Abstract
Objectives
People who inject drugs (PWID) are at high risk for acquiring hepatitis C virus (HCV), but many are unaware of their infection. HCV dried blood spot (DBS) testing increases case-finding in addiction services and prisons. We determine the cost-effectiveness of increasing HCV case-finding among PWID by offering DBS testing in specialist addiction services or prisons as compared to using venepuncture.
Design
Cost-utility analysis using a dynamic HCV transmission model among PWID, including: disease progression, diagnosis, treatment, injecting status, incarceration and addition services contact.
Setting
UK.
Intervention
DBS testing in specialist addiction services or prisons. Intervention impact was determined by a meta-analysis of primary data.
Primary and secondary outcome measures
Costs (in UK £, £1=US$1.60) and utilities (quality-adjusted life years, QALYs) were attached to each state and the incremental cost effectiveness ratio (ICER) determined. Multivariate uncertainty and one-way sensitivity analyses were performed.
Results
For a £20 000 per QALY gained willingness-to-pay threshold, DBS testing in addiction services is cost-effective (ICER of £14 600 per QALY gained). Under the base-case assumption of no continuity of treatment/care when exiting/entering prison, DBS testing in prisons is not cost-effective (ICER of £59 400 per QALY gained). Results are robust to changes in HCV prevalence; increasing PWID treatment rates to those for ex-PWID considerably reduces ICER (£4500 and £30 000 per QALY gained for addiction services and prison, respectively). If continuity of care is >40%, the prison DBS ICER falls below £20 000 per QALY gained.
Conclusions
Despite low PWID treatment rates, increasing case-finding can be cost-effective in specialist addiction services, and in prisons if continuity of treatment/care is ensured.
Keywords: HEALTH ECONOMICS
Article summary.
Article focus
We performed a cost-utility analysis of increasing hepatitis C virus (HCV) case-finding among people who inject drugs (PWID) by offering dried blood spot testing in specialist addiction services or prisons.
Key messages
Despite low PWID treatment rates, increasing case-finding for PWID can be cost-effective in specialist addiction services.
In prisons, the cost-effectiveness of HCV case-finding depends on adequate continuity of treatment/care between prison and the community, as many treatments are discontinued due to the short incarceration times.
Strengths and limitations of this study
We used a dynamic mathematical model of HCV transmission to capture the potential prevention benefits of treatment, which has been shown to increase the cost-effectiveness of HCV treatment for PWID.
Key limitations are the limited empirical data on PWID health utilities, treatment rates and intervention impact.
Introduction
In developed countries, the hepatitis C virus (HCV) is spread primarily through injecting drug use, with over 90% of new infections among people who inject drugs (PWID).1 However, diagnosis rates are low, with only half of the infected PWID in the USA and the UK diagnosed.2
The majority of HCV testing performed in the USA and the UK is through venepuncture, which is available in virtually all prisons,3 and addiction services (structured programmes providing pharmacological or non-pharmacological drug treatment in the community) either on site or by referral. However, testing opportunities among PWID may still be limited. This is because venous access can be poor and specialist staff (who may not be available at all potential testing sites) are required to take blood, which if only available in hospital phlebotomy services can increase stigma.4
Dried blood spot (DBS) testing is non-invasive and can be performed by clinical and non-clinical staff. Two UK studies5 6 showed that offering DBS testing within specialist addiction services and prisons led to a threefold to sixfold increase in HCV testing, and a recent systematic review identified DBS as the best available targeted intervention for increasing HCV case-finding among PWID.7 Hence, DBS testing could be an important component of any strategy attempting to scale up treatment provision for PWID, for both care and prevention.8
We perform a cost-utility analysis of introducing DBS testing among current and former PWID in specialist addiction services and prisons in the UK.5 Unlike previous economic evaluations of HCV testing in these settings,9 10 we incorporate a dynamic mathematical model to capture the potential prevention benefits of treatment, which can substantially increase the cost-effectiveness of HCV treatment for PWID.11 A dynamic model accounts for the individual and population benefits of treatment, as well as the dynamic nature of incarceration, especially among PWID. Our model is the first to explore the importance of continuity of care between prison and the community.
Methods
Mathematical model
An existing dynamic, deterministic model of HCV transmission, progression and HCV treatment was adapted to project the impact of introducing DBS testing in prisons and addiction services.11 See online supplementary appendix for details and model schematics. Briefly, the model stratifies by: injecting state (never PWID/PWID/ex-PWID); incarceration status (never/currently/formerly); contact with addiction services (in contact/not in contact); age ((15–19), (20–24), (25–29), (30–54), (55–64), (65–74), (75+)); HCV infection and disease progression (never infected, spontaneously cleared, mild HCV, moderate HCV, compensated cirrhosis, decompensated cirrhosis, hepatocellular carcinoma, liver transplant, post-transplant). HCV disease stages are further subdivided into undiagnosed or diagnosed, where those who are diagnosed can either be lost to follow-up, in referral (early/late), on antiviral treatment, sustained viral response (SVR) or non-SVR.
All PWID can acquire and transmit HCV, but imprisoned PWID only transmit HCV to other prisoners. We define ex-PWID as those who have permanently ceased injecting, and assume no ongoing transmission from non/ex-PWID. An individual's risk of acquiring HCV is proportional to the setting-specific HCV prevalence (prison/community). The model assumes a background rate of HCV testing for all PWID and ex-PWID in the community/prison, and in addiction services for PWID.
No UK data exist regarding continuity of care (treatment or referral) on prison entry/exit, but experts described difficulty in ensuring continuity on release (Eamonn O'Moore(Offender Health, UK Department of Health), Iain Brew(HMP Leeds) personal communication). Therefore, in our base case, we assume that those in treatment or referral become lost to follow-up upon entering/exiting prison, but can be retested/retreated.
Model fitting and base-case projections
For the probabilistic uncertainty analysis, 1000 parameter sets were sampled from each parameter uncertainty distribution in table 1 and online supplementary appendix tables 1 and 2. For the parameter set, the model was calibrated to UK epidemiological data on incarceration, injecting drug use, HCV prevalence and diagnosis. This was achieved through a multistep parameter sampling and model calibration process, utilising simplified models where possible to reduce computational time and to verify the full model predictions against simplified models. For details on the model calibration (including schematics and equations) and initialisation (see online supplementary appendix).
Table 1.
Intervention parameters
| Mean value | Distribution | Units | References | |
|---|---|---|---|---|
| Intervention effect (proportional change in testing rate) | ||||
| Addiction services | 3.6 (2.3–5.8) | Lognormal (μ=1.285, σ=0.239) | – | 5 |
| Prison | 2.6 (0.2–34.9) | Lognormal (μ=0.968, σ=1.317) | – | 5 |
| Intervention costs (addiction services) | ||||
| Organisation/coordination of training* | 2005.71 | Per health board | † | |
| Training session‡ | 135 | Per training session | † | |
| Attendees time§ | 1620 | Per training session | † | |
| Travel reimbursement for training leader¶ | 90.86 | Per training session | † | |
| Total cost per addiction services training | 3851.57 | Per training session | † | |
| Mean number tested | 40.3 | Per addiction service** | 5 | |
| Total intervention cost per test | 95.57 | Uniform±50% | Per test | |
| Intervention costs (prison) | ||||
| Organisation/coordination of training†† | 7020 | Per prison | † | |
| Training session‡ | 135 | Per prison | † | |
| Attendees time‡‡ | 405 | Per prison | † | |
| Travel reimbursement for training leader§§ | 127.20 | Per prison | † | |
| Total cost per prison training | 7687.20 | Per prison | † | |
| Mean number tested per prison | 116 | Per prison | 5 | |
| Total intervention cost per test | 66.27 | Uniform±50% | Per test | |
All cost estimates assume a staff-nurse cost per hour of £36 (median estimate for band 5 general practice nurse21).
*1 Nurse 2 days/week for 6 months for seven health boards. One training session per health board.
†Noel Craine, personal communication.
‡1 Nurse, half day.
§12 Nurses, half day.
¶1200 miles (£0.53 per mile) for travel to seven health boards.
**Assumed 1 addiction service per health board.
††1 Nurse full time for five prisons (1 training session per prison).
‡‡3 Nurses per prison, half day.
§§1200 miles (£0.53 per mile) for five prisons.
After calibration, for each of the 1000 parameter sets, the model was run with and without the intervention (‘intervention’ and ‘baseline’, respectively). We model an intervention of offering DBS testing in prison, compared to a baseline of current testing with venepuncture only. Additionally, we evaluate an intervention of offering DBS in specialist addiction services, compared to a baseline of current testing with venepuncture. The economic analysis was performed from a UK National Health Service perspective. Costs (in 2011 GBP, £1=US$1.55) and health utilities (in QALYs) were attached to each model compartment. Costs and QALYs were discounted at 3.5% per annum as per the National Institute for Health and Clinical Excellence guidelines, with a 100-year time horizon (to accrue individual and population benefits). The mean incremental cost-effectiveness ratio (ICER) was calculated and cost-effectiveness determined using the UK willingness-to-pay (WTP) threshold, estimated between £20 000 and £30 000 per QALY gained.12 The cost-effectiveness acceptability curves were constructed and univariate sensitivity analyses undertaken. Analysis of covariance (ANCOVA) methods were used to summarise the proportion of the variability in the incremental costs and QALYs explained by the uncertainty in input parameters.13
Parameters
All parameters can be found in table 1 and the online supplementary appendix tables 1–4.
Health state utilities
Uninfected utility values were taken from the UK population norms for non-PWID, and a large cross-sectional study of injectors in Scotland14 for current PWID. We assumed equal utilities for ex-PWID and non-PWID.10 Utilities for the HCV disease and treatment stages came from the UK HCV trials and economic evaluations15–17 and were used for ex-PWID. To derive PWID HCV utilities, non-PWID HCV utilities were rescaled by multiplying by the ratio of the uninfected PWID utility to the uninfected ex-PWID utility for the youngest age group. All states included disutilities with age.
No disutility was associated with testing in the base case. However, some evidence suggests that PWID may experience a disutility after positive HCV diagnosis.14 18 We explored the impact of a disutility (0.09,14 see online supplementary appendix) on diagnosis, which was fully regained with treatment SVR.
Health state and testing costs
Healthcare costs for HCV disease stages, antiviral treatment (pegylated interferon-α and ribavirin, pegIFN+RBV) and testing were taken from the UK economic analyses.15 16 19 20 Data on the yield (proportion tests Ab+) and prevalence in each setting were used to calculate the number of non-PWID tested for each PWID/ex-PWID (see online supplementary appendix). Costs were inflated to 2011 GBP using the Health and Community Hospital Service pay and prices index.21 Additional PWID treatment delivery costs were applied.11 We assumed that undiagnosed individuals would not incur HCV-related healthcare costs unless progressing to decompensated disease.9
HCV disease progression parameters
Transition rates between disease stages were taken from the UK economic evaluations.15–17 Although estimates were not PWID-specific, a recent meta-analysis suggests little evidence for differences in progression between PWID and non-PWID.22
HCV prevalence
PWID HCV chronic prevalence was estimated from the HCV antibody prevalence among PWID in England (45% (41% to 49%, 95%CI)23). As one-quarter of acute infections spontaneously clear, 24 we assume three-quarters of those who are ever exposed (antibody positive) are chronically infected, resulting in 35% chronic infection among PWID.
Incarceration duration
Incarceration duration for non-PWID and ex-PWID was age-stratified, with a mean of 8 months.25 However, PWID have shorter durations in custody than non-PWID.25–27 We used a 4-month PWID incarceration duration, based on an estimate for England and Wales.25 A recent study in Scotland reported a median sentence of 7.1 months in PWID,27 which, given that most prisoners serve approximately half their sentence,28 would also equate to a duration of 4 months.
Testing rates
The overall baseline PWID testing rate (mean 12% undiagnosed PWID per year) was estimated through fitting the model to the current proportion of diagnosed PWID (approximately 50%2). Data on the proportion of tests from each setting (29% and 12% of tests originated from addiction services and prisons, respectively) were used in combination with the model projected annual numbers of PWID in contact with each setting to calculate setting-specific testing rates (0.5% and 2% per month of undiagnosed PWID in contact with addiction services and prisons, respectively, see online supplementary appendix). We assume that ex-PWID are tested at equal rates to PWID in prison and in general community settings. We assumed all diagnostic tests to be 100% accurate due to the high sensitivity and specificity of DBS (99.6% sensitivity, 100% specificity in a setting with 50% prevalence29) and venepuncture assays,30 and because those who receive an initial positive test will receive additional tests before treatment.
Referral and treatment transition rates
The referral rate from testing services to secondary care (35%) was estimated from a UK study.31 Those not referred or not attending referral were considered to be ‘lost to follow-up’.
Approximately 50% of diagnosed ex-PWID in referral are treated within 2 years.31–33 Since many delay treatment, we assume that after 2 years, 10% of those in referral initiate treatment annually. Within prison, treatment rates are lower than in the community,31 34 although a recent UK prison audit found that 24% of those diagnosed were treated (Iain Brew(HMP Leeds), unpublished data). We therefore estimated halved treatment initiation rates in prison as compared to the community.
PWID treatment rates are unknown, but thought to be similarly low to other countries,35 36 with an estimated <1% of PWID treated annually (Graham Foster(Consultant Hepatologist), personal communication). Hence, if we assume that 1% of infected PWID are treated within 2 years, this equates to treating approximately 5.5% of those who attend referral (35% of the 50% diagnosed) within 2 years. After 2 years, 1% of those in referral are treated annually thereafter.
Intervention
The effect of introducing DBS was modelled by assuming a 3.6-fold increase in testing (2.2 to 5.8 CI) in addiction services, and a 2.6-fold increase in testing (0.1 to 34.9 CI) in prison, based on two multicentre studies (table 1 and online supplementary appendix). Intervention costs were determined from the study methods5 and in consultation with the authors (table 1).
Sensitivity analyses
We performed one-way sensitivity analyses on: time horizon (50/200 years), discount rates (3.5% costs/1.5% QALYs), PWID treatment rates (increased in each setting), PWID SVR rates (reduced by 20%), PWID HCV chronic prevalence (20%/50%), antiviral treatment (telaprevir/boceprevir for genotype 1 patients, see online supplementary appendix) and continuity of care for treatment/referral on entry/exit from prison (varied from 0% to 100%). We also explored the effect of assuming no prevention benefit (but allowing for reinfection) by permanently fixing the force of infection.
Results
Case finding in addiction services
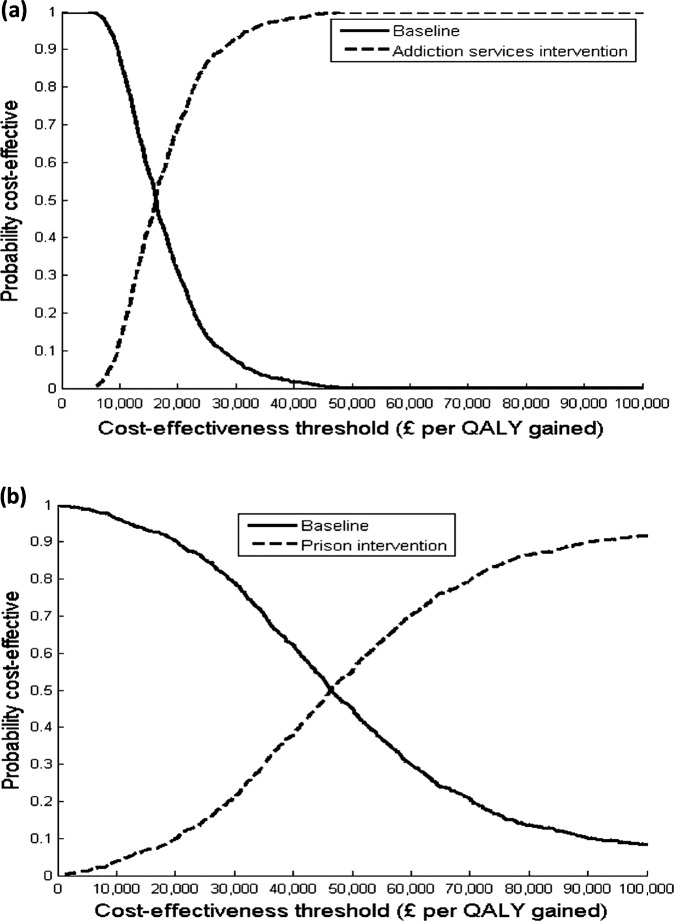
The incremental cost effectiveness ratio (ICER) of increasing case-finding in addiction services, by introducing DBS testing, was an estimated £14600 (US$22 630) per QALY gained in the base case (table 2). At a £20 000 or £30 000 WTP threshold, the intervention is likely to be cost-effective in 69% or 93%, respectively, of the simulations (figure 1A). Uncertainty in the intervention effect contributed to 86% and 58%, respectively, of the variation in incremental costs and QALYs. The remaining variation in incremental QALYs was mainly due to uncertainty in the treatment rates (22%) and health utilities (17%).
Table 2.
Cost-effectiveness results from the base-case intervention analyses
| Intervention location | Discounted costs (2011 £) (95% interval) | Discounted QALYs (95% interval) | Incremental costs (95% interval) | Incremental QALYs (95% interval) | ICER (£ per QALY gained) |
|---|---|---|---|---|---|
| Addiction services | |||||
| Baseline | 37 181 582 (19 384 816 to 67 271 249) | 5 354 331 (4 867 168 to 5 960 766) | – | – | – |
| Intervention | 38 099 060 (20 140 578 to 68 378 488) | 5 354 393 (4 867 206 to 5 960 853) | 917 478 (481 174 to 1 664 430) | 63 (19 to 153) | 14 632 |
| Prison | |||||
| Baseline | 37 181 582 (19 384 816 to 67 271 249) | 5 354 331 (4 867 168 to 5 960 766) | – | – | – |
| Intervention | 38 245 293 (19 852 634 to 68 601 970) | 5 354 349 (4 867 184 to 5 960 823) | 1 063 710 (−225 101 to 6 060 267) | 18 (−12 to to 75) | 59 418 |
Figure 1.
Base-case cost-effectiveness acceptability curves for the dried blood spot intervention. Results shown for the (A) addiction services and (B) prison interventions for various willingness-to-pay thresholds.
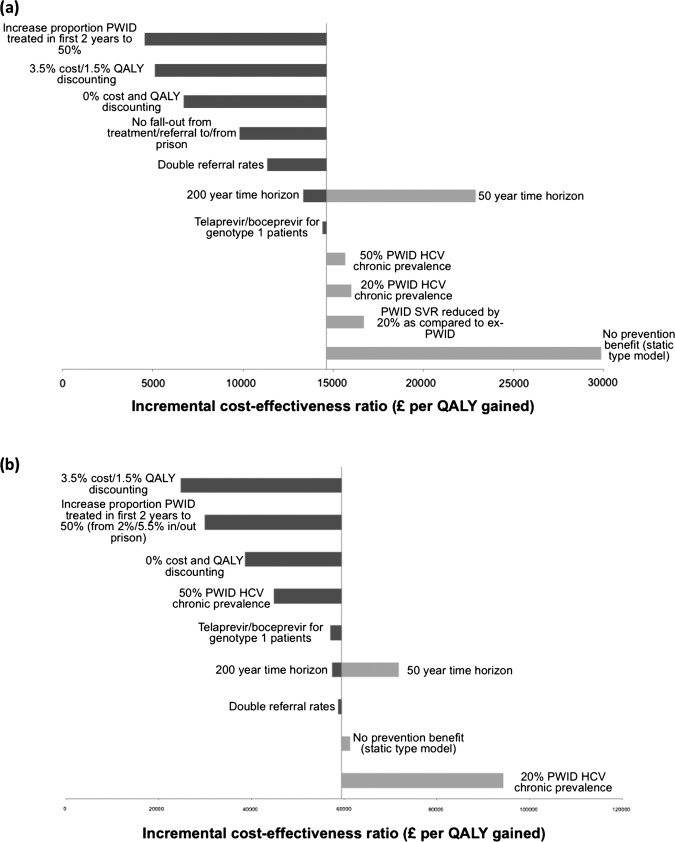
For most sensitivity analyses, the ICER remained below a £30 000 WTP threshold (figure 2A). Reducing the time horizon to 50 years increased the estimated ICER to £22 900 per QALY gained because fewer prevention benefits were accrued, whereas lengthening to 200 years increased the cost-effectiveness. Changing the discount rates to 3.5% costs/1.5% QALYs or no discounting decreased the estimated ICER to £5100 or £6700 per QALY gained, respectively. Variations in baseline HCV chronic prevalence had little effect (<10%). At lower prevalence (20%), identifying cases was more expensive but the prevention impact was greater due to the reduced reinfection risk, whereas the opposite occurred at higher prevalence (50%).
Figure 2.
Univariate sensitivity analyses on the mean incremental cost-effectiveness ratio (ICER). Results shown for the dried blood spot intervention in (A) addiction services and (B) prison. Vertical line represents the base-case ICER, estimated at (A) £14 600 per QALY gained and (B) £59 400 per QALY gained.
Increasing treatment rates increased the intervention's cost-effectiveness. If 50% (compared to 5.5% for base case) of PWID in referral initiated treatment within 2 years (a treatment rate achieved by one UK service,37) the ICER fell to £4500 per QALY gained. If SVR rates among PWID were 20% lower than in ex-PWID, the ICER increased by 14% (£16 700per QALY gained). Using telaprevir/boceprevir for genotype 1 patients minimally altered the ICER. Ignoring any prevention benefit doubled the ICER to £29 900 per QALY gained.
Only one sensitivity analysis substantially altered the cost-effectiveness conclusion. If a disutility was attached to diagnosis, the intervention resulted in negative incremental QALYs (due to low treatment rates) and was dominated (more expensive with fewer health benefits). However, even with this disutility, if treatment rates were increased to 50% of PWID in referral initiating treatment within 2 years, then the estimated ICER was £20 100 per QALY gained.
Case finding in prison
The ICER of increasing case-finding in prison, by introducing DBS testing, was estimated at £59 400 (US$92 070) per QALY gained (21% likely to be cost-effective at a £30 000 WTP threshold) in the base case (table 2 and figure 1B). Uncertainty in the intervention effect contributed to most (>85%) of the variation in incremental costs and QALYs.
The base-case conclusion was robust to most one-way sensitivity analyses (figure 2B)—including time horizon, discount rates, HCV prevalence and use of new treatments. If 50% of PWID in referral initiated treatment within 2 years, the ICER would be halved to just below £30 000 per QALY gained.
Introducing continuity of care (which measures the proportion of initiated treatments/referrals that are continued when entering/exiting prison) led to an increase in cost-effectiveness: from an ICER of £59 400 per QALY gained with 0% continuity to £10 400 per QALY gained with 100% continuity (figure 3). The ICER fell below £20 000 when >40% continuity of care was ensured; at 40% continuity, the intervention was 57% and 83% likely to be cost-effective at the £20 000 and £30 000 WTP thresholds, respectively. The level of continuity required for prison case-finding to be cost-effective also depended on the treatment rates. If the prison treatment rates were increased to equal those in the community (50%/5.5% of ex-PWID/PWID treated within 2 years of referral), then 35% of the continuity results in an ICER would be just below £20 000 per QALY gained. Increasing treatment rates further so that 50% of all referred prisoners initiate treatment within 2 years lowers the required continuity to 20% for an ICER below £20 000.
Figure 3.
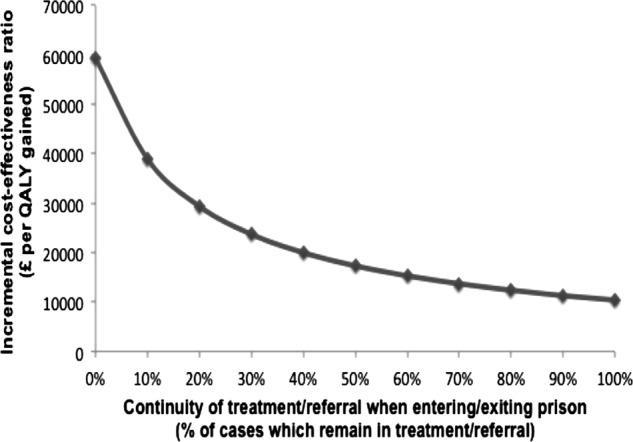
Incremental cost-effectiveness ratios for the prison intervention with varying continuity of care assumptions. Base-case scenario assumed 0% continuity.
Discussion
Main findings
Our results indicate that the introduction of dried blood spot testing for HCV case-finding is likely to be cost-effective under commonly used willingness-to-pay thresholds in the UK (£20 000–£30 000/QALY gained12) and the USA ($50 000/QALY gained38) in addiction services, but not in prison, unless a minimum level of continuity of care in treatment or referral between prison and the community can be ensured. Ignoring the prevention benefit doubles the ICER of the intervention in addiction services. In the base case, most PWID treatments initiated in prison were interrupted due to the lack of continuity of care and short PWID incarceration times (∼4 months) in the UK.25 27 Consequently, little prevention benefit was achieved from the prison intervention, with the results approaching the ‘static’ model. With the low base-case PWID treatment rates, the continuity required for DBS to be cost-effective was approximately 35–40% of the estimated treatment/referral rates, but if the treatment/referral rates increased, then lower levels of continuity would be cost-effective. Crucially, not all treatments need to be initiated or completed in prison, as only maintaining treatment or referral contact is necessary. Finally, both interventions are most cost-effective at higher treatment rates.
Strengths and limitations
The key strength of this analysis is that the model is dynamic, therefore capturing the prevention impact of case-finding and treatment. The main limitations are concerned with parameter uncertainty and lack of model heterogeneity. First, we based our increase in case-finding on the DBS intervention which, though empirically founded, was informed by relatively small UK studies, resulting in wide uncertainty around the effect estimates.
Second, the base case assumed comparatively low treatment rates for PWID, partly because the UK data on PWID treatment numbers are not available. This information is critical, as higher treatment rates increase the cost-effectiveness. This is especially important for prisons where treatment completion information was unavailable, yet strongly influenced cost-effectiveness. Additionally, even if treatment is interrupted, some may benefit from shortened treatment, which we did not incorporate.
Third, more data are needed to quantify PWID health utilities, which can be below the general population.39 Especially important is whether any transient or permanent disutility on HCV diagnosis occurs, as current data are weak and not based on prospective studies. Our projections indicate that if a disutility occurs, then higher treatment rates are required for case-finding to be cost-effective.
Fourth, the model did not incorporate other interventions or behaviours that may influence HCV risk or treatment uptake. However, modelling work has shown introducing risk heterogeneity does not substantially reduce intervention impact if PWID circulate between risk states,40 which is likely to occur as individuals move in/out of drug treatment and prison.
Fifth, the model was parameterised to the UK data, so our results are not necessarily applicable to other settings. However, our conclusions are robust to changes in HCV prevalence. Continuity of care could also be an issue in Australia, where PWID incarceration duration is similar to the UK.41 However, sentences are longer in the USA,42 so fewer treatments may be interrupted, and therefore case-finding in US prisons could be more cost-effective than our results indicate.
Our modelled UK treatment and HCV healthcare costs are within the range of those presented by recent US studies,43 44 with the exception of approximately threefold higher liver transplantation costs, which would increase the cost-effectiveness of case-finding in the USA. Testing costs were taken from UK economic evaluations; however, it is possible that a streamlined and experienced testing service could lower costs associated with staff time, thus increasing cost-effectiveness.
Sixth, we were unable to evaluate future interferon-free direct-acting antiviral therapies as information on treatment costs and health utilities is unavailable. These treatments will most likely have increased SVR (90% for all genotypes), shorter treatment durations (12–24 weeks), lower toxicity and simpler dosing regimes.45 Therapies with a shorter duration could not only increase the impact of testing and treatment in prison as more patients will be able to complete therapy prior to release, but also could potentially be more cost-effective depending on the ratio of additional costs to incremental impact.
Comparison with other studies
Two publications evaluated the cost-effectiveness of testing ex-PWID in prison, with ICERs varying from about £20 00010 to £55 0009 per QALY gained. Our results are consistent with Sutton et al,9 who used the same discount rates as our study did. However, we included the possible prevention impact of treating PWID, and unlike the previous studies, show how continuity of care between prison and the community can make case-finding cost-effective.
Three papers evaluated testing PWID in drug services.10 20 46 Differences in baseline assumptions led to varying ICERs from £28 10020 to £17 50010 46 per QALY gained. Our results for addiction services support those found in the latter studies.10 46 However, the intervention examined in these studies10 20 46 was one-off testing using a cohort model (with no evidence-based intervention effect) and neglected any prevention benefit.
Several US studies examined birth cohort screening for all people born in 1945–196544 47 or in 1946–197043 as compared to risk-based screening, reporting ICERs of $38 000 per QALY gained with direct-acting antivirals43 44 and $5 400–16 000 per QALY gained with pegIFN+RBV.44 47 Critically, the cost-effectiveness varies substantially by HCV prevalence,47 and the estimated US prevalence is higher than that of many other developed countries. Additionally, ICERs were generated given assumptions of higher treatment rates, as well as greater utility gains with SVR than we had considered. Importantly, our intervention targets PWID with a risk of transmitting infection to others, whereas birth cohort screening is likely to identify infections among ex-injectors and non-injecting populations, which will have little primary prevention impact.
Implications
Our cost-effectiveness work indicates that increasing HCV case-finding in addiction services can be cost-effective. However, the cost-effectiveness of prison case-finding interventions depends on adequate continuity of care with the community. Few settings have developed comprehensive strategies to address this issue, though New York recently initiated the Hepatitis C Continuity Program.48 In all settings, treatment uptake is critical: higher treatment rates prevent more disease transmission and increase the cost-effectiveness of case-finding interventions. If a disutility on diagnosis occurs, higher treatment rates would be necessary to ensure cost-effectiveness. Further empirical data are required on treatment uptake and changes in utilities following diagnosis and treatment in order to compare targeted case-finding with cohort models.
Supplementary Material
Acknowledgments
We would like to thank the following for their insights and/or data: Iain Brew, Eamonn O'Moore, Sarah Collins, Noel Craine, Graham Foster, Anjan Ghosh, Cathie Gillies, Vivian Hope, Scott McDonald, Fortune Ncumbe, Mary Ramsay, Katy Turner.
Footnotes
Contributors: NKM contributed to the study design, model development, analysis, manuscript drafting and editing. MH contributed to the study design, model development, analysis and manuscript editing. AM contributed to the analysis and manuscript editing. SJH contributed to the model parameterisation, analysis and manuscript editing. AT contributed to the model parameterisation and manuscript editing. PV contributed to the study design, model development, analysis and manuscript editing. All authors have read and approved the final version of the manuscript.
Funding: This work is produced by NM under the terms of the postdoctoral research training fellowship issued by the NIHR. The views expressed in this publication are those of the author and not necessarily those of the NHS, The National Institute for Health Research or the Department of Health. PV was supported by Medical Research Council New Investigator Award G0801627. MH received support from NIHR School of Public Health, Nationally Integrated Quantitative Understanding of Addiction Harm (NIQUAD) MRC addiction research cluster, and support of The Centre for the Development and Evaluation of Complex Interventions for Public Health Improvement (DECIPHer), a UKCRC Public Health Research: Centre of Excellence. Funding from the British Heart Foundation, Cancer Research UK, Economic and Social Research Council (RES-590–28–0005), Medical Research Council, the Welsh Assembly Government and the Wellcome Trust (WT087640MA), under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged.
Competing interests: MH, AM, AT, AND PV have no competing interests. NM has received an honorarium for speaking at a conference sponsored by Janssen. SH has received honoraria for speaking at conferences sponsored by MSD, Janssen, Gilead and Roche.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Nelson PK, Mathers BM, Cowle B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011;378:571–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Health Protection Agency Colindale. Hepatitis C in the UK 2011, July 2011.
- 3.Department of Health, HPA Prison Infection Prevention Team, Liver Strategy Team. National survey of hepatitis C services in prisons in England. July 2012.
- 4.Harris M, Rhodes T. Venous access and care: harnessing pragmatics in harm reduction for people who inject drugs. Addiction 2011;107:1090–6 [DOI] [PubMed] [Google Scholar]
- 5.Hickman M, McDonald T, Ali J, et al. Increasing the uptake of hepatitis C virus testing among injecting drug users in specialist drug treatment and prison settings by using dried blood spots for diagnostic testing: a cluster randomized controlled trial. J Viral Hepat 2008;15:250–4 [DOI] [PubMed] [Google Scholar]
- 6.Craine N, Parry J, O'Toole J, et al. Improving blood-borne viral diagnosis; clinical audit of the uptake of dried blood spot testing offered by a substance misuse service. J Viral Hepat 2009;16:219–22 [DOI] [PubMed] [Google Scholar]
- 7.Jones L, Bates G, McCoy E, et al. 2012. A systematic review of the effectiveness & cost-effectiveness of interventions aimed at raising awareness and engaging with groups who are at an increased risk of hepatitis B and C infection. http://www.nice.org.uk/nicemedia/live/11957/5946/5946.pdf.
- 8.Martin NK, Vickerman P, Foster GR, et al. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modelling analysis of its prevention utility. J Hepatol 2011;54:1137–44 [DOI] [PubMed] [Google Scholar]
- 9.Sutton AJ, Edmunds WJ, Sweeting MJ, et al. The cost-effectiveness of screening and treatment for hepatitis C in prisons in England and Wales: a cost-utility analysis. J Viral Hepat 2008;15:797–808 [DOI] [PubMed] [Google Scholar]
- 10.Castelnuovo E, Thompson-Coon J, Pitt M, et al. The cost-effectiveness of testing for hepatitis C in former injecting drug users. Health Technol Assess 2006;2006:32. [DOI] [PubMed] [Google Scholar]
- 11.Martin NK, Miners A, Vickerman P, et al. The cost-effectiveness of HCV antiviral treatment for injecting drug user populations. Hepatology 2012;55:49–57 [DOI] [PubMed] [Google Scholar]
- 12.NICE. Guide to the methods of technology appraisal, 2008.
- 13.Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford: Oxford University Press, 2006 [Google Scholar]
- 14.McDonald S, Hutchinson SJ, Palmateer N, et al. Decrease in health-related quality of life associated with awareness of hepatitis C virus infection among people who inject drugs in Scotland. J Hepatol 2013;58:460–6 [DOI] [PubMed] [Google Scholar]
- 15.Shepherd J, Jones J, Hartwell D, et al. Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaulation. Health Technol Assess 2007;11:1–224 [DOI] [PubMed] [Google Scholar]
- 16.Wright M, Grieve R, Roberts J, et al. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess 2006;10:1–113 [DOI] [PubMed] [Google Scholar]
- 17.Hartwell D, Jones J, Baxter L, et al. Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment, or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess 2011;15:1–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalgard O, Egeland A, Skaug K, et al. Health-related quality of life in active injecting drug users with and without chronic hepatitis C virus infection. Hepatology 2004;39:74–80 [DOI] [PubMed] [Google Scholar]
- 19.British Medical Association British National Formulary, number 62. BMJ Publishing Group, 2011 [Google Scholar]
- 20.Stein K, Dalziel K, Walker A, et al. Screening for hepatitis C in injecting drug users: a cost utility analysis. J Public Health 2004;26:61–71 [DOI] [PubMed] [Google Scholar]
- 21.Personal Social Services Research Unit. Unit Costs of Health and Social Care 2011: University of Kent, 2011.
- 22.John-Baptiste A, Krahn MD, Heathcote J, et al. The natural history of hepatitis C infection acquired through injection drug use: meta-analysis and meta-regression. J Hepatol 2010;53:245–51 [DOI] [PubMed] [Google Scholar]
- 23.Harris RJ, Ramsay M, Hope VD, et al. Hepatitis C prevalence in England remains low and varies by ethnicity: an updated evidence synthesis. Eur J Public Health 2012;22:187–92 [DOI] [PubMed] [Google Scholar]
- 24.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat 2006;13:34–41 [DOI] [PubMed] [Google Scholar]
- 25.Sutton AJ, Gay NJ, Edmunds WJ, et al. Modelling the hepatitis B vaccination programme in prisons. Epidemiol Infect 2006;134:231–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bird AG, Gore SM, Cameron S, et al. Anonymous HIV surveillance with risk factor elicitation at Scotland's largest prison, Barlinnie. AIDS 1995;9:801–8 [DOI] [PubMed] [Google Scholar]
- 27.Taylor A, Munro A, Allen E, et al. Low incidence of hepatitis C virus among prisoners in Scotland. Addiction 2013;108:1296–304 [DOI] [PubMed] [Google Scholar]
- 28.Ministry of Justice Offender Management Statistics Quarterly Bulletin, April to June 2011, England and Wales, 2011
- 29.Judd A, Parry J, Hickman M, et al. Evaluation of a modified commercial assay in detecting antibody to hepatitis C virus in oral fluids and dried blood spots. J Med Virol 2003;71:49–55 [DOI] [PubMed] [Google Scholar]
- 30.Colin C, Lanoir D, Touzet S, et al. Sensitivity and specificity of third-generation hepatitis C virus antibody detection assays: an analysis of the literature. J Viral Hepat 2001;8:87–95 [DOI] [PubMed] [Google Scholar]
- 31.Irving WL, Smith S, Cater R, et al. Clinical pathways for patients with newly diagnosed hepatitis C—what actually happens. J Viral Hepat 2006;13:264–71 [DOI] [PubMed] [Google Scholar]
- 32.Jowett SL, Agarwal K, Smith BC, et al. Managing chronic hepatitis C acquired through intravenous drug use. Q J Med 2001;94: 153–8 [DOI] [PubMed] [Google Scholar]
- 33.Foster G, Goldin RD, Main J, et al. Management of chronic hepatitis C: clinical audit of biopsy based management algorithm. BMJ 1997;315:453–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skipper C, Guy JM, Parkes J, et al. Evaluation of a prison outreach clinic for the diagnosis and prevention of hepatitis C: implications for the national strategy. Gut 2003;52:1500–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grebely J, Raffa JD, Lai C, et al. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat 2009;16:352–8 [DOI] [PubMed] [Google Scholar]
- 36.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of Hepatitis C treatment among injection drug users. J Comm Health 2008;33:126–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkinson M, Crawford V, Tippet A, et al. Community-based treatment for chronic hepatitis C in drug users: high rates of compliance with therapy despite ongoing drug use. Aliment Pharmacol Ther 2009;29:29–37 [DOI] [PubMed] [Google Scholar]
- 38.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res 2008;8:165–78 [DOI] [PubMed] [Google Scholar]
- 39.Kimber J, Copeland L, Hickman M, et al. Survival and cessation in injecting drug users: prospective observational study of outcomes and effect of opiate substitution treatment. BMJ 2010;340:c3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vickerman P, Martin N, Turner K, et al. Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in HCV prevalence? Model projections for different epidemic settings. Addiction 2012;107:1984–95 [DOI] [PubMed] [Google Scholar]
- 41.Miller E, Bi P, Ryan P. Hepatitis C virus infection in South Australian prisoners: seroprevalence, seroconversion, and risk factors. Int J Infect Dis 2009;13:201–8 [DOI] [PubMed] [Google Scholar]
- 42.West H, Sabol W, Greenman S.2010. Bureau of Justice Statistics Bulletin: Prisoners in 2009. http://bjs.ojp.usdoj.gov/content/pub/pdf/p09.pdf.
- 43.McGarry LJ, Pawar VS, Parekh HH, et al. Economic model of a birth cohort screening program for hepatitis C virus. Hepatology 2012;55:1344–55 [DOI] [PubMed] [Google Scholar]
- 44.Rein D, Smith B, Witenborn J, et al. The cost-effectiveness of birth-cohort screening for Hepatitis C antibody in US primary care settings. Ann Inter Med 2012;156:263–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dore GJ. The changing therapeutic landscape for hepatitis C. Med J Aust 2012;196:629–32 [DOI] [PubMed] [Google Scholar]
- 46.Thompson Coon J, Castelnuovo E, Pitt M, et al. Case finding for hepatitis C in primary care: a cost utility analysis. Fam Pract 2006;23:393–406 [DOI] [PubMed] [Google Scholar]
- 47.Coffin PO, Scott JD, Golden MR, et al. Cost-effectiveness and population outcomes of general population screening for Hepatitis C. Clin Infect Dis 2012;54:1259–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein SJ, Wright LN, Birkhead GS, et al. Promoting HCV treatment completion for prison inmates: New York State's Hepatitis C continuity program. Public Health Rep 2007;122(Suppl 2):83–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.