Abstract
Gamma interferon (IFN-γ) plays a significant role in the control of mycobacterial infections, including Mycobacterium avium subsp. paratuberculosis. However, the contribution of other immunoregulatory cytokines, such as interleukin-10 (IL-10) and transforming growth factor β (TGF-β), in Johne's disease has not been investigated as yet. In this study, we examined the effects of in vivo and in vitro infection with M. avium subsp. paratuberculosis on the production of IFN-γ, IL-10, and TGF-β by peripheral blood mononuclear cells (PBMC). We also examined the effects of exogenous IFN-γ, IL-10, and TGF-β on M. avium subsp. paratuberculosis survival in the cell cultures. PBMC obtained from naturally infected cows, regardless of their disease status, specifically upregulated IL-10 and TGF-β in culture supernatants in response to stimulation with live M. avium subsp. paratuberculosis. Nonstimulated PBMC recovered from subclinically infected animals secreted the lowest levels of TGF-β, but after stimulation with live M. avium subsp. paratuberculosis, TGF-β levels in the culture supernatants increased to levels similar to that produced by PBMC from healthy animals. The numbers of viable M. avium subsp. paratuberculosis recovered from cultures from naturally infected animals were higher than those from healthy cows after in vitro infection with M. avium subsp. paratuberculosis. The addition of exogenous IL-10 and TGF-β to PBMC isolated from healthy cows inhibited the bactericidal activity of these cells as evidenced by the increased number of viable M. avium subsp. paratuberculosis recovered from these cultures compared to cell cultures containing medium alone. These data suggest important immune regulatory roles for IL-10 and TGF-β during infection with M. avium subsp. paratuberculosis that may be directly related to their effects on macrophage activation and killing of M. avium subsp. paratuberculosis.
Johne's disease (paratuberculosis) typically progresses through three distinct stages (3). The division of these stages depends upon the existence of clinical signs and the correlation of these signs with the fecal shedding of Mycobacterium avium subsp. paratuberculosis. Accordingly, the first and second stages are considered subclinical stages where animals are asymptomatic. Animals may remain in these stages without developing clinical disease or might progress to the clinical stage after 3 to 4 years of infection. In the first subclinical stage, the animal is infected but shedding of M. avium subsp. paratuberculosis in the feces cannot be detected. This stage is followed by the second subclinical stage, the intermittent excretory phase. The third or clinical excretory stage is characterized by progressive symptoms associated with persistent shedding of high numbers of bacteria in the feces and severe diarrhea with concomitant weight loss. The stages of Johne's disease reflect an ongoing struggle within the host animal's immune system. Animals in the excretory subclinical stage are reported to have increased gamma interferon (IFN-γ) expression locally at the site of infection (24) and higher IFN-γ production in culture supernatants after stimulation of peripheral blood mononuclear cells (PBMC) with M. avium subsp. paratuberculosis antigens (23). As M. avium subsp. paratuberculosis-infected animals progress to a more clinical state, local and peripheral IFN-γ production declines (21, 23, 24).
After ingestion of M. avium subsp. paratuberculosis by the neonatal calf, the bacteria cross M cells lining the Peyer's patches and can be then engulfed by resident macrophages in the distal ileum where the bacteria can survive and replicate (15). It has been suggested that the host immune system responds to M. avium subsp. paratuberculosis infection by recruiting more macrophages and lymphocytes to the site and by activating immune cells, such as γδ T cells, CD4+ T cells, and cytolytic CD8+ T cells (5, 14). These cells interact directly with the infected macrophages through cell-to-cell contact or/and indirectly through production of cytokines, such as IFN-γ, transforming growth factor β (TGF-β), and interleukin-10 (IL-10), that cause macrophage activation and further activation of T cells (14). Therefore, it is important for macrophages to remain in contact with the proper immune cells and their products to overcome this type of infection.
It has been hypothesized that during the interaction between host immunity and M. avium subsp. paratuberculosis, a deviation from the proper immune response arises and disrupts the ability of the host to contain the disease. Although a mechanism has not been identified, the decrease in IFN-γ noted during the clinical stage signals a disruption in the effector phase of host immunity. Exogenous IFN-γ has been shown to stimulate monocytes to kill M. avium subsp. paratuberculosis (31). In other mycobacterial diseases, such as tuberculosis, a similar upregulation of IFN-γ production during the contained stage of the disease has been reported (8, 18). With regards to macrophage activation, the addition of recombinant human IFN-γ to monocyte cell cultures enhanced the ability of these cells to limit growth of Mycobacterium tuberculosis and Mycobacterium bovis (8, 18). Conversely, infection of IFN-γ knockout mice with a sublethal dose of M. bovis or M. tuberculosis resulted in increased mortality and increased bacterial numbers recovered from the spleen, liver, and lung (4, 7).
Recently, we found that the immunomodulatory cytokines TGF-β and IL-10 were upregulated in the tissues of cows with clinical Johne's disease (13). The presence of anti-inflammatory cytokines that modulate the effects of proinflammatory cytokines, such as IFN-γ, might be one mechanism that enhances the survival of mycobacteria within the host. In the present study, we investigated the effects of in vitro infection with M. avium subsp. paratuberculosis on the production of IFN-γ, IL-10, and TGF-β by PBMC isolated from healthy and naturally infected cows. We also investigated the effects of exogenous IFN-γ, IL-10, and TGF-β addition on the ability of monocyte-derived macrophages in unfractionated PBMC cultures to kill M. avium subsp. paratuberculosis. These studies indicate that IL-10 and TGF-β downregulate the bactericidal activity of monocyte-derived macrophages.
MATERIALS AND METHODS
Animals.
The animals used in this study were placed in three groups consisting of five noninfected healthy cows, four cows naturally infected with M. avium subsp. paratuberculosis but asymptomatic (i.e., subclinical), and five naturally infected cows with the clinical form of Johne's disease. Infection was monitored bacteriologically for the fecal shedding of M. avium subsp. paratuberculosis by standard culture methods (22). By definition, clinical animals were shedding more than 100 CFU per g of feces and presented with weight loss and intermittent diarrhea. Subclinically infected cows were shedding less than 10 CFU/g of feces. The noninfected control cows were characterized by repeated negative fecal cultures performed quarterly over a 3- to 5-year period. In addition, these animals were negative on any serologic assays (i.e., production of antibody specific for M. avium subsp. paratuberculosis and IFN-γ) performed during that period. All animals were housed in American Association for Accreditation of Laboratory Animal Care-accredited facilities, and all procedures performed on animals were approved by the Institution Animal Care and Use Committee (National Animal Disease Center [NADC], Ames, Iowa).
Blood collection, culture conditions, and sample collection.
Blood was collected from the jugular vein in 2× acid-citrate-dextrose (ACD) (1:10). PBMC were isolated from the buffy coat fractions of the peripheral blood as previously described (2). PBMC were resuspended in RPMI 1640 (Gibco, Grand Island, N.Y.) containing 10% fetal calf serum (Atlanta Biologics, Atlanta, Ga.), 100 U of penicillin G sodium (Gibco) per ml, 100 μg of streptomycin sulfate (Gibco) per ml, 0.25 μg of amphotericin B (Gibco) per ml, and 2 mM l-glutamine (Gibco).
Cells were cultured at 2 × 106/ml in 1-ml volumes in 24-well flat-bottomed plates (Nunc, Life Technologies) for 7 days at 39°C in 5% CO2 in a humidified atmosphere to allow monocytes in the unfractionated PBMC cultures to develop into macrophages. Additional control wells for each animal were set up to allow characterization of the unfractionated PBMC cultures (day 7) by flow cytometry. After nonadherent cells were removed from the plates, the wells were washed with cold 1× phosphate-buffered saline (PBS) to remove the adherent cells from the plates. The adherent cells were approximately 80 to 90% monocyte-derived macrophages by staining and were quantitated with a cell counter prior to the addition of live M. avium subsp. paratuberculosis. The plates were then centrifuged at 400 × g for 2 min, and the supernatants were removed without disturbing the cells in culture. Cells in duplicate wells were cultured with fresh medium containing 100 ng of human IL-10 (catalog no. 200-10; Peprotech, Rocky Hill, N.J.) per ml, 100 ng of bovine IFN-γ (generously donated by Novartis Animal Health, Basel, Switzerland) per ml, 10 ng of human TGF-β (catalog no. 100-21R; Peprotech) per ml, 100 ng of IL-10 per ml, and 10 ng of TGF-β per ml or cultured without cytokine stimulation. Cultured cells were incubated overnight (18 h) and then infected the next day with M. avium subsp. paratuberculosis strain 19698 (NADC) at a ratio of 10 bacteria per adherent PBMC (24). Replicate wells of in vitro-infected and noninfected cell cultures were also concurrently treated with 1 μg of lipopolysaccharide (LPS) (Escherichia coli O11:B4-W; Sigma, St. Louis, Mo.) per ml as a positive-control stimulator of macrophages in the study.
To assess cytokine production, cell culture supernatants were collected at 8 h, 3 days, or 6 days after in vitro infection and stored at −20°C prior to cytokine measurement. For cultures that had been infected with M. avium subsp. paratuberculosis, cells that were left in each well after the culture supernatant was collected were lysed and washed several times with distilled H2O, left overnight at room temperature (RT), and cultured on Herrold's egg yolk medium (HEYM).
Bacteria.
M. avium subsp. paratuberculosis strain 19698 (NADC) was grown in Middlebrook 7H9 broth (pH 6.0) supplemented with mycobactin J (2 mg/liter; Allied Monitor, Fayette, Mo.) and oleic acid-albumin-dextrose complex (Becton Dickinson Microbiology). The bacteria were harvested, washed three times with PBS (pH 7.4) (0.15 M), and resuspended in PBS to a final concentration of 109/ml as determined by the absorbance at 540 nm. Bacterial stocks were then frozen in PBS at −80°C until use in the experiments. Prior to in vitro infection, frozen bacterial stocks were thawed, and clumps were dispersed by brief sonication at 25 W for 40 s with a Tekmar sonic disturber (Lorton, Va). The viable cells in the frozen bacterial stocks were determined by culturing on HEYM. Viable cells in stocks were reduced after thawing and sonication to approximately 108 CFU/ml.
Assessment of cell phenotypes in the unfractionated PBMC prior to inoculation with live M. avium subsp. paratuberculosis.
To assess the effects of animal infection status on the cell phenotypes present in the unfractionated PBMC, flow cytometric analysis was performed on cells after 7 days in culture with no stimulation. Cells were stained with monoclonal antibodies (MAbs) for different cell surface molecules. A single-color flow cytometric analysis of T cells for the expression of CD4 (CACT138A), CD8α (CACT80A), or γδ TCR-N24 (GB21A) (VMRD, Pullman, Wash.) was performed. Briefly, 50 μl of anti-CD4, CD8, or γδ cell surface markers were incubated for 15 min at RT with 100 μl of 2 × 106 cells/ml at the working concentration of 7, 10, or 7 μg/ml, respectively. Cells were then washed once by centrifugation at 400 × g for 2 min. Bound markers were visualized by incubating cells for 15 min with 50 μl of phycoerythrin-conjugated goat anti-mouse immunoglobulin G1 (IgG1) (diluted 1:1,000) (Southern Biotechnology Associates, Inc., Birmingham, Ala.) for the mouse anti-bovine CD4 marker, anti-IgG2b (diluted 1:1,000) for the mouse anti-bovine γδ marker, and anti-IgM (diluted 1:2,000) for the mouse anti-bovine CD8 marker (Southern Biotechnology Associates, Inc.). Cells were then washed and incubated for 5 min with DAPI (4′,6-diamidino-2-phenylindole) (Sigma) to remove the dead cells by using a gate, washed, and resuspended in fluorescence-activated cell sorting buffer (PBS, 1% fetal calf serum, 0.1% sodium azide).
For analysis of B cells and cells bearing major histocompatibility complex class II (MHC-II) markers, a two-color flow cytometric analysis was performed. Briefly, 100-μl samples of cell cultures at 2 × 106 cells/ml were incubated for 15 min at RT with 50 μl of B-cell marker BAQ155A (7 μg/ml) (VMRD) and 50 μl of MHC-II marker CH34A (3.5 μg/ml) (VMRD). Cell surface markers were then visualized by incubating cells for 15 min with 50 μl of phycoerythrin-conjugated goat anti-mouse IgG2a (diluted 1:500) to detect bound anti-bovine MHC-II marker and 50 μl of fluorescein isothiocyanate- conjugated goat anti-mouse IgG1 (diluted 1:250) to detect the bound anti-bovine B-cell marker. Cells were then washed and resuspended in fluorescence-activated cell sorting buffer. To remove the dead cells from the flow analysis by using a gate, cells were incubated with DAPI (diluted 1:1,000) (10 mg/ml) (Sigma) before the final wash. Data from 5,000 events per sample were acquired (Cell Quest software; BD Biosciences, San Jose, Calif.) using flow cytometry (BD LSR system; BD Biosciences). For all analyses, only mononuclear cells that were DAPI negative were gated and analyzed for phenotypic marker expression by Flowjo software (Tree Star Inc, San Carlos, Calif.).
Assessment of M. avium subsp. paratuberculosis survival in the extended unfractionated PBMC cultures.
The number of viable M. avium subsp. paratuberculosis in each cell culture lysate and its corresponding cell culture supernatant from each treatment were cultured separately on HEYM. Growth was measured by counting the CFU after serial 10-fold dilutions on duplicate HEYM slants for each treated well. HEYM slants were incubated at 39°C for 12 weeks. CFU recovered from the cell culture lysates were added to the number of CFU recovered from the corresponding cell culture supernatants and represented the surviving M. avium subsp. paratuberculosis after each treatment. The number of CFU from the cell culture supernatants alone represented M. avium subsp. paratuberculosis that was either not phagocytized originally or was phagocytized and released by macrophages after cell death.
Measurement of IFN-γ, IL-10, and TGF-β production in cell culture supernatants by an enzyme-linked immunosorbent assay (ELISA).
Bovine IFN-γ was measured using the Bovigam test kit (CSL Veterinary Laboratories, Parkville, Victoria, Australia) as described by the manufacturer. To detect bovine IL-10, purified rat anti-human IL-10 capture MAb (JES3-19F1) (PharMingen, San Diego, Calif.) and biotinylated rat anti-human IL-10 detection MAb (JES3-12G8) (PharMingen) were used. Cross-reactivity of the human MAbs with bovine samples was evaluated by immunoblotting. Bovine test samples were prepared by stimulating bovine PBMC for 18 h with medium alone (nonstimulated) or concanavalin A (Sigma). Polyacrylamide gel electrophoresis was performed on the test samples and recombinant human IL-10 (rhIL-10) using 12% polyacrylamide gels. Electrophoretic transfer of proteins onto nitrocellulose filters (Schleicher & Schuell) was accomplished with the Bio-Rad Trans Blot Cell (Bio-Rad, Hercules, Calif.) in sodium phosphate buffer (25 mM) (pH 7.8) at 0.9 A for 90 min. After transfer, the filters were blocked with PBS plus 2% bovine serum albumin and 0.1% Tween 20. Commercial anti-human IL-10 MAbs (from Pharmingen or Peprotech) were diluted according to the manufacturer's recommendations (1:1,000) and incubated on the respective blots at RT for 2 h. After three washes in PBS plus 0.1% Tween 20, blots were incubated for 1.5 h with avidin-horseradish peroxidase (HRP). The blots were washed three times and developed for chemiluminescence using Supersignal detection reagents (Pierce Chemical Co., Rockford, Ill.). A band for the test samples and the rhIL-10 resolved at 20.7 kDa, indicative of positive reactivity with the anti-human IL-10 MAbs. Although a high-molecular-weight protein also resolved on the blots, it is presumed that this was protein from the FCS used to dilute the test samples and rhIL-10. This did not interfere with the ELISA, as a negative control (complete medium alone) was run during each immunoassay and low background absorbance was noted.
To perform the bovine IL-10 immunoassay, MaxiSorp microtiter plates (Nunc, Rochester, N.Y.) were coated with the capture MAb (100 μl per well at 2 μg/ml) overnight at 4°C. Plates were then blocked with 1% gelatin in PBS for 2 h at RT. Plates were then washed four times with PBS washing buffer containing 1% Tween 80. The diluted samples (1:1.5 in RPMI 1640) and serial twofold dilutions of rhIL-10 standard (starting at 20 ng/ml) were added in duplicate and incubated overnight at 4°C. Plates were then washed five times with washing buffer before incubating with the detection biotinylated anti-human IL-10 MAb (100 μl at 1 μg/ml) for 1 h at RT. Plates were washed five times with washing buffer, 100 μl of avidin-HRP conjugate (diluted 1:2,000) (PharMingen) was added to each well, and the plates were incubated for 30 min at RT. After another wash cycle, wells were incubated with substrate solution (40 mM ABTS [2,2′-azino-di-ethylbenzthiozoline-6-sulfonic acid] in citrate buffer [pH 4.0]), and color development was quantified after 10 min by measuring absorbance at 405 nm with a Wallac Victor 1420 multilabel counter ELISA plate reader (Perkin-Elmer, Gaithersburg, Md.).
An ELISA for measurement of TGF-β levels in culture supernatants was developed using anti-human TGF-β antibodies. The anti-human TGF-β antibodies were cross-reactive with bovine TGF-β due to the high level of conservation at the amino acid level between species reported. Previous studies have utilized anti-human TGF-β antibodies to detect bovine TGF-β activity (1, 10). TGF-β was detected by coating each well of MaxiSorp microtiter plates (Nunc) with 100 μl of anti-human TGF-β capture MAb (MAB240) (R&D Systems, Minneapolis, Minn.) at 2 μg/ml and then using biotinylated rat anti-human TGF-β MAb (BAF240) (R&D Systems) at 100 ng/ml as the detection MAb. The plates with the capture MAb were incubated overnight at RT, washed four times with PBS containing 0.5% Tween 20, and blocked with PBS containing 5% Tween 20, 5% sucrose, and 0.05% NaN3 (blocking buffer) for 2 h at RT. Diluted samples (1:6) or the rhTGF-β standards (5 to 0.078 ng/ml diluted in RPMI 1640) (Peprotech) were acidified to pH 2.0 with 1 N HCl for 10 min at RT to activate the latent TGF-β, and then samples were neutralized to pH 7.2 to 7.6 by 1.2 N NaOH-0.5 M HEPES. Activated samples were then added to plates coated with the TGF-β capture MAb within 5 min and incubated for 2 h at RT. Plates were then washed four times with washing buffer and incubated with TGF-β detection MAb for 2 h at RT. Plates were then washed four times and incubated with 100 μl of streptavidin-HRP (diluted 1:2,000) (PharMingen), and ABTS substrate solution was added to the plates as described above for the IL-10 ELISA. The concentration of cytokines for each of the assays was estimated from the standard curve generated using recombinant bovine IFN-γ (Novartis Animal Health), rhIL-10 (PharMingen), and rhTGF-β (Peprotech).
Statistical analysis.
Results were compared by using two-way analysis of variance, and significant differences among means were tested by Fisher protected least-significant-difference test using the Statview software package (Graphpad Software, Inc., San Diego, Calif.). For all tests and comparisons, only P values less than 0.05 were considered statistically significant.
RESULTS
Mononuclear cell profile in unfractionated PBMC culture.
After 7 days in culture, most of the adherent cells in culture assessed by light microscopy were stellate in shape, consistent with typical macrophage morphology. Flow cytometric analyses of the cellular phenotypes in the unfractionated PBMC after 7 days in culture for the three animal groups are presented in Table 1. Cows with subclinical M. avium subsp. paratuberculosis infection had more (P < 0.05) γδ T cells in their unfractionated cell cultures than healthy and clinically infected cows. MHC-II-positive B-cell-positive and MHC-II-negative B-cell-positive PBMC were higher (P < 0.05) in the clinically infected cows than in healthy or subclinically infected cows after 7 days in culture. Differences in CD4+ and CD8+ cell populations were not observed for animal groups in this time period.
TABLE 1.
Phenotypic analysis of unfractionated PBMC after 7 days in culturea
| Cow | % of cells (mean ± SEM) in cell typeb
|
|||||
|---|---|---|---|---|---|---|
| γδ T cells | CD4+ cells | CD8α+ cells | PBMC
|
|||
| MHC-II+ B cell+ | MHC-II+ B cell− | MHC-II− B cell+ | ||||
| Healthy (n = 4) | 20.09 ± 6.81 A | 28.23 ± 2.71 | 24.63 ± 0.84 | 14.38 ± 2.22 A | 17.88 ± 5.18 | 4.32 ± 2.12 A |
| Subclinically infected (n = 2) | 27.25 ± 0.25 A | 33.70 ± 9.70 | 19.65 ± 4.85 | 14.82 ± 4.98 A | 7.51 ± 1.05 | 5.05 ± 3.06 A |
| Clinically infected (n = 2) | 14.80 ± 1.00 B | 18.00 ± 6.30 | 10.72 ± 7.18 | 36.45 ± 1.95 B | 9.39 ± 4.91 | 15.30 ± 3.00 B |
Phenotypic analyses were performed 18 h after the replacement of the supernatant in culture with fresh medium and before the addition of M. avium subsp. paratuberculosis to the in vitro cultures.
The different letters after the values indicate statistically significant differences (P < 0.05) (values with the same letter were not significantly different).
Effects of infection status of cows and in vitro infection of PBMC cultures on IFN-γ, IL-10, and TGF-β production by PBMC.
Effects of animal infection status (i.e., not infected, subclinical, or clinical) and in vitro infection with M. avium subsp. paratuberculosis on the secretion of IFN-γ, IL-10, or TGF-β by PBMC is presented in Tables 2, 3, and 4, respectively. Infected animals (subclinical or clinical) had significantly higher (P < 0.05) IFN-γ production in noninfected culture supernatants than healthy noninfected controls (Table 2). In noninfected cell cultures isolated from clinically infected cows, IFN-γ production significantly (P < 0.05) decreased with time. In contrast, after in vitro infection with M. avium subsp. paratuberculosis, IFN-γ production remained fairly constant at all time points in PBMC cultures of subclinically and clinically infected cows. In PBMC cultures from healthy animals, the in vitro infection resulted in a continuous accumulation (P < 0.05) of IFN-γ production between 8 h and 6 days.
TABLE 2.
Effects of in vitro infection with live M. avium subsp. paratuberculosis on IFN-γ production in cell culture supernatants from healthy cows and cows with subclinical or clinical Johne's disease
| Disease status of cow (no. of cows) | M. paratuberculosis infection | Concn of IFN-γ produced (ng/ml) (mean ± SEM)a
|
|||
|---|---|---|---|---|---|
| 8 h | 3 days | 6 days | Avgb | ||
| Healthy (5) | No | 0.40 ± 0.17 *† | 0.06 ± 0.04 * | 0.89 ± 0.49 † | 0.45 ± 0.19 A |
| Yes | 0.92 ± 0.38 * | 4.85 ± 2.00 * | 8.73 ± 2.74 † | 4.83 ± 1.40 C | |
| Subclinical disease (4) | No | 3.20 ± 1.03 * | 1.44 ± 0.87 * | 2.36 ± 1.66 * | 2.33 ± 0.68 BD |
| Yes | 2.96 ± 0.26 * | 3.36 ± 0.88 * | 4.05 ± 2.00 * | 3.45 ± 0.68 BC | |
| Clinical disease (5) | No | 2.91 ± 0.32 * | 1.16 ± 0.34 † | 0.72 ± 0.45 † | 1.60 ± 0.32 D |
| Yes | 5.26 ± 0.73 * | 6.79 ± 1.49 * | 4.84 ± 1.73 * | 5.63 ± 0.77 C | |
PBMC culture medium was replaced with complete medium on day 6. On day 7 (18 h later), cells in culture were stimulated with live M. avium subsp. paratuberculosis at a bacterium-to-adherent cell ratio of 10:1. Culture supernatants were collected at 8 h, 3 days, and 6 days after in vitro infection and analyzed by an ELISA. The different symbols after the values indicate statistically significant differences over time (P < 0.05) (values with the same symbol were not significantly different).
Values in the Avg column are the mean concentration of IFN-γ produced for all time points in the medium control supernatants with or without in vitro infection with M. avium subsp. paratuberculosis by cells from healthy, subclinically infected, and clinically infected cows. The different letters after the values indicate statistically significant differences between the values for the treatment groups (P < 0.05) (values with the same letter were not significantly different).
TABLE 3.
Effects of in vitro infection with live M. avium subsp. paratuberculosis on IL-10 production in cell culture supernatants from healthy cows and cows with subclinical or clinical Johne's disease
| Disease status of cow (no. of cows) | M. avium subsp. paratuberculosis infection | Concn of IL-10 produced (ng/ml) (mean ± SEM)a
|
|||
|---|---|---|---|---|---|
| 8 h | 3 days | 6 days | Avgb | ||
| Healthy (5) | No | 4.72 ± 0.76 * | 3.24 ± 0.78 *† | 2.28 ± 0.22 † | 3.41 ± 0.45 A |
| Yes | 6.38 ± 1.5 * | 5.00 ± 1.63 * | 3.79 ± 0.61 * | 5.06 ± 0.79 AC | |
| Subclinical disease (4) | No | 4.59 ± 1.09 * | 5.37 ± 0.92 * | 3.30 ± 0.79 * | 4.42 ± 0.55 A |
| Yes | 13.76 ± 4.31 * | 5.54 ± 0.50 * | 8.18 ± 2.51 * | 9.16 ± 1.83 B | |
| Clinical disease (5) | No | 7.44 ± 2.067 * | 4.33 ± 0.75 *† | 2.51 ± 0.44 † | 4.76 ± 0.88 A |
| Yes | 10.51 ± 2.01 * | 10.33 ± 3.14 * | 4.6 ± 1.23 * | 8.48 ± 1.42 BC | |
PBMC culture medium was replaced with complete medium on day 6. On day 7 (18 h later), cells in culture were stimulated with live M. avium subsp. paratuberculosis at a bacteriaum-to-adherent cell ratio of 10:1. Culture supernatants were collected at 8 h, 3 days, and 6 days after in vitro infection and analyzed by an ELISA. The different symbols after the values indicate statistically significant differences over time (P < 0.05) (values with the same symbol were not significantly different).
Values in the Avg column are the mean concentration of IL-10 produced for all time points in the medium control supernatants with or without in vitro infection with M. avium subsp. paratuberculosis by cells from healthy, subclinically infected, and clinically infected cows. The different letters after the values indicate statistically significant differences between the values for the treatment groups (P < 0.05) (values with the same letter were not significantly different).
TABLE 4.
Effects of in vitro infection with M. avium subsp. paratuberculosis on TGF-β production in cell culture supernatants from healthy cows and cows with subclinical or clinical Johne's disease
| Disease status of cow (no. of cows) | M. avium subsp. paratuberculosis infection | Concn of TGF-β produced (ng/ml) (mean ± SEM)a
|
|||
|---|---|---|---|---|---|
| 8 h | 3 days | 6 days | Avgb | ||
| Healthy (5) | No | 7.18 ± 1.124 * | 9.97 ± 2.2 *† | 14.51 ± 3.10 † | 11.42 ± 1.74 A |
| Yes | 11.82 ± 2.12 * | 13.79 ± 3.02 * | 15.41 ± 2.51 * | 13.68 ± 1.48 AC | |
| Subclinical disease (4) | No | 6.77 ± 0.58 * | 7.27 ± 1.64 * | 7.64 ± 1.40 * | 7.22 ± 0.68 B |
| Yes | 10.39 ± 0.53 * | 13.78 ± 1.24 † | 12.3 ± 1.22 *† | 12.15 ± 0.69 A | |
| Clinical disease (5) | No | 8.90 ± 1.16 * | 11.62 ± 1.63 * | 12.86 ± 1.66 * | 11.13 ± 0.92 A |
| Yes | 14.57 ± 3.01 * | 16.09 ± 2.54 * | 21.41 ± 1.63 * | 17.35 ± 1.53 C | |
PBMC culture medium was replaced with complete medium on day 6. On day 7 (18 h later), cells in culture were stimulated with live M. avium subsp. paratuberculosis at a bacteriaum-to-adherent cell ratio of 10:1. Culture supernatants were collected at 8 h, 3 days, and 6 days after in vitro infection and analyzed by an ELISA. The different symbols after the values indicate statistically significant differences over time (P < 0.05) (values with the same symbol were not significantly different).
Values in the Avg column are the mean concentration of TGF-β produced for all time points in the medium control supernatants with or without in vitro infection with M. avium subsp. paratuberculosis by cells from healthy, subclinically infected, and clinically infected cows. The different letters after the values indicate statistically significant differences between the values for the treatment groups (P < 0.05) (values with the same letter were not significantly different).
In vitro production of IL-10 by PBMC from healthy and clinically infected animals decreased (P < 0.05) with time (8 h versus 6 days), while it remained fairly constant in cell cultures of the subclinically infected animals at all time points tested in this experiment (Table 3). After in vitro infection with live M. avium subsp. paratuberculosis, there was an increase in IL-10 production in the cultures containing cells recovered from naturally infected animals for most time points, resulting in significant upregulation after the exposure of cells from sensitized animals to the bacterium (Table 3). In contrast, only mild upregulation of IL-10 secretion was noted in cultures containing cells isolated from the healthy animals after in vitro challenge with M. avium subsp. paratuberculosis (Table 3).
The level of TGF-β production increased (P < 0.05) with time (8 h versus 6 days) in cell cultures obtained from healthy cows not infected with M. avium subsp. paratuberculosis (Table 4). Compared to healthy and clinically infected animals at day 6, TGF-β levels were significantly (P < 0.05) lower in noninfected cell culture supernatants from cows in the subclinical stage of the disease. After in vitro infection with M. avium subsp. paratuberculosis, there was a resultant upregulation (P < 0.05) of TGF-β production for both subclinically and clinically infected cows (Table 4), with the levels of TGF-β produced by cells from subclinically infected cows becoming similar to the levels produced by cells from healthy cows in noninfected cultures.
Effects of LPS stimulation on TGF-β, IL-10, and IFN-γ production by PBMC.
Cells in the unfractionated PBMC cultures were able to produce IFN-γ when stimulated with LPS (Fig. 1). The addition of LPS resulted in a significant (P < 0.05) boost in IFN-γ production in cell cultures from all animal groups (Fig. 1) with the most statistically significant results noted at 3 and 6 days of culture. While the addition of M. avium subsp. paratuberculosis to cell cultures caused an upregulation of IL-10 and TGF-β in the naturally infected animals, the addition of LPS as a positive stimulator of monocyte-derived macrophages in the unfractionated PBMC cultures did not affect IL-10 or TGF-β production (data not shown). The effects of costimulation with LPS and live M. avium subsp. paratuberculosis on cytokine production were not additive (data not shown).
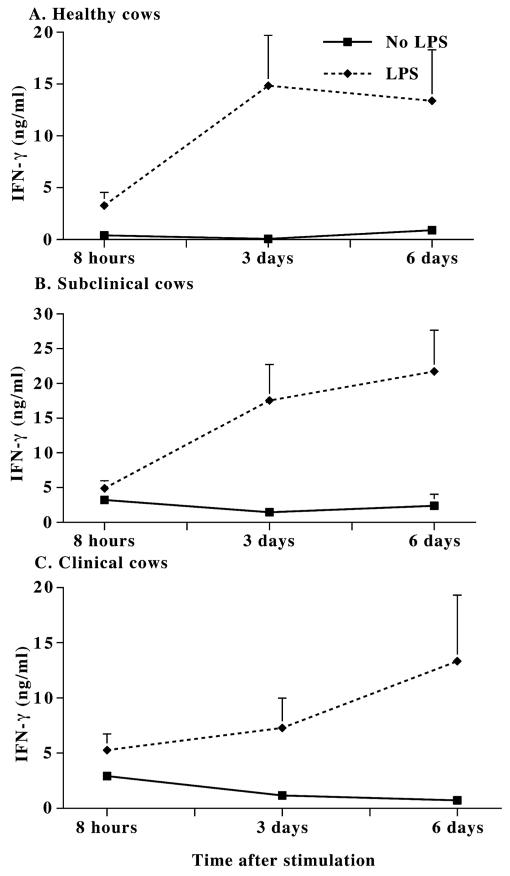
FIG. 1.
IFN-γ production in the unfractionated PBMC cultures of noninfected healthy cows (A) and cows with subclinical (B) and clinical (C) Johne's disease after LPS (E. coli O11:B4) stimulation. PBMC culture medium was replaced with complete medium at day 6. After 18 h (day 7), cells in culture were stimulated with LPS (1 μg/ml). Culture supernatants were collected at 8 h, 3 days, and 6 days after LPS stimulation and analyzed by an ELISA. The IFN-γ levels produced in the supernatants with or without LPS stimulation (means ± standard errors of the means [error bars]) are shown.
Effects of exogenous cytokines on M. avium subsp. paratuberculosis survival.
Regardless of the state of infection (i.e., subclinical versus clinical), PBMC from naturally infected animals had decreased (P < 0.05) capacity to kill M. avium subsp. paratuberculosis 6 days after in vitro infection (Fig. 2). There was no significant difference between the number of CFU recovered from subclinically and clinically infected animals (data not shown). Pretreating cultured PBMC isolated from healthy animals with exogenous IL-10 or TGF-β or a combination of both for 18 h before the PBMC were infected with M. avium subsp. paratuberculosis resulted in higher (P < 0.05) numbers of CFU recovered than medium control cultures (no added cytokines) by day 6 postinfection (Fig. 3). Pretreatment with IFN-γ had no significant effect on the number of viable M. avium subsp. paratuberculosis recovered from cell culture (Fig. 3), although the number of bacteria recovered tended to decrease compared to medium controls (no exogenous cytokine).
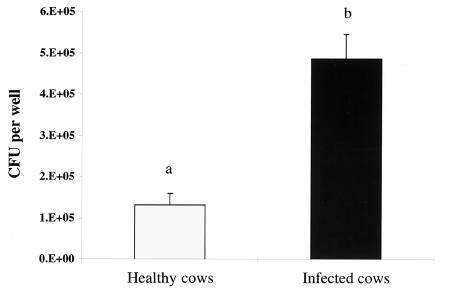
FIG. 2.
Bactericidal activity of the unfractionated PBMC cultures recovered from healthy cows (n = 5) or cows naturally infected with M. avium subsp. paratuberculosis (n = 9). PBMC culture medium was replaced with complete medium on day 6. After 18 h, cells in culture were infected with live M. avium subsp. paratuberculosis at a bacterium-to-adherent cell ratio of 10:1. Data shown are the numbers of viable bacteria represented by the mean numbers of CFU ± standard errors of the means (error bars) recovered from cell culture lysates and supernatants 6 days after in the vitro infection with M. avium subsp. paratuberculosis. The values for the two animal groups were statistically significantly different (P < 0.05) as indicated by the different letters over the bars. 1.E+05, 1 × 105.
FIG. 3.
Effects of exogenous cytokines on the bactericidal activity of unfractionated PBMC cultures from healthy noninfected cows (n = 5). After 6 days, PBMC culture supernatants were replaced with fresh medium containing recombinant IFN-γ (100 ng/ml), recombinant IL-10 (rIL-10) (100 ng/ml), rTGF-β (10 ng/ml), and rIL-10 (100 ng/ml) plus rTGF-β (10 ng/ml). The medium control was not stimulated with a cytokine. Eighteen hours later, cultures were infected with live M. avium subsp. paratuberculosis (10:1 bacterium-to-adherent cell ratio). Data shown are the numbers of viable bacteria (mean numbers of CFU ± standard errors of the means [error bars]) recovered from cell culture lysates and supernatants 6 days after in vitro infection with M. avium subsp. paratuberculosis. The values for cytokine treatment groups were statistically significantly different (P < 0.05) (values with the same letter were not significantly different). 1.E+05, 1 × 105.
Interaction of exogenous cytokines and infection on IFN-γ, IL-10, and TGF-β production.
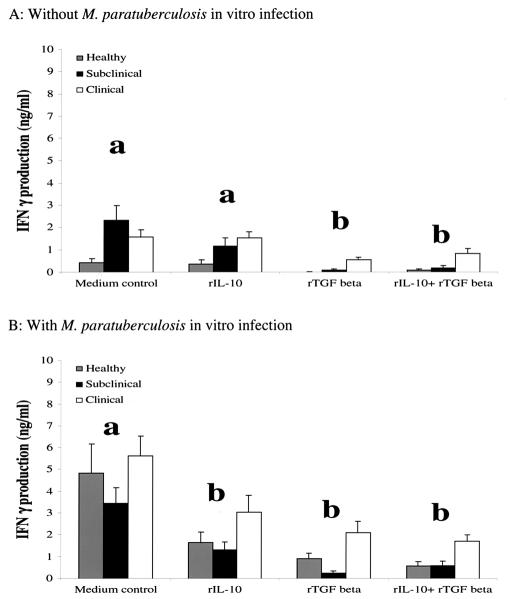
Pretreatment of PBMC with exogenous TGF-β significantly increased (P < 0.05) endogenous IL-10 production in all groups (Fig. 4). This effect was noted regardless of the incubation period, LPS stimulation, or in vitro infection with live M. avium subsp. paratuberculosis, so data points within each animal group were combined. Addition of exogenous IL-10 and TGF-β to PBMC cultures had significant (P < 0.05) downregulatory effects on IFN-γ production (Fig. 5). Production of IFN-γ was significantly (P < 0.05) reduced by the addition of TGF-β alone or in combination with IL-10 to PBMC regardless of animal group (Fig. 5A). In vitro infection with M. avium subsp. paratuberculosis resulted in an overall increase in IFN-γ production compared to noninfected PBMC cultures regardless of cytokine treatment or animal group (Fig. 5B). Similar to noninfected cell cultures, the addition of anti-inflammatory cytokines IL-10 and TGF-β to infected cell cultures resulted in a significant (P < 0.05) inhibition of IFN-γ production. The inhibitory effects of these anti-inflammatory cytokines on IFN-γ production was most noticeable (P < 0.05) in cell cultures obtained from subclinically infected animals, whereas cells isolated from clinically infected cows were the least (P < 0.05) affected (Fig. 5).
FIG. 4.
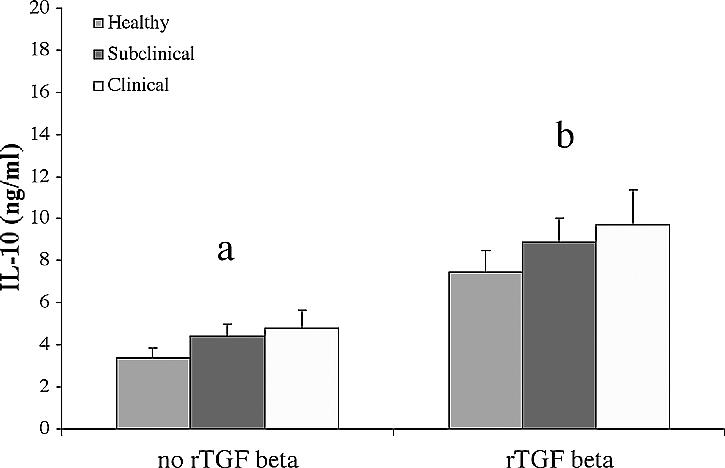
Overall effect of exogenous recombinant TGF-β (rTGF-β) on endogenous IL-10 production in cell culture supernatants. PBMC culture medium was replaced on day 6 with complete medium containing exogenous rTGF-β (10 ng/ml) or not stimulated with rTGF-β (medium control). Culture supernatants were collected 24 h, 4 days, or 7 days after cytokine stimulation and analyzed by an ELISA. All values were grouped together regardless of time, LPS stimulation, or M. avium subsp. paratuberculosis infection and are presented as the mean concentrations ± standard errors of the means (error bars). The values for the cells not stimulated or stimulated with rTGF-β were statistically significantly different (P < 0.05) as indicated by the different letters over the two groups of bars.
FIG. 5.
Inhibitory effects of exogenous anti-inflammatory cytokines (recombinant IL-10 [rIL-10] and rTGF-β) on IFN-γ production. PBMC culture medium was replaced on day 6 with fresh complete medium containing rIL-10 (100 ng/ml), rTGF-β (10 ng/ml), or rIL-10 (100 ng/ml) plus rTGF-β (10 ng/ml). The medium control was not stimulated with a cytokine. Cells in culture were either not infected (A) or infected with M. avium subsp. paratuberculosis on day 7 (B). Culture supernatants were collected 8 h, 3 days, or 6 days after in vitro infection and analyzed by an ELISA. All values were grouped together regardless of time and are presented as the levels of IFN-γ (mean concentrations ± standard errors of the means [error bars]) in the supernatants of healthy (n = 5), subclinically infected (n = 4), or clinically infected cows (n = 5). The values for cytokine treatment groups were statistically significantly different (P < 0.05) (values with the same letter were not significantly different).
DISCUSSION
Recently we found that IFN-γ expression in intestinal tissues was higher in cows with subclinical M. avium subsp. paratuberculosis infection than in clinically infected cows (13). Conversely, IL-10 and TGF-β gene expression was upregulated in cows with clinical disease. Previous studies have reported significant effects of exogenous IFN-γ on the ability of monocytes, monocyte-derived macrophages, or peritoneal macrophages to kill mycobacteria, including M. avium subsp. paratuberculosis (9, 20, 31). Through its capacity to activate macrophages and initiate induction of other cytokines, IFN-γ appears to play a significant role in controlling mycobacterial infections (18). It has been demonstrated that IL-10 and TGF-β actively interfere with IFN-γ production (19). Therefore, the primary goal of this study was to evaluate the effects of in vivo infection status of cows on the patterns of IFN-γ, IL-10, and TGF-β secretion at various time points of culture and correlating it with the ability of the cells to kill M. avium subsp. paratuberculosis. In addition, a critical component of this study was to assess inhibitory or stimulatory properties of these cytokines upon each another by adding exogenous IFN-γ, IL-10, and TGF-β to cell cultures.
Compared to noninfected cultures, in vitro challenge of cell cultures with live M. avium subsp. paratuberculosis resulted in enhanced production of IFN-γ in cell cultures from all animal groups, but effects were more marked for clinically infected and healthy animals than the subclinically infected cows. These data differ from what was previously reported by our laboratory where the amounts of IFN-γ secreted after stimulation with either an M. avium subsp. paratuberculosis whole-cell sonicate or live M. avium subsp. paratuberculosis was higher in PBMC cultures from subclinically infected cows than in cultures from healthy or clinically infected cows (21, 23). However, these studies utilized a short-term culture system (18 h) that was intended as a diagnostic tool for detection of infected cows rather than the extended cell culture system (7 to 12 days) utilized in the current study. It is apparent from the time course analysis performed in this study that cells from subclinically infected cows had a higher innate production of IFN-γ than cells isolated from the other two animal groups (2.33, 0.45, and 1.60 ng/ml for subclinically infected, healthy, and clinically infected cows, respectively). Data from our laboratory indicate that IFN-γ gene expression in short-term PBMC cultures (18 h) is higher for subclinically infected cows than for healthy control and clinically infected cows (data not shown). These data suggest that cells from subclinically infected cows may have been less able to respond to the stimulus of in vitro infection with live M. avium subsp. paratuberculosis than cells from the other two groups of cows.
The disparity in IFN-γ production between the two culture systems may indicate that after the development of monocytes into macrophages, antigen presentation and cytokine production from cultured macrophages and lymphocytes favor a Th1 response as represented by increased IFN-γ production, depending upon the disease state of the animal. The changes in IFN-γ secretion noted in the present study may imply that the control mechanism(s) for IFN-γ production is different in the three groups. This became most apparent after the addition of TGF-β or TGF-β plus IL-10 to cell cultures resulted in almost complete inhibition of IFN-γ secretion for healthy and subclinically infected cows yet only moderate reduction in IFN-γ was noted for clinically infected cows. Although infection of unfractionated PBMC with live M. avium subsp. paratuberculosis upregulated IFN-γ secretion in all animal groups, particularly healthy controls, the inhibitory effects of TGF-β and IL-10 were most predominant in the cell cultures from control and subclinically infected cows. It is possible that since IL-10 and TGF-β are already upregulated in clinically infected cows that cells in culture were somewhat refractory to the inhibitory effects observed in the other two groups. The higher percentages of γδ T cells noted in cultures from control and subclinically infected cows and the higher percentage of B cells noted in cultures from clinically infected cows might also explain some of the disparate results in IFN-γ production noted between animal groups in the present study.
It is well-documented that γδ T cells produce IFN-γ after exposure or sensitization to mycobacteria. Experimental depletion of γδ T cells from calves experimentally infected with M. bovis resulted in a decrease in early IFN-γ responses with a concomitant increase in IL-4 production (12). In addition, B cells are extensive producers of IL-10 and the higher number of B cells noted in cell cultures from clinically infected cows may have influenced the balance of cytokines by skewing it towards more IL-10 and less IFN-γ production. We had previously noted that clinically infected cows had higher numbers of B cells in PBMC, but the cells were refractory to antigen stimulation (28). Further studies are planned to investigate the roles of these cell populations in the pathogenesis of M. avium subsp. paratuberculosis infection and whether these cells are the source of inhibitory cytokines IL-10 and TGF-β.
Extended culture of unfractionated PBMC specifically upregulated IL-10 and TGF-β production after stimulation with live M. avium subsp. paratuberculosis but not in response to stimulation with LPS. Interestingly, the ability of M. avium subsp. paratuberculosis to upregulate IL-10 and TGF-β was noted in animals naturally infected with M. avium subsp. paratuberculosis regardless of the disease state but not from cells recovered from healthy animals. Therefore, in the absence of IL-10 and TGF-β upregulation, cells from all groups regardless of their infection status were capable of producing IFN-γ (e.g., LPS stimulation). This was also demonstrated after the addition of exogenous cytokines to the unfractionated PBMC culture. The interactions of TGF-β, IL-10, and IFN-γ confirmed that the anti-inflammatory cytokines (i.e., TGF-β and IL-10) downregulate IFN-γ production in cell cultures.
This is the first study to report significant effects of M. avium subsp. paratuberculosis infection on the production of both regulatory cytokines (IL-10 and TGF-β). One study recently reported higher IL-10 gene expression in PBMC isolated from cows with clinical paratuberculosis than in cells from healthy animals (6). In addition, upregulation of IL-10 gene expression was also noted in monocyte-derived macrophages obtained from healthy animals after stimulation with live M. avium subsp. paratuberculosis (29). Results noted in the present study are similar to those documented for other mycobacterial diseases, such as tuberculosis (11, 27). The cells of tuberculosis patients with advanced disease (disseminated infection) produced higher levels of TGF-β and IL-10 than cells from patients in the early stages of infection (contained infection) (8, 19, 27). In addition, M. tuberculosis infection of cell cultures from patients with tuberculosis further increased production of TGF-β and IL-10 (19). We also determined that the addition of exogenous TGF-β to unfractionated PBMC cultures potentiated IL-10 production. Similarly, TGF-β has been shown to upregulate IL-10 production in M. tuberculosis-infected cell cultures (19). When TGF-β and IL-10 were added together, they were found to synergistically downregulate IFN-γ production by T cells stimulated with M. tuberculosis purified protein derivative (19).
The decreased bactericidal activity of PBMC recovered from naturally infected animals correlated with the increased production of TGF-β and IL-10 in these cell cultures. In support of this observation, it was determined that the addition of these cytokines to cell cultures of healthy animals also increased the number of surviving M. avium subsp. paratuberculosis recovered compared to control cell cultures (no exogenous cytokines). These results are in agreement with previous reports evaluating the effects of TGF-β and IL-10 on the survival of M. tuberculosis (16, 26). Hirsch et al. (11) showed that incubation of monocyte cultures from healthy individuals with 10 ng of recombinant TGF-β per ml prior to infection with M. tuberculosis resulted in a threefold increase in the number of CFU recovered from cell culture lysates. A singular effect of IL-10 on mycobacterial survival was also demonstrated in a study in which intravenous inoculation of IL-10 transgenic mice with M. tuberculosis bacillus Calmette-Guérin (BCG) resulted in dissemination of the bacteria throughout the body for the course of the study (12 weeks) (17).
The addition of exogenous IFN-γ (100 ng) to monocyte-derived macrophages in the unfractionated PBMC culture did not decrease the number of viable bacteria recovered from cell culture after in vitro infection. This information is in contrast to data previously reported in which monocyte cultures activated with 1 μg of IFN-γ prior to infection resulted in decreased M. avium subsp. paratuberculosis viability (31). Therefore, it is possible that under our culture conditions, stimulation of the monocyte-derived macrophages in the presence of T cells in the unfractionated cell culture left macrophages refractory to further IFN-γ stimulation. A mechanism for this has not been elucidated, but a recent study indicated that M. tuberculosis can block the effects of IFN-γ by interfering with the IFN-γ signaling pathway (25). One important step in IFN-γ signaling is the translocation of STAT1 homodimers into the nucleus. This translocated dimer interacts with the basal transcriptional apparatus in the nucleus through the CBP/p300 family of transcriptional coactivators that are essential for effective IFN-γ signaling (30). The presence of M. tuberculosis disrupted this interaction and caused further disruption in the ability of IFN-γ-treated macrophages to kill other intracellular organisms, such as Toxoplasma gondii. Disruption of IFN-γ signaling may explain why elevated IFN-γ production in M. tuberculosis infection does not clear the infection. Perhaps the inhibition of macrophage function due to reduced IFN-γ is one mechanism by which mycobacteria avoid destruction.
In summary, IL-10 and TGF-β had inhibitory roles on the destruction of intracellular M. avium subsp. paratuberculosis, potentially through their effects on IFN-γ production. Therefore, upregulation of IL-10 and TGF-β even in the presence of high IFN-γ production (e.g., cells from naturally infected cows) likely resulted in less-effective killing of the M. avium subsp. paratuberculosis in these animals compared to healthy animals. Data from this study support a model in which the progression of Johne's disease from a subclinical to clinical stage is the result of an imbalance of cytokine production favoring TGF-β and IL-10 production over IFN-γ production. This imbalance might be due to the difference in the type of specific cells activated at each disease stage.
Acknowledgments
We thank Trudy Bosworth for technical assistance and Donnie Robinson and Tim Gogerty for excellent animal care.
Editor: F. C. Fang
REFERENCES
- 1.Borrelli, V., A. V. Sterpetti, P. Coluccia, B. Randone, A. Cavallaro, L. S. D'Angelo, and A. Cucina. 2001. Bimodal concentration-dependent effect of thrombin on endothelial cell proliferation and growth factor release in culture. J. Surg. Res. 100:154-160. [DOI] [PubMed] [Google Scholar]
- 2.Burton, J. L., and M. E. Kehrli, Jr. 1996. Effects of dexamethasone on bovine circulating T lymphocyte populations. J. Leukoc. Biol. 59:90-99. [DOI] [PubMed] [Google Scholar]
- 3.Cocito, C., P. Gilot, M. Coene, M. de Kesel, P. Poupart, and P. Vannuffel. 1994. Paratuberculosis. Clin. Microbiol. Rev. 7:328-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coussens, P. M. 2001. Mycobacterium paratuberculosis and the bovine immune system. Anim. Health Res. Rev. 2:141-161. [PubMed] [Google Scholar]
- 6.Coussens, P. M., C. J. Colvin, K. Wiersma, A. Abouzied, and S. Sipkovsky. 2002. Gene expression profiling of peripheral blood mononuclear cells from cattle infected with Mycobacterium paratuberculosis. Infect. Immun. 70:5494-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 8.Dlugovitzky, D., A. Torres-Morales, L. Rateni, M. A. Farroni, C. Largacha, O. Molteni, and O. Bottasso. 1997. Circulating profile of Th1 and Th2 cytokines in tuberculosis patients with different degrees of pulmonary involvement. FEMS Immunol. Med. Microbiol. 18:203-207. [DOI] [PubMed] [Google Scholar]
- 9.Flesch, I. E., J. H. Hess, I. P. Oswald, and S. H. Kaufmann. 1994. Growth inhibition of Mycobacterium bovis by IFN-gamma stimulated macrophages: regulation by endogenous tumor necrosis factor-alpha and by IL-10. Int. Immunol. 6:693-700. [DOI] [PubMed] [Google Scholar]
- 10.Ginjala, V., and R. Pakkanen. 1998. Determination of transforming growth factor-β1 (TGF-β1) and insulin-like growth factor (IGF-1) in bovine colostrum samples. J. Immunoassay 19:195-207. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch, C. S., T. Yoneda, L. Averill, J. J. Ellner, and Z. Toossi. 1994. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-β1. J. Infect. Dis. 170:1229-1237. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy, H. E., M. D. Welsh, D. G. Bryson, J. P. Cassidy, F. I. Forster, C. J. Howard, R. A. Collins, and J. M. Pollock. 2002. Modulation of immune responses to Mycobacterium bovis in cattle depleted of WC1+ γδ T cells. Infect. Immun. 70:1488-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalifeh, M. S., and J. R. Stabel. Upregulation of transforming growth factor-beta and interleukin-10 in cows with clinical Johne's disease. Vet. Immunol. Immunopathol., in press. [DOI] [PubMed]
- 14.Lee, H., J. R. Stabel, and M. E. Kehrli, Jr. 2001. Cytokine gene expression in ileal tissues of cattle infected with Mycobacterium paratuberculosis. Vet. Immunol. Immunopathol. 82:73-85. [DOI] [PubMed] [Google Scholar]
- 15.Momotani, E., D. L. Whipple, A. B. Thiermann, and N. F. Cheville. 1988. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet. Pathol. 25:131-137. [DOI] [PubMed] [Google Scholar]
- 16.Murray, P. J., L. Wang, C. Onufryk, R. I. Tepper, and R. A. Young. 1997. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J. Immunol. 158:315-321. [PubMed] [Google Scholar]
- 17.Murray, P. J., and R. A. Young. 1999. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect. Immun. 67:3087-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orme, I. M. 1993. Immunity to mycobacteria. Curr. Opin. Immunol. 5:497-502. [DOI] [PubMed] [Google Scholar]
- 19.Othieno, C., C. S. Hirsch, B. D. Hamilton, K. Wilkinson, J. J. Ellner, and Z. Toossi. 1999. Interaction of Mycobacterium tuberculosis-induced transforming growth factor β1 and interleukin-10. Infect. Immun. 67:5730-5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rook, G. A., J. Steele, M. Ainsworth, and B. R. Champion. 1986. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology 59:333-338. [PMC free article] [PubMed] [Google Scholar]
- 21.Stabel, J. R. 1996. Production of gamma-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. J. Vet. Diagn. Investig. 8:345-350. [DOI] [PubMed] [Google Scholar]
- 22.Stabel, J. R. 1997. An improved method for cultivation of Mycobacterium paratuberculosis from bovine fecal samples and comparison to three other methods. J. Vet. Diagn. Investig. 9:375-380. [DOI] [PubMed] [Google Scholar]
- 23.Stabel, J. R. 2000. Cytokine secretion by peripheral blood mononuclear cells from cows infected with Mycobacterium paratuberculosis. Am. J. Vet. Res. 61:754-760. [DOI] [PubMed] [Google Scholar]
- 24.Sweeney, R. W., D. E. Jones, P. Habecker, and P. Scott. 1998. Interferon-gamma and interleukin 4 gene expression in cows infected with Mycobacterium paratuberculosis. Am. J. Vet. Res. 59:842-847. [PubMed] [Google Scholar]
- 25.Ting, L. M., A. C. Kim, A. Cattamanchi, and J. D. Ernst. 1999. Mycobacterium tuberculosis inhibits IFN-γ transcriptional responses without inhibiting activation of STAT1. J. Immunol. 163:3898-3906. [PubMed] [Google Scholar]
- 26.Toossi, Z., and J. J. Ellner. 1998. The role of TGF β in the pathogenesis of human tuberculosis. Clin. Immunol. Immunopathol. 87:107-114. [DOI] [PubMed] [Google Scholar]
- 27.Toossi, Z., P. Gogate, H. Shiratsuchi, T. Young, and J. J. Ellner. 1995. Enhanced production of TGF-β by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculous granulomatous lung lesions. J. Immunol. 154:465-473. [PubMed] [Google Scholar]
- 28.Waters, W. R., J. R. Stabel, R. E. Sacco, J. A. Harp, B. A. Pesch, and M. J. Wannemuehler. 1999. Antigen-specific B-cell unresponsiveness induced by chronic Mycobacterium avium subsp. paratuberculosis infection of cattle. Infect. Immun. 67:1593-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss, D. J., O. A. Evanson, A. Moritz, M. Q. Deng, and M. S. Abrahamsen. 2002. Differential responses of bovine macrophages to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. Infect. Immun. 70:5556-5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, J. J., U. Vinkemeier, W. Gu, D. Chakravarti, C. M. Horvath, and J. E. Darnell, Jr. 1996. Two contact regions between Stat1 and CBP/p300 in interferon gamma signaling. Proc. Natl. Acad. Sci. USA 93:15092-15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao, B., M. T. Collins, and C. J. Czuprynski. 1997. Effects of gamma interferon and nitric oxide on the interaction of Mycobacterium avium subsp. paratuberculosis with bovine monocytes. Infect. Immun. 65:1761-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]