Abstract
Neurodegeneration and depression are two common co-morbid conditions, particularly within the aging population. Research has linked neuroinflammation as a major contributing factor to both of these diseases. The key to neuroinflammation effects on neurodegeneration and depression appears to lie within the dysregulation of the control and release of pro- and anti-inflammatory cytokines. This can come from an internal or external insult to the system, or from changes in the individual due to aging that culminate in immune dysregulation. The need to reduce neuroinflammation has led to extensive research into neuroprotectants. We discuss the efficacy found with nicotine, alcohol, resveratrol, curcumin, and ketamine. Our main focus will be on what research is telling us about the connections between neuroinflammation, neurodegeneration, and depression, and the hope that neuroprotectants research is giving people suffering from neurodegeneration and depression stemming from neuroinflammation. We will conclude by making suggestions for future research in this area.
1.0 Introduction
The aging baby-boomer population has encouraged an increase in research into age-related neurodegeneration and its causes. This research has led to discoveries that link neuroinflammation to not only neurodegeneration, but also depression. New hypotheses have been developed based on these findings, e.g., the inflammatory and neurodegenerative hypothesis of depression (Maes et al. 2009). However, the lingering question in this regard is the extent neuroinflammation plays in the etiology of neurodegenerative processes and neuropsychiatric disorders such as depression. In this review, we will present arguments in favor of a positive feedback loop between neuroinflammation, neurodegeneration, and depression. To do this we will focus on the relation between depression and neurodegeneration, what neuroinflammation is and its mechanisms, and end with a discussion of research investigating neuroprotectants with an eye towards the neuroinflammation, neurodegeneration, and depression feedback loop (Figure 1). It is important to note that such a review, far from being an exhaustive one, is best envisioned as a framework for further exploration of the interaction between neuroinflammation, neurodegeneration, and depression.
Figure 1.
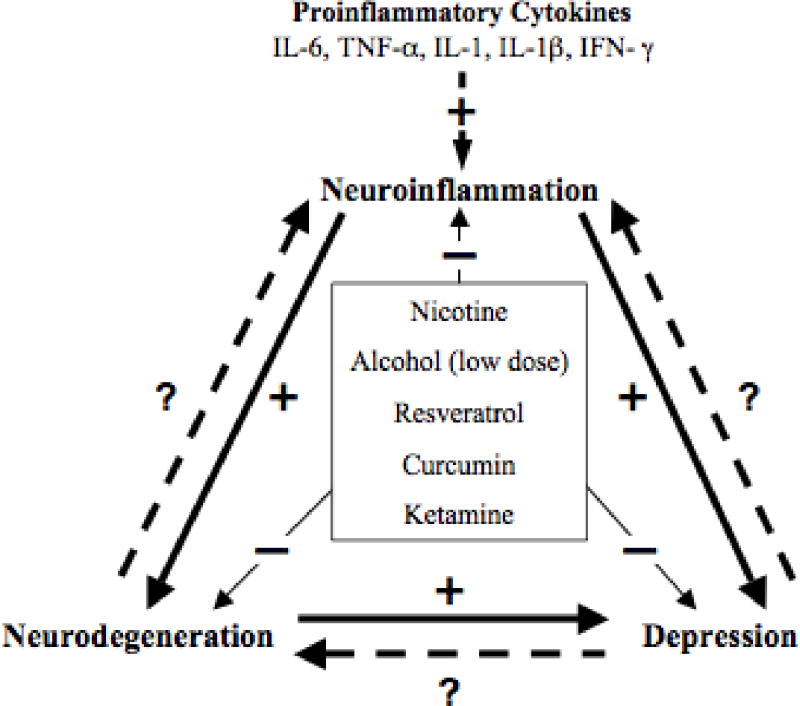
The cycle of neuroinflammation, neurodegeneration, and depression, including alterations by key neuroprotectants. Solid line with: (+) indicates a positive influence, (−) denotes inhibition or alleviation of inflammatory response, neurodegeneration and depression. Dashed line with (?) is an unknown or un-established effect.
2.0 Depression and Neurodegeneration
Mood disorders, such as depression, are chronic, severe, and often life-threatening illnesses. Although genetic factors likely play a major role in their etiology, research related to anatomical circuitry and biochemical abnormalities underlying their predisposition have shed light into their pathophysiology. Cumulative data indicate that impairments in cellular plasticity underlie the pathophysiology of severe mood disorders (Manji and Duman 2001). However, increasing evidence (discussed below) suggest that neurodegenerative processes, reflected in neuronal and glial cell atrophy or loss, may also be contributory factors to mood disorders.
Depression commonly occurs in neurodegenerative diseases such as Alzheimer's disease (de Souza et al. 2010; Raskind 2008; Teng et al. 2008; Zec and Burkett 2008), Parkinson's disease (Gómez-Esteban et al. 2009; Kulisevsky et al. 2008; Simuni and Sethi 2008; Stella et al. 2008), Lewy body disease (Fritze et al. 2011; Takahashi et al. 2009; Yamane et al. 2011), and Huntington's disease (Paulsen et al. 2005; Perlis et al. 2010), but it has been suggested that depression itself, particularly in late life, may be an indication of latent neurodegeneration (Burgut et al. 2006). An association has been seen between late-life depressed mood, anhedonia, apathy, anergia and higher lacunars volume in white matter, suggestive of a role of subcortical ischemic vascular disease in the pathogenesis of such late-life mood disorders (Lavretsky et al. 2008). Additionally, human imaging studies showing cellular loss in key brain regions such as prefrontal cortex and amygdala of patients with mood disorders causally links select reduction in brain volume to depression (Cotter et al. 2001; Rajkowska 2002; Rosso et al. 2005; Sheline et al. 1998). Depending on the region the cause for this reduction may be due to either neurodegeneration or reduction of neurogenesis. For example, there is indication of selective neuronal loss in the paraventricular nucleus of hypothalamus in patients suffering from major depression (Manaye et al. 2005), and depression in human subjects may be associated with a reduction in hippocampal volume (Czeh and Lucassen 2007; Sheline et al. 2003). Animal models of depression support human findings, reporting loss of hippocampal volume or neurodegeneration in selective brain areas. WKY rats, a putative animal model of depression, show reduced hippocampal volume compared to their control, the Wistar rats (Tizabi et al. 2010). They also show reduced number and size of their hypothalamic hypocretinergic neurons, which may underlie the disrupted sleep pattern associated with depressive characteristics in these rats (Allard et al., 2004). On the other hand, it is thought that depression induced by bulbectomy in mice is associated with neuronal loss in areas such as piriform cortex and posterolateral cortical amygdaloid nucleus (Jarosik et al. 2007), or glial cells in the prefrontal cortex (Banasr and Duman 2008).
Depression-associated volume reduction in the hippocampus appears to be more reflective of a reduction in neurogenesis rather than just neuronal atrophy or destruction (Zhao et al. 2008). This contention is further supported by the findings that treatment with antidepressants may promote neurogenesis, and thus normalize the hippocampal volume (Czeh and Lucassen 2007; Sheline et al. 2003). Chronic-mild-stress animal studies suggest that it is reduction in neurogenesis as opposed to a neurodegenerative process in the hippocampus that causes depression in adults (Sapolsky 2004; Toth et al. 2008), but the story is not that simple when considering stress-induced neuroinflammation (Kubera et al. 2011). It is important to note that most animal models of depression induce depression through stress, which in itself may increase the susceptibility of certain neurons to damage or death (McEwen 2008; McKernan et al. 2009). The hypothesis that stress, via activation of neuroendocrine system, neurotransmitter changes and particularly proinflammatory cytokines can induce neurodegeneration and contribute to pathology of depression led to the development of the cytokine hypothesis of depression, which has been consistently tested since the 1990s (e.g., Maes 1993, 1994, 1995, 1999, 2001; Maes et al. 1990, 1995; Qin et al. 2007; Raison et al. 2006; Schiepers et al. 2005; Song and Wang 2011). Stress induced cytokine release is not the only culprit in causing a reduction in neurogenesis. A baseline reduction in hippocampal brain derived neurotrophic factor (BDNF), a marker of neurogenesis, has been observed in WKY rat model of depression, suggestive of a reduced neurogenesis (Hauser et al. 2011). In the interest of brevity, we will primarily discuss neuroinflammation via cytokine function, and will not go in-depth into neuroendocrine and neurotransmitter involvement. However, we will discuss their roles where appropriate in relation to glial activation of cytokine release.
3.0 Role of Neuroinflammation
Neuroinflammation is defined as the brain's response to injury, infection or disease. The purpose of inflammation in general is to remove or inactivate potentially damaging agents or damaged tissue. This response is primarily mediated via one of two cell systems: glia of the central nervous system (CNS), and lymphocytes, monocytes, and macrophages of the hematopoietic system (Streit et al. 1999; Stoll and Jander 1999). Results of a number of studies suggest that patients who have major depressive disorder show alterations in immunologic markers including increases in proinflammatory cytokine activity and inflammation (Gold and Irwin 2009). Moreover, it has been proposed that chronic low-grade inflammation may result in changes in brain structure and synaptic plasticity leading to neurodegeneration (Hayley et al. 2005; Khairova et al. 2009; Leonard 2007; Maes et al. 2009). Such increases in neurodegeneration, coupled with a reduction in neuroprotection and neuronal repair due to increase in glucocorticoid levels may be the initial pathological markers of depression and a prelude to dementia, particularly in older people (Leonard 2007; Leonard and Myint 2009). Interestingly, neuroinflammation mediated by microglia and astrocytic activation can be suppressed by norepinephrine (NE). Hence, NE uptake inhibitors' therapeutic efficacy in depression may be partially related to NE-mediated anti-inflammatory effects (O'Sullivan et al. 2009). It is of importance to note that chronic stress may exacerbate the release of proinflammatory cytokines and hence precipitate depressive episodes (Maes et al. 2009). It has been shown that stress through its interaction with the immune system may increase the levels of proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 (see below). It is now well documented that neuroinflammation is actively involved in neurological diseases and disorders like Alzheimer's disease (Leonard and Myint 2006; Wuwongse et al. (2010), amyotrophic lateral sclerosis, epilepsy, Huntington's disease, multiple sclerosis, and Parkinson's disease (Hemmerle et al. 2012), and that often these diseases show co-morbid expression with depression. Thus, drugs that may interfere with detrimental consequences of stress on inflammatory pathways may offer novel treatments for mood disorders and subsequent neurodegenerative pathologies. Specific pro- and anti- inflammatory mediators will be discussed is more detail below.
4.0 Mechanisms of Neuroinflammation
As mentioned above, glial cells – particularly microglia – are the primary modulators of inflammation in the central nervous system (CNS: Block et al. 2007; Streit et al. 1999; Stoll and Jander 1999). Microglia constantly ‘survey’ their environment and with constitutively expressed surface receptors can trigger or amplify responses to a given insult (Aloisi 2001). Glial activation can be the result of local insult or a response to a systemic insult. However, a key point elaborated below, is that glial cell activation can quickly lead to the release of both pro- and antiinflammatory cytokines. The final effect of cytokine release is dependent on the balance between these opposing responses. In this vein it is postulated that glial activation initially serves a protective function, but that continuous activation can lead to exaggerated release of proinflammatory cytokines and hence neuronal damage. Labeling a given cytokine as pro- or anti-inflammatory can often be misleading, as the response they elicit can vary with exposure duration, target cell and the micro-environment that the cytokines are acting in (Dinarello 1997).
4.1 Cytokines
Cytokines are a large family of small signaling proteins secreted from various cell types that illicit varied biological activity. Key to this review, they induce both an anti- or proinflammatory response within the body. Anti-inflammatory cytokines are generally released to regulate proinflammatory cytokines. This regulation helps limit potentially damaging effects of prolonged or excess inflammation caused by the proinflammatory cytokines. However, dysregulation in cytokines can lead to insufficient mediation or inhibition of normal immune reaction leading to disease manifestation (Dinarello 1997, 1998; Kasai et al. 1997; Munoz et al. 1991; Rubio-Perez and Morillas-Ruiz 2012). For example, IL-1, IL-6, and TNF-α are released in response to disease threat or exotoxins such as lipopolysaccharide (LPS) and typically cause a low-level inflammatory response in order to combat the insult or threat (Barton 1997; Zhang et al. 2012). However, these three cytokines tend to be dualistic in their action. For example, IL-6 has acute proinflammatory action (Hurst et al. 2001; Barton et al. 1996), but can also act to attenuate or down-regulate synthesis of other proinflammatory cytokines (e.g., Interferon-gamma (IFN-γ), IL-1, and TNF-α: Barton 1997; Libert et al. 1994; Xing et al. 1998). Furthermore, it activates release of IL-1 receptor antagonist (IL-1ra) and IL-10, both of which possess potent anti-inflammatory properties (Steensberg et al. 2003).
Similar to IL-6, IL-1 and TNF-α also have acute proinflammatory actions, and dysregulation of production of one or more of these cytokines has been linked to Alzheimer's disease (Swardfager et al. 2010), Parkinson's disease (Nagatsu et al. 2000), cancer (Locksley et al. 2001), inflammatory bowel disease (Brynskov et al. 2002), and major depression (Dowlati et al. 2010; Howren et al. 2009). Furthermore, unregulated IL-1, IL-1β and TNF-α have been shown to impair neurogenesis, exacerbate cell death, and cause neurodegeneration (Goshen et al. 2008; Koo and Duman 2008; Patel et al. 2006; Viviani et al. 2006; Zou and Crews 2005). These effects appear to happen primarily through the cytokines' disruption of survival signaling pathways, caspase-dependant cascades, and alteration of normal receptor function (Patel et al. 2006; Viviani et al. 2006; Zou and Crews 2005). The dysfunction of these cytokines can often be attributed to immune dysregulation (discussed below).
Change in cytokine expression can also occur in the absence of pathology. The expressions of certain cytokines appear to increase as a function of age, suggesting another cause for age related dementia and depression. For example, aging patients without neurological diseases show a progressive increase in the expression of IL-1 and microglia activation (Roubenoff et al. 1998; Wilson et al. 2002), but this increase is still less than in patients with Alzheimer's disease (Griffin et al. 1989). IL-6 levels also increase in the mouse brain with advancing age (Godbout and Johnson 2004), and TNF-α gene expression is dramatically increased in the cerebellum of aged rats compared to young rats (Gemma et al. 2002). Research suggests that immune response-related molecules and their receptors expressed throughout the brain change with age and disrupt normal physiology, contributing to cognitive and behavioral dysfunction (Barrientos et al. 2002, 2010; Lynch 1998, 2002; Pugh et al. 1999, 2001). These changes are possibly due to dysfunction in communication between microglial and neurons caused by “microglial senescence” (Gemma et al. 2010; Streit and Xue 2010).
4.2 Immune dysregulation
The immune system is designed to help protect the body against disease, toxic agents, stress, and injury. Immune response to insult can vary, but inflammation is the first response to infection and injury as the body initiates a defense, mediated by cytokines, followed by the healing process. As discussed above, in a normal functioning system pro- and anti-inflammatory cytokines function in a regulatory loop. However, breakdown of the normal response by factors such as stress or disease can cause inflammation to become persistent and harmful (Gao and Hong 2008). For example, inflammation induced by LPS – simulating disease induced inflammation – causes long-term increase in TNF-α from brain microglia months after it has subsided in the periphery (Qin et al. 2007). Moreover, this increased proinflammatory response induced a delayed and progressive loss in dopaminergic neurons in the substantia nigra, similar to that seen in Parkinson's disease, suggesting that unregulated neuroinflammation could lead to neurodegeneration (Qin et al. 2007). Similarly, it was demonstrated that IFN-γ induces the enzyme indoleamine 2,3- dioxygenase (IDO), which causes reduction in tryptophan availability, leading to a reduction in serotonin synthesis in the brain (Wirleitner et al. 2003). Since serotonin has been directly implicated in depression and neurodegeneration, this provides further support that unchecked inflammatory response can play a significant role in such disorders.
LPS studies have shown that other proinflammatory cytokines (e.g., IL-6 and IL-1β) can remain elevated and induce symptoms of a syndrome termed “sickness behavior” (Kent et al. 1992; Qin et al. 2007). The symptoms that define sickness behavior vary and bear a strong similarity to depression, including reduction in locomotor activity, anhedonia, anorexia and cognitive disturbances, strengthening the suggestion that the inflammatory response could induce symptoms of depression (Kent et al. 1992; Maes et al. 1993; Yirmiya 1997). In the years since these initial studies, a number of findings have reinforced the idea that unregulated inflammation can lead to depression and possibly neurodegeneration. For example, administration of high levels of proinflammatory cytokines can cause changes in behavior similar to depression and that attenuation of inflammatory response reduces depressive symptoms (Capuron and Miller 2004; Pollak and Yirmiya 2002).
Stress is also known to alter immune functioning. Maes et al. (1998) were the first to show that psychological stress in humans induces an inflammatory response through production of proinflammatory cytokines, such as IFN-γ and TNF-α. Subsequent studies show this to be true in stressful situations as well (Shapira-Lichter et al. 2008; Steptoe et al. 2007). Animal studies also have demonstrated that stressors increase cytokine levels such as IL-1β and IL-6 in the blood and in various brain regions (Goshen et al. 2008; Ishikawa et al. 2001; Nguyen et al. 1998). Goshen et al. (2008) specifically showed that after chronic mild stress normal mice showed increased IL-1β in the hippocampus and depressive-like behavior, but IL-1 receptor deficient mice did not show such behavioral changes. Recently it has been shown that direct administration of TNF-α can also induce a depressive like state that can be blocked with the anti-TNF-α antibody (Kaster et al. 2012). Interestingly, it has been proposed that the action of some antidepressants may also be attributed to a reduction in pro-inflammatory cytokines (Pollak and Yirmiya 2002). For a more detailed review of the role of inflammation in depression see Zunszain et al. (2012).
5.0 Neuroprotectants
Neuroprotectants are drugs that act to protect against or help repair the damaging effects of an insult to the brain. Damage induced by acute disease, trauma or chronic disease (e.g., Alzheimer's and Parkinson's) can lead to cell death through necrosis, apoptosis, or autophagic pathways, some of which can be short term whereas others may cause progressive dysfunction. This variability means that it is unlikely that a single class of neuroprotectant therapy may afford total protection. Neuroprotectants also work through a number of mechanisms some of which may overlap. A major concern with therapeutic interventions is to ensure that targeting a specific pathway would not result in the activation of a compensatory mechanism(s) or increase in activity of one of the other pathways that could undermine the intended purpose (Eisenberg-Lerner et al. 2009; Yokoyama et al. 2008). For example, apoptosis and autophagy interact in several ways: antagonistically, as a back-up, or in some cooperative manner (see Eisenberg-Lerner et al. 2009). Hence, in some instances protection from apoptosis may lead to an increase in autophagy related cell death (Yokoyama et al. 2008).
Keeping this in mind, there is a lot of hope that regulating neuroinflammation mediated by microglia activation may help prevent or reverse depression and neurodegenerative diseases. A number of drugs have been tested over the years that act on different parts of the inflammatory pathway. Some of the more novel ones including, nicotine, alcohol, resveratrol, curcumin, and ketamine with potential dual application for depression and neurodegeneration will be briefly discussed here.
5.1 Nicotine
Nicotine addiction (from tobacco products) and depression are highly correlated, but the reasons are generally unclear. One possibly is that nicotine demonstrates antidepressant qualities, and often depression relapses when cessation is attempted, leading to “self-medication hypothesis” (Cook et al. 2007; Moreno-Coutiño et al. 2007; Spring et al. 2008). A second possibility is that excessive nicotine use induces depression itself, but this is only consistently seen in adolescence (Parrott 2003; Steuber and Danner 2006; Upadhyaya et al. 2002). Third, nicotine withdrawal induces depression, which likely contributes to the failure rate of smoking cessation (Borrelli et al. 1996; Covey et al. 1997; Edwards and Kendler 2011; Glassman et al. 2001; Tsoh et al. 2000). Preclinical as well as clinical studies suggest an antidepressant-like effect of nicotine. This effect could come from the euphoric effect experienced by new smokers (Pomerleau and Pomerleau 1992). However, it is clear that nicotine (from patch or smoking) has a direct effect in alleviating anhedonia and improving mood in depressed patients (Cook et al. 2007; McClernon et al. 2006; Salin-Pascual et al. 1995). In animal model studies, it is clear that nicotine reduces depressive-like symptoms such as helplessness and anhedonia (Djuric et al. 1999; Kalejaiye et al. 2011, 2012; Semba et al. 1998; Tizabi et al. 1999, 2000, 2009b, 2010). Therefore, depressed individuals may use nicotine as an anti-depressant at first, but continued use may worsen depression during use and withdrawal (Borrelli et al. 1996; Convey et al. 1997; Tsoh et al. 2000; Glassman et al. 2001). This varied relationship between nicotine and depression, may explain the mercurial relationship depressed patients have with nicotine usage (see also reviews by Philip et al. 2010, 2012)
Beyond its anti-depressant qualities, a number of epidemiological and empirical studies also suggest neuroprotective effects nicotine. An inverse relationship between Parkinson's disease and smoking has been consistently demonstrated in epidemiological studies (Baron 1996; Baumann et al. 1980; Dorn 1959; Nefzger et al. 1969; Ross and Petrovitch 2001; Thacker et al. 2007). In-vivo and in-vitro studies have shown that nicotine protects against nigrostriatal damage induced by various compounds. For example, in Parkinson's disease cell models nicotine protects against endogenous substances such as salsolinol and aminochrome that selectively damage dopaminergic cells (Copeland et al. 2005, 2007; Das and Tizabi 2009; Muñoz et al. 2012; Ramlochansingh et al. 2011), and delays Parkinson's disease-like symptoms induced by MPTP in non-human primates (Quik et al. 2006). Recently it has been suggested that nicotine protection against MPTP in a mouse model of Parkinson's disease is via inhibition of astrocyte activation (Liu et al. 2012). Beyond protection in Parkinson's disease models, nicotine has been seen in in-vitro studies (primary and immortal cell cultures) to protect against or attenuate toxicity induced by LPS, cytokines, glutamate, alcohol, N-methyl-D-aspartate (NMDA) and hypoxia (Dajas-Bailador et al. 2002; Guan et al. 2003; Hejmadi et al. 2003; Kihara et al. 1998; Liu and Zhao 2004; Park et al. 2007; Stevens et al. 2003; Tizabi et al. 2003, 2004, 2005).
The action of this protection is unclear, but it appears to be mediated by activation of multiple nicotinic receptors (Copeland et al. 2005, 2007; Dajas-Bailador et al. 2002; Hejmadi et al. 2003; Picciotto and Zoli 2008; Quik et al. 2009). The signal transduction mechanism(s) underlying the neuroprotection may involve direct or indirect nicotinic receptor mediated modulation of calcium and other anti-apoptotic mechanisms, but the exact mechanism and pathway is still unclear (Donnelly-Roberts et al. 1996; Kihara et al. 2001; Liu and Zhao 2004; Ren et al. 2005; Stevens et al. 2003; reviewed in Buckingham et al. 2009). Regardless of the pathway, it is clear that nicotine or nicotinic agonists may be suitable drugs for treating some neurodegenerative diseases, depression, or both. Important to this review, is that mediation may occur via regulation of neuroinflammation, a contention supported by a number of studies (Cui and Li 2010; Piao et al. 2009; Shi et al. 2009). More specifically, nicotine modulation of innate immune pathway, believed to be primarily mediated by alpha7 nicotinic receptors (Cui and Li 2010), immunosuppressive effects of nicotine (Piao et al. 2009), and attenuation of peripheral as well as central inflammation by nicotine (Piao et al. 2009; Shi et al. 2009) have been reported. Interestingly, vagus nerve modulation of the immune response may also be mediated through alpha7 nicotinic receptors (Ulloa 2005). It remains to be determined however, whether antidepressant effects of vagal stimulation observed in some patients may also be due to inflammatory modulation (Conway et al. 2011; Rizvi et al. 2011).
In addition to neurodegenerative and neuropsychiatric diseases, the anti-inflammatory effects of nicotine may also be applicable to variety of conditions including ulcerative colitis, septic kidney injury and obesity all of which can be precipitated or exacerbated by inflammatory processes (Chatterjee et al. 2012; Lakham and Kirchgessner 2011).
5.2 Alcohol
Depression and alcoholism have a high rate of co-morbidity. There are two primary viewpoints on how these diseases interact: First, chronic alcohol use may result in depressive-like characteristics, which may linger or worsen after cessation (Hodgins et al. 1995; Schulteis et al. 1995). This could be caused by significant interactions of chronic alcohol exposure with neurotransmitter systems that regulate mood (Dixit and Crum 2000; Getachew et al. 2008, 2010; Ratsma et al. 2002; Rozas 2009; Tupala and Tiihonen 2004). Second, depression precedes alcoholism, and may improve at first, but then worsens due to chronic alcohol intake (Dixit and Crum 2000; Rodgers et al. 2000; Spak et al. 2000). We have found that high doses of alcohol induce depressive-like behavior in normal rats and exacerbate that seen in Wistar-Kyoto (WKY) rats which exhibit innate depressive-like behavior (Getachew et al. 2010; Hauser et al. 2011). Moreover, these effects of alcohol can be blocked with prescribed antidepressants (Getachew et al. 2010; Hauser et al. 2011) and nicotine (Kalejaiye et al. 2012). On the other hand, we have also observed antidepressant-like effects of low alcohol doses in WKY rat model of depression (Kalejaiye et al., 2011; Tizabi et al. 2009a). Therefore, it is possible that the initial antidepressant effect of low doses of alcohol may contribute to eventual co-morbidity of alcoholism and depression.
Epidemiological studies show trends that light to moderate drinkers have reduced risk of dementia and cognitive decline in comparison to non-drinkers (reviewed Collins et al. 2009). Furthermore, given in moderate to low doses alcohol provides neuroprotection, this is likely because it dampens the inflammatory processes within the brain or in culture (Belmadani et al. 2001; Collins et al. 2000; Park et al. 2007). Some of these benefits can be attributed to anti-oxidant polyphenols (e.g., resveratrol in red wine: discussed below), but it is likely that alcohol in moderate levels has its own direct neuroprotective effect. Studies have shown that giving low doses of alcohol (ethanol) protects in-vitro and ex-vivo neural cultures exposed to toxins that cause neurodegeneration such as HIV-1 glycoprotein gp120 (gp120: Collins et al. 2000), homoquinolinic acid (Cebere and Liljequist 2003) and NMDA (Cebere and Liljequist 2003; Chandler et al. 1993; Wegelius and Korpi 1995). Similarly, pre-treatment of SH-SY5Y cells, a cell line commonly used to model nigral dopaminergic neurons for Parkinson's disease with ethanol caused attenuated salsolinol-induced toxicity (Ramlochansingh et al. 2011). The exact neuroprotective mechanism of low alcohol concentration is not known. It appears that several mechanisms may be at work, including alcohol causing increased release of heat shock proteins (Belmadani et al. 2004; Sivaswamy et al. 2010; reviewed in Collins et al. 2010; see also Hurley et al. 2012b). Our preliminary studies in cultured cells indicate protective effects of low alcohol concentrations against LPS and cytokine induced toxicity (Chin et al. 2012; Tizabi et al. 2012b), suggesting a possible anti-inflammatory effect of low alcohol concentration (Chin et al. 2012; Tizabi et al. 2012b). It remains to be determined whether low doses of alcohol could be promoted as neuroprotective or antidepressant based on reduction of neuroinflammation.
5.3 Resveratrol
A natural non-flavonoid polyphenol antioxidant, resveratrol (3, 4′, 5-trihydroxy-trans-stilbene) is a substance extracted from red grapes in the processing of wine, but it is also found in other fruit skins. The antidepressant-like effect of resveratrol has been suggested in a preclinical study where it was shown to alleviate depressive-like symptoms in mice (Xu et al. 2010). Although it is unknown how resveratrol antidepressant effect may be mediated, contribution of immune-mediated mechanism (i.e., anti-inflammatory effects) cannot be ruled out. Resveratrol has been shown to impedes cancer progression at various stages (Jang et al. 1997), reduce cardiovascular disease (Bradamante et al. 2004), diminish ischemic injuries (Sinha et al. 2002; Wang et al. 2002), and increase stress resistance while lengthening life (Valenzano et al. 2006). Furthermore, it has been shown in mice that resveratrol improves cognitive functioning by increasing insulin-like growth factor (IGF)-1 (Harada et al. 2011). Neuroprotection by resveratrol is also implied, as it reduces the risk of Alzheimer's (reviewed in Vingtdeux et al. 2008) and Parkinson's disease (Chen et al. 2007; Tredici et al. 1999). Recently, it has been suggested that these effects come from resveratrol mediation of neuroinflammation. Zhang et al. (2012) found that resveratrol significantly inhibited LPS-induced microglial activation, and hence production of TNF-α, IL-1β, and nitric oxide (another proinflammatory factor). Thus, resveratrol might represent a potential benefit for the treatment of inflammation-related neurological or neuropsychiatric diseases, particularly mood related disorders.
5.4 Curcumin
Curcumin, the active ingredient in turmeric (Curcuma longa), has been empirically shown to function as an antioxidant (Ruby et al. 1995; Sandur et al. 2007a,b; Sharma 1976), hepato- and nephro-protectant (Kiso et al. 1983; Singh and Sharma 2011; Venkatesan et al. 2000), antimicrobial (De et al. 2009; Wang et al. 2009), anti-inflammatory (Aggarwal and Harikumar 2009; Jurenka 2009), neuroprotectant (Singla and Dhawan 2012), and antidepressant. The antidepressant effects of curcumin have been primarily reported in stress-induced depression models (Bhutani et al. 2009; Kulkarni et al. 2008; Li et al. 2007; Xu et al. 2005a,b). We have recently observed a dose-dependent antidepressant-like effect of curcumin in WKY rats, a non-induced animal model of depression (Hurley et al. 2012a). Curcumin effectiveness against depression has been seen through several pathways. Curcumin increases biogenic amines (e.g., dopamine, serotonin, and norepinephrine) in the cortex and hippocampus after stress and olfactory bulbectomy induced depression-like behavior (Kulkarni et al. 2008; Xu et al. 2005a,b). We have seen an up-regulation of hippocampal BDNF following chronic curcumin treatment (Hurley et al. 2012a). Arora et al. (2011) used curcumin preventatively against pain-induced depression, and found increased biogenic amines. They also found reduction in mRNA and protein of pro-inflammatory cytokines (TNF-α and IL-1β: Abe et al. 1999, Arora et al. 2011) and proteins involved in apoptotic pathways (NF- κβ and caspase-3: Arora et al. 2011). Thus, multiple mechanisms may be contributing to the antidepressant-like effects of curcumin. However, the mediation of inflammation by curcumin through inhibition of cytokine functions, especially IL-1β and induction of nuclear factor (NF)-κB (Buhrmann et al. 2011) suggests curcumin as a potential multi-target drug to work against depression and neurodegeneration. In regard to neuroprotection, it has been observed that societies that widely use curcumin show reduced incidence of inflammation-influenced and cognitive function diseases (Aggarwal et al. 2007; Chandra et al. 2001; Ng et al. 2006; Vas et al. 2001). Curcumin has also been shown to ameliorate the adverse effects of the mutagenic neurotoxin N-methyl N-nitrosourea in cerebrum and cerebellum of mice (Singla and Dhawan 2012). The potential use of curcumin in neurodegenerative diseases, particularly Alzheimer's and Parkinson's disease has been recently reviewed (Darvesh et al. 2012).
5.5 Ketamine
Ketamine is a non-competitive NMDA receptor antagonist and a derivative of phencyclidine (PCP) that blocks the NMDA receptor (Harrison and Simmonds 1985). Ketamine and other NMDA receptor antagonists have been shown to produce both anxiolytic and antidepressant effects in preclinical studies (Berman et al. 2000; Zarate et al. 2006). Animal studies have borne out this effect: sub-anesthetic dose of ketamine caused an acute and sustained antidepressant-like effect in mice (Maeng et al. 2008) and a single dose of ketamine ameliorated behavioral despair for at least a week after its administration in Wistar rats (Yilmaz et al. 2002). We have also reported that administration of low ketamine doses lead to reduced depressive-like behavior as assessed in the forced swimming test while increasing AMPA/NMDA receptor density ratio in the hippocampus of WKY rats (Tizabi et al. 2012a). Clinical studies have shown significant reduction of depressive symptoms within 72 hours of ketamine administration (Berman et al. 2000; Zarate et al. 2006), and that a single sub-anesthetic dose of ketamine has rapid and sustained antidepressant effects in treatment-resistant patients suffering from major depressive disorder (Maeng et al. 2008).
Ketamine has also been shown to prevent endotoxin (Escherichia coli) induced-shock in rats by inhibiting release of plasma cytokines such as TNF-α and IL-6 and reducing inflammation (Taniguchi et al. 2001, 2004). LPS-induced expression of proinflammatory cytokines such as TNF-α, IL-6, iNOS, NF-κB, and activator protein (AP)-1 is also attenuated by ketamine, without effecting expression of anti-inflammatory cytokines like IL-10 (Helmer et al. 2003a,b). In-vitro, ketamine blocked LPS-induced IL-1β and IL-6 at low doses and TNF-α at higher dose due to inhibition of extracellular signal-regulated kinase (ERK1/2) phosphorylation (Chang et al. 2009). It is unknown if ketamine anti-inflammatory effect may be linked to its antidepressant effect, or if it may be an effective neuroprotectant. Preliminary studies do not support protection against apoptotic neurodegeneration (Ribeiro et al. 2012). On the contrary ketamine may cause neurodegeneration in the developing brain (Soriano et al. 2010; Zou et al. 2009; reviewed by Schifilliti et al. 2010). However, a critical consideration in these studies is the dose of ketamine used, as too high of a dose can cause addiction or toxicity. Future studies incorporating low ketamine doses could shed light on possible neuroprotectant effect of ketamine. Moreover, establishing the exact interaction of ketamine with inflammatory system and its possible application in depressive as well as neurodegenerative disorders remain to be determined.
6.0 Future considerations
Study of neurodegeneration and depression has revealed a common thread of neuroinflammation as a major contributing factor to their manifestation. There is hope in curbing the cycle of neuroinflammation, neurodegeneration, and depression through the use of neuroprotectants that act on that link (Fig 1). As we have discussed in this review, there is a lot of promise in neuroprotectants, especially naturally derived ones, that act to regulate cytokine release and reduce neuroinflammation and depression, while also blocking the neurodegenerative process. There is even more hope, as it is found that combining natural anti-inflammatories (e.g., curcumin and resveratrol) produce a multilevel mediation of inflammation, and therefore provide more chance of interfering with manifestation of neurodegeneration and/or depression (Csaki et al. 2009; Van der Schyf 2011). Nonetheless, the full story of how neuroinflammation, neurodegeneration and depression are linked and regulated remains to be elucidated. Therefore, future research needs to closely scrutinize these intriguing relationships particularly in reference to any novel intervention or pharmacotherapy.
Acknowledgments
Supported by: NIH/NIGMS (2 SO6 GM08016-39) and NIH-RCMI 2 G12 RR003048
References
- Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39:41–7. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- Allard JS, Tizabi Y, Shaffery JP, Trouth CO, Manaye K. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides. 2004;38:311–315. doi: 10.1016/j.npep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Arora V, Kuhad A, Tiwari V, Chopra K. Curcumin ameliorates reserpine-induced pain-depression dyad: behavioural, biochemical, neurochemical and molecular evidences. Psychoneuroendocrinology. 2011;36:1570–1581. doi: 10.1016/j.psyneuen.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA. Beneficial effects of nicotine and cigarette smoking: the real, the possible and the spurious. Br Med Bull. 1996;52:58–73. doi: 10.1093/oxfordjournals.bmb.a011533. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Watkins LR, Maier SF. Memory impairments in healthy aging: Role of aging-induced microglial sensitization. Aging Dis. 2010;1:212–231. [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Barton BE. IL-6: insights into novel biological activities. Clin Immunol Immunopathol. 1997;85:16–20. doi: 10.1006/clin.1997.4420. [DOI] [PubMed] [Google Scholar]
- Barton BE, Shortall J, Jackson JV. Interleukins 6 and 11 protect mice from mortality in a staphylococcal enterotoxin-induced toxic shock model. Infect Immun. 1996;64:714–718. doi: 10.1128/iai.64.3.714-718.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann RJ, Jameson HD, McKean HE, Haack DG, Weisberg LM. Cigarette smoking and Parkinson disease: 1. Comparison of cases with matched neighbors. Neurology. 1980;30:839–843. doi: 10.1212/wnl.30.8.839. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Kumar S, Schipma M, Collins MA, Neafsey EJ. Inhibition of amyloid-beta-induced neurotoxicity and apoptosis by moderate ethanol preconditioning. Neuroreport. 2004;15:2093–2096. doi: 10.1097/00001756-200409150-00019. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Zou JY, Schipma MJ, Neafsey EJ, Collins MA. Ethanol pre-exposure suppresses HIV-1 glycoprotein 120-induced neuronal degeneration by abrogating endogenous glutamate/Ca2+-mediated neurotoxicity. Neuroscience. 2001;104:769–781. doi: 10.1016/s0306-4522(01)00139-7. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bhutani MK, Bishnoi M, Kulkarni SK. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol Biochem Behav. 2009;92:39–43. doi: 10.1016/j.pbb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Niaura R, Keuthen NJ, Goldstein MG, DePue JD, Murphy C, Abrams DB. Development of major depressive disorder during smoking-cessation treatment. J Clin Psychiatry. 1996;57:534–538. doi: 10.4088/jcp.v57n1106. [DOI] [PubMed] [Google Scholar]
- Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004;22:169–188. doi: 10.1111/j.1527-3466.2004.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Brynskov J, Foegh P, Pedersen G, Ellervik C, Kirkegaard T, Bingham A, Saermark T. Tumor necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut. 2002;51:37–43. doi: 10.1136/gut.51.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham SD, Jones AK, Brown LA, Sattelle DB. Nicotinic acetylcholine receptor signaling: roles in Alzheimer's disease and amyloid neuroprotection. Pharmacol Rev. 2009;61:39–61. doi: 10.1124/pr.108.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrmann C, Mobasheri A, Busch F, Aldinger C, Stahlmann R, Montaseri A, Shakibaei M. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: role of the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2011;286:28556–28566. doi: 10.1074/jbc.M111.256180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgut FT, Benaur M, Hencliffe C. Late-life depression: a neuropsychiatric approach. Expert Rev Neurother. 2006;6:65–72. doi: 10.1586/14737175.6.1.65. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Cebere A, Liljequist S. Ethanol differentially inhibits homoquinolinic acid- and NMDA-induced neurotoxicity in primary cultures of cerebellar granule cells. Neurochem Res. 2003;28:1193–1199. doi: 10.1023/a:1024228412198. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Sumners C, Crews FT. Ethanol inhibits NMDA receptor-mediated excitotoxicity in rat primary neuronal cultures. Alcohol Clin Exp Res. 1993;17:54–60. doi: 10.1111/j.1530-0277.1993.tb00726.x. [DOI] [PubMed] [Google Scholar]
- Chandra V, Pandav R, Dodge HH, Johnston JM, Belle SH, DeKosky ST, Ganguli M. Incidence of Alzheimer's disease in a rural community in India: the Indo-US study. Neurology. 2001;57:985–989. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- Chang Y, Lee JJ, Hsieh CY, Hsiao G, Chou DS, Sheu JR. Inhibitory effects of ketamine on lipopolysaccharide-induced microglial activation. Mediators Inflamm. 2009;2009:705379. doi: 10.1155/2009/705379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin Y, Brown DO, Taylor RE, Tizabi Y. Protective effects of low alcohol concentrations against inflammatory-mediated toxicity in neuroblastoma-derived cells; Soc for Neuroscience Ann Meeting; 2012.2012. [Google Scholar]
- Chatterjee PK, Yeboah MM, Dowling O, Xue X, Powell SR, Al-Abed Y, Metz CN. Nicotinic acetylcholine receptor agonists attenuate septic acute kidney injury in mice by suppressing inflammation and proteasome activity. PLoS One. 2012;7:e35361. doi: 10.1371/journal.pone.0035361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LW, Wang YQ, Wei LC, Shi M, Chan YS. Chinese herbs and herbal extracts for neuroprotection of dopaminergic neurons and potential therapeutic treatment of Parkinson's disease. CNS Neurol Disord Drug Targets. 2007;6:273–281. doi: 10.2174/187152707781387288. [DOI] [PubMed] [Google Scholar]
- Chin Y, Brown DO, Taylor RE, Tizabi Y. Protective effects of low dose alcohol concentrations against inflammatory-mediated toxicity in neuroblastoma-derived cells; Soc for Neuroscience Ann Meeting; 2012; 2012. p. 866.09. [Google Scholar]
- Collins MA, Neafsey EJ, Mukamal KJ, Gray MO, Parks DA, Das DK, Korthuis RJ. Alcohol in moderation, cardioprotection, and neuroprotection: epidemiological considerations and mechanistic studies. Alcohol Clin Exp Res. 2009;33:206–219. doi: 10.1111/j.1530-0277.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, Wang K, Achille NJ, Mitchell RM, Sivaswamy S. Moderate ethanol preconditioning of rat brain cultures engenders neuroprotection against dementia-inducing neuroinflammatory proteins: possible signaling mechanisms. Mol Neurobiol. 2010;41:420–425. doi: 10.1007/s12035-010-8138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, Zou JY. HIV-I gpI20 neurotoxicity in brain cultures is prevented by moderate ethanol pretreatment. Neuroreport. 2000;11:1219–1222. doi: 10.1097/00001756-200004270-00015. [DOI] [PubMed] [Google Scholar]
- Conway CR, Sheline YI, Chibnall JT, Bucholz RD, Price JL, Gangwani S, Mintun MA. Brain blood-flow change with acute vagus nerve stimulation in treatment-refractory major depressive disorder. Brain Stimul. 2012;5:163–171. doi: 10.1016/j.brs.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology (Berl) 2007;192:87–95. doi: 10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- Copeland RL, Jr, Das JR, Kanaan YM, Taylor RE, Tizabi Y. Antiapoptotic effects of nicotine in its protection against salsolinol-induced cytotoxicity. Neurotox Res. 2007;12:61–69. doi: 10.1007/BF03033901. [DOI] [PubMed] [Google Scholar]
- Copeland RL, Jr, Leggett YA, Kanaan YM, Taylor RE, Tizabi Y. Neuroprotective effects of nicotine against salsolinol-induced cytotoxicity: implications for Parkinson's disease. Neurotox Res. 2005;8:289–293. doi: 10.1007/BF03033982. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Major depression following smoking cessation. Am J Psychiatry. 1997;154:263–265. doi: 10.1176/ajp.154.2.263. [DOI] [PubMed] [Google Scholar]
- Csaki C, Mobasheri A, Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther. 2009;11:R165. doi: 10.1186/ar2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui WY, Li MD. Nicotinic modulation of innate immune pathways via alpha7 nicotinic acetylcholine receptor. J Neuroimmune Pharmacol. 2010;5:479–488. doi: 10.1007/s11481-010-9210-2. [DOI] [PubMed] [Google Scholar]
- Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Soliakov L, Wonnacott S. Nicotine activates the extracellular signal-regulated kinase 1/2 via the alpha7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. J Neurochem. 2002;80:520–530. doi: 10.1046/j.0022-3042.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- Darvesh AS, Carroll RT, Bishayee A, Novotny NA, Geldenhuys WJ, Van der Schyf CJ. Curcumin and neurodegenerative diseases: a perspective. Expert Opin Investig Drugs. 2012;21(8):1123–40. doi: 10.1517/13543784.2012.693479. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Das JR, Tizabi Y. Additive protective effects of donepezil and nicotine against salsolinol-induced cytotoxicity in SH-SY5Y cells. Neurotox Res. 2009;16:194–204. doi: 10.1007/s12640-009-9040-2. [DOI] [PubMed] [Google Scholar]
- de Souza MB, de Lemos RR, da Cunha JE, de Lima Filho JL, de Oliveira JR. Searching for new genetic risk factors for neuropsychiatric disorders in expression databases. J Mol Neurosci. 2010;41:193–197. doi: 10.1007/s12031-009-9321-5. [DOI] [PubMed] [Google Scholar]
- De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB, Mukhopadhyay AK. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother. 2009;53:1592–1597. doi: 10.1128/AAC.01242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Role of pro- and anti-inflammatory cytokines during inflammation: experimental and clinical findings. J Biol Regul Homeost Agents. 1997;11:91–103. [PubMed] [Google Scholar]
- Dixit AR, Crum RM. Prospective study of depression and the risk of heavy alcohol use in women. Am J Psychiatry. 2000;157:751–758. doi: 10.1176/appi.ajp.157.5.751. [DOI] [PubMed] [Google Scholar]
- Djuric VJ, Dunn E, Overstreet DH, Dragomir A, Steiner M. Antidepressant effect of ingested nicotine in female rats of Flinders resistant and sensitive lines. Physiol Behav. 1999;67:533–537. doi: 10.1016/s0031-9384(99)00091-8. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Xue IC, Arneric SP, Sullivan JP. In vitro neuroprotective properties of the novel cholinergic channel activator (ChCA), ABT-418. Brain Res. 1996;719:36–44. doi: 10.1016/0006-8993(96)00063-7. [DOI] [PubMed] [Google Scholar]
- Dorn HF. Tobacco consumption and mortality from cancer and other diseases. Public Health Rep. 1959;74:581–593. [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Edwards AC, Kendler KS. Nicotine withdrawal-induced negative affect is a function of nicotine dependence and not liability to depression or anxiety. Nicotine Tob Res. 2011;13:677–685. doi: 10.1093/ntr/ntr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–975. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- Fritze F, Ehrt U, Hortobagyi T, Ballard C, Aarsland D. Depressive symptoms in Alzheimer's disease and Lewy body dementia: a one-year follow-up study. Dement Geriatr Cogn Disord. 2011;32:143–149. doi: 10.1159/000332016. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemma C, Bachstetter AD, Bickford PC. Neuron-Microglia Dialogue and Hippocampal Neurogenesis in the Aged Brain. Aging Dis. 2010;1:232–244. [PMC free article] [PubMed] [Google Scholar]
- Gemma C, Mesches MH, Sepesi B, Choo K, Holmes DB, Bickford PC. Diets enriched in foods with high antioxidant activity reverse age-induced decreases in cerebellar beta-adrenergic function and increases in proinflammatory cytokines. J Neurosci. 2002;22:6114–6120. doi: 10.1523/JNEUROSCI.22-14-06114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew B, Hauser SR, Taylor RE, Tizabi Y. Alcohol-induced depressive-like behavior is associated with cortical norepinephrine reduction. Pharmacol Biochem Behav. 2010;96:395–401. doi: 10.1016/j.pbb.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew B, Hauser SR, Taylor RE, Tizabi Y. Desipramine blocks alcohol-induced anxiety- and depressive-like behaviors in two rat strains. Pharmacol Biochem Behav. 2008;91:97–103. doi: 10.1016/j.pbb.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH, Covey LS, Stetner F, Rivelli S. Smoking cessation and the course of major depression: a follow-up study. Lancet. 2001;357:1929–1932. doi: 10.1016/S0140-6736(00)05064-9. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Interleukin-6 in the aging brain. J Neuroimmunol. 2004;147:141–144. doi: 10.1016/j.jneuroim.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Gold SM, Irwin MR. Depression and immunity: inflammation and depressive symptoms in multiple sclerosis. Immunol Allergy Clin North Am. 2009;29:309–320. doi: 10.1016/j.iac.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Esteban JC, Tijero B, Somme J, Bilbao I, Fernandez J, Boyero S, Velasco F, Lezcano E, Zarranz JJ. Application of depression criteria (DSM-IV) in patients with Parkinson's disease. Clin Neurol Neurosurg. 2009;111:665–669. doi: 10.1016/j.clineuro.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan ZZ, Yu WF, Nordberg A. Dual effects of nicotine on oxidative stress and neuroprotection in PC12 cells. Neurochem Int. 2003;43:243–249. doi: 10.1016/s0197-0186(03)00009-3. [DOI] [PubMed] [Google Scholar]
- Harada N, Zhao J, Kurihara H, Nakagata N, Okajima K. Resveratrol improves cognitive function in mice by increasing production of insulin-like growth factor-I in the hippocampus. J Nutr Biochem. 2011;22:1150–1159. doi: 10.1016/j.jnutbio.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Simmonds MA. Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. Br J Pharmacol. 1985;84:381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Taylor RE, Tizabi Y. Alcohol induced depressive-like behavior is associated with a reduction in hippocampal BDNF. Pharmacol Biochem Behav. 2011;100:253–258. doi: 10.1016/j.pbb.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S, Poulter MO, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135:659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Hejmadi MV, Dajas-Bailador F, Barns SM, Jones B, Wonnacott S. Neuroprotection by nicotine against hypoxia-induced apoptosis in cortical cultures involves activation of multiple nicotinic acetylcholine receptor subtypes. Mol Cell Neurosci. 2003;24:779–786. doi: 10.1016/s1044-7431(03)00244-6. [DOI] [PubMed] [Google Scholar]
- Helmer KS, Cui Y, Chang L, Dewan A, Mercer DW. Effects of ketamine/xylazine on expression of tumor necrosis factor-alpha, inducible nitric oxide synthase, and cyclo-oxygenase-2 in rat gastric mucosa during endotoxemia. Shock. 2003a;20:63–69. doi: 10.1097/01.shk.0000065766.72937.cf. [DOI] [PubMed] [Google Scholar]
- Helmer KS, Cui Y, Dewan A, Mercer DW. Ketamine/xylazine attenuates LPS-induced iNOS expression in various rat tissues. J Surg Res. 2003b;112:70–78. doi: 10.1016/s0022-4804(03)00138-0. [DOI] [PubMed] [Google Scholar]
- Hemmerle AM, Herman JP, Seroogy KB. Stress, depression and Parkinson's disease. Exp Neurol. 2012;233:79–86. doi: 10.1016/j.expneurol.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgins DC, el-Guebaly N, Armstrong S. Prospective and retrospective reports of mood states before relapse to substance use. J Consult Clin Psychol. 1995;63:400–407. doi: 10.1037//0022-006x.63.3.400. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hurley LL, Akinfiresoye L, Tizabi Y. Behavioral and neurotrophic effects of curcumin in a putative animal model of depression; Soc for Neuroscience Ann Meeting; 2012.2012a. [Google Scholar]
- Hurley LL, Taylor RE, Tizabi Y. Positive and negative effects of alcohol and nicotine and their interactions: a mechanistic review. Neurotox Res. 2012b;21:57–69. doi: 10.1007/s12640-011-9275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, Rose-John S, Fuller GM, Topley N, Jones SA. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa I, Kitamura H, Kimura K, Saito M. Brain interleukin-1 is involved in blood interleukin-6 response to immobilization stress in rats. Jpn J Vet Res. 2001;49:19–25. [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Jarosik J, Legutko B, Unsicker K, von Bohlen Und Halbach O. Antidepressant-mediated reversal of abnormal behavior and neurodegeneration in mice following olfactory bulbectomy. Exp Neurol. 2007;204:20–28. doi: 10.1016/j.expneurol.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- Kalejaiye O, Cortez L, Taylor RE, Tizabi Y. Effects of Alcohol and Nicotine Combination in a Rat Model of Depression; Soc for Neuroscience Ann Meeting; 2011; 2011. p. 794.08. [Google Scholar]
- Kalejaiye OO, Hurley LL, Taylor RE, Tizabi Y. Nicotine mitigates depressogenic effects of alcohol in Wistar rats; Soc for Neuroscience Ann Meeting; 2012; 2012. p. 665.03. [Google Scholar]
- Kasai T, Inada K, Takakuwa T, Yamada Y, Inoue Y, Shimamura T, Taniguchi S, Sato S, Wakabayashi G, Endo S. Anti-inflammatory cytokine levels in patients with septic shock. Res Commun Mol Pathol Pharmacol. 1997;98:34–42. [PubMed] [Google Scholar]
- Kaster MP, Gadotti VM, Calixto JB, Santos AR, Rodrigues AL. Depressive-like behavior induced by tumor necrosis factor-alpha in mice. Neuropharmacology. 2012;62:419–426. doi: 10.1016/j.neuropharm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Khairova RA, Machado-Vieira R, Du J, Manji HK. A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2009;12:561–578. doi: 10.1017/S1461145709009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Shibasaki H, Kume T, Akaike A. alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. J Biol Chem. 2001;276:13541–13546. doi: 10.1074/jbc.M008035200. [DOI] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Urushitani M, Sawada H, Kimura J, Kume T, Maeda T, Akaike A. Stimulation of alpha4beta2 nicotinic acetylcholine receptors inhibits beta-amyloid toxicity. Brain Res. 1998;792:331–334. doi: 10.1016/s0006-8993(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Kiso Y, Suzuki Y, Watanabe N, Oshima Y, Hikino H. Antihepatotoxic Principles of Curcuma longa Rhizomes1. Planta Med. 1983;49:185–187. doi: 10.1055/s-2007-969845. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:744–759. doi: 10.1016/j.pnpbp.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Kulisevsky J, Pagonabarraga J, Pascual-Sedano B, Gironell A, Garcia-Sanchez C, Martinez-Corral M. Motor changes during sertraline treatment in depressed patients with Parkinson's disease*. Eur J Neurol. 2008;15:953–959. doi: 10.1111/j.1468-1331.2008.02218.x. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Bhutani MK, Bishnoi M. Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacology (Berl) 2008;201:435–442. doi: 10.1007/s00213-008-1300-y. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A. Anti-inflammatory effects of nicotine in obesity and ulcerative colitis. J Transl Med. 2011;9:129. doi: 10.1186/1479-5876-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Zheng L, Weiner MW, Mungas D, Reed B, Kramer JH, Jagust W, Chui H, Mack WJ. The MRI brain correlates of depressed mood, anhedonia, apathy, and anergia in older adults with and without cognitive impairment or dementia. Int J Geriatr Psychiatry. 2008;23:1040–1050. doi: 10.1002/gps.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32:1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- Leonard BE, Myint A. The psychoneuroimmunology of depression. Hum Psychopharmacol. 2009;24:165–175. doi: 10.1002/hup.1011. [DOI] [PubMed] [Google Scholar]
- Li S, Wang C, Wang M, Li W, Matsumoto K, Tang Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007;80:1373–1381. doi: 10.1016/j.lfs.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Libert C, Takahashi N, Cauwels A, Brouckaert P, Bluethmann H, Fiers W. Response of interleukin-6-deficient mice to tumor necrosis factor-induced metabolic changes and lethality. Eur J Immunol. 1994;24:2237–2242. doi: 10.1002/eji.1830240945. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhao B. Nicotine attenuates beta-amyloid peptide-induced neurotoxicity, free radical and calcium accumulation in hippocampal neuronal cultures. Br J Pharmacol. 2004;141:746–754. doi: 10.1038/sj.bjp.0705653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hu J, Wu J, Zhu C, Hui Y, Han Y, Huang Z, Ellsworth K, Fan W. alpha7 nicotinic acetylcholine receptor-mediated neuroprotection against dopaminergic neuron loss in an MPTP mouse model via inhibition of astrocyte activation. J Neuroinflammation. 2012;9:98. doi: 10.1186/1742-2094-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Interleukin-1 beta exerts a myriad of effects in the brain and in particular in the hippocampus: analysis of some of these actions. Vitam Horm. 2002;64:185–219. doi: 10.1016/s0083-6729(02)64006-3. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Age-related impairment in long-term potentiation in hippocampus: a role for the cytokine, interleukin-1 beta? Prog Neurobiol. 1998;56:571–589. doi: 10.1016/s0301-0082(98)00054-9. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Maes M. The immunoregulatory effects of antidepressants. Hum Psychopharmacol. 2001;16:95–103. doi: 10.1002/hup.191. [DOI] [PubMed] [Google Scholar]
- Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- Maes M. Cytokines in major depression. Biol Psychiatry. 1994;36:498–499. doi: 10.1016/0006-3223(94)90652-1. [DOI] [PubMed] [Google Scholar]
- Maes M. A review on the acute phase response in major depression. Rev Neurosci. 1993;4:407–416. doi: 10.1515/revneuro.1993.4.4.407. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, Suy E, Vandervorst C, De Jonckheere C, Raus J. Immune disturbances during major depression: upregulated expression of interleukin-2 receptors. Neuropsychobiology. 1990;24:115–120. doi: 10.1159/000119472. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Scharpe S, Cooreman W, Uyttenbroeck W, Suy E, Vandervorst C, Calabrese J, Raus J, Cosyns P. Psychomotor retardation, anorexia, weight loss, sleep disturbances, and loss of energy: psychopathological correlates of hyperhaptoglobinemia during major depression. Psychiatry Res. 1993;47:229–241. doi: 10.1016/0165-1781(93)90081-q. [DOI] [PubMed] [Google Scholar]
- Maes M, Smith R, Scharpe S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology. 1995;20:111–116. doi: 10.1016/0306-4530(94)00066-j. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- Manaye KF, Lei DL, Tizabi Y, Davila-Garcia MI, Mouton PR, Kelly PH. Selective neuron loss in the paraventricular nucleus of hypothalamus in patients suffering from major depression and bipolar disorder. J Neuropathol Exp Neurol. 2005;64:224–229. doi: 10.1093/jnen/64.3.224. [DOI] [PubMed] [Google Scholar]
- Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull. 2001;35:5–49. [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Westman EC, Rose JE, Levin ED. Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind, placebo-controlled trial. Psychopharmacology. 2006;189:125–133. doi: 10.1007/s00213-006-0516-y. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan DP, Dinan TG, Cryan JF. “ Killing the Blues ”: a role for cellular suicide (apoptosis) in depression and the antidepressant response? Prog Neurobiol. 2009;88:246–263. doi: 10.1016/j.pneurobio.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Moreno-Coutino A, Calderon-Ezquerro C, Drucker-Colin R. Long-term changes in sleep and depressive symptoms of smokers in abstinence. Nicotine Tob Res. 2007;9:389–396. doi: 10.1080/14622200701188901. [DOI] [PubMed] [Google Scholar]
- Munoz C, Carlet J, Fitting C, Misset B, Bleriot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz P, Huenchuguala S, Paris I, Cuevas C, Villa M, Caviedes P, Segura-Aguilar J, Tizabi Y. Protective Effects of Nicotine Against Aminochrome-Induced Toxicity in Substantia Nigra Derived Cells: Implications for Parkinson's Disease. Neurotox Res. 2012;22:177–180. doi: 10.1007/s12640-012-9326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson's disease. J Neural Transm Suppl. 2000;(60):277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- Nefzger MD, Quadfasel FA, Karl VC. A retrospective study of smoking in Parkinson's disease. Am J Epidemiol. 1968;88:149–158. doi: 10.1093/oxfordjournals.aje.a120874. [DOI] [PubMed] [Google Scholar]
- Ng TP, Chiam PC, Lee T, Chua HC, Lim L, Kua EH. Curry consumption and cognitive function in the elderly. Am J Epidemiol. 2006;164:898–906. doi: 10.1093/aje/kwj267. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan JB, Ryan KM, Curtin NM, Harkin A, Connor TJ. Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: implications for depression and neurodegeneration. Int J Neuropsychopharmacol. 2009;12:687–699. doi: 10.1017/S146114570800967X. [DOI] [PubMed] [Google Scholar]
- Park HJ, Lee PH, Ahn YW, Choi YJ, Lee G, Lee DY, Chung ES, Jin BK. Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur J Neurosci. 2007;26:79–89. doi: 10.1111/j.1460-9568.2007.05636.x. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Cigarette-derived nicotine is not a medicine. World J Biol Psychiatry. 2003;4:49–55. doi: 10.3109/15622970309167951. [DOI] [PubMed] [Google Scholar]
- Patel HC, Ross FM, Heenan LE, Davies RE, Rothwell NJ, Allan SM. Neurodegenerative actions of interleukin-1 in the rat brain are mediated through increases in seizure activity. J Neurosci Res. 2006;83:385–391. doi: 10.1002/jnr.20735. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Nehl C, Hoth KF, Kanz JE, Benjamin M, Conybeare R, McDowell B, Turner B. Depression and stages of Huntington's disease. J Neuropsychiatry Clin Neurosci. 2005;17:496–502. doi: 10.1176/jnp.17.4.496. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Smoller JW, Mysore J, Sun M, Gillis T, Purcell S, Rietschel M, Nothen MM, Witt S, Maier W, Iosifescu DV, Sullivan P, Rush AJ, Fava M, Breiter H, Macdonald M, Gusella J. Prevalence of incompletely penetrant Huntington's disease alleles among individuals with major depressive disorder. Am J Psychiatry. 2010;167:574–579. doi: 10.1176/appi.ajp.2009.09070973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Carpenter LL, Tyrka AR, Price LH. Nicotinic acetylcholine receptors and depression: a review of the preclinical and clinical literature. Psychopharmacology. 2010;212:1–12. doi: 10.1007/s00213-010-1932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Carpenter LL, Tyrka AR, Price LH. The nicotinic acetylcholine receptor as a target for antidepressant drug development. Scientific World J. 2012;2012:104105. doi: 10.1100/2012/104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao WH, Campagnolo D, Dayao C, Lukas RJ, Wu J, Shi FD. Nicotine and inflammatory neurological disorders. Acta Pharmacol Sin. 2009;30:715–722. doi: 10.1038/aps.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for “ depression due to a general medical condition”, immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol. 2002;5:389–399. doi: 10.1017/S1461145702003152. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF. Euphoriant effects of nicotine in smokers. Psychopharmacology (Berl) 1992;108:460–465. doi: 10.1007/BF02247422. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Fleshner M, Watkins LR, Maier SF, Rudy JW. the immune system and memory consolidation: a role for the cytokine IL-1beta. Neurosci Biobehav Rev. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Wakins LR, Maier SF, Rudy JW. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behav Brain Res. 1999;106:109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Huang LZ, Parameswaran N, Bordia T, Campos C, Perez XA. Multiple roles for nicotine in Parkinson's disease. Biochem Pharmacol. 2009;78:677–685. doi: 10.1016/j.bcp.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Parameswaran N, McCallum SE, Bordia T, Bao S, McCormack A, Kim A, Tyndale RF, Langston JW, Di Monte DA. Chronic oral nicotine treatment protects against striatal degeneration in MPTP-treated primates. J Neurochem. 2006;98:1866–1875. doi: 10.1111/j.1471-4159.2006.04078.x. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sings the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry. 2002;7:281–292. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- Ramlochansingh C, Taylor RE, Tizabi Y. Toxic effects of low alcohol and nicotine combinations in SH-SY5Y cells are apoptotically mediated. Neurotox Res. 2011;20:263–269. doi: 10.1007/s12640-011-9239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA. Diagnosis and treatment of depression comorbid with neurologic disorders. Am J Med. 2008;121:S28–37. doi: 10.1016/j.amjmed.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Ratsma JE, Van Der Stelt O, Gunning WB. Neurochemical markers of alcoholism vulnerability in humans. Alcohol Alcohol. 2002;37:522–533. doi: 10.1093/alcalc/37.6.522. [DOI] [PubMed] [Google Scholar]
- Ren K, Puig V, Papke RL, Itoh Y, Hughes JA, Meyer EM. Multiple calcium channels and kinases mediate alpha7 nicotinic receptor neuroprotection in PC12 cells. J Neurochem. 2005;94:926–933. doi: 10.1111/j.1471-4159.2005.03223.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro PO, Valentim AM, Rodrigues P, Olsson IA, Antunes LM. Apoptotic neurodegeneration and spatial memory are not affected by sedative and anaesthetics doses of ketamine/medetomidine combinations in adult mice. Br J Anaesth. 2012;108:807–814. doi: 10.1093/bja/aes003. [DOI] [PubMed] [Google Scholar]
- Rizvi SJ, Donovan M, Giacobbe P, Placenza F, Rotzinger S, Kennedy SH. Neurostimulation therapies for treatment resistant depression: a focus on vagus nerve stimulation and deep brain stimulation. Int Rev Psychiatry. 2011;23:424–436. doi: 10.3109/09540261.2011.630993. [DOI] [PubMed] [Google Scholar]
- Rodgers B, Korten AE, Jorm AF, Jacomb PA, Christensen H, Henderson AS. Non-linear relationships in associations of depression and anxiety with alcohol use. Psychol Med. 2000;30:421–432. doi: 10.1017/s0033291799001865. [DOI] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H. Current evidence for neuroprotective effects of nicotine and caffeine against Parkinson's disease. Drugs Aging. 2001;18:797–806. doi: 10.2165/00002512-200118110-00001. [DOI] [PubMed] [Google Scholar]
- Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA. Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry. 2005;57:21–26. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998;53:M20–6. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- Rozas I. Improving antidepressant drugs: update on recently patented compounds. Expert Opin Ther Pat. 2009;19:827–845. doi: 10.1517/13543770902932934. [DOI] [PubMed] [Google Scholar]
- Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer's disease, role of cytokines. Scien World J. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- Salin-Pascual RJ, de la Fuente JR, Galicia-Polo L, Drucker-Colin R. Effects of transderman nicotine on mood and sleep in nonsmoking major depressed patients. Psychopharmacology (Berl) 1995;121:476–479. doi: 10.1007/BF02246496. [DOI] [PubMed] [Google Scholar]
- Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, Aggarwal BB. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane) Free Radic Biol Med. 2007a;43:568–580. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007b;28:1765–1773. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry. 2004;56:137–139. doi: 10.1016/j.biopsych.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schifilliti D, Grasso G, Conti A, Fodale V. Anaesthetic-related neuroprotection: intravenous or inhalational agents? CNS Drugs. 2010;24:893–907. doi: 10.2165/11584760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba J, Mataki C, Yamada S, Nankai M, Toru M. Antidepressant like effects of chronic nicotine on learned helplessness paradigm in rats. Biol Psychiatry. 1998;43:389–391. doi: 10.1016/s0006-3223(97)00477-0. [DOI] [PubMed] [Google Scholar]
- Shapira-Lichter I, Beilin B, Ofek K, Bessler H, Gruberger M, Shavit Y, Seror D, Grinevich G, Posner E, Reichenberg A, Soreq H, Yirmiya R. Cytokines and cholinergic signals co-modulate surgical stress-induced changes in mood and memory. Brain Behav Immun. 2008;22:388–398. doi: 10.1016/j.bbi.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Sharma OP. Antioxidant activity of curcumin and related compounds. Biochem Pharmacol. 1976;25:1811–1812. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Shi FD, Piao WH, Kuo YP, Campagnolo DI, Vollmer TL, Lukas RJ. Nicotinic attenuation of central nervous system inflammation and autoimmunity. J Immunol. 2009;182:1730–1739. doi: 10.4049/jimmunol.182.3.1730. [DOI] [PubMed] [Google Scholar]
- Simuni T, Sethi K. Nonmotor manifestations of Parkinson's disease. Ann Neurol. 2008;64(2):S65–80. doi: 10.1002/ana.21472. [DOI] [PubMed] [Google Scholar]
- Singh R, Sharma P. Hepatoprotective effect of curcumin on lindane-induced oxidative stress in male Wistar rats. Toxicol Int. 2011;18:124–129. doi: 10.4103/0971-6580.84264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singula N, Chawan DK. N-methyl N-nitrosourea induced functional and structural alterations in mice brain-role of curcumin. Neurotox Res. 2012;22(2):115–26. doi: 10.1007/s12640-011-9307-2. [DOI] [PubMed] [Google Scholar]
- Sinha K, Chaudhary G, Gupta YK. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002;71:655–665. doi: 10.1016/s0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- Sivaswamy S, Neafsey EJ, Collins MA. Neuroprotective preconditioning of rat brain cultures with ethanol: potential transduction by PKC isoforms and focal adhesion kinase upstream of increases in effector heat shock proteins. Eur J Neurosci. 2010;32:1800–1812. doi: 10.1111/j.1460-9568.2010.07451.x. [DOI] [PubMed] [Google Scholar]
- Song C, Wang H. Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:760–768. doi: 10.1016/j.pnpbp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Soriano SG, Liu Q, Li J, Liu JR, Han XH, Kanter JL, Bajic D, Ibla JC. Ketamine activates cell cycle signaling and apoptosis in the neonatal rat brain. Anesthesiology. 2010;112:1155–1163. doi: 10.1097/ALN.0b013e3181d3e0c2. [DOI] [PubMed] [Google Scholar]
- Spak L, Spak F, Allebeck P. Alcoholism and depression in a Swedish female population: co-morbidity and risk factors. Acta Psychiatr Scand. 2000;102:44–51. doi: 10.1034/j.1600-0447.2000.102001044.x. [DOI] [PubMed] [Google Scholar]
- Spring B, Cook JW, Appelhans B, Maloney A, Richmond M, Vaughn J, Vanderveen J, Hedeker D. Nicotine effects on affective response in depression-prone smokers. Psychopharmacology (Berl) 2008;196:461–471. doi: 10.1007/s00213-007-0977-7. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433–7. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- Stella F, Banzato CE, Barasnevicius Quagliato EM, Viana MA. Depression in patients with Parkinson's disease: impact on functioning. J Neurol Sci. 2008;272:158–163. doi: 10.1016/j.jns.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Steuber TL, Danner F. Adolescent smoking and depression: which comes first? Addict Behav. 2006;31:133–136. doi: 10.1016/j.addbeh.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Stevens TR, Krueger SR, Fitzsimonds RM, Picciotto MR. Neuroprotection by nicotine in mouse primary cortical cultures involves activation of calcineurin and L-type calcium channel inactivation. J Neurosci. 2003;23:10093–10099. doi: 10.1523/JNEUROSCI.23-31-10093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Xue QS. The Brain's Aging Immune System. Aging Dis. 2010;1:254–261. [PMC free article] [PubMed] [Google Scholar]
- Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer's disease. Biol Psychiatry. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Mizukami K, Yasuno F, Asada T. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9:56–61. doi: 10.1111/j.1479-8301.2009.00292.x. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Kanakura H, Takemoto Y, Yamamoto K. The antiinflammatory effects of ketamine in endotoxemic rats during moderate and mild hypothermia. Anesth Analg. 2004;98:1114–20. doi: 10.1213/01.ANE.0000100740.07331.A2. table of contents. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Shibata K, Yamamoto K. Ketamine inhibits endotoxin-induced shock in rats. Anesthesiology. 2001;95:928–932. doi: 10.1097/00000542-200110000-00022. [DOI] [PubMed] [Google Scholar]
- Teng E, Ringman JM, Ross LK, Mulnard RA, Dick MB, Bartzokis G, Davies HD, Galasko D, Hewett L, Mungas D, Reed BR, Schneider LS, Segal-Gidan F, Yaffe K, Cummings JL. Diagnosing depression in Alzheimer disease with the national institute of mental health provisional criteria. Am J Geriatr Psychiatry. 2008;16:469–477. doi: 10.1097/JGP.0b013e318165dbae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker EL, O'Reilly EJ, Weisskopf MG, Chen H, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 2007;68:764–768. doi: 10.1212/01.wnl.0000256374.50227.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Bhatti BH, Taylor RE. Antidepressant-like effects of low alcohol doses in an animal model of depression. Alc Clin Exp Res. 2009a;33(Suppl):157A. [Google Scholar]
- Tizabi Y, Al-Namaeh M, Manaye KF, Taylor RE. Protective effects of nicotine on ethanol-induced toxicity in cultured cerebellar granule cells. Neurotox Res. 2003;5:315–321. doi: 10.1007/BF03033151. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience. 2012a;213:72–80. doi: 10.1016/j.neuroscience.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Getachew B, Rezvani AH, Hauser SR, Overstreet DH. Antidepressant-like effects of nicotine and reduced nicotinic receptor binding in the Fawn-Hooded rat, an animal model of co-morbid depression and alcoholism. Prog Neuropsychopharmacol Biol Psychiatry. 2009b;33:398–402. doi: 10.1016/j.pnpbp.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Hauser SR, Tyler KY, Getachew B, Madani R, Sharma Y, Manaye KF. Effects of nicotine on depressive-like behavior and hippocampal volume of female WKY rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:62–69. doi: 10.1016/j.pnpbp.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Manaye KF, Smoot DT, Taylor RE. Nicotine inhibits ethanol-induced toxicity in cultured cerebral cortical cells. Neurotox Res. 2004;6:311–316. doi: 10.1007/BF03033441. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Manaye KF, Taylor RE. Nicotine blocks ethanol-induced apoptosis in primary cultures of rat cerebral cortical and cerebellar granule cells. Neurotox Res. 2005;7:319–322. doi: 10.1007/BF03033888. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E, Jr, Janowsky DS, Kling MA. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl) 1999;142:193–199. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Qualls Z, Brown DO, Chin Y, Hurley LL, Taylor RE. Neuroprotective effects of low alcohol concentration against LPS-induced toxicity in cutured cells. Alc Clin Exp Res. 2012b;36(6 Suppl):21A. [Google Scholar]
- Tizabi Y, Rezvani1 AH, Russell LT, Tyler KY, Overstreet DH. Depressive characteristics of FSL rats: involvement of central nicotinic receptors. Pharmacol Biochem Behav. 2000;66:73–77. doi: 10.1016/s0091-3057(00)00236-7. [DOI] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Tredici G, Miloso M, Nicolini G, Galbiati S, Cavaletti G, Bertelli A. Resveratrol, map kinases and neuronal cells: might wine be a neuroprotectant? Drugs Exp Clin Res. 1999;25:99–103. [PubMed] [Google Scholar]
- Tsoh JY, Humfleet GL, Munoz RF, Reus VI, Hartz DT, Hall SM. Development of major depression after treatment for smoking cessation. Am J Psychiatry. 2000;157:368–374. doi: 10.1176/appi.ajp.157.3.368. [DOI] [PubMed] [Google Scholar]
- Tupala E, Tiihonen J. Dopamine and alcoholism: neurobiological basis of ethanol abuse. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1221–1247. doi: 10.1016/j.pnpbp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- Upadhyaya HP, Deas D, Brady KT, Kruesi M. Cigarette smoking and psychiatric comorbidity in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2002;41:1294–1305. doi: 10.1097/00004583-200211000-00010. [DOI] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Van der Schyf CJ. The use of multi-target drugs in the treatment of neurodegenerative diseases. Expert Rev Clin Pharmacol. 2011;4:293–298. doi: 10.1586/ecp.11.13. [DOI] [PubMed] [Google Scholar]
- Vas CJ, Pinto C, Panikker D, Noronha S, Deshpande N, Kulkarni L, Sachdeva S. Prevalence of dementia in an urban Indian population. Int Psychogeriatr. 2001;13:439–450. doi: 10.1017/s1041610201007852. [DOI] [PubMed] [Google Scholar]
- Venkatesan N, Punithavathi D, Arumugam V. Curcumin prevents adriamycin nephrotoxicity in rats. Br J Pharmacol. 2000;129:231–234. doi: 10.1038/sj.bjp.0703067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, Dreses-Werringloer U, Zhao H, Davies P, Marambaud P. Therapeutic potential of resveratrol in Alzheimer's disease. BMC Neurosci. 2008;9(2):S6. doi: 10.1186/1471-2202-9-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Corsini E, Galli CL, Marinovich M. Cytokines role in neurodegenerative events. Toxicol Lett. 2004;149:85–89. doi: 10.1016/j.toxlet.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lu Z, Wu H, Lv F. Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int J Food Microbiol. 2009;136:71–74. doi: 10.1016/j.ijfoodmicro.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Wang Z, Huang Y, Zou J, Cao K, Xu Y, Wu JM. Effects of red wine and wine polyphenol resveratrol on platelet aggregation in vivo and in vitro. Int J Mol Med. 2002;9:77–79. [PubMed] [Google Scholar]
- Wegelius K, Korpi ER. Ethanol inhibits NMDA-induced toxicity and trophism in cultured cerebellar granule cells. Acta Physiol Scand. 1995;154:25–34. doi: 10.1111/j.1748-1716.1995.tb09882.x. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Wirleitner B, Neurauter G, Schrocksnadel K, Frick B, Fuchs D. Interferon-gamma-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr Med Chem. 2003;10:1581–1591. doi: 10.2174/0929867033457179. [DOI] [PubMed] [Google Scholar]
- Wuwongse S, Chang RC, Law AC. The putative neurodegenerative links between depression and Alzheimer's disease. Prog Neurobiol. 2010;91:362–375. doi: 10.1016/j.pneurobio.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav. 2005a;82:200–206. doi: 10.1016/j.pbb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ. The effects of curcumin on depressive-like behaviors in mice. Eur J Pharmacol. 2005b;518:40–46. doi: 10.1016/j.ejphar.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Xu Y, Wang Z, You W, Zhang X, Li S, Barish PA, Vernon MM, Du X, Li G, Pan J, Ogle WO. Antidepressant-like effect of trans-resveratrol: Involvement of serotonin and noradrenaline system. Eur Neuropsychopharmacol. 2010;20:405–413. doi: 10.1016/j.euroneuro.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Yamane Y, Sakai K, Maeda K. Dementia with Lewy bodies is associated with higher scores on the Geriatric Depression Scale than is Alzheimer's disease. Psychogeriatrics. 2011;11:157–165. doi: 10.1111/j.1479-8301.2011.00368.x. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Schulz D, Aksoy A, Canbeyli R. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol Biochem Behav. 2002;71:341–344. doi: 10.1016/s0091-3057(01)00693-1. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Barak O, Avitsur R, Gallily R, Weidenfeld J. Intracerebral administration of Mycoplasma fermentans produces sickness behavior: role of prostaglandins. Brain Res. 1997;749:71–81. doi: 10.1016/s0006-8993(96)01295-4. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Miyazawa K, Naito M, Toyotake J, Tauchi T, Itoh M, Yuo A, Hayashi Y, Georgescu MM, Kondo Y, Kondo S, Ohyashiki K. Vitamin K2 induces autophagy and apoptosis simultaneously in leukemia cells. Autophagy. 2008;4:629–640. doi: 10.4161/auto.5941. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, Manji HK, Charney DS. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163:153–155. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- Zec RF, Burkett NR. Non-pharmacological and pharmacological treatment of the cognitive and behavioral symptoms of Alzheimer disease. NeuroRehabilitation. 2008;23:425–438. [PubMed] [Google Scholar]
- Zhang F, Wang H, Wu Q, Lu Y, Nie J, Xie X, Shi J. Resveratrol Protects Cortical Neurons against Microglia-mediated Neuroinflammation. Phytother Res. 2012 doi: 10.1002/ptr.4734. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Zou X, Patterson TA, Divine RL, Sadovova N, Zhang X, Hanig JP, Paule MG, Slikker W, Jr, Wang C. Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J Dev Neurosci. 2009;27:727–731. doi: 10.1016/j.ijdevneu.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Zunszain PA, Hepgul N, Pariante CM. Inflammation and Depression. Curr Top Behav Neurosci. 2012 May 3; doi: 10.1007/7854_2012_211. Epub ahead of print. [DOI] [PubMed] [Google Scholar]


