Abstract
The synthesis of potassium trifluoro(N-methylheteroaryl)borates and their use in cross-coupling reactions with various aryl and heteroaryl halides to construct N-methyl heteroaryl-substituted aromatic and heteroaromatic compounds are reported.
Introduction
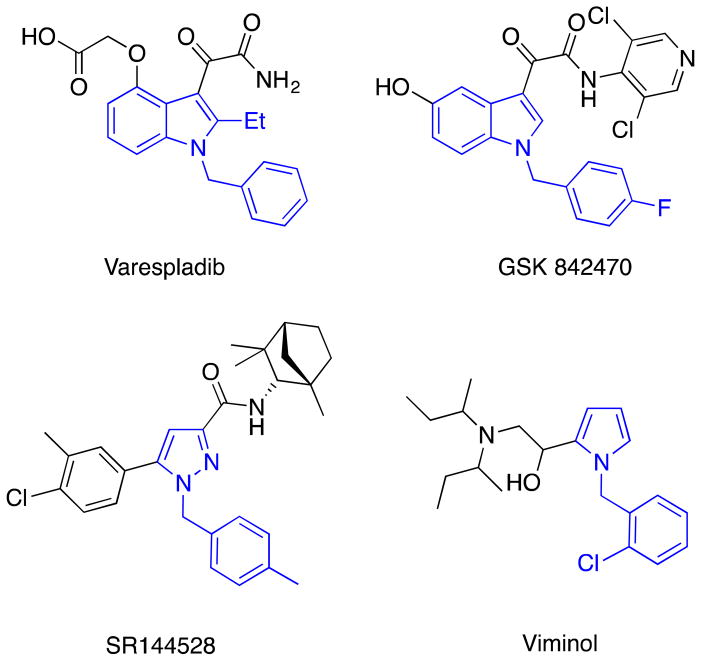
N-Heteroaromatics are ubiquitous components in natural products and pharmaceuticals. The indole scaffold, for instance, is considered a privileged substructure that is frequently utilized in the discovery of new drug candidates.1 Developing methods to build structural complexity by incorporating such N-heteroaromatics, therefore, is an area of great interest to the synthetic community. Among the structural moieties utilized, N-methyl heteroaryl-substituted arenes are one of the prominent targets because they are found in numerous biologically active compounds, including pharmaceutical products (Figure 1).2–5
Figure 1.
Drugs containing N-methyl heteroaryl-substituted arenes
Strategies commonly used in the literature to install the N-methyl heteroaryl-substituted aryl or heteroaryl moiety include alkylation of N-heteroaromatics with benzyl halides,6 reductive amination of aromatic aldehydes,7,8 and condensations of dicarbonyls (or their electronically similar analogs, e.g., ketonitriles) with benzylamines9 or benzyl hydrazines.10 Although these methods have been utilized extensively and effectively, some limitations exist because of the limited commercial availability of benzyl halides, aromatic aldehydes, benzylamines, and benzyl hydrazines.
A nontraditional approach employing a dissonant disconnect presents a new and complementary way of constructing N-methyl heteroaryl-substituted aromatic or heteroaromatic compounds. With the use of N-methyl heteroaryl nucleophilic agents in lieu of nitrogen nucleophiles, aryl and heteroaryl halides can be used as starting materials. Not only are there markedly more commercially available aryl and heteroaryl halides than the corresponding benzyl halides, aromatic aldehydes, benzylamines, and benzyl hydrazines combined,11 they are also generally less expensive, with aryl and heteroaryl chlorides being the least expensive and most commercially diverse among the halides.12 Thus, employing the strategic dissonant disconnect leads to more accessible starting materials, which can be used to create novel chemical space in a facile manner.
Transformations involving methylamino nucleophiles have been explored in our laboratory most relevantly in Suzuki-Miyaura cross-coupling of potassium N,N-dialkylaminomethyltrifluoroborates with aryl and/or heteroaryl bromides, chlorides, iodides, and mesylates.13–16 Aminomethyltrifluoroborates were synthesized from potassium chloromethyltrifluoroborate and secondary amines.13–16 Because the efficiency of the synthesis of aminomethyltrifluoroborates as well as the cross-coupling approach to aminomethyl-substituted aromatic and heteroaromatic compounds was demonstrated, we envisioned that the same approach could be applied to the N-alkylation of heteroaromatics.
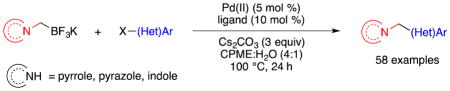
In this study, the potassium salts of trifluoro(N-methylpyrrolo)borate, trifluoro(N-methylpyrazolo)borate, and trifluoro(N-methylindolo)borate were synthesized and cross-coupled with aryl and heteroaryl halides.
Results and Discussion
The synthesis of potassium trifluoro(N-methylpyrazolo)borate (1a) was carried out under the conditions that were reported for the synthesis of N,N-dialkylaminomethyltrifluoroborates (eq 1).15
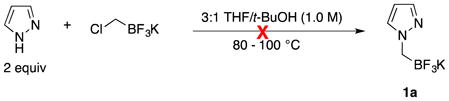 |
(1) |
The reaction did not go to completion (as judged by 19F NMR) despite using extended reaction times or elevated temperatures. In acetone and N,N-dimethylformamide (DMF), the reaction reached completion, but only after three days at 100 °C, and a mixture of side products that could not be characterized was observed owing to the prolonged reaction time.
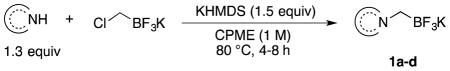
Unlike the case with secondary amines, addition of a base was necessary with pyrazole to effect the alkylation. After several bases and solvents were examined, improved reaction conditions for the synthesis of 1a were developed with the addition of KHMDS. An excess of the nucleophile (1.3 equiv) was used to consume chloromethyltrifluoroborate completely and simplify the purification process since two trifluoroborates are hard to separate. The developed reaction conditions were tested on other applicable N-heteroaromatics, and the isolated yields of the successful transformations are reported in Table 1.
Table 1.
Synthesis of Potassium Trifluoro(N-methylheteroaryl)borates
| ||||
|---|---|---|---|---|
| entry | nucleophile | product | % yield | |
| 1 |
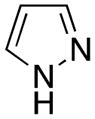
|
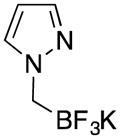
|
1a | 83a |
| 2 |
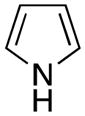
|
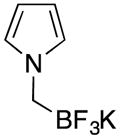
|
1b | 91 |
| 3 |
|
|
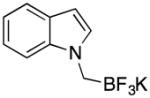
1c | 91 |
0.5 M
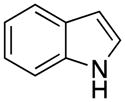
The limitations of this reaction were shown with substrates, which, upon deprotonation, can be alkylated at different positions. With indazole, two new peaks were observed in the 19F NMR spectrum, which, we conjectured, corresponded to two regioisomers that might have formed. Findings from similar reactions with indazole in the literature support this supposition.17 This substrate was not pursued to purification in favor of those that yielded a single product.
Although harsher reaction conditions are required with chloromethyltrifluoroborate in comparison to bromomethyltrifluoroborate, the KCl byproduct is more readily removed than KBr.15 After removal of the solvent in vacuo, the reaction mixture was suspended in hot acetone and filtered to remove the insoluble KCl. Crystallizing the products was difficult as oiling out was often observed, especially with the indole system (1c). After KCl was filtered off and the solvent was removed, the resulting oily residue containing unreacted indole and 1c was placed under high vacuum overnight. Adding ether as an antisolvent and then letting the resulting viscous solution sit at room temperature for 2~3 hours led to solids that gradually precipitated out of solution.
Next, the Suzuki-Miyaura cross-coupling of these potassium trifluoro(N-methylheteroaryl)borates was examined. Using p-chloroanisole and a stoichiometric amount of 1b as our model electrophile and nucleophile, respectively, we screened a wide variety of catalyst/ligand combinations, solvents, solvent to water ratios, bases and temperatures. The screening was performed on a millimolar scale as well as through microscale High Throughput Experimentation (HTE).18,19 The best coupling conditions were determined to be 5 mol % Pd(OAc)2, 10 mol % 2-dicyclohexylphosphino-2′6′-diisopropoxybiphenyl (RuPhos), and 4:1 CPME (cyclopentyl methyl ether)/H2O at 100 °C for 24 h, with 3 equivalents of Cs2CO3 as the base. Attempts to lower the catalyst loading resulted in significantly lowered yields. Using nearly stoichiometric amounts of the nucleophile (1.1 equiv), we successfully cross-coupled 1b with electron-rich, electron-neutral, and electron-poor aryl chlorides in moderate to excellent yields (Table 2, entries 1–18). Ketones, esters, nitriles, pyrroles, and nitro groups were tolerated, and fluorinated electrophiles were coupled in good yields. In addition, ortho-, meta-, and para-substituted derivatives, along with a more sterically-hindered substrate, were all effective coupling partners. Several heteroaryl chlorides were also coupled under the optimized conditions (Table 2, entries 19–22).
Table 2.
Cross-coupling of Potassium Trifluoro(N-methylpyrrolo)borate with Aryl and Heteroaryl Chlorides
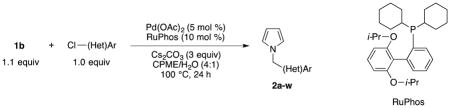
| |||
|---|---|---|---|
| entry | aryl chloride | product | % yield |
| 1 |
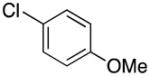
|
2a | 78 |
| 2 |
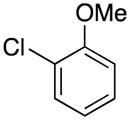
|
2b | 74 |
| 3 |
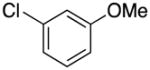
|
2c | 90 |
| 4 |
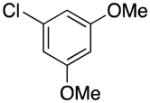
|
2d | 75 |
| 5 |
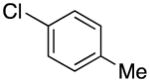
|
2e | 77 |
| 6 |
|
2f | 68 |
| 7 |
|
2g | 65 |
| 8 |
|
2h | 97 |
| 9 |
|
2i | 86 |
| 10 |
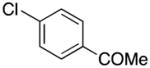
|
2j | 88 |
| 11 |
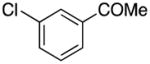
|
2k | 73 |
| 12 |
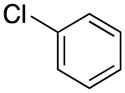
|
2l | 98 |
| 13 |
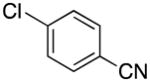
|
2m | 85 |
| 14 |
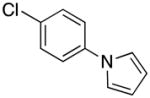
|
2n | 91 |
| 15 |
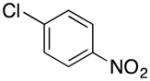
|
2o | 87 |
| 16 |
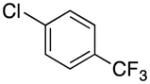
|
2p | 76 |
| 17 |
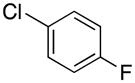
|
2q | 81 |
| 18 |
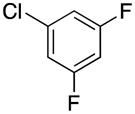
|
2r | 76 |
| 19 |
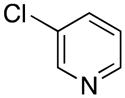
|
2s | 63 |
| 20 |
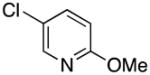
|
2t | 83 |
| 21 |
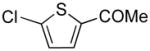
|
2u | 75 |
| 22 |
|
2v | 64 |
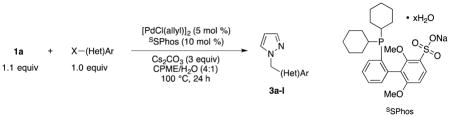
When the optimized conditions developed for 1b were applied to 1a with p-chloroanisole, only a small amount of the cross-coupled product was detected, and p-chloroanisole was mostly recovered. We performed another screening process using 1a and p-chloroanisole as the model system, and by switching the catalyst and ligand pair to [PdCl(allyl)]2 and sodium 2′-dicyclohexylphosphino-2,6-dimethoxy-1,1′-biphenyl-3-sulfonate hydrate (SSPhos), we were able to obtain the cross-coupled product in 56% yield (Table 3, entry 1). A slightly improved 68% yield was obtained with p-bromoanisole (Table 3, entry 1). Encouraged by this result, we cross-coupled 1a with both aryl chlorides and bromides, but a significant improvement in yield was observed only with 1-bromo-3,5-dimethoxybenzene (Table 3, entry 6). Using these conditions, we cross-coupled 1a with aryl electrophiles containing electron-rich, electron-poor, and electron-neutral groups, and nitrile, ketone, ester, and nitro groups were tolerated under the reaction conditions. Two nitrogen-containing heteroaryl chlorides were also cross-coupled successfully (Table 3, entries 10–11).
Table 3.
Cross-coupling of Potassium Trifluoro(N-methylpyrazolo)borate with Aryl and Heteroaryl Halides
| |||
|---|---|---|---|
| entry | aryl halide | product | % yield |
| 1 |
|
3a | X = Cl, 56 X = Br, 68 |
| 2 |
|
3b | X = Cl, 81 X = Br, 71 |
| 3 |
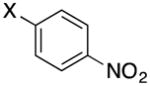
|
3c | X = Cl, 76 X = Br, 78 |
| 4 |
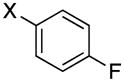
|
3d | X = Cl, 60a X = Br, 45 |
| 5 |
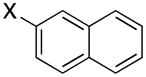
|
3e | X = Cl, 72 X = Br, 68 |
| 6 |
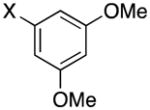
|
3f | X = Cl, 63 X = Br, 82 |
| 7 |
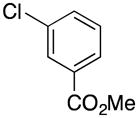
|
3g | 61 |
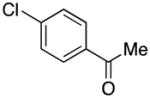
| 8 |
|
3h | 73 |
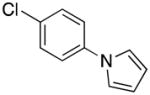
| 9 |
|
3i | 58 |
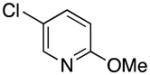
| 10 |
|
3j | 82 |
| 11 |
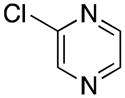
|
3k | 60 |
3 equiv of KOH used instead.
The conditions developed for the pyrrole system (1b) could be applied directly to the indole system (1c), which was coupled with p-chloroanisole in 73% yield without any further optimization. Using the same conditions, we cross-coupled 1c with a wide range of aryl chlorides in mostly good to excellent yields (Table 4). As seen with 1b, ortho-, meta-, para-substituted, as well as sterically hindered aryl chlorides cross-coupled successfully. The reaction conditions were tolerant of many functional groups, but no desired product was formed with aryl chlorides containing hydroxyl groups, primary amines, or Boc-protected secondary amines. A 4 mmol scale reaction was performed where 1 g of 1c was cross-coupled with 4-chlorobenzonitrile in 71% yield with only 1 mol % catalyst loading (Table 4, entry 4).
Table 4.
Cross-coupling of Potassium Trifluoro(N-methylindolo)borate with Aryl Chlorides
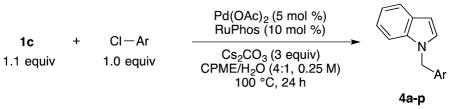
| |||
|---|---|---|---|
| entry | aryl chloride | product | % yield |
| 1 |
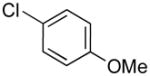
|
4a | 73 |
| 2 |
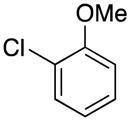
|
4b | 78 |
| 3 |
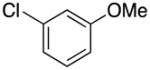
|
4c | 80 |
| 4 |
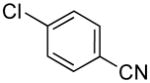
|
4d | 94 71a |
| 5 |
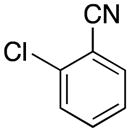
|
4e | 87 |
| 6 |
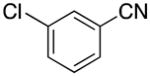
|
4f | 90 |
| 7 |
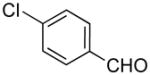
|
4g | 81 |
| 8 |
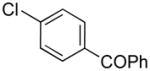
|
4h | 70 |
| 9 |
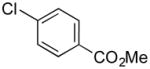
|
4i | 78 |
| 10 |
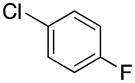
|
4j | 72 |
| 11 |
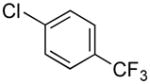
|
4k | 91 |
| 12 |
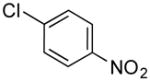
|
4l | 90 |
| 13 |
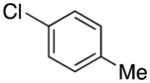
|
4m | 68 |
| 14 |
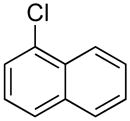
|
4n | 78 |
| 15 |
|
4o | 94 |
| 16 |
|
4p | 74 |
1 g of 1c was used with 1 mol % of Pd(OAc)2 and 2 mol % of RuPhos.
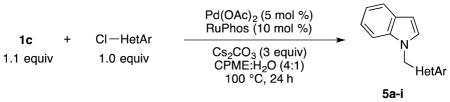
Efforts were then focused on heteroaryl cross-coupling. Diverse nitrogen-, oxygen-, sulfur-containing heteroaryl chlorides of various ring sizes cross-coupled in good to excellent yields (Table 5). Using 2.1 equiv of 1c, we were able to carry out two sequential cross-couplings with 2,6-dichloropyridine to afford the bis-cross-coupled product in 83% yield (Table 5, entry 9).
Table 5.
Cross-coupling of Potassium Trifluoro(N-methylindolo)borate with Heteroaryl Chlorides
| |||
|---|---|---|---|
| entry | heteroaryl chloride | product | % yield |
| 1 |
|
5a | 91 |
| 2 |
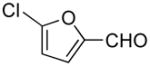
|
5b | 87 |
| 3 |
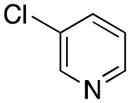
|
5c | 86 |
| 4 |
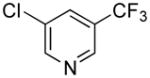
|
5d | 76 |
| 5 |
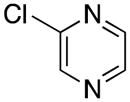
|
5e | 65 |
| 6 |
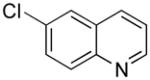
|
5f | 70 |
| 7 |
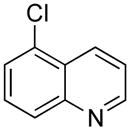
|
5g | 78 |
| 8 |
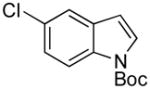
|
5h | 72 |
| 9 |
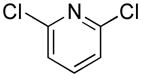
|
5i | 83a |
2.1 equiv of 1c was used to afford the bis-cross-coupled product.
Conclusion
A complementary approach to existing methods of constructing N-methyl heteroaryl-substituted aromatic and heteroaromatic compounds was developed. Unlike in the existing methods, the dissonant disconnect enables the use of aryl and heteroaryl halides as starting materials, which is advantageous because libraries of compounds can be easily created with a variety of accessible starting materials. Pyrrole-, pyrazole-, and indole-containing building blocks were synthesized, and it was demonstrated that they were viable cross-coupling partners. The reaction conditions developed with a mild base (Cs2CO3) were tolerant of many functional groups, and successful cross-coupling was achieved with a wide range of aryl halides. Several heteroaryl chlorides were cross-coupled effectively as well, with the most successful heteroaryl cross-coupling being obtained with (N-methylindolo)trifluoroborate.
Experimental Section
General Procedure for the Preparation of Potassium Trifluoro(N-methylheteroaryl)borates (1a–1c)
An oven-dried 10–20 mL microwave vial equipped with a stirrer bar was charged with N-heteroaromatic (6.5 mmol), potassium chloromethyltrifluoroborate (0.78 g, 5.0 mmol), KHMDS (1.49 g, 7.5 mmol), and sealed with a cap lined with a disposable PTFE septum. The vial was then evacuated under vacuum and purged with Ar (3 times). Anhydrous CPME (5 or 10 mL) was added via syringe. The reaction mixture was stirred and heated to 80 °C for 4–8 h until judged complete by 19F NMR. At this point the reaction mixture was quenched with H2O (2 mL) and transferred to a 100 mL round-bottom flask, and the volatiles were removed in vacuo. The crude solid was dried under high vacuum overnight before being dissolved in a solution of hot HPLC grade acetone, and the solution was filtered to remove KCl. The filtrate was concentrated in vacuo, dissolved in a minimal amount of hot acetone (7 mL), and precipitated by addition of Et2O (15 mL) to afford the desired product.
Potassium Trifluoro(N-methylpyrazolo)borate (1a)
white powder (0.78 g, 83%): mp 195–198 °C; IR (neat) 1084, 1059, 1047, 1011, 968, 787, 744, 721 cm−1; 1H NMR (500 MHz, DMSO) δ 7.50 (d, J = 1.0 Hz, 1H), 7.19 (s, 1H), 6.03 (t, J = 1.75 Hz, 1H), 3.05 (s, 2H); 13C NMR (125.8 MHz, DMSO) δ 136.8, 129.7, 104.0; 19F NMR (470.8 MHz, DMSO) δ −140.4; 11B NMR (400 MHz, DMSO) δ 3.13; HRMS (ESI-TOF) m/z calcd. for C4H5BN2F3− [M-K] − 149.0498, found 149.0501.
Potassium Trifluoro(N-methylpyrrolo)borate (1b)
pale yellow powder (0.85 g, 91%): mp 202–205 °C; IR (neat) 1065, 983, 782, 738, 707 cm−1; 1H NMR (500 MHz, DMSO) δ 6.56 (s, 2H), 5.76 (t, J = 2.0 Hz, 2H), 2.68 (s, 2H); 13C NMR (125.8 MHz, DMSO) δ 121.9, 105.7; 19F NMR (470.8 MHz, DMSO) δ −140.1; 11B NMR (400 MHz, DMSO) δ 3.23; HRMS (ESI-TOF) m/z calcd. for C5H6BNF3− [M-K] − 148.0545, found 148.0579.
Potassium Trifluoro(N-methylindolo)borate (1c)
white powder (1.08 g, 91%): mp 176–179 °C; IR (neat) 2969, 2904, 1463, 1337, 1302, 1255, 1075, 1036, 990, 789, 743, 648 cm−1; 1H NMR (300 MHz, DMSO) δ 7.51 (d, J = 7.8 Hz, 1H), 7.37–7.42 (m, 2H), 7.06 (t, J = 7.2, 1H), 6.95 (t, J = 7.2 Hz, 1H), 6.29 (d, J = 3.0 Hz, 1H), 3.04 (s, 2H); 13C NMR (75.4 MHz, DMSO) δ 137.6, 130.2, 128.0, 120.0, 119.9, 118.0, 110.4, 98.2; 19F NMR (282.4 MHz, DMSO) δ −139.1; 11B NMR (400 MHz, DMSO) δ 2.09; HRMS (ESI-TOF) m/z calcd. for C9H8BNF3− [M-K]− 198.0708, found 198.0702.
General Procedure for Cross-Coupling of Trifluoro(N-methylheteroaryl)borates with Aryl and Heteroaryl Halides (2a–5i)
A 10 mL microwave vial equipped with a stirrer bar was charged with trifluoro(N-methylheteroaryl)borate (0.55 mmol), Cs2CO3 (0.4887 g, 1.5 mmol, 3 equiv), (hetero)aryl halide (0.5 mmol), Pd(OAc)2 or [PdCl(allyl)]2 (0.025 mmol, 5 mol %), and RuPhos or SSPhos (0.05 mmol, 10 mol %) and then sealed with a cap lined with a disposable PTFE septum. The vial was then evacuated under vacuum and purged with Ar (3 times). Ar-purged CPME (2 mL) and H2O (0.5 mL) were added by syringe [(hetero)aryl halides that were liquids at room temperature were added by syringe], and the reaction mixture was stirred and heated at 100 °C for 24 h and then cooled to rt and diluted with H2O (5 mL). The reaction mixture was extracted with EtOAc (5 × 3 mL). The combined organics were dried (MgSO4), filtered through Celite, and concentrated in vacuo. All products were purified by column chromatography.
1-(4-Methoxybenzyl)-1H-pyrrole (2a)7
pale brown oil (73 mg, 78%); 1H NMR (CDCl3, 500 MHz) δ 7.09 (d, J = 8.5 Hz, 2H), 6.86 (d, J = 9.0 Hz, 2H), 6.68 (t, J = 2.0 Hz, 2H), 6.18 (t, J = 2.0 Hz, 2H), 5.01 (s, 2H), 3.80 (s, 3H); 13C NMR (CDCl3, 125.8 MHz) δ 159.4, 130.3, 128.8, 121.2, 114.3, 108.62, 55.5, 53.0.
1-(2-Methoxybenzyl)-1H-pyrrole (2b)8
pale yellow oil (69 mg, 74%); 1H NMR (CDCl3, 400 MHz) δ 7.23 (t, J = 8.0 Hz, 1H), 6.7 (d, J = 8.0 Hz, 2H), 6.79 (d, J = 7.2 Hz, 1H), 6.71 (d, J = 2.0 Hz, 2H), 6.71 (d, J = 2.0 Hz, 2H), 6.16 (d, J = 2.0 Hz, 2H), 5.07 (s, 2H), 3.84 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 156.5, 128.8, 128.2, 126.8, 121.3, 120.0, 110.1, 108.0, 55.3, 48.2.
1-(3-Methoxybenzyl)-1H-pyrrole (2c)20
red oil (84 mg, 90%); 1H NMR (CDCl3, 400 MHz) δ 7.21 (t, J = 8.0 Hz, 1H), 6.79 (d, J = 8.4 Hz, 1H), 6.69 (d, J = 7.6 Hz, 1H), 6.67 (d, J = 2.0 Hz, 2H), 6.62 (s, 1H), 6.17 (d, J = 2.0 Hz, 2H), 5.01 (s, 2H), 3.74 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 159.9, 139.7, 129.7, 121.1, 119.2, 112.9, 112.7, 108.5, 55.1, 53.2.
1-(3,5-Dimethoxybenzyl)-1H-pyrrole (2d)
colorless oil (81 mg, 75%); IR (KBr, neat) 2929, 2854, 1612, 1591, 1472, 1458, 1428, 1315, 1285, 1198, 1159, 1053, 1067, 926, 830, 736 cm−1; 1H NMR (CDCl3, 400 MHz) δ 6.99 (s, 2H), 6.37 (s, 1H), 6.26 (s, 2H), 6.19 (s, 2H), 5.00 (s, 2H), 3.75 (s, 6H); 13C NMR (CDCl3, 100 MHz) δ 161.0, 140.5, 121.2, 108.5, 104.9, 99.3, 55.3, 53.3; HRMS (ESI-TOF) m/z calcd. for C13H16NO2+ [M + H]+ 218.1181, found 218.1187.
1-(4-Methylbenzyl)-1H-pyrrole (2e)8
pale yellow oil (66 mg, 77%); 1H NMR (CDCl3, 400 MHz) δ 7.12 (d, J = 7.6 Hz, 2H), 7.01 (d, J = 7.6 Hz, 2H), 6.67 (d, J = 2.0 Hz, 2H), 6.17 (d, J = 1.6 Hz, 2H), 5.01 (s, 2H), 2.32 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 137.3, 135.1, 129.3, 127.0, 121.0, 108.3, 53.1, 21.0.
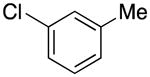
1-(3-Methylbenzyl)-1H-pyrrole (2f)20
red oil (58 mg, 68%), 1H NMR (CDCl3, 400 MHz) δ 7.19 (d, J = 7.6 Hz, 1H), 7.08 (d, J = 7.2 Hz, 1H), 6.94 (s, 1H), 6.91 (d, J = 7.6 Hz, 1H), 6.68 (d, J = 2.0 Hz, 2H), 6.18 (d, J = 2.0 Hz, 2H), 5.02 (s, 2H), 2.31 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 138.3, 138.0, 128.6, 128.4, 127.7, 124.1, 121.1, 108.4, 53.3, 21.4.
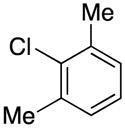
1-(2,6-Dimethylbenzyl)-1H-pyrrole (2g)
colorless oil (60 mg, 65%); IR (KBr, neat) 2925, 2855, 1594, 1466, 1379, 1271, 1120, 1085, 966, 767, 715 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.15 (t, J = 7.6 Hz, 1H), 7.06 (d, J = 7.6 Hz, 2H), 6.51 (d, J = 0.8 Hz, 2H), 6.11 (d, J = 1.2 Hz, 2H), 5.10 (s, 2H), 2.29 (s, 6H); 13C NMR (CDCl3, 100 MHz) δ 137.9, 132.7, 128.5, 128.2, 120.2, 107.8, 47.1, 19.6; HRMS (ESI-TOF) m/z calcd. for C13H16N+ [M + H]+ 186.1283, found 186.1291.
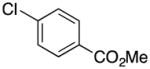
Methyl 4-((1H-Pyrrol-1-yl)methyl)benzoate (2h)21
colorless oil (104 mg, 97%); 1H NMR (CDCl3, 400 MHz) δ 7.98 (d, J = 8.0 Hz, 2H), 7.13 (d, J = 8.0 Hz, 2H), 6.68 (s, 2H), 6.21 (s, 2H), 5.11 (s, 2H), 3.89 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 166.6, 143.3, 129.9, 129.4, 126.6, 121.2, 108.8, 52.9, 52.1.
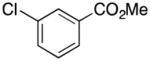
Methyl 3-((1H-Pyrrol-1-yl)methyl)benzoate (2i)
white solid (93 mg, 86%): mp 68–71 °C; IR (KBr, neat) 3127, 2925, 2854, 1711, 1593, 1500, 1430, 1299, 1281, 1200, 1113, 1091, 983, 733, 628 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.94 (d, J = 7.6 Hz, 1H), 7.86 (s, 1H), 7.38 (t, J = 7.6 Hz, 1H), 7.25 (d, J = 7.6 Hz, 1H), 6.68 (d, J = 2.0 Hz, 2H), 6.19 (d, J = 2.0 Hz, 2H), 5.09 (s, 2H), 3.90 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 166.7, 138.5, 131.4, 130.5, 128.8, 128.1, 121.0, 108.7, 52.9, 52.1; HRMS (ESI-TOF) m/z calcd. for C13H14NO2+ [M + H]+ 216.1025, found 216.1035.
1-(4-((1H-Pyrrol-1-yl)methyl)phenyl)ethanone (2j)
colorless oil (88 mg, 88%); IR (KBr, neat) 2925, 2856, 1730, 1683, 1610, 1497, 1415, 1359, 1265, 1180, 1087, 1017, 967, 815, 721 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.90 (d, J = 8.0 Hz, 2H), 7.16 (d, J = 8.0 Hz, 2H), 6.68 (s, 2H), 6.21 (s, 2H), 5.12 (s, 2H), 2.57 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 197.5, 143.5, 136.5, 128.8, 126.9, 121.2, 108.9, 52.9, 26.6; HRMS (ESI-TOF) m/z calcd. for C13H14NO+ [M + H]+ 200.1075, found 200.1073.
1-(3-((1H-Pyrrol-1-yl)methyl)phenyl)ethanone (2k)
white solid (73 mg, 73%): mp 58–60 °C; IR (KBr, neat) 3122, 2924, 2854, 1677, 1589, 1502, 1424, 1361, 1276, 1178, 1098, 1074, 953, 797, 732, 686, 595 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.85 (d, J = 7.6 Hz, 1H), 7.72 (s, 1H), 7.41 (t, J = 7.6 Hz, 1H), 7.26 (d, J = 8.0 Hz, 1H), 6.68 (d, J = 2.0 Hz, 2H), 6.20 (d, J = 2.0 Hz, 2H), 5.11 (s, 2H), 2.56 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 197.8, 138.8, 137.5, 131.5, 129.1, 127.7, 126.7, 121.0, 108.9, 52.9, 26.6; HRMS (ESI-TOF) m/z calcd. for C13H14NO+ [M + H]+ 200.1075, found 200.1077.
1-Benzyl-1H-pyrrole (2l)8
red oil (77 mg, 98%); 1H NMR (CDCl3, 400 MHz) δ 7.32–7.24 (m, 3H), 7.10 (d, J = 7.2 Hz, 2 H), 6.68 (s, 2H), 6.18 (s, 2H), 5.05 (s, 2H); 13C NMR (CDCl3, 100 MHz) δ 138.1, 128.7, 127.6, 126.9, 121.4, 108.4, 53.3.
4-((1H-Pyrrol-1-yl)methyl)benzonitrile (2m)9
pale pink solid (77 mg, 85%): mp 68–71 °C; 1H NMR (CDCl3, 500 MHz) δ 7.61 (d, J = 8.5 Hz, 2 H), 7.15 (d, J = 8.0 Hz, 2 H), 6.80 (t, J = 2.0 Hz, 2 H), 6.23 (t, J = 2.0 Hz, 2 H), 5.14 (s, 2 H); 13C NMR (CDCl3, 125.8 MHz) δ 143.9, 132.8, 127.4, 121.4, 118.7, 111.7, 109.4, 52.9.
1-(4-(1H-Pyrrol-1-yl)benzyl)-1H-pyrrole (2n)
white solid (101 mg, 91%): mp 87–90 °C; IR (KBr, neat) 3139, 3100, 2854, 2924, 1612, 1525, 1499, 1430, 1327, 1295, 1251, 1125, 1091, 1069, 1017, 921, 811, 731, 608 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.33 (d, J = 8.0 Hz, 2H), 7.15 (d, J = 8.0 Hz, 2H), 7.05 (s, 2H), 6.70 (s, 2H), 6.33 (s, 2H), 6.20 (s, 2H), 5.07 (s, 2H); 13C NMR (CDCl3, 100 MHz) δ 140.1, 135.5, 128.2, 121.0, 120.6, 119.2, 110.5, 108.7, 52.7; HRMS (ESI-TOF) m/z calcd. for C15H15N2+ [M + H]+ 223.1235, found 223.1232.
1-(4-Nitrobenzyl)-1H-pyrrole (2o)20
yellow oil (88 mg, 87%); 1H NMR (CDCl3, 400 MHz) δ 8.16 (dd, J = 8.0 Hz, 1.6 Hz, 2H), 7.19 (d, J = 8.0 Hz, 2H), 6.68 (d, J = 1.6 Hz, 2H), 6.23 (d, J = 1.6 Hz, 2H), 5.18 (s, 2H); 13C NMR (CDCl3, 100 MHz) δ 147.4, 145.7, 127.3, 123.9, 121.2, 109.3, 52.5.
1-(4-(Trifluoromethyl)benzyl)-1H-pyrrole (2p)
yellow oil (86 mg, 76%); IR (neat) 1497, 1420, 1324, 1163, 1122, 1088, 1067, 1018, 821, 725 cm−1; 1H NMR (CDCl3, 500 MHz) δ 7.58 (d, J = 8.0 Hz, 2H), 7.19 (d, J = 8.0 Hz, 2H), 6.70 (t, J = 2.0 Hz, 2H), 6.23 (t, J = 2.0 Hz, 2H), 5.14 (s, 2H); 13C NMR (CDCl3, 125.8 MHz) δ 142.5, 130.1 (q, J = 32.6 Hz), 127.2, 125.9 (q, J = 3.7 Hz), 124.2 (q, J = 272.2 Hz), 121.4, 109.2, 52.9; 19F NMR (CDCl3, 470.8 MHz) δ −62.60; HRMS (ESI-TOF) m/z calcd. for C12H11NF3+ [M + H]+ 226.0844, found 226.0830.
1-(4-Fluorobenzyl)-1H-pyrrole (2q)9
yellow oil (71 mg, 81%); 1H NMR (CDCl3, 500 MHz) δ 7.10–7.07 (m, 2H), 7.00 (t, J = 9.0 Hz, 2H), 6.67 (t, J = 2.0 Hz, 2H), 6.19 (d, J = 2.0 Hz, 2H), 5.03 (s, 2H); 13C NMR (CDCl3, 125.8 MHz) δ 162.2 (d, J = 239.6 Hz), 133.9, 128.6 (d, 9.1 Hz), 120.9, 115.5 (d, J = 22.5 Hz), 108.6, 52.5; 19F NMR (CDCl3, 470.8 MHz) δ −114.8.
1-(3,5-Difluorobenzyl)-1H-pyrrole (2r)
pale yellow oil (73 mg, 76%); IR (neat) 1624, 1591, 1495, 1452, 1352, 1308, 1274, 1113, 1096, 1073, 998, 869, 748, 729, 676, 640 cm−1; 1H NMR (CDCl3, 500 MHz) δ 6.74–6.69 (m, 3H), 6.60 (d, J = 6.5 Hz, 2H), 6.24 (t, J = 9.0 Hz, 2H), 5.06 (s, 2H); 13C NMR (CDCl3, 125.8 MHz) δ 163.2 (dd, J = 236.3 Hz, 12.9 Hz), 142.3 (t, J = 8.9 Hz), 121.1, 109.5 (dd, J = 13.6 Hz, 6.3 Hz), 109.1, 103.0 (t, J = 25.1 Hz), 52.4; 19F NMR (CDCl3, 470.8 MHz) δ −109.1; HRMS (ESI-TOF) m/z calcd. for C11H10NF2+ [M + H]+ 194.0781, found 194.0772.
3-((1H-Pyrrol-1-yl)methyl)pyridine (2s)
pale pink solid (50 mg, 63%): mp 54–57 °C; IR (neat) 1500, 1426, 1325, 1284, 1090, 795, 729, 516 cm−1; 1H NMR (CDCl3, 500 MHz) δ 8.54–8.48 (m, 2H), 7.36 (d, J = 8.0 Hz, 1H), 7.25–7.24 (m, 1H), 6.69 (t, J = 2 Hz, 2H), 6.21 (t, J = 2 Hz, 2H), 5.09 (s, 2H); 13C NMR (CDCl3, 125.8 MHz) δ 149.4, 148.7, 134.8, 133.9, 123.9, 121.2, 109.2, 50.9; HRMS (ESI-TOF) m/z calcd. for C10H11N2+ [M + H]+ 159.0922, found 159.0912.
5-((1H-Pyrrol-1-yl)methyl)-2-methoxypyridine (2t)
yellow oil (78 mg, 83%); IR (KBr, neat) 2926, 2853, 1610, 1574, 1492, 1442, 1394, 1332, 1309, 1281, 1258, 1126, 1087, 1067, 1024, 967, 828, 718 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.02 (s, 1H), 7.32 (d, J = 8.8 Hz, 1H), 6.69 (d, J = 8.4 Hz, 1H), 6.65 (s, 2H), 6.17 (s, 2H), 4.97 (s, 2H), 3.92 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 163.9, 145.5, 137.9, 126.2, 120.7, 111.2, 108.8, 53.4, 50.3; HRMS (ESI-TOF) m/z calcd. for C11H13N2O+ [M + H]+ 189.1028, found 189.1044.
1-(5-((1H-Pyrrol-1-yl)methyl)thiophen-2-yl)ethanone (2u)
pale yellow oil (77 mg, 75%); IR (KBr, neat) 2925, 2855, 1660, 1536, 1496, 1458, 1434, 1360, 1266, 1087, 1069, 1032, 967, 928, 810, 721, 703 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.52 (d, J = 3.2 Hz, 1H), 6.86 (d, J = 3.2 Hz, 1H), 6.71 (s, 2H), 6.19 (s, 2H), 5.21 (s, 2H), 2.50 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 190.4, 149.8, 144.0, 132.4, 126.3, 120.8, 109.2, 48.3, 26.5; HRMS (ESI-TOF) m/z calcd. for C11H12NOS+ [M + H]+ 206.0640, found 206.0642.
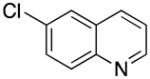
6-((1H-Pyrrol-1-yl)methyl)quinoline (2v)
yellow oil (67 mg, 64%); IR (neat) 1500, 1288, 1088, 831, 798, 724, 619 cm−1; 1H NMR (CDCl3, 500 MHz) δ 8.90 (m, 1H), 8.09–8.07 (m, 2H), 7.50–7.47 (m, 2H), 7.39 (q, J = 4.2 Hz, 1 H), 6.75 (s, 2H), 6.25 (s, 2H), 5.25 (s, 2H); 13C NMR (CDCl3, 125.8 MHz) δ 150.8, 148.0, 136.8, 136.2, 130.3, 128.7, 128.4, 125.6, 121.7, 121.5, 109.1, 53.4; HRMS (CI-TOF) m/z calcd. for C14H12N2 [M]+ 208.1000, found 208.0999.
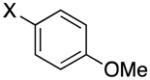
1-(4-(Methoxy)benzyl)-1H-pyrazole (3a)
pale yellow oil (52 mg, 56%); IR (neat) 1612, 1514, 1395, 1248, 1176, 1088, 1033, 822, 668 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.53 (s, 1H), 7.34 (s, 1H), 7.18 (d, J = 8.5 Hz, 2H), 6.78 (d, J = 8.5 Hz, 2H), 6.25 (s, 1H), 5.23 (s, 2H), 3.76 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 159.3, 139.3, 129.1, 128.8, 128.4, 114.0, 105.7, 55.3; HRMS (ESI-TOF) m/z calcd. for C11H13N2O+ [M + H]+ 189.1028, found 189.1022.
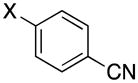
1-(4-Cyanobenzyl)-1H-pyrazole (3b)
pale yellow powder (74 mg, 81%): mp 73–75 °C; IR (neat) 2961, 2921, 2852, 2229, 1511, 1392, 1275, 1086, 1044, 966, 860, 828, 762, 746, 688, 616 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.60 (d, J = 8.0 Hz, 2H), 7.56 (s, 1H), 7.44 (s, 1H), 7.22 (d, J = 8.0 Hz, 2H), 6.31 (t, J = 4 Hz, 1H), 5.37 (s, 2H); 13C NMR (125.8 MHz, CDCl3) δ 142.0, 140.1, 132.4, 129.6, 127.7, 118.3, 111.7, 106.4, 55.0; HRMS (ESI-TOF) m/z calcd. for C11H10N3+ [M + H]+ 184.0875, found 184.0871.
1-(4-Nitrobenzyl)-1H-pyrazole (3c)
pale yellow powder (77 mg, 76%): mp 77–80 °C; IR (neat) 1607, 1517, 1396, 1340, 1312, 1275, 1088, 1108, 1047, 968, 918, 874, 860, 804, 753, 719, 641 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.19 (d, J = 8.5 Hz, 2H), 7.59 (s, 1H), 7.46 (s, 1H), 7.30 (d, J = 8.5 Hz, 2H), 6.34 (t, J = 4 Hz, 1H), 5.43 (s, 2H); 13C NMR (125.8 MHz, CDCl3) δ 147.5, 144.0, 140.35, 136.4, 129.7, 127.9, 124.0, 106.6, 54.9; HRMS (ESI-TOF) m/z calcd. for C10H10N3O2+ [M + H]+ 204.0773, found 204.0781.
1-(4-Fluorobenzyl)-1H-pyrazole (3d)
pale yellow oil (53 mg, 60%); IR (neat) 1607, 1510, 1395, 1223, 1158, 1088, 1048, 925, 824, 741, 616 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.56 (s, 1H), 7.38 (s, 1H), 7.18–7.21 (m, 2H), 7.01–7.04 (m, 2H), 6.29 (t, J = 4 Hz, 1H), 5.29 (s, 2H); 13C NMR (125.8 MHz, CDCl3) δ 163.3, 161.4 (d, J = 246 Hz), 139.6, 132.4, 129.3, 129.0, 115.7, 105.9, 55.0; 19F NMR (470.8 MHz, CDCl3) δ −114.2; HRMS (CI-TOF) m/z calcd. for C10H9N2F [M]+ 176.0750, found 176.0751.
1-(Naphthalene-2-ylmethyl)-1H-pyrazole (3e)
pale yellow powder (75 mg, 72%): mp 86–88 °C; IR (neat) 1510, 1445, 1396, 1283, 1087, 1051, 894, 824, 774, 762, 623 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.81–7.85 (m, 3H), 7.69 (s, 1H), 7.62 (s, 1H), 7.48–7.52 (m, 2H), 7.33–7.43 (m, 2H), 6.33 (m, 1H), 5.50 (s, 2H); 13C NMR (75.4 MHz, CDCl3) δ 139.5, 133.9, 133.2, 132.9, 129.1, 128.6, 127.8, 127.6, 126.6, 126.3, 126.1, 125.3, 105.9, 56.0; HRMS (ESI-TOF) m/z calcd. for C14H13N2+ [M + H]+ 209.1079, found 209.1080.
1-(3,5-Dimethoxybenzyl)-1H-pyrazole (3f)
pale yellow powder (68 g, 63%): mp 29–31 °C; IR (neat) 1611, 1594, 1477, 1429, 1397, 1360, 1314, 1203, 1167, 1087, 1062, 1046, 934, 821, 755, 642 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.62 (s, 1H), 7.49 (s, 1H), 6.39 (t, J = 2.4 Hz, 1H), 6.35 (s, 2H), 6.29 (s, 1H), 5.26 (s, 2H), 3.75 (s, 6H); 13C NMR (75.4 MHz, CDCl3) δ 161.0, 139.4, 138.9, 129.2, 105.9, 105.5, 99.7, 55.8; HRMS (ESI-TOF) m/z calcd. for C12H15N2O2+ [M + H]+ 219.1134, found 219.1132.
Methyl 3-((1H-Pyrazol-1-yl)methyl)benzoate (3g)
pale yellow oil (85 mg, 61%); IR (neat) 1720, 1433, 1395, 1286, 1200, 1107, 1084, 1048, 730, 623 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.96 (d, J = 6.9 Hz, 1H), 7.90 (s, 1H), 7.54 (s, 1H), 7.37–7.40 (m, 3H), 6.28 (t, J = 2.1 Hz, 1H), 5.34 (s, 2H), 3.88 (s, 3H); 13C NMR (75.4 MHz, CDCl3) δ 166.5, 139.7, 137.0, 131.9, 130.6, 129.2, 129.1, 128.8, 128.6, 106.1, 55.3, 52.0; HRMS (ESI-TOF) m/z calcd. for C12H13N2O2+ [M + H]+ 217.0977, found 217.0976.
1-(4-((1H-Pyrazol-1-yl)methyl)phenyl)ethan-1-one (3h)
pale yellow powder (77 mg, 73%): mp 79–81 °C; IR (neat) 1673, 1607, 1396, 1357, 1266, 1086, 1048, 964, 862, 755, 704 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.93 (d, J = 8.1 Hz, 2H), 7.57 (s, 1H), 7.43 (s, 1H), 7.26 (d, J = 8.4 Hz, 2H), 6.32 (s, 1H), 5.38 (s, 2H), 2.57 (s, 3H); 13C NMR (75.4 MHz, CDCl3) δ 197.3, 141.9, 139.8, 136.6, 129.4, 128.7, 127.4, 106.1, 55.2, 26.5; HRMS (ESI-TOF) m/z calcd. for C12H13N2O+ [M + H]+ 201.1028, found 201.1031.
1-(4-(1H-Pyrrol-1-yl)benzyl)-1H-pyrazole (3i)
pale yellow powder (88 mg, 58%): mp 75–77 °C; IR (neat) 1613, 1524, 1396, 1327, 1277, 1125, 1069, 1016, 920, 809, 757, 727, 643 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.59 (s, 1H), 7.43 (s, 1H), 7.38 (d, J = 8.5 Hz, 2H), 7.28 (d, J = 8.5 Hz, 2H), 7.08 (s, 2H), 6.36 (s, 2H), 6.32 (s, 1H), 5.35 (s, 2H); 13C NMR (125.8 MHz, CDCl3) δ 140.3, 139.6, 133.9, 129.1, 128.8, 120., 119.1, 110.5, 106.0, 55.2; HRMS (ESI-TOF) m/z calcd. for C14H14N3+ [M + H]+ 224.1188, found 224.1186.
5-((1H-Pyrazol-1-yl)methyl)-2-methoxypyridine (3j)
colorless oil (77 mg, 82%); IR (neat) 1611, 1571, 1492, 1395, 1290, 1221, 1088, 1025, 833, 753, 618 cm−1; 1H NMR (360 MHz, CDCl3) δ 8.09 (s, 1H), 7.53 (s, 1H), 7.47 (d, J = 6.1 Hz, 1H), 7.36 (s, 1H), 6.71 (d, J = 8.6 Hz, 1H), 6.27 (s, 1H), 5.24 (s, 2H), 3.92 (s, 3H); 13C NMR (90.5 MHz, CDCl3) δ 164.1, 146.1, 139.7, 138.4, 128.8, 124.9, 111.2, 106.1, 111.2, 106.1, 53.5, 52.9; HRMS (ESI-TOF) m/z calcd. for C10H12N3O+ [M + H]+ 190.0980, found 190.0976.
2-((1H-Pyrazol-1-yl)methyl)pyrazine (3k)
pale yellow oil (48 mg, 60%); IR (neat) 1514, 1405, 1281, 1088, 1053, 1019, 967, 835, 757, 643 cm−1; 1H NMR (360 MHz, CDCl3) δ 8.49–8.51 (m, 2H), 8.34 (s, 1H), 7.54–7.57 (m, 2H), 6.32 (s, 1H), 5.47 (s, 2H); 13C NMR (90.5 MHz, CDCl3) δ 151.9, 143.9, 143.6, 140.3, 129.9, 106.4, 55.1; HRMS (ESI-TOF) m/z calcd. for C8H9N4+ [M + H]+ 161.0827, found 161.0827.
1-(4-(Methoxy)benzyl)-1H-indole (4a)
pale yellow oil (86 mg, 73%); IR (neat) 1612, 1512, 1462, 1304, 1246, 1175, 1032, 821, 739 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.62 (d, J = 7.8 Hz, 1H), 7.27 (d, J = 8.2 Hz, 1H), 7.05–7.18 (m, 5H), 6.80 (d, J = 8.7 Hz, 2H), 6.50 (d, J = 3.8 Hz, 1H), 5.20 (s, 2H), 3.72 (s, 3H); 13C NMR (75.4 MHz, CDCl3) δ 159.0, 136.2, 129.4, 128.7, 128.1, 128.0, 121.5, 120.9, 119.4, 114.0, 109.6, 101.4, 55.2, 49.5; HRMS (ESI-TOF) m/z calcd. For C16H16NO+ [M + H]+ 238.1232, found 238.1237.
1-(2-Methoxybenzyl)-1H-indole (4b)
yellow powder (92 mg, 78%): mp 75–77 °C; IR (neat) 1599, 1492, 1460, 1437, 1360, 1302, 1248, 1115, 743 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.68 (d, J = 7.6 Hz, 1H), 7.38 (d, J = 7.9 Hz, 1H), 7.12–7.30 (m, 4H), 6.74–6.95 (m, 3H), 6.57 (d, J = 2.9 Hz, 1H), 5.37 (s, 2H), 3.92 (s, 3H); 13C NMR (75.4 MHz, CDCl3) δ 156.6, 136.3, 128.6, 128.5, 127.9, 125.8, 121.4, 120.7, 120.5, 119.2, 110.0, 109.7, 101.2, 55.2, 45.0; HRMS (ESI-TOF) m/z calcd. for C16H16NO+ [M + H]+ 238.1232, found 238.1232.
1-(3-Methoxybenzyl)-1H-indole (4c)
pale yellow oil (95 mg, 80%); IR (neat) 1600, 1587, 1510, 1489, 1462, 1335, 1262, 1147, 1049, 739 cm−1; 1H NMR (360 MHz, CDCl3) δ 7.63 (d, J = 8.2 Hz, 1H), 7.24 (d, J= 8.3 Hz, 1H), 7.06–7.18 (m, 4H), 6.75 (d, J = 8.1 Hz, 1H), 6.62 (m, 2H), 6.52 (d, J = 3.8 Hz, 1H), 5.22 (s, 2H), 3.67 (s, 3H); 13C NMR (75.4 MHz, CDCl3) δ 159.9, 139.2, 136.3, 129.7, 128.7, 128.2, 121.6, 120.9, 119.5, 119.0, 112.7, 112.6, 109.6, 101.7, 55.1, 49.9; HRMS (ESI-TOF) m/z calcd. for C16H16NO+ [M + H]+ 238.1232, found 238.1235.
1-(4-Cyanobenzyl)-1H-indole (4d)
pale yellow oil (70 mg, 94%); IR (neat) 2227, 1728, 1609, 1462, 1317, 1245, 1180, 818, 741 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.64 (d, J = 7.2 Hz, 1H), 7.47 (d, J = 8.1 Hz, 2H), 7.06–7.13 (m, 6H), 6.56 (d, J = 3.3 Hz, 1H), 5.30 (s, 2H); 13C NMR (75.4 MHz, CDCl3) δ 143.0, 136.0, 132.5, 128.7, 128.1, 127.1, 122.0, 121.1, 119.9, 118.5, 111.3, 109.3, 102.4, 49.5; HRMS (ESI-TOF) m/z calcd. for C16H13N2+ [M + H]+ 233.1079, found 233.1082.
1-(2-Cyanobenzyl)-1H-indole (4e)
pale yellow oil (101 mg, 87%); IR (neat) 2223, 1514, 1482, 1462, 1435, 1356, 1312, 1244, 1190, 740 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.69–7.74 (m, 2H), 7.35–7.46 (m, 2H), 7.15–7.29 (m, 4H), 6.83 (d, J = 7.8 Hz, 1H), 6.65 (d, J = 3.0 Hz, 1H), 5.58 (s, 2H); 13C NMR (90.5 MHz, CDCl3) δ 141.3, 136.1, 133.4, 132.9, 128.8, 128.3, 128.1, 127.4, 122.1, 121.2, 120.0, 117.2, 110.7, 109.4, 102.6, 48.1; HRMS (ESI-TOF) m/z calcd. for C16H13N2+ [M + H]+ 233.1079, found 233.1080.
1-(3-Cyanobenzyl)-1H-indole (4f)
pale yellow oil (105 mg, 90%); IR (neat) 2231, 1512, 1482, 1462, 1435, 1313, 1253, 1186, 741 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.74 (d, J = 7.5 Hz, 1H), 7.55 (d, J = 7.8 Hz, 1H), 7.15–7.42 (m, 7H), 6.65 (d, J = 3.0 Hz, 1H), 5.35 (s, 2H); 13C NMR (75.4 MHz, CDCl3) δ 139.2, 135.9, 131.2, 130.9, 129.9, 129.5, 128.8, 127.9, 122.0, 121.2, 119.9, 118.4, 112.8, 109.3, 102.4, 49.1; HRMS (ESI-TOF) m/z calcd. for C16H13N2+ [M + H]+ 233.1079, found 233.1077.
4-((1H-Indol-1-yl)methyl)benzaldehyde (4g)
pale yellow oil (95 mg, 81%); IR (neat) 1692, 1606, 1462, 1316, 1210, 1168, 1013, 848, 808, 741 cm−1; 1H NMR (300 MHz, CDCl3) δ 9.98 (s, 1H), 7.83 (d, J = 8.1 Hz, 2H), 7.69–7.72 (m, 1H), 7.16–7.27 (m, 6H), 6.64 (d, J = 3.9 Hz, 1H), 5.42 (s, 2H); 13C NMR (90.5 MHz, CDCl3) δ 191.6, 144.5, 136.2, 135.8, 130.2, 128.8, 128.2, 127.1, 126.8, 122.0, 121.2, 119.8, 109.5, 102.3, 49.8; HRMS (ESI-TOF) m/z calcd. for C16H14NO+ [M + H]+ 236.1075, found 236.1074.
(4-((1H-Indol-1-yl)methyl)phenyl)(phenyl)methanone (4h)
pale yellow powder (108 mg, 70%): mp 78–80 °C; IR (neat) 1655, 1605, 1462, 1317, 1276, 1179, 926, 743, 720, 704 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.63–7.70 (m, 5H), 7.50 (t, J = 7.5 Hz, 1H), 7.37–7.42 (m, 2H), 7.10–7.23 (m, 6H), 6.56 (d, J = 3.3 Hz, 1H), 5.30 (s, 2H); 13C NMR (75.4 MHz, CDCl3) δ 196.0, 142.2, 137.4, 136.8, 136.1, 132.4, 130.5, 129.9, 128.7, 128.2, 126.4, 121.8, 121.1, 119.7, 109.5, 102.1, 49.7; HRMS (ESI-TOF) m/z calcd. for C22H18NO+ [M + H]+ 312.1388, found 312.1383.
Methyl 4-((1H-Indol-1-yl)methyl)benzoate (4i)
pale yellow oil (103 mg, 78%); IR (neat) 1719, 1611, 1512, 1462, 1433, 1277, 1178, 1108, 1018, 740 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.04 (d, J = 8.5 Hz, 2H), 7.78 (d, J = 6.5 Hz, 1H), 7.23–7.30 (m, 3H), 7.17–722 (m, 3H), 6.68 (d, J = 3.5 Hz, 1H), 5.35 (s, 2H), 3.95 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 166.6, 142.7, 136.2, 130.0, 129.5, 128.8, 128.2, 126.5, 121.9, 121.1, 119.7, 109.6, 102.1, 52.0, 49.7; HRMS (ESI-TOF) m/z calcd. for C17H16NO2+ [M + H]+ 266.1181, found 266.1181.
1-(4-Fluorobenzyl)-1H-indole (4j)
pale yellow oil (80 g, 72%); IR (neat) 1604, 1509, 1483, 1462, 1335, 1315, 1221, 1157, 739 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.64 (d, J = 8.0 Hz, 1H), 6.90–7.24 (m, 8H), 6.54 (d, J =3.1 Hz, 1H), 5.22 (s, 2H); 13C NMR (75.4 MHz, CDCl3) δ 163.8, 160.5, 136.1, 133.2, 128.0–128.7 (q, J = 52.7 Hz ), 121.7, 121.0, 119.6, 115.7, 115.4, 109.5, 101.8, 49.3; 19F NMR (282.4 MHz, CDCl3) δ −114.7; HRMS (ESI-TOF) m/z calcd. for C15H13FN+ [M + H]+ 226.1032, found 226.1032.
1-(4-(Trifluoromethyl)benzyl)-1H-indole (4k)
pale yellow oil (125 mg, 91%); IR (neat) 1619, 1512, 1462, 1420, 1323, 1163, 1119, 1066, 1017, 821, 740, 716 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.77 (d, J = 7.8 Hz, 1H), 7.61 (d, J = 8.4 Hz, 2H), 7.27 (d, J = 5.8 Hz, 2H), 7.24–7.21 (m, 3H), 7.18 (d, J = 3.0, 1H), 6.68 (d, J = 3.0 Hz, 1H), 5.40 (s, 2H); 13C NMR (75.4 MHz, CDCl3) δ141.6, 136.1, 129.8 (q, J = 97.34 Hz), 128.7, 128.1, 126.8, 125.6 (q, J = 11.32 Hz), 122.1, 121.9, 121.1, 119.8, 109.4, 102.2, 49.5; 19F NMR(282.4 MHz, CDCl3) δ −62.4; HRMS (ESI-TOF) m/z calcd. for C16H13F3N+ [M + H]+276.1000, found 276.1009.
1-(4-Nitrobenzyl)-1H-indole (4l)
pale yellow oil (114 mg, 90%); IR (neat) 1605, 1518, 1462, 1342, 1318, 1253, 1181, 741 cm−1; 1H NMR (360 MHz, CDCl3) δ 8.11 (d, J = 8.7 Hz, 2H), 7.67 (d, J = 8.2 Hz, 1H), 7.10–7.17 (m, 6H), 6.59 (d, J = 3.2 Hz, 1H), 5.39 (s, 2H); 13C NMR (75.4 MHz, CDCl3) δ 147.5, 145.0, 136.1, 128.8, 128.0, 127.2, 124.0, 122.2, 121.3, 120.0, 109.3, 102.6, 49.4; HRMS (ESI-TOF) m/z calcd. for C15H13N2O2+ [M + H]+ 253.0993, found 253.0988.
1-(4-Methylbenzyl)-1H-indole (4m)
pale yellow oil (75 mg, 68%); IR (neat) 1514, 1481, 1462, 1316, 1252, 1183, 798, 738 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.64 (d, J = 7.8 Hz, 1H), 7.27 (d, J = 8.1 Hz, 1H), 7.06–7.21 (m, 5H), 7.00 (d, J = 8.1 Hz, 2H), 6.53 (d, J = 3.9 Hz, 1H), 5.25 (s, 2H), 2.29 (s, 3H); 13C NMR (75.4 MHz, CDCl3) δ137.2, 136.2, 134.4, 129.3, 128.6, 128.1, 126.7, 121.5, 120.8, 119.3, 109.6, 101.4, 49.8, 21.0; HRMS (ESI-TOF) m/z calcd. for C16H16N+ [M + H]+ 222.1283, found 222.1283.
1-(Naphthalene-1-ylmethyl)-1H-indole (4n)
pale yellow powder (100 mg, 78%): mp 64–66 °C; IR (neat) 1708, 1508, 1483, 1461, 1320, 1192, 1121, 1010, 741 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.93–7.95 (m, 2H), 7.84 (d, J = 8.1 Hz, 1H), 7.73 (d, J = 8.1 Hz, 1H), 7.55–7.60 (m, 2H), 7.35–7.40 (m, 2H), 7.17–7.28 (m, 2H), 7.09 (d, J = 3.0 Hz, 1H), 6.93 (d, J = 6.9 Hz, 1H), 6.61 (d, J = 3.0 Hz, 1H), 5.80 (s, 2H); 13C NMR (90.5 MHz, CDCl3) δ 136.5, 133.7, 132.5, 130.9, 128.9, 128.8, 128.4, 128.2, 126.6, 126.0, 125.6, 125.1, 122.6, 121.8, 121.1, 119.7, 109.6, 101.8, 47.8; HRMS (ESI-TOF) m/z calcd. for C19H16N+ [M + H]+ 258.1283, found 258.1282.
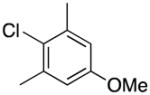
1-(4-Methoxy-2,6-dimethylbenzyl)-1H-indole (4o)
yellow powder (125 mg, 94%): mp 93–95 °C; IR (neat) 1604, 1460, 1318, 1294, 1193, 1144, 1061, 856, 739, 717 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.69 (d, J = 7.8 Hz, 1H), 7.52 (d, J = 8.2 Hz, 1H), 7.32–7.27 (m, 1H), 7.20–7.15 (m, 1H), 6.71–6.50 (m, 3H), 6.44 (d, J = 2.7, 1s), 5.22 (s, 2H), 3.85 (s, 3H), 2.27 (s, 6H); 13C NMR (75.4 MHz, CDCl3) δ 159.1, 139.6, 136.3, 128.8, 125.7, 124.2, 121.2, 120.8, 119.3, 113.7, 109.1, 100.8, 55.0, 43.3, 19.9; HRMS (ESI-TOF) m/z calcd. for C18H20NO+ [M + H]+ 266.1545, found 266.1545.
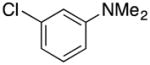
4-((1H-Indole-1-yl)methyl)-N,N-dimethylbenzeneamine (4p)
yellow oil (93 mg, 74%); IR (neat) 1602, 1499, 1459, 1437, 1352, 1315, 1184, 997, 738 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.75 (d, J = 8.4 Hz, 1H), 7.42 (d, J = 8.1 Hz, 1H), 7.17–7.28 (m, 4H), 6.71 (d, J = 8.4 Hz, 1H), 6.53–6.63 (m, 3H), 5.34 (s, 2H), 2.95 (s, 6H); 13C NMR (75.4 MHz, CDCl3) δ 150.8, 138.3, 136.4, 129.4, 128.6, 128.3, 121.5, 120.8, 119.3, 115.0, 111.7, 110.8, 109.7, 101.4, 50.4, 40.4; HRMS (ESI-TOF) m/z calcd. for C17H19N2+ [M + H]+ 251.1548, found 251.1554.
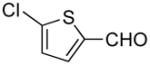
5-((1H-Indol-1-yl)methyl)thiophene-2-carbaldehyde (5a)
pale yellow powder (110 mg, 91%): mp 64–66 °C; IR (neat) 1652, 1508, 1461, 1433, 1314, 1225, 1184, 1044, 817, 746 cm−1; 1H NMR (300 MHz, CDCl3) δ 9.76 (s, 1H), 7.64 (d, J = 6.90 Hz, 1H), 7.52 (d, J = 3.6 Hz, 1H), 7.09–7.29 (m, 4H), 6.85 (d, J = 3.0 Hz, 1H), 6.56 (d, J = 2.7 Hz, 1H), 5.44 (s, 2H); 13C NMR (75.4 MHz, CDCl3) δ 182.6, 150.9, 143.1, 136.3, 135.7, 128.7, 127.5, 126.3, 122.1, 121.2, 120.0, 109.2, 102.7, 45.4; HRMS (ESI-TOF) m/z calcd. for C14H12NOS+ [M + H]+ 242.0640, found 242.0645.
5-((1H-Indol-1-yl)methyl)furan-2-carbaldehyde (5b)
pale yellow oil (97 mg, 87%); IR (neat) 1652, 1508, 1461, 1433, 1314, 1225, 1184, 1044, 817, 746 cm−1; 1H NMR (360 MHz, CDCl3) δ 9.58 (s, 1H), 7.64 (d, J = 7.2 Hz, 1H), 7.12–7.34 (m, 5H), 6.58 (d, J = 3.2 Hz, 1H), 6.20 (d, J = 3.6 Hz, 1H), 5.38 (s, 2H); 13C NMR (75.4 MHz, CDCl3) δ 177.4, 157.2, 136.2, 129.0, 127.8, 122.1, 121.2, 120.0, 117.2, 110.3, 109.1, 105.9, 102.7, 43.5; HRMS (ESI-TOF) m/z calcd. for C14H12NO2+ [M + H]+ 226.0868, found 226.0876.
1-(Pyridin-3-ylmethyl)-1H-indole (5c)
pale yellow oil (90 mg, 86%); IR (neat) 1718, 1676, 1462, 1427, 1315, 1258, 1177, 739 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.55 (s, 2H), 7.70 (d, J = 6.9 Hz, 1H), 7.12–7.33 (m, 6H), 6.60 (d, J = 3.6 Hz, 1H), 5.31 (s, 2H); 13C NMR (75.4 MHz, CDCl3) δ 148.8, 148.0, 136.0, 134.5, 133.1, 128.7, 127.8, 123.7, 121.9, 121.1, 119.7, 109.3, 102.2, 47.4; HRMS (ESI-TOF) m/z calcd. for C14H13N2+ [M + H]+ 209.1079, found 209.1079.
1-5-{[(Trifluoromethyl)pyridin-3-yl]methyl}-1H-indole (5d)
yellow oil (105 mg, 76%); IR (neat) 1680, 1607, 1583, 1462, 1336, 1316, 1131, 1087, 741 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.86 (s, 1H), 8.63 (s, 1H), 7.72 (d, J = 6.0 Hz, 1H), 7.65 (s, 1H), 7.14–7.27 (m, 4H), 6.67 (d, J = 3.3 Hz, 1H), 6.37 (s, 2H); 13C NMR (75.4 MHz, CDCl3) δ 151.4, 146.0, 145.9, 135.9, 133.4, 131.3, 131.2, 128.8, 127.6, 126.9, 126.5, 124.9, 122.2, 121.3, 120.0, 109.1, 102.9, 47.0; 19F NMR (282.4 MHz, CDCl3) δ −62.3; HRMS (ESI-TOF) m/z calc. for C15H12F3N2+ [M + H]+ 277.0953, found 277.0952.
1-(Pyrazin-2-ylmethyl)-1H-indole (5e)
pale yellow powder (67 mg, 65%): 71–73 °C; IR (neat) 1507, 1464, 1404, 1349, 1335, 1189, 1053, 1016, 743 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.56 (s, 1H), 8.49 (s, 1H), 8.18 (s, 1H), 7.70 (d, J = 8.4 Hz, 1H), 7.13–7.33 (m, 4H), 6.63 (d, J = 3.0 Hz, 1H), 5.50 (s, 2H); 13C NMR (75.4 MHz, CDCl3) δ 152.8, 144.0, 143.7, 143.0, 128.8, 127.9, 122.0, 121.1, 119.8, 109.2, 102.6, 49.9; HRMS (ESI-TOF) m/z calcd. for C13H12N3+ [M + H]+ 210.1030, found 210.1028.
6-((1H-Indol-1-yl)methyl)quinolone (5f)
pale yellow powder (70 mg, 70%): mp 59–61 °C; IR (neat) 1591, 1565, 1503, 1482, 1462, 1435, 1331, 1257, 1189, 828, 746 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.91 (d, J = 5.8 Hz, 1H), 8.11 (d, J = 8.7 Hz, 1H), 7.99 (d, J = 7.4 Hz, 1H), 7.74 (d, J = 6.9 Hz, 1H), 7.53 (d, J = 6.7 Hz, 1H), 7. 45 (s, 1H), 7.14–7.38 (m, 5H), 6.65 (d, J = 3.1 Hz, 1H), 5.49 (s, 2H); 13C NMR (75.4 MHz, CDCl3) δ 150.4, 147.7, 136.2, 135.8, 135.8, 130.0, 128.7, 128.2, 128.2, 128.1, 125.0, 121.8, 121.3, 121.0, 119.6, 109.5, 102.0, 49.8; HRMS (ESI-TOF) m/z calcd. for C18H15N2+ [M + H]+ 259.1235, found 259.1237.
5-((1H-Indol-1-yl)methyl)quinolone (5g)
pale yellow powder (100 mg, 78%): mp 111–114 °C; IR (neat) 1621, 1587, 1461, 1382, 1318, 1273, 1198, 824, 742 cm−1; 1H NMR (360 MHz, CDCl3) δ 9.58 (s, 1H), 8.60 (d, J = 6.1 Hz, 1H), 7.90 (d, J = 7.9 Hz, 1H), 7.71–7.75 (m, 2H), 7.41–7.46 (m, 1H) 7.19–7.31 (m, 3H), 7.06 (d, J = 2.8 Hz, 2H), 6.62 (d, J = 2.8 Hz, 1H), 5.68 (s, 2H); 13C NMR (90.5 MHz, CDCl3) δ 153.3, 143.8, 136.4, 133.5, 132.1, 128.9, 128.8, 128.7, 128.0, 127.8, 127.0, 122.0, 121.2, 119.9, 115.6, 109.5, 102.3, 47.0; HRMS (ESI-TOF) m/z calcd. for C18H15N2+ [M + H]+ 259.1235, found 259.1234.
tert-Butyl 5-((1H-Indol-1-yl)methyl)-1H-indole-1-carboxylate (5h)
red oil (125 mg, 72%); IR (neat) 1731, 1471, 1441, 1368, 1352, 1336, 1254, 1160, 1128, 1082, 1023, 738 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.15 (d, J = 8.4 Hz, 1H), 7.73 (d, J = 6.8 Hz, 1H), 7.65 (d, J = 3.6 Hz, 1H), 7.34–7.40 (m, 2H), 7.16–7.27 (m, 4H) 6.63 (d, J = 3.0 Hz, 1H), 6.53 (d, J = 3.6 Hz, 1H), 5.45 (s, 2H), 1.72 (s, 9H); 13C NMR (75.4 MHz, CDCl3) δ 149.5, 136.3, 134.5, 131.8, 130.8, 128.7, 128.2, 126.4, 123.1, 121.5, 120.9, 119.4, 119.2, 115.3, 109.7, 107.1, 101.5, 83.7, 50.1,28.1; HRMS (ESI-TOF) m/z calcd. for C22H23N2O2+ [M + H]+347.1760, found 347.1760.
2,6-bis((1H-Indol-1-yl)methyl)pyridine (5i)
pale yellow powder (140 mg, 83%): mp 92–94 °C; IR (neat) 1595, 1575, 1512, 1484, 1425, 1334, 1314, 1253, 1184, 1043, 737 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.71 (d, J = 7.2 Hz, 2H), 7.15–7.37 (m, 9H), 6.65 (d, J = 3.3 Hz, 2H), 6.57 (d, J = 7.8 Hz, 2H), 5.50 (s, 4H); 13C NMR (75.4 MHz, CDCl3) δ 157.3, 138.0, 136.1, 128.7, 128.3, 121.8, 121.0, 119.7, 119.3, 109.6, 102.1, 51.8; HRMS (ESI-TOF) m/z calc. for C23H20N3+ [M + H]+ 338.1579, found 338.1549.
Supplementary Material
Acknowledgments
This research was generously supported by a National Priorities Research Program (NPRP) grant from the Qatar National Research Fund (Grant no. 08-035-1-008) and by NIGMS (R01 GM081376). Dr. Rakesh Kohli (University of Pennsylvania) is acknowledged for obtaining the HRMS data.
Footnotes
Supporting Information Available: Copies of 1H, 13C, 19F, and 11B spectra for all compounds prepared by the method described. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Alves F, Barreiro E, Fraga C. Mini-Rev Med Chem. 2009;9:782–793. doi: 10.2174/138955709788452649. [DOI] [PubMed] [Google Scholar]
- 2.Arsenault BJ, Boekholdt SM, Kastelein JJP. Eur Heart J. 2011;32:923–926. doi: 10.1093/eurheartj/ehq385. [DOI] [PubMed] [Google Scholar]
- 3.Giembycz MA. Brit J Pharmacol. 2008;155:288–290. doi: 10.1038/bjp.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Brelière JC, Le Fur GL. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- 5.Bella DD, Ferrari V, Frigeni V, Lualdi P. Nature-New Biol. 1973;241:282–283. doi: 10.1038/newbio241282a0. [DOI] [PubMed] [Google Scholar]
- 6.Nyandege A, Kolanos R, Roth BL, Glennon RA. Bioorg Med Chem Lett. 2007;17:1691–1694. doi: 10.1016/j.bmcl.2006.12.089. [DOI] [PubMed] [Google Scholar]
- 7.Pahadi NK, Paley M, Jana R, Waetzig SR, Tunge JA. J Am Chem Soc. 2009;131:16626–16627. doi: 10.1021/ja907357g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deb I, Coiro DJ, Seidel D. Chem Commun. 2011;47:6473–6475. doi: 10.1039/c1cc11560j. [DOI] [PubMed] [Google Scholar]
- 9.D’Silva C, Walker DA. J Org Chem. 1998;3263:6715–6718. [Google Scholar]
- 10.Sidique S, Shiryaev SA, Ratnikov BI, Herath A, Su Y, Strongin AY, Cosford NDP. Bioorg Med Chem Lett. 2009;19:5773–5777. doi: 10.1016/j.bmcl.2009.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Compare 3,372,858 commercially available aryl and heteroaryl chlorides versus 265,129 corresponding commercially available benzyl halides, aromatic aldehydes, benzylamines, and benzyl hydrazines: SciFinder; Chemical Abstracts Service, Columbus, OH, 2010 (as of April 29, 2013).
- 12.Zhou Z, Xue W. J Organomet Chem. 2009;694:599–603. [Google Scholar]
- 13.Molander GA, Sandrock DL. Org Lett. 2007;9:1597–1600. doi: 10.1021/ol070543e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molander GA, Gormisky PE, Sandrock DL. J Org Chem. 2008;73:2052–2057. doi: 10.1021/jo800183q. [DOI] [PubMed] [Google Scholar]
- 15.Raushel J, Sandrock DL, Josyula KV, Pakyz D, Molander GA. J Org Chem. 2011;76:2762–2769. doi: 10.1021/jo2001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molander GA, Beaumard F. Org Lett. 2011;13:1242–1245. doi: 10.1021/ol200128y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slade DJ, Pelz NF, Bodnar W, Lampe JW, Watson PS. J Org Chem. 2009;74:6331–6334. doi: 10.1021/jo9006656. [DOI] [PubMed] [Google Scholar]
- 18.Dreher SD, Dormer PG, Sandrock DL, Molander GA. J Am Chem Soc. 2008;130:9257–9259. doi: 10.1021/ja8031423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dreher SD, Lim SE, Sandrock DL, Molander GA. J Org Chem. 2009;74:3626–3631. doi: 10.1021/jo900152n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CK, Jun JH, Yu JS. J Het Chem. 2000;37:15–24. [Google Scholar]
- 21.Striley CAF, Amer A, Zhang WY, Zimmer H, Lando JB. Phosphorus, Sulfur Silicon Relat Elem. 1996:115. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.