Abstract
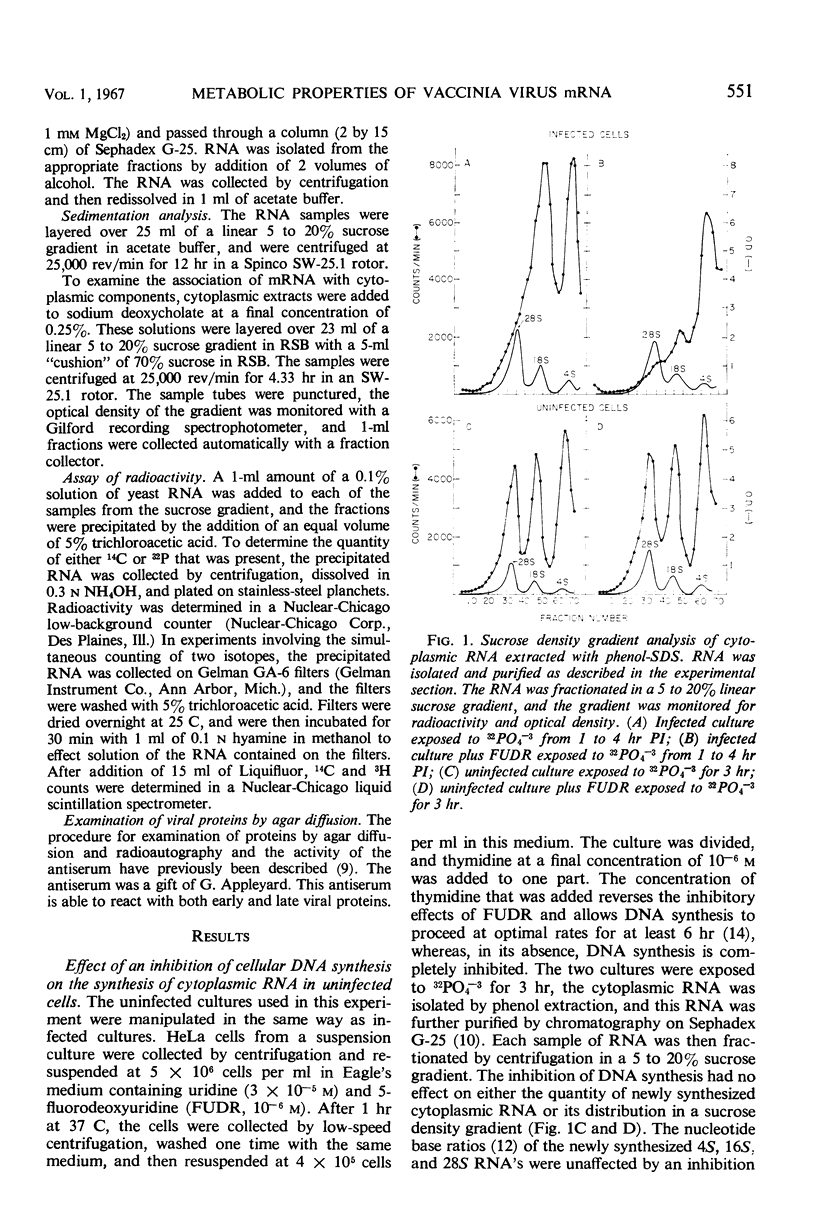
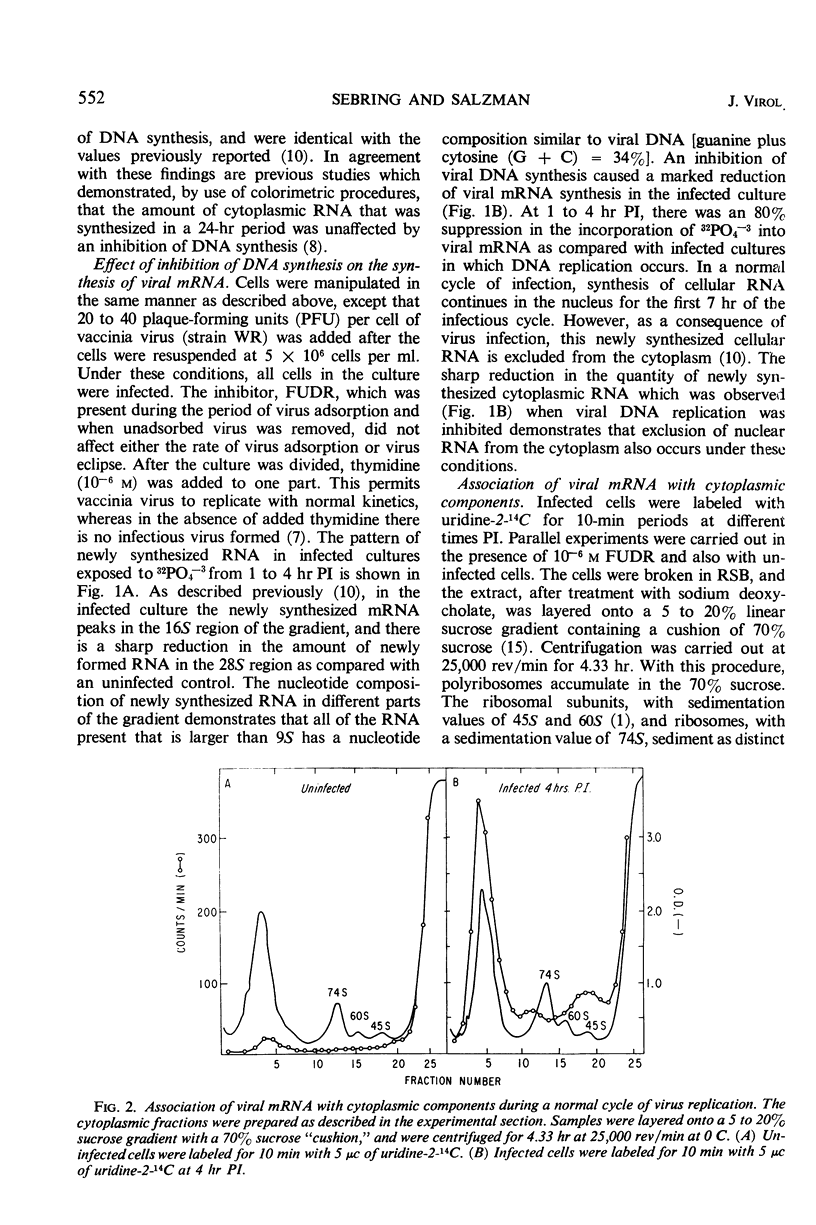
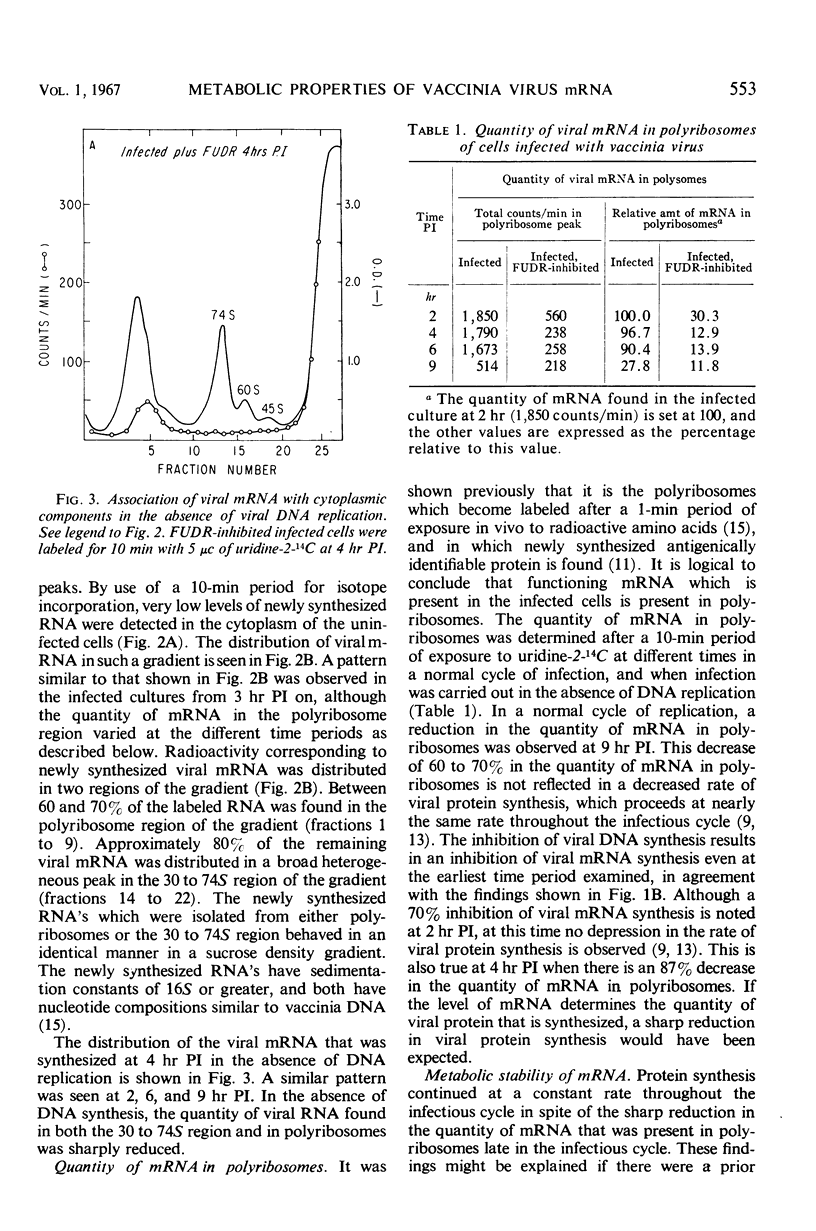
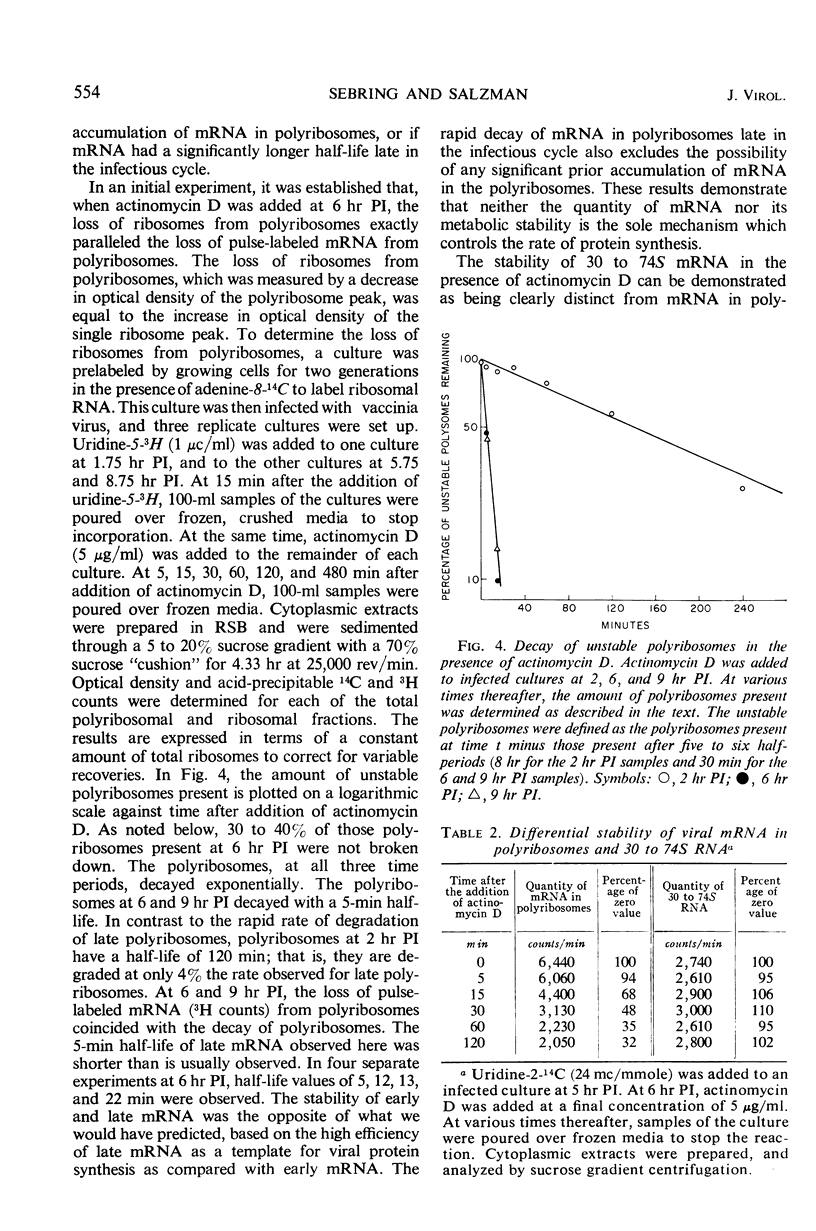
In vaccinia-infected cells, 60% of the viral messenger ribonucleic acid (mRNA) was associated with polyribosomes, and the remainder sedimented in a broad peak in the 30 to 74S region. The quantity of mRNA in polyribosomes was sharply reduced late in the infectious cycle [9 hr postinfection (PI)] to less than 30% of the 2-hr value. However, protein synthesis proceeded at a nearly constant rate from 2 to 13 hr PI. This ability of small quantities of late mRNA to support as much protein synthesis as do the much larger quantities of early mRNA was not due to an increase in stability, since late mRNA decays with a half-life of 13 min, whereas early mRNA has a half-life of 120 min. A similar decrease in viral mRNA synthesis without an accompanying decrease in viral protein synthesis was observed when deoxyribonucleic acid synthesis is inhibited. In contrast to the rapid decay of the late mRNA which was present in polyribosomes, the mRNA which sedimented in the 30 to 74S region remained unchanged even after a 2-hr period of exposure to actinomycin. The rate at which infected cells lose the capacity to synthesize specific viral proteins after exposure to actinomycin D was consistent with the half-life values of early and late mRNA that were observed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GIRARD M., LATHAM H., PENMAN S., DARNELL J. E. ENTRANCE OF NEWLY FORMED MESSENGER RNA AND RIBOSOMES INTO HELA CELL CYTOPLASM. J Mol Biol. 1965 Feb;11:187–201. doi: 10.1016/s0022-2836(65)80050-x. [DOI] [PubMed] [Google Scholar]
- Guthrie G. D., Buchanan J. M. Control of phage-induced enzymes in bacteria. Fed Proc. 1966 May-Jun;25(3):864–873. [PubMed] [Google Scholar]
- Joklik W. K., Becker Y. Studies on the genesis of polyribosomes. II. The association of nascent messenger RNA with the 40 S subribosomal particle. J Mol Biol. 1965 Sep;13(2):511–520. doi: 10.1016/s0022-2836(65)80113-9. [DOI] [PubMed] [Google Scholar]
- Joklik W. K. The poxviruses. Bacteriol Rev. 1966 Mar;30(1):33–66. doi: 10.1128/br.30.1.33-66.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCAUSLAN B. R. THE INDUCTION AND REPRESSION OF THYMIDINE KINASE IN THE POXVIRUS-INFECTED HELA CELL. Virology. 1963 Nov;21:383–389. doi: 10.1016/0042-6822(63)90199-5. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P., SEBRING E. D. The coupled formation of deoxyribonucleic acid, nuclear ribonucleic acid and protein in animal-cell cultures. Biochim Biophys Acta. 1962 Sep 17;61:406–413. doi: 10.1016/0926-6550(62)90143-3. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D. THE SYNTHESIS OF A DNA-LIKE RNA IN THE CYTOPLASM OF HELA CELLS INFECTED WITH VACCINIA VIRUS. J Mol Biol. 1964 Mar;8:405–416. doi: 10.1016/s0022-2836(64)80204-7. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P. The rate of formation of vaccinia deoxyribonucleic acid and vaccinia virus. Virology. 1960 Jan;10:150–152. doi: 10.1016/0042-6822(60)90015-5. [DOI] [PubMed] [Google Scholar]
- SCHARFF M. D., SHATKIN A. J., LEVINTOW L. ASSOCIATION OF NEWLY FORMED VIRAL PROTEIN WITH SPECIFIC POLYRIBOSOMES. Proc Natl Acad Sci U S A. 1963 Oct;50:686–694. doi: 10.1073/pnas.50.4.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEBRING E. D., SALZMAN N. P. AN IMPROVED PROCEDURE FOR MEASURING THE DISTRIBUTION OF P32O4--AMONG THE NUCLEOTIDES OF RIBONUCLEIC ACID. Anal Biochem. 1964 May;8:126–129. doi: 10.1016/0003-2697(64)90177-0. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J., SALZMAN N. P. Deoxyribonucleic acid synthesis in vaccinia virus-infected HeLa cells. Virology. 1963 Apr;19:551–560. doi: 10.1016/0042-6822(63)90050-3. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J., SEBRING E. D., SALZMAN N. P. VACCINIA VIRUS DIRECTED RNA: ITS FATE IN THE PRESENCE OF ACTINOMYCIN. Science. 1965 Apr 2;148(3666):87–90. doi: 10.1126/science.148.3666.87. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J. The formation of vaccinia virus protein in the presence of 5-fluorodeoxyuridine. Virology. 1963 Jun;20:292–301. doi: 10.1016/0042-6822(63)90118-1. [DOI] [PubMed] [Google Scholar]
- Salzman N. P., Sebring E. D. Sequential formation of vaccinia virus proteins and viral deoxyribonucleic acid replication. J Virol. 1967 Feb;1(1):16–23. doi: 10.1128/jvi.1.1.16-23.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subak-Sharpe H. An animal virus with DNA of high guanine + cytosine content which codes for S-RNA. J Mol Biol. 1965 Jul;12(3):924–928. doi: 10.1016/s0022-2836(65)80339-4. [DOI] [PubMed] [Google Scholar]



