Abstract
Individuals suffering from type 2 diabetes or obesity exhibit a significant increase in the incidence of various types of cancer. It is generally accepted that those conditions arise from overnutrition and a sedentary lifestyle, which lead to insulin resistance characterized by overproduction of insulin acting as a growth factor. There is a consensus based largely on epidemiological data that chronic overproduction of insulin is responsible for the increased incidence of cancer. A model system in culture of NIH 3T3 cells induces the collective effects of serum growth factors on progression through the stages of field cancerization. It shows that the driving force of progression is promotion of cell growth under selection at high cell density, with no requirement for exogenous carcinogenic agents. The early effect is gradual selection among many preexisting, low-penetrance preneoplastic mutations or stable epigenetic variants, followed by sporadic, high-penetrance transforming variants, all dependent on endogenous processes. The significance of the results for cancer in diabetic and obese individuals is that the initial stages of the process involve multiorgan metabolic interactions that produce a systemic insulin resistance with chronic overproduction of insulin and localized field cancerization. Hypomagnesemia is prevalent in the foregoing metabalo/systemic disorders, and may also provide a selective microenvironment for tumor development.
Keywords: hyperinsulinemia, preneoplasia, neoplastic transformation
The term “field cancerization” was introduced in 1953 to describe the microscopic observation of flat, epithelial, hyperplastic areas of grossly normal tissue in the margins of human oral cancers (1). Such areas had been reported in the margins of colorectal cancers a quarter century earlier (2) and had been described in detail as existing in a large number of squamous cell carcinomas of the skin (3). There was disagreement among pathologists whether the hyperplastic areas represented stimulation of the epithelium by secretion from the tumors or were an early stage of the onset of tumor development, but later genetic evidence supported the latter interpretation (4). Field cancerization was described in the margins of a wide variety of human cancers and was thought to be characteristic of them all (5).
The problem with the histological and genetic observations on cancerization fields in human cancers is that they were conducted after the tumor itself had grown to a size that was clinically apparent. In other words, the preneoplastic fields were already old, and it was not possible to determine the causes and dynamics of their formation. Indeed, very small foci of neoplastic cells could be seen scattered among hyperplastic and dysplastic cells that constituted the fields of oral carcinomas (1). The conventional concept of tumor development was based largely on experimental carcinogenesis of the skin, which evoked the model of initiation by carcinogenic chemicals. Initiation was generally considered to be a mutational event because a single exposure took effect immediately and remained functionally detectable for extended periods of time. The biological method of detection was repeated exposure of the initiated area with a promoter, which was itself noncarcinogenic. The experimental endpoint in vivo was the appearance of a tumor, and in vitro it was the appearance of transformed foci with little attention paid to the field stage of cancerization. However, the NIH 3T3 cell culture in serial passages of growth to confluence both started and continuously maintained in low serum concentrations displayed an extended period of gradually increasing saturation density before the appearance of very small foci in some cultures, as discussed in Results. Saturation density is the maximum population density of cells that can be achieved in a culture with a given concentration of serum and frequent changes of medium. It is directly proportional to the concentration of serum in the medium.
The entire panoply of neoplastic development consisted of gradual increases of saturation density, with each round of serial selection at confluence, followed by an increase in size, density, and number of transformed foci, the most advanced of which produce tumors in 19–25 d when injected into athymic mice (6). There is no involvement of initiating carcinogens in the process, which is represented as promotion by proliferation and selection of mutations and epigenetic changes for increased capacity of growth at high cell density. The question then arose, What is the significance of a cell culture model, with its absence of an initiating carcinogen and a possibly extended period of the preneoplastic field stage in human cancerization?
An answer was suggested by the increased incidence of cancer in humans with obesity and type 2 diabetes (7). The increase includes many different types of cancer and is related to a systemic condition called insulin resistance. Intramyocellular lipid accumulation plays an important role in causing insulin resistance in muscle of the offspring of parents with type 2 diabetes (8). Consumption of excess dietary energy results in obesity, often leading to type 2 diabetes, insulin resistance, and increased levels of circulating insulin (9). Although insulin is predominantly concerned with regulation of carbohydrate metabolism, it also acts as a growth factor, as is unequivocally shown in cell culture (10, 11).
There is a wide consensus that the activation of the hormones of the insulin and insulin-like growth factor axis associated with insulin resistance mediate the increase of a variety of cancers in obesity (12) and in type 2 diabetes (13, 14). Insulin resistance, which is commonly measured by a decrease of insulin-stimulated uptake of glucose, is associated with elevated serum insulin, glucose, nonesterified fatty acids, and triglycerides (15). Infusion of insulin into rats for 10 h increased the level of serum insulin to that seen in insulin resistance and increased the proliferation of normal colorectal cells in a dose-dependent manner (16). Infusion of glucose or triglycerides alone had no effect on proliferation, although they increased hyperinsulinemia when combined with insulin. Earlier experiments in which rats were initiated with a colorectal carcinogen and promoted with frequent injections of insulin yielded an increase of aberrant crypt foci (17, 18), which are precursors of colon cancer.
These experiments lent support to the role of hyperinsulinemia in promoting a role of insulin in colorectal cancer. However, most of the evidence for a major role of insulin in carcinogenesis associated with obesity and type 2 diabetes came from epidemiological studies. A meta-analysis of such studies of the association of colorectal cancer with circulating insulin concluded that they yielded conflicting or inconsistent results and that multiple factors are likely to underlie the influence of obesity on colorectal cancer (19, 20). For example, one epidemiological study reported that increased insulin and insulin growth factor 1 were each associated with colorectal cancer incidence, but they became insignificant when adjusted for each other (21). In contrast, endogenous estradiol levels were positively associated with colorectal cancer. More recent studies have revealed that even more growth factors such as cytokines, chemokines, and adipokines reinforce the complexity of the relationship between obesity and cancer (22, 23). It can be concluded that any chronic stimulation of proliferation by one or more growth factors in obesity or type 2 diabetes has the potential to promote tumor development. The best-known pathway is the overproduction of insulin via insulin resistance, but there are many more growth factors produced in the inflammatory response in tissues that accompany insulin resistance.
Given the large number of growth factors, hormones, cytokines, and adipokines, as well as their tissue receptors and binding proteins, it would be difficult, if not impossible, to identify them all and quantify their role in tumor promotion. There is, however, a simple operational alternative, which is to quantify the collective promoter activity in the serum of a cell culture system, which responds to such activity by progressing through all of the stages of field cancerization, including the preneoplastic stage, without exposing the cells to an initiating, presumably mutagenic, agent. A preliminary report on these findings has been published (24), and a more detailed, quantitative version is presented here, along with its significance for increased spontaneous tumor development in type 2 diabetes and obesity. The initial interest in this association was that both the neoplastic development of the cell culture process and the human disorders required only promotion by endogenous growth factors and selection, with no evidence for an initiating step by exogenous factors. The overall objective was to determine in cell culture the quantitative relations between growth factors and time to field cancerization, with its unique in vitro capacity to exhibit the preneoplastic state. That information would be applied to human cancer, which could provide information about the metabalo/systemic state that precedes even the local preneoplastic field stage of cancerization.
Results
The Saturation Densities of the First-Round Assay.
There was a linear relationship between serum concentration and saturation density, with a small rise between 2 and 3 wk of the cultures in 2% (vol/vol) and 10% (vol/vol) serum, as well as a slightly larger rise in 5% (vol/vol) serum (Table 1). The results indicate that relatively small differences in growth factor activity could be detected by saturation density, especially when assayed with a stepwise set of serum concentrations.
Table 1.
Saturation densities of first-round assay started with 105 cells
| Cell count (×105) |
||
| Serum, % | 2 wk | 3 wk |
| 2 | 4.3 ± 0.1 | 4.7 ± 0.1 |
| 5 | 9.6 ± 0.5 | 11.3 ± 0.4 |
| 10 | 21.4 ± 0.5 | 23.0 ± 0.9 |
NIH 3T3 cells were grown to confluence in 2%, 5%, and 10% calf serum in MCDB 402 medium for 2 and 3 wk. Some of the cultures were trypsinized and the cells counted for saturation density.
Effects of Variation in Serum Concentration and Time in First-Round Assay on Progression in Serial Second-, Third-, and Fourth-Round Assays Under Constant Conditions.
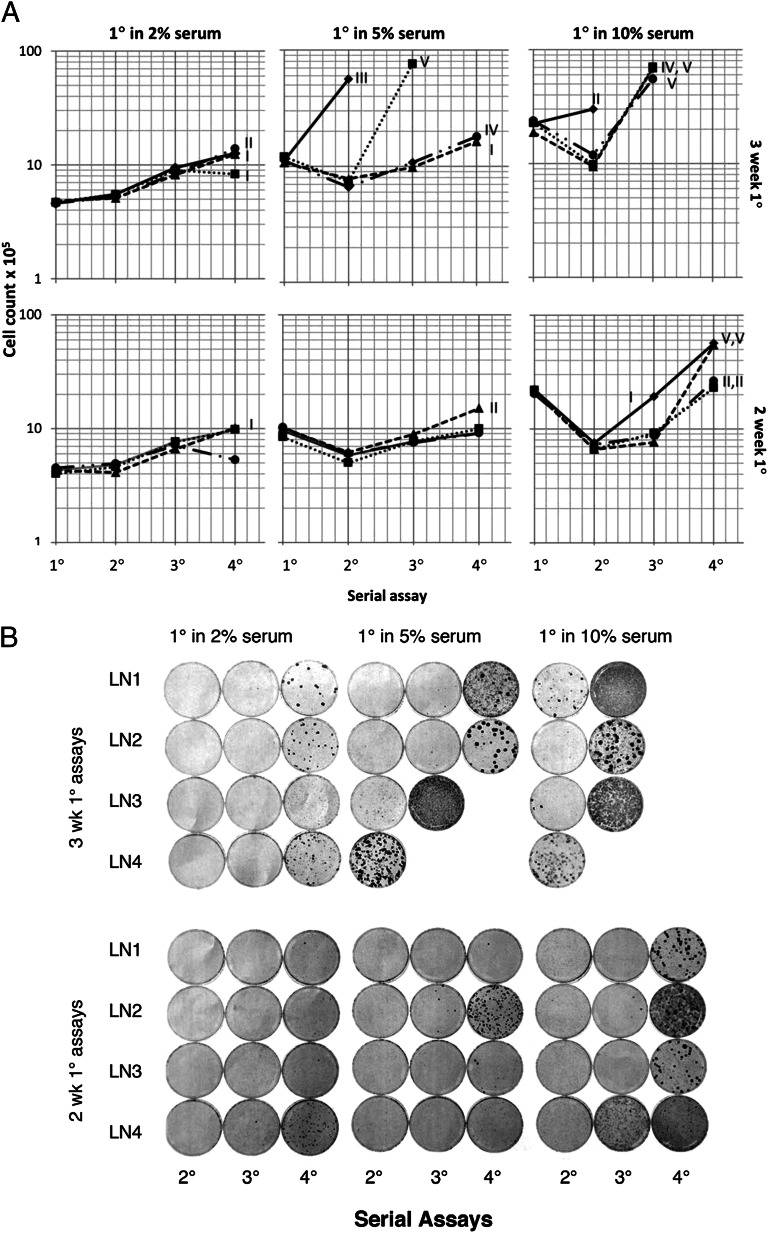
The trends in serial assays for the relationship between saturation density and transformation are shown in Fig. 1A. In each category, the higher the saturation densities in the first-round assay, the higher they remained during their further increase in the second-, third-, and fourth-round assays. The cultures derived from 3 wk in the first-round assay were in each case higher than those derived from their particular serum counterparts in the 2-wk first-round assay. In addition, the higher the serum concentration and consequent saturation density of the first-round assay, the higher the saturation densities of the second-, third-, and fourth-round assays in constant 2% serum concentration. The appearance of well-developed, transformed foci coincided with sharp increases in saturation density; overall rank order for the most part remained the same as that of the first-round assay saturation density. The early increases in saturation density were the same among all of the lineages within each serum and time category, but transformed foci appeared randomly over time (Fig. 1 A and B). The conclusion from these observations was that the early, gradual increases in saturation density arose by the selection of several, among many preexisting, heterogeneous mutations and epigenetic changes of low penetrance at high density. In contrast, the variants that produced transformed foci apparently arose from random, single, new variants of high penetrance. On the basis of the gradual, uniformly distributed increases of preneoplastic saturation density within each serum/time category, those changes would be classified as promotion, but in fact, promotion and newly minted focus-forming variants partially overlapped with each other.
Fig. 1.
(A) Saturation density of cultures in serial selection at high densities. NIH 3T3 cells were seeded in 4 lineages with 105 cells per culture in 2%, 5%, and 10% serum in MCDB 402 medium for 2 or 3 wk. The cultures were trypsinized and counted for the first-round (1°) assays and subcultured in 2% serum for 2 wk for the second-round (2°) assays. The procedures were repeated for the third- and fourth-round (3° and 4°) assays. Some sequential assays were terminated in the second- or third-round assays of cultures originating from first-round assays in 5% or 10% serum for 3 wk because it was judged that further assays would show confluent transformation. Transformation key: I, many tiny foci; II, small foci; III, medium size foci; IV, large foci; V, semiconfluent or confluent foci. Lineages: LN1, ●; LN2, ■ ; LN3, ▲ ; LN4, ◆. (B) Photograph of cultures in serial assays in A except for the absence of those in the first-round assay. B is part of the experiment in A and treated in the same way, except these cultures were fixed and stained overnight with 4% Giemsa.
Although there were only small differences in saturation densities between the 2- and 3-wk periods of the first-round assays started with the same concentration of serum (Table 1), they became larger differences in the subsequent serial assays. In an overall sense, the higher the saturation density, the earlier and larger the expression of transformed foci (Fig. 1 A and B). This showed that the successive assays revealed mutations and epigenetic changes that were hidden in the first-round assay. However, the neoplastic transformation itself was more difficult to quantify than saturation density because of its sporadic occurrence (Fig. 1B). Nevertheless, the two measurements, in combination, supplement each other in quantitating and visualizing the growth factor activity and consequent tumor-producing potential of serum.
Were it not for the varied serum concentrations of the first-round assay and subsequent serial passages under constant low serum concentrations and time of the latter, it would not be visibly apparent that there were significant increases in saturation density without foci. That finding indicated there were preneoplastic fields and that the entire process warranted the designation “field cancerization” (1, 3). The early fields are equivalent to the hyperplasia of in vivo neoplastic development. Those early fields would likely have been overshadowed by the large or confluent foci of the cultures derived from the 3-wk first-round assays in higher serum concentrations. It illustrates the difficulty of recognizing and properly identifying fields in the margins of excised human tumors, which represent the most protracted and incipient part of their development. They also constitute an increasingly permissive aspect of the whole culture in tumor progression, most likely arising from reduction in cell–cell adhesion and contact inhibition (25, 26).
Discussion
Addressing the problems of carcinogenesis, especially those of tumor virology in cell culture, opened those areas to methodologies that were not available to studies in vivo. An example is that of Rous sarcoma virology, which had moved forward slowly in vivo for years before the advent of a quantitative assay in cell culture for the Rous sarcoma virus (27). That assay had an enormous effect on our own understanding of oncogenes and neoplastic transformation of cells, which was facilitated by the simplicity of the system in which a single gene determines the transformation of a single cell and its descendants within a few days without intermediate steps (28). However, this very simplicity raises questions about its adequacy for characterizing the complex development of human cancer. The natural history of a solid human cancer spans decades and involves progressive stages of preneoplastic and neoplastic development (29). Recent genomic analysis indicates that there are between 1,000 and 10,000 somatic substitutions in most solid cancers, and as many as 100,000 in lung cancer and melanoma (30). There are in addition other types of genetic, as well as epigenetic, change that increase the complexity of the process. Just as was the case with Rous sarcoma virus (RSV), the understanding of human cancer should be facilitated by a quantitative cell culture assay that exhibits all stages of the process.
It is perhaps no accident that the NIH 3T3 cells were the vehicles for demonstrating a model for field cancerization in serial selection at high population density in varying serum concentrations (24). That same line of cells was unique, as the target for transformation by DNA from a human cancer, which indicates they were already close to transformation, needing only a mutated oncogene to take the cells over the top (31). In fact, some foci were clearly visible in the original NIH 3T3 cells in prolonged incubation at confluence in 10% serum (32). There was, however, no sign of transformation at confluence in several rounds of selection exclusively in 2% serum. The cells, however, did develop increasing degrees of saturation density, ultimately resulting in small, light foci in some of the cultures. That indicated they had undergone the early stages of preneoplastic field cancerization. This conclusion was reinforced by the finding that a single round of confluence in 10% serum magnified and accelerated transformed foci in serial rounds of selection at 2% serum. It also indicated that the initial incubation at 10% serum would not have led to the convincing demonstration of preneoplastic cancerization fields because the early occurrence of a few high-density foci by themselves could account for any increase in saturation density.
It is apparent that all stages of field cancerization were determined by the serum growth factor concentration and period of the first round of selection, despite the constant low serum and time of all of the subsequent serial rounds. Not only did the curves not converge in the later rounds of selection but they diverged from one another, indicating that the promoting effects of the first round were perpetuated and drove further promotion in the later rounds. Hence, stable variants constituted the bulk and cumulative nature of the changes. The slow gradual increase in saturation density derived from first-round assays in low and intermediate concentrations of serum for 2 wk indicates that there was selection of preexisting common genetic variants of small fitness gains. These conclusions match those obtained in a long-term evolution experiment with Drosophila, which were selected for accelerated development (33).
The near identity of all early increases in saturation density in all lineages within each serum concentration and period of time led to the conclusion that they resulted from a selection of multiple preexisting mutations. That presumed there was a high degree of heterogeneity in growth properties among clones of the original population. Such was indeed the case, as had been demonstrated in the original NIH 3T3 cell line (34) and in transformed cultures (35). However, the similarity of focal morphology produced from the same lineage of cultures indicated that they arose from a single cell.
The fact is that endogenous growth factors were responsible for producing the proliferation, but selection for growth at high density was the driving force. This was proven by the absence of progression in cultures growing exponentially in high serum concentration at low density for more than a year of rapid subculturing and the production of focus-formers in optimally selective low serum concentrations. It was also demonstrated in a single short-term experiment (36, 37). The conclusion that selection is generally the driving force in neoplastic development is reinforced by reports on human cancer by genetic methods (38, 39) and two differing models of mathematical analysis (40).
The exclusive involvement of endogenous growth factors in neoplastic development in cell culture drew attention to the increased incidence of cancer in obesity and type 2 diabetes, which derive from metabolic changes in the organism, such as the hyperinsulinemia of insulin resistance. Detailed analysis of Fig. 1 A and B shows that once the saturation density levels of the different combinations of the first-round assay reach a high level, the number of transformed cells increases rapidly with time at confluence. That demonstrates that whatever preventive or therapeutic measures are applied, they would be most effective early in the preneoplastic field stages of the process.
The evidence that both field cancerization in culture and high incidence of cancer in obesity and type 2 diabetes are driven by endogenous growth factors, with no role for exogenous carcinogenic chemicals, suggests that insulin resistance and systemic inflammation (22, 23), which operate at the level of the whole organism predisposing to cancer, may extend to healthy individuals as well. Support for this proposition is the marked increase of insulin resistance with age in lean, healthy individuals (41). That relation of insulin resistance with age of normal individuals would be consistent with the well-established increase of cancer with age, but the rate of its occurrence would remain lower than in obesity and type 2 diabetes. Hence, there is a need for epidemiological studies of the relation of insulin resistance to cancer in otherwise healthy people.
Increase of saturation density in culture moves study of the origin of cancer in vivo back in time to the broad, flat areas of hyperplasia that precede neoplasia. However, the connection between the cell culture results and the increased incidence of cancer in obesity and type 2 diabetes takes a further step back in time to the metabolic changes that involve the whole organism, which presently cannot be duplicated in cell culture. However, much remains to be done in quantifying field cancerization. In particular, the concentration of serum should be reduced to levels below those used here to determine the extent to which the field stage can be prolonged. One reason for doing so is that lymph, which is the equivalent of intercellular fluid, has much lower growth factor activity than serum (42), and extending the field stage could provide a better estimate of the time relationships of the preneoplastic to the neoplastic stages of cancer.
The methods used here for field cancerization could serve as a screen for the relationship between serum growth factor activity and the prospective incidence of cancer. The aim of the screen would be to undertake preventive measures such as lowering body mass index, increasing physical activity, and supplementing folic acid intake, along with other lifestyle changes (43), as early as possible to deter the development of cancer. Intensive counseling of people at high risk for type 2 diabetes with regard to a better diet and regular exercise reduced the incidence of the disease by 58% (44). That raises the question of whether such counseling would similarly reduce the incidence of cancer in people at high risk for the disease, as determined by the screen.
Subnormal concentrations of magnesium are prevalent in the diet, plasma, and cells of individuals with insulin resistance (45–49), type 2 diabetes (45, 49), obesity (48), and hypertension (45). In a recent study with more than 50,000 nondiabetic participants and 59 coauthors, a highly significant inverse relationship was found between dietary intake of magnesium and fasting insulin, indicative of insulin resistance (50). Insulin resistance is associated with an increase in plasma insulin (51), which of course promotes cancer (12, 14). It is therefore likely there is an association between hypomagnesemia and the increased incidence of cancer. This suggests the value of an epidemiological study on the relation of insulin resistance to the magnesium concentrations in the plasma of nondiabetic participants with a high risk for cancer. It has been shown that magnesium supplementation in the diet of patients with diabetes alleviated the symptoms of insulin resistance, and that it delayed the onset of diabetes in a rat diabetes model (45). Given that type 2 diabetes and cancer share the condition of insulin resistance, increased intake of magnesium might also prevent or slow progression to cancer. It should also be noted that magnesium is the second messenger for serum regulation of cell growth (52). Therefore, hypomagnesemia might provide the selective microenvironment for field cancerization in vivo.
Materials and Methods
Cells.
The NIH 3T3 line of mouse fibroblasts (53) was used to determine saturation density and transformability of cells in serial assays at confluence after exposure to different concentrations of serum for different periods of time. Those cells had undergone weekly passages of 400 cells, which gave rise to 200 colonies in 100-mm plastic culture dishes. The medium used for these passages was MCDB 402 (54), in 10% calf serum, which gave a 104-fold weekly increase in cell numbers with a doubling time of 12 h. They had been in weekly passages for 1 y.
There were several reasons for the choice of these cells. They appeared to be in early stages of neoplastic transformation, as indicated by the occasional appearance of a transformed focus when seeded at 105 per 60-mm dishes and grown to confluence for 14 d or more in 10% serum, but not in 2% serum. They were known for their unique sensitivity to the formation of multiple transformed foci when transfected with oncogenes (31). Finally, another sign of their preselection state of transformation was their saturation density in 2% serum, which was equal to that of the Swiss 3T3 line of cells in 10% serum (55). This early preneoplastic state in 2% serum was consistent with their progression to focus formation when they were serially selected for ability to multiply at high cell density.
Procedures.
The methodologies used in the experiment are described in the legends to Table 1 and Fig. 1A.
Acknowledgments
I thank Dorothy M. Rubin for correcting and typing the manuscript and Prof. Janet E. Rubin, University of North Carolina Medical School, for the graphs in Fig. 1A.
Footnotes
The author declares no conflict of interest.
References
- 1.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Lockhart-Mummery JP, Dukes MP. The precancerous changes in the rectum and colon. Surg Gynecol Obstet. 1928;46:591–596. [Google Scholar]
- 3.Willis RA. The mode of origin of tumors: Solitary localized squamous cell growths of the skin. Cancer Res. 1944;4:630–644. [Google Scholar]
- 4.Braakhuis BJM, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter’s concept of field cancerization: Evidence and clinical implications. Cancer Res. 2003;63(8):1727–1730. [PubMed] [Google Scholar]
- 5.Califano J, et al. Genetic progression model for head and neck cancer: Implications for field cancerization. Cancer Res. 1996;56(11):2488–2492. [PubMed] [Google Scholar]
- 6.Rubin AL, Arnstein P, Rubin H. Physiological induction and reversal of focus formation and tumorigenicity in NIH 3T3 cells. Proc Natl Acad Sci USA. 1990;87(24):10005–10009. doi: 10.1073/pnas.87.24.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taubes G. Cancer research. Unraveling the obesity-cancer connection. Science. 2012;335(6064):28–32, 30–32. doi: 10.1126/science.335.6064.28. [DOI] [PubMed] [Google Scholar]
- 8.Petersen KF, et al. Reversal of muscle insulin resistance by weight reduction in young, lean, insulin-resistant offspring of parents with type 2 diabetes. Proc Natl Acad Sci USA. 2012;109(21):8236–8240. doi: 10.1073/pnas.1205675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruce WR, Giacca A, Medline A. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 2000;9(12):1271–1279. [PubMed] [Google Scholar]
- 10.Rubin H. Antagonistic effects of insulin and cortisol on coordinate control of metabolism and growth in cultured fibroblasts. J Cell Physiol. 1977;91(2):249–259. doi: 10.1002/jcp.1040910210. [DOI] [PubMed] [Google Scholar]
- 11.Sanui H, Rubin AH. Membrane bound and cellular cationic changes associated with insulin stimulation of cultured cells. J Cell Physiol. 1978;96(3):265–278. doi: 10.1002/jcp.1040960302. [DOI] [PubMed] [Google Scholar]
- 12.Sridhar SS, Goodwin PJ. Insulin-insulin-like growth factor axis and colon cancer. J Clin Oncol. 2009;27(2):165–167. doi: 10.1200/JCO.2008.19.8937. [DOI] [PubMed] [Google Scholar]
- 13.Wideroff L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89(18):1360–1365. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: A review of the evidence. J Nutr. 2001;131(11) Suppl:3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 15.Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 16.Tran TT, et al. Hyperinsulinemia, but not other factors associated with insulin resistance, acutely enhances colorectal epithelial proliferation in vivo. Endocrinology. 2006;147(4):1830–1837. doi: 10.1210/en.2005-1012. [DOI] [PubMed] [Google Scholar]
- 17.Tran TT, Medline A, Bruce WR. Insulin promotion of colon tumors in rats. Cancer Epidemiol Biomarkers Prev. 1996;5(12):1013–1015. [PubMed] [Google Scholar]
- 18.Tran TT, et al. Direct measure of insulin sensitivity with the hyperinsulinemic-euglycemic clamp and surrogate measures of insulin sensitivity with the oral glucose tolerance test: Correlations with aberrant crypt foci promotion in rats. Cancer Epidemiol Biomarkers Prev. 2003;12(1):47–56. [PubMed] [Google Scholar]
- 19.Kabat GC, et al. A longitudinal study of serum insulin and glucose levels in relation to colorectal cancer risk among postmenopausal women. Br J Cancer. 2012;106(1):227–232. doi: 10.1038/bjc.2011.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabat GC, Rohan TE. Is elevated serum insulin a marker of increased risk of colorectal cancer? Colorectal Cancer. 2012;1(2):89–92. [Google Scholar]
- 21.Gunter MJ, et al. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res. 2008;68(1):329–337. doi: 10.1158/0008-5472.CAN-07-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11(12):886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 23.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339(6116):172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin H. Fields and field cancerization: The preneoplastic origins of cancer: Asymptomatic hyperplastic fields are precursors of neoplasia, and their progression to tumors can be tracked by saturation density in culture. Bioessays. 2011;33(3):224–231. doi: 10.1002/bies.201000067. [DOI] [PubMed] [Google Scholar]
- 25.Coman DR. Decreased mutual adhesiveness, a property of cells from squamous cell carcinomas. Cancer Res. 1944;4:625–629. [Google Scholar]
- 26.Rubin H. Cell-cell contact interactions conditionally determine suppression and selection of the neoplastic phenotype. Proc Natl Acad Sci USA. 2008;105(17):6215–6221. doi: 10.1073/pnas.0800747105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Temin HM, Rubin H. Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. Virology. 1958;6(3):669–688. doi: 10.1016/0042-6822(58)90114-4. [DOI] [PubMed] [Google Scholar]
- 28.Martin GS. The road to Src. Oncogene. 2004;23(48):7910–7917. doi: 10.1038/sj.onc.1208077. [DOI] [PubMed] [Google Scholar]
- 29.Farber E. The multistep nature of cancer development. Cancer Res. 1984;44(10):4217–4223. [PubMed] [Google Scholar]
- 30.Stratton MR. Exploring the genomes of cancer cells: Progress and promise. Science. 2011;331(6024):1553–1558. doi: 10.1126/science.1204040. [DOI] [PubMed] [Google Scholar]
- 31.Varmus HE. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]
- 32.Rubin H, Xu K. Evidence for the progressive and adaptive nature of spontaneous transformation in the NIH 3T3 cell line. Proc Natl Acad Sci USA. 1989;86(6):1860–1864. doi: 10.1073/pnas.86.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burke MK, et al. Genome-wide analysis of a long-term evolution experiment with Drosophila. Nature. 2010;467(7315):587–590. doi: 10.1038/nature09352. [DOI] [PubMed] [Google Scholar]
- 34.Grundel R, Rubin H. Maintenance of multiplication rate stability by cell populations in the face of heterogeneity among individual cells. J Cell Sci. 1988;91(Pt 4):571–576. doi: 10.1242/jcs.91.4.571. [DOI] [PubMed] [Google Scholar]
- 35.Grundel R, Rubin H. Effect of interclonal heterogeneity on the progressive, confluence-mediated acquisition of the focus-forming phenotype in NIH-3T3 populations. Cancer Res. 1991;51(3):1003–1013. [PubMed] [Google Scholar]
- 36.Rubin AL, Yao A, Rubin H. Relation of spontaneous transformation in cell culture to adaptive growth and clonal heterogeneity. Proc Natl Acad Sci USA. 1990;87(1):482–486. doi: 10.1073/pnas.87.1.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin H. Degrees and kinds of selection in spontaneous neoplastic transformation: An operational analysis. Proc Natl Acad Sci USA. 2005;102(26):9276–9281. doi: 10.1073/pnas.0503688102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krawczak M, et al. Somatic spectrum of cancer-associated single basepair substitutions in the TP53 gene is determined mainly by endogenous mechanisms of mutation and by selection. Hum Mutat. 1995;5(1):48–57. doi: 10.1002/humu.1380050107. [DOI] [PubMed] [Google Scholar]
- 39.Rodin SN, Rodin AS. Human lung cancer and p53: The interplay between mutagenesis and selection. Proc Natl Acad Sci USA. 2000;97(22):12244–12249. doi: 10.1073/pnas.180320897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schöllnberger H, Beerenwinkel N, Hoogenveen R, Vineis P. Cell selection as driving force in lung and colon carcinogenesis. Cancer Res. 2010;70(17):6797–6803. doi: 10.1158/0008-5472.CAN-09-4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen KF, et al. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin H, Nomura T. Use of lymph in cell culture to model hormonal and nutritional constraints on tumor growth in vivo. Cancer Res. 1987;47(18):4924–4931. [PubMed] [Google Scholar]
- 43.Willett WC. Balancing life-style and genomics research for disease prevention. Science. 2002;296(5568):695–698. doi: 10.1126/science.1071055. [DOI] [PubMed] [Google Scholar]
- 44.Marx J. Unraveling the causes of diabetes. Science. 2002;296(5568):686–689. doi: 10.1126/science.296.5568.686. [DOI] [PubMed] [Google Scholar]
- 45.Paolisso G, Barbagallo M. Hypertension, diabetes mellitus, and insulin resistance: The role of intracellular magnesium. Am J Hypertens. 1997;10(3):346–355. doi: 10.1016/s0895-7061(96)00342-1. [DOI] [PubMed] [Google Scholar]
- 46.Humphries S, Kushner H, Falkner B. Low dietary magnesium is associated with insulin resistance in a sample of young, nondiabetic Black Americans. Am J Hypertens. 1999;12(8 Pt 1):747–756. doi: 10.1016/s0895-7061(99)00041-2. [DOI] [PubMed] [Google Scholar]
- 47.Takaya J, Higashino H, Kobayashi Y. Intracellular magnesium and insulin resistance. Magnes Res. 2004;17(2):126–136. [PubMed] [Google Scholar]
- 48.Huerta MG, et al. Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care. 2005;28(5):1175–1181. doi: 10.2337/diacare.28.5.1175. [DOI] [PubMed] [Google Scholar]
- 49.Corica F, et al. Serum ionized magnesium levels in relation to metabolic syndrome in type 2 diabetic patients. J Am Coll Nutr. 2006;25(3):210–215. doi: 10.1080/07315724.2006.10719534. [DOI] [PubMed] [Google Scholar]
- 50.Hruby A, et al. Higher magnesium intake is associated with lower fasting glucose and insulin, with no evidence of interaction with select genetic loci, in a meta-analysis of 15 CHARGE Consortium Studies. J Nutr. 2013;143(3):345–353. doi: 10.3945/jn.112.172049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paolisso G, Ravussin E. Intracellular magnesium and insulin resistance: Results in Pima Indians and Caucasians. J Clin Endocrinol Metab. 1995;80(4):1382–1385. doi: 10.1210/jcem.80.4.7714114. [DOI] [PubMed] [Google Scholar]
- 52.Rubin H. Magnesium deprivation reproduces the coordinate effects of serum removal or cortisol addition on transport and metabolism in chick embryo fibroblasts. J Cell Physiol. 1976;89(4):613–625. doi: 10.1002/jcp.1040890418. [DOI] [PubMed] [Google Scholar]
- 53.Aaronson SA, Todaro GJ. Development of 3T3-like lines from Balb-c mouse embryo cultures: Transformation susceptibility to SV40. J Cell Physiol. 1968;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- 54.Shipley GD, Ham RG. Improved medium and culture conditions for clonal growth with minimal serum protein and for enhanced serum-free survival of Swiss 3T3 cells. In Vitro. 1981;17(8):656–670. doi: 10.1007/BF02628401. [DOI] [PubMed] [Google Scholar]
- 55.Holley RW, Kiernan JA. “Contact inhibition” of cell division in 3T3 cells. Proc Natl Acad Sci USA. 1968;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]