Abstract
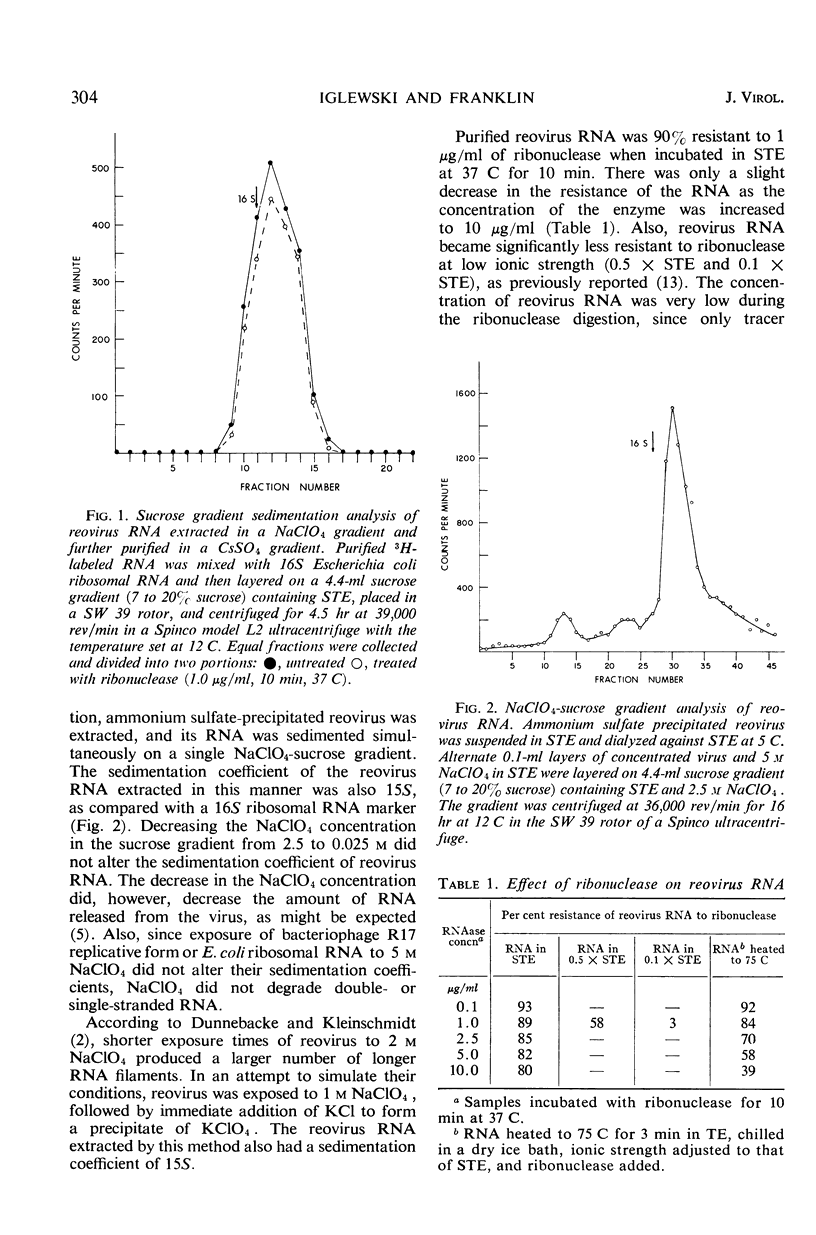
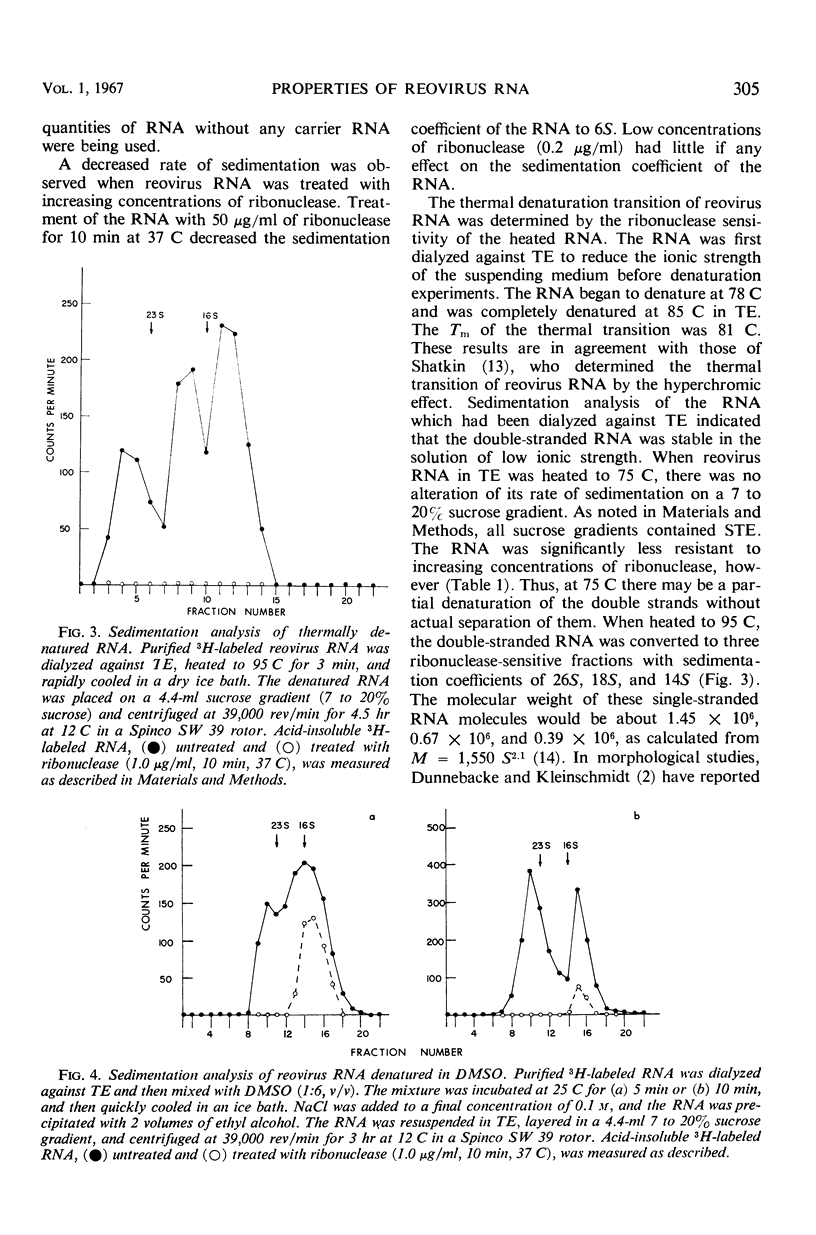
NaClO4 was employed in a technique for the rapid extraction of reovirus ribonucleic acid (RNA). The extracted RNA, which was purified in a Cs2SO4 equilibrium density gradient, had a buoyant density of 1.61 g/cm3 and a sedimentation coefficient of 15S in a 7 to 20% sucrose gradient. It was 90% resistant to ribonuclease in a solution of high ionic strength (0.1 m NaCl). The sensitivity of reovirus RNA to ribonuclease increased with decreasing ionic strength. The thermal denaturation transition of the RNA began at 78 C and was complete at 85 C. The Tm of the transition was 81 C in 0.01 m tris(hydroxymethyl)aminomethane buffer (pH 7.2) containing 0.001 m ethylenediaminetetraacetate. Thermal denaturation of reovirus RNA resulted in the formation of three ribonuclease-sensitive fractions. Denaturation at 25 C in the presence of dimethyl sulfoxide resulted in the formation of two ribonuclease-sensitive fractions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Replication of bacteriophage ribonucleic acid: some physical properties of single-stranded, double-stranded, and branched viral ribonucleic acid. J Virol. 1967 Feb;1(1):64–75. doi: 10.1128/jvi.1.1.64-75.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. A rapid technique for the preparation of purified bacteriophage DNA or RNA from crude lysates. Biochim Biophys Acta. 1965 Oct 11;108(2):318–319. doi: 10.1016/0005-2787(65)90020-1. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Effect of NaCIO-4 on bacteriophage: release of DNA and evidence for population heterogeneity. Virology. 1966 Apr;28(4):742–750. doi: 10.1016/0042-6822(66)90258-3. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., STOECKENIUS W. ELECTRON MICROSCOPE STUDIES ON REOVIRUS RNA. Proc Natl Acad Sci U S A. 1964 Dec;52:1449–1455. doi: 10.1073/pnas.52.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomatos P. J., Tamm I. THE SECONDARY STRUCTURE OF REOVIRUS RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):707–714. doi: 10.1073/pnas.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Penman S. The solvent denaturation of double-stranded RNA from poliovirus infected HeLa cells. Biochem Biophys Res Commun. 1966 May 25;23(4):557–560. doi: 10.1016/0006-291x(66)90765-0. [DOI] [PubMed] [Google Scholar]
- Kudo H., Graham A. F. Synthesis of reovirus ribonucleic acid in L cells. J Bacteriol. 1965 Oct;90(4):936–945. doi: 10.1128/jb.90.4.936-945.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERNER A. M. CONCENTRATION AND PURIFICATION OF VIRUSES WITH SPECIAL REFERENCE TO REOVIRUSES. Bacteriol Rev. 1964 Dec;28:391–396. doi: 10.1128/br.28.4.391-396.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh P. C., Soergel M. Growth characteristics of reovirus type 2: actinomycin D and the synthesis of viral RNA. Proc Natl Acad Sci U S A. 1965 Sep;54(3):857–863. doi: 10.1073/pnas.54.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. Inactivity of purified reovirus RNA as a template for E. coli polymerases in vitro. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1721–1728. doi: 10.1073/pnas.54.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]


