Abstract
Chronic beryllium disease (CBD) is an occupational lung disorder characterized by granulomatous inflammation and the accumulation of beryllium-responsive CD4+ T cells in the lung. These differentiated effector memory T cells secrete IL-2, IFN-γ, and TNF-α upon in vitro activation. Beryllium-responsive CD4+ T cells in the lung are CD28 independent and have increased expression of the coinhibitory receptor, programmed death 1, resulting in antigen-specific T cells that proliferate poorly yet retain the ability to express Th1-type cytokines. To further investigate the role of coinhibitory receptors in the beryllium-induced immune response, we examined the expression of CTLA-4 in blood and bronchoalveolar lavage cells from subjects with CBD. CTLA-4 expression was elevated on CD4+ T cells from the lungs of study subjects compared to blood. Furthermore, CTLA-4 expression was greatest in the beryllium-responsive subset of CD4+ T cells that retained the ability to proliferate and express IL-2. Functional assays show that the induction of CTLA-4 signaling in blood cells inhibited beryllium-induced T cell proliferation while having no effect on the proliferative capacity of beryllium-responsive CD4+ T cells in lung. Collectively, our findings suggest a dysfunctional CTLA-4 pathway in the lung and its potential contribution to the persistent inflammatory response that characterizes CBD.
Keywords: Human, T Cells, Cell Surface Molecules, Cytokines, Lung
Introduction
Chronic beryllium disease (CBD) is a granulomatous lung disease that develops in genetically susceptible individuals following workplace exposure to beryllium (1–3). Inflammation in the lung is the end-result of an adaptive immune response to beryllium presented by HLA-DP molecules containing a glutamic acid residue at the 69th position of the β-chain (4–6). CD4+ effector memory T (TEM) cells from blood and bronchoalveolar lavage (BAL) of CBD patients robustly secrete IL-2, IFN-γ, and TNF-α when stimulated with beryllium salts in vitro (7, 8). In vivo, beryllium-responsive TEM cells accumulate in the lung and produce these pro-inflammatory cytokines in response to persistent beryllium exposure, leading to alveolitis, granulomatous inflammation and ultimately, fibrosis (1–3).
CD4+ T cells from the lungs of CBD patients proliferate poorly in vitro yet retain the ability to secrete Th1-type cytokines in response to beryllium stimulation (9, 10), suggesting a dysfunctional phenotype with persistent inflammation despite the absence of CD28-mediated costimulation (10) and increased expression of programmed death 1 (PD-1), a coinhibitory receptor that regulates beryllium-induced T cell proliferation (11). Aside from PD-1, relatively little is known about negative regulators of inflammation in chronic inflammatory lung disorders. CTLA-4 is a coinhibitory receptor (12, 13) with similar functions to PD-1 (14–16) and antagonistic activities to CD28 (17–20). It is upregulated on CD4+ TEM cells from blood and heart tissue of individuals chronically infected with Trypanosoma cruzi (21). In addition, CTLA-4 and PD-1 are coexpressed on HIV-specific T cells from the blood of HIV-infected subjects (22, 23). These findings suggest that CTLA-4 plays a key role in the regulation of the T cell phenotype associated with chronic infections as well as other chronic inflammatory disorders.
To define the function of the CTLA-4 pathway in beryllium-induced disease, we examined the expression of CTLA-4 in blood and BAL cells of normal individuals, beryllium-sensitized (BeS) subjects and CBD patients. CTLA-4 expression was elevated on total CD4+ T cells from the lungs of study subjects compared to blood, regardless of disease classification. Furthermore, CTLA-4 expression was most elevated in beryllium-responsive CD4+ T cells that retained the ability to proliferate and express IL-2. Functional assays show that the induction of CTLA-4 signaling in blood cells inhibits beryllium-induced T cell proliferation while having no effect on the proliferative capacity of beryllium-responsive CD4+ T cells in the lung. Taken together, our findings suggest that the loss of CD28 on beryllium-responsive CD4+ T cells and the resultant ineffective CTLA-4 pathway in the lung contribute to persistent inflammation in CBD.
Materials and Methods
Study population
Forty-three patients with a diagnosis of CBD and sixteen BeS patients were enrolled in this study, along with eight healthy volunteers. The diagnosis of CBD was established using previously defined criteria, including the presence of granulomatous inflammation on lung biopsy and a positive proliferative response of blood and/or BAL T cells to beryllium sulfate (BeSO4) in vitro (24, 25). The diagnosis of beryllium sensitization was established based on a positive proliferative response of PBMCs to BeSO4 in vitro and the absence of granulomatous inflammation or other abnormalities on lung biopsy (26, 27). Active smokers were excluded from enrollment. Informed consent was obtained from each subject, and the protocol was approved by the Human Subject Institutional Review Boards at the University of Colorado Denver (Aurora, CO) and National Jewish Health (Denver, CO).
The demographics of the study subjects are shown in Table 1. No difference was seen in the age of the BeS and CBD patients enrolled in this study. The majority of BeS and CBD subjects were male. Six CBD patients were treated with oral glucocorticoids. All clinical beryllium lymphocyte proliferation tests (BeLPTs) were performed in the Advanced Diagnostics Laboratory at National Jewish Health. No difference in the blood BeLPT was seen between BeS and CBD patients. In contrast, a significant increase in the proliferation of BAL cells from CBD patients compared to BeS subjects in response to beryllium was seen; the median stimulation index for CBD patients was 8.3 (range 0.8 – 308) versus 1.2 (range 0.8 – 3.8; p < 0.001) for BeS subjects. CBD subjects had a statistically significant increase in the percentage of BAL lymphocytes (median, 15%; range, 1% – 82%) compared to BeS patients (median, 5%; range, 1% – 15%; p < 0.001).
Table 1.
Characteristics of the Study Population*
| Characteristics | Normal (n = 8) | BeS (n = 16) | CBD (n = 43) |
|---|---|---|---|
| Age (years) | 41 (24 – 56) | 60 (50 – 78) | 59 (43 – 85) |
| Gender (M/F) | 4/4 | 12/4 | 38/5 |
| Race (C/AA/O)† | 8/0/0 | 14/2/0 | 42/0/1 |
| Smoking status (CS/FS/NS)‡ | 0/8 | 0/4/12 | 0/18/25 |
| Industry of Exposure (Nuclear/Ceramic/Other) | 16/0/0 | 39/3/2 | |
| Treatment (None/Prednisone/Unknown) | 12/0/5 | 34/6/4 | |
| BeLPT, Stimulation Index | |||
| Blood | 1.8 (0.8 – 117) | 3.6 (0.7 –106) | |
| BAL | 1.2 (0.8 – 3.8) | 8.3 (0.8 – 308)¶ | |
| BAL cells | |||
| WBC count (x 106) | 33 (16 – 211) | 39 (7 – 121) | |
| Lymphocytes (%) | 5 (1 – 15) | 15 (1 – 82)¶ | |
Data expressed as median (range).
C = Caucasian; AA = African-American; O = Other
CS = Current smoker; FS = Former smoker; Never smoker.
p < 0.001
Preparation of peripheral blood and BAL cells for beryllium-induced cytokine production
PBMCs were isolated from heparinized blood by Ficoll-Hypaque density gradient separation, and bronchoscopy with BAL was performed as previously described (28, 29). PBMCs and BAL cells were stimulated under the following conditions: approximately 5 × 106 cells were resuspended in RPMI 1640 plus 10% heat-inactivated fetal bovine serum (Hyclone) in 12 × 75 mm culture tubes with anti-CD49d (BD Biosciences) in the presence of either medium alone, 100 μM BeSO4 (Brush Wellman), or staphylococcal enterotoxin B (SEB) for 6 hours at 37°C in a humidified 5% CO2 atmosphere. Brefeldin A (BD Biosciences) was added after 1 hour in culture.
Immunofluorescence staining of CD4+ T cells and monocytes/macrophages
PBMCs or BAL cells were washed, incubated with FcR-blocking reagent (Miltenyi Biotec) and stained with anti-CD4 (PerCP-Cy5.5, BD Biosciences), anti-CD3 (PE-Texas Red, Beckman Coulter), anti-CD8 (V-500, BD Biosciences), anti-CD279 (PD-1, FITC, BD Biosciences) and anti-CD28 (V-450, BD Biosciences) mAbs for 30 minutes at 4°C. Cells were washed with PBS containing 1% BSA, fixed, permeabilized, and stained with anti-CD152 (CTLA-4, PE, BD Biosciences), anti-IFN-γ (PE-Cy7, BD Biosciences), anti-IL-2 (AF-700, BioLegend), and anti-Ki-67 (AF-647, BD Biosciences) mAbs for 30 minutes at 4°C. For ex vivo staining, PBMCs and BAL cells were washed, blocked with FcR and stained with anti-CD45 (eFluor 605NC, eBiosciences), anti-CD14 (eFluor 450, eBiosciences), anti-CD80 (FITC, eBiosciences) and anti-CD86 (PerCP eFluor 710, eBiosciences) mAbs for 30 minutes at 4°C. Cells were washed and resuspended in 1% formaldehyde.
Proliferation assay
Proliferation assays were performed using PBMCs and BAL cells (0.5 – 1 × 106 cells/well) labeled with 5 μM CellTrace Violet (Invitrogen) (30, 31). CellTrace Violet-labeled cells were stimulated with 100 μM BeSO4 or medium. Wells of a twenty-four well plate were coated with 50μg/mL anti-CTLA-4 antibody (clone BNI3, BD Biosciences) or isotype control (IgG2A κ, BD Biosciences) and incubated overnight at 4°C (21). Wells were washed with PBS, and CellTrace Violet-labeled cells were added along with 100 μM BeSO4 where appropriate and incubated for 5 days at 37°C in a humidified 5% CO2 atmosphere. Cells were harvested, incubated with FcR-blocking reagent, stained with anti-CD3 and anti-CD4 and resuspended in 1% formaldehyde.
To detect CD69 expression and de novo Ki-67 expression in response to anti-CD3 stimulation, PBMCs were cultured as described above in wells of a 24-well plate coated with either medium or 0.1 μg/mL purified anti-human CD3 (clone OKT3, eBiosciences) for 6, 24, or 48 hours. Harvested cells were stained with mAbs directed against CD3, CD4, CD8, CD69 (FITC, BD Biosciences), and Ki-67 as described above.
Flow cytometry
Formaldehyde-fixed cells were analyzed using a LSRII flow cytometer (BD Immunocytometry Systems). The number of events collected ranged between 1 and 3 million. Electronic compensation was performed with antibody capture beads (BD Biosciences) stained separately with individual antibodies used in the test samples. Data files were analyzed using FlowJo software (Tree Star, Inc.), and biexponential scaling was used in all dot plots. Lymphocytes were gated based on their forward and side scatter profile. CD3+ T cells were selected, and the expression of CD4 and CD8 was analyzed in a bivariate dot plot. Since the frequency of beryllium-responsive CD4+ T cells in blood and BAL tends to be low, we only examined the expression of PD-1, CTLA-4, and Ki-67 on cytokine-producing cells (IFN-γ+, IL-2+ or both as described in the Results section) with frequencies greater than or equal to 0.04% for blood and 0.4% for BAL to ensure an adequate number of events for analysis as previously described (28–30). SEB-stimulated T cells were analyzed in a similar manner. Dividing cells were identified in proliferation experiments by gating on CellTrace Violetlo staining as compared to unstimulated control samples.
To control for the accuracy and precision of measurements taken during the course of the study, routine quality control was performed on the LSRII using the Cytometer Setup & Tracking (CS&T) feature within BD FACSDiva software version 6.1.2 (BD Biosciences). Voltage, laser delay and area scaling were determined using standardized CS&T beads (BD Biosciences), and settings were tracked over time. To verify the laser delay and area scaling determined by CS&T, a manual quality control (QC) using rainbow beads was performed daily. To further control for quantitative comparisons of surface and intracellular molecule expression levels, collection of data from healthy control subjects was interspersed throughout the study period with acquisition of data from study subjects.
Statistical Analysis
Kruskal-Wallis ANOVA, paired Student’s t-test, and Spearman correlation analysis were used to determine significance of differences between subject groups. A p value of < 0.05 was considered statistically significant.
Results
CTLA-4 expression is increased in the lung
To determine if CTLA-4 expression is increased in the setting of chronic lung inflammation, we initially analyzed its expression in blood and BAL CD4+ T cells. Freshly isolated PBMCs and BAL cells were obtained from CBD patients, BeS subjects and normal controls, and the mean fluorescence intensity (MFI) of CTLA-4 expression in total CD3+CD4+ T cells was determined by immunofluorescence staining and cytofluorographic analysis. Representative histograms of CTLA-4 expression on CD3+CD4+ T cells from the peripheral blood and BAL of a CBD subject are shown in Figure 1A. Overall, CTLA-4 expression was significantly increased in CD4+ T cells from the lung compared to blood, regardless of disease status (Figure 1B). For example, in CBD patients, CTLA-4 expression in BAL CD4+ T cells (median, 1007; range, 559–1246) was 1.7-fold higher than that seen in blood cells (median, 582; range, 310–1284; p < 0.001).
FIGURE 1.
CTLA-4 and CD28 expression in blood and BAL. Representative histograms of CTLA-4 (A) and CD28 (C) expression on CD3+CD4+ T cells from blood and BAL of a CBD patient are shown. Shaded histograms represent fluorescence minus one (FMO) staining for the corresponding antibody. Mean fluorescent intensity (MFI) is indicated. CTLA-4 (B) and CD28 (D) expression in CD4+ T cells from blood and BAL of normal subjects, BeS subjects and CBD patients is shown. (E) Representative density plots of the expression of CD28 and CLTA-4 on CD4+ T cells from blood and BAL of a CBD patient are shown. The numbers in the quadrants are the percentage of CD4+ T cells positive and negative for CTLA-4 and CD28 expression. (F) CTLA-4 expression in CD28+ and CD28− CD4+ T cells in blood and lung of CBD patients is shown. Data shown in B, D and F are expressed as the median MFI with median values indicated with solid lines. Statistical significance was determined using a Kruskal-Wallis ANOVA. Significance levels are indicated by asterisks (* p < 0.05, ** p < 0.01, *** p < 0.001).
Since CD28 signaling induces the expression of CTLA-4 (32) and CTLA-4 activation in conventional T cells negatively regulates the immune response largely by attenuating CD28-induced signaling (12, 13, 17, 18, 33, 34), we analyzed co-expression of CTLA-4 and CD28 on CD4+ T cells in blood and BAL. As shown in Figure 1C and D, a significant decrease in CD28 expression on a per cell basis as measured by MFI on CD4+ T cells in the lung compared to blood was seen, with a significant percentage of BAL CD4+ T cells having lost CD28 expression (Figure 1C) (10). Gating on CD28+CD4+ T cells, decreased expression of CD28 in the lung occurred in all study groups. When gating on CD28+ and CD28− T cells (representative example shown in Figure 1E), CTLA-4 expression was significantly higher on CD28+CD4+ T cells in blood and lung of CBD patients compared to their CD28− counterparts (Figure 1F). CTLA-4 was also significantly higher on CD28− T cells in BAL as compared to CD28+ T cells in blood (1.3-fold higher, p < 0.001), and similar findings were seen in normal control and BeS subjects (data not shown). Thus, CTLA-4 is upregulated on ex vivo BAL CD4+ T cells in the setting of CD28 downregulation, and this effect appears to be lung-specific as opposed to disease-specific since it occurs in all study subjects regardless of the presence of lung pathology.
Differential CTLA-4 expression on beryllium-responsive, Th1 cytokine-expressing CD4+ T cells
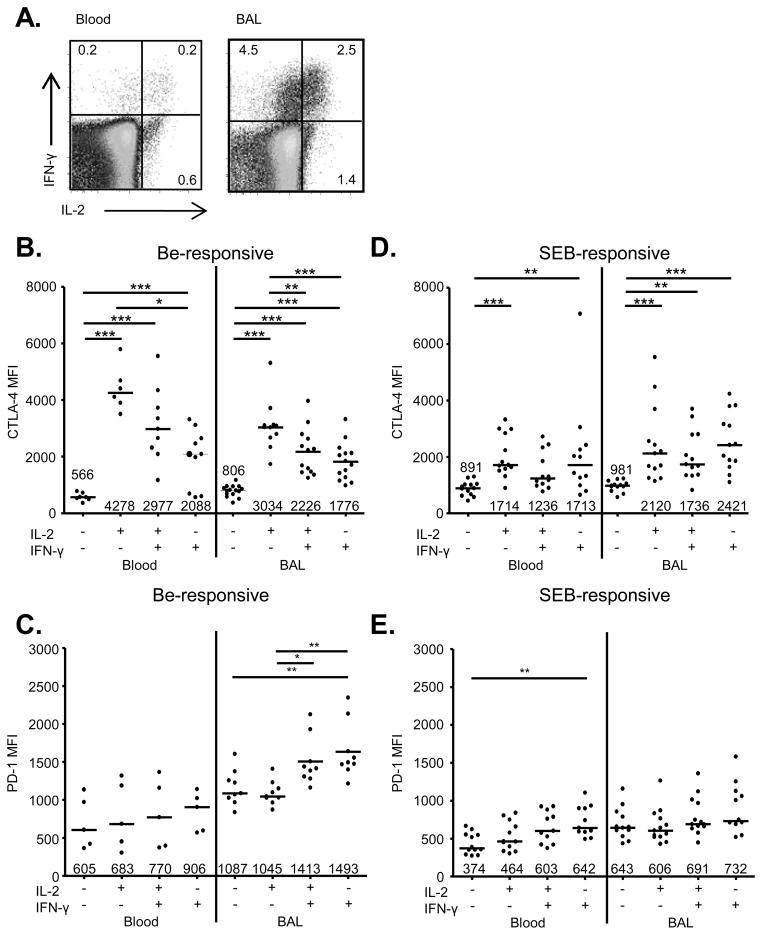
As beryllium-responsive TEM cells mature into their most terminally differentiated and least proliferative state, they lose the expression of IL-2 and gain the expression of IFN-γ (7, 9, 31). This expression pattern can be used to dissect beryllium-responsive CD4+ T cells into distinct subsets (Figure 2A), with the least differentiated subset expressing IL-2 alone (IL-2+IFN-γ−), followed by the upregulation of IFN-γ expression (IL-2+IFN-γ+), and finally with the most terminally differentiated subset marked by the loss of IL-2 expression (IL-2−IFN-γ+) (7, 9, 31). In order to determine if CTLA-4 expression is upregulated in the context of beryllium exposure, we measured CTLA-4 in these beryllium-responsive, IL-2- and IFN-γ-producing CD4+ T cell subsets in CBD subjects. PBMCs and BAL cells were stimulated with BeSO4 for 6 hours in culture followed by intracellular cytokine staining to identify the beryllium-responsive T cells in each patient. All subsets of cytokine-secreting, beryllium-responsive CD4+ T cells from blood and BAL expressed significantly higher levels of CTLA-4 compared to unstimulated cells (Figure 2B). In addition, beryllium-responsive CD4+ cells producing only IL-2 expressed higher levels of CTLA-4 than cells producing only IFN-γ in blood and BAL (Figure 2B). Within these distinct cytokine-secreting T cell subsets, CD4+ T cells in blood and BAL appear to downregulate CTLA-4 expression as they undergo further differentiation (Figure 2B). For example, median CTLA-4 expression in beryllium-responsive, IL-2−IFN-γ+ CD4+ T cells in blood (2088; range, 563–3313) was 2-fold lower compared to IL-2+IFN-γ− cells (4258; range, 3501–5793; p < 0.001) in blood. Similar findings were also seen in BAL, with a 1.7-fold difference in CTLA-4 expression between IL-2-only and IFN-γ-only expressing T cells (Figure 2B). However, unlike CTLA-4, PD-1 expression increased on terminally differentiated beryllium-responsive T cells in the lung (Figure 2C).
FIGURE 2.
CTLA-4 and PD-1 expression on beryllium-responsive, Th1-type cytokine-expressing CD4+ T cells in blood and BAL of CBD patients. (A) Representative density plots of beryllium-induced IL-2 and IFN-γ expression in blood and BAL cells from a CBD patient. The numbers in the quadrants of each density plot are the percentages of CD4+ T cells that express IFN-γ, IL-2 or both cytokines. CTLA-4 expression (MFI) in unstimulated (Un), beryllium-responsive (B) or SEB-responsive (D) IL-2+IFN-γ−, IL-2+IFN-γ+ and IL-2−IFN-γ+ CD4+ T cells from blood and BAL of CBD patients is shown. PD-1 expression (MFI) on unstimulated (Un), beryllium-responsive (C) or SEB-responsive (E) IL-2+IFN-γ−, IL-2+IFN-γ+ and IL-2−IFN-γ+ CD4+ T cells from blood and BAL of CBD patients is shown. Data shown in B-E are expressed as the median MFI with median values indicated with solid lines. Statistical significance was determined using a Kruskal-Wallis ANOVA. Vertical black line dividing each graph separates datasets into those compared with an ANOVA. Significance levels are indicated by asterisks (* p < 0.05, ** p < 0.01, *** p < 0.001).
To exclude the possibility that the increased CTLA-4 expression on less differentiated beryllium-responsive CD4+ T cells is due to T cell activation, we also stimulated PBMCs and BAL cells from CBD patients with SEB and analyzed intracellular cytokine, CTLA-4 and PD-1 expression. As shown in Figure 2D, CTLA-4 was upregulated in SEB-responsive cells expressing IL-2 and/or IFN-γ in both blood and BAL. However, the pattern seen in beryllium-responsive cells where differentiation to IFN-γ-only producing cells coincided with CTLA-4 downregulation was not seen in response to SEB stimulation. In addition, CTLA-4 expression was significantly increased in beryllium-responsive, IL-2-only-expressing CD4+ T cells in blood (p = 0.0007) and BAL (p = 0.04) compared to SEB-responsive IL-2-expressing T cells in the same compartments (Figure 2D). PD-1 expression was only significantly increased in IFN-γ only-expressing CD4+ T cells in blood compared to unstimulated cells (median 642 in IL-2−IFN-γ+ cells compared to 374 in IL-2−IFN-γ− cells) (Figure 2E). Thus, the differential pattern of CTLA-4 upregulation seen in Th1 cytokine-expressing beryllium-responsive CD4+ T cells is not simply a marker of T cell activation.
To examine whether differences in antigen-presenting cells (APCs) in blood and BAL could account for this differential CTLA-4 expression, cross-over experiments were performed with blood CD4+ T cells incubated with autologous APCs derived from either blood or BAL and BAL CD4+ T cells incubated with autologous blood or BAL APCs. No differences were seen in CTLA-4 or PD-1 expression or beryllium-induced cytokine expression by CD4+ T cells in the presence of different APC subsets (data not shown).
Beryllium-responsive, IFN-γ-expressing CD4+ T cells do not proliferate in the lungs of CBD patients
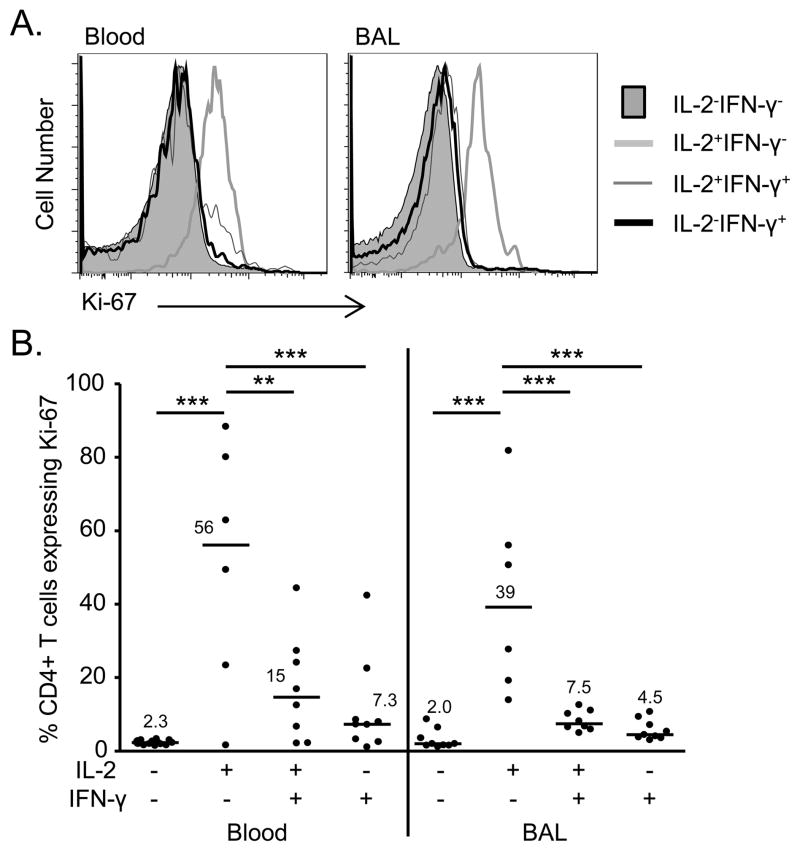
Despite the relatively poor proliferative capacity of some beryllium-responsive CD4+ T cells in vitro (9, 10), the proliferative status of the various populations of beryllium-responsive, memory CD4+ T cell subsets in vivo is unknown. Ki-67 is a nuclear protein often analyzed as a marker of in vivo proliferation (35). When cells transition from a resting state, Ki-67 is exposed on the surface of chromosomes and can be detected using standard methods of intracellular flow cytometry (35, 36). To validate that Ki-67 expression is not induced during short-term stimulation, PBMCs were stimulated with plate-bound anti-CD3 for 0, 6, 24, and 48 hours and analyzed for changes in Ki-67 expression. As shown in Supplemental Figure 1A, no differences in the percentage of Ki-67+ T cells were detected at 6 hours with anti-CD3 stimulation compared to no stimulation (median 2.8% compared to 1.4%). Conversely, expression of the recent activation marker CD69 was significantly increased as early as 6 hours after anti-CD3 stimulation (median 7.8% compared to 1.9% at time 0, p = 0.01) (Supplemental Figure 1B).
Following 6 hour stimulation with BeSO4 in culture, Ki-67 expression was analyzed in beryllium-responsive T cell subsets from blood and BAL in order to determine which beryllium-responsive, Th1-cytokine expressing memory T cells were undergoing in vivo proliferation. Figure 3A shows representative histogram overlays of Ki-67 expression in these subsets from blood and lung. Only those cells exclusively producing IL-2 in the blood and BAL were undergoing in vivo proliferation (Figure 3B). The median percentage of proliferating (Ki-67+) IL2+IFN-γ− cells in the blood was 56% (range, 1.6–88%) compared to 15% (range, 2.1–27%) of IL-2+IFN-γ+ cells (p < 0.01) and 7.3% (range 1.1–42%) of IL-2−IFN-γ+ cells (p < 0.001). In BAL, 39% (range 14–56) of IL-2+IFN-γ−CD4+ T cell expressed Ki-67 cells compared to 7.5% (range, 5–13; p < 0.001) of IL-2+IFN-γ+ cells and 4.4% (range, 3–11; p < 0.001) of IFN-γ-only expressing T cells. Thus, these data suggest that Ki-67 detected in beryllium-responsive cells at 6 hours reflects their in vivo proliferative status and that the gain of IFN-γ expression by these cells is associated with a loss of proliferation (Figure 3B).
FIGURE 3.
Proliferative status of beryllium-responsive T cell subsets in blood and BAL of CBD patients. (A) Representative histograms of Ki-67 detected in unstimulated or BeSO4-responsive IL-2+IFN-γ−, IL-2+IFN-γ+ and IL-2−IFN-γ+ CD4+ T cells from blood and BAL of a CBD patient is shown (B) Ki-67 detected in CD4+ T cells in blood and BAL CD4+ T cells from CBD patients with the following Th1 cytokine expression patterns is shown: IL-2−IFN-γ−, IL-2+IFN-γ−, IL-2+IFN-γ+ and IL-2−IFN-γ+. Data are expressed as the median mean fluorescent intensity (MFI) with median values indicated with solid lines. Statistical significance was determined using a Kruskal-Wallis ANOVA. Significance levels are indicated by asterisks (** p < 0.01, *** p < 0.001).
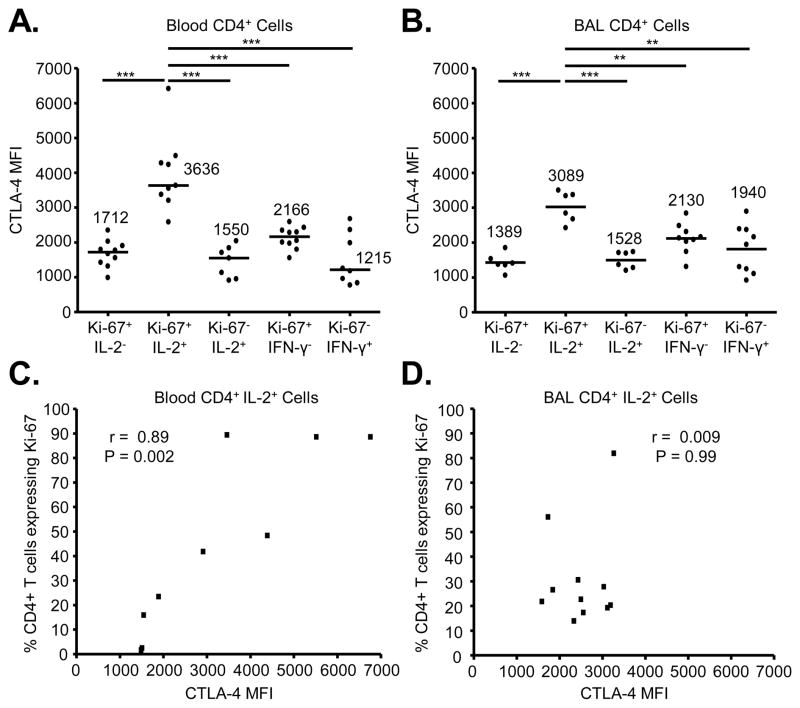
CTLA-4 expression is highest on proliferating, IL-2-secreting CD4+ T cells in CBD patients
We next directly compared the expression of CTLA-4 on populations of proliferating and cytokine-producing CD4+ T cells from the blood and lungs of CBD patients. Gating on CD4+ T cells, we analyzed CTLA-4 expression based on in vivo proliferation and beryllium-induced Th1 cytokine production. CTLA-4 expression was highest on blood and BAL T cells expressing Ki-67 and IL-2 (Figure 4A and 4B). Median CTLA-4 expression in blood Ki-67+IL-2+ cells was 3636 (range, 2584–6411) and was 1.7 to 3-fold higher compared to all other groups (p < 0.001). Median CTLA-4 expression in BAL Ki-67+IL-2+ cells was 3089 (range, 2420–3497) and was 1.5- to 2.2-fold higher compared to all other groups (p < 0.01 or < 0.001). On the other hand, PD-1 expression was highest on non-proliferating, IFN-γ-secreting cells in the lung (data not shown).
FIGURE 4.
CTLA-4 expression in beryllium-responsive CD4+ T cells in blood and BAL in relation to proliferation and IL-2 expression. (A and B) CTLA-4 expression in beryllium-responsive CD4+ T cells from blood and BAL of CBD patients based on the presence or absence of Ki-67, IL-2 and IFN-γ expression is shown. Data shown in A and B are expressed as the median MFI with median values indicated with solid lines. Statistical significance was determined using a Kruskal-Wallis ANOVA. Significance levels are indicated by asterisks (** p < 0.01, *** p < 0.001). (C and D) Correlation between CTLA-4 expression (MFI) on beryllium-responsive, IL-2-expressing CD4+ T cells from the blood (n = 9) and BAL (n = 11) of CBD patients and in vivo proliferation as measured by intracellular Ki-67 staining is shown.
Based on our findings of increased CTLA-4 and Ki-67 expression on beryllium-responsive, IL-2-expressing CD4+ T cells in blood and BAL of CBD patients, we correlated CTLA-4 and Ki-67. As shown in Figure 4C, a significant positive correlation was noted between Ki-67 and CTLA-4 expression on IL-2+ CD4+ T cells in blood (r = 0.89, p = 0.002) (Figure 4C). In the lung, however, no correlation between CTLA-4 expression and proliferation of IL-2+ cells (r = 0.009, p = 0.99) was seen (Figure 4D), due to the low percentage of proliferating cells in the lung and the variability of CTLA-4 expression in these cells.
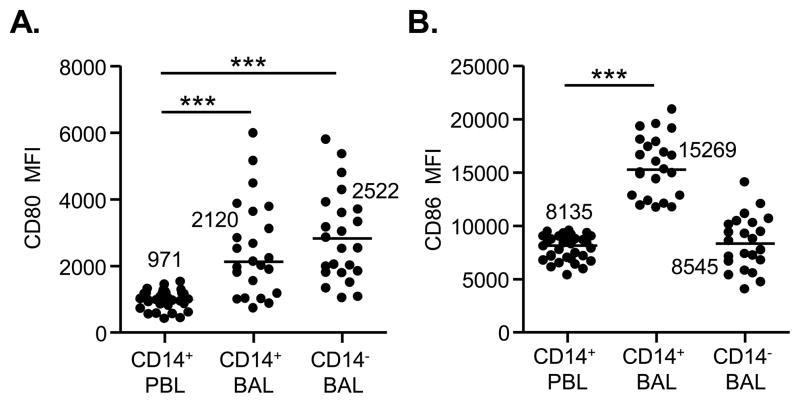
Increased expression of CD80 and CD86 on CD14+ cells in the lungs of CBD patients
Suppression of CTLA-4-expressing T cells requires that the CTLA-4 ligands (CD80 and CD86) are expressed on APCs. To examine this, CTLA-4 ligand expression was measured by flow cytometry on monocytes/macrophages, defined by characteristic forward and side scatter profiles and CD14 expression, from blood and BAL of CBD patients. CD80 expression on CD14+ and CD14− cells within the macrophage gate of BAL cells was significantly greater than on CD14+ monocytes in blood (Figure 5A). Median CD80 expression was 2120 (range, 713–5970) on CD14+ BAL cells and 2522 (range, 1025–5779) on CD14− BAL cells compared to 971 (range, 401–1518; p < 0.001) for CD14+ cells in blood (Figure 5A). Conversely, CD86 expression on BAL cells was only significantly increased on CD14+ cells in the macrophage gate compared to CD14+ cells in blood (Figure 5B). These data demonstrate that CD14+ cells within the macrophage gate of BAL of CBD patients have significantly higher levels of CTLA-4 ligands than those in the blood, suggesting that this pathway is upregulated in the lung.
FIGURE 5.
Expression of CTLA-4 ligands is elevated on APCs in the lung of CBD patients. CD80 (A) and CD86 (B) expression was evaluated on large cells (based on FSC and SSC profile) from the peripheral blood (n = 18) and BAL (n = 11) of CBD patients. Median values are indicated with solid lines. Statistical significance was established using the Mann-Whitney test. Significance levels are indicated by asterisks (*** p < 0.001).
CTLA-4 is not functional in the lungs of CBD patients
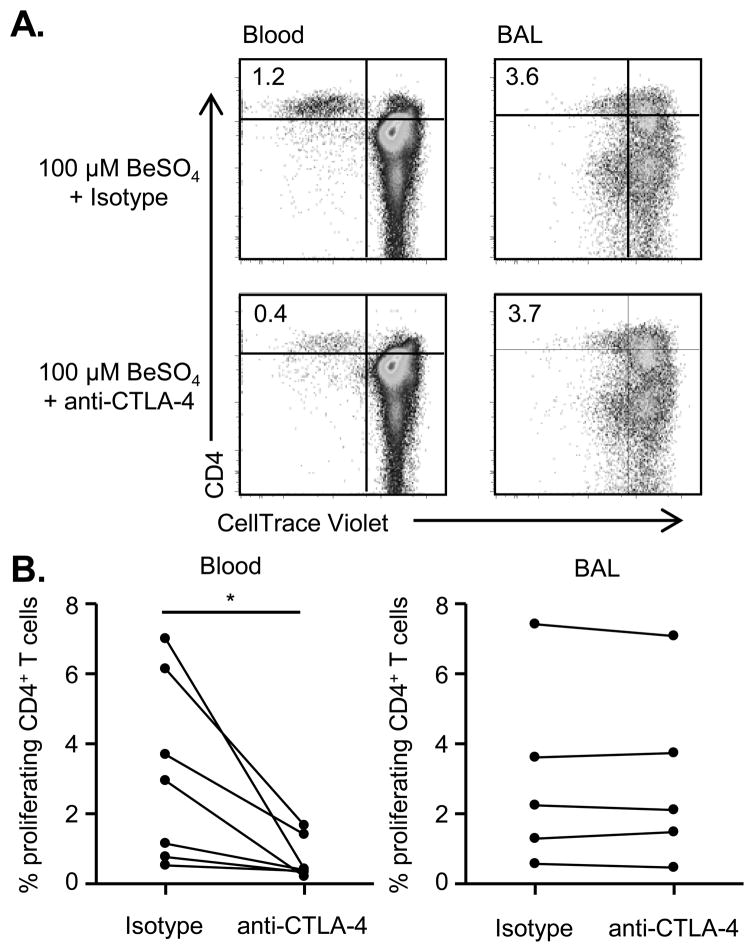
Our data show that CTLA-4 and PD-1 have opposite expression patterns on beryllium-responsive CD4+ T cells in blood and BAL despite the similarity of their proposed functions as inhibitory receptors (14–16). We have previously shown that blocking PD-1 engagement with its ligands in culture restored the proliferative capacity of beryllium-responsive lung T cells (11), indicating that the PD-1 pathway is active in the lung of CBD patients. To investigate the functionality of the CLTA-4 pathway, we cross-linked CTLA-4 to induce signaling (21, 33, 37) and measured beryllium-induced proliferation in culture, with the expected finding being a decrease in proliferation if the CTLA-4 pathway is active. Cells from CBD patients were labeled with CellTrace Violet and stimulated with BeSO4 in the presence of plate-bound anti-CTLA4 or isotype control. After 5 days, CD3+CD4hi cells from these cultures were examined for loss of CellTrace Violet as a measurement of beryllium-induced T cell proliferation. Representative examples of proliferating CD4+ T cells (CellTrace VioletloCD4hi) from blood and BAL are shown in Figure 6A. Overall, cross-linking CTLA-4 significantly reduced beryllium-induced CD4+ T cell proliferation in blood (p = 0.032) (Figure 6B, left panel) while having no effect on the proliferation of beryllium-stimulated BAL cells (p = 0.63) (Figure 6B, right panel). Collectively, these data suggest that the CTLA-4 pathway in the lungs of CBD patients is dysfunctional despite the upregulation of CTLA-4 on beryllium-specific CD4+ T cells and its ligands on APCs in the BAL.
FIGURE 6.
Beryllium-induced proliferation in the presence of activating anti-CTLA-4 antibody. (A) Representative density plots of the percentage of CellTrace ViolotloCD3+CD4hi T cells from blood and BAL of a CBD subject after 5 days of stimulation with 100 μM BeSO4 in the presence of either plate-bound, cross-linking CTLA-4 or isotype control is shown. (B) Summary data showing the percentage of CellTrace ViolotloCD3+CD4hi T cells in the presence and absence of CTLA-4 cross-linking (n = 7 for blood and n = 5 for BAL). Statistical comparisons were made using a paired Student’s T test, and significance levels are indicated by asterisks (* p < 0.05).
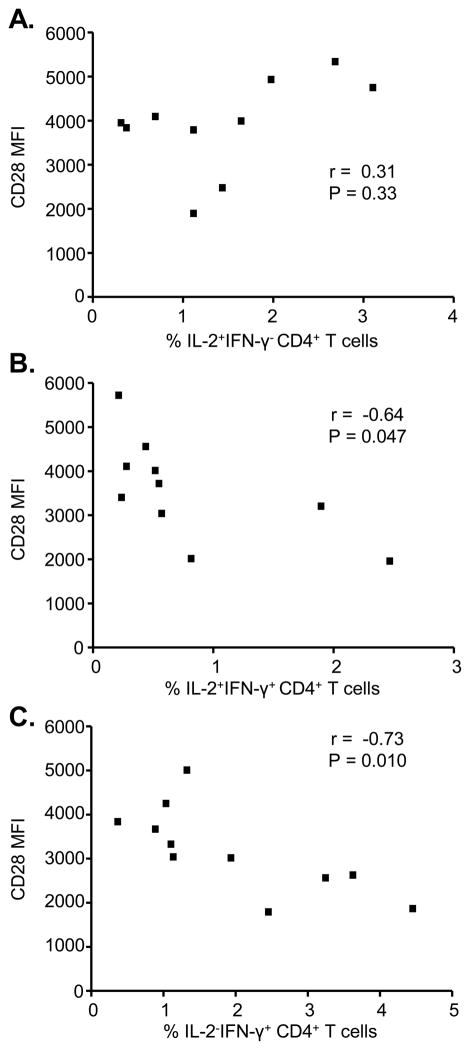
Loss of CD28 expression inversely correlates with an increased frequency of IFN-γ-expressing CD4+ T cells in the lung
Since a primary function of CTLA-4 is inhibition of the CD28 signaling cascade (33), our data suggest that the inability of CTLA-4 to downregulate beryllium-induced T cell proliferation and IFN-γ expression in the lung is related to the independence of beryllium-responsive CD4+ T cells in BAL from CD28-mediated costimulation (10). To determine whether the failure of CTLA-4 to downregulate IFN-γ production is related to decreased CD28 expression, we correlated CD28 expression and the percentage of CD4+ T cells expressing IL-2 alone, IL-2 and IFN-γ, or IFN-γ alone in CBD patients. No correlation was seen between CD28 expression and the percentage of IL-2 only expressing cells (r = 0.31, p = 0.33, Figure 7A). However, there was a slight negative correlation between CD28 levels and percentage of cells producing both IL-2 and IFN-γ (r = −0.64, p = 0.047, Figure 7B) and a strong negative correlation with CD4+ T cells expressing IFN-γ alone (r = −0.73, p = 0.010, Figure 7C). Conversely, no correlation was seen in the blood between CD28 expression and the percentage of beryllium-responsive, cytokine-secreting T cells (IL-2+IFN-γ−, r = 0.033, p = 0.95; IL-2+IFN-γ+, r = −0.086, p = 0.92; IL-2−IFN-γ+, r = −0.62, p = 0.12) (data not shown). Collectively, these data support an inverse relationship between CD28 and IFN-γ expression on beryllium-responsive CD4+ T cells in the lung, contributing to persistent lung inflammation even in the setting of enhanced CTLA-4 expression.
FIGURE 7.
Correlation between CD28 expression and IFN-γ expression by beryllium-responsive CD4+ T cells in the lung. CD28 expression (MFI) was correlated with (A) the percentage of IL-2+IFN-γ− (n = 10), (B) the percentage of IL-2+IFN-γ+ (n = 10), and (C) the percentage of IL-2−IFN-γ+ (n = 11) beryllium-responsive CD4+ T cells in the BAL of CBD patients.
Discussion
CBD represents an important model of persistent antigen exposure and is characterized by the accumulation of large numbers of beryllium-responsive CD4+ T cells in the lung (1, 2, 7). Consequently, Th1-type cytokine secretion by lung T cells plays a central role in the immunopathogenesis of beryllium-induced disease. The regulation of the immune response and the subsequent inflammation in the lung that results from persistent antigen exposure is not well understood. We have previously shown that beryllium-responsive CD4+ T cells in the lung express predominantly an effector memory phenotype (7, 31), are CD28 independent (10), express CD57 (a marker of senescence) (38), and upregulate PD-1 (11), a CD28 family member that serves to negatively regulate T cell function. The PD-1 pathway is active in CBD and contributes to the downregulation of proliferation in response to chronic beryllium exposure (11). The present study extends our previous findings by showing for the first time that another coinhibitory receptor, CTLA-4, is up-regulated on beryllium-responsive CD4+ T cells in blood and lung of CBD patients. In contrast to blood cells, induction of CTLA-4 signaling fails to block beryllium-induced CD4+ T cell proliferation, suggesting a differential functional capacity of CTLA-4 between antigen-specific T cells in blood and lung.
CD28 and CTLA-4 are homologous receptors that share ligands despite having opposite effects on cellular activation (12, 13, 17, 18, 33, 34). CD28 costimulates T cell activation while CTLA-4 inhibits the T cell response through a blockade of CD28 signaling and activation of CTLA-4-specific signaling pathways (33). We have previously shown that beryllium-specific CD4+ T cells in the lung downregulate CD28 and no longer require CD28-mediated costimulation (10, 39), findings consistent with TEM cells in other immune-mediated diseases (40–42). Despite the decreased expression of CD28 on CD4+ T cells in lung compared to blood, CTLA-4 expression on lung T cells was increased. In blood and BAL, CD28− T cells had lower CTLA-4 levels than cells expressing CD28, but CTLA4 was still elevated in lung T cells compared to cells in the blood, suggesting that loss of CD28 expression precedes loss of CTLA-4 in the lung and that even though beryllium-specific TEM cells in the lung are independent of CD28, CTLA-4 expression is still induced.
Due to the inhibitory function of CTLA-4, we were initially surprised that beryllium-responsive, Ki-67+IL-2+CD4+ T cells in both blood and lung expressed the highest levels of CTLA-4. However, previous studies have shown that the induction of CTLA-4 expression requires CD28 signaling, IL-2 production, and entry into the cell cycle (13, 32, 43). This is also consistent with published reports of memory T cells containing a relatively large pool of intracellular CTLA-4 (44). The requirement of IL-2 and cell cycle entry for CTLA-4 expression provides an explanation for the highest expression of CTLA-4 on beryllium-responsive T cells in blood that are proliferating and expressing IL-2. We have previously shown that a properly functioning PD-1 pathway is likely contributing to the diminished proliferative capacity of antigen-responsive T cells in the lung (11). However, the presence of elevated CTLA-4 expression on beryllium-responsive, IFN-γ-expressing T cells in the lung raises the possibility of an impaired function of this inhibitory receptor to turn off inflammatory cytokine production.
PD-1 and CTLA-4 are both coinhibitory receptors expressed following T cell activation and function to negatively regulate the resulting immune response (14–16). While these receptors are proposed to have similar functional outcomes (i.e., downregulation of proliferation, cytokine production, cell survival), it is evident that they utilize distinct signaling pathways to accomplish non-redundant immunomodulatory effects (16). In response to chronic antigen exposure, both of these inhibitory receptors are upregulated (21, 23, 45), contributing to a state of cellular senescence. In this state, cells are more susceptible to death and fail to proliferate or produce cytokines (46). HIV-specific T cells upregulate a series of coinhibitory molecules when chronically stimulated, which results in the inability to mount an effective response against the virus (23, 45). Blocking these receptors restored effector functions to the cells and led to an improved antiviral response. CBD also results in chronic immune activation, due to persistent exposure to beryllium. Therefore, we originally expected to see PD-1 and CTLA-4 upregulated on the same T cells in the lungs of CBD patients. Instead, we saw an opposite expression pattern, indicating that the expression of each receptor has a distinct role in regulating the immune response to chronic beryllium exposure in the lung.
In this regard, PD-1 predominantly functions to block T cell proliferation of beryllium-responsive cells, having little role in downregulating IFN-γ expression. Our data support this contention with blockade of the PD-1 pathway restoring proliferation of beryllium-responsive lung T cells while having no effects on intracellular IFN-γ expression (11). Aside from regulating proliferation, one of the major functions of CTLA-4 is to control the secretion of cytokines by activated cells (42–45). In murine models of autoimmunity, blockade of CTLA-4 engagement rapidly induced the onset of diabetes in NOD mice (47, 48) and increased the severity of experimental autoimmune encephalomyelitis (EAE) in EAE-susceptible and -resistant mouse strains (49, 50). Part of the mechanism for the exacerbated disease severity resulting from a lack of CTLA-4 function was increased secretion of IFN-γ by antigen-specific T cells (48, 50). Our data raise the possibility that the continued secretion of IFN-γ by non-proliferating beryllium-responsive CD4+ T cells in the CBD lung is due to the loss of CD28 expression and a resulting ineffective CTLA-4 pathway. In CBD, the continued presence of IFN-γ promotes chronic lung inflammation, including the development of granulomas and fibrosis (1–3).
Our data raise the possibility that a normal consequence of memory cell transition to the most terminally differentiated state in a target organ is a loss of CTLA-4 function. The active mechanism by which CTLA-4 downregulates T cell activation is direct inhibition of the CD28 signaling pathway (17–20). Memory T cells become less dependent on CD28 costimulation as they become more differentiated (40–42), and work from this laboratory has shown beryllium-specific TEM cells in the lung no longer require CD28 costimulation, and a subset of these cell have lost CD28 expression (10). Here, we extend those findings by showing that loss of CD28 directly correlates with increased IFN-γ expression by beryllium-responsive CD4+ T cells in the BAL. Therefore, as memory cells differentiate and lose signaling through CD28, our data suggest that CTLA-4-mediated signaling pathway is incomplete and cannot downregulate cytokine production. Since beryllium-specific cells in the blood of CBD patients remain CD28-dependent and are less differentiated than their counterparts in lung (7), this could explain the differences in CTLA-4 function between blood and lung observed in this study. We believe that our findings have important implication for other target organ-specific immune-mediated diseases, including cancer, where CTLA-4 blocking antibodies are being clinically used with limited success (51).
In conclusion, our data support the following model of continued inflammation due to chronic beryllium exposure in the lung. In the presence of active alveolitis, beryllium-responsive CD4+ T cells are recruited to lung and undergo progressive T cell differentiation, resulting in a loss of CD28 expression and less IL-2 secretion on a per cell basis than their blood counterparts (39, 52). In the process of trafficking to the lung and loss of CD28 expression, beryllium-responsive CD4+ T cells differentiate to gain IFN-γ expression and eventually lose the ability to secrete IL-2. CTLA-4 expression increases on proliferating, IL-2-expressing, beryllium-responsive CD4+ T cells and decreases as cells cease to divide and upregulate PD-1. Therefore, we propose that the major consequence of the loss of CD28 dependence in the lung is an ineffective CTLA-4 signaling pathway, resulting in continued IFN-γ secretion by proliferation-incompetent CD4+ T cells, persistent lung inflammation and eventually lung fibrosis.
Supplementary Material
Abbreviations used in this paper
- BAL
bronchoalveolar lavage
- BeS
beryllium-sensitized
- BeSO4
beryllium sulfate
- CBD
chronic beryllium disease
- MFI
mean fluorescence intensity
- PD-1
programmed death 1
- TEM
effector memory T cell
Footnotes
This work is supported by the following NIH grants: HL62410, HL102245 and ES011810 (to APF), ES011810 (to LAM), and the Clinical & Translational Sciences Institute (UL1 TR000154) from the National Center for Advancing Translational Sciences.
References
- 1.Amicosante M, Fontenot AP. T cell recognition in chronic beryllium disease. Clin Immunol. 2006;121:134–143. doi: 10.1016/j.clim.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Fontenot AP, Maier LA. Genetic susceptibility and immune-mediated destruction in beryllium-induced disease. Trends Immunol. 2005;26:543–549. doi: 10.1016/j.it.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Sawyer RT, Maier LA. Chronic beryllium disease: an updated model interaction between innate and acquired immunity. Biometals. 2011;24:1–17. doi: 10.1007/s10534-010-9376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai S, Murphy GA, Crawford F, Mack DG, Falta MT, Marrack P, Kappler JW, Fontenot AP. Crystal structure of HLA-DP2: Implications for chronic beryllium disease. Proc Natl Acad Sci U S A. 2010;107:7425–7430. doi: 10.1073/pnas.1001772107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontenot AP, Torres M, Marshall WH, Newman LS, Kotzin BL. Beryllium presentation to CD4+ T cells underlies disease susceptibility HLA-DP alleles in chronic beryllium disease. Proc Natl Acad Sci U S A. 2000;97:12717–12722. doi: 10.1073/pnas.220430797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falta MT, Bowerman NA, Dai S, Kappler JW, Fontenot AP. Linking genetic susceptibility and T cell activation in beryllium-induced disease. Proc Am Thorac Soc. 2010;7:126–129. doi: 10.1513/pats.201002-022RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontenot AP, Canavera SJ, Gharavi L, Newman LS, Kotzin BL. Target organ localization of memory CD4+ T cells in patients with chronic beryllium disease. J Clin Invest. 2002;110:1473–1482. doi: 10.1172/JCI15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tinkle SS, Kittle LA, Schumacher BA, Newman LS. Beryllium induces IL-2 and IFN-γ in berylliosis. J Immunol. 1997;158:518–526. [PubMed] [Google Scholar]
- 9.Pott GB, Palmer BE, Sullivan AK, Silviera L, Maier LA, Newman LS, Kotzin BL, Fontenot AP. Frequency of beryllium-specific, TH1-type cytokine-expressing CD4+ T cells in patients with beryllium-induced disease. J Allergy Clin Immunol. 2005;115:1036–1042. doi: 10.1016/j.jaci.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Fontenot AP, Gharavi L, Bennett SR, Canavera SJ, Newman LS, Kotzin BL. CD28 costimulation independence of target organ versus circulating memory antigen-specific CD4+ T cells. J Clin Invest. 2003;112:776–784. doi: 10.1172/JCI18317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer BE, Mack DG, Martin AK, Gillespie M, Mroz MM, Maier LA, Fontenot AP. Up-regulation of programmed death-1 expression on beryllium-specific CD4+ T cells in chronic beryllium disease. J Immunol. 2008;180:2704–2712. doi: 10.4049/jimmunol.180.4.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- 13.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 14.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 15.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. Embo J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 19.Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, Gregson BP, June CH, Linsley PS. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci U S A. 2002;99:11790–11795. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baroja ML, Madrenas J. Viewpoint: therapeutic implications of CTLA-4 compartmentalization. Am J Transplant. 2003;3:919–926. doi: 10.1034/j.1600-6143.2003.00182.x. [DOI] [PubMed] [Google Scholar]
- 21.Arguello RJ, Albareda MC, Alvarez MG, Bertocchi G, Armenti AH, Vigliano C, Meckert PC, Tarleton RL, Laucella SA. Inhibitory receptors are expressed by Trypanosoma cruzi-specific effector T cells and in hearts of subjects with chronic Chagas disease. PLoS One. 2012;7:e35966. doi: 10.1371/journal.pone.0035966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, Baker B, Zhu B, Le Gall S, Waring MT, Ahern R, Moss K, Kelleher AD, Coffin JM, Freeman GJ, Rosenberg ES, Walker BD. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 23.Kassu A, Marcus RA, D’Souza MB, Kelly-McKnight EA, Golden-Mason L, Akkina R, Fontenot AP, Wilson CC, Palmer BE. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. J Immunol. 2010;185:3007–3018. doi: 10.4049/jimmunol.1000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman LS, Kreiss K, King TE, Jr, Seay S, Campbell PA. Pathologic and immunologic alterations in early stages of beryllium disease: re-examination of disease definition and natural history. Am Rev Respir Dis. 1989;139:1479–1486. doi: 10.1164/ajrccm/139.6.1479. [DOI] [PubMed] [Google Scholar]
- 25.Rossman MD, Kern JA, Elias JA, Cullen MR, Epstein PE, Preuss OP, Markham TN, Daniele RP. Proliferative response of bronchoalveolar lymphocytes to beryllium. Ann Intern Med. 1988;108:687–693. doi: 10.7326/0003-4819-108-5-687. [DOI] [PubMed] [Google Scholar]
- 26.Newman LS. Significance of the blood beryllium lymphocyte proliferation test. Environ Health Perspect. 1996;104:953–956. doi: 10.1289/ehp.96104s5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mroz MM, Kreiss K, Lezotte DC, Campbell PA, Newman LS. Reexamination of the blood lymphocyte transformation test in the diagnosis of chronic beryllium disease. J Allergy Clin Immunol. 1991;88:54–60. doi: 10.1016/0091-6749(91)90300-d. [DOI] [PubMed] [Google Scholar]
- 28.Fontenot AP, Kotzin BL, Comment CE, Newman LS. Expansions of T-cell subsets expressing particular T cell receptor variable regions in chronic beryllium disease. Am J Respir Cell Mol Biol. 1998;18:581–589. doi: 10.1165/ajrcmb.18.4.2981. [DOI] [PubMed] [Google Scholar]
- 29.Fontenot AP, Falta MT, Freed BM, Newman LS, Kotzin BL. Identification of pathogenic T cells in patients with beryllium-induced lung disease. J Immunol. 1999;163:1019–1026. [PubMed] [Google Scholar]
- 30.Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J Immunol. 2005;175:8415–8423. doi: 10.4049/jimmunol.175.12.8415. [DOI] [PubMed] [Google Scholar]
- 31.Fontenot AP, Palmer BE, Sullivan AK, Joslin FG, Wilson CC, Maier LA, Newman LS, Kotzin BL. Frequency of beryllium-specific, central memory CD4+ T cells in blood determines proliferative response. J Clin Invest. 2005;115:2886–2893. doi: 10.1172/JCI24908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alegre ML, Noel PJ, Eisfelder BJ, Chuang E, Clark MR, Reiner SL, Thompson CB. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–4770. [PubMed] [Google Scholar]
- 33.Carreno BM, Bennett F, Chau TA, Ling V, Luxenberg D, Jussif J, Baroja ML, Madrenas J. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression. J Immunol. 2000;165:1352–1356. doi: 10.4049/jimmunol.165.3.1352. [DOI] [PubMed] [Google Scholar]
- 34.Blair PJ, Riley JL, Levine BL, Lee KP, Craighead N, Francomano T, Perfetto SJ, Gray GS, Carreno BM, June CH. CTLA-4 ligation delivers a unique signal to resting human CD4 T cells that inhibits interleukin-2 secretion but allows Bcl-XL induction. J Immunol. 1998;160:12–15. [PubMed] [Google Scholar]
- 35.Luporsi E, Andre F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F, Tubiana-Mathieu N, Sigal-Zafrani B, Arnould L, Gompel A, Egele C, Poulet B, Clough KB, Crouet H, Fourquet A, Lefranc JP, Mathelin C, Rouyer N, Serin D, Spielmann M, Haugh M, Chenard MP, Brain E, de Cremoux P, Bellocq JP. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 132:895–915. doi: 10.1007/s10549-011-1837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez F, Belloc F, Lacombe F, Dumain P, Reiffers J, Bernard P, Boisseau MR. Modalities of synthesis of Ki67 antigen during the stimulation of lymphocytes. Cytometry. 1991;12:42–49. doi: 10.1002/cyto.990120107. [DOI] [PubMed] [Google Scholar]
- 37.Anderson DE, Bieganowska KD, Bar-Or A, Oliveira EM, Carreno B, Collins M, Hafler DA. Paradoxical inhibition of T-cell function in response to CTLA-4 blockade; heterogeneity within the human T-cell population. Nat Med. 2000;6:211–214. doi: 10.1038/72323. [DOI] [PubMed] [Google Scholar]
- 38.Palmer BE, Mack DG, Martin AK, Maier LA, Fontenot AP. CD57 expression correlates with alveolitis severity in subjects with beryllium-induced disease. J Allergy Clin Immunol. 2007;120:184–191. doi: 10.1016/j.jaci.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Mack DG, Lanham AK, Palmer BE, Maier LA, Watts TH, Fontenot AP. 4-1BB enhances proliferation of beryllium-specific T cells in the lung of subjects with chronic beryllium disease. J Immunol. 2008;181:4381–4388. doi: 10.4049/jimmunol.181.6.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lovett-Racke AE, Trotter JL, Lauber J, Perrin PJ, June CH, Racke MK. Decreased dependence of myelin basic protein-reactive T cells on CD28- mediated costimulation in multiple sclerosis patients. A marker of activated/memory T cells. J Clin Invest. 1998;101:725–730. doi: 10.1172/JCI1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markovic-Plese S, Cortese I, Wandinger KP, McFarland HF, Martin R. CD4+CD28− costimulation-independent T cells in multiple sclerosis. J Clin Invest. 2001;108:1185–1194. doi: 10.1172/JCI12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7− CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang XB, Zheng CY, Giscombe R, Lefvert AK. Regulation of surface and intracellular expression of CTLA-4 on human peripheral T cells. Scand J Immunol. 2001;54:453–458. doi: 10.1046/j.1365-3083.2001.00985.x. [DOI] [PubMed] [Google Scholar]
- 44.Jago CB, Yates J, Camara NO, Lechler RI, Lombardi G. Differential expression of CTLA-4 among T cell subsets. Clin Exp Immunol. 2004;136:463–471. doi: 10.1111/j.1365-2249.2004.02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A, Wilson CC, Connick E, Palmer BE. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179:1979–1987. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 46.Sachdeva M, Fischl MA, Pahwa R, Sachdeva N, Pahwa S. Immune exhaustion occurs concomitantly with immune activation and decrease in regulatory T cells in viremic chronically HIV-1-infected patients. J Acquir Immune Defic Syndr. 54:447–454. doi: 10.1097/QAI.0b013e3181e0c7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luhder F, Hoglund P, Allison JP, Benoist C, Mathis D. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998;187:427–432. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eggena MP, Walker LS, Nagabhushanam V, Barron L, Chodos A, Abbas AK. Cooperative roles of CTLA-4 and regulatory T cells in tolerance to an islet cell antigen. J Exp Med. 2004;199:1725–1730. doi: 10.1084/jem.20040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karandikar NJ, Vanderlugt CL, Walunas TL, Miller SD, Bluestone JA. CTLA-4: a negative regulator of autoimmune disease. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurwitz AA, Sullivan TJ, Sobel RA, Allison JP. Cytotoxic T lymphocyte antigen-4 (CTLA-4) limits the expansion of encephalitogenic T cells in experimental autoimmune encephalomyelitis (EAE)-resistant BALB/c mice. Proc Natl Acad Sci U S A. 2002;99:3013–3017. doi: 10.1073/pnas.042684699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okada R, Kondo T, Matsuki F, Takata H, Takiguchi M. Phenotypic classification of human CD4+ T cell subsets and their differentiation. Int Immunol. 2008;20:1189–1199. doi: 10.1093/intimm/dxn075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.