Abstract
Background
Triiodothyronine (T3) has many effects on the heart, and marked changes in cardiac function and structure occur in patients with (subclinical) thyroid disease. We investigated whether between-subject variation in thyroid hormone levels within the euthyroid range is also associated with heart rate and echocardiographic heart function and structure.
Methods
Subjects were selected from the Asklepios study (n=2524), a population-representative random sample of patients aged between 35 and 55 years, free from overt cardiovascular disease at baseline. Analyses were restricted to 2078 subjects (1013 women and 1065 men), not using antihypertensive or thyroid medication nor having antithyroperoxidase antibody levels above clinical cut-off or thyrotropin (TSH) levels outside the reference range. All subjects were phenotyped in-depth and underwent comprehensive echocardiography, including diastolic evaluation. Thyroid function parameters were determined by automated electrochemiluminescence.
Results
Heart rate was robustly positively associated with (quartiles of) free T3 (FT3) and T3, both in subjects with TSH levels within reference (0.27–4.2 μU/L) and in narrow TSH range (0.5–2.5 μU/L; p<0.0001). FT3 and T3 were negatively associated with left ventricular (LV) end-diastolic volume but positively associated with relative wall thickness. Total T3 (TT3) was associated with enhanced ventricular contraction (as assessed by tissue Doppler imaging). Free thyroxine, FT3, and TT3 were positively associated with late ventricular filling, and TT3 was associated with early ventricular filling.
Conclusion
We have demonstrated a strong positive association between thyroid hormone levels within the euthyroid range and heart rate, and more subtle effects on cardiac function and structure. More specifically, we suggest a smaller LV cavity size (with increased relative wall thickness), an enhanced atrial and ventricular contraction, and LV relaxation with higher circulating thyroid hormones. These results illustrate that variation in thyroid hormone levels, even within the reference range, exerts effects on the heart.
Introduction
Triiodothyronine (T3) is known to exert various actions on the cardiovascular system, involving cardiac contractility, electrophysiological function, and cardiac structure (1–3). With regard to these contractile effects, it has positive inotropic, chronotropic, and lusitropic effects, which means that it stimulates the rate and force of systolic contraction and the rate of diastolic relaxation (1,2).
Overt hyperthyroidism increases the resting heart rate, carries a risk of (atrial) arrhythmias, and augments cardiac contractility and output. When the duration of this hyperthyroidism is prolonged, the increased protein synthesis in cardiac myocytes can lead to concentric cardiac hypertrophy (1,2,4). A thyroid hormone deficit, in contrast, decreases cardiac contractility and delays diastolic relaxation (4,5). Moreover, structural changes such as asymmetric septal hypertrophy (6), cardiomegaly (2), and pericardial effusion (7) have been reported.
Subclinical (SC) thyroid disease has also been associated with altered cardiac function and structure. Studies in SC hypothyroidism have reported an impairment of both systolic and diastolic function, as well as a slightly higher left ventricular (LV) mass, although this mass was still within the reference range (8,9). Conversely, in SC hyperthyroidism, increases in LV systolic function have been observed (10,11). Whether this is also reflected in cardiac hypertrophy is still a matter of debate (10–14). Of note, two studies in patients with iatrogenic SC hyperthyroidism have shown diastolic LV dysfunction, which was (at least in part) reversible after restoration of a euthyroid state (14,15).
The heart is one of the most thyroid hormone-responsive tissues in the body, and is responsive to minimal but persistent changes in circulating thyroid hormone levels (3,16). Accordingly, it is plausible that variance in thyroid hormone levels even within the euthyroid range could influence heart rate, cardiac function, and structure. However, to our present knowledge, only two studies covering this topic exist: one smaller study performed in hypertensive subjects (17), and a larger study from the Framingham cohort, where subjects were categorized according to thyrotropin (TSH) levels but without determination of free thyroid hormone levels (11). The present study therefore investigated whether between-subjects variation in thyroid hormone levels within the euthyroid range affects heart rate, function, and structure in a population of healthy middle-aged men and women, free of overt cardiovascular disease.
Materials and Methods
Study population
The Asklepios study is an ongoing population study focused on the interplay of aging, cardiovascular hemodynamics, and the emergence of cardiovascular disease. Study design, inclusion and exclusion criteria, study components, and baseline characteristics of the population have been described in detail before (18). The cohort (n=2524; 50.5% women) is a population-representative random sample of the general population aged between 35 and 55 years, free from overt cardiovascular disease at baseline. The data of the present investigation were acquired during the first measuring round (2002–2004). The study was approved by the medical ethical committee of Ghent University Hospital, Belgium. All participants gave written informed consent prior to enrollment. Data collection and measurements have been described previously (18–22).
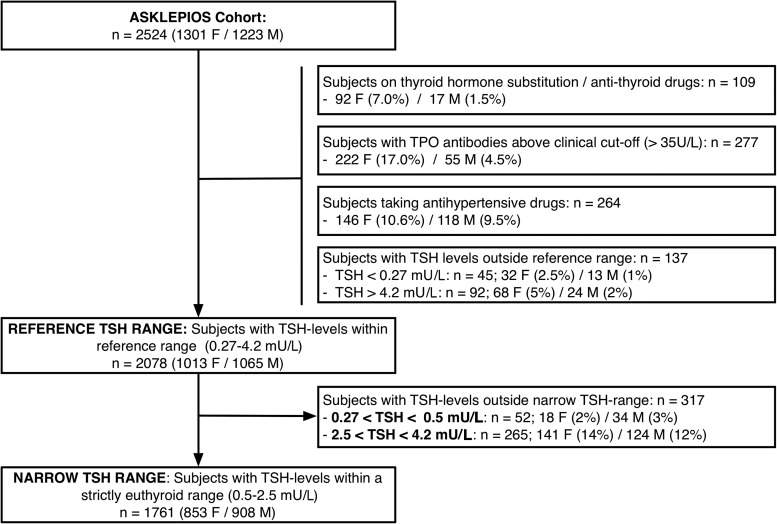
For subject selection in this paper, we refer to Figure 1. Individuals receiving thyroid hormone substitution or antithyroid drugs, having antithyroperoxidase antibodies (TPOAbs) above clinical cut-off, taking antihypertensive drugs, or with TSH levels outside the reference range of our laboratory (0.27–4.2 mU/L) were excluded from the analyses, leaving 2078 subjects (“reference TSH range”). As a sensitivity analysis, we performed some analyses only in subjects within a strictly euthyroid range (0.5–2.5 mU/L), leaving 1761 subjects (“narrow TSH range”).
FIG. 1.
Flowchart for inclusion and exclusion of the study population. TSH, thyrotropin.
Measurements
Measurements were (after 15 minutes of rest, informed consent, and review of questionnaire data in a temperature-controlled environment): (i) basic clinical data, (ii) blood sampling followed by a short rest, and (iii) and echocardiographic examination. Blood pressure was recorded using bilateral triplicate measurements (one-minute intervals) on a rested, sitting subject using a validated oscillometric Omron HEM-907 device (Omron Healthcare Co. Ltd., Kyoto, Japan). Blood-pressure values for these six readings were averaged, and the mean value was used throughout the study. A digital 12-lead (Schiller AT-104PC, Schiller AG, Baar, Switzerland) electrocardiogram (ECG) was recorded and reviewed by a physician. Heart rate was recorded eight times throughout the examination period. The mean value of these eight measurements was calculated and used for analyses throughout the study, except for analyses on cardiac function, where heart rate at the time of echocardiographic measurement was used.
The subjects underwent a resting echocardiographic examination (VIVID 7, GE Vingmed Ultrasound, Horten, Norway). Analysis of the ECG-gated cineloops or images was performed offline by a single, blinded, measurement-dedicated reader. All variables were measured on at least three cardiac cycles; reported measurements are averaged values. LV internal dimensions were measured at end diastole (LVEDD) and end systole (LVESD). Wall thickness (interventricular septal diameter [IVSD] and posterior wall thickness [PW]) were measured at end diastole according to the American Society of Echocardiography leading-edge convention (23,24). LVEDD was used to calculate LV mass by a necropsy-validated formula (25). Standard two-dimension volumetric methods were used to calculate ejection fraction (EF; Teichholz formula). Systolic (s′), early (e′) and late (a′) diastolic mitral annulus velocities were recorded by pulsed wave tissue Doppler imaging (TDI; sample volume located at the medial mitral annulus) (26). Isovolumic relaxation time (IVRT) was measured using continuous wave Doppler recordings as the interval from the closure spike of the aortic valve to onset of mitral flow. PW Doppler recordings of transmitral flow velocities were obtained between the tips of the mitral valve leaflets in the apical four-chamber view. Early (E) and late (A) diastolic components were recorded (18,27).
Biochemical determinations
Blood samples were obtained at different times during the day after six hours of fasting and refraining from smoking. All serum samples were stored at −80° until batch analysis. Thyroid hormone function tests (TSH, FT3, FT4, and TT3) and TPOAbs were determined using immuno-electrochemiluminescence (Roche reagents) on Cobas 411 (Roche Diagnostics GmbH, Mannheim, Germany). The intra- and interassay CV% were less than 10% for all measurements. The time of the day (morning, afternoon, early evening) did not influence free thyroid hormone levels. However, TSH levels were higher when blood was drawn in the afternoon or early evening (mean TSH levels 1.54, 1.67, 1.98 mU/L respectively; ANOVA p<0.001).
Statistical analysis
TSH, FT3, FT4, and TT3 were analyzed both as a continuous variable and using quartiles (quartiles TSH women: 0.27–1.08, 1.09–1.54, 1.55–2.08, 2.09–4.2 mU/L; quartiles TSH men: 0.27–1.03, 1.04–1.47, 1.48–1.99, 2.00–4.2 mU/L; quartiles FT3 women: <2.80, 2.81–3.00, 3.01–3.24, >3.24 pg/mL; quartiles FT3 men: <3.12, 3.13–3.36, 3.37–3.58, >3.59 pg/mL; quartiles FT4 women: <1.15, 1.16–1.25, 1.26–1.35, >1.36 ng/dL; quartiles FT4 men: <1.23, 1.24–1.34, 1.35–1.45, >1.46 ng/dL, quartiles TT3 women: <104.5, 105–119, 119.5–139, >140 ng/dL; quartiles TT3 men: <106, 106.5–117, 118–128, >129 ng/dL). All statistical analyses (linear regression analysis and one-way ANOVA) were performed using SPSS version 19 (SPSS Inc., Chicago, IL). Since men and women were analyzed together to increase the power, analyses were first adjusted for sex. Additional adjustment for age, height, weight, and current smoking was performed in model 1. In a second model, we further adjusted for heart rate and systolic blood pressure, except for LV structure where adjustment for heart rate and systolic blood pressure was done sequentially (model 2). For diastolic function, we additionally adjusted for LVEDD and relative wall thickness (model 3). We also checked for interactions between thyroid hormones and heart rate. Statistical significance was assumed for p-values less than 0.05. Data are given as mean±SD for normally distributed data or median (interquartile range) for skewed data, unless noted otherwise.
Results
Descriptives
Descriptives for the general characteristics, TSH and thyroid hormone levels, and echocardiographic measures of LV function and structure are shown in Table 1. Subjects on thyroid hormone substitution, antithyroid drugs, or antihypertensive medication, and with TPOAbs above clinical cut-off or with TSH levels outside the reference range were excluded from the present analysis, as stated in the methods section, leaving 2078 subjects (see Fig. 1).
Table 1.
Descriptives for the Study Population (n=2078)
| Variable | Women (n=1013) | Men (n=1065) |
|---|---|---|
| General characteristics | ||
| Age (years) | 45 [40–50] | 46 [41–51] |
| Height (cm) | 163±6 | 176±7 |
| Weight (kg) | 65.4±11.6 | 81.1±12.2 |
| BMI (kg/m2) | 24.5±4.2 | 26.2±3.6 |
| Smoking (active/ex/never; %) | 19/21/60 | 22/36/42 |
| TSH and thyroid hormones | ||
| TSH (mU/L) | 1.54 [1.08–2.08] | 1.47 [1.03–2.04] |
| FT4 (ng/dL) | 1.25 [1.15–1.35] | 1.34 [1.23–1.44] |
| FT3 (pg/mL) | 3.0 [2.8–3.2] | 3.4 [3.1–3.6] |
| TT3 (ng/dL) | 120 [105–140] | 118 [107–129] |
| Blood pressure and heart rate | ||
| Systolic BP (mm Hg) | 121±13 | 130±12 |
| Diastolic BP (mm Hg) | 77±9 | 82±9 |
| Heart rate (average; bpm) | 68±9 | 64±10 |
| Echocardiographic parameters | ||
| Systolic function | ||
| Ejection fraction (%) | 67±8 | 65±8 |
| s′ (mm/s) | 80±11 | 79±12 |
| LVESD (mm) | 28±4 | 32±4 |
| Diastolic function | ||
| E (cm/s) | 79±14 | 71±13 |
| A (cm/s) | 62±11 | 59±10 |
| e′ (mm/s) | 96±21 | 87±18 |
| a′ (mm/s) | 86±15 | 92±15 |
| LV structure | ||
| LV mass (g) | 121±29 | 177±39 |
| LVMI (g/m2) | 71±14 | 90±18 |
| LVEDD (mm) | 45±4 | 49±4 |
| Relative wall thickness | 0.36±0.06 | 0.39±0.07 |
Subjects on thyroid or antihypertensive medication, with antithyroperoxidase antibodies above clinical cut-off, and with TSH levels outside the reference range were excluded from further analyses. Data are mean±SD or medians [first, third quartile] in case of non-Gaussian distribution. Conversion factor for FT3 from pg/mL to pmol/L and for TT3 from ng/dL to nmol/L is ×0.0154; conversion factor for FT4 from ng/dL to pmol/L is ×12.87.
T3, triiodothyronine; TSH, thyrotropin; FT4, free thyroxine; FT3, free T3; TT3, total T3; LVESD, left ventricular end-systolic diameter; s′, systolic mitral annulus velocity; E, early diastolic transmitral flow velocity; A, late diastolic transmitral flow velocity; e′, pulsed-wave tissue Doppler early diastolic component; a′, pulsed-wave tissue Doppler late diastolic component; LVMI, left ventricular mass index; LVEDD, left ventricular end-diastolic diameter.
Men have higher FT3 and FT4 levels (p<0.0001), lower T3 levels (p<0.0001), and slightly lower TSH levels (nonsignificant, p=0.08) compared to women. In women, TT3 and FT3 were positively related to the use of oral contraceptive therapy (β=0.6, p<0.0001; β=0.15, p<0.0001 respectively).
Mean heart rate levels were on average 4±0.4 beats per minute lower in men than women (p<0.0001) and comparable with mean heart-rate levels in the EPIC-Norfolk study (28). Current smokers have higher levels of FT3 (mean difference 0.18±0.03; p<0.001), TT3 (mean difference 0.10±0.01, p<0.001), and FT4 (mean difference 0.03±0.01; p=0.006), but no differences in TSH levels were detected. Therefore, smoking status was taken into account in the adjustment.
Systolic blood pressure was positively associated with FT3 (β=0.12, p<0.001), TT3 (β=0.13, p<0.001), and TSH (β=0.09, p<0.001), but not with FT4 (p=0.8).
Associations between thyroid hormone status and heart rate
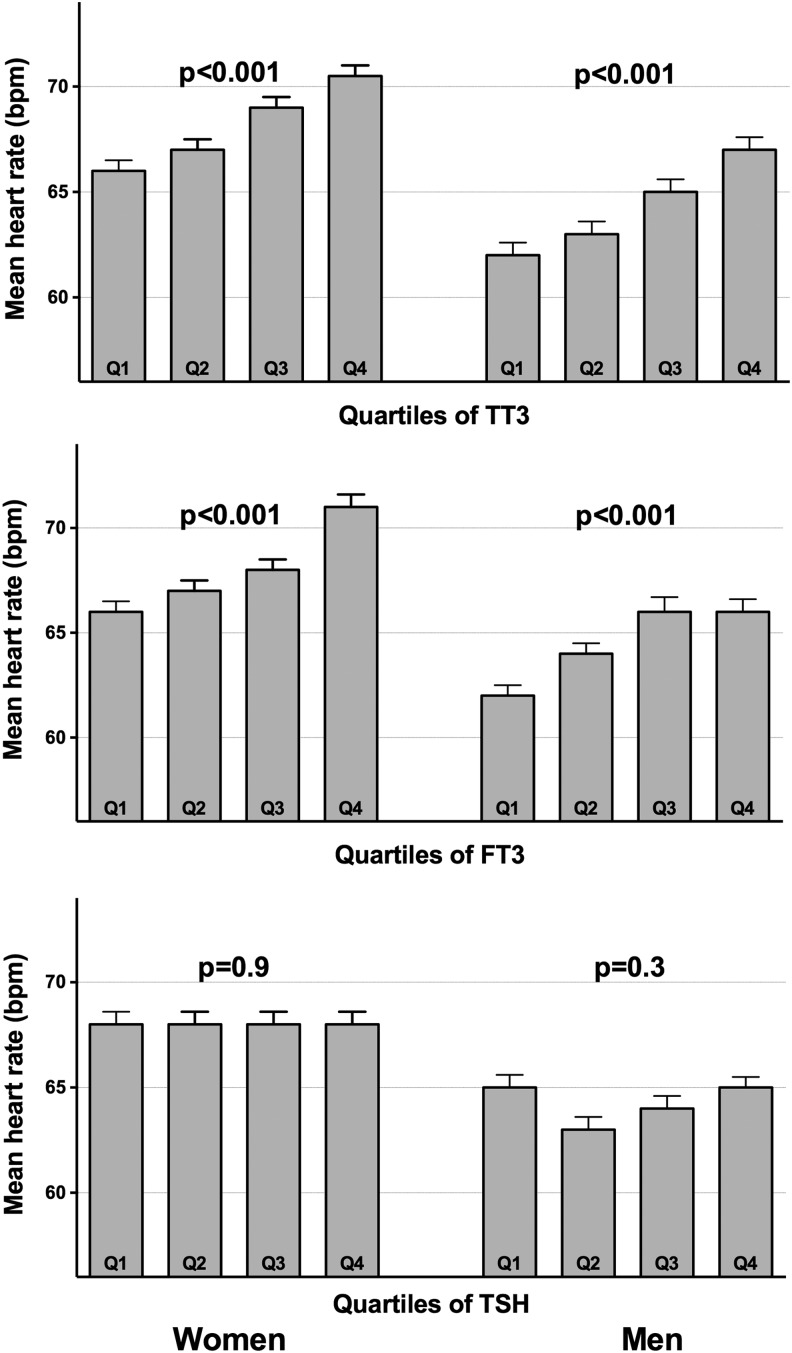
In Figure 2, associations between quartiles of FT3, TT3, and TSH and heart rate for subjects with TSH within the reference range are shown. Mean heart rate increased significantly with increasing quartiles of FT3 and T3, both in men and in women (mean difference±five beats per minute; ANOVA p<0.0001). When associations between circulating thyroid hormone levels and heart rate were explored in a linear model, we observed again a highly significant positive association between FT3 and heart rate, both in a model that only adjusted for sex (β=0.19, p<0.001) as in model 1 (adjusted for sex, age, height, weight, and active smoking; β=0.17, p<0.001). This association remained highly significant when analyses were restricted to subjects within the narrow TSH range (model 1: β=0.16, p<0.001). Comparable associations were observed between heart rate and TT3 concentrations: (β=0.21, p<0.001) in a model only adjusting for sex and (β=0.18, p<0.001) and in model 1 (adjusted for sex, age, height, weight, and active smoking). Also, when restricting the analyses to subjects within the narrow TSH range, the association with TT3 remained highly significant (model 1: β=0.18, p<0.001). No significant associations were observed between heart rate and TSH or FT4 levels.
FIG. 2.
Mean heart rate according to quartiles of FT3, TT3, and TSH (within reference range for TSH). A higher quartile of FT3 is associated with a gradual increase in heart rate; a higher quartile of TSH is not. p-Values result from analysis of variance (overall difference between categories). Error bars represent standard error of the mean. T3, triiodothyronine; FT3, free T3; TT3, total T3.
Associations between thyroid hormones and systolic function
Ejection fraction was not associated with free thyroid hormone levels, TT3, or TSH. FT3 was positively associated with the peak velocity of systolic annular motion s′ (FT3: β=0.06, p=0.01) and negatively with end-systolic diameter (LVESD; β=−0.05, p=0.04) in a sex-adjusted model. However, these associations became insignificant after additional adjustment for age, height, weight, and smoking or when considering the subgroup within the narrow TSH range. TT3 was also positively related to the peak velocity of systolic annular motion s′ (TT3: β=0.13, p<0.0001), and this association remained significant after adjustment for age, height, weight, and smoking, and when limiting analyses to subjects within the strict TSH range. TT3 was not associated with LVESD or EF.
Associations between thyroid hormones and diastolic function
In Table 2, associations between the main diastolic parameters derived from transmitral flow (E and A) and mitral annulus motion (e′ and a′) and thyroid hormone levels and TSH are shown. The most important finding was the positive association between circulating thyroid hormones (FT4, TT3, and FT3) and A and a′, parameters of late ventricular filling (reflecting atrial contraction), in several adjusted models. Besides, positive associations between TT3 levels and parameters of early ventricular filling were observed. In contrast, we did not observe these associations for free thyroid hormones. Interestingly, TSH was inversely related to early diastolic myocardial relaxation as evaluated by e′. Reported associations between FT3, FT4, and TT3 and parameters of diastolic function remained significant when limiting analyses to subjects within narrow TSH levels (data not shown). The associations between FT4 and late ventricular filling and the associations between TT3 and early ventricular filling appeared to be independent from the effects of thyroid hormone on heart rate or systolic blood pressure. A negative association was further observed between IVRT and FT3 (β=−0.06, p=0.007) and TT3 (β=−0.10, p<0.0001) in a sex-adjusted model and for TT3 also in models 1–3. However, for FT3 or other thyroid parameters, no associations with IVRT were observed after further adjustment in models 1–3 (data not shown).
Table 2.
Associations Between TSH and Thyroid Hormone Concentrations and Parameters of Diastolic Left Ventricular Function
| |
E (mm/s) |
A (mm/s) |
e′ (mm/s) |
a′ (mm/s) |
||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| TSH (mU/L) | ||||||||
| Sex-adjusted | −0.02 | 0.5 | 0.007 | 0.8 | −0.05 | 0.01 | 0.008 | 0.7 |
| Model 1 | −0.02 | 0.4 | 0.001 | 0.9 | −0.05 | 0.006 | −0.001 | 0.9 |
| Model 2 | −0.02 | 0.3 | −0.02 | 0.2 | −0.04 | 0.03 | −0.02 | 0.4 |
| Model 3 | −0.03 | 0.2 | −0.02 | 0.2 | −0.05 | 0.009 | −0.02 | 0.4 |
| FT4 (ng/dL) | ||||||||
| Sex-adjusted | −0.02 | 0.4 | 0.005 | 0.8 | 0.04 | 0.06 | 0.03 | 0.2 |
| Model 1 | −0.04 | 0.06 | 0.05 | 0.01 | −0.008 | 0.6 | 0.07 | 0.002 |
| Model 2 | −0.03 | 0.1 | 0.04 | 0.03 | −0.006 | 0.8 | 0.06 | 0.004 |
| Model 3 | −0.04 | 0.09 | 0.04 | 0.03 | −0.01 | 0.6 | 0.06 | 0.005 |
| FT3 (pg/mL) | ||||||||
| Sex-adjusted | 0.02 | 0.4 | 0.06 | 0.01 | 0.02 | 0.5 | 0.05 | 0.05 |
| Model 1 | −0.03 | 0.2 | 0.08 | <0.0001 | −0.04 | 0.06 | 0.08 | <0.0001 |
| Model 2 | −0.02 | 0.5 | 0.01 | 0.6 | −0.02 | 0.4 | 0.02 | 0.4 |
| Model 3 | −0.01 | 0.5 | 0.009 | 0.7 | −0.2 | 0.4 | 0.02 | 0.4 |
| TT3 (ng/dL) | ||||||||
| Sex-adjusted | 0.07 | <0.0001 | 0.11 | <0.0001 | 0.06 | 0.009 | 0.05 | 0.02 |
| Model 1 | 0.04 | 0.04 | 0.10 | <0.0001 | 0.04 | 0.03 | 0.06 | 0.007 |
| Model 2 | 0.07 | 0.001 | 0.02 | 0.2 | 0.07 | <0.0001 | 0.007 | 0.7 |
| Model 3 | 0.07 | 0.001 | 0.02 | 0.2 | 0.06 | 0.001 | 0.008 | 0.7 |
Results from linear regression analysis according to several models: a sex-adjusted model; a model with additional adjustment for age, height, weight, and active smoking; a model with additional adjustment for systolic blood pressure and heart rate; and a model with additional adjustment for LVEDD and relative wall thickness. Parameters of diastolic function (E, A, e′, and a′) were used as dependent variables; TSH, FT4, FT3 and TT3 were independent variables.
Associations between thyroid hormones and LV structure
Associations between free thyroid hormones, TT3, and TSH and two parameters of left ventricular structure (LVEDD and relative wall thickness) are shown in Table 3. Free and total T3 were negatively associated with LVEDD and positively associated with relative wall thickness in several models. Associations with relative wall thickness disappeared after adjustment for blood pressure.
Table 3.
Associations Between TSH and Thyroid Hormones and Parameters of Left Ventricular Structure
| |
TSH (mU/L) |
FT4 (ng/dL) |
FT3 (pg/mL) |
TT3 (ng/dL) |
||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| LVEDD (mm) | ||||||||
| Sex-adjusted | −0.01 | 0.5 | −0.05 | 0.015 | −0.04 | 0.046 | −0.04 | 0.05 |
| + Adjustment for age, height, weight, and smoking | −0.02 | 0.4 | −0.03 | 0.2 | −0.05 | 0.009 | −0.06 | 0.002 |
| + Adjustment for systolic blood pressure | −0.02 | 0.3 | −0.03 | 0.2 | −0.06 | 0.005 | −0.06 | 0.001 |
| Relative wall thickness | ||||||||
| Sex-adjusted | 0.01 | 0.5 | −0.02 | 0.4 | 0.07 | 0.006 | 0.06 | 0.005 |
| + Adjustment for age, height, weight, and smoking | 0.01 | 0.6 | 0.01 | 0.6 | 0.06 | 0.007 | 0.04 | 0.09 |
| + Adjustment for systolic blood pressure | −0.002 | 0.9 | 0.01 | 0.6 | 0.04 | 0.06 | 0.02 | 0.5 |
Results from linear regression analysis according to several models: a sex-adjusted model; a model with additional adjustment for age, height, weight, and active smoking; and a model with additional adjustment for systolic blood pressure. Parameters of LV structure (LVEDD and relative wall thickness) were used as dependent variables; TSH, FT4, FT3, and TT3 were independent variables.
Discussion
In the present study, we investigated whether between-subjects variation in thyroid hormone levels within the reference range affects heart rate, cardiac structure, and function in a population-based sample of apparently healthy middle-aged euthyroid men and women. Our study shows a gradual and robust positive association between FT3 levels and heart rate, even in a strictly defined euthyroid population. At a structural level, the data suggest a decreasing cavity size (LVEDD) with increased relative wall thicknesses with higher circulating thyroid hormones. Although no associations are found with EF, TT3 is associated with enhanced LV systolic function (as assessed by more sensitive tissue Doppler imaging, s′). With regard to diastolic function, higher (free) thyroid hormone levels are related to higher A and a′ and higher TT3 levels to higher E and e′. These data suggest an enhanced atrial contraction (mirroring the effects in the LV) and enhanced LV relaxation with higher thyroid hormone levels.
This study is the first to explore associations between thyroid hormone levels and heart rate, cardiac function, and structure in a large, carefully selected group of healthy, nonhypertensive euthyroid middle-aged subjects. The strengths of this study are the extensive cardiac characterization of a rather large population, together with the determination of TSH as well as circulating free thyroid hormones, the last being in contrast with a previous study, performed in the Framingham cohort (11). As methodological issues in the determination of FT3, for example due to changes in binding proteins or analytical problems, have been described, total T3 levels were also determined. Furthermore, a (strictly) euthyroid population was selected, excluding subjects with thyroid medication, positive TPO antibodies, or TSH levels outside the (strict) reference range. Finally, we studied a population without clinically overt presence of atherosclerosis and excluded subjects with drug-treated hypertension from our analyses, contrary to the recently published study of Iida et al. (17).
We have shown positive associations between heart rate and FT3. Sinus tachycardia and decreased heart rate variability are the most common cardiovascular signs of (SC) hyperthyroidism (29–32). Also, a positive association between FT3 and heart rate in thyroid hormone resistance was observed (30). This is in accordance with the known positive chronotropic effect of T3 through binding to TRα, the predominant thyroid receptor in myocardial tissue (2,33), but we are the first to prove this effect within a strictly euthyroid range.
Extending our observation of a positive association between FT3 and heart rate, we investigated whether circulating thyroid hormone levels within the reference range were associated with cardiac function as well, and whether these associations were heart rate–dependent. In hyperthyroid patients, there is a consistent increase of LV systolic function at rest (2–4). Indeed, we found a positive association between TT3 levels and s′, a sensitive parameter of systolic function.
Diastolic dysfunction is reported both in (SC) hypothyroidism (8,9,33) as well as in SC hyperthyroidism (14,15). Nevertheless, theoretically, T3 exerts a beneficial effect on diastolic function. Two opposing effects can govern this association. First, T3 improves myocardial relaxation by affecting the sarcoplasmic reticulum proteins, calcium-activated ATP-ase, and phospolamban (34). Second, a higher heart rate (>90 beats per minute) counteracts this improvement of diastolic function by reducing total diastolic filling time, and thus leads to a greater dependence on atrial systole (33). Moreover, in longstanding SC hyperthyroidism, this potential favorable effect on diastolic function can be counteracted by the concomitant ventricular hypertrophy induced by the chronically increased cardiac workload (29). As both thyroid hormone deficit as well as excess have been associated with diastolic dysfunction, interpretation of our data is difficult. However, the data are suggestive of a positive contractile effect at the atrial level (positive effects on A and a′), and possibly an enhanced ventricular relaxation (as TSH was inversely and TT3 positively related to early diastolic myocardial relaxation, evaluated by e′ and E) with higher thyroid hormone levels.
Finally, thyroid hormone status can affect LV structure. In longstanding hyperthyroidism, a compensated concentric cardiac hypertrophy occurs, caused by an increased protein synthesis in cardiac myocytes (1). However, changes in heart structure are also possible in SC thyroid disease. A modestly higher LV mass was reported in SC hypothyroidism (8,9). Long-term SC hyperthyroidism consequent to levothyroxine treatment can lead to a higher LV mass as well (14,29), yet this higher LV mass has not been observed in all studies on endogenous SC hyperthyroidism (10–13), due to varying duration of thyroid hormone excess. Only one other study on the effects of thyroid hormone status within the reference range on LV mass exists. Iida et al. (17) have very recently reported on linear relationships between peripheral thyroid hormones (positive) and TSH levels (negative) within the reference range and LVMI in a hypertensive population. Besides, TSH and FT3 levels were associated with changes in LV geometric pattern. (17). In our study, which excluded subjects with drug-treated hypertension, we still observed associations between thyroid hormones and LV structure. A negative association between FT3 and LVEDD together with a positive association between FT3 and relative wall thickness were observed in our study, suggesting a fine balance between higher wall thicknesses but smaller cavity size (LVEDD) with higher circulating FT3 levels. These findings seem to fit within the known framework of findings in more disturbed thyroid function. Both hyper- and hypothyroidism could thus lead to hypertrophy; the former through wall thickening (once the diminution of LVEDD would plateau quite quickly), the latter through chamber enlargement (the main driver of LV mass). These findings suggest that optimal cardiac effects of circulating thyroid hormones operate within very tight, counterbalancing boundaries, with both hypo- and hyperfunction leading to hypertrophy.
Noteworthy are our observations of sex differences in heart rate and thyroid function tests. We observed a small but significant sex difference in mean heart rate of four beats per minute, with men having lower heart rates than women. Previous studies on this topic, both older and more recent studies, showed almost the same trend; namely, slightly lower mean heart rates in men compared to women (difference between three and six beats per minute) (28,35–39). Regarding the sex differences in thyroid function tests, we observed higher FT3 and FT4 levels, and slightly lower TSH and TT3 levels in men than in women. Studies on sex differences in thyroid hormone levels are scarce, and results are conflicting (40–42), possibly because observations result from cohorts with diverging age ranges. However, our results in middle-aged subjects are in agreement with observations from Gullo et al. (43) and Kratzsch et al. (44).
Several limitations should be acknowledged. First, this is a cross-sectional study, so no causal inferences can be made. Second, heart rate was not recorded by 24-hour Holter electrocardiography but was averaged from eight different recordings throughout the examination, establishing an averaged heart rate over a one-hour period. Finally, blood samples were taken at different times of the day. This might have led to significant variation in TSH levels, acknowledging that the diurnal variation in TSH concentrations approximates 50% (45). The time of blood sampling indeed had an influence on TSH levels but not on FT3, T3, and FT4 concentrations. Therefore, it is unlikely that time of the day influenced the observed associations with free thyroid hormones.
In conclusion, in a well-defined general population sample, excluding clinical thyroid disease, confounding antihypertensive treatment and elevated anti-TPO levels, we have demonstrated a highly significant association of between-subject variation in FT3 and T3 levels within the euthyroid range and heart rate. Also, we observed more discrete effects on systolic and diastolic function and on LV structure. More specifically, the data suggest a smaller LV cavity size (with increased relative wall thickness), an enhanced atrial and ventricular contraction, and an enhanced LV relaxation with higher circulating thyroid hormones. This illustrates that variation of thyroid hormone levels, even within a very strict euthyroid range, exerts effects on the heart. However, the degree of CV functional changes seen across the “euthyroid” thyroid function test spectrum all remain within the boundaries of “normal function” and thus cannot automatically be proposed to have predictable clinical significance.
Acknowledgments
The Asklepios Study is supported by a Fonds voor Wetenschappelijk Onderzoek–Vlaanderen FWO research grant G.0427.03 and G.0838.10N, and for this study also by grant G0867.11. The Asklepios Study is indebted to Frida Brusselmans, Femke Van Hoeke, and Bianca Leydens, and the residents and general practitioners of Erpe-Mere and Nieuwerkerken for their invaluable help in completing the study. The authors also wish to thank Magda Becqué, Eric Vandersypt, Kathelyne Mertens, and Kaatje Toye for determining the (free) thyroid hormones, TSH, and anti-TPO values.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Dillmann W. Cardiac hypertrophy and thyroid hormone signaling. Heart Fail Rev. 2010;15:125–132. doi: 10.1007/s10741-008-9125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahaly GJ. Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704–728. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- 3.Klein I. Ojamaa K. Thyroid hormone-targeting the heart. Endocrinology. 2001;142:11–12. doi: 10.1210/endo.142.1.7986. [DOI] [PubMed] [Google Scholar]
- 4.Klein I. Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725–1735. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]
- 5.Tiryakioglu SK. Tiryakioglu O. Ari H. Basel MC. Ozkan H. Bozat T. Left ventricular longitudinal myocardial function in overt hypothyroidism: a tissue Doppler echocardiographic study. Echocardiography. 2010;27:505–511. doi: 10.1111/j.1540-8175.2009.01043.x. [DOI] [PubMed] [Google Scholar]
- 6.Santos AD. Miller RP. Mathew PK. Wallace WA. Cave WT., Jr Hinojosa L. Echocardiographic characterization of the reversible cardiomyopathy of hypothyroidism. Am J Med. 1980;68:675–682. doi: 10.1016/0002-9343(80)90253-3. [DOI] [PubMed] [Google Scholar]
- 7.Ladenson PW. Recognition and management of cardiovascular disease related to thyroid dysfunction. Am J Med. 1990;88:638–641. doi: 10.1016/0002-9343(90)90532-i. [DOI] [PubMed] [Google Scholar]
- 8.Aghini-Lombardi F. Di Bello V. Talini E. Di Cori A. Monzani F. Antonangeli L. Palagi C. Caraccio N. Grazia Delle DM. Nardi C. Dardano A. Balbarini A. Mariani M. Pinchera A. Early textural and functional alterations of left ventricular myocardium in mild hypothyroidism. Eur J Endocrinol. 2006;155:3–9. doi: 10.1530/eje.1.02174. [DOI] [PubMed] [Google Scholar]
- 9.Di Bello V. Monzani F. Giorgi D. Bertini A. Caraccio N. Valenti G. Talini E. Paterni M. Ferrannini E. Giusti C. Ultrasonic myocardial textural analysis in subclinical hypothyroidism. J Am Soc Echocardiogr. 2000;13:832–840. doi: 10.1067/mje.2000.106397. [DOI] [PubMed] [Google Scholar]
- 10.Iqbal A. Schirmer H. Lunde P. Figenschau Y. Rasmussen K. Jorde R. Thyroid stimulating hormone and left ventricular function. J Clin Endocrinol Metab. 2007;92:3504–3510. doi: 10.1210/jc.2007-0727. [DOI] [PubMed] [Google Scholar]
- 11.Pearce EN. Yang Q. Benjamin EJ. Aragam J. Vasan RS. Thyroid function and left ventricular structure and function in the Framingham Heart Study. Thyroid. 2010;20:369–373. doi: 10.1089/thy.2009.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorr M. Wolff B. Robinson DM. John U. Ludemann J. Meng W. Felix SB. Volzke H. The association of thyroid function with cardiac mass and left ventricular hypertrophy. J Clin Endocrinol Metab. 2005;90:673–677. doi: 10.1210/jc.2004-1554. [DOI] [PubMed] [Google Scholar]
- 13.Dorr M. Ittermann T. Aumann N. Obst A. Reffelmann T. Nauck M. Wallaschofski H. Felix SB. Volzke H. Subclinical hyperthyroidism is not associated with progression of cardiac mass and development of left ventricular hypertrophy in middle-aged and older subjects: results from a 5-year follow-up. Clin Endocrinol (Oxf) 2010;73:821–826. doi: 10.1111/j.1365-2265.2010.03882.x. [DOI] [PubMed] [Google Scholar]
- 14.Smit JW. Eustatia-Rutten CF. Corssmit EP. Pereira AM. Frolich M. Bleeker GB. Holman ER. van der Wall EE. Romijn JA. Bax JJ. Reversible diastolic dysfunction after long-term exogenous subclinical hyperthyroidism: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2005;90:6041–6047. doi: 10.1210/jc.2005-0620. [DOI] [PubMed] [Google Scholar]
- 15.Taillard V. Sardinoux M. Oudot C. Fesler P. Rugale C. Raingeard I. Renard E. Ribstein J. du Cailar G. Early detection of isolated left ventricular diastolic dysfunction in high-risk differentiated thyroid carcinoma patients on TSH-suppressive therapy. Clin Endocrinol (Oxf) 2011;75:709–714. doi: 10.1111/j.1365-2265.2011.04138.x. [DOI] [PubMed] [Google Scholar]
- 16.Biondi B. Palmieri EA. Lombardi G. Fazio S. Effects of subclinical thyroid dysfunction on the heart. Ann Intern Med. 2002;137:904–914. doi: 10.7326/0003-4819-137-11-200212030-00011. [DOI] [PubMed] [Google Scholar]
- 17.Iida M. Yamamoto M. Ishiguro Y. Yamazaki M. Honjo H. Kamiya K. Thyroid hormone within the normal range is associated with left ventricular mass in patients with hypertension. J Am Soc Hypertens. 2012;6:261–269. doi: 10.1016/j.jash.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Rietzschel ER. De Buyzere ML. Bekaert S. Segers P. De Bacquer D. Cooman L. Van Damme P. Cassiman P. Langlois M. van Oostveldt P. Verdonck P. De Backer G. Gillebert TC. Rationale, design, methods and baseline characteristics of the Asklepios Study. Eur J Cardiovasc Prev Rehabil. 2007;14:179–191. doi: 10.1097/HJR.0b013e328012c380. [DOI] [PubMed] [Google Scholar]
- 19.Ruige JB. Rietzschel ER. De Buyzere ML. Bekaert S. Segers P. De Bacquer D. De Backer G. Gillebert TC. Kaufman JM. Modest opposite associations of endogenous testosterone and oestradiol with left ventricular remodelling and function in healthy middle-aged men. Int J Androl. 2011;34:e587–e593. doi: 10.1111/j.1365-2605.2011.01191.x. [DOI] [PubMed] [Google Scholar]
- 20.Hermeling E. Vermeersch SJ. Rietzschel ER. De Buyzere ML. Gillebert TC. van de Laar RJ. Ferreira I. Hoeks AP. Van Bortel LM. Reneman RS. Segers P. Reesink KD. The change in arterial stiffness over the cardiac cycle rather than diastolic stiffness is independently associated with left ventricular mass index in healthy middle-aged individuals. J Hypertens. 2012;30:396–402. doi: 10.1097/HJH.0b013e32834e4b75. [DOI] [PubMed] [Google Scholar]
- 21.Ernande L. Bergerot C. Rietzschel ER. De Buyzere ML. Thibault H. Pignonblanc PG. Croisille P. Ovize M. Groisne L. Moulin P. Gillebert TC. Derumeaux G. Diastolic dysfunction in patients with type 2 diabetes mellitus: is it really the first marker of diabetic cardiomyopathy? J Am Soc Echocardiogr. 2011;24:1268–1275. doi: 10.1016/j.echo.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Chirinos JA. Rietzschel ER. De Buyzere ML. De Bacquer D. Gillebert TC. Gupta AK. Segers P. Arterial load and ventricular-arterial coupling: physiologic relations with body size and effect of obesity. Hypertension. 2009;54:558–566. doi: 10.1161/HYPERTENSIONAHA.109.131870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devereux RB. Roman MJ. Liu JE. Lee ET. Wang W. Fabsitz RR. Welty TK. Howard BV. An appraisal of echocardiography as an epidemiological tool. The Strong Heart Study. Ann Epidemiol. 2003;13:238–244. doi: 10.1016/s1047-2797(02)00264-8. [DOI] [PubMed] [Google Scholar]
- 24.Schiller NB. Shah PM. Crawford M. DeMaria A. Devereux R. Feigenbaum H. Gutgesell H. Reichek N. Sahn D. Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 25.Devereux RB. Alonso DR. Lutas EM. Gottlieb GJ. Campo E. Sachs I. Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 26.Rietzschel ER. Langlois M. De Buyzere ML. Segers P. De Bacquer D. Bekaert S. Cooman L. van Oostveldt P. Verdonck P. De Backer GG. Gillebert TC. Oxidized low-density lipoprotein cholesterol is associated with decreases in cardiac function independent of vascular alterations. Hypertension. 2008;52:535–541. doi: 10.1161/HYPERTENSIONAHA.108.114439. [DOI] [PubMed] [Google Scholar]
- 27.Chirinos JA. Segers P. De Buyzere ML. Kronmal RA. Raja MW. De Bacquer D. Claessens T. Gillebert TC. John-Sutton M. Rietzschel ER. Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010;56:91–98. doi: 10.1161/HYPERTENSIONAHA.110.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfister R. Michels G. Sharp SJ. Luben R. Wareham NJ. Khaw KT. Resting heart rate and incident heart failure in apparently healthy men and women in the EPIC-Norfolk study. Eur J Heart Fail. 2012;14:1163–1170. doi: 10.1093/eurjhf/hfs104. [DOI] [PubMed] [Google Scholar]
- 29.Biondi B. Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 30.Kahaly GJ. Matthews CH. Mohr-Kahaly S. Richards CA. Chatterjee VK. Cardiac involvement in thyroid hormone resistance. J Clin Endocrinol Metab. 2002;87:204–212. doi: 10.1210/jcem.87.1.8170. [DOI] [PubMed] [Google Scholar]
- 31.Kaminski GW. Makowski K. Michalkiewicz D. Kowal J. Ruchala M. Szczepanek E. Gielerak G. The influence of subclinical hyperthyroidism on blood pressure, heart rate variability and incidence of arrhythmia. Thyroid. 2012;22:454–460. doi: 10.1089/thy.2010.0333. [DOI] [PubMed] [Google Scholar]
- 32.Petretta M. Bonaduce D. Spinelli L. Vicario ML. Nuzzo V. Marciano F. Camuso P. De Sanctis V. Lupoli G. Cardiovascular haemodynamics and cardiac autonomic control in patients with subclinical and overt hyperthyroidism. Eur J Endocrinol. 2001;145:691–696. doi: 10.1530/eje.0.1450691. [DOI] [PubMed] [Google Scholar]
- 33.Biondi B. Palmieri EA. Lombardi G. Fazio S. Effects of thyroid hormone on cardiac function: the relative importance of heart rate, loading conditions, and myocardial contractility in the regulation of cardiac performance in human hyperthyroidism. J Clin Endocrinol Metab. 2002;87:968–974. doi: 10.1210/jcem.87.3.8302. [DOI] [PubMed] [Google Scholar]
- 34.Klein I. Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 35.Kannel WB. Kannel C. Paffenbarger RS., Jr Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 36.Mensink GB. Hoffmeister H. The relationship between resting heart rate and all-cause, cardiovascular and cancer mortality. Eur Heart J. 1997;18:1404–1410. doi: 10.1093/oxfordjournals.eurheartj.a015465. [DOI] [PubMed] [Google Scholar]
- 37.de Silva P. Antelmi I. Vincenzi MA. Andre CD. Artes R. Jose GC. Jose MA. Influence of age, gender, and serum triglycerides on heart rate in a cohort of asymptomatic individuals without heart disease. Int J Cardiol. 2005;105:152–158. doi: 10.1016/j.ijcard.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 38.Tverdal A. Hjellvik V. Selmer R. Heart rate and mortality from cardiovascular causes: a 12 year follow-up study of 379,843 men and women aged 40–45 years. Eur Heart J. 2008;29:2772–2781. doi: 10.1093/eurheartj/ehn435. [DOI] [PubMed] [Google Scholar]
- 39.Umetani K. Singer DH. McCraty R. Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31:593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- 40.Andersen S. Bruun NH. Pedersen KM. Laurberg P. Biologic variation is important for interpretation of thyroid function tests. Thyroid. 2003;13:1069–1078. doi: 10.1089/105072503770867237. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Sagrado M. Martin-Gil FJ. Population-specific reference values for thyroid hormones on the Abbott ARCHITECT i2000 analyzer. Clin Chem Lab Med. 2004;42:540–542. doi: 10.1515/CCLM.2004.091. [DOI] [PubMed] [Google Scholar]
- 42.Hollowell JG. Staehling NW. Flanders WD. Hannon WH. Gunter EW. Spencer CA. Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 43.Gullo D. Latina A. Frasca F. Le Moli R. Pellegriti G. Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One. 2011;6:e22552. doi: 10.1371/journal.pone.0022552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kratzsch J. Fiedler GM. Leichtle A. Brugel M. Buchbinder S. Otto L. Sabri O. Matthes G. Thiery J. New reference intervals for thyrotropin and thyroid hormones based on National Academy of Clinical Biochemistry criteria and regular ultrasonography of the thyroid. Clin Chem. 2005;51:1480–1486. doi: 10.1373/clinchem.2004.047399. [DOI] [PubMed] [Google Scholar]
- 45.Fisher DA. Physiological variations in thyroid hormones: physiological and pathophysiological considerations. Clin Chem. 1996;42:135–139. [PubMed] [Google Scholar]