Abstract
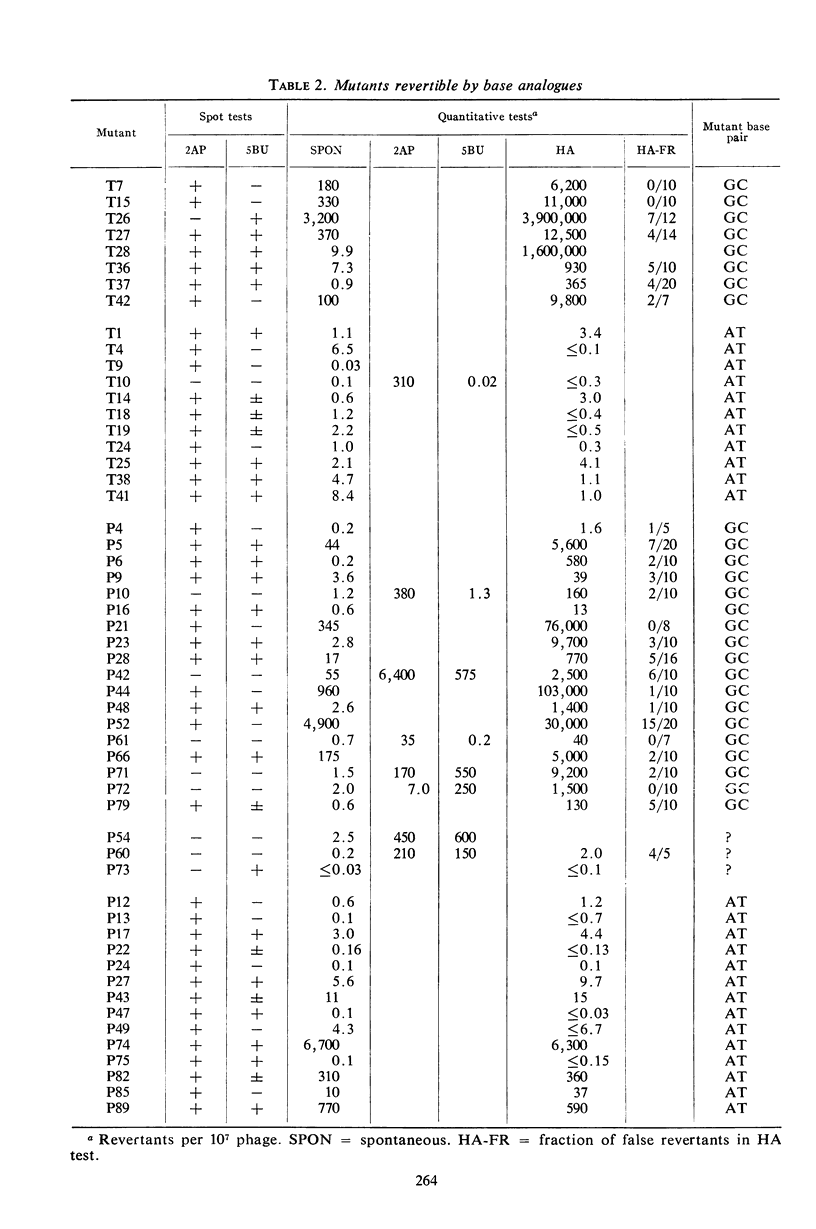
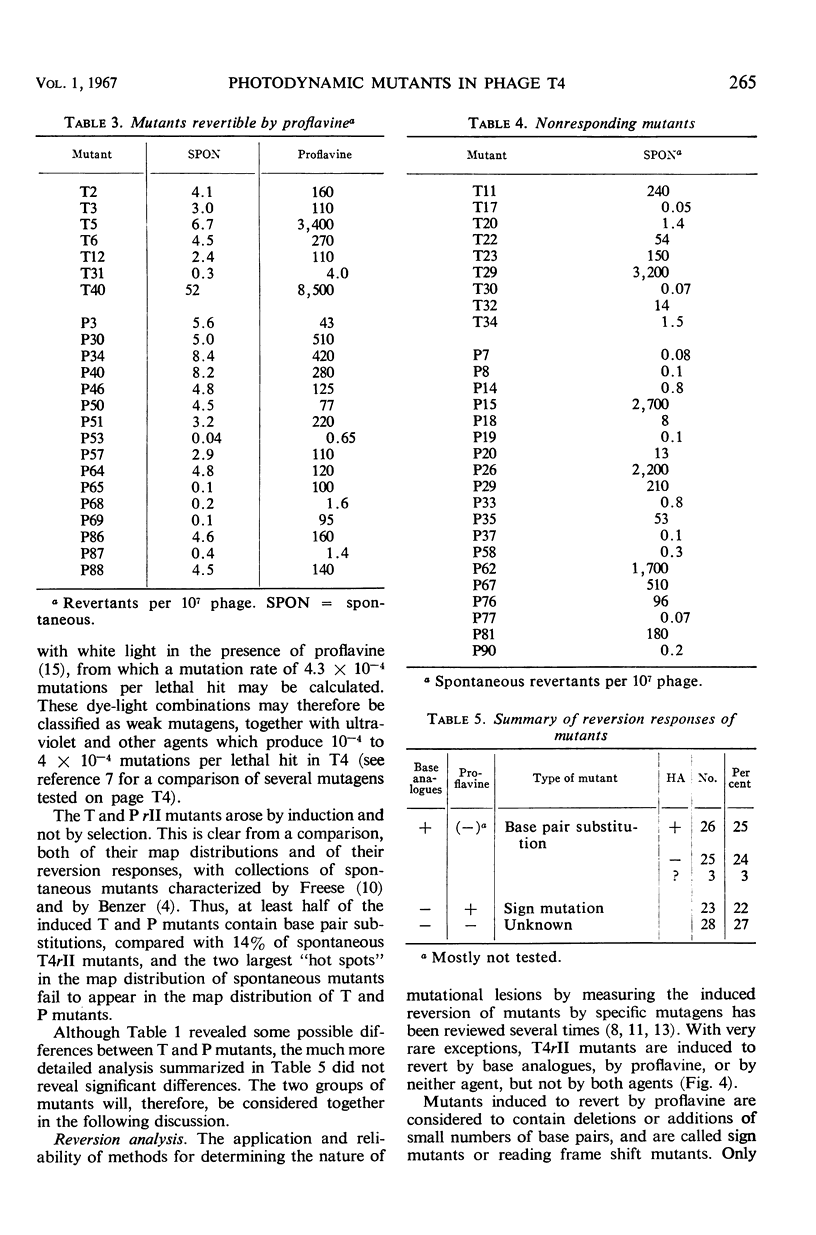
About 4 × 10−4r mutants were induced per lethal hit, a frequency characteristic of weak mutagens. Collections of mutants produced in the presence of either dye were indistinguishable in most of their properties. The rII mutants differed sharply from spontaneous mutants in their mutational spectra (fine scale map distribution) and their reversion responses to specific mutagens. Few or none of the induced mutants were induced to revert with proflavine (sign mutants; reading frame shift mutants). A majority were induced to revert with base analogues (base pair substitution mutants), and about half of these also responded to the hydroxymethylcytosine-specific agent hydroxylamine. A large minority of the mutants reverted spontaneously but failed to respond either to proflavine or to base analogues. We believe these mutants, as well as some of the mutants which did respond to base analogues, to be transversions (base pair substitutions which reverse the purine-pyrimidine orientation).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellin J. S., Grossman L. I. Photodynamic degradation of nucleic acids. Photochem Photobiol. 1965 Jan;4(1):45–53. doi: 10.1111/j.1751-1097.1965.tb05724.x. [DOI] [PubMed] [Google Scholar]
- Benzer S. ON THE TOPOGRAPHY OF THE GENETIC FINE STRUCTURE. Proc Natl Acad Sci U S A. 1961 Mar;47(3):403–415. doi: 10.1073/pnas.47.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W., McGuire J. Characteristics of mutations appearing spontaneously in extracellular particles of bacteriophage T4. Genetics. 1967 Mar;55(3):387–398. doi: 10.1093/genetics/55.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W. Spontaneous mutations accumulating in bacteriophage T4 in the complete absence of DNA replication. Proc Natl Acad Sci U S A. 1966 Apr;55(4):738–743. doi: 10.1073/pnas.55.4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W. Ultraviolet mutagenesis in bacteriophage T-4. I. Irradiation of extracellular phage particles. J Bacteriol. 1966 May;91(5):1775–1780. doi: 10.1128/jb.91.5.1775-1780.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESE E., BAUTZ E., FREESE E. B. The chemical and mutagenic specificity of hydroxylamine. Proc Natl Acad Sci U S A. 1961 Jun 15;47:845–855. doi: 10.1073/pnas.47.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESE E. On the molecular explanation of spontaneous and induced mutations. Brookhaven Symp Biol. 1959 Nov;NO:63–75. [PubMed] [Google Scholar]
- Ritchie D. A. Mutagenesis with light and proflavine in phage T4. II. Properties of the mutants. Genet Res. 1965 Nov;6(3):474–478. doi: 10.1017/s0016672300004353. [DOI] [PubMed] [Google Scholar]
- SIMON M. I., VAN VUNAKIS H. The photodynamic reaction of methylene blue with deoxyribonucleic acid. J Mol Biol. 1962 Jun;4:488–499. doi: 10.1016/s0022-2836(62)80104-1. [DOI] [PubMed] [Google Scholar]
- SIMON M. I., VANVUNAKIS H. THE DYE-SENSITIZED PHOTOOXIDATION OF PURINE AND PYRIMIDINE DERIVATIVES. Arch Biochem Biophys. 1964 Apr;105:197–206. doi: 10.1016/0003-9861(64)90253-x. [DOI] [PubMed] [Google Scholar]
- SUSSENBACH J. S., BERENDS W. PHOTODYNAMIC DEGRADATION OF GUANINE. Biochim Biophys Acta. 1965 Jan 11;95:184–185. doi: 10.1016/0005-2787(65)90226-1. [DOI] [PubMed] [Google Scholar]
- SUSSENBACH J. S., BERENDS W. PHOTOSENSITIZED INACTIVATION OF DEOXYRIBONUCLEIC ACID. Biochim Biophys Acta. 1963 Sep 17;76:154–156. [PubMed] [Google Scholar]


