Abstract
Progesterone, an agonist for the progesterone receptor (PR), can be an efficacious and well-tolerated treatment in endometrial cancer. The clinical use of progesterone is limited due to the lack of biomarkers that predict hormone sensitivity. Despite its efficacy in cancer therapy, mechanisms and site of action for progesterone remain unknown. Using an in vivo endometrial cancer mouse model driven by clinically relevant genetic changes but dichotomous responses to hormonal therapy, we demonstrate that signaling through stromal PR is necessary and sufficient for progesterone anti-tumor effects. Endometrial cancers resulting from epithelial loss of PTEN (PTENKO) were hormone sensitive and had abundant expression of stromal PR. Stromal deletion of PR as a single genetic change in these tumors induced progesterone resistance indicating that paracrine signaling through the stroma is essential for the progesterone therapeutic effects. A hormone refractory endometrial tumor with low levels of stromal PR developed when activation of KRAS was coupled with PTEN-loss (PTENKO/Kras). The innate progesterone resistance in PTENKO/Kras tumors stemmed from methylation of PR in the tumor microenvironment. Add-back of stromal PR expressed from a constitutively active promoter sensitized these tumors to progesterone therapy. Results demonstrate that signaling through stromal PR is sufficient for inducing hormone responsiveness. Our findings suggest that epigenetic de-repression of stromal PR could be a potential therapeutic target for sensitizing hormone refractory endometrial tumors to progesterone therapy. Based on these results, stromal expression of PR may emerge as a reliable biomarker in predicting response to hormonal therapy.
Introduction
Typically, antagonists of steroid receptors are exploited in the therapy of hormonally regulated carcinomas (1-3). Endometrial cancer is unique in that agonistic actions of progesterone exert anti-tumor effects. Uterine cancers are the most prevalent gynecologic malignancy in the western world with a rising incidence in the U.S. (4). Most uterine tumors arise from the endometrium, a hormonally regulated cell layer composed of epithelium and stroma. Endometrioid carcinomas, characterized by crowded disorganized epithelial glands with few intervening stroma, are the most common subtype of these tumors (5). Some of these cancers originate from excess proliferation induced by imbalances in estrogen and progesterone. Activation of oncogenes or loss of tumor suppressors initiate other tumors that are fueled by hormonal imbalances (6). Although hormonal therapy is successfully used in treatment and chemoprevention of breast (1, 2) and prostate adenocarcinomas (3), it is less widely embraced in therapy of endometrial cancers.
In the normal endometrium, prolonged exposure to unopposed estrogen can cause endometrial hyperplasia and cancer (7-9). Administration of high dose progesterone induces thinning of the normal endometrial lining (10). Given that progesterone causes atrophy of the normal endometrium, it is administered clinically as a single agent in the therapy of endometrial cancer. Despite five decades of clinical use, the anti-tumor mechanisms and site of action for progesterone therapy remain unknown. Molecular mechanisms underlying progesterone resistance or sensitivity are also poorly understood.
Progesterone is well tolerated, easily administered and has minimal side effects. Subsets of patients respond to progesterone while others have progression of disease while on hormonal therapy (11). Response rates to progesterone range from 11-50% in primary and recurrent disease (12). Despite its efficacy, hormonal therapy is not commonly administered in treatment of endometrial cancer primarily because patients with hormone sensitive or resistant disease cannot be prospectively identified. Standard therapy of endometrial cancer involves removal of reproductive organs, at times coupled with adjuvant radiation or chemotherapy (13). While this approach may be curative, it causes loss of fertility and can induce life-long side effects. Patients with metastatic disease often succumb to the cancer despite aggressive treatments (14). Discovery of reliable biomarkers that predict responsiveness to progesterone therapy and molecular mechanisms that dictate hormone sensitivity or resistance could broaden the application of hormonal therapy to endometrial cancer patients.
A challenge in endometrial cancer research is the perception of the endometrium as a homogeneous tissue. The endometrium is composed of epithelium and stroma, two distinct cell types with unique functions and responsiveness to steroid hormones (15, 16). To study contributions of each cell type in tumor initiation and progression, we established an in vivo endometrial regeneration model from dissociated epithelial and stromal populations (17). This model provides a unique tool for induction of concomitant but separate genetic changes in these two compartments (17) an experimental approach not achievable with existing endometrial cancer models. Here we utilized our dual compartment regeneration system as an in vivo pre-clinical platform for testing responsiveness to hormonal therapy in endometrial tumors generated from clinically relevant genetic changes. Tumors resulting from epithelial loss of PTEN were exquisitely progesterone sensitive, while tumors resulting from activation of KRAS concomitant with PTEN loss were completely progesterone resistant. Using these endometrial cancer models with dichotomous responses to hormonal therapy, we demonstrate that signaling through stromal progesterone receptor is necessary and sufficient for anti-tumor effects of progesterone therapy.
Material and Methods
Animals
WT C57BL/6, PtenloxP/loxP (C;129S4-Ptentm1Hwu/J), KrasLSL-G12D/+ (B6.129S4-Krastm4Tyj/J) and CB17Scid/Scid mice were from Jackson Laboratory. PRloxP/loxP (PRCE) mice were from Dr. Luisa Iruela-Arispe. PtenloxP/loxP KrasLSL-G12D/+ mice were generated by crossing the KrasLSL-G12D allele into the PtenloxP/loxP background. Mice were maintained in accordance with University of California Los Angeles (UCLA), Division of Laboratory Animal Medicine (DLAM) guidelines. All animal experiments were approved by the UCLA Animal Research Committee.
Lentivirus Constructs and Preparation
The Cre-RFP (17), Cre-GFP (18), GFP (19) lentiviral constructs have been previously described. Generation of the hPR-GFP and inducible Cre-shPTEN-GFP constructs is outlined in supplementary methods. Lentivirus production, titering, and infection of cells were performed as previously described (20).
Preparation of endometrial stroma and epithelia
Mouse neonatal uterine stroma and adult endometrial epithelial were prepared as previously described (17). Endometrial epithelial were isolated to purity by FACS sorting as reported (21). To obtain adult endometrial epithelia, mouse uteri were dissected, cut into fragments and incubated in 1% trypsin for 45min at 4°C. Luminal epithelia were separated from underlying stroma with a fine forceps and digested with 0.8 mg/ml collagenase in DMEM/10% FBS with 5 μg/ml insulin and 0.5 mg/ml DNase for 1.5h at 37C. Digested fractions were passed through 22-gauge syringes and filtered through 40 μm cell strainers to yield single cell suspensions. Cells were stained on ice with shaking for 15 min for fluorescent activator cell sorting (FACS). Endometrial epithelia marked by Trop1+CD90−CD45−CD31−Ter119− were FACS isolated using a BD FACSAria II flow cytometer. Antibodies used for FACS are listed in Supplementary Tables.
Tumor Generation
Endometrial tumor generation was performed as previously described (17). In some experiments endometrial epithelium or stroma were infected with lentivirus as described in Supplementary methods. For all experiments, approximately 125,000 prepared epithelial and 200,000 stromal cells were mixed, re-suspended in collagen (BDBiosciences; 354236) and dispensed into grafts. Endometrial grafts were implanted under the kidney capsule of oophorectomized CB17Scid/Scid mice and regenerated for 6-8 weeks with an estrogen pellet (60-d time release, 0.72-mg β-estradiol/pellet, SE-121 Innovative Research of America). Tumors generated with 100% efficiency using this 1:1 to 1:2 epithelial to stromal cell ratio.
Hormone Therapy
An eight week course of progesterone therapy was administered by subcutaneous implantation of a time release pellet (60-d time release, 100 mg progesterone/pellet; SP-131 Innovative Research of America). Placebo treatment in control mice was achieved by implantation of placebo pellets (60-d time release, 100 mg progesterone placebo/pellet; SC-111 Innovative Research of America). Estrogen pellets (60-d time release, 0.72-mg β-estradiol/pellet, SE-121 Innovative Research of America) were replenished at the start of progesterone therapy, unless otherwise noted. All surgical procedures were performed under the UCLA DLAM regulations. Serum hormone levels in mice were verified using estradiol EIA and progesterone EIA kits (Cayman Chemical).
Immunohistochemistry
Immunohistochemistry was performed as previously described (17) using antibodies listed in Supplementary Tables. To quantify expression of Ki67, TUNEL and cleaved caspase-3, 5-10 high-power fields of view were scored per sample and averaged.
Isolation of epithelia and stroma from regenerated tumors
PTENKO or PTENKO/Kras color-marked tumors were minced, and digested in 1 mg/ml each of collagenase and dispase in DMEM/10% FBS with 5 μg/ml insulin and 0.5 mg/ml DNase for 2 hr at 37 C. Resulting cells were passed through a 22 gauge syringe to yield a single cell suspension that was visualized and sorted using the BD FACSAria II.
Quantitative PCR
Quantitative-PCR was performed as previously described (21). RNA for QPCR was extracted from isolated tumor cell fractions using the Allprep DNA/RNA Micro kit (Qiagen). Data were normalized to the housekeeping gene Gapdh. Primers are outlined in Supplementary Tables.
Western Blot
Western blotting of isolated cell fractions was performed as previously described (21) using antibodies outlined in Supplementary Tables.
Methylation Specific PCR and Bisulfite Sequencing
DNA extracted from isolated tumor cell fractions was analyzed by methylation specific PCR and bisulfate sequencing using previously reported protocols (22) with minor modifications. Detailed descriptions and a list of the primers used are presented in supplementary methods.
Statistical analysis
Results are expressed as mean ± SD. To determine significance, comparisons were performed using a two-tailed t test for groups of two or one-way ANOVA with Tukey honestly significant difference criterion for three or more groups.
Results
Progesterone therapy effectively treated endometrial tumors resulting from cell autonomous loss of PTEN
Mutations in the PTEN tumor suppressor gene are reported in up to 79% of endometrioid endometrial cancers (23) where PTEN expression is lost predominantly in the tumor epithelium (24). Given the prevalence of this genetic change, defining whether PTEN status is a biomarker of response to hormonal therapy could be a valuable clinical tool. Results of retrospective clinical studies on this subject vary widely, with some studies reporting PTEN loss as a positive predictor (25, 26) while others report loss of PTEN as a negative predictor of response to progesterone (27, 28). A problem in these studies is that tumors examined may contain other genetic alterations in addition to PTEN loss. We hypothesized that tumors resulting from epithelial loss of PTEN, as a single genetic change, would be sensitive to hormonal therapy.
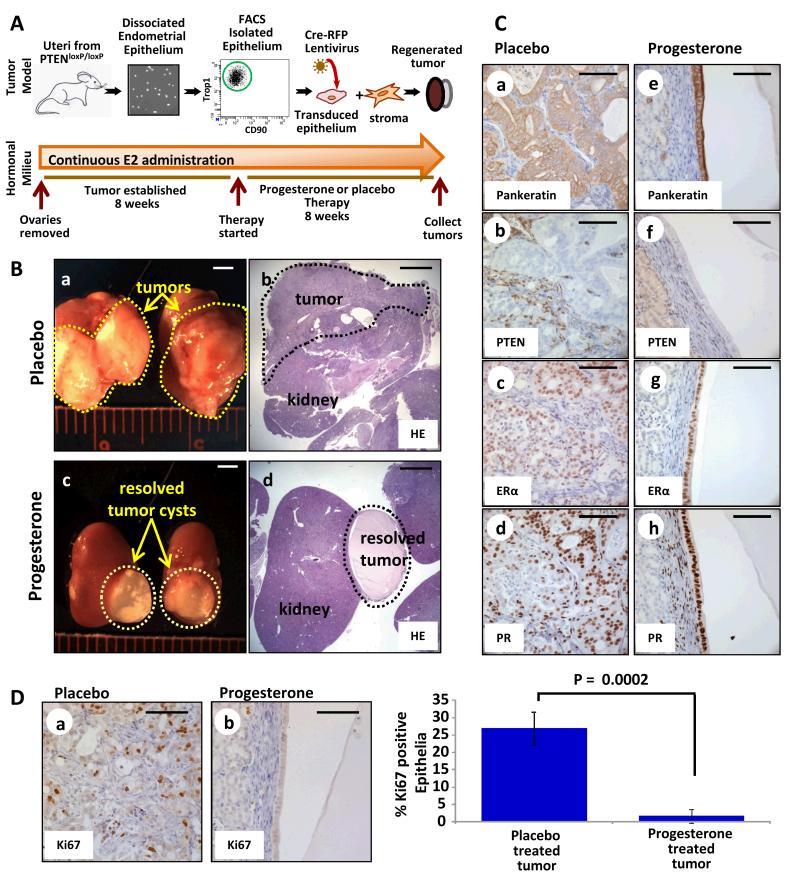
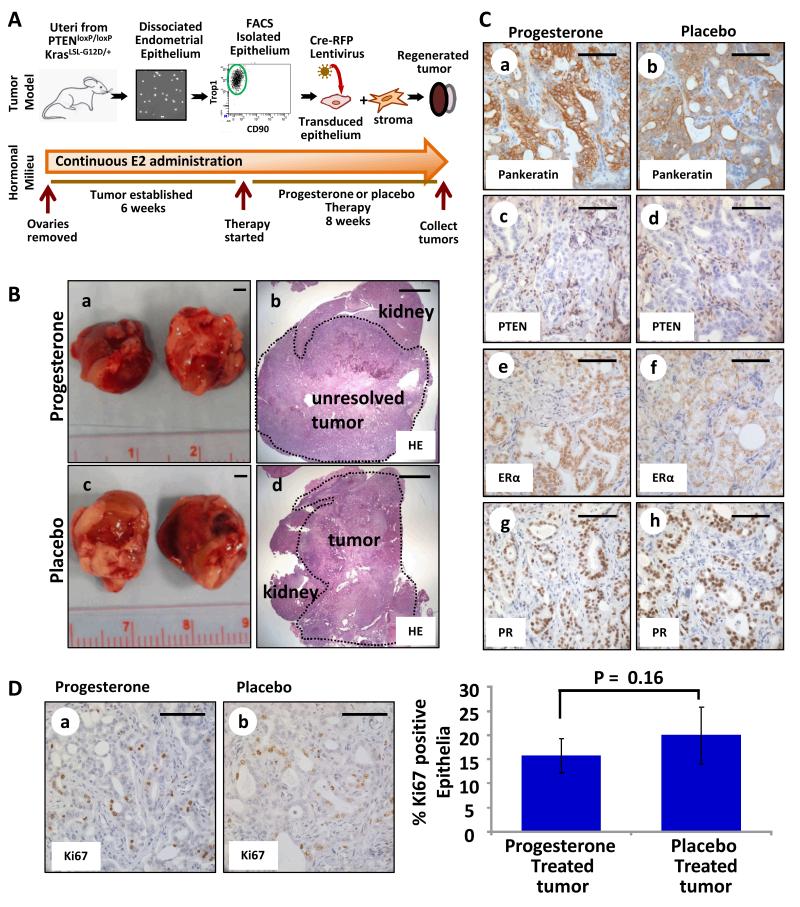
To address this hypothesis, the effects of progesterone therapy on PTEN-null tumors were examined using an in vivo model system (Fig. 1A). This model (17) mimics epithelial specific loss of PTEN seen in human endometrial cancers (24) unlike existing eutopic mouse models where Pten is deleted in both the epithelium and stroma (29-31). Endometrial epithelia were FACS isolated from uteri of PtenloxP/loxP mice and infected with lentivirus expressing red fluorescent protein (RFP) and Cre recombinase (17), resulting in deletion of Pten (Fig. 1A). These epithelia were combined with WT stroma and regenerated in the sub-renal space of oophorectomized mice supplemented with estrogen via implantation of an 8 week time-release 17β-estradiol pellet (17) (Fig. 1A). At 8 weeks, one mouse was sacrificed to confirm establishment of PTEN-null endometrioid endometrial tumors (PTENKO) that had a high proliferation index (Supplementary Fig. S1A&B). Given that endometrial cancer commonly occurs in patients with high estrogen levels (32), tumor bearing mice were re-implanted with new estradiol pellets to maintain a hyper-estrogenic state. Simultaneously half of these mice were implanted with progesterone pellets while the other half were treated with placebo for 8 weeks (Fig. 1A). Measurement of serum hormone levels confirmed high circulating levels of estrogen and progesterone in progesterone treated mice but only high levels of estrogen in the placebo group (Supplementary Fig. S1C). Large tumors were attached to the kidneys of placebo treated mice (Fig. 1B a&b), while only small cysts were found on kidneys of progesterone treated animals (Fig. 1B c&d). The histology of placebo treated PTENKO tumors was endometrioid with epithelial-specific loss of PTEN (Fig. 1C a&b and Supplementary Fig. S1D). Progesterone treated PTENKO endometrial tumors resolved and remaining tissue was primarily a simple cyst lined with normal appearing PTEN-null epithelium (Fig. 1C e&f and Supplementary Fig. S1D). Abundant expression of epithelial and stromal estrogen receptor α (Esr1 or ERα), and progesterone receptor (Pgr or PR) was detected in both progesterone and placebo treated tissue (Fig. 1C c,d,g&h). Progesterone therapy significantly diminished proliferation of PTEN-null epithelium as measured by Ki67 (Mki67) expression (Fig. 1D).
Figure 1. Progesterone therapy resulted in resolution of endometrial tumors initiated by epithelial loss of PTEN (PTENKO).
(A) Schema for in vivo therapy. (B) Large tumors were found in placebo treated mice (n=8) (a&b). Resolution of tumors was noted with progesterone therapy (n=8) (c&d). (C) Residual cysts in progesterone treated mice contained a single epithelial layer compared to persistent tumor epithelia in placebo treated controls (e vs. a). PTEN was absent in all epithelia (b&f). Epithelial and stromal ERα (c&g) and PR (d&h) were detected with progesterone or placebo. (D) A decrease in proliferating epithelia was detected upon progesterone administration (a vs. b). Scale bars equal 2 mm in B, and 100 μm in C&D.
Our findings demonstrate that endometrial tumors resulting from epithelial loss of PTEN are exquisitely sensitive to progesterone hormonal therapy. Observations in this mouse model suggest that loss of Pten as a single genetic change in the tumor epithelia should be investigated as a potential biomarker of response to progesterone therapy in patients with endometrial cancer.
Hormone mediated resolution of PTEN null endometrial tumors is time dependent but occurs efficiently in a short period
The time required for resolution of endometrial cancers treated with progesterone therapy varies widely (33). In some patients, tumors resolve with a few weeks of therapy, while others require more than six months of progesterone administration to demonstrate a clinical response (33). Many factors could contribute to this variation: dose and mode of drug administration, genetic heterogeneity in endometrial cancers and variations in the patient’s endogenous hormonal milieu. An advantage in our model is the homogenous genetic background of tumors and the host mice bearing tumors. Also, we are able to pharmacologically control the experimental hormonal milieu. This model enables us to systematically investigate the response of PTENKO tumors to hormonal therapy. We asked if hormonal resolution of tumors in our model is a kinetic process, occurring rapidly or incrementally.
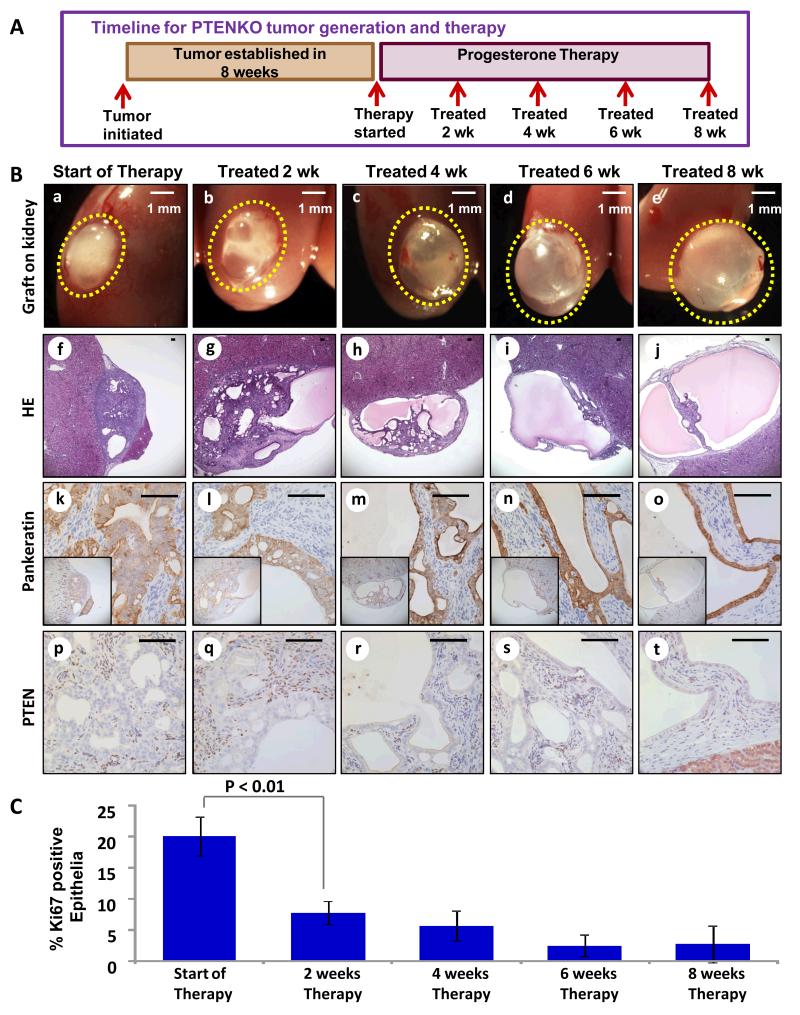
To assess kinetics of tumor response, mice bearing PTENKO carcinomas were treated with progesterone for 2, 4, 6 and 8 weeks (Fig. 2A). Tumors were examined grossly and histologically at each time point (Fig. 2B). Proliferation of tumor epithelia was measured and quantified using Ki67 (Fig. 2C and Supplementary Fig. S2A), while apoptotic cell death was measured with TUNEL assay and expression of cleaved caspase 3 (Supplementary Fig. S2B). Progesterone therapeutic effects were observed as early as 2 weeks, evidenced by formation of cystic regions in the tumor (Fig. 2B b,g,l vs. a,f,k) coupled with a significant decrease in proliferation (Fig. 2C and Supplementary Fig. S2A b vs. a). Progressive tumor clearance was detected at 4 (Fig. 2B c,h,m) and 6 weeks (Fig. 2B d,i,n). Although the tumor proliferation plateaued between 2 and 6 weeks (Fig. 2C), apoptotic cell death rose significantly during this time period (Supplementary Fig. S2B b-d and g-i). Complete tumor resolutions was observed at 8 weeks (Fig. 2B e,j,o). Cessation of progesterone therapy at this time point resulted in tumor recurrence in a hyper-estrogenic state (Supplementary Fig. S2C). Epithelial-specific PTEN loss was verified in tissue harvested at all time points (Fig. 2B p-t).
Figure 2. Resolution of PTENKO tumors is gradual but complete within 8 weeks.
(A) Established PTENKO tumors were treated with estrogen and progesterone for 2, 4, 6 or 8 weeks (n=4 at each time point). (B) Tumor clearing was detected over time (a-e). Histologic analysis confirmed resolution of tumor mass (f-j). The histology of epithelia marked by pankeratin demonstrated resolution of tumor epithelia into normal appearing glands (k-o). Epithelial loss of PTEN was confirmed (p-t). (C) A progressive decrease in the proliferation of tumor epithelia was observed throughout therapy. Scale bars are 100 μm except where noted.
Effective tumor therapy requires a shift in the balance between cellular proliferation and cell death. We observed a biphasic response to progesterone in this kinetic experiment. In the first therapeutic phase cell proliferation ceased while in the second phase cell death ensued, resulting in resolution of the remaining tumor cells. Findings here demonstrate that progesterone anti-tumor effects in PTENKO tumors are quick but kinetic resulting from a combination of decreased proliferation and increased cell death.
Co-administration of estrogen was essential for progesterone mediated anti-tumor effects in PTEN null endometrial tumors
While some patients with endometrial cancer have normal levels of circulating estrogen, many have a hyper-estrogenic state resulting from a variety of causes (32). Thus far, we administered continuous estrogen during progesterone therapy to mimic a hyper-estrogenic state. Estrogen alone promotes progression of endometrial cancer; therefore, in clinical practice progesterone is administered without estrogen. To replicate standard practice, we tested the efficacy of progesterone without estrogen in therapy of PTENKO tumors.
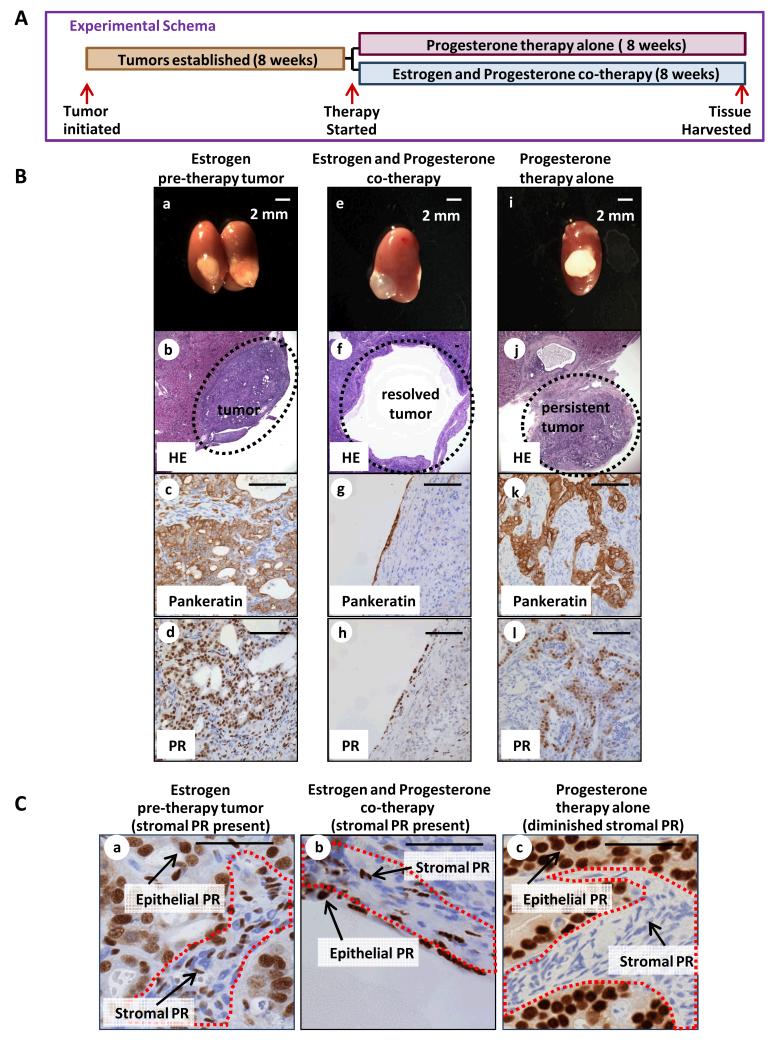
Mice bearing PTENKO tumors were divided into two cohorts (Fig. 3A). In half the mice, existing estrogen pellets were removed and progesterone pellets were implanted (progesterone therapy alone) (Fig. 3A). In the second group the existing estrogen pellets were removed but new estrogen and progesterone pellets were implanted (estrogen and progesterone co-therapy) (Fig. 3A). Prior to initiating treatment, establishment of pre-therapy tumors was confirmed (Fig. 3B a-c). Co-therapy with estrogen and progesterone resolved PTENKO endometrial tumors demonstrated by development of cysts (Fig. 3B e-g) with decreased epithelial proliferation compared to pre-therapy tumors (Supplementary Fig. S3A). Surprisingly, PTENKO tumors treated with progesterone alone persisted (Fig. 3B i-k) and had a proliferation index comparable to pre-therapy tumors (Supplementary Fig. S3A). ERα was expressed in the epithelia and stroma of all PTENKO tumors (Supplementary Fig. S3B). Notably, PTENKO tumors treated with progesterone alone had significantly diminished stromal PR (Fig. 3 Cc) compared to estrogen and progesterone co-treated (Fig. 3 Cb) and pre-therapy counterparts (Fig. 3 Ca). Abundant expression of epithelial PR was detected in all conditions (Fig. 3B d,h,l and Fig. 3C).
Figure 3. Effective therapy with progesterone requires co-administration of estrogen.
(A) Established PTENKO tumors were treated with: (1) estrogen with progesterone co-therapy (n=4) or (2) progesterone therapy alone (n=8). (B) In the absence of estrogen, progesterone hormonal therapy failed to resolved PTENKO tumors. Establishment of tumor was confirmed prior to therapy (a-d). Resolution of tumors was observed with estrogen and progesterone co-therapy (e-h). Persistence of the tumor was noted with progesterone therapy alone (i-l). (C) Lower levels of stromal PR were detected in tumors treated with progesterone alone (c vs. a&b). Scale bars equal 100 μm except where noted.
Findings here demonstrate that co-administration of estrogen with progesterone is required in hormonal therapy of PTENKO tumors. Although in clinical practice progesterone is administered without estrogen, in fact many patients with endometrial carcinomas are often obese and have elevated levels of endogenous estrogen due to the activity of aromatase in adipocytes (32, 34). Thus in many patients progesterone is acting in a high estrogenic hormonal milieu similar to that used in our experiments. Differences in the hormonal milieu of endometrial cancer patients may account for the wide variation in time required to achieve therapeutic responses (33).
Stromal deletion of progesterone receptor is sufficient to convert a hormone sensitive tumor to a hormone refractory cancer
Despite its efficacy, the mechanisms of progesterone action and signaling in endometrial tumors have not been systematically investigated. Progesterone is the agonist for the progesterone receptor (35). PR is expressed in the normal endometrial epithelium and stroma. With progression to cancer expression of PR can become deregulated and lost in either cellular compartment (36). Given that PR is the primary target for progesterone, its expression has been examined as a predictor of response to hormonal therapy in endometrial cancer. In most studies expression of PR correlated with better response to hormonal therapy, but some PR negative tumors also respond to progesterone (37, 38). A problem in these studies is that PR was examined in tumor lysates or histologic sections without carefully assessing its epithelial and stromal expression (37, 38). It is unclear whether epithelial or stromal PR signaling mediates effects of progesterone therapy. We observed that PTENKO tumors became progesterone resistant with diminution of stromal PR (Fig. 3Bl and Fig. 3Cc). We hypothesized that progesterone therapeutic effects in endometrial cancer are mediated through PR signaling in the stroma of the tumor microenvironment.
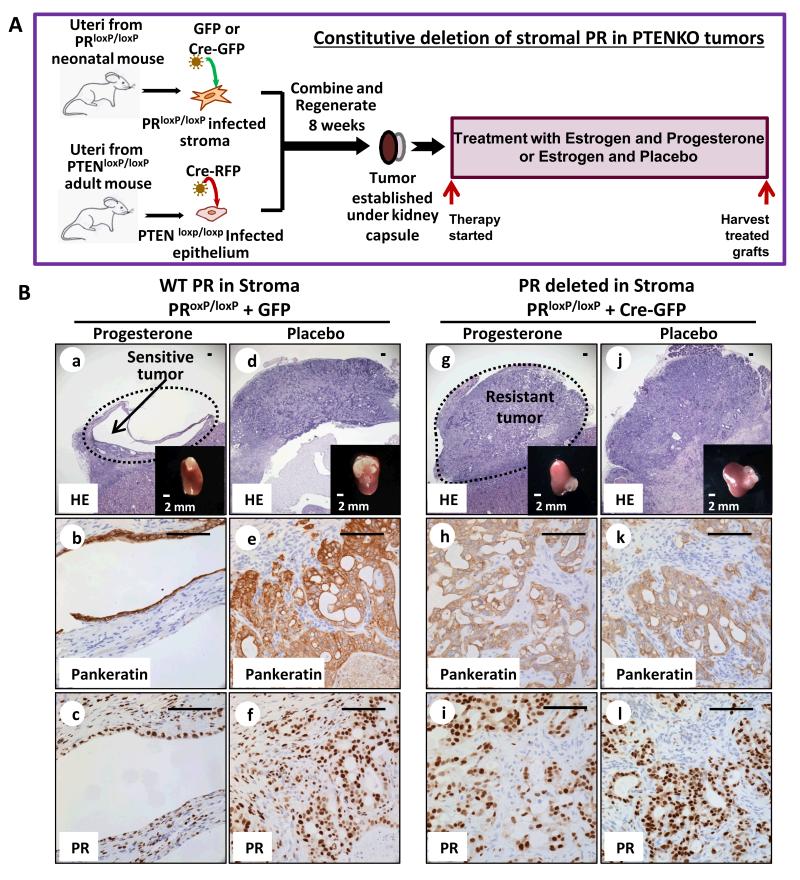
To address this question, PR was selectively deleted in the stroma of PTENKO endometrial tumors. Neonatal PRloxP/loxP (39) endometrial stromal cells were infected with Cre-expressing or control lentivirus marked with green fluorescent protein (GFP) and cultured short-term (Fig. 4A). Expression of Cre in these cells resulted in loss of PR-A and PR-B (Supplementary Fig. S4A). Combinations of FACS isolated PR deleted (Cre-GFP) or PR WT (GFP) stroma with PTENKO epithelia were implanted in mice supplemented with estrogen throughout the course of this experiment (Fig. 4A & Supplementary Fig. S4B). After tumors were established, mice were treated with progesterone or placebo (Fig. 4A). In tumors with stroma expressing WT PR (GFP infected stroma), resolution of tumor was detected after progesterone treatment (Fig. 4B a&b) while tumors persisted in placebo treated controls (Fig. 4B d&e). A significant drop in proliferation index was noted concomitant with tumor resolution with progesterone therapy (Supplementary Fig. S4C). Abundant expression of stromal PR was detected in this cohort (Fig. 4B c&f). When PR was deleted in the stroma of PTENKO tumors (Cre-GFP infected stroma), progesterone treated tumors persisted and resembled placebo treated counterparts (Fig. 4B g&h vs. j&k). The proliferation index of epithelia in progesterone and placebo treated PTENKO PR deleted tumors was equivalent (Supplementary Fig. S4C). Loss of PR in the stroma of these tumors was confirmed by immunohistochemistry (Fig. 4B i&l). Our results show that one genetic change, deletion of stromal PR, was sufficient to convert hormone sensitive PTENKO tumors to hormone refractory endometrial cancers (Fig. 4B). Conversely, epithelial specific deletion of PR in PTENKO tumors did not impact their response to progesterone therapy (Supplementary Fig. S4D).
Figure 4. Progesterone therapeutic effects are mediated through the stromal progesterone receptor.
(A) Strategy for deletion of PR in the stroma of PTENKO tumors. (B) Stromal loss of PR induced progesterone resistance. PTEN null endometrial tumors with stromal PR expression regressed with progesterone therapy (n=6) (a-c) but persisted in the placebo treated group (n=6) (d-f). Conversely, when stromal PR was deleted, PTENKO endometrial tumors did not respond to progesterone (n=8) (g-i) and the histology was similar to placebo treated controls (n=8) (j-l). Cre-GFP induced loss of stromal PR was confirmed (i&l vs. c&f). Scale bars are 100 μm except where noted.
These results demonstrate that signaling through stromal PR is essential for mediating anti-tumor effects of hormonal therapy in endometrial cancer. In developmental tissue recombination studies stromal PR signaling decreased estrogen mediated DNA synthesis in endometrial epithelia (40). During pregnancy, signaling through stromal PR inhibits endometrial epithelial proliferation to support fetal implantation (41). Our results demonstrate that signaling through stromal PR decreases epithelial tumor proliferation and induces apoptotic cell death in endometrial tumor tissue. We suspect that mediators of the anti-tumor effects of progesterone signaling from stroma are paracrine secreted growth factors.
Endometrial tumors driven by concomitant loss of PTEN and Kras activation are refractory to progesterone therapy
Epithelial Pten loss as a single genetic change was a positive predictor of response to hormonal therapy in our model. Activation of KRAS is a common mutation co-existing with PTEN loss in 21% of endometrial cancers (23). We asked if the addition of KRAS activation to PTENKO tumors would alter the susceptibility of resulting endometrial cancers to hormonal therapy.
Endometrial epithelia from PtenloxP/loxP KrasLSL-G12D/+ mice were infected with Cre-RFP resulting in loss of PTEN with activation of KRAS (PTENKO/Kras) (Fig. 5A). These cells were combined with WT stroma and placed in the in vivo regeneration model (Fig. 5A). Within six weeks, combinations of PTENKO/Kras epithelium and WT stroma gave rise to endometrioid endometrial tumors that invaded into the renal parenchyma (Supplementary Fig. S5A&B). Mice harboring endometrial tumors were treated with progesterone or placebo (Fig. 5A). Unlike progesterone sensitive PTENKO tumors, no response to hormonal therapy was observed in PTENKO/Kras tumors (Fig. 5B vs. Fig. 1B). These tumors persisted on the kidney, did not resolve despite co-administration of progesterone and estrogen and appeared similar to placebo treated controls (Fig. 5B a&b vs. c&d). Histology (Fig. 5C a vs. b and Supplementary Fig. S5C) and proliferation indices (Fig. 5D) were equivalent in progesterone and placebo treated PTENKO/Kras tumors. Loss of PTEN (Fig. 5C c&d) and activation of the RAS pathway (Supplementary Fig. S5D) was confirmed in tumor epithelia. Obvious differences in the epithelial vs. stromal distribution of ERα and PR (Fig. 5C e-h) were not detected in progesterone or placebo treated cohorts. Importantly, almost all PTENKO/Kras tumor stroma appeared PR negative in both hormonal conditions while abundant expression of epithelial PR was detected (Fig. 5C g&h).
Figure 5. Activation of Kras concomitant with PTEN loss (PTENKO/Kras) caused progesterone resistance.
(A) Schema for generation and hormonal therapy of PTENKO/Kras tumors (B) Despite co-administration of estrogen and progesterone PTENKO/Kras tumors did not resolve (n=8) (a&b) and were similar to placebo treated counterparts (n=8) (c&d). (C) No obvious differences in histology was detected with progesterone treatment (a vs. b). PTEN was absent in tumor epithelia (c&d). The hormone receptor expression was similar between progesterone and placebo treated tumors (e&g vs. f&h). (D) A high proliferation index was detected in tumors despite hormonal therapy (a vs. b). Scale bars are equal to 2 mm in B, and 100 μm in C&D.
Activation of KRAS concomitant with cell autonomous loss of PTEN resulted in hormone refractory endometrial cancers. Detection of KRAS and PTEN mutations are feasible with current clinical technologies. Given our findings, the status of both these genes should be tested in cohorts of endometrial cancer patients as potential biomarkers for assessing response to progesterone therapy.
The expression of progesterone receptor is diminished by methylation in the stroma of PTENKO/Kras progesterone resistant tumors
Our data demonstrates that stromal PR signaling is essential in hormone mediated resolution of PTENKO endometrial tumors (Fig. 4). Activation of KRAS in conjunction with PTEN loss resulted in hormone refractory cancers containing predominantly PR negative stroma (Fig. 5C g&h). We hypothesized that stromal PR protein levels would be decreased in PTENKO/Kras tumors as a result of epigenetic modification.
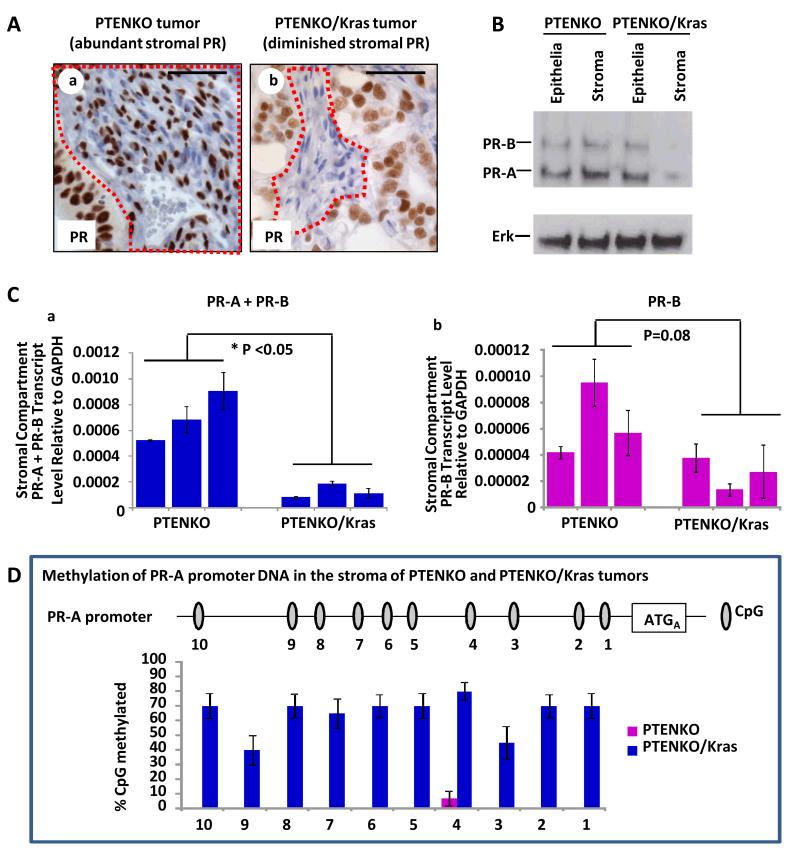
PR expression in the epithelium and stroma of PTENKO and PTENKO/Kras tumors was examined. Based on immunohistochemistry, much lower levels of PR were detected in the stroma but not epithelia of PTENKO/Kras compared to PTENKO tumors (Fig. 6A). Next, differences in epithelial and stromal PR protein and transcript were quantified in these carcinomas. Tumor stroma was color marked via infection with GFP expressing lentivirus (Supplementary Fig. S6A) while tumor epithelia were marked with RFP during Cre lentiviral infection. PTENKO and PTENKO/Kras tumors were harvested, dissociated into single cells and sorted by FACS (Supplementary Fig. S6A). Clear RFP (epithelial) and GFP (stromal) cellular populations could be visualized (Supplementary Fig. S6A). A western blot of isolated epithelia and stroma confirmed a significant decrease only in the stromal PR expression of PTENKO/Kras progesterone refractory tumors (Fig. 6B and Supplementary Fig. S6B).
Figure 6. Stromal PR is diminished in PTENKO/Kras progesterone resistant tumors by methylation.
(A) Abundant PR was detected in the epithelium and stroma of PTENKO tumors (a). Diminution in stromal PR was detected in PTENKO/Kras tumors (b). (B) Western blot confirmed a decrease in stromal PR of PTENKO/Kras tumors. ERK was a loading control. (C) Q-PCR revealed a significant decrease in PR-A+ PR-B transcript in tumor stroma of PTENKO/Kras compared to PTENKO tumors (a). A non-significant decrease in levels of PR-B transcript was detected (b). (D) Schematic of the PR-A promoter with CpG islands is shown. Based on bisulfite sequencing, a higher percentage of the PR-A promoter CpG islands were methylated in tumor stroma of PTENKO/Kras compared to PTENKO tumors. Three independent tumors for each group were analyzed. Scale bars equal 50 μm.
A potential mechanism for regulation of PR expression is epigenetic and through DNA methylation (42). Methylation of the PR gene (Pgr) has been reported in human endometrial cancer but was not specifically examined in stromal or epithelial compartments (43). Given these previous findings we first examined PR transcript levels in the stroma of PTENKO/Kras compared to PTENKO tumors. There are two PR isoforms, PR-A and PR-B (44). PR-A is the dominant form of PR in the endometrium (45). Both transcripts overlap such that all nucleotides in PR-A are shared with PR-B (44) (Supplementary Fig. S6C), consequently PR-A transcripts cannot be measured directly. Message levels of PR-A+PR-B or PR-B alone were determined in stroma and epithelia isolated from progesterone sensitive and refractory tumors (Fig. 6C and Supplementary Fig. S6C). Decreased transcript levels of PR-A+PR-B were detected in stroma but not the epithelium of PTENKO/Kras tumors compared to their hormone sensitive PTENKO counterparts (Fig. 6Ca and Supplementary Fig. S6C). Decreased PR-B transcript levels were also observed in PTENKO/Kras tumor stroma, but this was not statistically significant (Fig. 6Cb). Results suggest that transcriptional modulation of PR primarily occurred through repression of PR-A. Methylation specific PCR was performed to examine DNA methylation in the PR-A promoter (Supplementary Fig. S6D). A greater proportion of methylated PR-A DNA was detected in the stroma of PTENKO/Kras tumors (Supplementary Fig. S6E). Bisulfite sequencing confirmed methylation of DNA at the PR-A promoter. Minimal methylation of the PR-A promoter was detected in PTENKO tumor stroma, while the majority of PR-A promoter CpG islands were methylated in the stroma of PTENKO/Kras tumors (Fig. 6D). These findings confirm that DNA methylation of PR-A decreases transcription and thus expression of PR in the stroma of progesterone refractory PTENKO/Kras tumors.
In this model, activation of KRAS with PTEN-loss in tumor epithelium may lead to the secretion of paracrine factors that modulate the epigenetic landscape of surrounding tumor stroma. This signaling cascade from epithelium to stroma could trigger a series of events ultimately resulting in methylation of the PR gene. Methylation of PR in hormone refractory endometrial tumors may be among one of the mechanisms causing repression of PR transcription and expression in tumor stroma.
Over-expression of exogenous PR in tumor stroma sensitized PTENKO/Kras tumors to hormonal therapy
Progesterone resistant PTENKO/Kras tumors had decreased expression of stromal PR due to silencing of the PR gene in the tumor microenvironment. We hypothesized that loss of stromal PR is a key mechanism leading to hormone resistance in endometrial tumors. If this hypothesis is correct, re-expression of PR in the tumor stroma would be sufficient to sensitize PTENKO/Kras tumors to hormonal therapy.
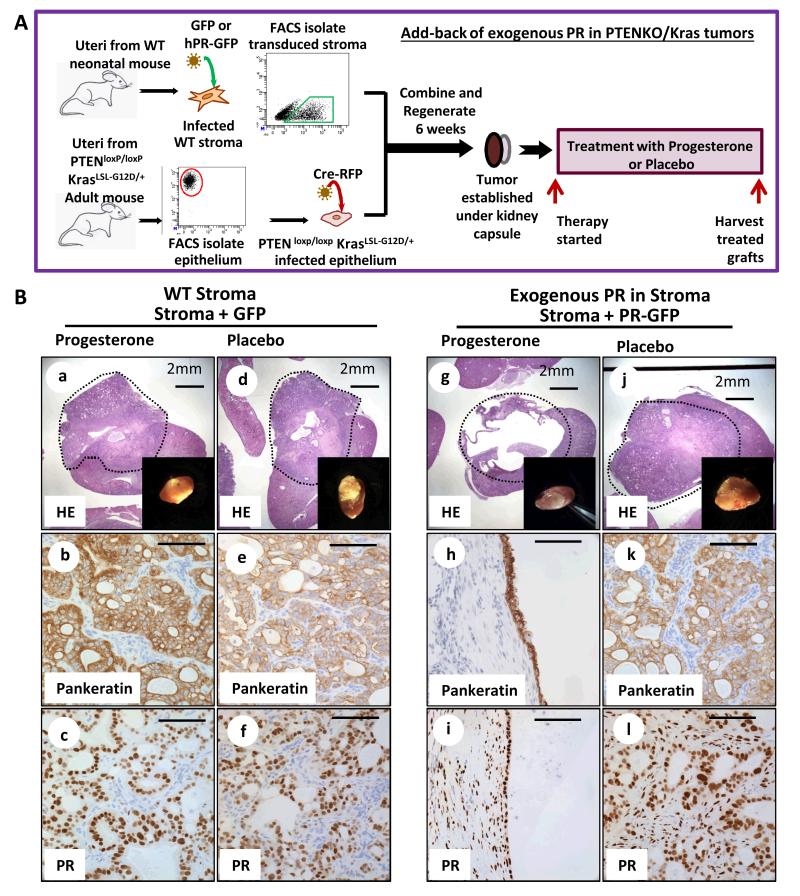
To address this question the human progesterone receptor (hPR) was cloned into a GFP expressing lentiviral vector (Supplementary Fig. S7Aa). Wild type neonatal stroma was infected with hPR-GFP or GFP control lentivirus (Fig. 7A). Over-expression of PR-A and PR-B was confirmed (Supplementary Fig. S7A b&c). Stroma expressing GFP or hPR-GFP was FACS isolated, combined with Cre-RFP infected PtenloxP/loxP KrasLSL-G12D/+ epithelia and regenerated in vivo (Fig. 7A). This resulted in activation of KRAS and PTEN-loss in tumor epithelium with or without over-expression of human PR in the stroma. After 6 weeks, tumor histology was similar in pre-therapy PTENKO/Kras cancers regardless of PR over-expression in the stroma (Fig. S7B). Mice were treated with estrogen plus progesterone co-therapy (progesterone) or estrogen plus placebo (placebo) (Fig. 7A). Tumors with WT GFP stroma were large and persisted in both progesterone and placebo treated cohorts (Fig. 7B a&b vs. d&e). Stromal PR was significantly diminished in these tumors (Fig. 7B c&f). In contrast, when exogenous hPR was expressed in tumor stroma the majority of PTENKO/Kras tumor tissue resolved with progesterone administration (Fig. 7B g&h and Supplementary Fig. S7D a&b). This effect was mediated by progesterone signaling as placebo treated tumors expressing stromal hPR persisted (Fig. 7B j&k and Supplementary Fig. S7D c&d). Unlike PTENKO/Kras with WT-GFP stroma, tumors with hPR-GFP stroma had abundant expression of PR in the tumor microenvironment (Fig. 7B c&f vs. i&l). Deletion of PTEN and RAS pathway activation was confirmed in tumor epithelia (Supplementary Fig. S7C).
Figure 7. Expression of exogenous progesterone receptor in the stroma of PTENKO/Kras tumors shifted their biologic behavior from hormone refractory to hormone sensitive cancers.
(A) Experimental strategy for over-expression of PR in the stroma of PTENKO/Kras tumors. (B) Over expression of human PR in the stroma of PTENKO/Kras tumors facilitated tumor resolution in response to progesterone therapy. PTENKO/Kras tumors expressing GFP in the stroma were resistant to progesterone therapy (n=2) (a-c) and resembled placebo treated counterparts (n=2) (d-f). Tumors over-expressing stromal PR responded to progesterone (n=3) evidenced by formation of cysts attached to the kidney (g-i) compared to placebo (j-l) treated controls (n=3). Scale bars equal 100μm except where noted.
In PTENKO tumors we demonstrated that expression of stromal PR was essential for progesterone mediated resolution of the carcinoma (Fig. 4). Here we demonstrate that re-expression of PR in the tumor stroma is sufficient to sensitize the hormone refractory PTENKO/Kras endometrial tumors to progesterone therapy (Fig. 7). Collectively our findings demonstrate that signaling through stromal PR is sufficient and necessary for mediating anti-tumor effects of progesterone therapy in endometrial cancer.
Discussion
The prevailing dogma in clinical practice assumes that progesterone exerts its therapeutic effects through tumor epithelia, which are the more abundant cell type in endometrial carcinomas. Studies have attempted to assess PR expression as a predicative biomarker for response to progesterone therapy with limited success (37, 38). Here we demonstrate that progesterone hormonal therapy works by paracrine signaling through PR in the endometrial tumor microenvironment. Loss of stromal PR in PTENKO hormone sensitive tumors induced progesterone resistance. Activation of KRAS concomitant with PTEN-loss initiated progesterone resistant endometrial tumors that had measurable loss of stromal PR. Add-back of exogenous PR in the stroma of hormone refractory tumors was sufficient to induce sensitivity to progesterone therapy. For the first time we demonstrate that signaling through the stromal PR axis is sufficient and necessary for successful resolution of endometrial tumors with progesterone.
Cross-talk between epithelium and stroma is essential for neoplastic transformation in many hormonally regulated tissues (18, 20, 46, 47). One way this cross-talk occurs is through reciprocal signals regulating hormone receptor expression or activity in each cellular compartment. Previously we demonstrated that cancer initiating fibroblast growth factor signals originating from stroma increased androgen receptor levels in prostate tumor epithelia, resulting in androgen-independent growth (20). Here we show that oncogenic insults in endometrial tumor epithelia decreased levels of PR in tumor stroma inducing de novo progesterone resistance. Epigenetic modification of the tumor microenvironment has emerged as a potential regulator of tumor initiation and propagation in breast (48) and prostate (18) cancers. Our results show that epigenetic regulation of tumor stroma can induce drug resistance. Defining mechanisms and site of origin for innate or acquired resistance to hormonal therapy in human endometrial cancer trials will have immense translational application.
We find that endometrial tumors resulting solely from epithelial PTEN loss are highly sensitive to progesterone anti-tumor effects. These results contradict findings from a Pten+/- transgenic model (29), but there are two major differences in the studies. In our model, Pten is deleted only in tumor epithelia emulating the epithelial-specific loss of PTEN seen in human endometrial cancers. Conversely, in Pten+/- transgenic mice Pten is deleted in tumor epithelia and stroma. In both models, mice are oophorectomized and treated with progesterone. But in our study, estrogen is co-administered with progesterone resulting in successful hormonal therapy of PTEN-null tumors. The discrepancy in progesterone sensitivity in these two models could be explained by stromal Pten status and the absence or presence of estrogen during treatment.
Although loss of PTEN function is the most common genetic change in endometrial cancer, in 21% of cases it occurs in conjunction with KRAS activation (23). Despite the prevalence of this co-existing genetic alteration, previous studies have not assessed the sensitivity of endometrial tumors resulting from PTEN-loss and KRAS activation to hormonal therapy. When epithelial activation of KRAS was coupled with PTEN loss, stromal progesterone receptor expression was drastically reduced resulting in progesterone resistance. In another Pten/Kras transgenic endometrial cancer model (30), decreased expression of PR was reported. However, this study did not assess sensitivity of these tumors to progesterone or examine PR distribution in tumor epithelium or stroma. The lack of response to progesterone therapy in PTENKO/Kras tumors in our model may explain why many PTEN-null endometrial cancers in clinical series are refractory to progesterone therapy (27, 28). Co-existence of other genetic changes, such as activation of KRAS, in these tumors may induce progesterone resistance. In a mouse pancreatic tumor model epithelial specific activation of KRAS could alter the tumor microenvironment by non-cell autonomous mechanisms (49). Similarly, we suspect that activation of KRAS in PTEN-null tumor epithelia results in secretion of paracrine factors that can modulate the endometrial tumor microenvironment ultimately silencing PR expression.
This study reveals three imminently testable biomarkers for progesterone sensitivity to be assessed in future clinical trials. These biomarkers include PTEN and KRAS status in the tumor epithelia and expression of PR in tumor stroma. Molecular analysis of tumors with reliable biomarkers of response to hormonal therapy can individualize treatment options for the 49,000 U.S. women diagnosed with endometrial cancer annually (4). Stromal loss of PR through epigenetic silencing was a key mechanism inducing progesterone resistance in our model. Importantly, stromal re-expression of PR in hormone refractory cancers sensitized these tumors to progesterone therapeutic effects. This finding may have critical clinical implications as it demonstrates that modulation of the tumor microenvironment can reverse hormone resistance in endometrial tumors. In future work we will test if stromal specific delivery of DNA methyltransferase inhibitors may be an effective way to re-sensitize hormone refractory endometrial cancers to progesterone therapy.
Supplementary Material
Acknowledgements
We thank Drs. Steve Jacobson, Jiaoti Huang and Jianyu Rao for technical advice. SM is supported by a VA CDA-2 Award, Scholars in Translational Medicine Program, Mary Kay Award, the CDU/UCLA NIH/NCI Grant #U54-CA-143931 award, a Sidney Kimmel Foundation award, the UCLA Cancer Research Coordinating Committee grant and the Concern foundation.
References
- 1.Puhalla S, Bhattacharya S, Davidson NE. Hormonal therapy in breast cancer: a model disease for the personalization of cancer care. Molecular oncology. 2012;6:222–36. doi: 10.1016/j.molonc.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabriel EM, Jatoi I. Breast cancer chemoprevention. Expert review of anticancer therapy. 2012;12:223–8. doi: 10.1586/era.11.206. [DOI] [PubMed] [Google Scholar]
- 3.Hammerer P, Madersbacher S. Landmarks in hormonal therapy for prostate cancer. BJU international. 2012;110(Suppl 1):23–9. doi: 10.1111/j.1464-410X.2012.11431.x. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 5.Azueta A, Gatius S, Matias-Guiu X. Endometrioid carcinoma of the endometrium: pathologic and molecular features. Semin Diagn Pathol. 2010;27:226–40. doi: 10.1053/j.semdp.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol. 2006;24:4783–91. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- 7.Acken HS., Jr. Estrogen replacement therapy. Obstetrics and gynecology. 1969;34:46–9. [PubMed] [Google Scholar]
- 8.Martin L, Finn CA, Trinder G. Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. The Journal of endocrinology. 1973;56:133–44. doi: 10.1677/joe.0.0560133. [DOI] [PubMed] [Google Scholar]
- 9.Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293:1164–7. doi: 10.1056/NEJM197512042932302. [DOI] [PubMed] [Google Scholar]
- 10.Charles D. Iatrogenic Endometrial Patterns. J Clin Pathol. 1964;17:205–12. doi: 10.1136/jcp.17.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Seminars in reproductive medicine. 2010;28:81–90. doi: 10.1055/s-0029-1242998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decruze SB, Green JA. Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. Int J Gynecol Cancer. 2007;17:964–78. doi: 10.1111/j.1525-1438.2007.00897.x. [DOI] [PubMed] [Google Scholar]
- 13.Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012;379:1352–60. doi: 10.1016/S0140-6736(12)60442-5. [DOI] [PubMed] [Google Scholar]
- 14.Rauh-Hain JA, Del Carmen MG. Treatment for advanced and recurrent endometrial carcinoma: combined modalities. Oncologist. 2010;15:852–61. doi: 10.1634/theoncologist.2010-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–34. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- 16.Franco HL, Rubel CA, Large MJ, Wetendorf M, Fernandez-Valdivia R, Jeong JW, et al. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012;26:1218–27. doi: 10.1096/fj.11-193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Memarzadeh S, Zong Y, Janzen DM, Goldstein AS, Cheng D, Kurita T, et al. Cell-autonomous activation of the PI3-kinase pathway initiates endometrial cancer from adult uterine epithelium. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1012548107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zong Y, Huang J, Sankarasharma D, Morikawa T, Fukayama M, Epstein JI, et al. Stromal epigenetic dysregulation is sufficient to initiate mouse prostate cancer via paracrine Wnt signaling. Proc Natl Acad Sci U S A. 2012;109:E3395–404. doi: 10.1073/pnas.1217982109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xin L, Teitell MA, Lawson DA, Kwon A, Mellinghoff IK, Witte ON. Progression of prostate cancer by synergy of AKT with genotropic and nongenotropic actions of the androgen receptor. Proc Natl Acad Sci U S A. 2006;103:7789–94. doi: 10.1073/pnas.0602567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Memarzadeh S, Xin L, Mulholland DJ, Mansukhani A, Wu H, Teitell MA, et al. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell. 2007;12:572–85. doi: 10.1016/j.ccr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janzen DM, Cheng D, Schafenacker AM, Paik DY, Goldstein AS, Witte ON, Jaroszewicz A, et al. Estrogen and Progesterone Together Expand Murine Endometrial Epithelial Progenitor Cells. Stem Cells. 2012;31:808–22. doi: 10.1002/stem.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wargon V, Fernandez SV, Goin M, Giulianelli S, Russo J, Lanari C. Hypermethylation of the progesterone receptor A in constitutive antiprogestin-resistant mouse mammary carcinomas. Breast Cancer Res Treat. 2011;126:319–32. doi: 10.1007/s10549-010-0908-x. [DOI] [PubMed] [Google Scholar]
- 23.Rudd ML, Price JC, Fogoros S, Godwin AK, Sgroi DC, Merino MJ, et al. A unique spectrum of somatic PIK3CA (p110alpha) mutations within primary endometrial carcinomas. Clin Cancer Res. 2011;17:1331–40. doi: 10.1158/1078-0432.CCR-10-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–30. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 25.Orbo A, Rise CE, Mutter GL. Regression of latent endometrial precancers by progestin infiltrated intrauterine device. Cancer Res. 2006;66:5613–7. doi: 10.1158/0008-5472.CAN-05-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng W, Baker HE, Mutter GL. Involution of PTEN-null endometrial glands with progestin therapy. Gynecol Oncol. 2004;92:1008–13. doi: 10.1016/j.ygyno.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Minaguchi T, Nakagawa S, Takazawa Y, Nei T, Horie K, Fujiwara T, et al. Combined phospho-Akt and PTEN expressions associated with post-treatment hysterectomy after conservative progestin therapy in complex atypical hyperplasia and stage Ia, G1 adenocarcinoma of the endometrium. Cancer letters. 2007;248:112–22. doi: 10.1016/j.canlet.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Milam MR, Soliman PT, Chung LH, Schmeler KM, Bassett RL, Jr., Broaddus RR, et al. Loss of phosphatase and tensin homologue deleted on chromosome 10 and phosphorylation of mammalian target of rapamycin are associated with progesterone refractory endometrial hyperplasia. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2008;18:146–51. doi: 10.1111/j.1525-1438.2007.00958.x. [DOI] [PubMed] [Google Scholar]
- 29.Fyles A, Wood G, Li M, Manoukian AS, Gowing K, Khokha R, et al. Neither ovariectomy nor progestin treatment prevents endometrial neoplasia in pten+/− mice. Gynecol Oncol. 2008;108:395–401. doi: 10.1016/j.ygyno.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 30.Kim TH, Wang J, Lee KY, Franco HL, Broaddus RR, Lydon JP, et al. The Synergistic Effect of Conditional Pten Loss and Oncogenic K-ras Mutation on Endometrial Cancer Development Occurs via Decreased Progesterone Receptor Action. J Oncol. 2010:139087. doi: 10.1155/2010/139087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joshi A, Ellenson LH. Adenovirus mediated homozygous endometrial epithelial Pten deletion results in aggressive endometrial carcinoma. Exp Cell Res. 2011;317:1580–9. doi: 10.1016/j.yexcr.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cramer DW. The epidemiology of endometrial and ovarian cancer. Hematol Oncol Clin North Am. 2012;26:1–12. doi: 10.1016/j.hoc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheeler DT, Bristow RE, Kurman RJ. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am J Surg Pathol. 2007;31:988–98. doi: 10.1097/PAS.0b013e31802d68ce. [DOI] [PubMed] [Google Scholar]
- 34.Cleland WH, Mendelson CR, Simpson ER. Effects of aging and obesity on aromatase activity of human adipose cells. J Clin Endocrinol Metab. 1985;60:174–7. doi: 10.1210/jcem-60-1-174. [DOI] [PubMed] [Google Scholar]
- 35.Schrader WT, O’Malley BW. Progesterone-binding components of chick oviduct. IV. Characterization of purified subunits. The Journal of biological chemistry. 1972;247:51–9. [PubMed] [Google Scholar]
- 36.Snijders MP, de Goeij AF, Koudstaal J, Thunnissen EB, de Haan J, Bosman FT. Oestrogen and progesterone receptor immunocytochemistry in human hyperplastic and neoplastic endometrium. J Pathol. 1992;166:171–7. doi: 10.1002/path.1711660214. [DOI] [PubMed] [Google Scholar]
- 37.Markman M. Hormonal therapy of endometrial cancer. Eur J Cancer. 2005;41:673–5. doi: 10.1016/j.ejca.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Thigpen JT, Brady MF, Alvarez RD, Adelson MD, Homesley HD, Manetta A, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. J Clin Oncol. 1999;17:1736–44. doi: 10.1200/JCO.1999.17.6.1736. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto-Partyka MK, Lydon JP, Iruela-Arispe ML. Generation of a mouse for conditional excision of progesterone receptor. Genesis. 2006;44:391–5. doi: 10.1002/dvg.20227. [DOI] [PubMed] [Google Scholar]
- 40.Kurita T, Young P, Brody JR, Lydon JP, O’Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 1998;139:4708–13. doi: 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331:912–6. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lapidus RG, Ferguson AT, Ottaviano YL, Parl FF, Smith HS, Weitzman SA, et al. Methylation of estrogen and progesterone receptor gene 5′ CpG islands correlates with lack of estrogen and progesterone receptor gene expression in breast tumors. Clin Cancer Res. 1996;2:805–10. [PubMed] [Google Scholar]
- 43.Sasaki M, Dharia A, Oh BR, Tanaka Y, Fujimoto S, Dahiya R. Progesterone receptor B gene inactivation and CpG hypermethylation in human uterine endometrial cancer. Cancer Res. 2001;61:97–102. [PubMed] [Google Scholar]
- 44.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–14. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O’Malley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–55. doi: 10.1210/rp.57.1.339. [DOI] [PubMed] [Google Scholar]
- 46.Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–71. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanwar PS, Kaneko-Tarui T, Zhang L, Tanaka Y, Crum CP, Teixeira JM. Stromal liver kinase B1 [STK11] signaling loss induces oviductal adenomas and endometrial cancer by activating mammalian Target of Rapamycin Complex 1. PLoS genetics. 2012;8:e1002906. doi: 10.1371/journal.pgen.1002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu M, Yao J, Cai L, Bachman KE, van den Brule F, Velculescu V, et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nature genetics. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 49.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–70. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.