Abstract
Although fear directs adaptive behavioral responses, how aversive cues recruit motivational neural circuitry is poorly understood. Specifically, while it is known that dopamine (DA) transmission within the nucleus accumbens (NAc) is imperative for mediating appetitive motivated behaviors, its role in aversive behavior is controversial. It has been proposed that divergent phasic DA transmission following aversive events may correspond to segregated mesolimbic dopamine pathways; however, this prediction has never been tested. Here, we used fast-scan cyclic voltammetry to examine real-time DA transmission within NAc core and shell projection systems in response to a fear-evoking cue. In male Sprague Dawley rats, we first demonstrate that a fear cue results in decreased DA transmission within the NAc core, but increased transmission within the NAc shell. We examined whether these changes in DA transmission could be attributed to modulation of phasic transmission evoked by cue presentation. We found that cue presentation decreased the probability of phasic DA release in the core, while the same cue enhanced the amplitude of release events in the NAc shell. We further characterized the relationship between freezing and both changes in DA as well as local pH. Although we found that both analytes were significantly correlated with freezing in the NAc across the session, changes in DA were not strictly associated with freezing while basic pH shifts in the core more consistently followed behavioral expression. Together, these results provide the first real-time neurochemical evidence that aversive cues differentially modulate distinct DA projection systems.
Introduction
Fear-evoking cues in the environment powerfully shape our behavior. While there is an extensive literature surrounding how fearful cues engage the amygdala and related circuitry to generate reactive defensive behaviors (for review, see (LeDoux, 2000; Pape and Pare, 2010; Maren, 2011), there has been less investigation regarding how fearful cues impact motivational neural circuitry. This is notable because fear can be highly motivating. For example, it is adaptive for fearful stimuli to alter motivated behavior or foster active avoidance responding (Kelley et al., 2005). It is well known that appetitive goal-directed behavior is mediated by phasic dopamine (DA) transmission within mesolimbic systems (Mirenowicz and Schultz, 1996; Schultz, 2007; Wanat et al., 2009), and mesolimbic DA is also critical for processing aversive information (Pezze and Feldon, 2004; Faure et al., 2008; Fadok et al., 2009, 2010; Darvas et al., 2011; Valenti et al., 2011; Zweifel et al., 2011). For example, DA transmission within the NAc is critical for the modulation of conditioned fear, demonstrated by aversive latent inhibition(Young et al., 1993; Gray et al., 1997). However, the specific involvement of phasic DA transmission in aversive motivation remains a matter of considerable debate.
While early studies suggested that aversive cues strictly decrease DA neuron firing (Mirenowicz and Schultz, 1996; Ungless et al., 2004), more recent studies suggest that anatomically heterogeneous subpopulations of DA neurons respond with both increases and decreases in firing to aversive stimuli (Brischoux et al., 2009; Matsumoto and Hikosaka, 2009; Bromberg-Martin et al., 2010a,b). These subpopulations contribute to neurochemically and functionally distinct projection systems that are difficult to study electrophysiologically (Bannon and Roth, 1983; Deutch and Roth, 1990; Di Chiara and Bassareo, 2007; Ikemoto, 2007; Belin and Everitt, 2008; Liu et al., 2008; Aragona et al., 2009). Here, we use fast-scan cyclic voltammetry (FSCV) to measure subsecond fluctuations in DA concentration within distinct mesolimbic projection systems (Ikemoto, 2007; Lammel et al., 2008), terminating in either the nucleus accumbens (NAc) core or shell while freely behaving rats were presented with a fear-evoking cue. Although FSCV has been used previously to explore regional differences in DA transmission in response to both cued and unconditioned appetitive stimuli (Aragona et al., 2009; Brown et al., 2011), studies that have used FSCV to explore aversive stimulus processing have focused on primary aversive stimuli (Roitman et al., 2008; Budygin et al., 2012). However, cues and the unconditioned stimuli they predict drive differential responses in DA neurons (Matsumoto and Hikosaka, 2009). Thus, DA transmission dynamics within motivational circuits underlying learned aversive cues must be elucidated.
Here, we provide the first real-time electrochemical data demonstrating that a fear cue causes regionally specific changes in both phasic DA transmission and local pH levels. Presentation of the aversive cue decreases DA transmission and causes a basic pH shift within the NAc core but increases DA transmission without altering pH levels within the NAc shell. Together, these findings significantly extend our understanding of how aversive cues recruit motivational circuits, speaking to a long-standing controversy regarding how aversive stimuli alter DA transmission.
Materials and Methods
Animals.
Twenty-two male Sprague Dawley rats weighing 280–320 g were used as subjects (Core Conditioned: n = 5; Shell Conditioned: n = 6; Core Control: n = 6; Shell Control: n = 5). Rats were individually housed with food and water available ad libitum, and were maintained on a 14–10 h light/dark cycle. All experiments were conducted during the animals' dark cycle. All procedures were performed in accordance with guidelines approved by the University of Michigan University Committee on Use and Care of Animals.
Surgery.
Before behavioral manipulations, rats received FSCV surgery (Aragona et al., 2008, Day et al., 2010; Wheeler et al., 2011; McCutcheon et al., 2012). Rats were anesthetized with ketamine (90 mg/kg, i.m.) and xylazine (10 mg/kg, i.m.). A guide cannula (Bioanalytical Systems) was placed over the NAc core (anteroposterior (A/P) + 1.7, mediolateral (M/L) +1.3; relative to bregma) or NAc shell (A/P + 1.7, M/L +0.8; relative to bregma) and an Ag/AgCl reference electrode was implanted in contralateral cortex (A/P −0.8, M/L −4.0; relative to bregma). A bipolar stimulating electrode (Plastics One) was positioned over the ventral tegmental area (VTA; A/P + 3.8, M/L +0.8; relative to the intra-aural line) and secured in a dorsoventral (D/V) position that yielded maximum electrically stimulated (60 pulses, 60 Hz, 120 μA) DA release within the ventral striatum (typically −8.6 mm D/V relative to brain surface). The stimulating electrode was used at the end of behavioral testing (see below) to evoke a range of electrically stimulated DA release magnitudes needed for statistical analysis; i.e., to construct “training sets” needed to perform principal component analysis (Heien et al., 2005; Keithley et al., 2011).
Behavioral testing.
Following surgery, rats were allowed 5–7 recovery days before receiving pavlovian fear conditioning. The conditioning and extinction phases of the behavioral testing were conducted in distinct observation chambers (Med Associates) that differed in visual and tactile cues. Consistent with previous studies (Orsini et al., 2011), Pavlovian fear conditioning consisted of five pairings of an auditory stimulus (conditioned stimulus [CS] 10 s, 2 kHz, 80 dB) coterminating with an aversive footshock (unconditioned stimulus [US]; 2 s, 1 mA) with a 60 s intertrial interval. Control rats received either tone- or footshock-alone training (Arcediano et al., 2005) to control for nonassociative factors related to conditioning. Freezing served as the behavioral index of conditional fear during both the conditioning and expression sessions. During conditioning, behavioral chambers rested on load-cell platforms that recorded chamber displacement in response to animals' locomotor activity. One minute after the final shock presentation, animals were transported back to their home cages and ∼24 h later, rats were transported to a novel environment for voltammetric measures during a tone-alone test session.
The tone-alone test session, or tone-test, was conducted in a modified Med Associates chamber that allowed for voltammetry recordings (Aragona et al., 2009). Before behavioral testing, an appropriately sensitive electrode was selected (see below). Once an electrode that detected naturally occurring phasic DA release events (i.e., transients) was identified (see below), we performed a “context switch,” which entailed turning white room lights off and red room lights on, and briefly moving the rat from the chamber to remove a padded floor to reveal a grid floor. This context switch was necessary to reestablish robust locomotor behavior that ensured the ability to detect cue-evoked freezing. Immediately following the context change voltammetry recordings commenced. After a 3 min baseline period, rats were given 15 tone-alone presentations (10 s, 2 kHz, 80 dB) with a 1 min intertrial interval. Behavior was recorded and hand scored off-line by experimenters blind to condition (core or shell and control or conditioned). At least two experimenters provided behavioral scoring and achieved an inter-rater reliability of 95%.
FSCV.
During behavioral conditions described above, FSCV was used to measure phasic changes in DA and local pH. For each subject, a fresh glass-encased carbon-fiber microelectrode was lowered into the NAc core or shell (Phillips et al., 2003a; Aragona et al., 2008). Electrode construction and calibration were conducted in the same manner as described in our previous studies (Aragona et al., 2009), with the additional use of a novel flow cell, which was used for in vitro calibrations (Sinkala et al., 2012). Epoxy was used to create a more robust and reliable seal between the carbon fiber and glass casing to ensure clean and stable recordings. Electrodes were positioned within the NAc core or shell where naturally occurring DA transients were reliably detected, as reported in many previous FSCV studies (this is now a standard operating procedure) (Wightman et al., 2007; Aragona et al., 2008; Roitman et al., 2008). Consistent with previous studies, if the electrode did not detect a high DA “transient” frequency, it was withdrawn and another inserted until DA transients were reliably detected (Aragona et al., 2008). We selected highly sensitive electrodes across all subjects as previous studies have shown that this is necessary to detect decreases in transient frequency and the associated decrease in time averaged [DA] (Roitman et al., 2008; Aragona et al., 2009). Once stable DA transients were detected, the environmental context was altered by changing the visual and tactile elements of the chamber, as described above, and FSCV recordings began. Following FSCV recordings during the behavioral study, DA release was evoked in the freely moving subject by electrical stimulation of the ventral tegmental area (Phillips et al., 2003a; Stuber et al., 2005) for use in principle component analyses to convert the recorded current into [DA] and pH units (Heien et al., 2004; Keithley et al., 2011).
To verify the identity of the electro-active analytes, current values were converted to [DA] and pH units using principal component analysis (Heien et al., 2004; Keithley et al., 2011). Detection and analysis of DA have been described in detail previously (Heien et al., 2005; Aragona et al., 2008). Consistent with previous FSCV studies (Day et al., 2007; Aragona et al., 2009), mean changes in [DA] were calculated relative to a zero point determined by the background subtraction applied to the raw data. Background subtraction was performed at the lowest current value within the 10 s preceding cue onset separately for each tone presentation (Roitman et al., 2008; Aragona et al., 2009). Phasic changes in [DA] were determined within a 30 s sampling period that was centered around tone duration, with a 10 s baseline, 10 s recording during CS presentation, and a 10 s post-CS period. Transients were identified using MiniAnalysis (Synaptosoft) as previously described (Aragona et al., 2008). A transient was defined as a fivefold or greater increase in [DA] relative to the root mean square noise value taken from the same electrode (Heien et al., 2005).
Characterization of DA and pH changes.
FSCV allows detection of local changes in pH that frequently accompany DA transmission (Venton et al., 2003; Heien et al., 2005; Roitman et al., 2008) and these two analytes are easily distinguished by cyclic voltammogram (CV) analysis, in which background subtracted changes in current detected at the carbon-fiber electrode are plotted against the applied potential (i.e., the voltage ramp from −0.4 V to 1.3V and back; described in detail elsewhere; Heien et al., 2005) and time. For each time point, the change in current (resulting from the oxidation and reduction of the neurochemical species) as a function of the applied potential may be determined. This represents the CV for a given neurochemical species and the shape of the CV reveals its identity (Fig. 1a, i–iii, insets) (Phillips et al., 2003a; Heien et al., 2005). This is conveniently visualized in color plots, wherein changes in current are plotted in false color against both time and applied voltage.
Figure 1.
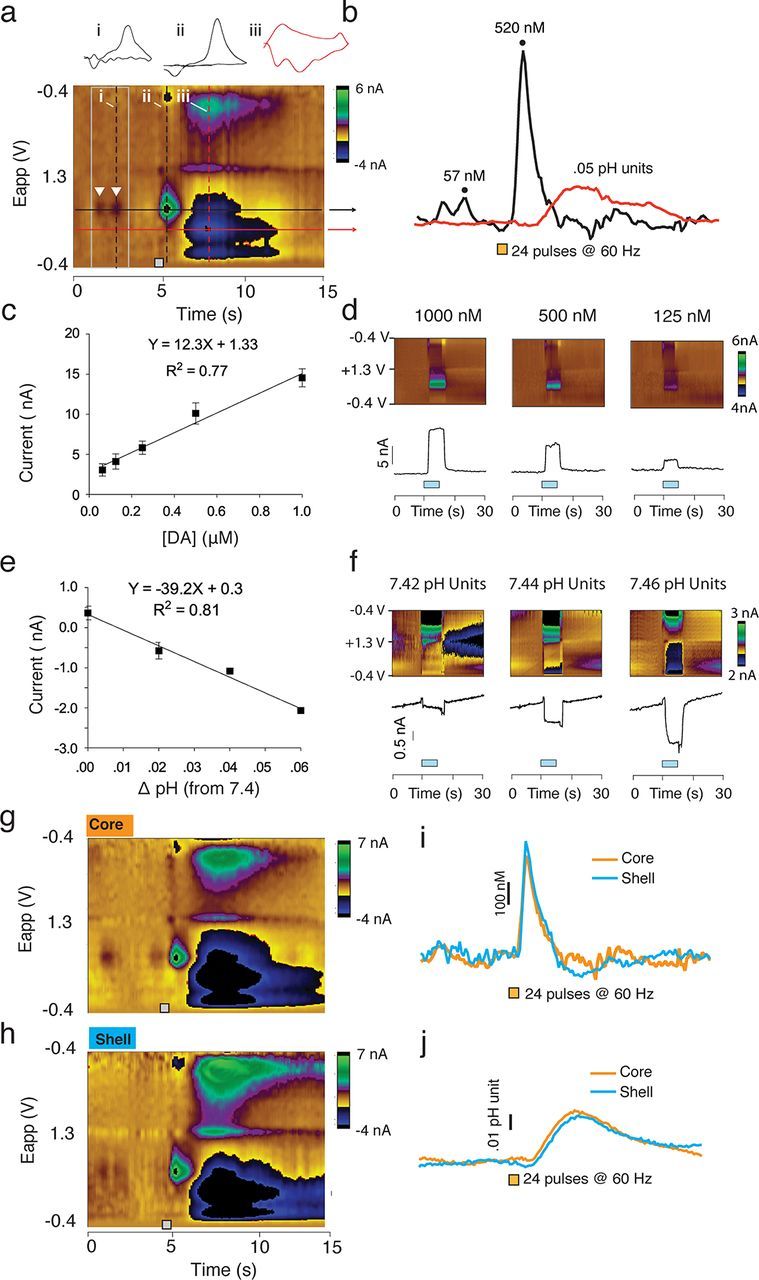
Changes in [DA] and pH values are characterized based on stimulation both in vivo and in vitro. a, Representative color plot showing current changes observed at the recording electrode plotted against the applied voltage and time. Analytes (DA and pH) are identified based on specific oxidation-reduction profiles. The holding potential applied to the carbon-fiber electrode (−0.4 V) was ramped to +1.3 V and back to 0.4 V at a rate of 10 Hz. This example shows two spontaneous DA transients, indicated by white inverted triangles. Electrical stimulation (24 pulses, 60 Hz) of the VTA results in a near instantaneous surge in [DA] and a more delayed and long-lasting basic shift in pH. Vertical dashed lines on the color plot indicate the time point of representative cyclic voltammograms, shown above. Horizontal black (DA) and red (pH) lines indicate the applied voltage at the point of maximum oxidation, used to identify neurochemical species. b, Traces for Δ[DA] and pH units were obtained by using principal components analysis. Δ[DA] for the first transient observed in a and the stimulation is shown, as well as peak change in pH units. c, Calibration curve for DA. Known concentrations of DA were used to determine the average current evoked in a standard set of electrodes. The slope of the resulting trend line was then used as the calibration factor for conversion of current to DA concentration. d, Representative color plots and current traces recorded in vitro during the application of specific DA concentrations. Blue bars indicate infusion of DA. e, Calibration curve for pH. Current changes were again recorded for known basic shifts in pH. The resulting slope was again used as the calibration factor. f, Representative color plots and current traces recorded in vitro during the application of basic pH shifts. Blue bars indicate the duration of the infusion. g–j, Representative color plots and traces of electrical stimulation (24 pulses, 60 Hz) of DA afferents to the NAc core (g, i, j) and shell (h–j). These plots indicate similar DA and pH responses within the core and shell.
Electrical stimulation of DA afferents reliably alters both DA release and local changes in pH (Phillips et al., 2003a; Heien and Wightman, 2006). In this example (Fig. 1a), two naturally occurring DA transients (at random, unpredictable times) were detected (emphasized by inverted white triangles) before electrical stimulation. The CV corresponding to the second transient is shown above the color plot (Fig. 1a, i) and its peak magnitude is shown in the [DA] trace (Fig. 1b). Upon electrical stimulation of dopaminergic afferents, a near instantaneous increase in current is detected at the peak oxidational potential for DA (∼0.65 V of the voltage ramp; Fig. 1a, ii to inspect the CV of electrically evoked DA release). Current at this point in the voltage ramp (Fig. 1a, black line) is converted to [DA] [Figure 1b; described in detail elsewhere (Heien et al., 2005; Keithley et al., 2011)]. In contrast to electrically evoked changes in DA transmission, local changes in pH follow electrical stimulation by a delay of several seconds (Fig. 1a,b) and its peak change in current is primarily evident at ∼0.4 V in the applied waveform (Fig. 1a, iii). Conversion from current to pH units (Fig. 1b, red line) has been described in detail elsewhere (Heien et al., 2005; Roitman et al., 2008).
Current changes that are identified as DA or pH, based on statistically significant concordance between the CVs of naturally occurring signals and CVs of electrically evoked signals, were converted into concentration measures using calibration factors obtained from in vitro electrode characterization (Fig. 1c–f). Numerous electrodes were tested against a series of known DA (Fig. 1c,d) and pH values (Fig. 1e,f) to determine the average change in current following administration of known values of [DA] or pH units. The slopes of these resulting curves were subsequently used as the calibration factor for converting analyte-evoked current into [DA] or pH units. Consistent with previous reports (Roitman et al., 2008; Aragona et al., 2009), there were no differences in stimulated release of DA or pH between the NAc core and shell, as seen in representative traces from each region (Fig. 1g–j). It should be noted that, as with all FSCV studies, these DA and pH values are Δ[DA] and pH over the lowest current point in each trace. Thus, they do not represent absolute levels of DA or pH, but rather changes over an arbitrary zero point.
Percentage change in [DA] was calculated for each tone presentation over a 30 s sampling window (10 s pretone period, 10 s tone presentation, and 10 s post-tone period) as follows:
 |
Similarly, fold-change in pH units was calculated for each tone presentation as follows:
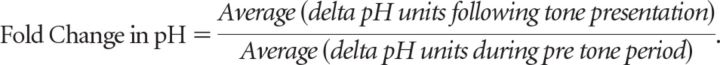 |
Here, “following tone presentation” indicates the time period including the tone presentation and the post-tone period, or the 20 s following tone onset.
Histology.
Following behavioral and neurochemical testing, animals were euthanized with an overdose of ketamine. To allow for histological verification of electrode placements, either electrolytic lesions were made at the same microdrive setting used for the experiment (Fig. 1) or another established method was used wherein the A/P and M/L coordinates are very clearly identified and the D/V coordinate was “reconstructed” based on the known relationship between the settings of the microdrive and how far this lowers the electrode (Robinson et al., 2002).
Statistics.
Analysis of freezing, both between groups and across the session was performed using two-way repeated-measures ANOVA with Bonferroni corrected post hoc tests. Changes in [DA] as well as pH units were extracted using principal components regression as previously described (Heien et al., 2004; Aragona et al., 2008; Keithley et al., 2011). CS-evoked changes in [DA] calculated as percentage change in [DA] following tone onset and transient amplitude were assessed using one-way repeated-measures ANOVA and Bonferroni corrected post hoc comparisons. Binned [DA], pH, and transient probability data were analyzed using a linear-mixed model regression (Aragona et al., 2008) due to its superior ability to handle repeated-measures data (Verbeke, 2009). For these analyses, post-tone bins were compared with a pretone baseline value with time as a repeated fixed factor and region as a fixed factor. Changes in [DA], pH, and transient frequency and amplitude were calculated in five-tone blocks, and statistical analyses were performed on the resulting data. All data are presented as mean ± SEM unless otherwise noted. A p value < 0.05 was considered significant. Linear mixed-model regressions were performed in SPSS version 19 for Windows; all other statistics were performed in Prism version 5.0 for Mac OSX.
Results
Histology and behavior
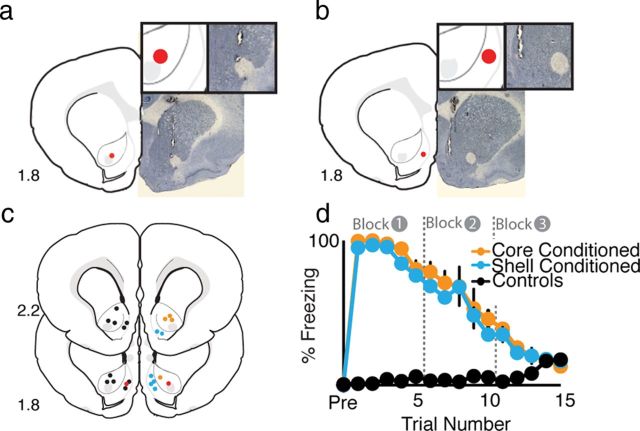
One day following fear conditioning, rats were moved to a novel environment and FSCV was used to measure real-time DA transmission (Fig. 1) within the NAc core (Fig. 2a,c) or shell (Fig. 2b,c) (Rebec et al., 1997; Robinson and Wightman, 2004; Aragona et al., 2009). Three minutes after the context shift, rats were presented with 15 CS-alone presentations (at 1 min intervals) while real-time neurochemical measurements were continued. Freezing behavior did not differ between conditioned groups (F values < 1.0, Fig. 2d) but was significantly higher than controls (main effect of group, F(1,15) = 332.6, p< 0.001) until the final block of tones during which there was no difference in behavior between conditioned and control animals (p > 0.05; Fig. 2d). Rats that had previously received paired CS–US presentations showed nearly 100% freezing behavior during block 1 (first five tone presentations) (Fig. 2d). During the next block of five tone presentations (block 2), freezing behavior began to decline and returned to baseline levels by the third block of tone presentations (Fig. 2d). Control rats that received only tone (CS-alone) or shock (US-alone) presentation training did not show freezing behavior during the tone test (Fig. 2d). It should be noted that control animals show a slight upward trend in freezing toward the end of the session; however, this was not significantly different from freezing levels earlier in the session and was not likely due to fear-evoked freezing responses but rather generalized decreases in locomotor activity in unconditioned animals following a prolonged period of time sitting within the chamber. Histological verification of electrode placements was performed at the end of the experiment (see Materials and Methods; Fig. 2a–c).
Figure 2.
Histology and fear expression during recording sessions. a, Example of histological verification of recording site in the NAc core (b) and within the NAc shell. c, Diagrammatic representation of electrode placements for all animals within the NAc. Core-conditioned placements are indicated in orange (n = 5), Shell-Conditioned placements are indicated in cyan (n = 6), and placements for control animals are shown in black (n = 11). Although the placements are distributed throughout both cerebral hemispheres here for clarity, recordings from all animals were taken from the same hemisphere. Red indicates the examples shown in a and b. d, Percentage freezing during fear expression for core-conditioned, shell-conditioned, and control animals. There was no difference between conditioned groups in fear expression. Unconditioned rats did not freeze during CS presentation. All conditioned rats showed robust freezing that attenuated as the session progressed. By the end of the session, conditioned rats showed no more freezing than control rats.
Fear-evoking cues diminished DA release within the NAc core but enhanced DA release within the NAc shell
After appropriate electrodes were selected, real-time [DA] and local pH changes were measured across the context switch and throughout the behavioral testing session. Although previous FSCV studies have demonstrated that presentation of novel stimuli (Robinson and Wightman, 2004) and entry into novel environments can increase DA transmission (Rebec et al., 1997), this context change did not significantly alter phasic DA transmission (transient frequency during final 3 min before context switch = 10.68 ± 0.60 and during the initial 3 min following the context switch = 10.81 ± 0.65; t(10) = −196, p = 0.85). This baseline measure of transient frequency is consistent with previous FSCV studies (Cheer et al., 2004; Aragona et al., 2008; Sombers et al., 2009). Indeed, the detection of basal transients before experimental manipulation (Wightman et al., 2007) is essential to assure electrode fidelity and increases consistency across experimenters. Moreover, it is essential to allow decreases in DA transmission to be detected using FSCV (see below). These, among other critical features of basal transient detection, have contributed to it becoming a standard operating procedure in FSCV data acquisition when acute electrode implantation is employed on the day of the experiment.
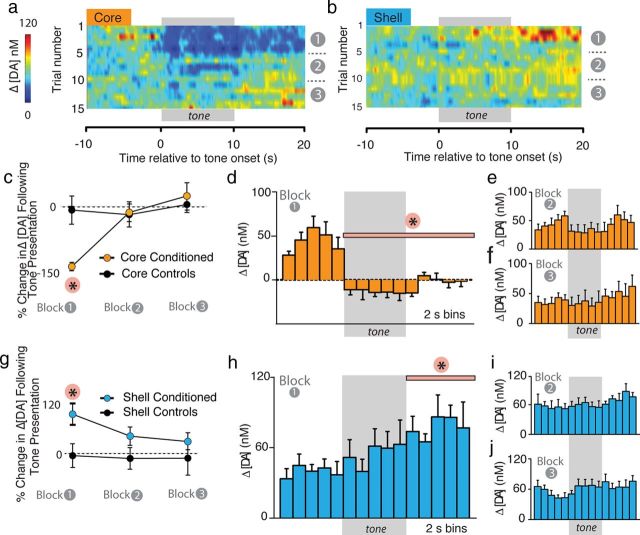
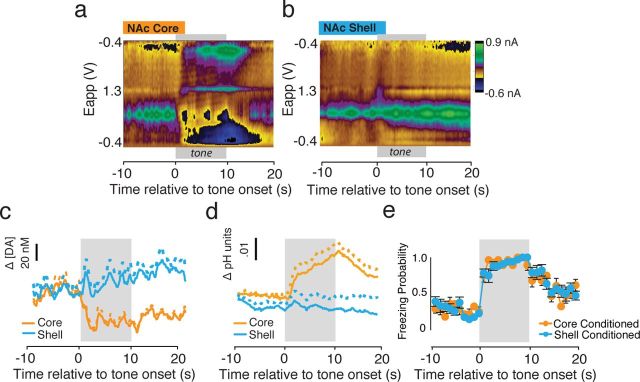
Although rats in both the core-conditioned and shell-conditioned groups showed similar behavioral patterns during the tone test (Fig. 2d), presentation of the CS caused dramatically different neurochemical changes within these NAc subregions (Figs. 3, 6). For analysis of fear-evoked changes in [DA], we constructed heatmaps to examine how average [DA] changed with CS presentation across the session in both the core (Fig. 3a; n = 5) and the shell (Fig. 3b; n = 6). Within the NAc core, cue presentation caused a rapid and prolonged decrease in [DA] (Fig. 3a). Quantification of the percentage change in CS-evoked Δ[DA] within the NAc core showed that CS presentation dramatically decreased [DA] within conditioned, but not control, subjects only within the first block of trials (Fig. 3c). Two-way ANOVA analysis of core-conditioned versus core control groups revealed a significant interaction between time and group (F(2,16) = 17.3, p < 0.01). Post hoc tests revealed a significant difference between core-conditioned animals and core-control animals only during block 1 (i.e., the first five trials, p < 0.01). We next analyzed the resulting concentration data in 2 s bins for each block of trials, collapsed across five tones in each of our behaviorally designated blocks for both the NAc core and shell. Within the NAc core, CS-presentation caused a significant decrease in [DA] relative to the pre-CS period that was rapid and long lasting selectively during block 1 (Fig. 3d; F(10, 23.7)=10.32, p < 0.01 for block 1). No significant changes in [DA] were observed during block 2 (Fig. 3e; F(10, 31.4) < 1.0, p = 0.71) or block 3 (Fig. 3f; F(10, 32.5) < 1.0, p = 0.73).
Figure 3.
Fear cues cause decreased DA transmission in the NAc core but enhanced DA transmission in the NAc shell. a, b, Heatmaps depicting trial-by-trial Δ[DA] across all 15 cue presentations in the core (n = 5) and shell (n = 6), respectively. Tone presentation was immediately followed by a dramatic decrease in [DA] within the NAc core, but within the shell, [DA] was gradually increased following cue offset. In both regions, the effect attenuated as the session progressed. c, Percentage change in mean [DA] in the NAc core in conditioned versus unconditioned animals in response to CS presentations. A significant decrease in [DA] was observed in response to CS presentations during the first five tone block. d–f, Δ[DA] within the NAc core relative to CS presentation across each block of trials. [DA] was calculated in 2 s bins. Tone presentation causes an immediate and significant decrease in [DA] during block 1 (d) that attenuated during subsequent blocks (e, f). g, Percentage change in mean [DA] in the NAc shell in conditioned versus unconditioned animals in response to CS presentations. A significant increase in [DA] was observed in response to the CS during the first five tone block. h–j, Δ[DA] within the NAc shell relative to CS presentation across each block of trials. Tone presentation resulted in a gradual increase in [DA] that was significant relative to pretone [DA] following cue offset during block 1 (h). There was no significant change following tone presentation in block 2 (i) or 3 (j). *p < 0.05.
Figure 6.
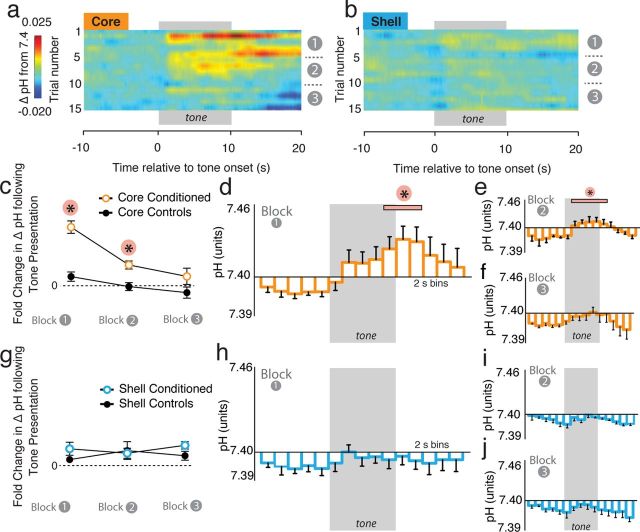
Fear cues cause basic shifts in pH within the NAc core but not within the NAc shell. a, b, Heatmaps depicting trial-by-trial pH shifts across all 15 cue presentations in the core (n = 5) and shell (n = 6), respectively. Tone presentation was followed by a basic pH shift within the NAc core, but not within the shell. The basic shift within the NAc core remained prominent through blocks 1 and 2 and attenuated during block 3. c, Fold-change in average pH in the NAc core in conditioned versus unconditioned animals in response to CS presentations. A significant increase in pH was observed in response to CS presentations during the first and second five tone blocks. d–f, ΔpH units within the NAc core relative to CS presentation across each block of trials. pH was calculated in 2 s bins. Cue presentation causes a significant increase in pH during block 1 (d) and 2 (e) that attenuated during the final blocks (f). g, Fold-change in mean pH in the NAc shell in conditioned versus unconditioned animals in response to CS presentations. There was no difference in pH changes within any block between conditioned and unconditioned animals. h–j, ΔpH within the NAc shell relative to CS presentation across each block of trials. Tone presentation did not significantly alter pH shifts in the NAc shell during blocks 1 (h), 2 (i) or 3 (j). *p < 0.05.
In stark contrast to the NAc core, CS presentation increased [DA] within the NAc shell, although still selectively within the first block of trials (Fig. 3b,g); note the robust average increase during block 1 depicted in the heat map. Quantification of percentage changes shows a main effect of group (F(2,32) = 10.6, p < 0.01) and post hoc tests confirm that [DA] was significantly increased within the NAc shell in conditioned, but not control, animals only within the first block of trials (Fig. 3g; p < 0.05). Heatmaps illustrate the “diffuse” (i.e., poorly time locked to the CS) increase in the NAc shell following CS presentation early during fear expression (Fig. 3b). However, the increase was strongest at cue offset. Specifically, analysis of binned concentration data revealed an increase in [DA] following CS presentation during block 1 that was only significant during the final five bins (i.e., following tone offset, p < 0.05, Fig. 3h). However, we still refer to this as a diffuse increase because it was not nearly as robustly or reliably associated with any temporal attributes of cue presentation, compared with that seen within the core. However, it should be noted that the first bin showing a significant increase in [DA] was the bin immediately following cue offset. This effect, however, is an average effect and was not consistently observed across subjects, and animals showed no difference in behavior at cue offset (i.e., they still showed robust freezing during early CS presentations). Taken together, this suggests that the increase is unlikely to serve as an emotional “relief” signal at the omission of an expected aversive footshock (Ikemoto and Panksepp, 1999).
No significant changes in [DA] were observed during block 2 or block 3 (Fig. 3i,j; F < 1.0, p > 0.05). Importantly, there was also a lack of a CS-evoked response in control rats (Fig. 3c,g). This suggests that the changes in [DA] in conditioned animals were due to the CS–US association and not to sensitization of the DA system due to prior experience with the tone or shock. This is consistent with previous reports from other groups that unconditioned tone presentations do not evoke changes in dopamine release (Phillips et al., 2003b; Robinson and Wightman, 2004).
Cue-evoked changes in DA release within both the NAc core and shell can be attributed to altered phasic transmission dynamics
To determine whether aversive cue-evoked changes are mediated by specific changes in phasic DA transmission dynamics, we assessed how the aversive CS altered the probability and magnitude of DA transients. In FSCV measures, average [DA] is comprised of time-averaged DA transients (Roitman et al., 2008) superimposed on basal extrasynaptic [DA] levels that are not detected by this technique (Venton et al., 2003; Arbuthnott and Wickens, 2007). As described previously (Aragona et al., 2008, 2009) we analyzed the probability of the occurrence of transients to approximate changes in the number of phasic release events and transient amplitude to estimate changes in the magnitude of DA released.
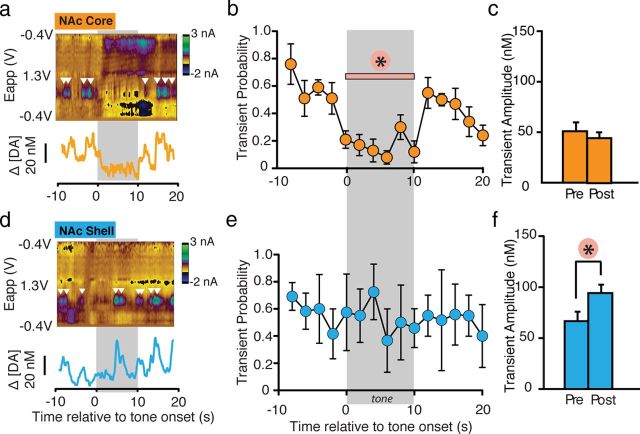
A representative color plot and DA concentration trace within the NAc core (Fig. 4a) and shell (Fig. 4d) following the presentation of an aversive CS shows that before CS presentation, transients were observed consistently during the baseline period in both the NAc core and shell (baseline probability: core = 0.55 ± 0.07 and shell = 0.63 ± 0.04, t(9)=0.02, p=0.9). Within the NAc core, CS presentation caused a significant decrease in transient probability (F(10, 25.8) = 6.51, p < 0.001; Fig. 4a,b), suggesting that the CS-evoked decrease in [DA] within the NAc core is due to a decrease in the occurrence of phasic release events. In contrast, presentation of the same CS did not alter transient probability within the NAc shell (Fig. 4d,e) (F(10, 30.3) = 1.67, p = 0.14). Analysis of transient amplitude, on the other hand, showed that while CS presentation did not alter release magnitude within NAc core (t(5) = 0.78, p = 0.48; Fig. 4c), there was a significant increase in transient amplitude (t(5) = 9.01, p < 0.01) following CS onset within the NAc shell (Fig. 4f). These data suggest the CS-evoked increase in [DA] described above within the NAc shell is due to a cue-evoked increase in the magnitude of phasic DA release events. Furthermore, it is important to note that, with FSCV, only acute, background subtracted effects are seen. Thus, it is possible that the phasic increase in the shell we report here may be an underestimation of the overall increase in [DA] occurring within the NAc shell, due to the differential nature of the technique.
Figure 4.
Changes in [DA] in response to the fear-evoking CS are attributable to changes in the probability and amplitude of DA release events. a, d, Representative transient traces. White inverted triangles indicate transients. The panel underneath each color plot shows the converted [DA] traces. b, e, Transient probability calculated during 2 s bins for block 1 within the NAc core (i.e. averaged over first five tones for n = 5; 25 traces all together) and shell (i.e. averaged over first five tones for n = 6; 30 traces). Tone onset caused a significant decrease in transient probability that lasted the duration of the tone presentation, but returned to pretone levels following tone offset in the NAc core. Transient probability did not change with tone presentation in the NAc shell. c, f, Average transient amplitude before and following cue onset in the NAc core and shell. Cue onset did not alter transient amplitude in the NAc core, but increased transient amplitude in the NAc shell. *p < 0.05.
Fear-evoked changes in DA transmission in the NAc core but not shell are accompanied by basic shifts in pH
As CS-related changes in DA transmission in the both the NAc core and shell were selective to the first five-tone block, further analysis focused on this block of CS presentations. Subregion differences in the neurochemical responses to aversive cues are obvious upon inspection of average color plots for the NAc core (Fig. 5a) and shell (Fig. 5b) for the first block of CS presentations. Before CS onset, [DA] and pH levels were equivalent within the NAc core and shell (DA: t(51) = 0.16, p = 0.87; pH: t(50) = 0.51, p = 0.63) (Fig. 5c,d). However, at CS onset, [DA] rapidly decreased within the NAc core (Fig. 5a,b; statistically described elsewhere) and this decrease was followed by a long-lasting basic pH shift (Fig. 5a,d; t(23) = 2.62, p < 0.05). Within the NAc shell, it is evident that the increase in [DA] by the fear CS (Fig. 3) is quite diffuse and robustly not time locked to tone presentation (Fig. 5b,c). Moreover, in contrast to the NAc core, there was no change in local pH within the NAc shell following presentation of the aversive cue (t(27) = 0.73, p = 0.47)(Fig. 5b,d). These differences in core-shell pH shifts were not attributable to differences in overall pH responsiveness between these regions (Fig. 1g–j; Roitman et al., 2008). Thus, presentation of the fear CS alters local pH selectively within the NAc core, which is the region in which cue-evoked decreases in DA transmission are strongly time locked to stimulus presentation. A similar relationship between a basic pH shift and a time-locked decrease in [DA] has also been shown after presentations of an unconditioned aversive taste stimulus (intra-oral infusion of quinine) (Roitman et al., 2008). However, while the quinine-evoked decrease in [DA] was similarly associated with a basic pH shift, this neurochemical response occurred within the NAc shell whereas the aversive CS (present study) resulted in this [DA]/basic pH alteration within the NAc core.
Figure 5.
Cue onset is accompanied by basic pH shifts within the NAc core. a, b, Average color plots for the first block of CS presentations in the NAc core (n = 5; a) and shell (n = 6; b) were generated to illustrate the raw changes in neurochemical activity associated with tone presentation (gray bar). Within the NAc core, cue onset is accompanied by a delayed but lasting basic pH shift. No visible pH shifts were detected in the shell. c, Quantification of changes in [DA] in core and shell following cue onset during block 1. Cue presentation altered both core and shell [DA]. d, Quantification of pH changes during block 1 within the NAc core and shell. Cue presentation results in a significant basic pH shift within the NAc core, but does not cause any change in pH within the NAc shell. e, Within-trial probability of freezing across block 1 for both core-conditioned and shell-conditioned rats. Cue onset causes rapid increases in freezing probability that last throughout the tone presentation and attenuate slowly following tone-offset.
To more thoroughly explore how DA and pH changes related to fear expression, we analyzed within-trial trajectories of freezing for block 1 (Fig. 5e). For each trial, we assessed freezing across the 30 s sampling window used for neurochemical analyses. This showed that freezing has a rapid onset following cue presentation and slow attenuation following cue offset, demonstrating that the neurochemical dynamics that best mirror the behavior occur within the NAc core and not the NAc shell.
Cue-evoked basic pH shifts within the NAc core but not shell track freezing behavior
Given that fear cues caused a strictly region-specific shift in local pH, we analyzed this in more detail. Specifically, we (in the same manner that we used for the [DA] data) compared alterations in cue-evoked changes in pH units across blocks of fear expression.
We constructed heatmaps to study how cue-evoked pH changes within the NAc core and shell evolve across the 15 tone session (Fig. 6a,b). Within the NAc core (Fig. 6a), cue presentation was followed by a basic shift in pH that lasted through blocks 1 and 2 but diminished through block 3. Quantification of the fold-change in pH from the pretone period to the post-tone period in conditioned animals showed that cue presentation resulted in a significant increase in pH (i.e., a basic shift) within the NAc core during both the first and second blocks in conditioned, but not control, subjects (Fig. 6c). Two-way ANOVA analysis of core-conditioned versus control groups showed significant main effects of group (F(1,18) = 53.96, p < 0.001)and time (F(2, 18) = 20.10, p < 0.001) and revealed an interaction between group and time (F(2, 16) = 5.59, p = 0.013). Post hoc tests showed a significant difference between core-conditioned animals and control animals during both block1 (p < 0.001) and block 2 (p < 0.05). For block 3, there was no difference between conditioned and control subjects (p > 0.05). We then analyzed each five tone block of pH data in 2 s bins (Fig. 6d–f). Within the NAc core, cue presentation caused a significant basic shift in pH units during both blocks 1 (Fig. 6d; F(10, 23.7) = 5.94, p < 0.001) and 2 (Fig. 6e; F(10, 32.7) = 6.10, p < 0.001). We observed no significant changes in block 3 (Fig. 6f; F < 1.0). It should be noted that basic pH shifts within the core are significant during blocks 1 and 2, but not 3, which mirrors freezing behavior in conditioned animals (Fig. 2d).
In contrast to the NAc core, CS presentation did not alter pH within the NAc shell (Fig. 6b). Quantification of the fold-change in pH following tone presentation confirms this observation from the heatmaps and shows no changes in pH within the NAc shell in conditioned or control animals (Fig. 6g). This was further confirmed by examining the binned pH data for each 5-tone block (Fig. 6h–j). No significant cue-evoked changes in pH were observed in block 1 (Fig. 6h), block 2 (Fig. 6i), or block 3 (Fig. 6j) within the NAc shell.
Changes in DA transmission are related to but not caused by changes in locomotor activity
As the NAc has been implicated in the generation of general locomotor activity, we assessed whether changes in [DA] and pH within the NAc core and shell were correlated with the behavioral output quantified in this study, freezing. Given that CS-evoked decreases in [DA] within the NAc core occur consistently only during early trials, when fear expression is high, it is tempting to expect that there may be a strong, and perhaps causal, relationship between changes in [DA] and freezing in this region. However, as demonstrated by a representative rat (Fig. 7a,b), which showed equivalent freezing behavior on individual trials within the first and second block of trials and yet showed a robust decrease in [DA] in block 1 (trial 2) (Fig. 7a) and no change in [DA] in block 2 (trial 8) (Fig. 7b), this is clearly not the case. This suggests that changes in [DA] were not directly causal with respect to changes in behavior.
Figure 7.
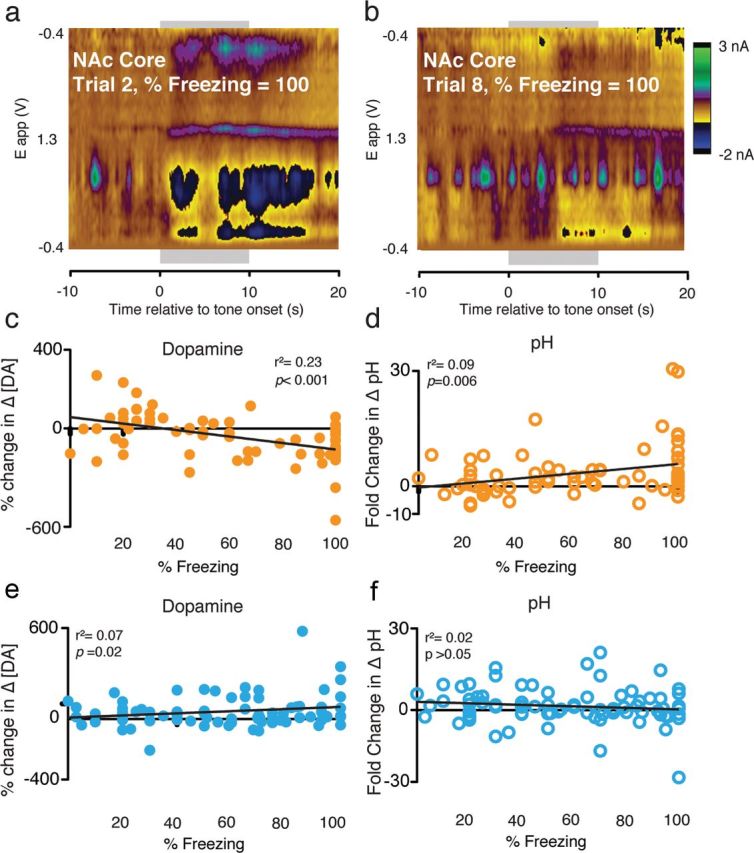
Analysis of the relationship between locomotor activity and neurochemical changes. a, b, Representative traces from a single animal that displayed identical freezing behavior on two trials during block 1 (a) and block 2 (b) illustrate that [DA] does not strictly follow locomotor activity. c, Quantification of the correlation between percentage change in [DA] and freezing in the NAc core shows a significant correlation between behavioral expression and changes in dopamine. d, Quantification of the correlation between pH and freezing revealed a modest but significant relationship in the NAc core. e, f, Conversely, no relationships were observed between changes in [DA] (e) or pH (f) and freezing within the NAc shell.
However, quantification of the relationship between DA and freezing showed that, despite the absence of a strongly causal relationship between changes in [DA] within the NAc and freezing, there was a relationship between behavioral output and DA neurotransmission. Specifically, there was a significant correlation between changes in [DA] and freezing across all trials in the NAc core (r2 = 0.23, p < 0.01)(Fig. 7c) and shell (r2 = 0.07, p < 0.05) (Fig. 7e). This is consistent with our general observation that changes in [DA] were greatest at the beginning of the test session, when freezing was highest, and extinguished as the session proceeded.
We also observed a modest but significant correlation between changes in basic pH and freezing in the NAc core (r2 = 0.09, p < 0.05; Fig. 7d) but not the NAc shell (r2 = 0.03, p = 0.27; Fig. 7f). This relationship between freezing and basic pH changes within the NAc core may be of interest because it has been suggested that changes in pH correspond to increased postsynaptic neural activity, with alkaline changes in pH corresponding with increased metabolic activity within the structure (Roitman et al., 2008; Ariansen et al., 2012). The correlation between pH and locomotor activity, taken together with the change in DA transmission during the initial block of trials, may indicate that the NAc core is involved in organizing behavioral outputs in response to aversive stimuli. These data show that changes in DA transmission only occur in response to early trials, when reorganization of behavioral strategies in response to learned fear information would be expected, while other measures of neural activity (i.e., pH) may track the behavioral output.
Discussion
The present study demonstrates, for the first time, that fear-evoking stimuli differentially alter phasic DA transmission across NAc subregions. Fear cues decreased [DA] within the NAc core but increased [DA] within the NAc shell. These regionally specific changes are attributable to changes in phasic DA transmission, as the aversive cue decreased the probability of DA transients within the NAc core but increased the magnitude of transients within the NAc shell. Moreover, changes in [DA] are not strongly causally attributed to the cessation of locomotor activity. Thus, rather than DA coding a unitary motivational signal, multiple mesolimbic DA systems may process distinct aspects of motivated behavior, including states associated with fear-evoking cues (Ikemoto, 2007; Aragona et al., 2009; Bromberg-Martin et al., 2010b; Lammel et al., 2011).
Aversive cues decrease DA transmission specifically in the NAc Core
Here, we demonstrate that conditioned aversive cues evoke a rapid and prolonged decrease in [DA] in the NAc core that is attributable to decreased DA release probability. This is consistent with many previous studies that have shown that acute aversive stimuli cause phasic decreases in DA neuron firing (Mirenowicz and Schultz, 1996; Ungless et al., 2004) and one recent study demonstrated that the duration of DA neuron inhibitions is associated with a behavioral measure of conditioned fear (Mileykovskiy and Morales, 2011). Although it has been suggested that phasic decreases in DA transmission may code negative motivational value evoked by aversive stimuli (Bromberg-Martin et al., 2010b), the present study provides the first evidence regarding the forebrain locus for this decrease. Specifically, diminished DA neuron firing, and the subsequent decrease in [DA], appears to have functional effects within the NAc core.
While we know relatively little, mechanistically, about phasic decreases in DA activity, recent work demonstrating that VTA-GABA cells are involved in conditioned place aversion (Tan et al., 2012) and in disrupting reward-seeking behaviors (van Zessen et al., 2012) suggests that these cells may play an important role in modulating DA release to aversive cues and events. Interestingly, there is compelling evidence that aversive motivational signals are encoded by habenular cells (Matsumoto and Hikosaka, 2009; Hong et al., 2011) and it has been suggested that their inhibitory projections to DA neurons may be driven by these VTA-GABAergic cells. Future work will be needed regarding how interactions among these neural populations may allow for the DA signals we observe here to arise.
Importantly, decreased [DA] within the NAc core does not appear to be uniquely involved in representing aversive stimuli. Previous studies have shown that aversive taste cues cause phasic decreases in [DA] within the NAc shell (Roitman et al., 2008; Wheeler et al., 2011). Thus, phasic DA transmission within both the NAc core and shell appear capable of encoding motivational value. These core-shell differences in aversive cue modulation of DA may represent differences in how unconditioned versus conditioned stimuli are represented in these circuits. Recent work characterizing how phasic DA release within the NAc supports appetitive motivational signals has demonstrated that while unconditioned rewards elicit strong DA release within the NAc shell (Aragona et al., 2008, 2009), reward predictive cues preferentially increase DA release within the NAc core (Day et al., 2007; Aragona et al., 2009; Wanat et al., 2010; Flagel et al., 2011). The aversive cues previously used to alter DA transmission within the NAc have been tastants that have caused decreased DA transmission in the NAc shell (e.g., Roitman et al., 2008, Wheeler et al., 2011). Taken together with previous appetitive and aversive work, our data suggest that phasic signaling within the NAc core may represent a common mode of transmission underlying incentive motivational responses especially for conditioned stimuli (Robinson and Berridge, 2001; Berridge, 2007). The directionality of the shift (i.e., increases or decreases) may encode motivational value, or may rather reflect the organization of behavioral strategies into active (e.g., reward seeking, avoidance) or passive (e.g., freezing) forms, respectively.
Theories of striatal activity posit that the striatum is critical for behavioral selection, and it has been suggested that enhanced phasic DA signals are critical for identifying and reinforcing actions to organize novel and adaptive behavioral strategies (Redgrave et al., 2008). Likewise, phasic decreases in DA release may allow reorganization of behavioral strategies to support cessation of any activity that might lead to an aversive outcome via the indirect pathway through the basal ganglia (Kelley et al., 2005; Kravitz et al., 2012). The results presented here are consistent with these ideas, as the decreases observed here are specific to initial tone presentations, when behavioral strategies are selected as well as the period when the animals are experiencing the most heightened aversive motivational states.
Moreover, the specificity of the decrease to the NAc core, as well as the fact that the decrease was only present during early CS presentations, likely explains why this effect was missed by many previous microdialysis studies (Wilkinson et al., 1998; Levita et al., 2002; Martinez et al., 2008). The subsecond temporal resolution of FSCV allows for phasic neurochemical changes to be detected and therefore better reveals the nature of how DA transmission is altered by environmental cues in real-time.
Cue-evoked increases in DA transmission in the NAc Shell
Interestingly, the current study also demonstrates that aversive cues increase DA transmission in a regionally specific manner. Indeed, many studies spanning multiple techniques have recently supported this claim (Brischoux et al., 2009; Matsumoto and Hikosaka, 2009; Lammel et al., 2011; Zweifel et al., 2011). Consistent with several previous microdialysis studies (Kalivas and Duffy, 1995; Ikemoto and Panksepp, 1999; Pezze et al., 2001; Martinez et al., 2008), the present study demonstrates that pavlovian aversive cues enhance DA release specifically within the NAc shell. However, it is unclear what this increased [DA] in the NAc shell is encoding. It is well established that phasic increases in [DA] within the NAc shell are associated with primary rewards (Di Chiara and Bassareo, 2007; Roitman et al., 2008). Thus, it is possible that increased [DA] following a fearful cue reflects a “relief” signal when the expected footshock is absent (Ikemoto and Panksepp, 1999). This is consistent with the timing of the increase we report, which is significant immediately following cue offset, although there is a gradual but not significant increase to that point throughout tone presentation that would not be explained by a relief signal (Fig. 3b,h). Alternatively, the enhanced DA could represent a prediction error signal, independent of any hedonic experiences, in that rats are expecting to receive a footshock following CS presentation but do not receive it. Although electrophysiological correlates of prediction errors suggest that this should have a more precise temporal relationship to the tone, the diffuse nature may be explained by internal timing errors due to the long duration of the tone and the relatively few conditioning trials. It is perhaps more likely that when cues increase [DA] regardless of their valence, the increased DA transmission codes general motivational salience, which may involve either the fear that the cue predicts or the lack of expected footshock animals experience (Faure et al., 2008; Bromberg-Martin et al., 2010b; Richard and Berridge, 2011). This is consistent with previous studies showing that increasing DA transmission within the NAc shell increases motivational responding (Ito et al., 2000; Wyvell and Berridge, 2001; Parkinson et al., 2002; Berridge, 2007).
Presentation of the aversive cue increased [DA] within the NAc shell by increasing the amplitude of phasic DA release events suggesting that [DA] is increased by an enhanced magnitude in the amount of DA released per event (Aragona et al., 2009; Jones et al., 2010). This may indicate an increase in DA neurons participating in a release event (Hyland et al., 2002). It has been shown that aversive stimuli often drive enhanced excitation of VTA DA neurons at stimulus offset, which may contribute to the increase we report here (Brischoux et al., 2009; Wang and Tsien, 2011). Moreover, recent work suggests that stressors potentiate shell-projecting DA neurons and increase their burst firing, which suggests that these neurons may be recruited during an aversive behavioral state (Valenti et al., 2011). Further, it has been proposed that this modulation of DA neuron excitability is mediated by the ventral hippocampus, a structure critical for fear-associated learning and memory (Adhikari et al., 2010; Orsini et al., 2011). Future studies will need to determine if and how this circuit is active during aversive cue presentations, and whether presentation of an aversive stimulus increases phasic transmission in other brain regions such as the prefrontal cortex (Lammel et al., 2011).
Conclusion
Understanding how aversive information modulates DA signaling within the NAc provides a window into how the brain perceives and processes motivational information. The present study demonstrates the importance of phasic DA signaling within distinct mesolimbic circuits for processing aversive stimuli. Moreover, this study uses real-time neurochemical measures in freely moving animals to address a long-standing controversy regarding how fear cues alter dopamine transmission in the NAc. We demonstrate that DA transmission does not reflect aversive cues by a simple increase or decrease, but rather the nature of transmission depends on the specific mesolimbic pathway from which the neurochemical signaling is occurring.
Footnotes
This work was supported by National Institutes of Health (R01MH065961, S.M.), National Science Foundation (0953106, B.J.A.), and a Rackham Regents Fellowship (A.B.). We wish to thank Drs. Joshua Berke, Richard Keithley, Jeremy Day, Caitlin Orsini, and Vedran Lovic as well as Kirsten Porter-Stransky, Katherine Prater, Brandy Rademacher, and Ramzy Khabbaz for technical assistance and comments during the assembly of this manuscript.
References
- Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Wightman RM, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30:1889–1899. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott GW, Wickens J. Space, time and dopamine. Trends Neurosci. 2007;30:62–69. doi: 10.1016/j.tins.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Arcediano F, Escobar M, Miller RR. Bidirectional associations in humans and rats. J Exp Psychol Anim Behav Process. 2005;31:301–318. doi: 10.1037/0097-7403.31.3.301. [DOI] [PubMed] [Google Scholar]
- Ariansen JL, Heien ML, Hermans A, Phillips PE, Hernadi I, Bermudez MA, Schultz W, Wightman RM. Monitoring extracellular pH, oxygen, and dopamine during reward delivery in the striatum of primates. Front Behav Neurosci. 2012;6:36. doi: 10.3389/fnbeh.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon MJ, Roth RH. Pharmacology of mesocortical dopamine neurons. Pharmacol Rev. 1983;35:53–68. [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Distinct tonic and phasic anticipatory activity in lateral habenula and dopamine neurons. Neuron. 2010a;67:144–155. doi: 10.1016/j.neuron.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010b;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci. 2011;34:1997–2006. doi: 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, Park J, Bass CE, Grinevich VP, Bonin KD, Wightman RM. Aversive stimulus differentially triggers subsecond dopamine release in reward regions. Neuroscience. 2012;201:331–337. doi: 10.1016/j.neuroscience.2011.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Fadok JP, Palmiter RD. Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response. Learn Mem. 2011;18:136–143. doi: 10.1101/lm.2041211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Wightman RM, Carelli RM. Phasic nucleus accumbens dopamine release encode effort- and delay-related costs. Biol Psychiatry. 2010;68:306–309. doi: 10.1016/j.biopsych.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–403. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn't do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Dickerson TM, Palmiter RD. Dopamine is necessary for cue-dependent fear conditioning. J Neurosci. 2009;29:11089–11097. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Darvas M, Dickerson TM, Palmiter RD. Long-term memory for pavlovian fear conditioning requires dopamine in the nucleus accumbens and basolateral amygdala. PLoS ONE. 2010;5:e12751. doi: 10.1371/journal.pone.0012751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J Neurosci. 2008;28:7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Moran PM, Grigoryan G, Peters SL, Young AM, Joseph MH. Latent inhibition: the nucleus accumbens connection revisited. Behav Brain Res. 1997;88:27–34. doi: 10.1016/s0166-4328(97)02313-9. [DOI] [PubMed] [Google Scholar]
- Heien ML, Wightman RM. Phasic dopamine signaling during behavior, reward, and disease states. CNS Neurol Disord Drug Targets. 2006;5:99–108. doi: 10.2174/187152706784111605. [DOI] [PubMed] [Google Scholar]
- Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31:11457–11471. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Day JJ, Aragona BJ, Wheeler RA, Wightman RM, Carelli RM. Basolateral amygdala modulates terminal dopamine release in the nucleus accumbens and conditioned responding. Biol Psychiatry. 2010;67:737–744. doi: 10.1016/j.biopsych.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Keithley RB, Takmakov P, Bucher ES, Belle AM, Owesson-White CA, Park J, Wightman RM. Higher sensitivity dopamine measurements with faster-scan cyclic voltammetry. Anal Chem. 2011;83:3563–3571. doi: 10.1021/ac200143v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Levita L, Dalley JW, Robbins TW. Nucleus accumbens dopamine and learned fear revisited: a review and some new findings. Behav Brain Res. 2002;137:115–127. doi: 10.1016/s0166-4328(02)00287-5. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Shin R, Ikemoto S. Dual role of medial A10 dopamine neurons in affective encoding. Neuropsychopharmacology. 2008;33:3010–3020. doi: 10.1038/npp.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70:830–845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez RC, Oliveira AR, Macedo CE, Molina VA, Brandão ML. Involvement of dopaminergic mechanisms in the nucleus accumbens core and shell subregions in the expression of fear conditioning. Neurosci Lett. 2008;446:112–116. doi: 10.1016/j.neulet.2008.09.057. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Beeler JA, Roitman MF. Sucrose-predictive cues evoke greater phasic dopamine release than saccharin-predictive cues. Synapse. 2012;66:346–351. doi: 10.1002/syn.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskiy B, Morales M. Duration of inhibition of ventral tegmental area dopamine neurons encodes a level of conditioned fear. J Neurosci. 2011;31:7471–7476. doi: 10.1523/JNEUROSCI.5731-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Heidbreder CA, Feldon J, Murphy CA. Selective responding of nucleus accumbens core and shell dopamine to aversively conditioned contextual and discrete stimuli. Neuroscience. 2001;108:91–102. doi: 10.1016/s0306-4522(01)00403-1. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Robinson DL, Stuber GD, Carelli RM, Wightman RM. Real-time measurements of phasic changes in extracellular dopamine concentration in freely moving rats by fast-scan cyclic voltammetry. Methods Mol Med. 2003a;79:443–464. doi: 10.1385/1-59259-358-5:443. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003b;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic dopamine signals? Brain Res Rev. 2008;58:322–339. doi: 10.1016/j.brainresrev.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear. J Neurosci. 2011;31:12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Wightman RM. Nomifensine amplifies subsecond dopamine signals in the ventral striatum of freely-moving rats. J Neurochem. 2004;90:894–903. doi: 10.1111/j.1471-4159.2004.02559.x. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Heien ML, Wightman RM. Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci. 2002;22:10477–10486. doi: 10.1523/JNEUROSCI.22-23-10477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Sinkala E, McCutcheon JE, Schuck MJ, Schmidt E, Roitman MF, Eddington DT. Electrode calibration with a microfluidic flow cell for fast-scan cyclic voltammetry. Lab Chip. 2012;12:2403–2408. doi: 10.1039/c2lc40168a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005;30:853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, Deisseroth K, Tye KM, Lüscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Valenti O, Lodge DJ, Grace AA. Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci. 2011;31:4280–4289. doi: 10.1523/JNEUROSCI.5310-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Michael DJ, Wightman RM. Correlation of local changes in extracellular oxygen and pH that accompany dopaminergic terminal activity in the rat caudate-putamen. J Neurochem. 2003;84:373–381. doi: 10.1046/j.1471-4159.2003.01527.x. [DOI] [PubMed] [Google Scholar]
- Verbeke G. Linear mixed models for longitudinal data. New York: Springer; 2009. [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, Phillips PE. Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev. 2009;2:195–213. doi: 10.2174/1874473710902020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Kuhnen CM, Phillips PE. Delays conferred by escalating costs modulate dopamine release to rewards but not their predictors. J Neurosci. 2010;30:12020–12027. doi: 10.1523/JNEUROSCI.2691-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DV, Tsien JZ. Convergent processing of both positive and negative motivational signals by the VTA dopamine neuronal populations. PLoS ONE. 2011;6:e17047. doi: 10.1371/journal.pone.0017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biol Psychiatry. 2011;69:1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Heien ML, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Humby T, Killcross AS, Torres EM, Everitt BJ, Robbins TW. Dissociations in dopamine release in medial prefrontal cortex and ventral striatum during the acquisition and extinction of classical aversive conditioning in the rat. Eur J Neurosci. 1998;10:1019–1026. doi: 10.1046/j.1460-9568.1998.00119.x. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM, Joseph MH, Gray JA. Latent inhibition of conditioned dopamine release in rat nucleus accumbens. Neuroscience. 1993;54:5–9. doi: 10.1016/0306-4522(93)90378-s. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM, Allen JM, Mizumori SJ, Bonci A, Palmiter RD. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 2011;14:620–626. doi: 10.1038/nn.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]