the (1[rarrow]3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41:654–659. doi: 10.1086/432470. [DOI] [PubMed] [Google Scholar]
- 85.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, Kontoyiannis DP, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Perl TM, Oster RA, Schuster MG, Walker R, Walsh TJ, Wannemuehler KA, Chiller TM. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50:1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 86.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46:201–211. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 87.Patterson JE. Epidemiology of fungal infections in solid organ transplant patients. Transpl Infect Dis. 1999;1:229–236. doi: 10.1034/j.1399-3062.1999.010402.x. [DOI] [PubMed] [Google Scholar]
- 88.Penack O, Rempf P, Graf B, Blau IW, Thiel E. Aspergillus galactomannan testing in patients with long-term neutropenia: implications for clinical management. Ann Oncol. 2008;19:984–989. doi: 10.1093/annonc/mdm571. [DOI] [PubMed] [Google Scholar]
- 89.Perfect JR. Aerosolized antifungal prophylaxis: the winds of change? Clin Infect Dis. 2008;46:1409–1411. doi: 10.1086/586740. [DOI] [PubMed] [Google Scholar]
- 90.Perfect JR, Dodds Ashley E, Drew R. Design of aerosolized amphotericin b formulations for prophylaxis trials among lung transplant recipients. Clin Infect Dis. 2004;39(Suppl 4):S207–210. doi: 10.1086/421958. [DOI] [PubMed] [Google Scholar]
- 91.Perfect JR, Marr KA, Walsh TJ, Greenberg RN, DuPont B, de la Torre-Cisneros J, Just-Nubling G, Schlamm HT, Lutsar I, Espinel-Ingroff A, Johnson E. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis. 2003;36:1122–1131. doi: 10.1086/374557. [DOI] [PubMed] [Google Scholar]
- 92.Pflugfelder SC, Flynn HW, Jr, Zwickey TA, Forster RK, Tsiligianni A, Culbertson WW, Mandelbaum S. Exogenous fungal endophthalmitis. Ophthalmology. 1988;95:19–30. doi: 10.1016/s0161-6420(88)33229-x. [DOI] [PubMed] [Google Scholar]
- 93.Price TH. Granulocyte transfusion in the G-CSF era. Int J Hematol. 2002;76(Suppl 2):77–80. doi: 10.1007/BF03165092. [DOI] [PubMed] [Google Scholar]
- 94.Pujol I, Guarro J, Gene J, Sala J. In-vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J Antimicrob Chemother. 1997;39:163–167. doi: 10.1093/jac/39.2.163. [DOI] [PubMed] [Google Scholar]
- 95.Raad II, Hachem RY, Herbrecht R, Graybill JR, Hare R, Corcoran G, Kontoyiannis DP. Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin Infect Dis. 2006;42:1398–1403. doi: 10.1086/503425. [DOI] [PubMed] [Google Scholar]
- 96.Reitz BA, Wallwork JL, Hunt SA, Pennock JL, Billingham ME, Oyer PE, Stinson EB, Shumway NE. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med. 1982;306:557–564. doi: 10.1056/NEJM198203113061001. [DOI] [PubMed] [Google Scholar]
- 97.Reuben A, Anaissie E, Nelson PE, Hashem R, Legrand C, Ho DH, Bodey GP. Antifungal susceptibility of 44 clinical isolates of Fusarium species determined by using a broth microdilution method. Antimicrob Agents Chemother. 1989;33:1647–1649. doi: 10.1128/aac.33.9.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rijnders BJ, Cornelissen JJ, Slobbe L, Becker MJ, Doorduijn JK, Hop WC, Ruijgrok EJ, Lowenberg B, Vulto A, Lugtenburg PJ, de Marie S. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trial. Clin Infect Dis. 2008;46:1401–1408. doi: 10.1086/586739. [DOI] [PubMed] [Google Scholar]
- 99.Rippon JW, Larson RA, Rosenthal DM, Clayman J. Disseminated cutaneous and peritoneal hyalohyphomycosis caused by Fusarium species: three cases and review of the literature. Mycopathologia. 1988;101:105–111. doi: 10.1007/BF00452895. [DOI] [PubMed] [Google Scholar]
- 100.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 101.Rodriguez CA, Lujan-Zilbermann J, Woodard P, Andreansky M, Adderson EE. Successful treatment of disseminated fusariosis. Bone Marrow Transplant. 2003;31:411–412. doi: 10.1038/sj.bmt.1703857. [DOI] [PubMed] [Google Scholar]
- 102.Ruiz-Cendoya M, Marine M, Guarro J. Combined therapy in treatment of murine infection by Fusarium solani. J Antimicrob Chemother. 2008;62:543–546. doi: 10.1093/jac/dkn215. [DOI] [PubMed] [Google Scholar]
- 103.Ruiz-Cendoya M, Marine M, Rodriguez MM, Guarro J. Interactions between triazoles and amphotericin B in treatment of disseminated murine infection by Fusarium oxysporum. Antimicrob Agents Chemother. 2009;53:1705–1708. doi: 10.1128/AAC.01606-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sabatelli F, Patel R, Mann PA, Mendrick CA, Norris CC, Hare R, Loebenberg D, Black TA, McNicholas PM. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob Agents Chemother. 2006;50:2009–2015. doi: 10.1128/AAC.00163-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sander A, Beyer U, Amberg R. Systemic Fusarium oxysporum infection in an immunocompetent patient with an adult respiratory distress syndrome (ARDS) and extracorporal membrane oxygenation (ECMO) Mycoses. 1998;41:109–111. doi: 10.1111/j.1439-0507.1998.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 106.Selleslag D. A case of fusariosis in an immunocompromised patient successfully treated with liposomal amphotericin B. Acta Biomed. 2006;77(Suppl 2):32–35. [PubMed] [Google Scholar]
- 107.Seyfarth F, Ziemer M, Sayer HG, Burmester A, Erhard M, Welker M, Schliemann S, Straube E, Hipler UC. The use of ITS DNA sequence analysis and MALDI-TOF mass spectrometry in diagnosing an infection with Fusarium proliferatum. Exp Dermatol. 2008;17:965–971. doi: 10.1111/j.1600-0625.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 108.Silveira FP, Kwak EJ, Paterson DL, Pilewski JM, McCurry KR, Husain S. Post-transplant colonization with non-Aspergillus molds and risk of development of invasive fungal disease in lung transplant recipients. J Heart Lung Transplant. 2008;27:850–855. doi: 10.1016/j.healun.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 109.Singh N, Husain S. Aspergillus infections after lung transplantation: clinical differences in type of transplant and implications for management. J Heart Lung Transplant. 2003;22:258–266. doi: 10.1016/s1053-2498(02)00477-1. [DOI] [PubMed] [Google Scholar]
- 110.Spader TB, Venturini TP, Cavalheiro AS, Mahl CD, Mario DN, Lara VM, Santurio J, Alves SH. In vitro interactions between amphotericin B and other antifungal agents and rifampin against Fusarium spp. Mycoses. 2009 Sep 16; doi: 10.1111/j.1439-0507.2009.01773.x. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 111.Spanakis EK, Aperis G, Mylonakis E. New agents for the treatment of fungal infections: clinical efficacy and gaps in coverage. Clin Infect Dis. 2006;43:1060–1068. doi: 10.1086/507891. [DOI] [PubMed] [Google Scholar]
- 112.Stanzani M, Tumietto F, Vianelli N, Baccarani M. Update on the treatment of disseminated fusariosis: Focus on voriconazole. Ther Clin Risk Manag. 2007;3:1165–1173. [PMC free article] [PubMed] [Google Scholar]
- 113.Stelzmueller I, Lass-Floerl C, Geltner C, Graziadei I, Schneeberger S, Antretter H, Mueller L, Zelger B, Singh N, Pruett TL, Margreiter R, Bonatti H. Zygomycosis and other rare filamentous fungal infections in solid organ transplant recipients. Transpl Int. 2008;21:534–546. doi: 10.1111/j.1432-2277.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- 114.Sturm AW, Grave W, Kwee WS. Disseminated Fusarium oxysporum infection in patient with heatstroke. Lancet. 1989;1(8644):968. doi: 10.1016/s0140-6736(89)92560-9. [DOI] [PubMed] [Google Scholar]
- 115.Tezcan G, Ozhak-Baysan B, Alastruey-Izquierdo A, Ogunc D, Ongut G, Yildiran ST, Hazar V, Cuenca-Estrella M, Rodriguez-Tudela JL. Disseminated fusariosis caused by Fusarium verticillioides in an acute lymphoblastic leukemia patient after allogeneic hematopoietic stem cell transplantation. J Clin Microbiol. 2009;47:278–281. doi: 10.1128/JCM.01670-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tortorano AM, Prigitano A, Dho G, Esposto MC, Gianni C, Grancini A, Ossi C, Viviani MA. Species distribution and in vitro antifungal susceptibility patterns of 75 clinical isolates of Fusarium spp. from northern Italy. Antimicrob Agents Chemother. 2008;52:2683–2685. doi: 10.1128/AAC.00272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Transplantation Services, Lung Transplantation Program. UPMC; Pittsburgh, PA, USA: [Accessed on April 12, 2010]. University of Pittsburgh Medical Center Web site. [ http://www.upmc.com/services/transplantationservices/typesoftransplant/lungtransplant/Pages/default.aspx. [Google Scholar]
- 118.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 119.Unilateral lung transplantation for pulmonary fibrosis. Toronto Lung Transplant Group. N Engl J Med. 1986;314:1140–1145. doi: 10.1056/NEJM198605013141802. [DOI] [PubMed] [Google Scholar]
- 120.U S Transplants Performed at Massachusetts General Hospital: January 1, 1988–August 31, 2009. U. S. Department of Health & Human Services Web site; [Accessed on April 12, 2010]. [ http://optn.transplant.hrsa.gov/latestData/stateData.asp?type=center. [Google Scholar]
- 121.Valentine NB, Wahl JH, Kingsley MT, Wahl KL. Direct surface analysis of fungal species by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun Mass Spectrom. 2002;16:1352–1357. doi: 10.1002/rcm.721. [DOI] [PubMed] [Google Scholar]
- 122.Veglia KS, Marks VJ. Fusarium as a pathogen. A case report of Fusarium sepsis and review of the literature. J Am Acad Dermatol. 1987;16:260–263. [PubMed] [Google Scholar]
- 123.Velasco E, Martins CA, Nucci M. Successful treatment of catheter-related fusarial infection in immunocompromised children. Eur J Clin Microbiol Infect Dis. 1995;14:697–699. doi: 10.1007/BF01690877. [DOI] [PubMed] [Google Scholar]
- 124.Walsh TJ, Groll A, Hiemenz J, Fleming R, Roilides E, Anaissie E. Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect. 2004;10(Suppl 1):48–66. doi: 10.1111/j.1470-9465.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 125.Walsh TJ, Pappas P, Winston DJ, Lazarus HM, Petersen F, Raffalli J, Yanovich S, Stiff P, Greenberg R, Donowitz G, Schuster M, Reboli A, Wingard J, Arndt C, Reinhardt J, Hadley S, Finberg R, Laverdiere M, Perfect J, Garber G, Fioritoni G, Anaissie E, Lee J. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med. 2002;346:225–234. doi: 10.1056/NEJM200201243460403. [DOI] [PubMed] [Google Scholar]
- 126.Welham KJ, Domin MA, Johnson K, Jones L, Ashton DS. Characterization of fungal spores by laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2000;14:307–310. doi: 10.1002/(SICI)1097-0231(20000315)14:5<307::AID-RCM823>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 127.Wheeler MS, McGinnis MR, Schell WA, Walker DH. Fusarium infection in burned patients. Am J Clin Pathol. 1981;75:304–311. doi: 10.1093/ajcp/75.3.304. [DOI] [PubMed] [Google Scholar]
- 128.White PL, Perry MD, Barnes RA. An update on the molecular diagnosis of invasive fungal disease. FEMS Microbiol Lett. 2009;296:1–10. doi: 10.1111/j.1574-6968.2009.01575.x. [DOI] [PubMed] [Google Scholar]
- 129.Wickern GM. Fusarium allergic fungal sinusitis. J Allergy Clin Immunol. 1993;92:624–625. doi: 10.1016/0091-6749(93)90087-v. [DOI] [PubMed] [Google Scholar]
- 130.Young NA, Kwon-Chung KJ, Kubota TT, Jennings AE, Fisher RI. Disseminated infection by Fusarium moniliforme during treatment for malignant lymphoma. J Clin Microbiol. 1978;7:589–594. doi: 10.1128/jcm.7.6.589-594.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang N, O_Donnell K, Sutton DA, Nalim FA, Summerbell RC, Padhye AA, Geiser DM. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J Clin Microbiol. 2006;44:2186–2190. doi: 10.1128/JCM.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


