Abstract
Identification and characterization of genes that contribute to infection with Borrelia burgdorferi and, of those, genes that are targets of host responses is important for understanding the pathogenesis of Lyme disease. The complement-independent bactericidal monoclonal antibody (MAb) CB2 recognizes a carboxy-terminal, hydrophilic epitope of the outer surface protein B (OspB). CB2 kills B. burgdorferi by an unknown bactericidal mechanism. Upon binding of CB2 to OspB, differentially expressed gene products may be responsible for, or associated with, the death of the organism. A time course of the response of B. burgdorferi to CB2 was completed to analyze the differential gene expression in the bacteria over a period of visual morphological changes. Bacteria were treated with a sublethal concentration in which spirochetes were visibly distressed by the antibody but not lysed. Preliminary whole-genome DNA arrays at various time points within 1 h of incubation of B. burgdorferi with the antibody showed that most significant changes occurred at 25 min. Circular plasmid 32 (cp32)-encoded genes were active in this period of time, including the blyA homologs, phage holin system genes. DNA array data show that three blyA homologs were upregulated significantly, ≥2 standard deviations from the mean of the log ratios, and a P value of ≤0.01. Quantitative real-time PCR analysis verified blyA and blyB upregulation over an 18- to 35-min time course. The hypothesis to test is whether the killing mechanism of CB2 is through uncontrolled expression of the blyA and blyB phage holin system.
Lyme disease is the predominant arthropod vector-borne disease in the United States, with an increase in cases worldwide (1). The spirochete Borrelia burgdorferi is the causative agent of Lyme disease in North America (4, 9). Although the complete genome of B. burgdorferi has been sequenced, potential virulence factors are lacking in this organism (11, 37, 59). Therefore, it is crucial to identify and characterize other genes that may contribute to infection, genes that may contribute to the homeostasis of the organism, and genes that are the targets of host responses.
B. burgdorferi expresses numerous outer surface lipoproteins (Osps) throughout its life cycle. Specifically, OspA and OspB are cotranscribed by a two-gene operon on a 49-kb linear plasmid, lp54 (5). Upon blood feeding, OspA and OspB are downregulated, whereas OspC is upregulated (20, 27, 40, 72, 73). Antibodies appear to be a major form of host defense against this extracellular organism. Borreliae are susceptible to antibodies within the midgut prior to transmission to the host (6, 36, 50, 68, 94). In this context, complement-independent bactericidal monoclonal antibodies (MAbs) have been described (19, 21, 22, 34, 64-67, 74, 77). The murine MAb CB2 is a complement-independent immunoglobulin G1 (IgG1) directed against the carboxy terminus of OspB. Both whole CB2 and its Fab fragments exhibit bactericidal properties (21).
The epitope for CB2 is in a hydrophilic region of OspB, and the lysine at position 253 is required for antibody recognition and subsequent killing (22, 64). CB2 results in lysis of the outer membrane of the spirochete in the complete absence of complement. The bactericidal mechanism of CB2 is unknown. One possibility is that binding of CB2 to OspB can lead to the differential expression of Borrelia genes in response to this antibody, which could have a role in or be associated with the death of the organism. DNA microarrays and whole-genome DNA array membranes serve as significant instruments to investigate the responses of bacteria to changing environments (7, 14, 25, 44, 54, 55, 62, 63, 69, 85). DNA array methods were chosen to investigate the response of B. burgdorferi to CB2 in order to yield a specific gene expression profile. For the present study we used whole DNA genome arrays and quantitative real-time PCR to determine whether sublethal concentrations of the CB2 antibody induced transcriptional changes in B. burgdorferi. Plasmid-encoded genes appeared to be activated, including a system potentially engaged in lytic function.
MATERIALS AND METHODS
Experimental design.
Differential gene expression of B. burgdorferi was analyzed in response to a sublethal concentration of CB2. Various amounts of CB2 were analyzed for an optimal sublethal concentration. RNA from B. burgdorferi was isolated at various time points up to 1 h (5, 20, 25, and 60 min) and used to create cDNA for use on a whole Borrelia genome DNA array membrane (54). Array results were validated by quantitative real-time PCR of selected differentially expressed genes and randomly chosen stable genes for controls.
B. burgdorferi strains, culture conditions, and antibodies.
B. burgdorferi strain B31 (high passage) was grown in BSK-H medium (Sigma, St. Louis, Mo.) at 33°C and was enumerated by dark-field microscopy. The plasmid content of the B31 strain used for all experiments was determined by PCR with previously designed primers (33). The following plasmids are present in this strain: lp54, cp26, lp17, lp28-1, lp38, lp5, and cp32-1-3-4-6-8. Affinity-purified murine MAb CB2, an IgG1κ to OspB, was used and has been described previously (19, 21, 22, 34, 51).
Antibody treatment and subsequent RNA isolation.
Borreliae were harvested from a 20-ml culture in the mid to late logarithmic phase of growth (7 × 107 cells per ml) at 7,000 × g and washed once with BSK-H. Spirochetes were resuspended in 1 ml of BSK-H at 33°C; two 400-μl aliquots served as an untreated control and a CB2-treated experimental fraction. Equivalent aliquots (5.6 × 108 spirochetes) received CB2 at final concentrations of 2, 20, or 200 μg/ml. Untreated control samples received comparable amounts of phosphate buffer alone (0.02 M sodium phosphate [pH 7.0]). After CB2 was added, samples were incubated at 33°C for 5, 20, and 60 min. RNA was isolated from the spirochetes in the 400-μl sample by using TRI-Reagent LS (Molecular Research Center, Inc., Cincinnati, Ohio). TRI-Reagent LS was designed for immediate lysis of spirochetes in liquid samples, hence the small (400 μl) volume. The RNA was run through an RNeasy Mini-Column (Qiagen, Inc., Valencia, Calif.) and resuspended in 30 μl of RNase-free water. RNA integrity was verified by agarose gel electrophoresis and quantified by spectrophotometry. A260/A280 ratios consistently ranged between 1.8 and 2.0. Separate RNA isolations were completed for each individual experiment.
In other experiments, B. burgdorferi was grown to a concentration of 108 cells per ml in a volume of 50 ml of BSK-H each for untreated control and for CB2-treated (20 μg/ml) samples. Both untreated and CB2-treated samples were incubated at 33°C for 25 min. RNA was isolated by using TRI-Reagent (Molecular Research Center, Inc.). RNA was verified as described previously.
Direct enumeration of spirochetes after antibody treatment was done by assessing motility under dark-field microscopy. In addition, samples were recultured in BSK-H to determine viability after 60 min of CB2 treatment. Borreliae were allowed to recover at 33°C for 5 days. Bacteria were enumerated daily thereafter to assess recovery.
HS and FT procedures.
The spirochete pellet was resuspended in 5 ml of prewarmed 33°C BSK-H. Twelve 400-μl aliquots served as untreated control, heat shock (HS) and freeze-thaw (FT) fractions (carried out in quadruplicate). Untreated control samples were placed at 33°C for 60 min. HS samples were placed at 60°C for 60 min. FT samples were placed in liquid nitrogen for 10 s, followed by 5 min at 37°C. The FT procedure was repeated nine times over 60 min.
PCR amplification and slot blot.
flaB DNA was obtained from B. burgdorferi MedImmune genomic DNA (a gift from Patricia Rosa, Rocky Mountain Laboratory, National Institutes of Health). Oligonucleotide primers for flaB (BB0147) were described previously (54). All PCRs were completed in 200-μl tubes in a GeneAmp PCR System 9600 (Perkin-Elmer, Norwalk, Conn.). Each 50-μl final volume reaction contained 1 μl of DNA template (100 ng/μl); 1× PCR buffer (10 mM Tris-HCl, 1.5 mM MgCl2, 50 mM KCl [pH 8.3]); dATP, dCTP, dGTP, and dTTP (10 mM each); BB0147FWD and BB0147REV (1 μM each); and 2 U of TaqDNA polymerase (Roche, Indianapolis, Ind.). PCR conditions included 1 cycle at 95°C for 5 min and 30 cycles of denaturation at 95°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 1 min, followed by a second extension cycle at 72°C for 15 min. Amplified flaB PCR product was cleaned and concentrated via the Wizard PCR Preps DNA purification system (Promega, Madison, Wis.). For construction of the slot blot (Schleicher & Schuell, Keene, N.H.), a charged nylon membrane was initially soaked in 0.4 M Tris-HCl (pH 7.5) for 5 min. DNA was denatured for 10 min at room temperature in a solution of 0.25 N NaOH-0.5 M NaCl. Each slot received 76 ng of flaB DNA. The membrane was neutralized in a solution of 0.5 M NaCl-0.5 M Tris-HCl (pH 7.5). DNA was fixed to the membrane in a UV StrataLinker 1800 (Stratagene, La Jolla, Calif.). The membrane was dried at room temperature and stored until probed with 33P-labeled cDNA.
cDNA probe synthesis.
A cDNA probe was created for hybridization to the DNA array membranes as described previously, with some modifications (54). Briefly, for both untreated and CB2-treated conditions, a 15-μl reaction mixture contained 5 μg of RNA and 1 μl of 3′ open reading frame (ORF) B. burgdorferi cDNA labeling primers (Sigma-Genosys, Inc., Woodlands, Tex.). Amplifications were carried out in a PTC-1152 MiniCycler (MJ Research, Waltham, Mass.) with the parameters set at 90°C for 2 min, followed by a ramp to 42°C over 20 min. A final volume of 30 μl containing 1× reaction buffer (50 mM Tris-HCl, 8 mM MgCl2, 30 mM KCl, 1 mM dithiothreitol [pH 8.5]), 50 U of avian myeloblastosis virus reverse transcriptase, deoxynucleoside triphosphates (333 μM dCTP, 333 μM dGTP, and 333 μM dTTP; Roche), and 20 μCi of [α-33P]dATP (ICN, Costa Mesa, Calif.) was added to the reaction mixture. This reaction was incubated at 42°C for 2.5 h in the MiniCycler. Unincorporated radiolabeled nucleotide was removed from the labeled cDNA by use of a Panorama Sephadex G-25 column, based on the manufacturer's protocol (Sigma-Genosys). Radioactivity was measured in duplicate, both before and after removal of unincorporated label, in a Wallac 1409 DSA liquid scintillation counter (Perkin-Elmer, Gaithersburg, Md.), and the incorporation was calculated according to the following formula: percent incorporation = (incorporated disintegrations per minute × volume/total disintegrations per minute × volume) × 100.
Membrane hybridization and array image analysis.
The B. burgdorferi whole-genome DNA array used for these experiments was created by a consortium of laboratories in conjunction with Sigma-Genosys (54). Membranes were neutralized in 35-by-300-mm hybridization bottles (Robbins Scientific, Sunnyvale, Calif.) in 250 ml of 2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) for 25 min and in subsequent experiments membranes were neutralized for 5 min. Membranes were prehybridized in 5 ml of 65°C hybridization solution (Sigma-Genosys) containing 100 μg of salmon testes DNA (Sigma-Genosys)/ml for 60 min. The entire radiolabeled cDNA sample was added to 10 ml of hybridization solution containing 100 μg of salmon testes DNA/ml, denatured in a 95°C water bath for 10 min, and then added to the membranes and allowed to hybridize for 16 to 18 h at 65°C in a model 2000 Micro-Hybridization incubator (Robbins Scientific). The cDNA was removed from the bottles, and 40 ml of wash solution (0.5× SSPE, 2% sodium dodecyl sulfate [SDS]) was added. Bottles were inverted 10 times at room temperature, and the wash solution was decanted. This step was repeated twice. After the third wash, 80 ml of wash solution was added, and the bottles were incubated at room temperature in the hybridization oven for 20 min. Membranes were exposed to a 35- by 43-cm PhosphorImager screen (Molecular Dynamics, Sunnyvale, Calif.) for 2 to 3 days depending on the percent incorporation of 33P label in the cDNA. Screens were viewed in a Storm 860 PhosphorImager (Molecular Dynamics) by using ImageQuant 1.2 software. Pixel densities for each spot were generated with ArrayVision software version 6.0.
Membranes were stripped in roller bottles initially with 250 ml of 0.4 M NaOH, followed by incubation with stripping solution (250 ml of 200 mM Tris-HCl-0.1× SSPE-0.2% SDS [pH 7.0]) each for 30 min at 45°C with rotation. This procedure was followed by incubation with 250 ml of stripping solution at room temperature in the hybridization oven for 30 min with moderate rotation. Membranes were exposed to the PhosphorImager screen (as before) overnight to ensure that the stripping was successful, so membranes could be reprobed.
Data processing.
ArrayVision software (Imaging Research, St. Catharines, Ontario, Canada) was used to generate raw data from each untreated and CB2-treated replicate (54). Briefly, an initial gene filter created a threshold for each gene individually based on the local background of each spot (89). Only genes with raw data values ≥2 standard deviations (SD) above the local background were further analyzed (23). The raw data of each individual spot were divided by the total raw data of all spots to get a percent intensity. The data were analyzed in Excel workbooks. To determine which genes were differentially expressed, we used the criteria for significance developed by Conway et al. for Escherichia coli DNA array membranes (13, 23, 86; unpublished data [http://www.ou.edu/microarray]). Differentially expressed genes were required to pass two sets of statistical criteria to be considered significant. The first criterion required that changes in each gene be ≥2 SD above or below the mean of the log ratio of the individual spot intensity of the CB2-treated replicate versus that of the untreated control. Log ratio plots were created based on the following formula: log [(percent spot intensity of the CB2-treated gene X)/(percent spot intensity of the untreated control gene X)]. For the second criterion, genes were required to pass an unpaired two-tailed Student t test with a P value of ≤0.01 (>99% probability).
Differentially expressed genes were further analyzed for paralogous family members and structural motifs, domains and regions by using TIGR and other protein databases (http://www.tigr.org, http://www.ch.embnet.org/software/TMPRED_form.html, http://smart.embl-heidelberg.de/, and http://psort.nibb.ac.jp/form.html).
Quantitative real-time PCR.
This procedure was used for the validation of whole-genome DNA array experiments comparing untreated control versus CB2-treated samples. For cDNA synthesis, 5 μg of total RNA was used as described previously, with genome-directed primers (2, 62, 84). Genome-directed primers were used to generate cDNA from untreated control RNA samples and from RNA samples treated with 20 μg of CB2/ml at the designated time points. The resulting cDNA sample was diluted 1:100 for use in the real-time PCR. LightCycler-FastStart DNA Master SYBR I Green (Roche) reaction mix was used in the experimental procedure with the LightCycler (Roche) based on the manufacturer's protocol. Cycle parameters were as follows: preincubation, 1 cycle at 95°C for 5 min; amplification (quantification), 40 cycles at 95°C for 15 s, 52°C for 5 s; and 72°C for 6 s; melting curve, 95°C for 0 s, 65°C for 15 s, and 72°C for 0 s; and cooling, 1 cycle at 40°C for 30 s. A region of the 16S rRNA was used as an internal control for all data as shown previously by Revel et al. (62). GAPDH (glyceraldehyde-3-phosphate dehydrogenase; BB0057) and flaB were also used as secondary controls for some genes. Oligonucleotide primers for real-time PCR were designed by using MacVector software (Kodak International Biotechnologies, Inc., New Haven, Conn.) (Table 1).
TABLE 1.
Oligonucleotide primers used for quantitative real-time PCR
| ORFa | Primer sequence (5′-3′)
|
|
|---|---|---|
| Forward | Reverse | |
| 16S | CACGCAGTGTCGCTCCG | TACGGGAGGCAGCAGCTAAG |
| BB0011 | AAGAGCCGCTTGGACATTCG | ATGCCTGCTATTTTAGAAAGAGTG |
| BB0057 | CTGTGCTCAGGAAGGCATCAGAAAC | CAAGACCATCAACAATAGAAGAATG |
| BB0143 | ATGAACATTTTTAAAATTTTA | TTTGATTAATCTTTTTTTAAATTC |
| BB0147 | GCTTCTGATGATGCTGCTGG | ATGTGCCGTTACCTGATTGAACTGCC |
| BB0369 | TGTGGGTGCTTGTCAAAAAAAG | GGCAGATTTAGTATTTACAGC |
| BB0377 | CATACAAAACTCAACCCTGGC | CGGTCCAAACTTCATTATTTCTAAG |
| BB0516 | ATGACAGTGACAAACATAAACAACAC | AGTGAGTCTATTTTTCCGCTGG |
| BB0603 | CAACGATTTATTGAGCCCAAC | CGCTGCTTTTGAGATGTGTCC |
| BBA30 | AGCGACCTGGCAGAAGAAAGCACTAC | CTGTGGTGGGAATCAATAAAAGAAC |
| BBB19 | GAGGTTGAAGCGTTGCTGTC | TCCATTGTGATTATTTTCGGTATC |
| BBD18 | GGGGGCATAAAAGGAAC | CACCGGTTTGCATATTAAC |
| BBI07 | GTGCAGCTACTAAAAGGCGCT | ATGTAACTTAATTAAGTTTTTAAT |
| BB131 | ATACAGATGATGAAACAAGAGC | TAAACGATAGTTGGAATCCGC |
| BBI39 | TGAAAAAGAAGCCGAAAAGTTG | GTAAGCGTCAATGGTTGCG |
| BBM35 | CTGGAGTTGAGTTTGATTGG | GGCTTTTCTCTTTTTTTATTTTCG |
| BBP08 | GTGTGGGGGTTGTCTAATG | TTCGGTCTTTTGGTTCATAG |
| BBP10 | GGGTTTAAGGCATATGCAAC | TACACCGGCTCTATAATTATG |
| BBP13 | CGAATTCTATGTGATTTTGGAT | CAAAAGCAACAAAATCACCAAC |
| BBP23b | ATGGATACTATTAAATTAACCG | ATCTCTTTTTTTAATGTGATTTT |
| BBP24c | AAGGATTCTTTTTGGTTTATG | AGTGCCGCATTTATTGCTGG |
| BBP25 | GTTTAGGACTTAATTTACTATC | GTCAGTAGAGATAGCCTC |
| BBT03 | CGATACACTGGTCGCAAGAATAAC | GGGACATTTTACGATTACTTTTGG |
Boldface ORFs represent genes used for real-time PCR concordance data.
blyA primers recognize all B. burgdorferi B31 paralogy alignment GBB family 109 members due to their 100% identity.
blyB primers recognize all B. burgdorferi B31 paralogy alignment GBB family 111 members due to their high conserved homology.
SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting.
Borreliae were harvested and prepared as described above for RNA isolation. Hanks balanced salt solution (Gibco, Carlsbad, Calif.) was used for washes and final resuspension. Eight 400-μl replicates of both untreated control samples and samples treated with 20 μg of CB2/ml were prepared for each 5-, 27-, 35-, and 60-min time point. After each time point, samples were centrifuged at 10,000 × g for 45 s. The supernatant was removed and pooled for concentration to a volume of 0.2 ml by using a Centricon YM-3, with a nominal molecular mass limit of 3 kDa, according to the manufacturer's protocol (Millipore, Bedford, Mass.). Each pellet was resuspended in 100 μl of Hanks balanced salt solution and pooled. The total protein concentration of both supernatant and pellet fractions was determined with the BCA protein assay kit (Pierce, Rockford, Ill.). In addition, gels were also run with equivalent volume ratios. Minislab 16.5% acrylamide gels (8 by 9 by 0.1 cm) were cast with a 1.5-mm 10-well comb, and samples (250 ng/well) were applied and run for 1.5 h at 20 mA per gel (52). E. coli strain MM294 harboring pTG3, containing the blyAB operon, was used as a marker, as described previously (26, 42). Gels were stained by using Silver Stain-Plus based on the manufacturer's protocol (Bio-Rad, Hercules, Calif.). Some gels were transferred overnight to Immobilon-PSQ (Millipore) in CAPS transfer buffer (10% methanol, 20% 0.05 M 3-cyclohexyl-amino-1-propane sulfonic acid [CAPS] [pH 11]) at 30 mA and blocked in 2% casein blocking solution. The membranes were probed with rabbit anti-BlyA peptide antiserum in casein blocking solution. The secondary antibody was alkaline phosphatase-conjugated goat anti-rabbit whole-molecule immunoglobulin G (IgG) (Sigma). Bands were visualized with nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate; KPL, Gaithersburg, Md.). Both BlyA antibody and MM294-pTG3 were a generous gift of Donald Oliver, Wesleyan University (42).
RESULTS
B. burgdorferi strain B31 treated with 20 μg of CB2/ml proves to be the optimal, sublethal concentration for assessing differential gene expression.
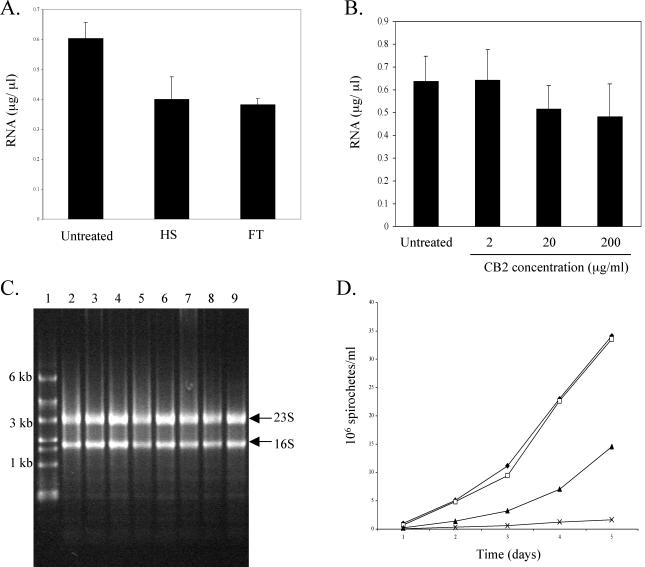
Prior to utilizing the whole-genome DNA arrays for the effect of CB2 on Borrelia gene expression, the desired antibody concentration used for treating the spirochetes was determined. To examine the feasibility of harvesting RNA under lethal or near-lethal conditions, B. burgdorferi were subjected to either HS or FT conditions, followed by RNA isolation. Both HS and FT treatments had marked effects on the numbers and morphology of spirochetes compared to the untreated control (data not shown). In line with these effects, RNA isolated from both HS and FT samples was 33% less concentrated than that obtained from untreated samples (Fig. 1A). These data served as a reference to assess the effects of CB2 on our ability to obtain RNA from the treated organisms.
FIG. 1.
Verification of 20 μg of CB2/ml as the optimal sublethal concentration. (A) Borrelia RNA concentration after HS or FT conditions. Error bars represent the mean of triplicate values ± the standard error. (B) Borrelia RNA concentration after treatment with various CB2 concentrations for 60 min. (C) 1% agarose gel electrophoresis of RNA extracts shown in panel B. Lane 1, RNA ladder; lanes 2 and 3, untreated; lanes 4 and 5, 2 μg of CB2/ml; lanes 6 and 7, 20 μg of CB2/ml; lanes 8 and 9, treatment with 200 μg of CB2/ml. Arrows indicate rRNA subunits. (D) Recovery of Borrelia after treatment with 2 (□), 20 (▴), or 200 (×) μg of CB2/ml or no treatment (♦).
Preliminary experiments were done to determine the antibody concentration and time needed for gene expression. Response of B. burgdorferi to CB2 was analyzed with increasing 10-fold CB2 concentrations (2, 20, and 200 μg/ml) at a 60-min time point to determine the concentration that affected but did not obliterate the organisms. The RNA yield showed a slight decrease as CB2 concentration increased in comparison to the untreated control (Fig. 1B). However, agarose gel electrophoresis of representative samples verified RNA quality and showed that the harvested RNA was not degraded under these conditions (Fig. 1C). In fact, RNA isolated from B. burgdorferi treated with the various CB2 concentrations did not yield concentrations of RNA lower than that observed with the HS and FT conditions. Spirochete recovery experiments were performed in which 400 μl of each sample (untreated or 2, 20, or 200 μg of CB2/ml) was added to fresh BSK-H. The spirochetes were then counted for 5 days to track growth patterns (Fig. 1D). The 2-μg/ml CB2 concentration had little or no effect on the growth of the bacteria. The sample treated with 200 μg of CB2/ml showed considerable delayed recovery, whereas the 20-μg/ml concentration of CB2 displayed a balance between having an effect on the organism and their potential for recovery. For this reason, we chose the CB2 concentration of 20 μg/ml. Spirochete viability was determined through direct enumeration after treatment of 20 μg of CB2/ml at 20 min under dark-field microscopy. The mean percentage of motile organisms after this treatment was 81.3 (±4.26 [standard error]) from three separate enumerations. To further verify the 20 μg of CB2/ml as the optimal sublethal concentration, flaB slot blot analysis of each cDNA sample was carried out. flaB was chosen as the optimal gene to analyze because its mRNA transcript should be unaffected by CB2. Each RNA sample (untreated or 2, 20, or 200 μg of CB2/ml) was used to create radiolabeled cDNA, which was then hybridized to flaB spotted on membrane strips. At each CB2 concentration, the density of the spotted flaB transcript remained unaffected compared to the untreated control (data not shown). Slot blot data of flaB proved that transcript was present and constant at all tested CB2 concentrations. This experiment also provided evidence for stable mRNA despite the concentration of CB2 and its effect on the organisms. All subsequent experiments were carried out by using a final CB2 concentration of 20 μg/ml.
DNA array experiments with borreliae incubated with CB2.
DNA array membranes were probed with radiolabeled cDNA from separate RNA isolations of untreated spirochetes and spirochetes treated with 20 μg of CB2/ml at various times ranging from 5 min to 1 h. Spots that did not pass the initial background filter (see methods) were not further analyzed but cannot be excluded from potential involvement given their low expression level.
DNA array data at various time points provided a preliminary assessment of gene activity. These data served as a basis for subsequent experiments at a relevant time point.
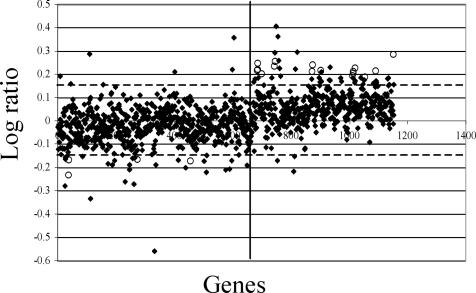
Transcriptome analysis of cultures (5 × 109 spirochetes) grown in 50-ml volumes were subsequently treated with 20 μg of CB2/ml for 25 min. This DNA array experiment was carried out twice with the same RNA sample to create cDNA for both experiments (Fig. 2). This method allowed a “snapshot” of gene activity to be created because the same RNA sample was used in creating cDNA for both membrane hybridizations. A constant pH and temperature of 33°C were maintained throughout the experiment. Figure 2 illustrates the log ratio plots of all of the genes analyzed, and Table 2 lists the respective differentially expressed genes that met the statistical requirements (≥2 SD from the mean of the log ratios and of P value of ≤0.01).
FIG. 2.
Spot graph log ratio plot analysis of Borrelia gene expression from array raw data of untreated versus CB2-treated (20 μg/ml) condition at 25 min. Dashed horizontal lines represent 2 SD above or below the mean of the log ratio plot. Solid vertical lines separate chromosomal (left) from plasmid (right) genes. Highlighted spots (○) represent genes that are significant by both criteria (see also Table 2).
TABLE 2.
DNA array differential gene expression of untreated borreliae versus borreliae treated with 20 μg of CB2/ml at 25 min
| Gene | Putative identification | Log ratio valuea | Fold induction | Location | Paralogous family |
|---|---|---|---|---|---|
| BB0055 | triose phosphate isomerase | −0.23276 | −1.71 | Main chromosome | |
| BB0056 | phosphoglycerate kinase | −0.16784 | −1.47 | Main chromosome | |
| BB0354 | hypothetical protein | −0.16727 | −1.47 | Main chromosome | |
| BB0603 | membrane associated protein, p66 | −0.17337 | −1.49 | Main chromosome | |
| BBA39 | hypothetical protein | 0.214931 | 1.64 | lp54 | 147 |
| BBA40 | hypothetical protein | 0.220437 | 1.66 | lp54 | 148 |
| BBA41 | conserved hypothetical protein | 0.245679 | 1.76 | lp54 | 149 |
| BBA57 | hypothetical protein | 0.202197 | 1.59 | lp54 | |
| BBD12 | hypothetical protein | 0.233454 | 1.71 | lp17 | |
| BBD18 | hypothetical protein | 0.253342 | 1.79 | lp17 | |
| BBL25 | conserved hypothetical protein | 0.211128 | 1.63 | cp32-8 | 112 |
| BBL26 | conserved hypothetical protein | 0.23918 | 1.73 | cp32-8 | 143 |
| BBM25 | conserved hypothetical protein | 0.214581 | 1.64 | cp32-6 | 112 |
| BBP23 | pore forming hemolysin, BlyA | 0.193051 | 1.56 | cp32-1 | 109 |
| BBP25 | conserved hypothetical protein | 0.211147 | 1.63 | cp32-1 | 112 |
| BBP26 | conserved hypothetical protein | 0.202929 | 1.60 | cp32-1 | 143 |
| BBP34 | conserved hypothetical protein | 0.226465 | 1.68 | cp32-1 | 80 |
| BBQ33 | conserved hypothetical protein | 0.187693 | 1.54 | lp56 | 143 |
| BBR23 | pore forming hemolysin, BlyA | 0.212907 | 1.63 | cp32-4 | 109 |
| BBU04 | conserved hypothetical protein | 0.283792 | 1.92 | lp21 | 57 |
Criteria for significance: ≥2 SD above or below the log ratio mean (±0.16), with a Student t test P value of ≤0.01.
Four chromosomal genes were downregulated in response to CB2 at 25 min. BB0055 and BB0056, triosephosphate isomerase and phosphoglycerate kinase, respectively, are located immediately upstream of BB0057 (GAPDH). All three genes are glycolytic pathway enzymes involved in energy metabolism and are in an operon (39). BB0354 is characterized by The Institute for Genomic Research (www.tigr.org) as a hypothetical protein but shares 31% identity and 58% similarity to a Fusobacterium nucleatum integral membrane protein (FN1300). The membrane-associated protein BB0603, p66 or Oms66, was also downregulated in this experiment. This protein has been characterized as a porin and as a protective immunogen (8, 17, 18, 28, 35, 56, 76). Sixteen (80%) of the differentially expressed genes were plasmid encoded. Analysis of the results obtained with this array (Table 2) again showed a marked activity of genes encoded by cp32. Cluster analysis of these 20 genes, many of which are hypothetical proteins, would not yield significant data when separated based on functional category. These genes were analyzed for unique motifs, regions, and/or domains by using TIGR, TMPRED, PSORT, and SMART protein databases. No obvious trends were observed with these analyses. However, paralogous family groupings showed three paralogs from both the cp32-encoded family 112 and 143 genes that are upregulated in response to CB2 (Table 2). BBA39, BBA40, and BBA41, in a possible operon with BBA38 on lp54, were all upregulated in response to CB2. There is a perfect −10 consensus and a partially conserved −35 consensus sequence upstream of BBA38. In addition, blyA homologs from the cp32 plasmids were associated with CB2 treatment in both experimental procedures, as BBO23, BBP23, and BBR23 were upregulated.
blyA, a holin system gene, was differentially expressed in two independent experiments at two different time points (20 and 25 min) and therefore was chosen for further analysis. blyA homologs and genes in the operon (BBP23 to BBP26) were differentially expressed along with cp32 plasmid activity at 25 min, and we attribute this effect to the action of CB2.
Quantitative real-time PCR analysis of blyA and blyB and other genes validate array results.
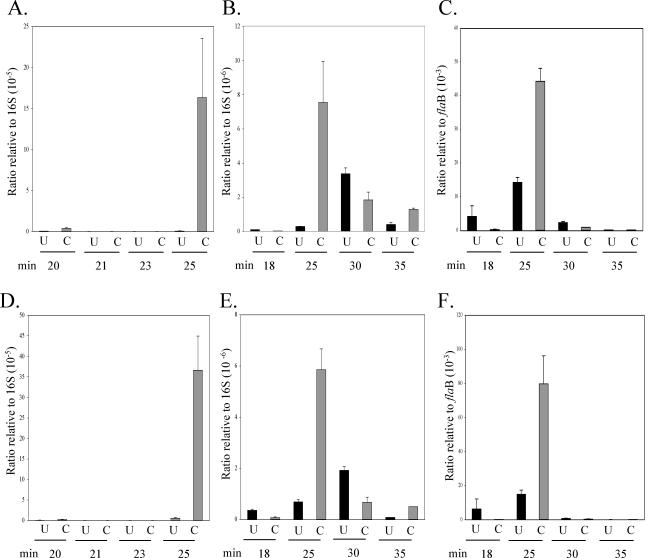
Both blyA and blyB displayed similar trends of gene expression in untreated versus CB2-treated samples, as would be expected from genes encoded in an operon. Because neither blyA nor blyB displayed a change in gene expression at 5 or 60 min in preliminary arrays, we performed real-time PCR analysis at these time points and confirmed the earlier results with a −1.39-fold and −1.03-fold change in blyA expression at 5 and 60 min, respectively, when normalized to the 16S ribosomal subunit. In similar experiments, blyB had a −1.4-fold and −1.06-fold change in gene expression at 5 and 60 min, respectively. However, at 20 min blyA was found to be upregulated, on average 76-fold, whereas blyB was upregulated 56-fold, relative to either 16S or GAPDH as internal controls. Two separate time courses were further analyzed to see exactly when blyA and blyB gene homologs were active with four separate RNA samples. The first time course of 20, 21, 23, and 25 min shows both blyA and blyB to be upregulated significantly at 25 min in the CB2-treated sample compared to the untreated control (Fig. 3A and D). The second time course of 18, 25, 30, and 35 min again illustrates 25 min as the prominent time point for blyA and blyB gene activity (Fig. 3B and E). For the second time course, blyA and blyB were also normalized to flaB, and a similar trend in gene expression was observed (Fig. 3C and F). It is clear that both blyA and blyB are upregulated significantly, with the 25-min time point as the critical peak time. Evidence for this upregulation comes from significant concordance between array and quantitative real-time PCR data.
FIG. 3.
Real-time PCR analysis of blyA (A to C) and blyB (D to F) in two different time courses. A, B, D, and E are ratios relative to the 16S ribosomal subunit; C and F are ratios relative to flaB. Black bars (U) represent untreated samples; gray bars (C) represent samples treated with 20 μg of CB2/ml. Error bars represent the mean of three replicates ± the standard error.
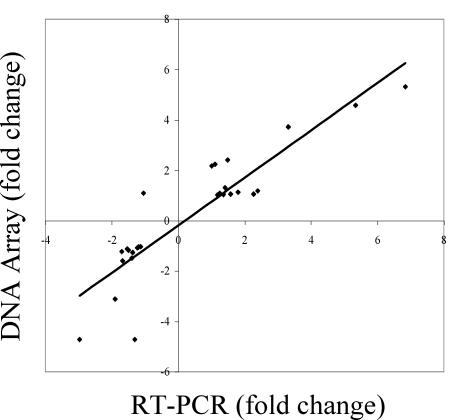
To validate the analyses of the array, 16 genes at various time points for a total of 28 triplicate quantitative real-time PCR reactions were completed. Unchanged genes in untreated control versus CB2-treated samples, as determined by previous array analysis, were randomly selected as negative controls for real-time PCR. Selected genes included BB0011, BB0057, BB0147, BB0377, BB0516, BB0615, BB0663, BBA30, BBB19, BBP10, and BBM35. Real-time PCR investigation of these genes, along with genes analyzed over time, and several differentially expressed genes was significantly consistent with array predictions with a correlation coefficient value of 0.90 (r2 = 0.81) (Fig. 4).
FIG. 4.
Concordance of differential gene expression between DNA array and quantitative real time-PCR Analysis. Fold change values for negative controls and differentially expressed genes were generated by comparing untreated to CB2-treated samples. In all cases, 16S ribosomal subunit was used as the internal control for real-time PCR (RT-PCR). Correlation coefficient = 0.90; trend line was generated with linear regression analysis.
BlyA protein expression in untreated Borrelia samples and in Borrelia samples treated with 20 μg of CB2/ml.
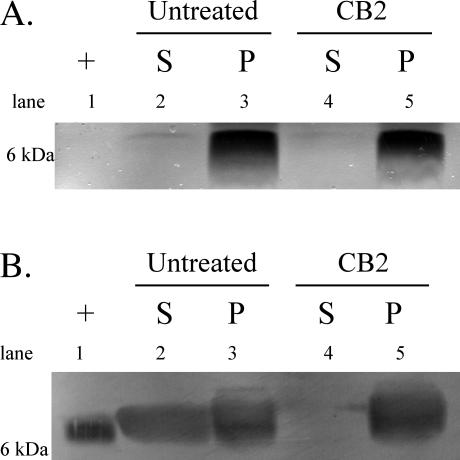
blyA and blyB were transcriptionally active and differentially expressed in response to CB2 at a critical 25-min time point. The translation of these genes was next analyzed via SDS-PAGE silver stain and Western blot with anti-BlyA peptide antiserum (Fig. 5). Untreated and CB2-treated samples were harvested at 5, 27, 35, and 60 min. Each time point showed the same trend in BlyA expression. Both pellet fractions contained similar amounts of BlyA, whereas there was clearly an absence of BlyA in the CB2 supernatant compared to the untreated control (Fig. 5B).
FIG. 5.
Representative SDS-PAGE gels of samples were collected at 5, 27, 35, or 60 min; the 35-min time point is shown. Silver stain (A) and anti-BlyA Western blot (B) of untreated samples and samples treated with 20 μg of CB2/ml. S, supernatant; P, pellet. Lane 1, MM294-pTG3, positive BlyA control; lanes 2 and 3, untreated Borrelia; lanes 4 and 5, CB2-treated Borrelia samples.
DISCUSSION
The goal of the present study was to identify genes differentially expressed in B. burgdorferi treated with CB2. CB2 is bactericidal to borreliae in the absence of complement (19, 21, 22, 34, 51). Although the characteristics of the antibody-antigen complex have been investigated, the mechanism of action of this antibody remains unknown. Therefore, we used whole-genome DNA array analysis to determine whether differential gene expression is involved in or related to CB2 killing of B. burgdorferi. We chose a sublethal CB2 concentration of 20 μg/ml for the arrays. Our approach provided evidence for the possibility that the upregulation of blyA and blyB, as evidenced by both the array and real-time PCR data, may play a role in the antibody mediated destruction of the organism. The holin-like genes of B. burgdorferi, blyA and blyB, were originally classified as hemolysins (42). These genes have subsequently been characterized as prophage-encoded holin genes (26). Analysis of bacteriophages associated with B. burgdorferi CA11.2A showed that they package cp32 DNA (31). Both blyA and blyB homologs are part of a four-gene operon located on cp32 and lp56 (11, 12, 55, 57, 83). The lytic action of CB2 may be an indirect result based on upregulation of the holin genes. Thus, these findings have generated our working hypothesis that the binding of CB2 to OspB may trigger upregulation of these holin genes at their critical concentration. Upon activation, they could effectively create holes in the bacterial membrane, causing lysis of the spirochete. It has been argued that the presence of low-level expression of blyAB in B. burgdorferi is inconsistent with their putative function as holins, since expression of these lethal molecules would need to be tightly regulated (55). It is possible that the low-level expression may account for the speed with which these molecules can act, or that there is a need for further holin functional activation. Our data show endogenous BlyA expression in untreated control samples. As determined by Western blotting, the presence of BlyA in the untreated control and its absence in the CB2-treated supernatant sample can have two possible explanations. First, the BlyA present in the untreated supernatant fraction could be the result of constitutive BlyA expression that ceases as the antibody has an effect on the cells until there is no further protein secretion. Alternatively, BlyA may be constitutively expressed in an inactive form. Second, CB2 itself could interfere with the translation or secretion of BlyA so that it is not expressed or remains associated with the dying bacteria. To our knowledge, this represents the first evidence of BlyA presence in the Borrelia supernatant fraction. Previously, Guina and Oliver (42) and Damman et al. (26) had shown BlyA to be present in the supernatant S100 fraction but only after sonication of the E. coli and B. burgdorferi, respectively.
E. coli mRNA has a half-life of ∼1 min; therefore, the timing chosen for DNA array experiments is crucial for the bacteria and for bacteriophage lysis (3, 24, 41, 87, 91, 92). Studies in E. coli show that the holin genes control host lysis (91). It has been proposed that lysis is a result of holin concentrations reaching a critical level that ultimately leads to disruption of the host membrane (41, 88, 92). Possibly the binding of CB2 to OspB causes indirect upregulation of the holin genes such that the critical concentration of blyA and blyB is reached at or ca. 25 min, and thus the host cell lyses. Holin genes appear to have tightly regulated expression that is sensitive to time and concentration (38, 41, 61, 93). Hence, if several time points had not been chosen, the blyA and blyB expression changes might have been overlooked. Likewise, if a different gene exhibited the same strict timing trend, but at a time point not analyzed in these experiments, its peak of activity may have been missed. DNA array methods have limitations in the study of transient gene expression.
The greatest differential expression in all of our arrays and real-time PCRs was seen in genes encoded by cp32. The B. burgdorferi genome contains many linear and circular plasmids. Many are essential for persistence and infection of the organism (11, 60, 71). DNA array data from CB2 treated cells showed notable cp32 activity. There are seven coexisting homologs of cp32 in B. burgdorferi B31 that also contain homologous regions to linear plasmid 56 (lp56) (10-12, 29, 30, 75, 80-83, 95). All Borrelia paralogs are spotted on the DNA arrays; this leads to cross-hybridization when a gene is differentially expressed (7, 11, 37, 54, 55, 57). Prior B. burgdorferi DNA arrays using various experimental approaches have shown upregulation of cp32 encoded genes to a greater or lesser extent (7, 54, 55). Here we show that activation of this plasmid can occur very quickly and timing of gene expression may be critical to the action of the gene products.
Of the 16 differentially expressed plasmid genes, 8 were cp32 plasmid-encoded genes. Of the cp32 active genes, seven genes were of the three-gene blyA/blyB operon, two of which were blyA genes themselves (BBP23 and BBR23). Furthermore, lp54 contains an insertion of cp32 in the region between BBA38 and BBA56 (11, 55). The DNA array data also showed an upregulation of four genes in this plasmid region (BBA39, BBA40, BBA41, and BBA57). This provides additional support to the significance of not only plasmid genes but also cp32 in particular in the effect of CB2.
Only four chromosomal genes were differentially expressed in the DNA array, all of which were downregulated. Of these, two genes are glycolytic pathway enzymes involved in energy metabolism (39). Also downregulated was BB0603 (p66 or Oms66), which is thought to be a porin and also functions as an immunogen (8, 17, 18, 28, 35, 56, 76). In contrast, Brooks et al. found BB0603 to be upregulated in response to mammalian host factors (7). The downregulation of glycolytic enzymes and a putative porin could be consistent with a death signal.
The transcriptome of our arrays can be compared to other array data obtained by the profiles of temperature-induced changes in B. burgdorferi or the results obtained by host-adapted spirochetes (7, 55, 62). The results from Ojaimi et al., Brooks et al., and the present study show the majority of differentially expressed genes (63, 65, and 80%, respectively) were plasmid encoded. Conversely, Revel et al. found approximately equal chromosome and plasmid differential gene expression. Despite this, all four independent DNA array experiments produced results noting evident cp32 activity. Specifically, Ojaimi et al. showed upregulation (1.5- to 2-fold) of 13 of the putative Borrelia hemolysins, including blyA/B, at 35°C (55). Ojaimi et al. also show upregulation of family 143 genes at 35°C (55). Our experiments likewise demonstrate upregulation of family 112 and 143 genes but in response to CB2. Both family 112 and 143 are located immediately downstream of blyA and blyB.
Numerous factors such as temperature, pH, and bacterial concentration affect gene expression in bacteria. Characteristic bacterial stress responses involve products such as auxiliary sigma factors, RecA in the SOS response and HS proteins (Hsps). E. coli and Bacillus subtilis both respond to different stresses through activation of sigma factors, such as rpoS and σB, respectively (43, 45-47, 53, 58, 85). Temperature shift experiments analyzing the HS response of spirochetes indicated induction of two Hsps, GroEL and DnaK (15, 16, 19, 78). In borreliae, RpoS is involved in stationary-phase response and control of group I-like genes such as OspC and DbpA (32, 49, 90). In our DNA array experiments, spirochetes were concentrated and then subjected to a bactericidal antibody; however, no stress response genes, sigma factors, Hsps, or quorum-sensing regulon genes were differentially expressed compared to the untreated control. However, the antibody incubation times used (up to 1 h) may be insufficient for eliciting a stress response or activating quorum-sensing regulon genes (48, 70, 79). Likewise, adjustment of the bacteria to CB2 in our experimental conditions may have bypassed the stress response. Therefore, the response to CB2 treatment is not consistent with a conventional bacterial stress response.
The results of the present study have shown that CB2 causes an upregulation of both blyA and blyB at 25 min. Whether a full packaged phage is produced remains unknown, but this will be further investigated. Possibly, the binding of CB2 to OspB induces a stress cascade that activates cp32 plasmids. The function and relevance of OspB is not known; therefore, it is not extreme to speculate that OspB could be involved in a bacterial stress response cascade leading to a lethal disturbance of the outer membrane.
Acknowledgments
This study was supported by PHS grant AI-27044 and by a grant from the Mathers Foundation to J.L.B. D.R.A. was supported in part by grants 0030130N from the American Heart Association and RR-15564 from the NIH, and C.S.B. was supported by Molecular Pathogenesis training grant AI-07364.
We appreciate the gift of an antibody to BlyA from Donald Oliver of Wesleyan University. We are also grateful for helpful discussions with Christian Eggers, University of Connecticut Health Sciences Center.
Editor: J. T. Barbieri
REFERENCES
- 1.Anonymous. 2001. Lyme disease-United States, 1999. Morb. Mortal. Wkly. Rep. 50:181-185. [PubMed] [Google Scholar]
- 2.Arfin, S. M., A. D. Long, E. T. Ito, L. Tolleri, M. M. Riehle, E. S. Paegle, and G. W. Hatfield. 2000. Global gene expression profiling in Escherichia coli K-12: the effects of integration host factor. J. Biol. Chem. 275:29672-29684. [DOI] [PubMed] [Google Scholar]
- 3.Baracchini, E., and H. Bremer. 1987. Determination of synthesis rate and lifetime of bacterial mRNAs. Anal. Biochem. 167:245-260. [DOI] [PubMed] [Google Scholar]
- 4.Benach, J. L., E. M. Bosler, J. P. Hanrahan, J. L. Coleman, G. S. Habicht, T. F. Bast, D. J. Cameron, J. L. Ziegler, A. G. Barbour, W. Burgdorfer, R. Edelman, and R. A. Kaslow. 1983. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 308:740-742. [DOI] [PubMed] [Google Scholar]
- 5.Bergstrom, S., V. G. Bundoc, and A. G. Barbour. 1989. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol. Microbiol. 3:479-486. [DOI] [PubMed] [Google Scholar]
- 6.Bockenstedt, L. K., E. Hodzic, S. Feng, K. W. Bourrel, A. de Silva, R. R. Montgomery, E. Fikrig, J. D. Radolf, and S. W. Barthold. 1997. Borrelia burgdorferi strain-specific Osp C-mediated immunity in mice. Infect. Immun. 65:4661-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunikis, J., L. Noppa, Y. Ostberg, A. G. Barbour, and S. Bergstrom. 1996. Surface exposure and species specificity of an immunoreactive domain of a 66-kilodalton outer membrane protein (P66) of the Borrelia spp. that cause Lyme disease. Infect. Immun. 64:5111-5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease-a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 10.Caimano, M. J., X. Yang, T. G. Popova, M. L. Clawson, D. R. Akins, M. V. Norgard, and J. D. Radolf. 2000. Molecular and evolutionary characterization of the cp32/18 family of supercoiled plasmids in Borrelia burgdorferi 297. Infect. Immun. 68:1574-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 12.Casjens, S., R. van Vugt, K. Tilly, P. A. Rosa, and B. Stevenson. 1997. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J. Bacteriol. 179:217-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang, D. E., D. J. Smalley, and T. Conway. 2002. Gene expression profiling of Escherichia coli growth transitions: an expanded stringent response model. Mol. Microbiol. 45:289-306. [DOI] [PubMed] [Google Scholar]
- 14.Chuang, S. E., D. L. Daniels, and F. R. Blattner. 1993. Global regulation of gene expression in Escherichia coli. J. Bacteriol. 175:2026-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cluss, R. G., and J. T. Boothby. 1990. Thermoregulation of protein synthesis in Borrelia burgdorferi. Infect. Immun. 58:1038-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cluss, R. G., A. S. Goel, H. L. Rehm, J. G. Schoenecker, and J. T. Boothby. 1996. Coordinate synthesis and turnover of heat shock proteins in Borrelia burgdorferi: degradation of DnaK during recovery from heat shock. Infect. Immun. 64:1736-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coburn, J., W. Chege, L. Magoun, S. C. Bodary, and J. M. Leong. 1999. Characterization of a candidate Borrelia burgdorferi β3-chain integrin ligand identified using a phage display library. Mol. Microbiol. 34:926-940. [DOI] [PubMed] [Google Scholar]
- 18.Coburn, J., and C. Cugini. 2003. Targeted mutation of the outer membrane protein P66 disrupts attachment of the Lyme disease agent, Borrelia burgdorferi, to integrin αvβ3. Proc. Natl. Acad. Sci. USA 100:7301-7306. [DOI] [PMC free article] [PubMed]
- 19.Coleman, J. L., and J. L. Benach. 1992. Characterization of antigenic determinants of Borrelia burgdorferi shared by other bacteria. J. Infect. Dis. 165:658-666. [DOI] [PubMed] [Google Scholar]
- 20.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Degen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89:1111-1119. [DOI] [PubMed] [Google Scholar]
- 21.Coleman, J. L., R. C. Rogers, and J. L. Benach. 1992. Selection of an escape variant of Borrelia burgdorferi by use of bactericidal monoclonal antibodies to OspB. Infect. Immun. 60:3098-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coleman, J. L., R. C. Rogers, P. A. Rosa, and J. L. Benach. 1994. Variations in the ospB gene of Borrelia burgdorferi result in differences in monoclonal antibody reactivity and in production of escape variants. Infect. Immun. 62:303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conway, T., B. Kraus, D. L. Tucker, D. J. Smalley, A. F. Dorman, and L. McKibben. 2002. DNA array analysis in a Microsoft Windows environment. BioTechniques 32:110-119. [DOI] [PubMed] [Google Scholar]
- 24.Conway, T., and G. K. Schoolnik. 2003. Microarray expression profiling: capturing a genome-wide portrait of the transcriptome. Mol. Microbiol. 47:879-889. [DOI] [PubMed] [Google Scholar]
- 25.Cummings, C. A., and D. A. Relman. 2000. Using DNA microarrays to study host-microbe interactions. Emerg. Infect. Dis. 6:513-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damman, C. J., C. H. Eggers, D. S. Samuels, and D. B. Oliver. 2000. Characterization of Borrelia burgdorferi BlyA and BlyB proteins: a prophage-encoded holin-like system. J. Bacteriol. 182:6791-6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Silva, A. M., S. R. Telford III, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Defoe, G., and J. Coburn. 2001. Delineation of Borrelia burgdorferi p66 sequences required for integrin αIIbβ3 recognition. Infect. Immun. 69:3455-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eggers, C. H., M. J. Caimano, M. L. Clawson, W. G. Miller, D. S. Samuels, and J. D. Radolf. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochaete. Mol. Microbiol. 43:281-295. [DOI] [PubMed] [Google Scholar]
- 30.Eggers, C. H., S. Casjens, S. F. Hayes, C. F. Garon, C. J. Damman, D. B. Oliver, and D. S. Samuels. 2000. Bacteriophages of spirochetes. J. Mol. Microbiol. Biotechnol. 2:365-373. [PubMed] [Google Scholar]
- 31.Eggers, C. H., and D. S. Samuels. 1999. Molecular evidence for a new bacteriophage of Borrelia burgdorferi. J. Bacteriol. 181:7308-7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elias, A. F., J. L. Bono, J. A. Carroll, P. Stewart, K. Tilly, and P. Rosa. 2000. Altered stationary-phase response in a Borrelia burgdorferi rpoS mutant. J. Bacteriol. 182:2909-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elias, A. F., P. E. Stewart, D. Grimm, M. J. Caimano, C. H. Eggers, K. Tilly, J. L. Bono, D. R. Akins, J. D. Radolf, T. G. Schwan, and P. Rosa. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escudero, R., M. L. Halluska, P. B. Backenson, J. L. Coleman, and J. L. Benach. 1997. Characterization of the physiological requirements for the bactericidal effects of a monoclonal antibody to OspB of Borrelia burgdorferi by confocal microscopy. Infect. Immun. 65:1908-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Exner, M. M., X. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2000. Protection elicited by native outer membrane protein Oms66 (p66) against host-adapted Borrelia burgdorferi: conformational nature of bactericidal epitopes. Infect. Immun. 68:2647-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fikrig, E., S. W. Barthold, F. S. Kantor, and R. A. Flavell. 1990. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science 250:553-556. [DOI] [PubMed] [Google Scholar]
- 37.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 38.Garrett, J. M., and R. Young. 1982. Lethal action of bacteriophage λ S gene. J. Virol. 44:886-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gebbia, J. A., P. B. Backenson, J. L. Coleman, P. Anda, and J. L. Benach. 1997. Glycolytic enzyme operon of Borrelia burgdorferi: characterization and evolutionary implications. Gene 188:221-228. [DOI] [PubMed] [Google Scholar]
- 40.Gilmore, R. D., Jr., and J. Piesman. 2000. Inhibition of Borrelia burgdorferi migration from the midgut to the salivary glands following feeding by ticks on OspC-immunized mice. Infect. Immun. 68:411-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grundling, A., M. D. Manson, and R. Young. 2001. Holins kill without warning. Proc. Natl. Acad. Sci. USA 98:9348-9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guina, T., and D. B. Oliver. 1997. Cloning and analysis of a Borrelia burgdorferi membrane-interactive protein exhibiting hemolytic activity. Mol. Microbiol. 24:1201-1213. [DOI] [PubMed] [Google Scholar]
- 43.Haldenwang, W. G. 1995. The sigma factors of Bacillus subtilis. Microbiol. Rev. 59:1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hatfield, G. W., S. P. Hung, and P. Baldi. 2003. Differential analysis of DNA microarray gene expression data. Mol. Microbiol. 47:871-877. [DOI] [PubMed] [Google Scholar]
- 45.Hecker, M., W. Schumann, and U. Volker. 1996. Heat-shock and general stress response in Bacillus subtilis. Mol. Microbiol. 19:417-428. [DOI] [PubMed] [Google Scholar]
- 46.Helmann, J. D., M. F. Wu, P. A. Kobel, F. J. Gamo, M. Wilson, M. M. Morshedi, M. Navre, and C. Paddon. 2001. Global transcriptional response of Bacillus subtilis to heat shock. J. Bacteriol. 183:7318-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hengge-Aronis, R. 1993. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell 72:165-168. [DOI] [PubMed]
- 48.Hubner, A., A. T. Revel, D. M. Nolen, K. E. Hagman, and M. V. Norgard. 2003. Expression of a luxS gene is not required for Borrelia burgdorferi infection of mice via needle inoculation. Infect. Immun. 71:2892-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hubner, A., X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. USA 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson, R. C., C. Kodner, and M. Russell. 1986. Passive immunization of hamsters against experimental infection with the Lyme disease spirochete. Infect. Immun. 53:713-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katona, L. I., S. Ayalew, J. L. Coleman, and J. L. Benach. 2000. A bactericidal monoclonal antibody elicits a change in its antigen, OspB of Borrelia burgdorferi, that can be detected by limited proteolysis. J. Immunol. 164:1425-1431. [DOI] [PubMed] [Google Scholar]
- 52.Laemmli, U. K., and M. Favre. 1973. Maturation of the head of bacteriophage T4. I. DNA packaging events. J. Mol. Biol. 80:575-599. [DOI] [PubMed] [Google Scholar]
- 53.Loewen, P. C., B. Hu, J. Strutinsky, and R. Sparling. 1998. Regulation in the rpoS regulon of Escherichia coli. Can. J. Microbiol. 44:707-717. [DOI] [PubMed] [Google Scholar]
- 54.Ojaimi, C., C. Brooks, D. Akins, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katonah, J. Radolf, M. Caimano, J. Skare, K. Swingle, S. Sims, and I. Schwartz. 2002. Borrelia burgdorferi gene expression profiling with membrane-based arrays. Methods Enzymol. 358:165-177. [DOI] [PubMed] [Google Scholar]
- 55.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ornstein, K., Y. Ostberg, J. Bunikis, L. Noppa, J. Berglund, R. Norrby, and S. Bergstrom. 2002. Differential immune response to the variable surface loop antigen of P66 of Borrelia burgdorferi sensu lato species in geographically diverse populations of Lyme borreliosis patients. Clin. Diagn. Lab. Immunol. 9:1382-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Porcella, S. F., T. G. Popova, D. R. Akins, M. Li, J. D. Radolf, and M. V. Norgard. 1996. Borrelia burgdorferi supercoiled plasmids encode multicopy tandem open reading frames and a lipoprotein gene family. J. Bacteriol. 178:3293-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Price, C. W., P. Fawcett, H. Ceremonie, N. Su, C. K. Murphy, and P. Youngman. 2001. Genome-wide analysis of the general stress response in Bacillus subtilis. Mol. Microbiol. 41:757-774. [DOI] [PubMed] [Google Scholar]
- 59.Purser, J. E., M. B. Lawrenz, M. J. Caimano, J. K. Howell, J. D. Radolf, and S. J. Norris. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48:753-764. [DOI] [PubMed] [Google Scholar]
- 60.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramanculov, E., and R. Young. 2001. Functional analysis of the phage T4 holin in a lambda context. Mol. Genet. Genomics 265:345-353. [DOI] [PubMed] [Google Scholar]
- 62.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richmond, C. S., J. D. Glasner, R. Mau, H. Jin, and F. R. Blattner. 1999. Genome-wide expression profiling in Escherichia coli K-12. Nucleic Acids Res. 27:3821-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sadziene, A., and A. G. Barbour. 1996. Experimental immunization against Lyme borreliosis with recombinant Osp proteins: an overview. Infection 24:195-202. [DOI] [PubMed] [Google Scholar]
- 65.Sadziene, A., A. G. Barbour, P. A. Rosa, and D. D. Thomas. 1993. An OspB mutant of Borrelia burgdorferi has reduced invasiveness in vitro and reduced infectivity in vivo. Infect. Immun. 61:3590-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sadziene, A., M. Jonsson, S. Bergstrom, R. K. Bright, R. C. Kennedy, and A. G. Barbour. 1994. A bactericidal antibody to Borrelia burgdorferi is directed against a variable region of the OspB protein. Infect. Immun. 62:2037-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sadziene, A., P. A. Rosa, P. A. Thompson, D. M. Hogan, and A. G. Barbour. 1992. Antibody-resistant mutants of Borrelia burgdorferi: in vitro selection and characterization. J. Exp. Med. 176:799-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schaible, U. E., M. D. Kramer, K. Eichmann, M. Modolell, C. Museteanu, and M. M. Simon. 1990. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc. Natl. Acad. Sci. USA 87:3768-3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schena, M., D. Shalon, R. W. Davis, and P. O. Brown. 1995. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270:467-470. [DOI] [PubMed] [Google Scholar]
- 70.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwan, T. G., W. Burgdorfer, and C. F. Garon. 1988. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect. Immun. 56:1831-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scriba, M., J. S. Ebrahim, T. Schlott, and H. Eiffert. 1993. The 39-kilodalton protein of Borrelia burgdorferi: a target for bactericidal human monoclonal antibodies. Infect. Immun. 61:4523-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simpson, W. J., C. F. Garon, and T. G. Schwan. 1990. Borrelia burgdorferi contains repeated DNA sequences that are species specific and plasmid associated. Infect. Immun. 58:847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skare, J. T., T. A. Mirzabekov, E. S. Shang, D. R. Blanco, H. Erdjument-Bromage, J. Bunikis, S. Bergstrom, P. Tempst, B. L. Kagan, J. N. Miller, and M. A. Lovett. 1997. The Oms66 (p66) protein is a Borrelia burgdorferi porin. Infect. Immun. 65:3654-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sole, M., C. Bantar, K. Indest, Y. Gu, R. Ramamoorthy, R. Coughlin, and M. T. Philipp. 1998. Borrelia burgdorferi escape mutants that survive in the presence of antiserum to the OspA vaccine are killed when complement is also present. Infect. Immun. 66:2540-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stamm, L. V., F. C. Gherardini, E. A. Parrish, and C. R. Moomaw. 1991. Heat shock response of spirochetes. Infect. Immun. 59:1572-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stevenson, B., and K. Babb. 2002. LuxS-mediated quorum sensing in Borrelia burgdorferi, the Lyme disease spirochete. Infect. Immun. 70:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stevenson, B., S. Casjens, R. van Vugt, S. F. Porcella, K. Tilly, J. L. Bono, and P. Rosa. 1997. Characterization of cp18, a naturally truncated member of the cp32 family of Borrelia burgdorferi plasmids. J. Bacteriol. 179:4285-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stevenson, B., S. F. Porcella, K. L. Oie, C. A. Fitzpatrick, S. J. Raffel, L. Lubke, M. E. Schrumpf, and T. G. Schwan. 2000. The relapsing fever spirochete Borrelia hermsii contains multiple, antigen-encoding circular plasmids that are homologous to the cp32 plasmids of Lyme disease spirochetes. Infect. Immun. 68:3900-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stevenson, B., K. Tilly, and P. A. Rosa. 1996. A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J. Bacteriol. 178:3508-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stevenson, B., W. R. Zuckert, and D. R. Akins. 2000. Repetition, conservation, and variation: the multiple cp32 plasmids of Borrelia species. J. Mol. Microbiol. Biotechnol. 2:411-422. [PubMed] [Google Scholar]
- 84.Talaat, A. M., P. Hunter, and S. A. Johnston. 2000. Genome-directed primers for selective labeling of bacterial transcripts for DNA microarray analysis. Nat. Biotechnol. 18:679-682. [DOI] [PubMed] [Google Scholar]
- 85.Tao, H., C. Bausch, C. Richmond, F. R. Blattner, and T. Conway. 1999. Functional genomics: expression analysis of Escherichia coli growing on minimal and rich media. J. Bacteriol. 181:6425-6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tucker, D. L., N. Tucker, and T. Conway. 2002. Gene Expression profiling of the pH response in Escherichia coli. J. Bacteriol. 184:6551-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang, I.-N., D. E. Dykhuizen, and L. B. Slobodkin. 1996. The evolution of phage lysis timing. Evol. Ecol. 10:545-558. [Google Scholar]
- 88.Wang, I. N., D. L. Smith, and R. Young. 2000. Holins: the protein clocks of bacteriophage infections. Annu. Rev. Microbiol. 54:799-825. [DOI] [PubMed] [Google Scholar]
- 89.Wei, Y., J. M. Lee, C. Richmond, F. R. Blattner, J. A. Rafalski, and R. A. LaRossa. 2001. High-density microarray-mediated gene expression profiling of Escherichia coli. J. Bacteriol. 183:545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470-1479. [DOI] [PubMed] [Google Scholar]
- 91.Young, I., I. Wang, and W. D. Roof. 2000. Phages will out: strategies of host cell lysis. Trends Microbiol. 8:120-128. [DOI] [PubMed] [Google Scholar]
- 92.Young, R. 2002. Bacteriophage holins: deadly diversity. J. Mol. Microbiol. Biotechnol. 4:21-36. [PubMed] [Google Scholar]
- 93.Young, R. 1992. Bacteriophage lysis: mechanism and regulation. Microbiol. Rev. 56:430-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhong, W., T. Stehle, C. Museteanu, A. Siebers, L. Gern, M. Kramer, R. Wallich, and M. M. Simon. 1997. Therapeutic passive vaccination against chronic Lyme disease in mice. Proc. Natl. Acad. Sci. USA 94:12533-12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zuckert, W. R., and J. Meyer. 1996. Circular and linear plasmids of Lyme disease spirochetes have extensive homology: characterization of a repeated DNA element. J. Bacteriol. 178:2287-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]