Abstract
Outcome prediction of traumatic brain injury (TBI) patients with severe disorders of consciousness (DOC) at the end of their time in an intensive care setting is important for clinical decision making and counseling of relatives, and constitutes a major challenge. Even the question of what constitutes an improved outcome is controversially discussed. We have conducted a retrospective cohort study for the rehabilitation dynamics and outcome of TBI patients with DOC. Out of 188 patients, 37.2% emerged from a minimally conscious state (MCS) and 16.5% achieved at least partial functional independence after a mean observation period of 107 days (range 1–399 days). This reflects that emergence from MCS is much easier to achieve than functional independence. Logistic regression analysis identified age and level of consciousness upon admission to neurorehabilitation as independent prognostic factors for both outcomes. The group who reached at least partial functional independence started to improve significantly more than the corresponding outcome group by post-injury week 7, and the average time to reach this functional status was 18 weeks. In contrast, the group who emerged from MCS started to improve after 6 weeks. The longest delay between brain injury and the beginning of functional improvement (measured by biweekly Functional Independence Measure [FIM] scores) still compatible with reaching at least partial functional independence was 18 weeks. In conclusion, despite a strong negative selection, a substantial proportion of severe TBI patients with DOC achieve functional improvements or at least emerge from MCS within the inpatient rehabilitation phase. In order to avoid self-fulfilling prophecies in decision making, it is important to be aware of the fact that the beginning of clinical improvement may take several months after brain injury. In this study, separation of both of the functional outcome groups started by 7 weeks post-injury.
Key words: clinical course, recovery of consciousness, rehabilitation outcome, TBI
Introduction
Traumatic brain injury (TBI) affects millions of people throughout the world, and is a leading cause for morbidity and mortality, especially in young adults.1 It is estimated that ∼10% of TBI cases are severe.2 Disorders of consciousness (DOC) are the clinical hallmark of severe TBI. Whereas many comatose patients regain consciousness in the first days and weeks after injury, some remain either in a vegetative state (VS; complete unawareness of self and environment; proposed new terminology: unresponsive wakefulness syndrome) or in a minimally conscious state (MCS; limited conscious interaction with the environment).3–6
Predicting the outcome of patients who remain in a VS or MCS at the end of their time in an intensive care setting is a major challenge. It is, however, very important for counseling and expectation management of the affected families and relatives.7,8 Based on this prognosis, medical professionals and families may decide to either limit/withdraw life-sustaining therapy or to pursue maximum medical care and neurorehabilitation.7,8 This decision-making process carries the risk of self-fulfilling prophecies.9
Age, low Glasgow Coma Scale (GCS) motor score, absence of pupillary response, and CT characteristics have been established as independent prognostic factors in patients with severe or moderate TBI upon admission to intensive care units (ICU).10 Also, analogous to patients with anoxic encephalopathy, bilateral absence of cortical responses of early somatosensory evoked potentials (SEP) during the first week post-injury has been shown to have high specificity to predict functional dependence.11,12 When the perspective is shifted from ICU admission to neurorehabilitation admission of TBI patients with DOC, data from the National Institute on Disability and Rehabilitation Research (NIDRR) TBI Model Systems Programs have shown that patients show functional improvement not only during the early recovery phase but also throughout the following years.13 An important issue in such prognosis studies are the definitions of outcome categories. It may be too simplistic to base improved outcome solely on functional aspects and independence in activities of daily living (ADL), as quality of life (QOL) comprises many more aspects.14 For a TBI patient who has remained in the VS for several weeks or months, it may be favorable to regain consciousness and communication skills in order to participate in family life, whereas functional independence may be out of reach.15
We have analyzed the clinical course and rehabilitation outcome of a large cohort of patients with DOC after severe TBI in order to provide further data for expectation management and informed decision making.
Methods
Study design and setting
This is a retrospective cohort study of consecutive severe TBI patients with impaired consciousness, who were discharged from a specialized neurorehabilitation center in southern Germany between January 1, 2005 and December 31, 2010. Patients were identified by a review of patient charts. Study data were collected from electronic patient files. Inclusion criteria were acute TBI, sustained DOC upon admission with lack of command following, direct referral from the acute setting ICU to the rehabilitation center, residence in Germany or Austria and German language skills (for follow-up), and availability for biweekly, prospectively collected clinical patient assessments throughout the course of the inpatient rehabilitation treatment. The institutional review board of the medical faculty of the University of Munich approved the retrospective data analysis. The study is in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Data collection procedures
All TBI patients at the neurorehabilitation center had biweekly standardized clinical assessments by trained hospital personnel. This standard assessment is a requirement of German health insurance companies for the treatment of severely brain injured patients. Data were entered prospectively into the clinical patient management system. As these assessments are standard procedures for all patients, assessors were blinded with respect to the later scientific data use.
Outcome measures
We chose to use two different levels of improved outcome, one addressing functional aspects, the other focusing on regaining higher levels of consciousness. Thereby emergence from MCS is easier to achieve than at least partial functional independence. Emerging from MCS is a prerequisite for achieving at least partial functional independence. In the first model, the overall functional outcomes were rated with the Glasgow Outcome Scale (GOS), which is one of the most widely used measures for classifying functioning in TBI survivors, both by acute care and rehabilitation specialists.16,17 In this study, the GOS was rated for each patient at admission and at discharge retrospectively, using discharge letters from the ICU and the neurorehabilitation center, respectively. The GOS includes five outcome categories: 1=dead, 2=vegetative (cannot interact, unresponsive), 3=severely disabled (can follow commands, cannot live independently), 4=moderately disabled (can live independently, reduced work capacity), and 5=good recovery (can work). In this study, we used a GOS of 4 or 5 to define TBI patients with a good outcome. This cutoff point (GOS≥4) is in accordance with previous studies, and addresses the functional aspect of outcome, as patients reaching those scores are able to live independently.8,18 In several studies, the GOS has proven its practicability and usefulness in assessing outcomes in patients with moderate and severe TBI.19
The German version of the Coma Remission Scale (CRS) is a behavioral test to quantify levels of consciousness and ranges from 0 (deep coma) to 24 (able to use objects purposefully, recognition of familiar people) points comprising six subcategories: alertness and attention, motor response, response to acoustical stimuli, response to visual stimuli, response to tactile stimuli, and verbal response.20 In contrast to the JFK Coma Recovery Scale –Revised (CRS-R)4 there are no strictly defined cutoff points in the separate subscales to classify a patient as being in MCS or emerged from MCS. Nevertheless, both scales comprise very similar items. Therefore, patients reaching a full CRS score of 24 are considered as having emerged from MCS, based on meeting at least one of the two criteria proposed for emergence from MCS by the CRS-R.4 This is a much lower threshold to achieve than at least partial functional independence. Emergence from MCS can be seen as a sequential marker for reaching at least partial functional independence. Even though both outcome measures can be seen as part of a continuous outcome spectrum, we chose to calculate statistical models for each of them separately.
For this further outcome model, the group reaching the better outcome category was defined by the maximum CRS score (24 points). All patients not reaching 24 points in the CRS were categorized as not emerging from MCS. As a consequence, this definition also rates those patients who remain dependent functionally but who have emerged from the MCS and are able to use objects purposefully, as having an improved outcome in respect to their level of consciousness. This dichotomization takes into account that a good QOL is not necessarily dependent on functional status, as it was shown in the case of patients in the locked-in syndrome.15 The temporal pattern of CRS improvements were analyzed by determining the week during which the first significant CRS increase occurred (Fig. 1b). The start of clinical improvement was defined as an increase of at least 10% of the maximum score, that is, of ≥2 CRS points compared with the initial scores. This definition was chosen only to give a rough estimate of the starting point of increase within the group who emerged from MCS.
FIG. 1.
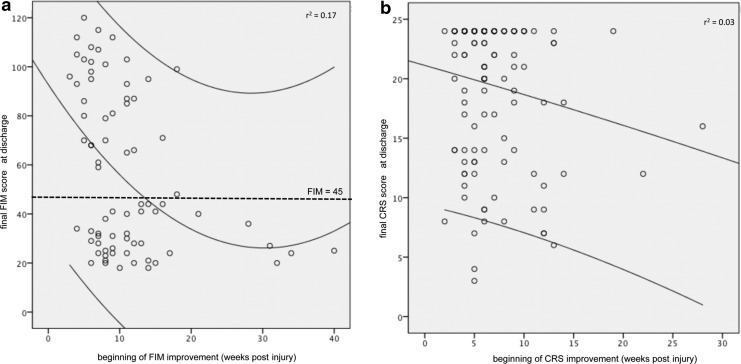
(a) Scatter plot for the correlation between the start of Functional Independence Measure (FIM) improvement (increase of ≥10%) measured in weeks post-injury and final FIM scores at discharge. The quadratic regression line (center curved line; r2=0.17) is shown together with the 95% confidence intervals (outer curved lines). The dotted line represents the level of 45 FIM points, which is the minimal clinical important difference (MCID) for the FIM. (b) Scatter plot for the correlation between the beginning of Coma Remission Scale (CRS) improvement (increase of ≥10%) measured in weeks post-injury and final CRS scores at discharge from neurorehabilitation. The upper line is the regression line (r2=0.03), the lower line represents the lower border of the 95% confidence interval. The upper border is not shown in this figure.
Clinical course of functional abilities
The Functional Independence Measure (FIM) was developed to uniformly assess severity of patient disability and medical rehabilitation functional outcome.21 The FIM includes 18 items in six subscales: self care, sphincters, mobility, communication, psychosocial, and cognition. Each item is rated on a seven level scale (1=patient needs total assistance, to 7=patient is completely independent). The minimal clinically important difference (MCID) for the FIM is estimated at 27, that is, only FIM increases above this threshold are noticed by patients as a relevant functional improvement.22 For the FIM, good reliability was found.23 The FIM at discharge had previously been shown to be an independent predictor of the 6 month outcome in TBI patients.24,25 According to the CRS, we analyzed the temporal pattern of FIM improvements by determining the week during which the FIM increased 10% of the maximum score, that is, ≥13 FIM points compared with the initial scores (Fig. 1a). As in the case of the temporal pattern of the CRS, this cutoff point was defined only to give a rough estimate of the starting point of functional recovery within the group who reached at least partial functional independence. Because for the FIM there are no strictly defined cutoff points to identify an improved functional outcome category, and the GOS is one of the most widely used measures to assess functional outcome in TBI survivors,8,18,19 we used the GOS instead as the functional outcome determinant. The FIM, however, was used to describe temporal patterns of functional abilities during neurorehabilitation.
Independent variables
All variables reaching or approaching significance in an univariate logistic regression model were used for multivariate regression modeling. If there were high intercorrelations between specific variables, the G-statistic was used to decide which of the variables were included in the multivariate model to improve the goodness of fit. Potential outcome predictors were: age, sex, cause of TBI (falls, road traffic accident), VS at admission to rehabilitation, infratentorial lesion, need for ventriculoperitoneal (VP) shunting and craniectomy, length of stay (LOS) in the ICU, FIM and CRS scores at admission, time to emergence from MCS, SEP bilaterally absent, and additional traumatic subarachnoid hemorrhage (SAH).
All patients received median nerve SEP recording within the first 2 weeks of admission to neurorehabilitation, using a standard clinical protocol. Cortical responses after 20 ms (N20) were rated as either bilaterally absent (“malignant”) or not absent (even if only unilaterally present and/or pathological; “non-malignant”).
Statistical analysis
For multivariate logistic regression analyses, the sample was dichotomized into patients who emerged from MCS and patients who did not, and patients who reached at least partial functional independence and patients who remained functionally dependent at the time of discharge from neurorehabilitation.
For a description of the temporal patterns during inpatient neurorehabilitation, clinical scores (FIM/ CRS) were analyzed by Kaplan–Meier analysis.
To test for significant differences between the corresponding groups, a χ2 test was used for nominal and ordinal variables, and a t-test was used for continuous variables. All statistical tests were two sided.
The level of significance was set at p<0.05. SYSTAT 11 (SYSTAT Software, Inc., 2004) and SPSS 20 (IBM® SPSS® Statistics 20, 2011) were used for statistical analyses and plotting.
Results
Patient characteristics and overall outcome
Out of a total of 687 TBI patients during the 5 year observation period, 41.5% had TBI as the main diagnosis and severe DOC. Of those, 66.0% (n=188) were available for analyses. The remaining patients were not directly referred to our center after ICU (32.3%), lacked clinical scoring data (0.02%), or lived abroad (1.5%).
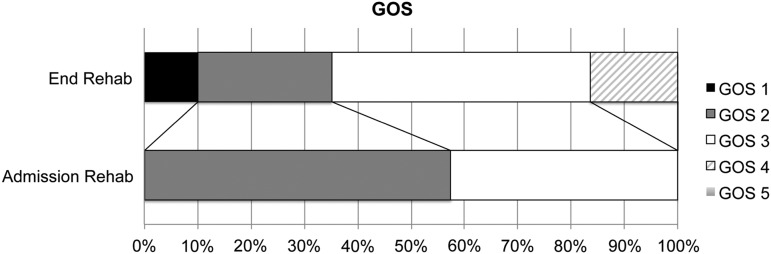
Demographic and clinical patient characteristics per outcome group are shown in Table 1. Out of the 188 patients, 16.5% reached at least partial functional independence (GOS≥4) at the end of inpatient neurorehabilitation (mean observation period: 107 days, range 1–399 days), 37.2% emerged from MCS (CRS=24 points), and 10.1% of patients died during neurorehabilitation after a mean of 128 days post-injury. In Table 1, only the locations the patients were most often discharged to are specified. The remaining patients were discharged to other rehabilitation centers, back to acute care settings in cases with complications, or to specialized small group housing environments for patients in a vegetative state. Changes between GOS scores at admission and at discharge are shown in Figure 2.
Table 1.
Patient Characteristics for the Group as a Whole and Dichotomized into Patients Who Reached at Least Partial Functional Independence and Patients Who Remained Functionally Dependent as well as into Patients Who Emerged from MCS and Patients Who Did Not at Discharge, Respectively
| |
|
Level of functioning |
Level of consciousness |
||
|---|---|---|---|---|---|
| Factor | All patients | (GOS≥4) | (GOS<4) | (CRS=24) | (CRS<24) |
| n | 188 | 31 | 157 | 70 | 118 |
| Age | 53±22 | 40±19* | 55±22 | 46±22* | 57±22 |
| % Male | 72 | 77 | 74 | 77 | 73 |
| Cause of TBI | |||||
| Falls | 105 | 11* | 94 | 29* | 76 |
| Traffic accidents | 74 | 20* | 54 | 38* | 36 |
| % VSa | 57 | 48 | 60 | 50* | 63 |
| % infratentorialb | 50 | 52 | 52 | 54 | 50 |
| % VP shunt | 22 | 6* | 25 | 9* | 31 |
| % craniectomy | 42 | 19* | 48 | 30* | 51 |
| LOS ICUc range |
32±36 6-322 |
22±9 | 33±38 | 25±15 | 36±43 |
| LOS rehab.d range |
107±73 1-399 |
128±62* | 103±74 | 123±63* | 97±77 |
| % Discharge | |||||
| Home | 28 | 42* | 28 | 43* | 21 |
| Nursing facility | 36 | 13* | 43 | 19* | 49 |
| Other/ Died in rehab | 36 | 45* | 29 | 38* | 30 |
| FIMe admission | 18±1 | 18±1 | 18±1 | 18±1 | 18±1 |
| FIM discharge | 38±30 | 95±15* | 27±17 | 67±32* | 20±5 |
| CRSf admission | 11±5 | 14±5* | 11±5 | 14±5* | 10±5 |
| CRS discharge | 18±7 | 24±0* | 17±7 | 24±0* | 14±6 |
Vegetative state (VS)at admission to neurorehabilitation; binfratentorial lesion; cLength of stay intensive care unit (days); dLength of stay neurorehabilitation (days); eFunctional Independence Measure (FIM); fGerman version of the Coma Remission Scale (CRS).
Significantly different from the corresponding group.
MCS, minimally conscious state; GOS, Glasgow Outcome Score; TBI, traumatic brain injury; VP, ventriculoperitoneal; LOS, length of stay; ICU, intensive care unit.
FIG. 2.
Changes in Glasgow Outcome Scale (GOS) outcome categories between admission to neurorehabilitation and inpatient discharge.
Clinical dynamics during neurorehabilitation
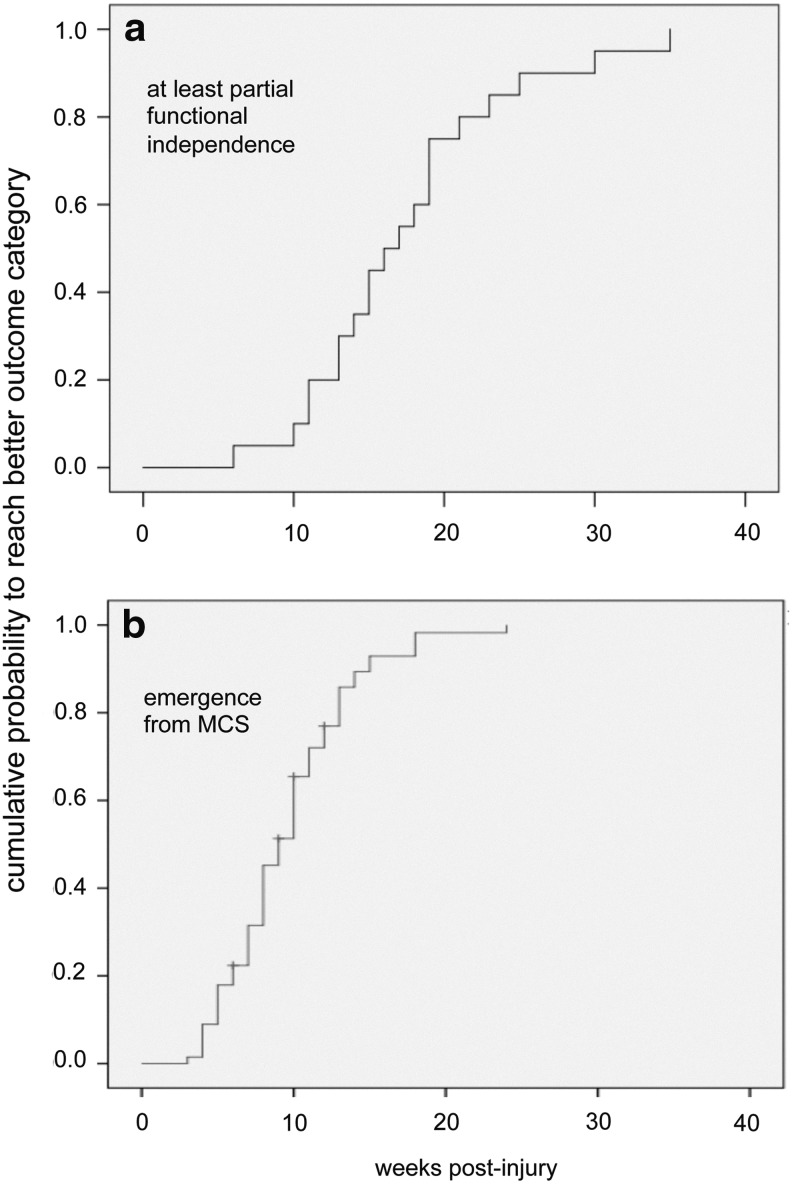
Patients who were at least partially functionally independent at the time of discharge reached this outcome category after 18±7 weeks, those who emerged from MCS after 9±4 weeks (p<0.01; t test). For both groups reaching the better categories (according to GOS and CRS scores, respectively) Kaplan–Meier plots for the cumulative probability of reaching the better outcome categories are shown in Figure 3.
FIG. 3.
Cumulative probability of the groups reaching the better outcome categories for reaching at least partial functional independence (a) and emergence from minimally conscious state (MCS) (b) in dependency of the length of stay during neurorehabilitation.
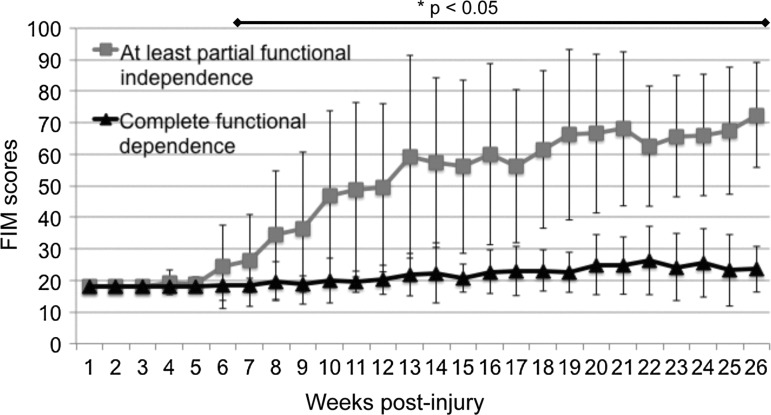
Biweekly standardized clinical scoring of the FIM showed that the group who reached at least partial functional independence started to separate from the corresponding outcome group by post-injury week 7 (Fig. 4). There was a significant correlation between time from injury to improvement and final FIM scores at discharge. The earlier the improvement began, the higher the discharge FIM scores were (Pearson correlation coefficient r=−0.37; p<0.01). The longest delay to the start of FIM improvement in a patient, who surpassed the MCID, was 18 weeks after injury (Fig. 1a). The longest delay still compatible with at least partial functional independence (GOS≥4) at discharge was also 18 weeks.
FIG. 4.
Dynamics of functional status measured by the Functional Independence Measure (FIM) throughout neurorehabilitation. The group reaching at least partial functional independence statistically starts to separate from the corresponding outcome group by week 7 (p<0.05).
On the other hand, when looking at the outcome in respect to the level of consciousness, the group who emerged from MCS already had higher CRS scores at admission than the group who did not (14±5 vs. 9±5 points; p<0.001; two sided t test). However, given these different consciousness starting levels, the temporal dynamics of both groups were the same. The group who emerged from MCS started to improve by at least 10% (i.e., two points on the CRS) after 6±3 weeks, whereas scores of the group not emerging from MCS started to rise after 7±4 weeks (p=0.1, t test). The longest individual delay until CRS improvement still compatible with maximum CRS scores at discharge was 19 weeks (Fig. 1b).
Regression analysis and prognostic markers
Multivariate binary logistic regression analysis was performed for both outcome measures (GOS and CRS). Age (1.05 odds ratio [OR], 1.02–1.09 95% confidence interval [CI]), CRS scores at admission (0.95 OR, 0.82–1.09 95% CI), time to emerge from MCS (1.35 OR, 1.11–1.65 CI), and previous decompressive craniectomy (4.70 OR, 1.14–19.38 95% CI) were strong functional outcome predictors (see also Table 2 for results of the univariate regression model). For the level of consciousness at discharge, age (1.02 OR, 1.00–1.04 95% CI), CRS scores at admission (0.85 OR, 0.79–0.91 95% CI), length of ICU stay (1.02 OR, 1.00–1.05 CI), VP shunting (3.92 OR, 1.37–11.26 95% CI), and falls as cause for TBI (2.01 OR, 1.12–7.58 CI) were independent predictors (see also Table 2 for results of the univariate regression model). As Table 2 shows, length of ICU stay does not reach but approaches significance in both univariate models (functional: p=0.11; in respect to the level of consciousness: p=0.07). A G-statistic revealed that this variable added significant improvements to the functional multivariate model (G=14.2, 1 df, p<0.001) but not to the model predicting the level of consciousness (G=0.52, 1 df, p>0.50). Interestingly, bilateral absence of N20 cortical SEP responses was not an independent outcome predictor in either of the two models (Table 2). The specificity of this malignant SEP test results to predict functional dependence or not emerging from MCS were 83% and 60%, respectively.
Table 2.
Odds Ratios and 95% Confidence Intervals of Univariate Potential Outcome Factors To Predict the Level of Functional Status and the Level of Consciousness
| |
Level of functioning |
Level of consciousness |
||
|---|---|---|---|---|
| Factor | Odds Ratio | (95% CI) | Odds Ratio | (95% CI) |
| Age | 1.03* | (1.01–1.05) | 1.02* | (1.00–1.03) |
| Sex | ||||
| Male vs. female | 0.83 | (0.33–2.06) | 0.80 | (0.40–1.59) |
| Cause of TBI | ||||
| Falls vs. traffic accidents | 3.17* | (1.41–7.10) | 2.77* | (1.48–5.17) |
| VSa present | 1.71 | (0.79–3.70) | 2.72 | (1.48–5.02) |
| Infratentorial lesion | 1.04 | (0.48–2.25) | 1.24 | (0.68–2.27) |
| Need for VP shunting | 4.96* | (1.13–21.72) | 4.68* | (1.86–11.80) |
| Need for craniectomy | 3.81* | (1.48–9.80) | 2.41* | (1.29–4.51) |
| LOS ICUb | 1.03 | (0.99–1.06) | 1.02* | (1.00–1.03) |
| CRSc admission | 0.86* | (0.79–0.94) | 0.87* | (0.81–0.92) |
| FIM admission | 0.74 | (0.44–1.23) | 0.73 | (0.45–1.19) |
| SEP bilaterally absent | 1.03 | (0.31–3.47) | 1.14 | (0.45–2.90) |
| Traumatic SAH | 1.22 | (0.56–2.27) | 1.53 | (0.83–2.81) |
| Time to emergence from MCS | 1.21* | (1.05–1.41) | - | - |
Odds ratios >1 describes variables promoting being in the better outcome category.
Vegetative state at admission to neurorehabilitation; bLength of stay in intensive care unit (days); cGerman version of the coma remission scale.
Reaching significance in an univariate model.
CI, confidence interval; TBI, traumatic brain injury; VP, ventriculoperitoneal; FIM, Functional Independence Measure; SEP, somatosensory evoked potentials; SAH, subarachnoid hemorrhage; MCS, minimally conscious state.
Discussion
This cohort study focuses on the inpatient rehabilitation outcome of TBI patients with sustained severe disturbances of consciousness at the time of admission to neurorehabilitation. The better outcome category was defined in terms of both functional aspects and emergence from MCS. It must be noted that emerging from MCS is a prerequisite to reaching at least partial functional independence, that is, all patients who have reached at least partial functional independence also have emerged from MCS earlier, but not vice versa. Most studies assessing outcome after TBI only focus on the functional status or physical autonomy of patients, possibly ignoring that the definition of a “better outcome” depends upon the individual perspective.8,18 Even very simple communication skills may be of invaluable importance in regaining aspects of QOL such as social support.15 Although the analysis was retrospective, clinical scoring data was elicited prospectively. Within a mean observation period of 15.3 weeks, 16.5% of patients achieved at least partial functional independence and 37.2% emerged from MCS. As indicated by the different rates in outcome in respect to functional status and level of consciousness, the threshold for emerging from MCS is lower for severely affected TBI patients. Average FIM scores at discharge were 38±30 points. To date, the most comprehensive outcome analysis of this patient population stems from the recent report of the NIDRR TBI Model Systems (TBIMS) Program, which prospectively analyzed inpatient rehabilitation and long-term outcomes of 396 and 108 patients, respectively.13 Inclusion criteria of this study were similar to ours, focusing on patients without command-following abilities. However, whereas we used a standardized behavioral assessment tool, the German CRS, this prospective study used a qualitative approach to identify patients. Patients in the TBIMS study had a 47 day rehabilitation LOS, which is considerably shorter than the 107 days in our study. However, in their study, 68% of patients regained consciousness. Their median FIM at discharge was 43 points. Despite a shorter rehabilitation treatment period, the functional outcome and the rate of patients regaining consciousness were higher than in our analysis. This underscores the high disease severity in our patients compared with other study populations. This is also reflected by the fact that 57% of our patients were in a VS upon rehabilitation admission and that 42% had to undergo decompressive craniectomy to relieve intractable intracranial hypertension. Other studies report highly variable recovery of consciousness rates between 14% and 95% in TBI patients.6,26–28 This variability is likely to stem from heterogeneous inclusion criteria, follow-up periods, and outcome measures used.
In contrast to the recent TBIMS study, we provided biweekly information about functional status and consciousness throughout the inpatient treatment phase. This allowed for detailed analysis of the temporal patterns and dynamics of clinical improvement. The clinical course of those patients who will go on to reach at least partial functional independence starts to separate from the corresponding group after a mean of 7 weeks post-injury, and the average time to reach at least partial functional independence is 18 weeks. No patients who started to significantly improve their FIM later than 18 weeks post-injury became at least partially functional independent (Fig. 1a). Recovery of consciousness begins earlier than functional improvement and maximum CRS scores are achieved after a mean of 9 weeks. However, the “slowest” patient's trajectory within the group who emerged from MCS began to improve by week 19. These results impressively show that the potential for recovery should not be underestimated. In fact, recovery may not start for 4–5 months, especially for younger patients who were not in a VS at admission to neurorehabilitation.
It must not be overlooked that inpatient neurorehabilitation may be considered futile by some neurointensivists or insurance regulations in severely brain-injured patients with prolonged coma, VS, or MCS.13,29 Given this notion, it is noteworthy that a substantial subgroup of patients improved significantly, even up to the point of functional independence.
The current German diagnosis-related group (DRG) catalogue defines the upper limit of the rehabilitation LOS for these patients as 27 days (OPS 8-552; early neurological rehabilitation complex treatment; www.g-drg.de) for the defined DRGs (longer LOS leads to hospital-specific daily rates).
This amounts to ∼8–10 weeks post-injury when combined with the average 4 weeks of previous intensive care treatment. Looking at the clinical dynamics of our patients, it becomes evident that a substantial number of patients begin to improve later than 8–10 weeks after their injury (Fig. 1b). This means that at the time when hospitals and health insurance companies must decide about an extension of inpatient rehabilitation and ask for an assessment of the patients' rehabilitation potential, they may be misguided if they are relying only on measurable score improvements at that time. Consequently, patients may be discharged prematurely and be deprived of further specialized treatment.29
Regression analysis identified age and levels of consciousness upon admission to neurorehabilitation as independent prognostic factors for both outcome definitions (reaching at least partial functional independence and emerging from MCS). It is not surprising that older patients and patients with a higher degree of unconsciousness fare worse during the course of inpatient rehabilitation. This is in line with previous studies and confirms clinical experience and intuition.10,18 We additionally found that the need for decompressive craniectomy to treat intracranial hypertension during the ICU phase and the need for introduction of VP shunting are strong negative predictors for functional outcome and the level of consciousness at discharge.
We were especially interested in the role that malignant SEP test results might play in outcome prediction in our cohort, that is, absence of bilateral cortical N20 responses. In comatose cardiac arrest survivors, this finding predicts an unfavorable outcome with very high specificity, even if this may be a bit lower than previously believed.9,30–32
In unconscious TBI patients, malignant SEP results have been reported to predict a failure to regain consciousness with high specificity between 90% and 100%.11,12 To our surprise, bilateral loss of cortical N20 responses of median nerve SEPs was not an independent outcome predictor in our sample (functional: 0.97 OR,0.23–3.28 95%CI; in respect to level of consciousness: 1.14 OR, 0.45–2.90 95% CI). In fact, it only had a specificity of 83% to predict functional dependence, and a specificity of 60% to predict non-emergence from MCS. This is an important finding for clinical practice, as such supposedly malignant SEP results may dramatically influence medical decision making on the ICU, and often lead to withdrawal of life-sustaining therapy.7 This carries the potential danger of a self-fulfilling prophecy, which is also relevant for severely affected TBI patients.33,34
Limitations
The main limitation of our study is the retrospective analysis design, even though we could depend on prospectively elicited data. We have, therefore, initiated a multicenter prospective observation trial to determine TBI patient outcome using a high methodical standard.35
Another weakness of our study design is the fact that the observation period is rather short, because we focused on inpatient rehabilitation outcome. We now know that TBI patients with DOC have a much longer potential for clinically relevant improvement than was previously thought.13,27,28 Therefore, we are almost certain to underestimate the amount of clinical improvement in our cohort, because we were not able to obtain sufficient post-rehabilitation follow-up data.
Conclusion
In conclusion, a significant proportion of patients with very severe TBI and DOC achieve either partial or full functional independence or emergence from MCS during inpatient rehabilitation. Age, the degree of DOC at rehabilitation admission, and the need for neurosurgical procedures are important rehabilitation outcome predictors. For clinical decision making, it is important to be aware of the fact that some patients within the better outcome category may require up to 5 months before showing signs of improvement.
Acknowledgments
This study was supported by a grant (#2011013) of the ZNS Hannelore Kohl Stiftung foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Tagliaferri F. Compagnone C. Korsic M. Servadei F. Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. (Wien) 2006;148:255–268. doi: 10.1007/s00701-005-0651-y. [DOI] [PubMed] [Google Scholar]
- 2.Corrigan J.D. Selassie A.W. Orman J.A. The epidemiology of traumatic brain injury. J. Head Trauma Rehabil. 2010;25:72–80. doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- 3.The Multi-Society Task Force on PVS (1994) Medical aspects of the persistent vegetative state (1) N. Engl. J. Med. 330:1499–1508. doi: 10.1056/NEJM199405263302107. [DOI] [PubMed] [Google Scholar]
- 4.Giacino J.T. Ashwal S. Childs N. Cranford R. Jennett B. Katz D.I. Kelly J.P. Rosenberg J.H. Whyte J. Zafonte R.D. Zasler N.D. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- 5.Laureys S. Celesia G.G. Cohadon F. Lavrijsen J. Leon–Carrion J. Sannita W.G. Sazbon L. Schmutzhard E. von Wild K.R. Zeman A. Dolce G. Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med. 2010;8:68. doi: 10.1186/1741-7015-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz D.I. Polyak M. Coughlan D. Nichols M. Roche A. Natural history of recovery from brain injury after prolonged disorders of consciousness: outcome of patients admitted to inpatient rehabilitation with 1–4 year follow-up. Prog. Brain Res. 2009;177:73–88. doi: 10.1016/S0079-6123(09)17707-5. [DOI] [PubMed] [Google Scholar]
- 7.Geocadin R.G. Buitrago M.M. Torbey M.T. Chandra–Strobos N. Williams M.A. Kaplan P.W. Neurologic prognosis and withdrawal of life support after resuscitation from cardiac arrest. Neurology. 2006;67:105–108. doi: 10.1212/01.wnl.0000223335.86166.b4. [DOI] [PubMed] [Google Scholar]
- 8.Yuan F. Ding J. Chen H. Guo Y. Wang G. Gao W.W. Chen S.W. Tian H.L. Predicting outcomes after traumatic brain injury: the development and validation of prognostic models based on admission characteristics. J. Trauma Acute Care Surg. 2012;73:137–145. doi: 10.1097/TA.0b013e31824b00ac. [DOI] [PubMed] [Google Scholar]
- 9.Young G.B. Clinical practice. Neurologic prognosis after cardiac arrest. N. Engl. J. Med. 2009;361:605–611. doi: 10.1056/NEJMcp0903466. [DOI] [PubMed] [Google Scholar]
- 10.Murray G.D. Butcher I. McHugh G.S. Lu J. Mushkudiani N.A. Maas A.I. Marmarou A. Steyerberg E.W. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J. Neurotrauma. 2007;24:329–337. doi: 10.1089/neu.2006.0035. [DOI] [PubMed] [Google Scholar]
- 11.Houlden D.A. Taylor A.B. Feinstein A. Midha R. Bethune A.J. Stewart C.P. Schwartz M.L. Early somatosensory evoked potential grades in comatose traumatic brain injury patients predict cognitive and functional outcome. Crit. Care Med. 2010;38:167–174. doi: 10.1097/CCM.0b013e3181c031b3. [DOI] [PubMed] [Google Scholar]
- 12.Robinson L.R. Micklesen P.J. Tirschwell D.L. Lew H.L. Predictive value of somatosensory evoked potentials for awakening from coma. Crit. Care Med. 2003;31:960–967. doi: 10.1097/01.CCM.0000053643.21751.3B. [DOI] [PubMed] [Google Scholar]
- 13.Nakase–Richardson R. Whyte J. Giacino J.T. Pavawalla S. Barnett S.D. Yablon S.A. Sherer M. Kalmar K. Hammond F.M. Greenwald B. Horn L.J. Seel R. McCarthy M. Tran J. Walker W.C. Longitudinal outcome of patients with disordered consciousness in the NIDRR TBI Model Systems Programs. J. Neurotrauma. 2012;29:59–65. doi: 10.1089/neu.2011.1829. [DOI] [PubMed] [Google Scholar]
- 14.Berger E. Leven F. Pirente N. Bouillon B. Neugebauer E. Quality of Life after traumatic brain injury: A systematic review of the literature. Restor. Neurol. Neurosci. 1999;14:93–102. [PubMed] [Google Scholar]
- 15.Lulé D. Zickler C. Hacker S. Bruno M.A. Demertzi A. Pellas F. Laureys S. Kubler A. Life can be worth living in locked-in syndrome. Prog. Brain Res. 2009;177:339–351. doi: 10.1016/S0079-6123(09)17723-3. [DOI] [PubMed] [Google Scholar]
- 16.Woischneck D. Firsching R. Efficiency of the Glasgow Outcome Scale (GOS)-Score for the long-term follow-up after severe brain injuries. Acta Neurochir. Suppl. 1998;71:138–141. doi: 10.1007/978-3-7091-6475-4_41. [DOI] [PubMed] [Google Scholar]
- 17.Jennett B. Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 18.Steyerberg E.W. Mushkudiani N. Perel P. Butcher I. Lu J. McHugh G.S. Murray G.D. Marmarou A. Roberts I. Habbema J.D. Maas A.I.R. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:e165. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson S.I. Housley A.M. Jones P.A. Slattery J. Miller J.D. Glasgow Outcome Scale: an inter-rater reliability study. Brain Inj. 1993;7:309–317. doi: 10.3109/02699059309034957. [DOI] [PubMed] [Google Scholar]
- 20.Voss A. Standards der neurologischen-neurochirurgischen Frührehabilitation. Ein Konzept der Arbeitsgemeinschaft Neurologisch-Neurochirurgische Frührehabilitation. In: Wild V, editor; Janzik KHH, editor. Spectrum der Neurorehabilitation: Frührehabilitation; Rehabilitation. Bern/w.eu/ New York: Zuckerschwerdt; 1993. pp. 112–120. [Google Scholar]
- 21.Granger C.V. The emerging science of functional assessment: our tool for outcomes analysis. Arch. Phys. Med. Rehabil. 1998;79:235–240. doi: 10.1016/s0003-9993(98)90000-4. [DOI] [PubMed] [Google Scholar]
- 22.Beninato M. Gill–Body K.M. Salles S. Stark P.C. Black–Schaffer R.M. Stein J. Determination of the minimal clinically important difference in the FIM instrument in patients with stroke. Arch. Phys. Med. Rehabil. 2006;87:32–39. doi: 10.1016/j.apmr.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton B.B. Laughlin J.A. Fiedler R.C. Granger C.V. Interrater reliability of the 7-level functional independence measure (FIM) Scand. J. Rehabil. Med. 1994;26:115–119. [PubMed] [Google Scholar]
- 24.Gabbe B.J. Williamson O.D. Cameron P.A. Dowrick A.S. Choosing outcome assessment instruments for trauma registries. Acad. Emerg. Med. 2005;12:751–758. doi: 10.1197/j.aem.2005.03.527. [DOI] [PubMed] [Google Scholar]
- 25.Gabbe B.J. Simpson P.M. Sutherland A.M. Williamson O.D. Judson R. Kossmann T. Cameron P.A. Functional measures at discharge: are they useful predictors of longer term outcomes for trauma registries? Ann. Surg. 2008;247:854–859. doi: 10.1097/SLA.0b013e3181656d1e. [DOI] [PubMed] [Google Scholar]
- 26.Dubroja I. Valent S. Miklic P. Kesak D. Outcome of post-traumatic unawareness persisting for more than a month. J. Neurol. Neurosurg. Psychiatry. 1995;58:465–466. doi: 10.1136/jnnp.58.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estraneo A. Moretta P. Loreto V. Lanzillo B. Santoro L. Trojano L. Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology. 2010;75:239–245. doi: 10.1212/WNL.0b013e3181e8e8cc. [DOI] [PubMed] [Google Scholar]
- 28.Luaute J. Maucort–Boulch D. Tell L. Quelard F. Sarraf T. Iwaz J. Boisson D. Fischer C. Long-term outcomes of chronic minimally conscious and vegetative states. Neurology. 2010;75:246–252. doi: 10.1212/WNL.0b013e3181e8e8df. [DOI] [PubMed] [Google Scholar]
- 29.Murray L.S. Teasdale G.M. Murray G.D. Jennett B. Miller J.D. Pickard J.D. Shaw M.D. Achilles J. Bailey S. Jones P. Kelly D. Lacey J. Does prediction of outcome alter patient management? Lancet. 1993;341:1487–1491. doi: 10.1016/0140-6736(93)90631-p. [DOI] [PubMed] [Google Scholar]
- 30.Wijdicks E.F. Hijdra A. Young G.B. Bassetti C.L. Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 31.Zandbergen E.G. Hijdra A. Koelman J.H. Hart A.A. Vos P.E. Verbeek M.M. de Haan R.J. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology. 2006;66:1–12. doi: 10.1212/01.wnl.0000191308.22233.88. [DOI] [PubMed] [Google Scholar]
- 32.Bender A. Howell K. Frey M. Berlis A. Naumann M. Buheitel G. Bilateral loss of cortical SSEP responses is compatible with good outcome after cardiac arrest. J Neurol. 2012;259:2481–2483. doi: 10.1007/s00415-012-6573-8. [DOI] [PubMed] [Google Scholar]
- 33.Turgeon A.F. Lauzier F. Simard J.F. Scales D.C. Burns K.E. Moore L. Zygun D.A. Bernard F. Meade M.O. Cong Dung T.C. Ratnapalan M. Todd S. Harlock J. Fergusson D.A. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ. 2011;183:1581–1588. doi: 10.1503/cmaj.101786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemphill J.C., 3rd White D.B. Clinical nihilism in neuroemergencies. Emerg Med Clin North Am. 2009;27(27–37):vii–viii. doi: 10.1016/j.emc.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grill E. Klein A.-M. Arndt M. Bodrozic L. Herzog J. Howell K. Jox R. Koenig E. Mansman U. Müller F. Müller T. Nowak D. Straube A. Bender A. Rationale, design and preliminary results of the prospective German registry of outcome in patients with severe disorders of consciousness following acute brain injury (KOPF-R) Arch Phys Med Rehabil. 2013 doi: 10.1016/j.apmr.2012.10.040. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]