Abstract
Neurons in the suprachiasmatic nucleus (SCN) display coordinated circadian changes in electrical activity that are critical for daily rhythms in physiology, metabolism, and behavior. SCN neurons depolarize spontaneously and fire repetitively during the day and hyperpolarize, drastically reducing firing rates, at night. To explore the hypothesis that rapidly activating and inactivating A-type (IA) voltage-gated K+ (Kv) channels, which are also active at subthreshold membrane potentials, are critical regulators of the excitability of SCN neurons, we examined locomotor activity and SCN firing in mice lacking Kv1.4 (Kv1.4−/−), Kv4.2 (Kv4.2−/−), or Kv4.3 (Kv4.3−/−), the pore-forming (α) subunits of IA channels. Mice lacking either Kv1.4 or Kv4.2 α subunits have markedly shorter (0.5 h) periods of locomotor activity than wild-type (WT) mice. In vitro extracellular multi-electrode recordings revealed that Kv1.4−/− and Kv4.2−/− SCN neurons display circadian rhythms in repetitive firing, but with shorter periods (0.5 h) than WT cells. In contrast, the periods of wheel-running activity in Kv4.3−/− mice and firing in Kv4.3−/− SCN neurons were indistinguishable from WT animals and neurons. Quantitative real-time PCR revealed that the transcripts encoding all three Kv channel α subunits, Kv1.4, Kv4.2, and Kv4.3, are expressed constitutively throughout the day and night in the SCN. Together, these results demonstrate that Kv1.4- and Kv4.2-encoded IA channels regulate the intrinsic excitability of SCN neurons during the day and night and determine the period and amplitude of circadian rhythms in SCN neuron firing and locomotor behavior.
Introduction
The suprachiasmatic nucleus (SCN) acts as a master circadian pacemaker driving daily rhythms in physiology and behavior (Dibner et al., 2010; Welsh et al., 2010). SCN neurons undergo changes in membrane potential and repetitive firing with a near 24 h period (Schwartz and Zimmerman, 1991; Welsh et al., 1995; Colwell, 2011). During the day, SCN neurons depolarize and fire action potentials repetitively; during the night, however, SCN neurons hyperpolarize and rarely fire. These daily rhythms in membrane potential and spontaneous firing depend on (Liu et al., 1997; Herzog et al., 1998; Albus et al., 2002; Nakamura et al., 2002) and modulate a transcription-translation feedback loop (Lundkvist and Block, 2005; Nitabach et al., 2005; Brown and Piggins, 2007). Currently, the mechanisms linking the daily changes in gene expression and membrane excitability are poorly understood.
The resting and active membrane properties of SCN neurons are determined by the interaction of multiple ionic conductances that function at the resting potential (Pennartz et al., 2002; Häusser et al., 2004; Jackson et al., 2004). The spontaneous daytime depolarization in membrane potential of SCN neurons is accompanied by an increase in input resistance (de Jeu et al., 1998, 2002; Kuhlman and McMahon, 2004) suggesting that decreased subthreshold K+ conductance(s) mediate the daytime depolarization and the increased firing of action potentials. Conversely, the nighttime hyperpolarization is associated with a decrease in input resistance, consistent with increased subthreshold K+ conductance(s). In addition, injection of depolarizing current converts SCN neurons from the electrically quiet nighttime state to regular firing, further supporting the hypothesis that subthreshold K+ channels are crucial regulators of the excitability of SCN neurons (Kuhlman and McMahon, 2004, 2006). Although specific roles for various K+ currents in determining the excitability of SCN neurons have been proposed (Kononenko et al., 2008; Colwell, 2011), exploring these hypotheses directly has been hindered by a lack of knowledge about the channel proteins responsible for individual currents and the limited availability of selective channel blockers.
A-type (IA) voltage-gated K+ (Kv) channels activate and inactivate rapidly on membrane depolarization and, on hyperpolarization, recover rapidly from inactivation (Connor and Stevens, 1971a; Birnbaum et al., 2004; Jerng et al., 2005; Covarrubias et al., 2008). In many neurons, these properties impact repetitive firing rates (Connor and Stevens, 1971b; Kang et al., 2000; Kim et al., 2005; Yuan et al., 2005; Khaliq and Bean, 2008). In some cells, IA channels are active at subthreshold membrane potentials, influencing cell input resistances and excitability (de Jeu et al., 2002; Yuan et al., 2005). IA is readily detected in SCN neurons and has been suggested to function in the regulation of repetitive firing rates (Huang, 1993; Bouskila and Dudek, 1995; Alvado and Allen, 2008). Using mice harboring targeted disruptions in the genes encoding the voltage-gated K+ (Kv) channel pore-forming (α) subunits, Kcna4 (Kv1.4−/−), Kcnd2 (Kv4.2−/−), or Kcnd3 (Kv4.3−/−) (Norris and Nerbonne, 2010), we directly tested the necessity of these α subunits in the generation of IA in SCN neurons and in regulating circadian rhythms in SCN neuron firing and locomotor behavior.
Materials and Methods
Animals.
Mice were maintained on a C57BL/6 background in the Danforth and Medical School animal facilities at Washington University. The four genotypes of mice used in this study were wild-type (WT) mice and mice harboring targeted genetic disruptions of the Kcna4 (Kv1.4−/−) (London et al., 1998), Kcnd2 (Kv4.2−/−) (Guo et al., 2005), or Kcnd3 (Kv4.3−/−) (Niwa et al., 2008) locus. All procedures were approved by the Animal Care and Use Committee of Washington University and conformed to US National Institutes of Health guidelines.
Behavioral recordings.
Adult (8- to 10-week-old) Kv1.4−/− (n = 17), Kv4.2−/− (n = 17), Kv4.3−/− (n = 6), and WT (n = 19) male mice were housed individually in cages equipped with a running wheel in light-tight chambers illuminated with fluorescent bulbs (2.4 ± 0.5 × 1018 photons/s*m2; General Electric). Running-wheel activity was recorded in 6 min bins (ClockLab software; Actimetrics) for 5–10 d in a 12 h light (L)/dark (D) cycle (lights on at 7:00 A.M.), 11–12 d in constant (DD), 10–18 d in the LD cycle (lights on at 7:00 A.M.), 15–16 d in a 6 h delayed LD cycle (lights on at 1:00 P.M.), and finally for 15–17 d in a 6 h advanced LD cycle (lights on at 7:00 A.M.).
The period of behavioral rhythmicity of each mouse was determined using χ2 periodogram analysis (Sokolove and Bushell, 1978) from continuous recordings of 10 d in DD (ClockLab software). Rhythmicity was considered statistically significant if the χ2 periodogram value exceeded the 99.9% confidence interval (Qp value). Additionally, the phase angle of entrainment in LD, number of days to re-entrain after shifts in the LD cycle, and total daily activity counts in DD were calculated for each mouse (ClockLab).
Cell culture and multi-electrode array recordings.
SCNs were explanted from 3- to 7-d-old Kv1.4−/−, Kv4.2−/−, Kv4.3−/−, or WT mice housed in a 12 h LD cycle. Genotypes were confirmed by PCR of tail DNA. For dispersed cultures, 4–6 tissue punches (400 μm in diameter), containing the SCN, were obtained from 200-μm-thick coronal slices, and neurons were enzymatically dissociated using papain, as previously described (Herzog et al., 1998). Viable neurons were plated at a density of at least 10,000 neurons/mm2 onto poly-d-lysine and laminin-coated multi-electrode arrays according to published methods (Aton et al., 2005) (60 10 μm diameter electrodes; Multichannel Systems). We maintained dispersed neuronal cultures in 1 ml of CO2-buffered DMEM (Sigma-Aldrich) medium with 10% fetal calf serum at 37°C with 5% CO2 for 2 weeks and then recorded extracellular action potentials for at least 5 d as described previously (Aton et al., 2005). Action potentials were digitized in real time (MC-Rack Software; Multichannel Systems) and discriminated off-line using principal component analysis (Offline Sorter; Plexon). Firing rates were binned in 10 min intervals (NeuroExplorer; Plexon).
Firing rate rhythms were evaluated by a Fast Fourier transformation as previously described (Aton et al., 2005). Data with a relative amplitude <0.2 defined neurons with a statistically significant circadian rhythm. Peak and trough firing rates were compared for the four genotypes. We used the Rayleigh test (Batschelet et al., 1981) to determine whether the times of peak firing for groups of SCN neurons were clustered or uniformly distributed.
Electrophysiological recordings from acute brain slices.
Brain slices were prepared from 3- to 6-week-old WT, Kv1.4−/−, Kv4.2−/−, and Kv4.3−/− mice using standard procedures (Aton et al., 2005). Briefly, mice housed in a 12 h LD cycle, with lights on at 7:00 A.M., were deeply anesthetized with 1.25% Avertin (2,2,2-tribromoethanol and tert-amyl alcohol in 0.9% NaCl; 0.025 ml/g body weight) between 12:00 and 2:00 P.M. and then perfused transcardially with ice-cold cutting solution containing the following (in mm): 240 sucrose, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, and 7 MgCl2, saturated with 95% O2/5% CO2 before cooling. The brains were rapidly removed and placed in oxygenated ice-cold cutting solution. Coronal slices (350 μm) containing the SCN were cut on a Leica VT1000 S vibrating blade microtome (Leica Microsystems). Slices were incubated in a holding chamber with oxygenated artificial CSF (ACSF) containing the following (in mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1 MgCl2, and dextrose 25 at room temperature for at least 30 min before transfer to the recording chamber. Recordings were obtained from SCN neurons in the acute slice between 1:00 and 5:00 P.M.
Whole-cell voltage-clamp recordings were obtained at room temperature (22−24°C) from visually identified SCN neurons using differential interference contrast with infrared microscopy. Data were collected using a Multiclamp 700B patch-clamp amplifier interfaced with a Digidata 1332 and the pCLAMP 9 software (Molecular Devices) to a Gateway computer. Series resistances were compensated electronically by ∼90%. Signals were acquired at 20 kHz and filtered at 10 kHz before digitization and storage. The recording pipette solution contained the following (in mm): 130 KCl, 10 HEPES, 10 glucose, 0.83 CaCl2, and 2.6 BAPTA, and 3 MgATP and 0.5 NaGTP were added the day of recording, pH 7.4 (300 mOsm). Tetraethylammonium (3 mm), CdCl2 (0.1 mm), and tetrodotoxin (100 nm) were added to the ACSF immediately before recordings. All reagents were from Sigma unless otherwise noted.
The rapidly activating and rapidly inactivating Kv current, IA, was isolated by a two-step voltage protocol, using previously described procedures (Norris and Nerbonne, 2010). Briefly, total whole-cell Kv currents were first evoked in response to 4 s depolarizing voltage steps to potentials between −40 and +40 mV (in 10 mV increments) from a holding potential of −70 mV. A prepulse paradigm that included a brief (60 ms) step to −10 mV before the 4 s depolarizing voltage steps to potentials between −40 and +40 mV (in 10 mV increments) was then used. Off-line subtractions of the currents evoked with the prepulse from the currents evoked without the prepulse were performed to isolate IA. The steady-state outward Kv current (ISS) was measured as the current remaining at the end of the 4 s depolarizing steps. Data were compiled and analyzed using ClampFit (Molecular Devices), Microsoft Excel, and Prism (GraphPad Software).
Quantitative real-time PCR.
Eight-week-old C57BL/6 mice were housed 12 h LD cycle for a week and then switched to constant darkness for 48 h. Mice were killed at specific circadian times (CT0, 4, 8, 12, 16, and 20; n = 4 per CT) on the third day of constant darkness and SCN punches (400 μm in diameter) were collected. Total RNA was isolated from the SCN and RNA concentrations were determined by optical density measurements. The expression levels of genes encoding the Kv4.2 (Kcnd2), Kv4.3 (Kcnd3), and Kv1.4 (Kcna4) α subunits, as well as the endogenous control gene hypoxanthine guanine phosphoribosyl transferase (HPRT) were determined using Taqman-based real-time PCR in a two-step process as described previously (Yang et al., 2010); experiments were conducted on a 7900HT Sequence Detection System (Applied Biosystems). Data were analyzed using the threshold cycle relative quantification method and were normalized to the expression value for HPRT in the same sample (Schmittgen and Livak, 2008) and evaluated for rhythmicity using COSOPT (Abraham et al., 2005). The primers sequences used to detect transcript expression were as follows: Kv4.2 (Kcnd2): 5′-TGAATCACGTTTGTGTCATTAGTGA and 5′-TTCAACTTGCGCTCATCTTAGG; Kv4.3 (Kcnd3): 5′-GCCGCAGCACCTAGTCGTT and 5′-CACCACGTCGATGATACTCATGA; Kv1.4 (Kcna4): 5′ AGAGGCGGATGAACCCACTA and 5′ GCCCACCAAAACGCATCT; and HPRT: 5′-TGAATCACGTTTGTGTCATTAGTGA and 5′-TTCAACTTGCGCTCATCTTAGG.
Results
The period of locomotor activity is altered in Kv1.4−/− and Kv4.2−/− mice
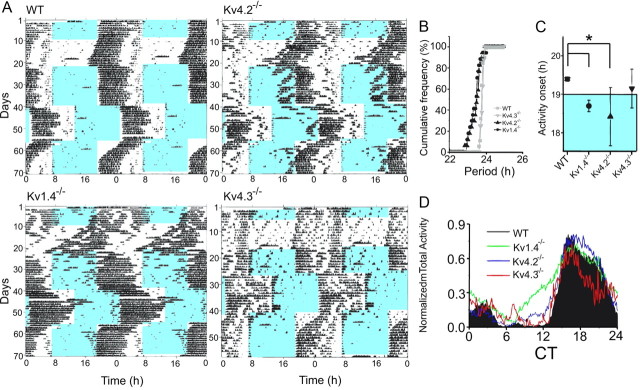
Representative recordings of wheel-running activity per unit time (actograms) in WT, Kv1.4−/−, Kv4.2−/−, and Kv4.3−/− mice are presented in Figure 1A. In constant darkness (DD), Kv1.4−/− and Kv4.2−/− mice displayed locomotor activity patterns with shorter circadian periods than WT or Kv4.3−/− mice (one-way ANOVA, p < 0.001; 23.8 ± 0.02 h for WT and 23.8 ± 0.04 h for Kv4.3−/−; 23.4 ± 0.07 h for Kv1.4−/− and 23.5 ± 0.07 h for Kv4.2−/−, mean ± SEM). Over 50% of the Kv1.4- or Kv4.2-deficient mice had periods shorter than WT mice (Fig. 1B). In addition, when housed in a 12 h LD cycle, Kv1.4−/− and Kv4.2−/− mice initiated daily wheel running ∼0.5 h earlier (Fig. 1C,D; one-way ANOVA, p < 0.04) than WT mice.
Figure 1.
The period of circadian locomotor behavior is reduced in Kv1.4−/− and Kv4.2−/− mice. A, Representative recordings of wheel-running activity of WT, Kv1.4−/−, Kv4.2−/−, and Kv4.3−/− mice over 72 consecutive days in different LD cycles (blue and white backgrounds, respectively). Each line plots wheel revolutions per minute over a 48 h period; data from the subsequent days are plotted on the line below. B, The cumulative distribution of the dominant periods reveals that nearly 80% of the Kv1.4−/− (n = 17) and Kv4.2−/− (n = 17) mice had shorter periods in constant darkness than WT (n = 19) or Kv4.3−/− (n = 6) mice. Kv1.4−/− (n = 17) and Kv4.2−/− (n = 17) mice also started running earlier in an LD cycle than WT (n = 19) or Kv4.3−/− (n = 6) mice; mean ± SEM time of onset of activity for each genotype is plotted in C. Normalized averaged total activity plots (D) also reveal the elevated activity of Kv1.4−/− (n = 17) and Kv4.2−/− (n = 17) during the subjective day compared with WT (n = 19) or Kv4.3−/− (n = 6) mice. All genotypes showed increased activity following cage changes (e.g., on days 19, 31, 45, and 59 in the WT trace).
The Kv1.4−/− and Kv4.2−/− mice also required fewer days to establish a stable phase relationship to the time of daily light onset following a 6 h advance in the light cycle (one-way ANOVA, p < 0.004; Fig. 2). It is also of interest to note that the Kv1.4−/− mice showed more total daily wheel running than WT (one-way ANOVA, p < 0.003), Kv4.2−/− or Kv4.3−/− mice, primarily due to an increase in the amount of time they were active each night) (Figs. 1A,D, 2; one-way ANOVA, p < 0.003). In marked contrast to the Kv1.4−/− and the Kv4.2−/− mice, the circadian period, phase angle of entrainment, ability to adjust to shifts in light schedule, and the daily wheel-running activity of Kv4.3−/− mice were statistically indistinguishable from WT mice.
Figure 2.
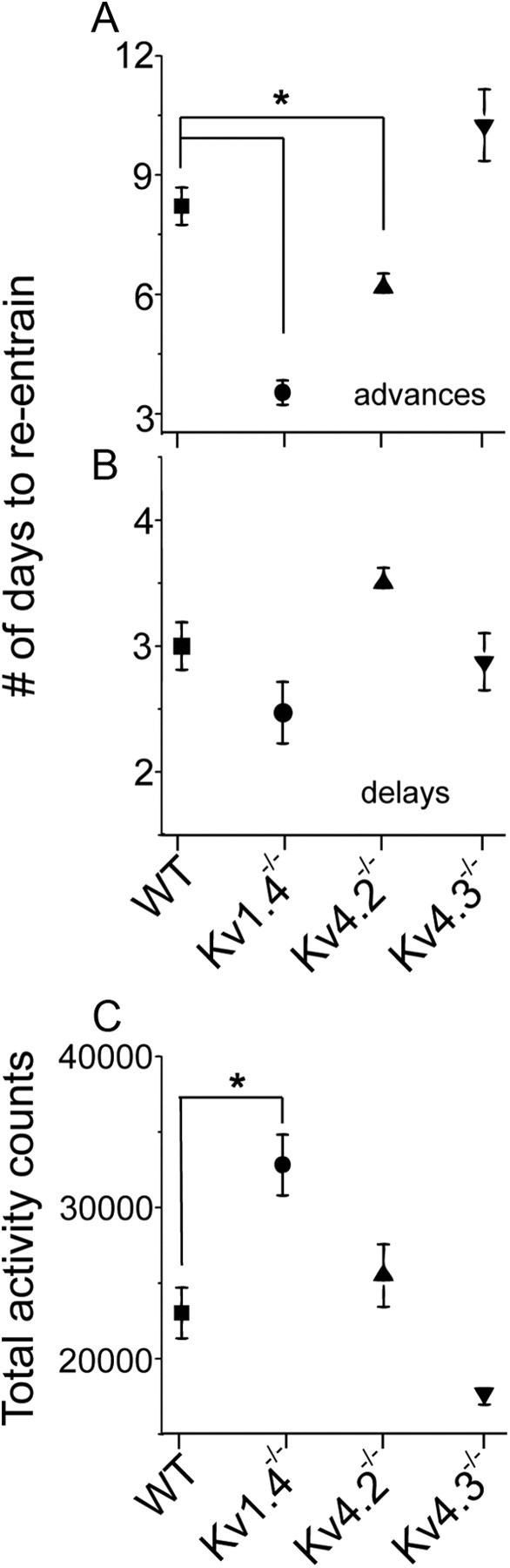
Loss of Kv1.4 and Kv4.2 channels affects resynchronization rates and total locomotor activity. A, Mice lacking Kv1.4-encoded (n = 17) or Kv4.2-encoded (n = 17) IA channels entrained faster when the light cycle was advanced by 6 h. B, In contrast, entrainment of Kv1.4−/− and Kv4.2−/− animals was indistinguishable from WT (n = 19) and Kv4.3−/− (n = 6) when the light cycle was delayed by 6 h. C, Kv1.4−/− mice consistently showed higher total wheel-running activity compared with the other genotypes. Data shown are means ± SEM.
IA densities are reduced in Kv1.4−/− and Kv4.2−/− mice
The observation that the alterations in the circadian patterns in wheel-running behavior of the Kv1.4−/− and Kv4.2−/− mice, compared with WT mice, were similar suggests that Kv1.4- and Kv4.2-encoded IA channels play similar roles in determining the onset and the period of circadian locomotor rhythms. Quantitative real-time PCR (qRT-PCR) analyses of mRNA transcripts from SCN samples revealed that the Kv1.4, Kv4.2, and Kv4.3 transcripts were readily detected in the mouse SCN. In contrast with Per2, the expression levels of the three Kv α subunits did not vary significantly with CT (Fig. 3; COSOPT test, p > 0.3).
Figure 3.
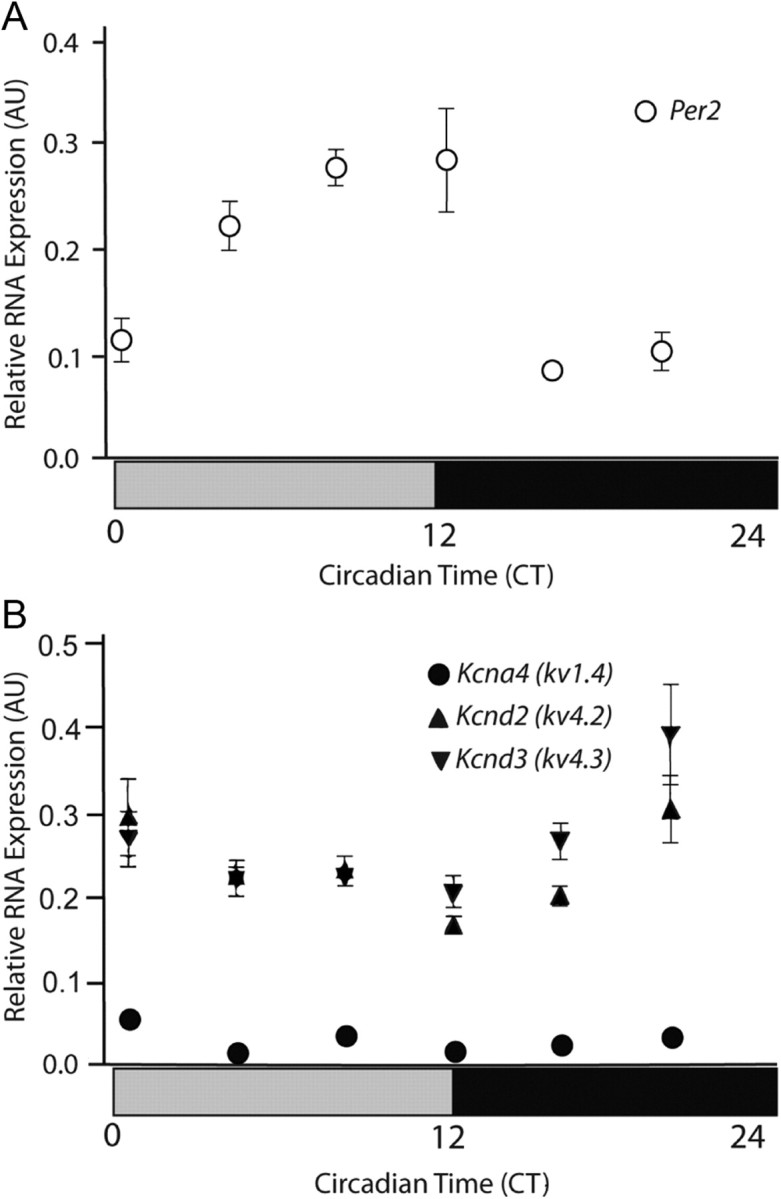
Kv channel α subunit expression levels in the SCN do not vary with CT. Transcript levels of Per2 (A) and of the IA channel pore-forming (α) subunits, Kcnd2, Kcnd3, and Kcna4 (B), were examined in the SCN as a function of CT. Transcript levels in each sample were determined by qRT-PCR and normalized to the Hprt transcript, as described previously (see Materials and Methods). Mice were maintained in constant darkness for two days before beginning these experiments. SCN tissue samples were collected (and frozen for subsequent RNA isolations) at the times indicated. Data are presented as means ± SEM (n = 4 mice per time point).
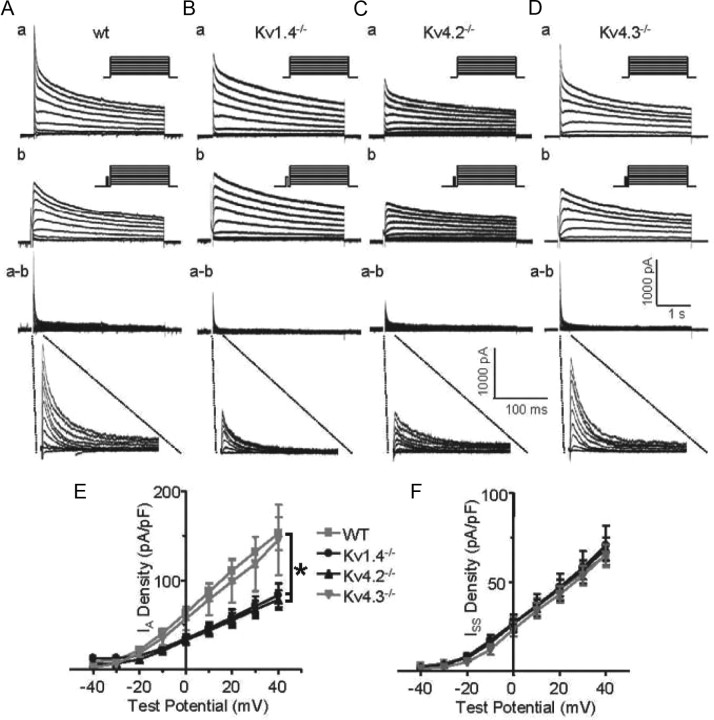
Whole-cell voltage-clamp recordings from SCN neurons in acute brain slices demonstrated that IA was present in all SCN cells examined, consistent with previous reports (Bouskila and Dudek, 1995; Itri et al., 2010), and that the densities of the currents were similar among SCN neurons. Voltage-clamp recordings from Kv1.4−/− and Kv4.2−/− neurons revealed that the loss of Kv1.4 or Kv4.2 attenuated mean IA densities (Student's t test, p < 0.001) by ∼50% (Fig. 4). The mean IA density in Kv4.3−/− SCN neurons, in contrast, was not significantly different from WT neurons, suggesting that Kv4.3 α subunits are not required for the generation of functional IA channels in SCN neurons.
Figure 4.
IA densities are reduced in Kv4.2−/− and Kv1.4−/− SCN neurons. Aa–Da, Representative whole-cell Kv currents, recorded in response to voltage steps to potentials ranging from −40 to +40 mV (in 10 mV increments) from a holding potential of −70 mV in WT (Aa), Kv4.2−/− (Ba), Kv4.3−/− (Ca), and Kv1.4−/− (Da) SCN neurons, are displayed. In each cell, outward Kv currents evoked at the same test potentials were also recorded following a brief prepulse to −20 mV to inactivate IA. The amplitudes of IA in individual cells of each genotype were then obtained by digital off-line subtraction (a-b) of the recordings with the prepulse (b) from the recordings without the prepulse (a); the subtracted records are also shown on an unexpanded time scale to facilitate direct comparisons. Mean (±SEM) IA (E) and steady-state Kv current (ISS) densities (F) in WT (n = 10), Kv4.2−/−(n = 20), Kv4.3−/− (n = 5), and Kv1.4−/− (n = 14) SCN neurons are plotted as function of test potential. *Values indicated are significantly different at the p < 0.001.
Firing rates of Kv1.4−/− and Kv4.2−/− SCN neurons are increased and circadian periods are shorter
To determine whether the markedly shortened periods in wheel-running activity seen in mice lacking either Kv1.4 or Kv4.2 relate to changes in circadian rhythms in SCN neuron firing, we measured the functional consequences of disrupting IA on the firing rates of isolated SCN neurons directly. Using multi-electrode arrays, we observed circadian patterns of electrical activity in SCN neurons from WT, Kv1.4−/−, Kv4.2−/−, and Kv4.3−/− mice (Fig. 5A), although the circadian periods of firing of Kv1.4−/− and Kv4.2−/− SCN neurons were significantly shorter (0.5 h) than in WT or Kv4.3−/− SCN neurons (Fig. 5A; one-way ANOVA, p < 0.03). The measured periods were 23.9 ± 0.1 h (mean ± SEM) for WT, 23.7 ± 0.2 h for Kv4.3−/−, 23.4 ± 0.1 h for Kv1.4−/−, and 22.7 ± 0.2 h for Kv4.2−/− neurons. The magnitude of this effect (0.5 h) is similar to the shortening of the period of locomotor activity seen in Kv1.4−/− and Kv4.2−/− mice (Fig. 1). Analyses of these data also revealed that the circadian periods of firing were shorter in only ∼40% of the Kv1.4−/− or Kv4.2−/− SCN neurons, whereas the remaining cells had circadian periods similar to WT or Kv4.3−/− cells (Fig. 5B; Kolmogorov–Smirnov test, p < 0.02).
Figure 5.
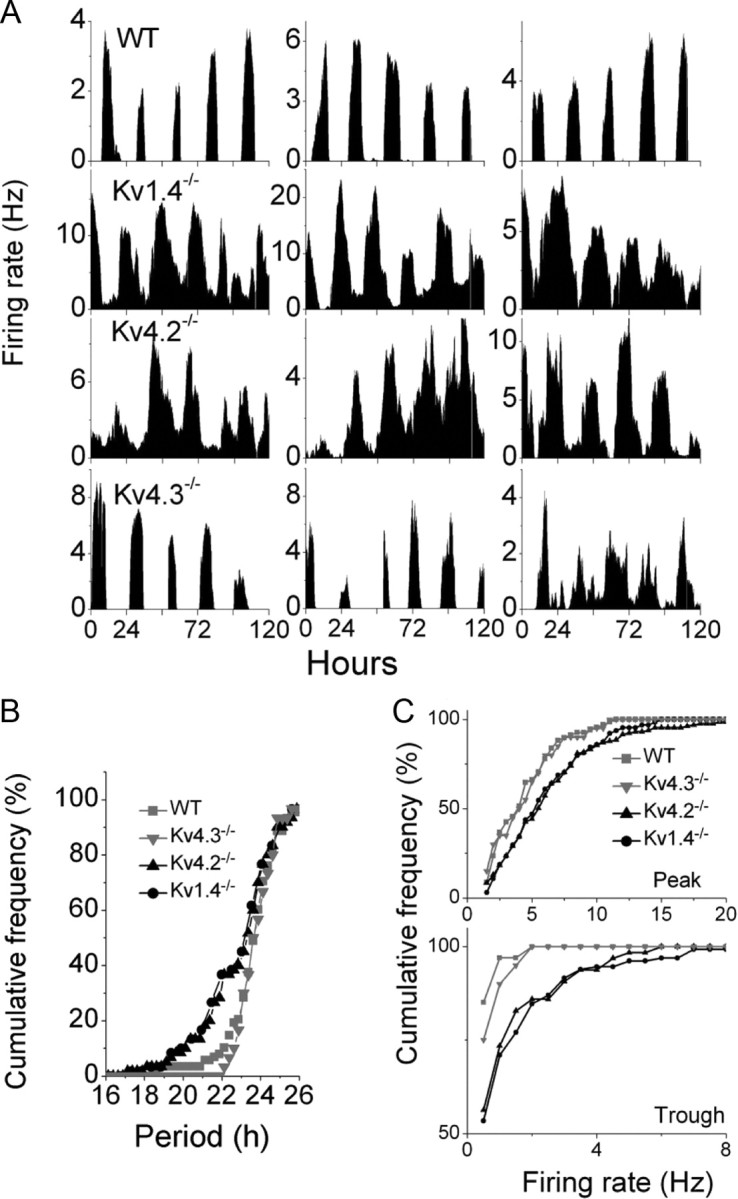
Kv1.4- and Kv4.2-encoded IA channels set the period and amplitude of circadian firing patterns in SCN neurons. Discharge profiles were recorded continuously over 5 d from dispersed SCN neurons as described previously (see Materials and Methods). A, Representative recordings from three SCN neurons of each of the four genotypes are illustrated. The circadian firing periods of individual cells were measured and averaged over the 5 d of recordings, and cumulative frequency plots of the measured mean values for each of the four genotypes are presented in B. Nearly 40% of Kv1.4−/− (n = 200) and Kv4.2−/− (n = 130) SCN neurons displayed markedly shorter circadian firing periods than WT (n = 106) or Kv4.3−/− (n = 30) SCN neurons. C, Plotting the cumulative distribution of mean peak and trough firing rates illustrates the marked increases in both (peak and trough) firing rates in Kv1.4−/− (n = 200) and Kv4.2−/− (n = 130) compared with WT (n = 106) or Kv4.3−/− (n = 30) SCN neurons.
Kv1.4−/− or Kv4.2−/− SCN neurons also fired at higher frequencies during both the subjective night and day than either WT or Kv4.3−/− SCN neurons significantly (Fig. 5C; Kolmogorov–Smirnov test, p < 0.01). The grouping of the daily peaks in the firing rates among Kv1.4−/− or Kv4.2−/− SCN neurons, however, was not significantly different from WT neurons (Fig. 6; Rayleigh test, p > 0.05).
Figure 6.
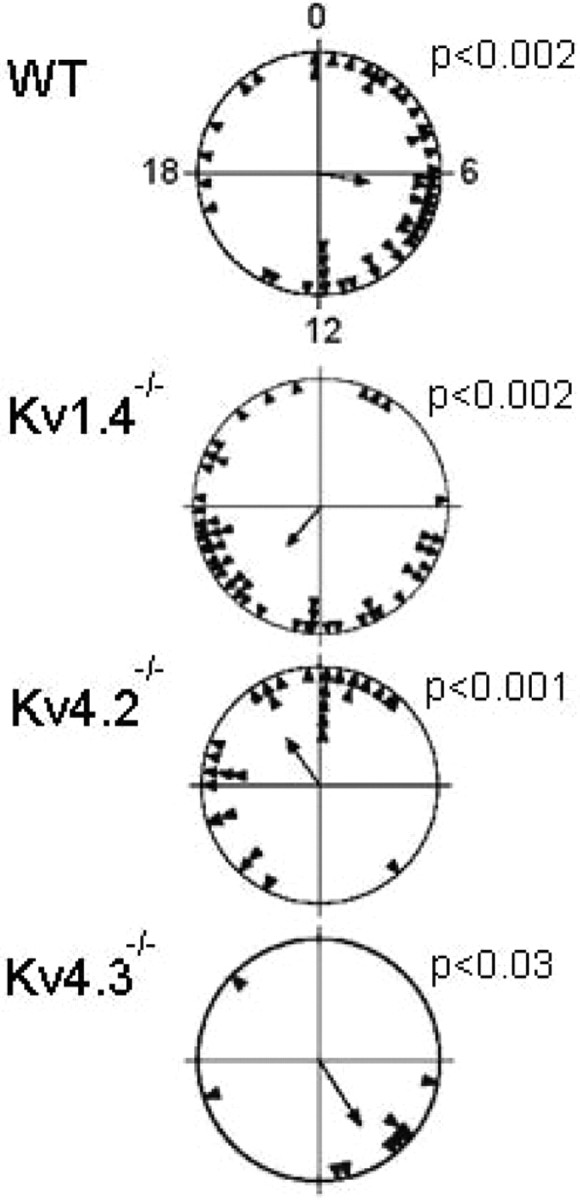
Circadian periods of individual SCN neurons are synchronized to each other independent of genotype. Rayleigh plots show the time of peak firing for each neuron (filled triangle) on a representative recording day. The Rayleigh statistic calculates the mean phase of the population of neurons (arrow) and the probability that the measured phases were uniformly distributed (p > 0.05). For these representative cultures from each of the four genotypes, the Rayleigh statistic indicates that the phases of the individual neurons were not random and were similarly distributed, suggesting that the loss of Kv1.4, Kv4.2, or Kv4.3 did not impact the synchronization of circadian firing patterns among SCN neurons.
Discussion
Together, the findings here demonstrate that the Kv1.4 and Kv4.2 α subunits encode IA channels in the SCN that control the intrinsic excitability, set the circadian firing period, and modulate the firing rates of SCN neurons. The voltage-clamp experiments revealed that the loss of either Kv1.4 or Kv4.2 reduces IA density in SCN neurons by ∼50%. Although Kv4.3 mRNA is expressed in the SCN and the Kv4.3 protein can associate and form heteromultimeric channels with Kv4.2 (Guo et al., 2002) and has been shown to encode IA channels in cortical pyramidal neurons (Norris and Nerbonne, 2010), the loss of Kv4.3 expression had no measureable effects on IA densities, electrical activity, or circadian rhythms in SCN neurons.
Circadian locomotor activity is altered in Kv1.4−/− and Kv4.2−/− mice
The results presented here demonstrate that the loss of either Kv1.4- or Kv4.2-encoded IA channels has very striking effects on the circadian period of behavioral rhythms in locomotor activity. Interestingly, loss of either Kv1.4 or Kv4.2 has a much larger effect on the circadian period than the loss of other genes, including some canonical clock genes, such as Clock (DeBruyne et al., 2007). In addition, the 0.5 h shortening of the period of circadian rhythms in locomotor activity of mice lacking either Kv1.4 or Kv4.2 contrasts with the recently reported 0.5 h lengthening of the period of circadian behavior in mice lacking one copy of Scn1a, which encodes the voltage-gated sodium channel α subunit, Nav1.1 (Han et al., 2012). The results here also contrast markedly with the results of several previous studies conducted on mice with other channel deficiencies, including mice lacking the large conductance, Ca2+ and voltage-dependent, BK (Kcnma1); K+ channel subunit (Meredith et al., 2006); the voltage-gated Ca2+ channel subunit, Cav2.2 (Cacna1b) (Beuckmann et al., 2003); or the Kv channel subunits, Kv3.1 and Kv3.2 (Kcnc1 and Kcnc2) (Kudo et al., 2011). In contrast with the findings here for Kv1.4−/− and Kv4.2−/− mice, negligible changes in circadian period were reported in BK-, Cav2.2-, or Kv3.1/Kv3.2-deficient mice, suggesting that Kv1.4- and Kv4.2-encoded IA channels subserve unique and important roles in modulating the functioning of the SCN and in regulating circadian biology.
Kv1.4- and Kv4.2-encoded IA channels regulate the intrinsic excitability of SCN neurons
The finding that both peak and trough firing rates were increased in Kv1.4−/− and Kv4.2−/− SCN neurons indicates that Kv1.4- and Kv4.2-encoded IA channels play critical roles in regulating the intrinsic excitability of SCN neurons during the day and during the night, i.e., throughout the circadian cycle. In addition, the qRT-PCR analyses revealed that the expression levels of the transcripts encoding these subunits do not vary with CT, consistent with previously published in situ hybridization (Lein et al., 2007) and DNA microarray data (Panda et al., 2002; Kasukawa et al., 2011). It has, however, recently been reported that IA density is higher during the day than at night in a subpopulation of SCN neurons (Itri et al., 2010). It is certainly possible that IA densities are altered in SCN neurons as a result of circadian regulation of the transcripts encoding critical accessory or regulatory proteins. Interestingly, and consistent with the hypothesis that accessory subunits could play a critical role, it was recently reported that the circadian regulation of Kv4.2-encoded Ito,f channels in mouse ventricular myocytes reflects circadian changes in the accessory Kv channel interacting protein, KChIP2, not in the Kv4.2 α subunit (Jeyaraj et al., 2012). Alternatively, post-transcriptional mechanisms, such as changes in Kv α subunit protein expression, localization and/or degradation, or altered interactions with other Kv α subunits, accessory subunits, or other regulatory proteins (Covarrubias et al., 2008; Norris et al., 2010) could mediate circadian changes in IA densities in SCN neurons. Additional experiments aimed at exploring each of these possibilities directly will be of considerable interest.
As classically described, the properties of rapid activation and inactivation position IA as a critical regulator of interspike interval and firing frequency (Connor and Stevens, 1971a,b). In addition, in some neurons, IA has been shown to contribute to resting, subthreshold K+ conductance, influencing input resistances, and excitability thresholds (de Jeu et al., 2002; Yuan et al., 2005). These unique properties, together with variable dendritic expression patterns, allow IA channels to play important roles in modulating the responses to synaptic inputs and to influence synaptic integration and neuronal output properties (Birnbaum et al., 2004; Jerng et al., 2004). In spontaneously active neurons, like those in the SCN, therefore, IA channels would be expected to function to regulate excitability and the initiation of firing by opposing membrane depolarizations, resulting from the closing/opening of other channels, as well as influence the voltage trajectory during the interspike interval, which will regulate repetitive firing rates (Connor and Stevens, 1971b; Kang et al., 2000; Kim et al., 2005; Yuan et al., 2005; Khaliq and Bean, 2008) Although IA was previously hypothesized to be critical in regulating the transition from the silent night state to the spontaneously active day state (Kim and Dudek, 1993; Bouskila and Dudek, 1995), the results presented here demonstrate that Kv1.4- and Kv4.2-encoded IA channels are instead critical in determining the threshold for firing of SCN neurons during the day and during the night. Other, yet to be identified subthreshold K+ channels, therefore, must be involved in determining the transitions between the night and day patterns of electrical activity in the SCN.
The role of IA in setting the circadian period of rhythmic firing seems likely to be intrinsic to individual SCN neurons. The experiments here demonstrated that dispersed Kv1.4−/− and Kv4.2−/− SCN neurons remain synchronized in the timing of daily maximum and minimum firing rates. In addition, Kv1.4−/− and Kv4.2−/− SCN neurons display a shortened circadian period in vitro that is very similar to the shortened period observed in locomotor behavior in Kv1.4−/− and Kv4.2−/− mice. The change in period observed at the cellular level supports the hypothesis that membrane excitability likely is an integral part of the core circadian clock (Nitabach et al., 2002, 2005; Lundkvist and Block, 2005). We conclude that IA functions to reduce the intrinsic excitability of SCN neurons, thereby contributing to the lengthening of circadian periods of firing and behavior.
The results presented here also hint at a novel role for Kv1.4 outside of the SCN. The increased duration of daily locomotion of mice lacking Kv1.4, for example, could reflect alterations in sleep–wake cycles, specifically reductions in daily sleep duration. Because the durations in daily firing patterns in SCN neurons deficient for Kv1.4 were not significantly different from WT SCN neurons, the behavioral phenotype may result from the functioning of Kv1.4-encoded IA channels outside the SCN. Together with previous studies linking Kv channel functioning to sleep in mice and in flies (Vyazovskiy et al., 2002; Cirelli et al., 2005; Douglas et al., 2007; Espinosa et al., 2008; Koh et al., 2008), the results here support the interesting possibility that modulation of Kv channels (and other conductances that regulate intrinsic neuronal excitability) play critical roles in both circadian biology and sleep.
In summary, these results demonstrate that, in the SCN, Kv1.4 and Kv4.2, but not Kv4.3, are critical subunits for the generation of rapidly activating and inactivating K+ currents that regulate the excitability, firing rate, and, consequently, the circadian period of firing and locomotor behavior.
Footnotes
This work was supported by the National Institute of Mental Health (R01-MH063104 to E.D.H.), the National Heart Lung and Blood Institute (R01-HL034161 to J.M.N.), and the National Institute of Neurological Disorders and Stroke (R21-NS065295 to J.M.N. and F32-NS065581, an individual National Research Service Award, to Y.C.). A.J.N. was supported by an Institutional Training Grant (T32-EY013360) from the National Eye Institute. We thank Rebecca Mellor and Tatiana Simon for expert technical assistance and Mr. Rick Wilson for maintaining and genotyping the mice used in the studies detailed here.
The authors declare no competing financial interests.
References
- Abraham U, Prior JL, Granados-Fuentes D, Piwnica-Worms DR, Herzog ED. Independent circadian oscillations of Period1 in specific brain areas in vivo and in vitro. J Neurosci. 2005;25:8620–8626. doi: 10.1523/JNEUROSCI.2225-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albus H, Bonnefont X, Chaves I, Yasui A, Doczy J, van der Horst GT, Meijer JH. Cryptochrome-deficient mice lack circadian electrical activity in the suprachiasmatic nuclei. Curr Biol. 2002;12:1130–1133. doi: 10.1016/s0960-9822(02)00923-5. [DOI] [PubMed] [Google Scholar]
- Alvado L, Allen CN. Tetraethylammonium (TEA) increases the inactivation time constant of the transient K(+) current in suprachiasmatic nucleus neurons. Brain Res. 2008;1221:24–29. doi: 10.1016/j.brainres.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batschelet E, Sibson R, Cohen JE. Circular statistics in biology. In: Sibson R, Cohen JE, editors. Mathematics in biology. New York: Academic; 1981. pp. 31–54. [Google Scholar]
- Beuckmann CT, Sinton CM, Miyamoto N, Ino M, Yanagisawa M. N-Type calcium channel {alpha}1b subunit (cav2.2) knock-out mice display hyperactivity and vigilance state differences. J Neurosci. 2003;23:6793–6797. doi: 10.1523/JNEUROSCI.23-17-06793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev. 2004;84:803–833. doi: 10.1152/physrev.00039.2003. [DOI] [PubMed] [Google Scholar]
- Bouskila Y, Dudek FE. A rapidly activating type of outward rectifier K+ current and A- current in rat suprachiasmatic nucleus neurones. J Physiol. 1995;488:339–350. doi: 10.1113/jphysiol.1995.sp020970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Piggins HD. Electrophysiology of the suprachiasmatic circadian clock. Prog Neurobiol. 2007;82:229–255. doi: 10.1016/j.pneurobio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, Tononi G. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–1092. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci. 2011;12:553–569. doi: 10.1038/nrn3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971a;213:21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol. 1971b;213:31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias M, Bhattacharji A, De Santiago-Castillo JA, Dougherty K, Kaulin YA, Na-Phuket TR, Wang G. The neuronal Kv4 channel complex. Neurochem Res. 2008;33:1558–1567. doi: 10.1007/s11064-008-9650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruyne JP, Weaver DR, Reppert SM. Peripheral circadian oscillators require CLOCK. Curr Biol. 2007;17:R538–R539. doi: 10.1016/j.cub.2007.05.067. [DOI] [PubMed] [Google Scholar]
- de Jeu M, Hermes M, Pennartz C. Circadian modulation of membrane properties in slices of rat suprachiasmatic nucleus. Neuroreport. 1998;9:3725–3729. doi: 10.1097/00001756-199811160-00028. [DOI] [PubMed] [Google Scholar]
- De Jeu M, Geurtsen A, Pennartz C. A Ba(2+)-sensitive K(+) current contributes to the resting membrane potential of neurons in rat suprachiasmatic nucleus. J Neurophysiol. 2002;88:869–878. doi: 10.1152/jn.2002.88.2.869. [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Douglas CL, Vyazovskiy V, Southard T, Chiu SY, Messing A, Tononi G, Cirelli C. Sleep in Kcna2 knockout mice. BMC Biol. 2007;5:42. doi: 10.1186/1741-7007-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa F, Torres-Vega MA, Marks GA, Joho RH. Ablation of Kv3.1 and Kv3.3 potassium channels disrupts thalamocortical oscillations in vitro and in vivo. J Neurosci. 2008;28:5570–5581. doi: 10.1523/JNEUROSCI.0747-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Li H, Aimond F, Johns DC, Rhodes KJ, Trimmer JS, Nerbonne JM. Role of heteromultimers in the generation of myocardial transient outward K+ currents. Circ Res. 2002;90:586–593. doi: 10.1161/01.res.0000012664.05949.e0. [DOI] [PubMed] [Google Scholar]
- Guo W, Jung WE, Marionneau C, Aimond F, Xu H, Yamada KA, Schwarz TL, Demolombe S, Nerbonne JM. Targeted deletion of Kv4.2 eliminates I(to,f) and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ Res. 2005;97:1342–1350. doi: 10.1161/01.RES.0000196559.63223.aa. [DOI] [PubMed] [Google Scholar]
- Han S, Yu FH, Schwartz MD, Linton JD, Bosma MM, Hurley JB, Catterall WA, de la Iglesia HO. Proc Natl Acad Sci U S A Plus: NaV1.1 channels are critical for intercellular communication in the suprachiasmatic nucleus and for normal circadian rhythms. Proc Natl Acad Sci U S A. 2012;109:E368–E377. doi: 10.1073/pnas.1115729109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusser M, Raman IM, Otis T, Smith SL, Nelson A, du Lac S, Loewenstein Y, Mahon S, Pennartz C, Cohen I, Yarom Y. The beat goes on: spontaneous firing in mammalian neuronal microcircuits. J Neurosci. 2004;24:9215–9219. doi: 10.1523/JNEUROSCI.3375-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- Huang RC. Sodium and calcium currents in acutely dissociated neurons from rat suprachiasmatic nucleus. J Neurophysiol. 1993;70:1692–1703. doi: 10.1152/jn.1993.70.4.1692. [DOI] [PubMed] [Google Scholar]
- Itri JN, Vosko AM, Schroeder A, Dragich JM, Michel S, Colwell CS. Circadian regulation of a-type potassium currents in the suprachiasmatic nucleus. J Neurophysiol. 2010;103:632–640. doi: 10.1152/jn.00670.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Yao GL, Bean BP. Mechanism of spontaneous firing in dorsomedial suprachiasmatic nucleus neurons. J Neurosci. 2004;24:7985–7998. doi: 10.1523/JNEUROSCI.2146-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004;27:343–369. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Jerng HH, Kunjilwar K, Pfaffinger PJ. Multiprotein assembly of Kv4.2, KChIP3 and DPP10 produces ternary channel complexes with ISA-like properties. J Physiol. 2005;568:767–788. doi: 10.1113/jphysiol.2005.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaraj D, et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–99. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Huguenard JR, Prince DA. Voltage-gated potassium channels activated during action potentials in layer V neocortical pyramidal neurons. J Neurophysiol. 2000;83:70–80. doi: 10.1152/jn.2000.83.1.70. [DOI] [PubMed] [Google Scholar]
- Kasukawa T, Masumoto KH, Nikaido I, Nagano M, Uno KD, Tsujino K, Hanashima C, Shigeyoshi Y, Ueda HR. Quantitative expression profile of distinct functional regions in the adult mouse brain. PloS one. 2011;6:e23228. doi: 10.1371/journal.pone.0023228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq ZM, Bean BP. Dynamic, nonlinear feedback regulation of slow pacemaking by A-type potassium current in ventral tegmental area neurons. J Neurosci. 2008;28:10905–10917. doi: 10.1523/JNEUROSCI.2237-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Wei DS, Hoffman DA. Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. J Physiol. 2005;569:41–57. doi: 10.1113/jphysiol.2005.095042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Dudek FE. Membrane properties of rat suprachiasmatic nucleus neurons receiving optic nerve input. J Physiol. 1993;464:229–243. doi: 10.1113/jphysiol.1993.sp019632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K, Joiner WJ, Wu MN, Yue Z, Smith CJ, Sehgal A. Identification of SLEEPLESS, a sleep-promoting factor. Science. 2008;321:372–376. doi: 10.1126/science.1155942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko NI, Honma S, Dudek FE, Honma K. On the role of calcium and potassium currents in circadian modulation of firing rate in rat suprachiasmatic nucleus neurons: multielectrode dish analysis. Neurosci Res. 2008;62:51–57. doi: 10.1016/j.neures.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Kudo T, Loh DH, Kuljis D, Constance C, Colwell CS. Fast delayed rectifier potassium current: critical for input and output of the circadian system. J Neurosci. 2011;31:2746–2755. doi: 10.1523/JNEUROSCI.5792-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, McMahon DG. Rhythmic regulation of membrane potential and potassium current persists in SCN neurons in the absence of environmental input. Eur J Neurosci. 2004;20:1113–1117. doi: 10.1111/j.1460-9568.2004.03555.x. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, McMahon DG. Encoding the ins and outs of circadian pacemaking. J Biol Rhythms. 2006;21:470–481. doi: 10.1177/0748730406294316. [DOI] [PubMed] [Google Scholar]
- Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell. 1997;91:855–860. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- London B, Wang DW, Hill JA, Bennett PB. The transient outward current in mice lacking the potassium channel gene Kv1.4. J Physiol. 1998;509:171–182. doi: 10.1111/j.1469-7793.1998.171bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist GB, Block GD. Role of neuronal membrane events in circadian rhythm generation. Methods Enzymol. 2005;393:623–642. doi: 10.1016/S0076-6879(05)93033-4. [DOI] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura W, Honma S, Shirakawa T, Honma K. Clock mutation lengthens the circadian period without damping rhythms in individual SCN neurons. Nat Neurosci. 2002;5:399–400. doi: 10.1038/nn843. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Holmes TC, Blau J. Membranes, ions, and clocks: testing the Njus-Sulzman-Hastings model of the circadian oscillator. Methods Enzymol. 2005;393:682–693. doi: 10.1016/S0076-6879(05)93036-X. [DOI] [PubMed] [Google Scholar]
- Niwa N, Wang W, Sha Q, Marionneau C, Nerbonne JM. Kv4.3 is not required for the generation of functional Ito,f channels in adult mouse ventricles. J Mol Cell Cardiol. 2008;44:95–104. doi: 10.1016/j.yjmcc.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris AJ, Nerbonne JM. Molecular dissection of I(A) in cortical pyramidal neurons reveals three distinct components encoded by Kv4.2, Kv4.3, and Kv1.4 alpha-subunits. J Neurosci. 2010;30:5092–5101. doi: 10.1523/JNEUROSCI.5890-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris AJ, Foeger NC, Nerbonne JM. Neuronal voltage-gated K(+) (Kv) channels function in macromolecular complexes. Neurosci Lett. 2010;486:73–77. doi: 10.1016/j.neulet.2010.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature. 2002;416:286–290. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Zimmerman P. Lesions of the suprachiasmatic nucleus disrupt circadian locomotor rhythms in the mouse. Physiol Behav. 1991;49:1283–1287. doi: 10.1016/0031-9384(91)90364-t. [DOI] [PubMed] [Google Scholar]
- Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978;72:131–160. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Deboer T, Rudy B, Lau D, Borbély AA, Tobler I. Sleep EEG in mice that are deficient in the potassium channel subunit K.v. 3.2. Brain Res. 2002;947:204–211. doi: 10.1016/s0006-8993(02)02925-6. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiology. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang KC, Foeger NC, Marionneau C, Jay PY, McMullen JR, Nerbonne JM. Homeostatic regulation of electrical excitability in physiological cardiac hypertrophy. J Physiol. 2010;588:5015–5032. doi: 10.1113/jphysiol.2010.197418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Burkhalter A, Nerbonne JM. Functional role of the fast transient outward K+ current IA in pyramidal neurons in (rat) primary visual cortex. J Neurosci. 2005;25:9185–9194. doi: 10.1523/JNEUROSCI.2858-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]