Abstract
Prey traits linking consumer diversity to ecosystem function remain poorly understood. On tropical coral reefs, herbivores promote coral dominance by suppressing competing macroalgae, but the roles of herbivore identity and diversity, macroalgal defenses, and their interactions in affecting reef resilience and function are unclear. We studied adjacent pairs of no-take marine reserves and fished areas on reefs in Fiji, and found that protected reefs supported 7–17x greater biomass and 2–3x higher species richness of herbivorous fishes, and 3–11x more live coral cover than did fished reefs. In contrast, macroalgae were 27–61x more abundant and 3–4x more species rich on fished reefs. When we transplanted seven common macroalgae from fished reefs into reserves they were rapidly consumed, suggesting that rates of herbivory (ecosystem functioning) differed inside versus outside reserves.
We then video recorded feeding activity on the same seven macroalgae when transplanted into reserves, and assessed the functional redundancy versus complementarity of herbivorous fishes consuming these macroalgae. Of 29 species of larger herbivorous fishes on these reefs, only four species accounted for 97% of macroalgal consumption. Two unicornfish consumed a range of brown macroalgae, a parrotfish consumed multiple red algae, and a rabbitfish consumed a green alga, with almost no diet overlap among these groups. The two most chemically rich, allelopathic algae were each consumed by a single, but different, fish species. This striking complementarity resulted from herbivore species differing in their tolerances to macroalgal chemical and structural defenses.
A model of assemblage diet breadth based on our feeding observations predicted that high browser diversity would be required for effective control of macroalgae on Fijian reefs. In support of this model, we observed strong, negative relationships between herbivore diversity and macroalgal abundance and diversity across the six study reefs. Our findings indicate that the total diet breadth of the herbivore community and the probability of all macroalgae being removed from reefs by herbivores increases with increasing herbivore diversity, but that a few critical species drive this relationship. Therefore, interactions between algal defenses and herbivore tolerances create an essential role for consumer diversity in the functioning and resilience of coral reefs.
Keywords: chemical ecology, functional diversity, herbivory, marine protected area, phase shift, plant-animal interactions
Introduction
Biodiversity promotes the function, stability, and productivity of ecosystems, as well as the services they provide to human societies (Balvanera et al. 2006, Cardinale et al. 2006, Worm et al. 2006). Positive effects of biodiversity on ecosystem function may result from the increasing probability of including a particular species with a disproportionately large impact (the selection effect), or the inclusion of multiple species with complementary and additive impacts on ecosystems processes (the complementarity effect) as communities increase in species richness (Loreau and Hector 2001). As such, elucidation of the functional roles of species in natural communities is critical for understanding links between biodiversity and ecosystem function, and for determining if species-specific or diversity-oriented management approaches are most effective for maintaining ecosystem processes (Duffy 2009, O’Gorman et al. 2011). Our present understanding of how biodiversity affects ecosystem processes generally comes from small-scale experiments utilizing a relatively limited number of species from lower trophic levels (Balvanera et al. 2006, Duffy et al. 2007). Thus, the functional diversity of consumers and effects of consumer diversity on ecosystem processes in natural communities remain poorly understood (Duffy 2002, Balvanera et al. 2006).
Consumers often have cascading effects on the structure and function of terrestrial, aquatic, and marine ecosystems (Pace et al. 1999, Estes et al. 2011). On coral reefs, intense grazing by herbivores can remove >90% of daily primary production (Hatcher and Larkum 1983, Carpenter 1986), preventing the proliferation of macroalgae that reduce coral survival, growth, and reproduction (Birrell et al. 2008, Hughes et al. 2010). Herbivory thus facilitates coral reefs by promoting the resilience of foundation species (Hughes et al. 2007, Mumby et al. 2007). Intense top-down pressure on coral reefs has selected for macroalgae that produce a wide range of chemical and structural defenses, and in turn, for consumers that have counter-adaptations to tolerate these defenses (Schupp and Paul 1994, Hay 1997). Varied prey defenses and herbivore tolerances could create a role for feeding complementarity, and thus consumer diversity, in the suppression of macroalgae on coral reefs, but mechanistic studies demonstrating these relationships are lacking. In general, interactions between consumer and prey functional traits that link consumer diversity with the top-down control of ecosystems remain unclear (Hillebrand and Cardinale 2004, Ives et al. 2005, Duffy et al. 2007, Edwards et al. 2010).
Coral reef herbivores can be broadly classified into functional groups of (a) grazers (i.e., species that consume the epilithic algal matrix (EAM) from the substratum, thereby keeping the community in an early successional stage largely devoid of upright macroalgae) and (b) browsers (i.e., species that remove large, established macroalgae) (Bellwood et al. 2004, Burkepile and Hay 2010). Functional differences among grazers and browsers are well described (Bellwood et al. 2004), but levels of complementary versus redundant feeding within functional groups are poorly understood. Recent field studies have elucidated the functional roles of some macroalgal browsers (e.g., Burkepile and Hay 2008, 2010, Hoey and Bellwood 2009), but few studies have examined responses of browsing herbivores to the diverse array of macroalgae that commonly characterize degraded reefs (Mantyka and Bellwood 2007, Burkepile and Hay 2008). Studies in marine soft sediment (Duffy et al. 2003), terrestrial shrub (Rogosic et al. 2006), and aquatic rocky reef communities (Duponchelle et al. 2005) suggest that consumer complementarity could be an important mechanism linking consumer diversity to ecosystem function, but the mechanistic basis producing such patterns are rarely explored (Byrnes et al. 2006).
On many tropical reefs experiencing coral decline, overfishing of consumers lessens top-down control, triggering phase shifts from coral toward macroalgae (Hughes et al. 2010). Once abundant, macroalgae reduce coral survival and recruitment via competition (Hughes et al. 2007, Diaz-Pulido et al. 2010, Rasher and Hay 2010), forming ecological feedbacks that further limit coral recovery and reinforce the dominance of macroalgae (Mumby and Steneck 2008, Hughes et al. 2010). However, the mechanisms and outcomes of algal-coral competition on degraded reefs are species-specific, and vary in part due to the unique traits of algal and coral species (Rasher et al. 2011). A clearer understanding of the functional roles of herbivores as algal browsers, of macroalgae as coral competitors, and of macroalgae as herbivore prey is needed for effective management of reef resilience (Bellwood et al. 2004, Hughes et al. 2010).
Here, we determined (a) the structure of herbivorous fish and benthic communities within a series of paired fished and protected reefs in Fiji, (b) whether herbivores within reserves are capable of suppressing an array of macroalgae that dominated fished reefs and that had variable effects on coral fitness, (c) the functional identities and redundancies of herbivores removing these macroalgae, (d) whether variable consumer tolerances to prey defenses play a role in generating browser complementarity, and thus (e) how consumer diversity and prey defenses interact to affect the process of herbivory on coral reefs.
Methods
Study site characteristics
This study was conducted on reef flats within paired fished and protected areas (i.e., no-take marine reserves) adjacent to Namada, Vatu-o-lailai, and Votua villages along the Coral Coast of Viti Levu, Fiji. Surveys of fish and benthic communities were conducted in July-August 2012, and feeding assays were conducted in May-June 2010 and 2011. Reserves are located along an 11 km stretch of fringing reef, each being separated by a minimum distance of 3.3 km and a maximum of 7.6 km. Established in 2002–2003, the reserves are characterized by high coral cover (~38–56%), low macroalgal cover (~1–3%), and a high biomass and diversity of herbivorous fishes (see Results). Between reserves, the reef flat is subject to artisanal fishing at all trophic levels; these adjacent fished areas (“non-reserves”) are characteristic of degraded reefs with high macroalgal cover (~49–91%), low coral cover (4–16%) and a low biomass and diversity of herbivorous fishes (see Results). Paired protected and fished areas were separated by 300–500 m.
We surveyed the benthic community structure of protected and fished reefs at each village using 30 m point-intercept transects (n=20 reef−1 location−1) that were non-overlapping and located haphazardly near the center of each area. The presence of hard corals, soft corals, sponges, crustose coralline algae (CCA), turf algae >0.5 cm, turf algae <0.5 cm, cyanobacteria, fleshy macroalgae (to genus) or sand was surveyed at 0.5 m intervals along each transect (1200 points reef−1). If more than one species was present under a single point, both were counted, so cover could exceed 100%. To focus exclusively on hard substratum biota, we subtracted points on sand before calculating the percent coverage of each benthic organism on each transect.
Herbivorous fishes in each protected and fished area were surveyed using 30 × 5 m belt transects (n=12 reef−1 location−1) that were non-overlapping and located haphazardly near the center of each area. A single snorkeler surveyed all nominally herbivorous fishes (the parrotfishes (Labridae), surgeonfishes (Acanthuridae), rabbitfishes (Siganidae) and chubs (Kyphosidae)) within the 5 m band of each transect, scoring fish identity and size (within 5 cm size classes). Biomass of each fish was calculated using published length-weight relationships.
Macroalgal consumption and identification of key herbivores
To determine the susceptibility of macroalgae to herbivore removal, we collected seven common macroalgae from non-reserve reefs at Votua village (the brown algae Sargassum polycystum, Turbinaria conoides, Padina boryana, and Dictyota bartayresiana, the red algae Amphiroa crassa and Galaxaura filamentosa, and the green alga Chlorodesmis fastigiata), deployed them within the three no-take reserves, and assessed loss of mass relative to caged controls over 48 h. We used these macroalgae because: (1) several dominate cover on the non-reserve reefs (see Results), (2) they encompass a range of taxonomic, morphological, and functional forms, and (3) they show a broad range of competitive impacts on corals (Hughes et al. 2007, Diaz-Pulido et al. 2010, Rasher and Hay 2010, Rasher et al. 2011).
We removed excess water from each alga using a salad spinner (10 revolutions), selected thalli of each species to roughly standardize visual apparency between species, weighed each alga, and arranged one thallus of each of the seven species in random order ~7 cm apart on a 60 cm section of 3-stranded nylon rope. Paired treatment (exposed to herbivores) and control (caged) ropes were assembled in the same manner (n=12 pairs reef−1). Standardizing apparency generated initial masses (g; mean ± SE) of: S. polycystum (2.35 ± 0.08), T. conoides (3.50 ± 0.11), P. boryana (2.36 ± 0.09), D. bartayresiana (6.13 ± 0.16), C. fastigiata (4.55 ± 0.21), G. filamentosa (1.60 ± 0.07), and A. crassa (0.90 ± 0.04).
We deployed paired treatment and control ropes in interconnected networks of reef flat pools accessible to herbivores during both low (~1.5 m depth) and high (~2.5 m depth) tidal periods. Treatment ropes were attached to dead coral fragments and deployed on the substratum at 5–7 m intervals. Each paired control was deployed in a wire mesh cage (65 × 10 × 10 cm; 1 cm2 mesh), and placed within 1 m of its paired treatment rope. We deployed assays during calm conditions. After 48 h, we bagged ropes in situ, returned them to the lab, and spun and weighed each alga as described above. The mass consumed was calculated using the formula: [Ti × (Cf/Ci)]−Tf, where Ti and Tf were the initial and final masses (respectively) of a treatment alga exposed to herbivores, and Ci and Cf were the initial and final masses (respectively) of its paired control. Percentage consumed was calculated to facilitate comparisons among species.
To identify the herbivores consuming macroalgae, we deployed the same seven algae in front of remote video cameras within each of the three reserves between 0800–1400 h, during low tide. For each assay, we deployed three individual thalli of each algal species in a conspecific group (standardizing visual apparency within and between species), and configured conspecific groups randomly among four parallel 60 cm ropes, held flush to the substrate with steel bars. Tripods with cameras, and ropes with algae, were deployed for three consecutive days prior to the experiment to acclimatize fish to the presence of the experimental array.
Feeding assays (n=3 reserve−1) were deployed within a 1 m2 quadrat and filmed for 1 h. We repeated each 1 h feeding assay at the same locations in each reserve over five consecutive days to capture effects of roving herbivores that fluctuate in space and time. This produced 15 feeding trials reserve−1. Assays were conducted sequentially across reserves over a 3 wk period.
Videoed feeding observations were assessed for nominally herbivorous fishes (sensu Choat et al. 2002). Juvenile fishes (<10 cm) and pomacentrids were not scored due to the difficulty of accurately assessing their impacts on the macroalgae. For each herbivore visit to the 1 m2 quadrat, we recorded herbivore species, size, and the number of bites taken from each macroalga and from the epilithic algal matrix (EAM) on the substratum within the 1 m2 area. If multiple rapid bites from an herbivore were not discernable, they were treated as one bite, but this occurred infrequently. Scoring was terminated when ~75% of any macroalgal species was removed; at this point, the relative availability of each algal species was unacceptably skewed and could impact the relative preferences of herbivores. Because most assays lasted less than 30 min before some algal species was 75 % removed, only the first 30 min of each video was analyzed.
Do algal chemical defenses determine differential feeding among herbivores?
To assess the role of algal chemical defenses in generating complimentary feeding among herbivores, we extracted the hydrophobic chemicals from algae avoided by particular herbivores, and coated these extracts onto an alga targeted by each respective herbivore (Meyer et al. 1994). We exhaustively extracted C. fastigiata, A. crassa, G. filamentosa, and D. bartayresiana with 100% methanol, dried each extract using rotary evaporation, and partitioned each extract between water and ethyl acetate. The hydrophobic (ethyl acetate) fraction of each extract was retained, dried using rotary evaporation, and stored at −5°C until used in feeding assays.
The unicornfish Naso lituratus and Naso unicornis did not consume the green alga C. fastigiata or the red algae A. crassa and G. filamentosa, but readily consumed the brown alga P. boryana. To test if the avoided algae possessed chemical defenses against N. lituratus and N. unicornis, we suspended each algal extract in ether and coated a natural volumetric concentration of the extract on five blades (a 2.04 ± 0.03 ml volumetric equivalent, mean ± SE, n=10) of blotted and pre-weighed P. boryana. We allowed the ether to evaporate, and inserted these blades, each 5 cm apart, on a 60 cm section of 3-stranded rope (n=15 ropes extract−1). Paired control ropes were assembled in the same manner, but P. boryana was coated with ether alone.
The parrotfish Chlorurus sordidus did not consume the brown alga D. bartayresiana or the green alga C. fastigiata, and consumed little of the red alga G. fastigiata. To test if these algae were chemically defended against C. sordidus, we conducted chemical defense assays as described above, but using the red alga A. crassa (a preferred prey of C. sordidus, but not of other fishes) as our bioassay organism. Assays to test the tolerance of Siganus argenteus to chemical defenses were not conducted, because the only alga it consumed (C. fastigiata) was filamentous and held too much water for our hydrophobic extracts to adhere to its surfaces.
Assays involving coated P. boryana were conducted within Votua’s marine reserve, during low tides (~1.5–2 m depth) between 0800–1400 h, over three days (i.e., one algal extract day−1). Paired treatment and control ropes were deployed 0.5–0.75 m apart on a small coral colony on the substratum. Because feeding on P. boryana was rapid (~10 min pair−1), treatment and control ropes were deployed one pair at a time, monitored, and recollected when approximately 50% of the total algal mass (treatment and control combined) was consumed. Subsequent replicates were deployed 2–5 m from the previous location (n=15 pairs extract−1). Post-assay ropes were bagged in the field, and blotted and re-weighed at the laboratory to determine the mass of treatment versus control consumed. Given the short duration of each assay and our visual assessment that algal portions were not lost to processes other than herbivory, caged controls were not deployed. We observed browsing by only N. lituratus and N. unicornis.
Due to lower browsing rates on A. crassa, we deployed paired A. crassa treatment and control ropes in Votua’s marine reserve for 24 h during a period of calm seas. Pairs were deployed within 0.5–0.75 m of each other, with replicate pairs separated by 5–7 m. Ropes were re-collected, blotted, and re-weighed as above.
Statistical analyses
Our benthic and herbivorous fish community data violated parametric assumptions. Thus, to evaluate differences among groups between paired reserve and non-reserves sites we used Mann-Whitney U tests. For these analyses we adjusted our significance level using a Bonferonni correction, in light of the large number of pair-wise contrasts performed (Quinn and Keogh 2002). We also evaluated the average diversity of macroalgal and herbivore species at each site by calculating their Shannon’s H in each transect.
We examined relationships between herbivore and macroalgal diversity and abundance in our field survey data using linear regression. For each analysis, a simple linear model was initially fitted to the data. We compared the fit of this model to the estimated best curvilinear model (e.g., second-order polynomial or exponential) using Akaike’s Informative Criteria (AICc), and retained the best-fit model. Our model selections were corroborated using extra sum-of-squares F-tests (Quinn and Keogh 2002). Regression analysis and fit tests where conducted using GraphPad Prism (v. 6.00).
When multiple macroalgae (i.e., treatments) are simultaneously offered to herbivores in multiple-choice feeding assays, herbivore preferences for an alga and their rate of grazing on each alga may depend on the identity and quantity of other available resources; thus the treatments may not be independent, violating the assumptions of ANOVA procedures (Roa 1992, Lockwood 1998). Alternative procedures are available to address this issue, such as Lockwood’s (1998) modification of the multivariate Hotelling’s T2 test, or the rank-based Friedman’s test (Roa 1992, Lockwood 1998). Data from our field herbivory assays could not be transformed to meet multivariate assumptions; we therefore analyzed them with Friedman’s tests (Roa 1992, Lockwood 1998). Significant differences were further evaluated using Friedman’s post-hoc multiple comparisons tests.
For each video assay, we summed the bites taken by each fish species on each alga and scaled them to rates h−1. Because videoed feeding assays (n=3 reserve−1 day−1) were conducted in the same physical locations within each reserve on sequential days, multiple assays at each feeding station within a reserve may not be independent. We therefore used each station as an independent replicate and averaged results from each feeding station over the five days (i.e., n=3 reserve−1). Replicates from each reserve were pooled for analysis. Feeding data from videos could not be transformed to meet multivariate assumptions, so we conducted rank-based Friedman’s tests for each of the four dominant browsing herbivores; collectively these species accounted for 97% of all recorded bites on macroalgae. Grazing rates for the four dominant fishes that fed primarily from the EAM were also analyzed using Friedman’s tests.
Diet breadth was evaluated for each of the four species of browsing herbivores. For each, we calculated Levins’ B (a metric of diet richness and evenness) using the proportions of their total bites taken on each of the seven macroalgae deployed in our 45 video assays. We then calculated the total diet breadths of hypothetical herbivore communities comprised of increasing species richness. For those, we calculated the cumulative diet breadth of each possible multi-species combination (i.e., summing bite count data for all species in a combination and calculating a cumulative Levins’ B). We evaluated the relationship between Levins’ B and herbivore species richness using the same regression procedure as we used for our field survey data (see above).
Data regarding the effects of algal extracts on herbivore feeding could not be transformed to meet the assumptions of paired t-tests. We therefore evaluated these data with Quade’s tests (Roa 1992). Friedman’s and Quade’s tests were performed using the program “R” (version 2.13.2; R Development Core Team 2011) and Friedman’s post-hoc analyses were conducted using the R package “agricolae” (v. 1.0–9; de Mendiburu 2010).
Results
Community structure and rates of herbivory across sites
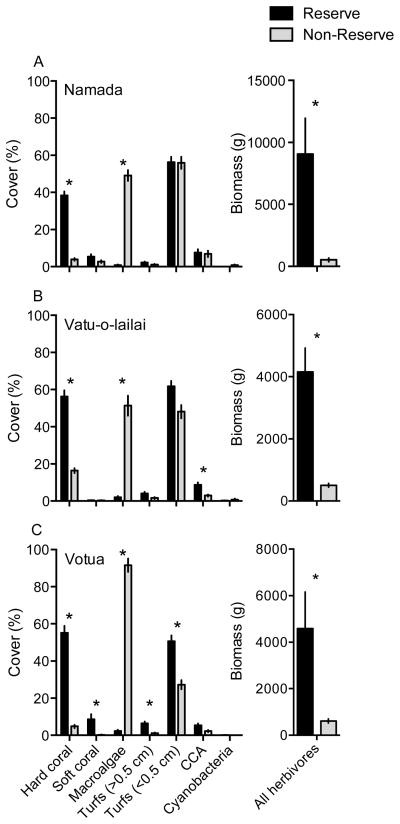
Benthic community structure, herbivore biomass, and herbivore species richness differed dramatically between adjacent protected and fished reefs. When compared to fished reefs, reserves supported 3–11 fold higher coral cover, 7–17 fold greater biomass of herbivorous fishes, and 2–3.3 times more species of herbivorous fishes (Fig. 1, Table A1). Conversely, the abundance of large macroalgae was 27–61 fold higher (49–91% versus 0.8–2.4% cover) and macroalgal species richness was 2.6–3.6 times greater in the non-reserves (Fig. 1, Table 1). Brown macroalgae of the genera Sargassum, Turbinaria, Hormophysa, and Dictyota accounted for the vast majority (87–94%) of macroalgal cover on non-reserve reefs, with several less common macroalgae comprising the other 6–13% (Table 1).
Figure 1.
Abundance (% cover; mean ± SE) of common benthic organisms and biomass (g (150 m2) −1; mean ± SE) of herbivorous fishes inside (black bars) and outside (gray bars) of no-take marine reserves at (A) Namada, (B) Vatu-o-lailai, and (C) Votua villages. * indicates a significant difference between reserve and non-reserve values at a location according to a Bonferonni corrected Mann-Whitney U test. Note scale differences between y-axes. n=20 transects reef−1 location−1 (benthos) or n=12 transects reef−1 location−1 (fish).
Table 1.
Macroalgal abundance by genus (% cover; mean ± SE) inside and outside of no-take marine reserves at Namada, Vatu-o-lailai, and Votua villages (n=20 transects reef−1 location−1).
| Namada | Vatu-o-lailai | Votua | ||||
|---|---|---|---|---|---|---|
| Macroalgae | % cover (mean ± SE) | % cover (mean ± SE) | % cover (mean ± SE) | |||
| Reserve | Non-Reserve | Reserve | Non-Reserve | Reserve | Non-Reserve | |
| Sargassum | 0.0 ± 0.0 | 22.6 ± 2.0 | 0.0 ± 0.0 | 26.3 ± 4.0 | 0.0 ± 0.0 | 57.3 ± 2.7 |
| Turbinaria | 0.0 ± 0.0 | 14.1 ± 1.6 | 0.1 ± 0.1 | 21.8 ± 1.8 | 1.2 ± 0.3 | 0.3 ± 0.2 |
| Dictyota | 0.0 ± 0.0 | 6.3 ± 1.5 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 13.6 ± 2.7 |
| Hormophysa | 0.0 ± 0.0 | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 8.4 ± 1.4 |
| Padina | 0.0 ± 0.0 | 1.2 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.9 ± 0.4 |
| Galaxaura | 0.2 ± 0.1 | 0.5 ± 0.2 | 0.0 ± 0.0 | 0.7 ± 0.2 | 0.4 ± 0.2 | 0.6 ± 0.2 |
| Amphiroa | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.4 ± 0.6 | 0.7 ± 0.3 | 0.0 ± 0.0 | 1.7 ± 0.4 |
| Liagora | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 3.8 ± 0.7 |
| Chlorodesmis | 0.6 ± 0.2 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.2 ± 0.2 |
| Halimeda | 0.1 ± 0.1 | 1.7 ± 0.3 | 0.0 ± 0.0 | 0.8 ± 0.3 | 0.0 ± 0.0 | 2.3 ± 0.7 |
| Other | 0.0 ± 0.0 | 2.2 ± 0.5 | 0.3 ± 0.2 | 1.0 ± 0.3 | 0.8 ± 0.2 | 2.3 ± 0.7 |
When transplanted from non-reserves into reserves, the brown algae S. polycystum, T. conoides, P. boryana, and D. bartayresiana were rapidly consumed (86–100% 48 h−1) by herbivorous fishes, while the green alga C. fastigiata and the red algae G. filamentosa and A. crassa were consumed at appreciable, but significantly lower, rates (5–54% 48 h−1) (Fig. 2).
Figure 2.
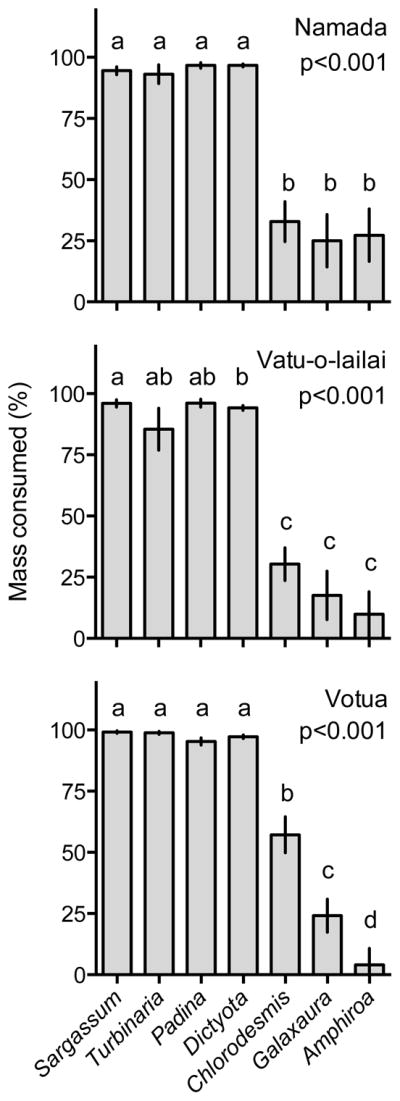
Mass of macroalgae removed (% 48 h−1; mean ± SE) by herbivorous fishes when macroalgae common to degraded reefs were deployed in no-take marine reserves at Namada, Vatu-o-lailai, and Votua villages. Evaluated by Friedman’s tests and post-hoc comparisons. Letters indicate significant groupings. n=12 reserve−1.
Identities of macroalgal browsers versus substratum grazers
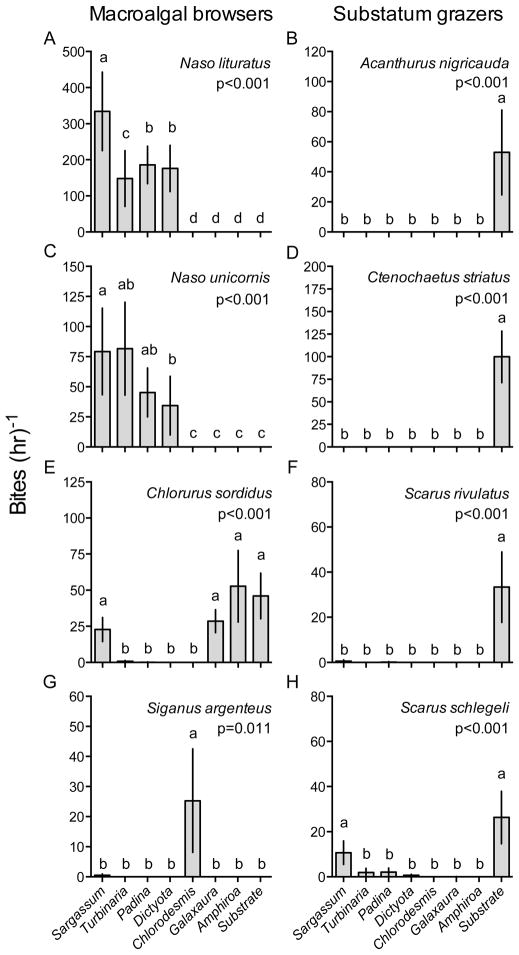
We quantified 19,757 fish bites on the seven species of macroalgae in our videoed feeding assays. Four herbivore species accounted for 97% of all bites. The unicornfishes N. lituratus and N. unicornis fed primarily on the brown macroalgae S. polycystum, T. conoides, P. boryana, and D. bartayresiana, with relative feeding on these algae differing somewhat between fishes (Fig. 3A&C). The parrotfish C. sordidus (IP) fed on the red algae A. crassa and G. filamentosa, with some feeding on S. polycystum but at modest rates relative to Naso species (Fig. 3E). The rabbitfish S. argenteus consumed the green alga C. fastigiata; it consumed no other alga (Fig. 3G).
Figure 3.
Bite rates h−1 (mean ± SE) of (A, C, E, G) dominant browsers on seven macroalgae common to degraded reef habitats, or of (B, D, F, H) dominant grazers on the epilithic algal matrix (EAM) on the substrata. Data for each consumer were analyzed separately by a Friedman’s test and post-hoc comparison. Letters indicate significant groupings. Scarids were all initial phase (IP). Note scale differences between y-axes. n=9.
With the exception of C. sordidus, the major consumers of macroalgae did not graze the EAM. However, other herbivore species took 4,999 bites from the EAM, with 98% of all bites being by only five species - the parrotfishes C. sordidus (IP), Scarus rivulatus (IP), and Scarus schlegeli (IP), and the surgeonfishes Ctenochaetus striatus and Acanthurus nigricauda (Fig. 3B, D, F, H). S. rivulatus, C. striatus, and A. nigricauda grazed the EAM almost exclusively, and in preference to all macroalgae (Fig. 3B, D, F). S. schlegeli fed from both S. polycystum (or its epiphytes) and the EAM at low rates (Fig. 3H), while C. sordidus grazed the EAM, A. crassa, G. filamentosa, and S. polycystum at similar rates (Fig. 3E).
Consumer diversity, ecosystem function, and the mechanism connecting them
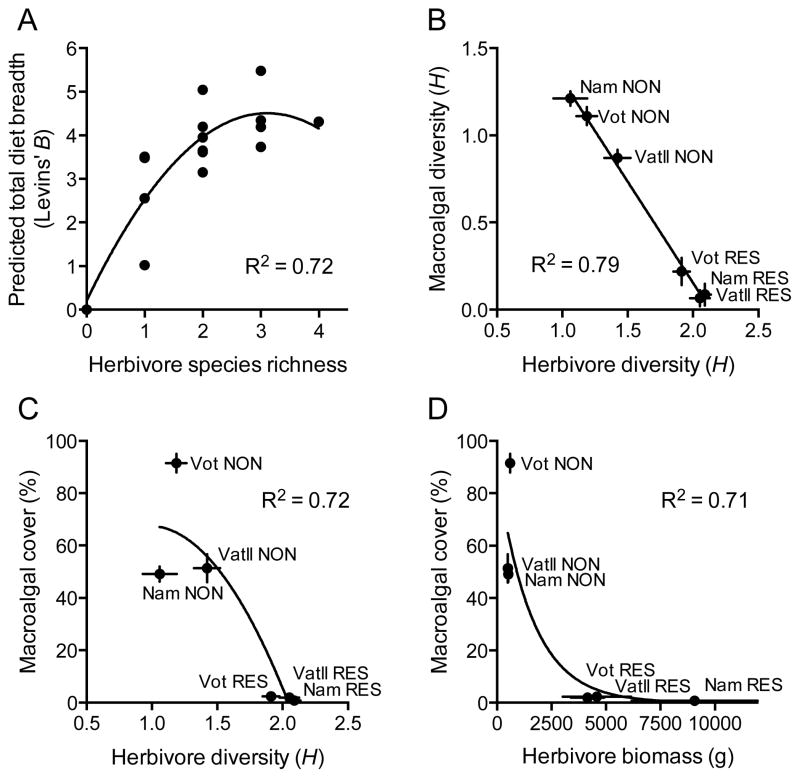
Herbivorous fishes exhibited strong feeding complementarity within a function group (macroalgal browsers) and between functional groups (browsers versus grazers), allowing no algae to escape attack from all consumers (Fig. 3). Such complementarity may make herbivore diversity a requirement if herbivores are to effectively remove a diversity of macroalgae from reefs. Consistent with this notion, the total breadth and evenness (Levins’ B) of macroalgae utilized by hypothetical communities of browsing fishes increased as a saturating function (R2=0.72) of increasing herbivore species richness in our empirically-derived model (Fig. 4A). Consistent with this model, macroalgal diversity in the field displayed a striking negative, linear relationship (R2=0.79) with herbivorous fish diversity across the six sites we investigated (Fig. 4B). Moreover, macroalgal abundance displayed a negative, curvilinear relationship with herbivorous fish diversity (R2=0.72; Fig. 4C) and a negative, non-linear relationship with herbivorous fish biomass (R2=0.71; Fig. 4D) across the six sites.
Figure 4.
(A) Predicted total diet breadth (Levins’ B) of the macroalgal browser guild as a function of herbivore species richness, based on empirical bite rate values for each species. (B) Relationship between mean macroalgal diversity and mean herbivorous fish diversity (Shannon’s H; ± SE) at each of the six study sites. (C) Relationship between mean macroalgal abundance (% cover; ± SE) and mean herbivorous fish diversity (Shannon’s H; ± SE) at each of the six study sites. (D) Relationship between mean macroalgal abundance (% cover; ± SE) and mean herbivorous fish biomass (g (150 m2) −1; ± SE) at each of the six study sites. Best-fit linear (A–C) or non-linear (D) regressions were selected following an analysis of fit using AICc criteria and extra sum-of-squares F-tests (Quinn and Keogh 2002). n=20 transects reef−1 location−1 for macroalgal data; n=12 transects reef−1 location−1 for herbivore data.
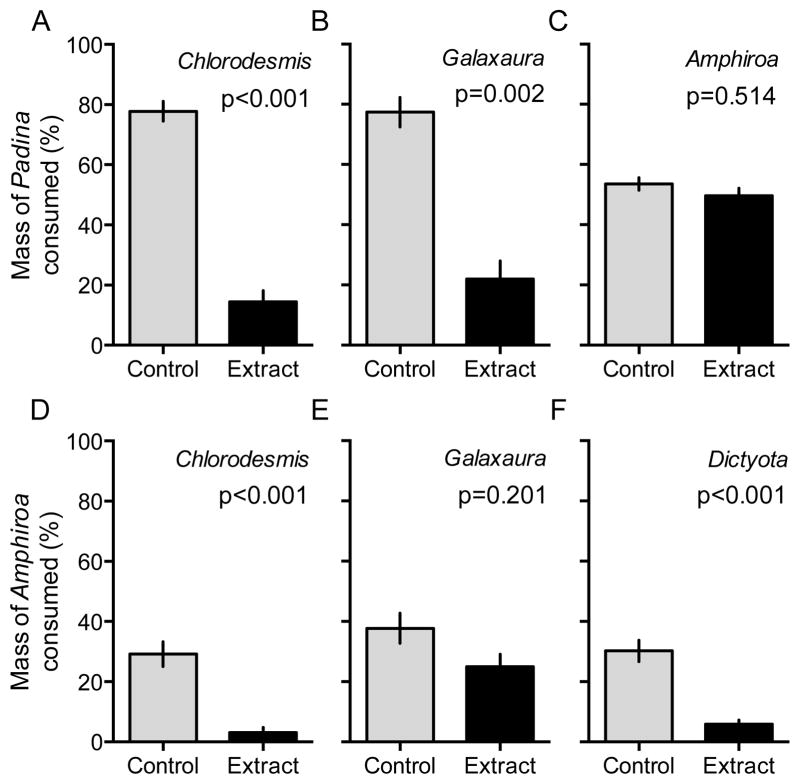
When hydrophobic extracts of C. fastigiata and G. filamentosa were coated at natural concentration onto P. boryana, both extracts significantly deterred browsing by N. lituratus and N. unicornis (p≤0.002; Fig. 5A&B). In contrast, the extract of the avoided, but heavily calcified A. crassa did not deter browsing by Naso spp. (p=0.51; Fig. 5C).
Figure 5.
(A–C) Percent mass of Padina boryana consumed (mean ± SE) by the fishes Naso lituratus and Naso unicornis, or (D–F) percent mass of Amphiroa crassa consumed (likely by Chlorurus sordidus), when coated with hydrophobic extracts of algae avoided by each respective herbivore versus paired control algae coated only with solvent. Evaluated by Quade’s tests. n=15 extract−1.
The hydrophobic extracts of C. fastigiata and D. bartayresiana deterred fish feeding on A. crassa (a preferred prey of C. sordidus; p<0.001; Fig. 5D&F). In contrast, the extract of G. filamentosa (an alga consumed by C. sordidus, but at low rates) did not significantly deter browsing (p=0.20) despite grazing declining by ~34% (Fig. 5E).
Discussion
Although dramatic phase shifts are less common on Pacific than Caribbean reefs (Hughes et al. 2010) and it has been suggested that Indo-Pacific reefs are more robust and would have to be severely stressed to undergo Caribbean-like phase shifts (Roff and Mumby 2012), we found striking differences in benthic and herbivore community structure between three pairs of adjacent Fijian reefs that were fished versus protected (Fig. 1, Table 1, Table A1). Fished reefs exhibited Caribbean-like degradation in that hard substrates were macroalgal dominated (~49–91% cover; Table 1) and coral poor (~4–16% cover; Fig. 1). Moreover, these reefs supported only 6–13% of the herbivorous fish biomass of adjacent reefs that were protected (Table A1). In contrast, on protected reefs corals were abundant (~38–56% cover; Fig. 1) and macroalgae were rare (~0.8–2.4% cover; Table 1), and herbivorous fish biomass and species richness were 7–17 fold and 2–3.3 fold greater, respectively, than on fished reefs (Table A1). The pairs of fished and protected reefs we studied are on the same contiguous coastline, are separated by only ~500 m, and differ primarily in access to artisanal fishers using hand lines, spears, and nets. We could find no other examples of such dramatic effects of reef protection on such small spatial scales. Reserves are adjacent to villages that enforce protection, so compliance is high, which may contribute to these striking differences.
When we transplanted common macroalgae from fished areas into protected areas, they were rapidly consumed (Fig. 2), suggesting that reduced herbivory in fished areas might have generated the differences in macroalgal abundance between non-reserves and reserves. Videos of transplanted macroalgae showed that of the 29 larger herbivorous fish species occupying these sites (Table A1), four species were responsible for 97% of bites taken on macroalgae within the reserves. Three of these four herbivores displayed feeding complementarity (Fig. 3). The unicornfishes N. lituratus and N. unicornis fed almost exclusively on brown macroalgae, while the parrotfish C. sordidus fed primarily on red algae and the rabbitfish S. argenteus was the sole consumer of a green alga (Fig. 3). These four species of browsing herbivores were of low biomass or absent in fished areas (Table A1).
Brown macroalgae commonly bloom on reefs following herbivore loss or removal (e.g., Lewis 1986, Hughes et al. 2007), and brown macroalgae comprised 87–94% of the macroalgal cover on the three fished reefs we studied (Table 1). N. lituratus and N. unicornis represented only 7% of the herbivorous fish species richness and 32% of the herbivorous fish biomass within the reserves (Table A1), but accounted for almost all (94%) of the feeding on the four abundant brown macroalgae we transplanted (Fig. 3). Similarly, the parrotfish C. sordidus represented only 3% of the herbivorous fish species richness and 9% of the herbivorous fish biomass within the reserves but was responsible for 100% of browsing on the red algae G. filamentosa and A. crassa; the rabbitfish S. argenteus accounted for less than 1% of the total herbivore biomass but 100% of the feeding on C. fastigiata. Given this strong complementarity and species-specificity of feeding, removal of established macroalgae will depend not only on total herbivore abundance and species identity, but also the mix of herbivore species present in the ecosystem (see also Bellwood et al. 2004, Burkepile and Hay 2008, 2010). It is well established that some fishes primarily consume small turfs and sediments comprising the epilithic algal matrix while other fishes browse macroalgae (e.g., Choat et al. 2002, 2004), and thus that the grazers responsible for suppressing macroalgal recruitment differ from the browsers responsible for removing established macroalgae (Bellwood et al. 2004, Burkepile and Hay 2010). However the extreme variance in feeding among macroalgal browsers has rarely been addressed.
Based on the strong browser complementarity we observed in these experiments, and on complementarity noted among other fishes in the Caribbean (Burkepile and Hay 2008, 2010) and on the Great Barrier Reef (Mantyka and Bellwood 2007), we predicted that not just herbivore biomass, but herbivore diversity per se, could be crucial to the effective control of macroalgal abundance and diversity on coral reefs (Fig. 4A). Consistent with this model, we found strong, negative associations between herbivorous fish diversity and macroalgal abundance and diversity across the six reefs we studied (Fig. 4). Our results thus highlight the critical roles that consumer diversity and functional complementarity play in promoting the control of macroalgae on coral reefs. A role for herbivore diversity and feeding complementarity is also suggested by comparative studies conducted across 92 reefs in Australia (Cheal et al. in press).
The complementarity we observed among browsers was driven, in large part, by differential herbivore tolerances to algal chemical defenses (Fig. 5). As such, a diverse group of specific browsers will be required to remove the broad range of differentially defended macroalgae that occur on degraded reefs (Fig. 1–5, Table 1). Both laboratory and field experiments (Paul et al. 1990, Hay et al. 1994, Meyer et al. 1994, Schupp and Paul 1994, Burkepile and Hay 2008, 2010) support these findings, indicating that differential tolerances to chemical and mineral defenses may be common among herbivores on both Pacific and Caribbean reefs. Although we demonstrate a role of prey chemical defenses in generating complementarity (Fig. 5), prey morphological, structural, and nutritional traits may also be important (Hay et al. 1994, Hay 1997, Clements et al. 2009). For example, avoidance of A. crassa by both Naso species was not explained by defensive chemistry (Fig. 5C). In this case, avoidance is likely due to the heavy calcification of A. crassa, which serves as a structural defense or as a mineral defense that buffers the acidic gut that several surgeonfish depend on for digestion of algal tissues (Hay et al. 1994, Schupp and Paul 1994). Interactions between prey chemical, nutritional, and structural traits and herbivore gut physiology likely play an important role in diet partitioning among reef herbivores (Schupp and Paul 1994, Choat et al. 2002, 2004, Clements et al. 2009) and in producing patterns of complementary feeding. However, our finding that prey chemical defenses play a major role in creating feeding complementarity appears to be the first experimental evidence that interactions between prey defenses and consumer tolerances underlie, in part, the complementarity effect driving the relationship between consumer diversity and coral reef function. Complex interactions between consumer and prey diversity could dictate the strength of top-down control in many other ecosystems (Hillebrand and Cardinale 2004, Edwards et al. 2010), yet few field studies have explicitly addressed this concept (Ives et al. 2005, Duffy et al. 2007).
There is considerable debate over the effectiveness of marine protected areas as tools for restoring reef structure and function (e.g., Stockwell et al. 2009, Selig and Bruno 2010). The relationships we describe here between herbivore diversity and macroalgal defenses may help to explain why some reefs have failed to recover from phase shifts to macroalgae, despite years to decades of protection (Ledlie et al. 2007, Huntington et al. 2011, but see Mumby and Harborne 2010). Our study suggests that if re-establishing herbivore communities on degraded reefs contain functionally similar herbivores with limited diet breadths, they will be unlikely to effectively remove a diverse array of macroalgae possessing varied defensive traits (Fig. 5).
On the reefs we studied, the diversity and biomass of herbivorous fishes was higher on protected reefs (Table A1) and these differences in herbivore communities were associated with increased browsing, a rarity of macroalgae, and a higher abundance of corals. In 2004, shortly after no-take reserves were established at our study sites, coral cover was low and did not differ between fished and protected reefs (~7% on all reefs due to a bleaching event), and macroalgal cover was 35–45% in both the fished and protected areas (unpublished data, V. Bonito, personal comm.). However, eight years later the reserves have abundant and species-rich herbivore assemblages, low macroalgal cover, and high coral cover (Fig. 1, Table A1). In contrast, the fished areas continue to lack both biomass and diversity of herbivores, are dominated by macroalgae, and have a low abundance of corals (Tables 1 and A1, Fig. 1). Thus, management efforts to increase reef resilience must ensure that reefs not only harbor adequate herbivore biomass, but also certain critical macroalgal browsers, and the right species mix of browsers and grazers. This critical mix of herbivores is needed to both limit algal proliferation on substrata not already occupied by macroalgae (Paddack et al. 2006) and to remove chemically and structurally defended macroalgae already on the reef (Burkepile and Hay 2008, 2010, Cheal et al. in press).
Previous studies identified the unicornfish N. unicornis as the most important consumer of Sargassum on the Great Barrier Reef (Hoey and Bellwood 2009, 2010). We expand on these findings by demonstrating that N. unicornis and N. lituratus are the primary consumers of a diverse range of brown macroalgae (including the chemically rich and allelopathic D. bartayresiana) that dominate the fished reefs at our study sites (Fig. 1, Table 1). Given the significant feeding impact of these fishes on brown algae throughout the Pacific (Choat et al. 2002, Hoey and Bellwood 2009), targeted management of N. lituratus and N. unicornis might facilitate the reversal of phase shifts where brown algae have bloomed and might improve coral resilience on degraded Pacific reefs.
In addition to the dominant brown algae, our study also included other broadly distributed and common macroalgae that can directly damage corals via allelopathic competition. G. filamentosa and C. fastigiata are two of the most allelopathic macroalgae on Pacific reefs, and A. crassa is variably allelopathic, harming some corals but not others (Rasher and Hay 2010, Rasher et al. 2011). These algae are of low palatability (Fig. 2&3) and two of the three are chemically defended against some herbivores (Fig. 5, Paul et al. 1990, Meyer et al. 1994), yet they rarely, if ever, dominate reefs that lack significant herbivore populations. Such patterns are in contrast to the fast growing, non-allelopathic, and palatable brown algae that dominate the non-reserve reefs at our study sites (Table 1), and suggest that macroalgae may experience complex trade-offs between growth, anti-herbivore defense, and allelopathic potency when competing for space under reduced herbivory.
In addition to browsers of macroalgae, we also documented five species of herbivores grazing the EAM growing on the substratum (Fig. 3). C. sordidus and S. schlegeli fed on both the EAM and on some macroalgae, but the other common herbivores fed only from either the EAM alone or only on macroalgae (Fig. 3). For fishes feeding on the EAM, differentiation of bites between the components of the EAM (small algal turfs, crustose coralline algae, sediments, detritus, cyanobacteria, etc.) could not be determined due to the mixture of these foods within the matrix and the resolution of our video assays. Thus, we could not assess complementarity versus redundancy within this functional group. However, gut content studies of these herbivores show different items in the gut (Choat et al. 2002, 2004), suggesting that complementarity may occur among these species as well, or that they feed from different surfaces or locations, either of which could make diversity within this group important in preventing phase shifts to macroalgae by keeping substrates in early successional stages.
Consumers impact ecosystem function through top-down forcing (Ives et al. 2005, Duffy et al. 2007, Estes et al. 2011), and consumers are likely more vulnerable to localized extinction than primary producers (Duffy 2003), yet our understanding of how consumer diversity affects real-world ecosystems is limited (Duffy 2009). Our study demonstrates the importance of consumer diversity and feeding complementarity to the critical ecosystem process of herbivory on a coral reef, but also reveals a strikingly limited redundancy of macroalgal consumers feeding within a diverse natural assemblage. Our findings have worrisome conservation implications, because critical ecosystem processes may rapidly deteriorate when only one, or a few, key consumers are locally extirpated (Estes et al. 2011, O’Gorman et al. 2011; Bellwood et al. 2012). Improved knowledge of the functional roles of consumers, the prey traits affecting consumer feeding choices, and the interaction of prey defenses and consumer tolerances appears critical for informed management to maintain the structure and function of natural ecosystems.
Acknowledgments
We thank the Fijian government and the Korolevu-i-wai district elders for collection and research permissions, P. Skelton (University of South Pacific) for identifying macroalgae, V. Bonito for logistical support, J. Stachowicz for comments on the manuscript, and C. Clements, I. Markham, R. Lasley-Rasher, and R. Simpson for field assistance. Support came from the National Science Foundation (OCE 0929119 and DGE-0114400), the National Institutes of Health (U01-TW007401), and the Teasley Endowment to the Georgia Institute of Technology.
Description of Ecological Archives Materials
Appendix A. A table listing the biomass of each herbivorous fish species observed inside and outside of no-take marine reserves at three locations in Fiji.
Contributor Information
Douglas B. Rasher, Email: doug.rasher@gatech.edu.
Andrew S. Hoey, Email: andrew.hoey@my.jcu.edu.au.
Mark E. Hay, Email: mark.hay@biology.gatech.edu.
References
- Balvanera P, Pfisterer AB, Buchmann N, He JS, Nakashizuka T, Raffaelli D, Schmid B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecology Letters. 2006;9:1146–1156. doi: 10.1111/j.1461-0248.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- Bellwood DR, Hughes TP, Folke C, Nystrom M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- Bellwood DR, Hoey AS, Hughes TP. Human activity selectively impacts the ecosystem roles of parrotfishes on coral reefs. Proceedings of the Royal Society B: Biological Sciences. 2012;279:1621–1629. doi: 10.1098/rspb.2011.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell CL, McCook LJ, Willis BL, Diaz-Pulido GA. Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanography and Marine Biology: An Annual Review. 2008;46:25–64. [Google Scholar]
- Burkepile DE, Hay ME. Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proceedings of the National Academy of Sciences (USA) 2008;105:16201–16206. doi: 10.1073/pnas.0801946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkepile DE, Hay ME. Impact of herbivore identity on algal succession and coral growth on a Caribbean reef. Plos One. 2010;5:e8963. doi: 10.1371/journal.pone.0008963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes J, Stachowicz JJ, Hultgren KM, Hughes AR, Olyarnik SV, Thornber CS. Predator diversity strengthens trophic cascades in kelp forests by modifying herbivore behaviour. Ecology Letters. 2006;9:61–71. doi: 10.1111/j.1461-0248.2005.00842.x. [DOI] [PubMed] [Google Scholar]
- Cardinale BJ, Srivastava DS, Duffy JE, Wright JP, Downing AL, Sankaran M, Jouseau C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443:989–992. doi: 10.1038/nature05202. [DOI] [PubMed] [Google Scholar]
- Carpenter RC. Partitioning herbivory and its effects on coral reef algal communities. Ecological Monographs. 1986;56:345–363. [Google Scholar]
- Cheal AJ, Emslie MJ, MacNeil MA, Miller I, Sweatman H. Spatial variation in the functional characteristics of herbivorous fish communities and the resilience of coral reefs. Ecological Applications. doi: 10.1890/11-2253.1. In press. [DOI] [PubMed] [Google Scholar]
- Choat JH, Clements KD, Robbins WD. The trophic status of herbivorous fishes on coral reefs - I: Dietary analyses. Marine Biology. 2002;140:613–623. [Google Scholar]
- Choat JH, Robbins WD, Clements KD. The trophic status of herbivorous fishes on coral reefs - II. Food processing modes and trophodynamics. Marine Biology. 2004;145:445–454. [Google Scholar]
- Clements KD, Raubenheimer D, Choat JH. Nutritional ecology of marine herbivorous fishes: ten years on. Functional Ecology. 2009;23:79–92. [Google Scholar]
- de Mendiburu F. agricolae: Statistical procedures for agricultural research. R package version 1.0–9. 2010 URL http://CRAN.R-project.org/package=agricolae.
- Diaz-Pulido G, Harii S, McCook LJ, Hoegh-Guldberg O. The impact of benthic algae on the settlement of a reef-building coral. Coral Reefs. 2010;29:203–208. [Google Scholar]
- Duffy JE. Biodiversity and ecosystem function: the consumer connection. Oikos. 2002;99:201–219. [Google Scholar]
- Duffy JE. Biodiversity loss, trophic skew and ecosystem functioning. Ecology Letters. 2003;6:680–687. [Google Scholar]
- Duffy JE. Why biodiversity is important to the functioning of real-world ecosystems. Frontiers in Ecology and the Environment. 2009;7:437–444. [Google Scholar]
- Duffy JE, Richardson JP, Canuel EA. Grazer diversity effects on ecosystem functioning in seagrass beds. Ecology Letters. 2003;6:637–645. [Google Scholar]
- Duffy JE, Cardinale BJ, France KE, McIntyre PB, Thebault E, Loreau M. The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecology Letters. 2007;10:522–538. doi: 10.1111/j.1461-0248.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- Duponchelle F, Ribbink AJ, Msukwa A, Mafuka J, Mandere D, Bootsma H. Food partitioning within the species-rich benthic fish community of Lake Malawi, East Africa. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:1651–1664. [Google Scholar]
- Edwards KF, Aquilino KM, Best RJ, Sellheim KL, Stachowicz JJ. Prey diversity is associated with weaker consumer effects in a meta-analysis of benthic marine experiments. Ecology Letters. 2010;13:194–201. doi: 10.1111/j.1461-0248.2009.01417.x. [DOI] [PubMed] [Google Scholar]
- Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, Marquis RJ, Oksanen L, Oksanen T, Paine RT, Pikitch EK, Ripple WJ, Sandin SA, Scheffer M, Schoener TW, Shurin JB, Sinclair ARE, Soule ME, Virtanen R, Wardle DA. Trophic downgrading of planet Earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- Hatcher BG, Larkum AWD. An experimental analysis of factors controlling the standing crop of the epilithic algal community on a coral reef. Journal of Experimental Marine Biology and Ecology. 1983;69:61–84. [Google Scholar]
- Hay ME. The ecology and evolution of seaweed-herbivore interactions on coral reefs. Coral Reefs. 1997;16:S67–S76. [Google Scholar]
- Hay ME, Kappel QE, Fenical W. Synergisms in plant defenses against herbivores: interactions of chemistry, calcification, and plant quality. Ecology. 1994;75:1714–1726. [Google Scholar]
- Hillebrand H, Cardinale BJ. Consumer effects decline with prey diversity. Ecology Letters. 2004;7:192–201. [Google Scholar]
- Hoey AS, Bellwood DR. Limited functional redundancy in a high diversity system: Single species dominates key ecological process on coral reefs. Ecosystems. 2009;12:1316–1328. [Google Scholar]
- Hoey AS, Bellwood DR. Cross-shelf variation in browsing intensity on the Great Barrier Reef. Coral Reefs. 2010;29:499–508. [Google Scholar]
- Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Current Biology. 2007;17:360–365. doi: 10.1016/j.cub.2006.12.049. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Graham NAJ, Jackson JBC, Mumby PJ, Steneck RS. Rising to the challenge of sustaining coral reef resilience. Trends in Ecology & Evolution. 2010;25:633–642. doi: 10.1016/j.tree.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Huntington BE, Karnauskas M, Lirman D. Corals fail to recover at a Caribbean marine reserve despite ten years of reserve designation. Coral Reefs. 2011;30:1077–1085. [Google Scholar]
- Ives AR, Cardinale BJ, Snyder WE. A synthesis of subdisciplines: predator-prey interactions, and biodiversity and ecosystem functioning. Ecology Letters. 2005;8:102–116. [Google Scholar]
- Ledlie MH, Graham NAJ, Bythell JC, Wilson SK, Jennings S, Polunin NVC, Hardcastle J. Phase shifts and the role of herbivory in the resilience of coral reefs. Coral Reefs. 2007;26:641–653. [Google Scholar]
- Lewis SM. The role of herbivorous fishes in the organization of a Caribbean reef community. Ecological Monographs. 1986;56:183–200. [Google Scholar]
- Lockwood JR. On the statistical analysis of multiple-choice feeding preference experiments. Oecologia. 1998;116:475–481. doi: 10.1007/s004420050612. [DOI] [PubMed] [Google Scholar]
- Loreau M, Hector A. Partitioning selection and complementarity in biodiversity experiments. Nature. 2001;412:72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- Mantyka CS, Bellwood DR. Macroalgal grazing selectivity among herbivorous coral reef fishes. Marine Ecology Progress Series. 2007;352:177–185. [Google Scholar]
- Meyer KD, V, Paul J, Sanger HR, Nelson SG. Effects of seaweed extracts and secondary metabolites on feeding by the herbivorous surgeonfish Naso lituratus. Coral Reefs. 1994;13:105–112. [Google Scholar]
- Mumby PJ, Hastings A, Edwards HJ. Thresholds and the resilience of Caribbean coral reefs. Nature. 2007;450:98–101. doi: 10.1038/nature06252. [DOI] [PubMed] [Google Scholar]
- Mumby PJ, Steneck RS. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends in Ecology & Evolution. 2008;23:555–563. doi: 10.1016/j.tree.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Mumby PJ, Harborne AR. Marine reserves enhance the recovery of corals on Caribbean reefs. Plos One. 2010;5:e8657. doi: 10.1371/journal.pone.0008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Gorman EJ, Yearsley JM, Crowe TP, Emmerson MC, Jacob U, Petchey OL. Loss of functionally unique species may gradually undermine ecosystems. Proceedings of the Royal Society B-Biological Sciences. 2011;278:1886–1893. doi: 10.1098/rspb.2010.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace ML, Cole JJ, Carpenter SR, Kitchell JF. Trophic cascades revealed in diverse ecosystems. Trends in Ecology & Evolution. 1999;14:483–488. doi: 10.1016/s0169-5347(99)01723-1. [DOI] [PubMed] [Google Scholar]
- Paddack MJ, Cowen RK, Sponaugle S. Grazing pressure of herbivorous coral reef fishes on low coral-cover reefs. Coral Reefs. 2006;25:461–472. [Google Scholar]
- Paul VJ, Nelson SG, Sanger HR. Feeding preferences of adult and juvenile rabbitfish Siganus argenteus in relation to chemical defenses of tropical seaweeds. Marine Ecology Progress Series. 1990;60:23–34. [Google Scholar]
- Quinn GP, Keough MJ. Experimental design and data analysis for biologists. Cambridge University Press; Cambridge: 2002. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. [Google Scholar]
- Rasher DB, Hay ME. Chemically rich seaweeds poison corals when not controlled by herbivores. Proceedings of the National Academy of Sciences (USA) 2010;107:9683–9688. doi: 10.1073/pnas.0912095107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasher DB, Stout EP, Engel S, Kubanek J, Hay ME. Macroalgal terpenes function as allelopathic agents against reef corals. Proceedings of the National Academy of Sciences (USA) 2011;108:17726–17731. doi: 10.1073/pnas.1108628108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa R. Design and analysis of multiple-choice feeding preference experiments. Oecologia. 1992;89:509–515. doi: 10.1007/BF00317157. [DOI] [PubMed] [Google Scholar]
- Roff G, Mumby PJ. Global disparity in the resilience of coral reefs. Trends in Ecology & Evolution. 2012;27:404–413. doi: 10.1016/j.tree.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Rogosic J, Estell RE, Skobic D, Martinovic A, Maric S. Role of species diversity and secondary compound complementarity on diet selection of Mediterranean shrubs by goats. Journal of Chemical Ecology. 2006;32:1279–1287. doi: 10.1007/s10886-006-9084-1. [DOI] [PubMed] [Google Scholar]
- Selig ER, Bruno JF. A global analysis of the effectiveness of marine protected areas in preventing coral loss. Plos One. 2010;5:e9278. doi: 10.1371/journal.pone.0009278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp PJ, V, Paul J. Calcium carbonate and secondary metabolites in tropical seaweeds: variable effects on herbivorous fishes. Ecology. 1994;75:1172–1185. [Google Scholar]
- Stockwell B, Jadloc CRL, Abesamis RA, Alcala AC, Russ GR. Trophic and benthic responses to no-take marine reserve protection in the Philippines. Marine Ecology-Progress Series. 2009;389:1–15. [Google Scholar]
- Worm B, Barbier EB, Beaumont N, Duffy JE, Folke C, Halpern BS, Jackson JBC, Lotze HK, Micheli F, Palumbi SR, Sala E, Selkoe KA, Stachowicz JJ, Watson R. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]