Abstract
Lupus nephritis is an immune complex GN that develops as a frequent complication of SLE. The pathogenesis of lupus nephritis involves a variety of pathogenic mechanisms. The extrarenal etiology of systemic lupus is based on multiple combinations of genetic variants that compromise those mechanisms normally assuring immune tolerance to nuclear autoantigens. This loss of tolerance becomes clinically detectable by the presence of antinuclear antibodies. In addition, nucleic acids released from netting or apoptotic neutrophils activate innate and adaptive immunity via viral nucleic acid-specific Toll-like receptors. Therefore, many clinical manifestations of systemic lupus resemble those of viral infection. In lupus, endogenous nuclear particles trigger IFN-α signaling just like viral particles during viral infection. As such, dendritic cells, T helper cells, B cells, and plasma cells all contribute to the aberrant polyclonal autoimmunity. The intrarenal etiology of lupus nephritis involves antibody binding to multiple intrarenal autoantigens rather than the deposition of circulating immune complexes. Tertiary lymphoid tissue formation and local antibody production add to intrarenal complement activation as renal immunopathology progresses. Here we provide an update on the pathogenic mechanisms that lead to lupus nephritis and provide the rationale for the latest and novel treatment strategies.
SLE is a chronic autoimmune disease characterized by loss of tolerance against nuclear autoantigens, lymphoproliferation, polyclonal autoantibody production, immune complex disease, and multiorgan tissue inflammation.1,2 SLE used to be referred to as a complex autoimmune disease of unknown etiology; however, during the last decade, a multidisciplinary approach to SLE research has built a more concise view of its pathogenesis and for lupus nephritis (LN). Here we briefly summarize an updated working model of SLE and LN, which provides a rationale for novel therapies.
Extrarenal Pathogenic Mechanisms of LN
Cell Death and Dead Cell Handling
SLE develops from a loss of self-tolerance to ubiquitous nuclear autoantigens, which is a result of an immunization process. This observation implies two notions (Figure 1 and Table 1). First, autoreactive, long-lived plasma cells, and memory T cells memorize their immunization against nuclei. These cells cannot be deleted by current immunosuppressive therapies; hence, current treatments may suppress disease activity but do not cure SLE.2,3 Second, the nuclear antigens used for immunization had to be accessible to antigen-presenting cells, a process that is normally avoided by the homeostatic mechanism of rapid dead cell clearance. In fact, SLE develops in individuals with unfortunate combinations of genetic variants that, among other immunoregulatory defects, compromise those mechanisms that normally assure low levels of chromatin in extracellular compartments, particularly mutations that alter apoptosis,4,5 the opsonization of dead cells by complement, or their removal by phagocytes.6 Neutrophils undergo NETosis, which releases nucleosomes into the extracellular extracellular space.7–10 This finding recently revealed an unexpected role of neutrophils in SLE.11 But how do dead cell clearance defects lead to SLE?
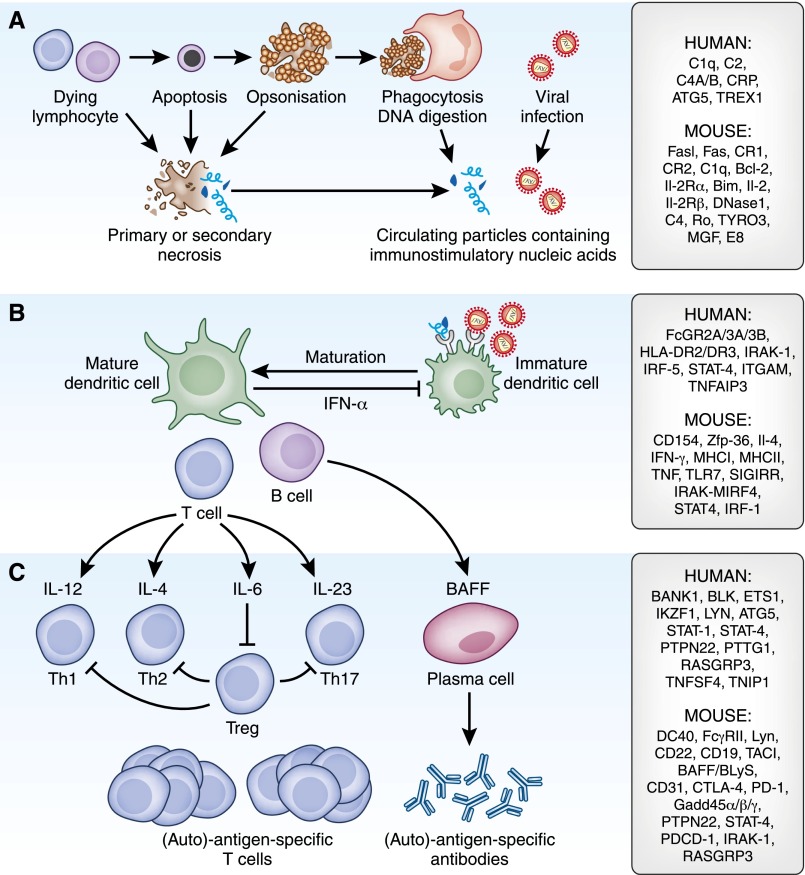
Figure 1.
Pathomechanisms of LN outside the kidney. (A) Genetic variants of homeostatic cell death (i.e., Fas variants) and the rapid clearance of dead cell corpses (e.g., C3/4 variants or DNAses variants) result either in secondary necrosis or incomplete chromatin digestion, which both promote the exposure of nuclear particles to the immune system. (B) Nuclear particles resemble viral particles and activate the same viral nucleic acid recognition receptors on antigen-presenting cells. Genetic variants of those signaling elements are recognized to be risk factors for SLE. The activation of antigen-presenting cells changes (by costimulation) the immune interpretation of concomitantly presented antigens of the same particle. (C) Polyclonal lymphocyte expansion has multiple effects on the disease process and genetic variants further affect the differentiation of T helper cells. The complex regulation of lymphocyte activation and expansion is affected by multiple genetic variants. The susceptibility genes and genes/molecules that are involved within each biologic pathway are listed to the right: C1q, C2, C4A/B, C-reactive protein (CRP), α-glucoside transporter 5 (ATG5), three prime repair exonuclease 1 (TREX1), B cell CLL/lymphoma 2 (Bcl-2), IL-2 receptor α (IL-2Rα), tyrosine-protein kinase receptor 3 (TYRO3), mast/stem cell growth factor (MGF), Fcγ receptor (FcGR), HLA IL-1 receptor–associated kinase (HLA IRAK), IFN regulatory factor (IRF), signal transducer and activator of transcription (STAT), integrin αM (ITGAM), TNF α-induced protein 3 (TNFAIP3), zinc finger protein 36 (Zfp-36), IL-4, IFN-γ, MHCI, MHCII, TNF, TLR7, single Ig and Toll-IL 1 receptor (SIGIRR), B cell scaffold protein with ankyrin repeats 1 (BANK1), B lymphoid tyrosine kinase (BLK), IKAROS family zinc finger 1 (IKZF1), protein tyrosine phosphatase, nonreceptor type 22 (PTPN22), pituitary tumor-transforming 1 (PTTG1), RAS guanyl releasing protein 3 (RASGRP3), TNF (ligand) superfamily, member 4 (TNFSF4), TNFAIP3-interacting protein 1 (TNIP1), transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI), BAFF/BLyS, cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1/PDCD-1), and Gadd45 (activated by the stress-inducible GADD45).
Table 1.
Pathomechanisms of LN inside the kidney
| Glomerular Pathology | Tubulointerstitial Pathology |
|---|---|
| Mesangial and subendothelial, immune complex deposits, complement activation | Immune complex deposits in periglomerular vessels |
| Fc, Toll-like, and complement receptor activation | Complement activation |
| Activation of renal cells and infiltrating leukocytes (subepithelial IC causes LN class V and podocyte injury with massive proteinuria) | Activation of endothelial cells, luminal adhesion molecules |
| Local cytokine expression | Leukocyte recruitment |
| Recruitment of leukocytes | Local antibody production by B cells including tertiary lymphoid organ formation |
| Proliferation of endothelial and mesangial cells | Cytotoxic and Th17 T cells |
| Filtration barrier damage causing proteinuria and hematuria | Proapoptotic cytokines |
| Renal cell necrosis causing focal scaring | Proximal tubular cell damage causing proteinuria |
| Proliferation of parietal epithelial cells and crescent formation | Tubular/vascular atrophy |
| Periglomerular inflammation | Hypoxia → inflammation |
| Global glomerulosclerosis | Insufficient tubular and vascular repair plus ischemia promotes interstitial fibrosis |
Induction of Antiviral Immunity
A delay of dead cell removal leads to degeneration of its components, which compromises those elements that normally distinguish self-nucleic acids from viral nucleic acids.12,13 For example, nature developed the methylation of DNA and RNA as a way to inhibit RNA and DNA recognition by Toll-like receptors (TLRs) 3, 7, and 9, a set of endosomal viral nucleic acid recognition receptors that trigger antiviral immunity during viral infection.14 Therefore, in SLE patients, nuclear particles are taken as viral particles that contain some protein component (antigen) as well as some immunostimulatory nucleic acid (immune adjuvant; Figure 1).
During evolution, our immune system was primed to mount potent antiviral immunity upon the recognition of viral particles, a response that is initiated against the components of virus-like nuclear particles in SLE patients. For example, ribonucleoprotein, U1snRNP, ligates TLR7 to induce type I IFN release in plasmacytoid dendritic cells,15 a process that is tightly controlled by IL-1 receptor–associated kinase-M.16 RNA immune complexes activate B cells to produce antinuclear antibodies,17 which is controlled by the TIR8 gene encoding for the SIGIRR protein.18,19 Nucleosomal DNA or DNA within immune complexes can activate TLR9 on plasmacytoid dendritic cells and drive B cell proliferation.20 Blockade of TLR7, TLR9, or both abrogates type I IFN induction, SLE, and LN in mice.21–23 This (pseudo)antiviral immune response involves all antigen-presenting cells, particularly dendritic cells and B cells, but only plasmacytoid dendritic cells secrete large amounts of type I IFNs to set off an antiviral immune response.5,24 The signature for IFN receptor–dependent secondary gene expression includes multiple antiviral and proliferative genes such as IFIT1, MX1, MX2, ISG15, and the OAS gene family, the IFN regulatory factors IRF7 and IRF5, as well as proinflammatory chemokines CXCL10 and CXCL5, which altogether account for the nonspecific symptoms shared by viral infections and SLE, such as fever, fatigue, arthralgia, and myalgia (Figure 2).25
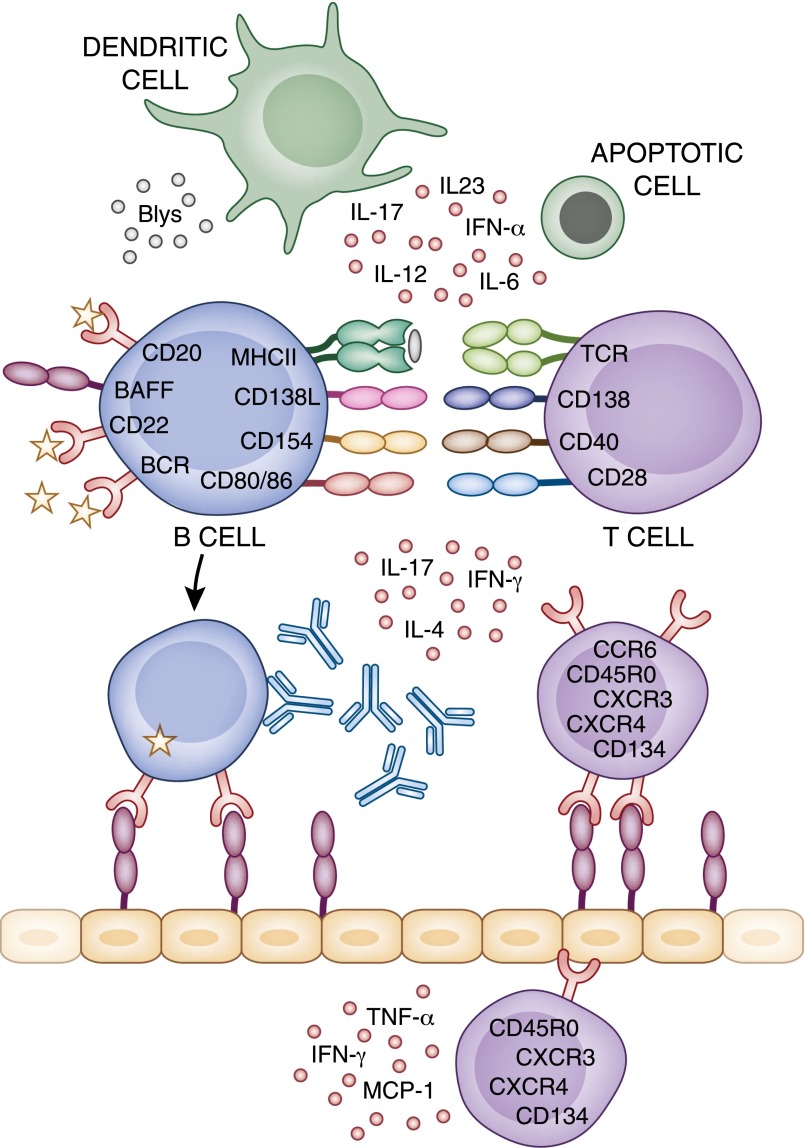
Figure 2.
Therapeutic targets for LN. Aberrant immunity in SLE involves many different cell types and cytokine mediators, which could be suitable therapeutic targets. MCP-1, monocyte chemotactic protein-1; CCR, CC chemokine receptor; CXCR, C-X-C chemokine receptor.
Aberrant Lymphocyte Proliferation
Dendritic cells and B cells both have the capacity to process antigens and present antigens to T cells and they can substitute each other for this purpose.26 Dendritic cells have a limited life span but their persistent activation by lupus autoantigens by TLR7 and TLR9 enhances their survival and renders them resistant to glucocorticoid-induced death.21 Persistent activation of antigen-presenting cells turns the interpretation of autoantigens from immune ignorance and lymphocyte anergy into lymphocyte activation and proliferation, which can overcome the functional unresponsiveness or anergy of mature autoreactive B cells.27 Murine double minute-2 is one of these mitogenic factors that is specifically induced by DNA recognition.28 Murine double minute-2 neutralizes p53-dependent cell cycle arrest, which explains the mitogenic effect of endogenous DNA or DNA viruses on autoreactive lymphocytes in SLE. Other lymphocyte mitogens are B lymphocyte stimulator (BlyS, also named B cell activating factor [BAFF]) and a proliferation-inducing ligand (APRIL), which regulate B cell differentiation and Ig class switching.29 The two APRIL/BLyS receptors transmembrane activator and CAML-interactor (TACI) and B cell maturation protein promote plasma cell survival; therefore, they are currently considered as potential therapeutic targets in SLE.30
Furthermore, TNF-like weak inducer of apoptosis, a proinflammatory cytokine of the TNF superfamily, regulates multiple processes in autoimmunity and tissue inflammation through activation of the fibroblast growth factor–inducible 14 receptor. B cell maturation toward autoantibody-producing plasma cells involves IFN-related factor-4.31
Environmental Triggers of SLE Activity
Viral infections induce IFN-α release, which triggers antiviral immunity as well as lupus disease activity.25 Bacterial infections have a nonspecific immunostimulatory effect, which involves a transient expansion of autoreactive lymphocyte clones. Furthermore, bacterial products stimulate intrarenal immune cells and renal cells, which can trigger a transient aggravation of proteinuria and kidney damage. Another environmental trigger of SLE activity is ultraviolet light, which induces an increase in the load of dead cells by causing keratinocyte death.32 In patients with a significant dead cell clearance defect, this process will lead to increased levels of extracellular nuclear material, and additional exposure of autoantigens and autoadjuvants to the immune system.1 Drug-induced SLE involves inhibition of methyl-transferases, a process that enhances the unmasking of endogenous nucleic acids and the activation of TLR7 and TLR9.33,34 Progesterone and estrogens stimulate the sex hormone–dependent immunoregulatory pathways.35
Together, SLE develops from a peculiar combination of genetic variants that impair those mechanisms that normally prevent the exposure of nuclear particles to the immune system and their capacity to activate viral recognition nucleic acid receptors (Figure 1). Therefore, an IFN-α–dependent (pseudo)antiviral immune response accounts for those nonspecific SLE symptoms that are shared with viral infections. The autoadjuvant activity of endogenous nucleic acids promotes an adaptive immune response against the components of the nuclear particle, a process identical to vaccination. This implies the expansion of T and B cell clones with specificities for predominantly nuclear autoantigens that account for the production of antinuclear antibodies, immune complex disease, and T cell–dependent tissue damage. Hormonal and environmental stimuli can enhance these processes at different levels.
Intrarenal Pathogenic Mechanisms of LN
Immune Complex-Mediated Renal Immunopathology
The nonspecific activation of autoreactive B cells explains the polyclonal autoantibody response leading to the diagnostic hallmark of LN, the full house pattern of IgM, IgA, and IgG deposits.36 However, antibody-deficient mice still develop LN; therefore, B cells have pathogenic effects beyond antibody production,37 including autoantigen presentation to activate autoreactive T cells and local proinflammatory effects.26 Immune complexes deposit in the mesangium or the subendothelial and subepithelial spaces or in peritubular capillaries depending on the quality of the autoantibodies, the duration, and severity of LN.38 This implies that immune complex formation in the mesangium causes class I and II lesions, subendothelial immune complex formation in class III and IV lesions, and subepithelial immune complexes in class V lesions as well as the overlapping forms III/IV and IV/V.39 The traditional concept that circulating immune complexes in lupus passively deposit in the kidney has been challenged by novel data.40,41 Glomerular immune complexes rather form in situ by secondary binding to nucleosomes from renal cells.42 Another potential intrarenal source of nucleosomes are neutrophils upon NETosis,11 due to the release of neutrophil extracellular traps that is initiated by anti-LL37 antibodies.7–10 Heparin modulates the intrarenal effect of chromatin either by enhancing the DNA-I–dependent chromatin degradation or by preventing chromatin binding to the glomerular basement membrane.43 Anti-DNA antibodies activate endothelial and mesangial cells through different mechanisms. For example, antibodies are directly taken up inside renal cells.41 This process involves cross-reactivity with α-actinin or annexin II on mesangial cells,44,45 but this concept could not be confirmed by recent studies.46,47 In addition, intrarenal immune complex deposits activate complement,36 which demonstrates the dual role of complement factors in LN.48 Complement deficiency impairs opsonization and removal of lupus autoantigens from the extracellular space, whereas complement factors also directly cause immune complex–related renal inflammation and immunopathology.49,50 That immune complexes activate glomerular cells by Fc receptor (FcR) ligation is also well established even though the data accumulated over the last few decades remain complex.51 Subepithelial immune complex deposits lead to secondary membranous GN and nephrotic syndrome by damaging podocytes.
Intrarenal Activation of TLRs and IFN Signaling
The nucleic acid component of immune complexes also activates intrarenal inflammation by TLRs in intrarenal macrophages and dendritic cells.52 In addition, immunostimulatory nucleic acids activate glomerular endothelium, mesangial cells, and macrophages to produce large amounts of proinflammatory cytokines and IFN-α and IFN-β.53–59 The functional significance of this intraglomerular IFN signaling is poorly understood but seems to contribute to renal damage in LN and should trigger the formation of tubuloreticular structures or inclusions that represent an ultrastructural characteristic of IFN signaling.56,60 Together, the ligation of TLRs, complement receptors, and FcRs activates renal cells to release proinflammatory cytokines and chemokines, and induces the luminal expression of selectins and adhesion molecules inside the microvasculature.61,62
Chemokine-Mediated Recruitment of Different Leukocyte Subsets
Cytotoxic T cells, Th17 T cells, as well as B cells infiltrate the kidney in LN.24 The members of the chemokine family specifically direct different leukocyte subsets by distinct chemokine receptors into different renal compartments.63 For example, the chemokine CCL2 recruits CCR2+ proinflammatory macrophages and T cells into the glomerulus and the tubulointerstitium,64,65 whereas CCR1+ cells only recruit to the interstitial compartment and not to the glomerulus in LN.66 Other leukocyte subsets involve other chemokines and chemokine receptors for their recruitment into the kidney.63,67 Leukocyte recruitment is tightly regulated. For example, renal cells produce pentraxin-3, which has the potential to directly inhibit leukocyte recruitment by interfering with P-selectin on the surface of activated endothelial cells.68,69 In lupus-like autoimmunity of C57BL/6lpr/lpr mice, the role of pentraxin-3 seems to be organ specific, because it suppresses lymphocyte recruitment to the lungs but not to the kidney.70 Infiltrating leukocytes form de novo perivascular tertiary lymphoid organs inside the kidney, which involve the clonal expansion and ongoing somatic hypermutation of B cells in proximity to T cell aggregates.71 Such B cells undergo intrarenal proliferation and activation, which contributes to local inflammation and tissue pathology in addition to their role for systemic and intrarenal autoantibody production.26,72,73 These data provide a rationale for B cell–targeted therapies in LN.74,75 T cell infiltrates also contribute to immunopathology in LN, particularly IL-17 producing CD3+/CD4+ or CD3+CD4/8−/− T cells.76 Macrophages also contribute to renal damage, particularly F4/80(hi)/CD11c(int)Gr1(lo)/Ly6C(lo)/VLA4(lo)/MHCII(hi)/CD43(lo)/CD62L(lo) macrophages.77,78
Maladaptive Tissue Repair Contributes to CKD Progression
Damage to renal parenchymal cells triggers healing responses that contribute to renal pathology. Focal tuft necrosis is followed by a migration of parietal epithelial cells in the glomerular tuft, where they produce extracellular matrix contributing to FSGS progressing to global glomerulosclerosis.79 During this process, the parietal cells maintain their polarized epithelial phenotype and lay down extracellular matrix on top of the podocytes.79 In addition, cellular glomerular crescent formation results from activation of parietal epithelial cells that fill the urinary space by uncoordinated proliferation.80,81 This process can be triggered by glomerular basement membrane breaks that allow plasma leakage into Bowman’s space, where mitogenic plasma components such as fibrinogen trigger hyperproliferation of the parietal epithelial cells.82 At later stages, the parietal epithelial cells lose their polarity and produce matrix all around themselves, which creates honeycomb matrix deposits in Bowman’s space that turn cellular crescents into fibrocellular crescents with glomerulosclerosis, also referred to as class VI LN.
The Present and Future of LN Therapy
Nonselective Immunosuppressants
Steroids, cyclophosphamide, azathioprine, and mycophenolate mofetil remain first-line therapeutics for treatment of LN. These nonselective immunosuppressants have much improved the response rates of acute manifestations and the overall mortality of SLE; however, the long-term outcomes of LN have not further improved during the last 30 years.83 High-dose steroids and cyclophosphamide are frequently associated with severe side effects, and infections contribute to the overall mortality in SLE. The same applies to the other nonselective immunosuppressants. For example, in the ALMS (Apreva Lupus Management) trial, infection-related mortality was even higher in patients treated with mycophenolate mofetil than in the group treated with high-dose cyclophosphamide. Reducing the drug dose was the first strategy to limit side effects, and some researchers wonder whether oral cyclophosphamide therapy is no longer needed.84 In addition, some studies suggest that Caucasian patients may no longer need high-dose cyclophosphamide, because the Euro-Lupus trial demonstrated favorable long-term outcomes with much lower doses of cyclophosphamide.85 Other immunosuppressants like dihydroorotate dehydrogenase inhibitors add to the current choices but are still nonspecific.86,87 However, lupus drug developers continue to search for new drugs that more specifically modulate the aberrant immunity in SLE with fewer side effects.
Novel Moieties that Target Specific Leukocyte Subsets
One of the current strategies to specifically interfere with systemic autoimmunity in SLE is to use cell type–specific drugs. For example, great hope was put into the strategy of B cell–directed therapy as B cells are the source of autoantibody production.88 Meanwhile, it is clear that B cells in SLE also contribute to autoimmunity and tissue inflammation in many other ways, which increases the hope that depleting or modulating B cells would result in major benefits in SLE and LN.89 This led to the development of the fully humanized anti-CD20 antibody (rituximab), the anti-CD22 antibody (epratuzumab), and to anti-BlyS (belimumab). Because the randomized placebo-controlled Lupus Nephritis Assessment with Rituximab trial failed to demonstrate a benefit of add-on rituximab for the induction therapy of incident LN class III/IV/V, this approach to B cell depletion still has questions.90,91 At about the same time, the Exploratory Phase II/III SLE Evaluation of Rituximab trial also failed to demonstrate benefits on nonrenal lupus.91 However, uncontrolled studies on refractory LN still document 75% responder rates and many specialists continue using rituximab successfully for these patients.92
BLyS blockade is another promising strategy to target B cell proliferation. A large randomized placebo-controlled trial suggested that add-on belimumab on top of standard maintenance therapy can significantly improve persistent disease activity up to 72 weeks.93 This study led to the US Food and Drug Administration and European Medicines Agency approval of belimumab for nonrenal lupus in the United States and Europe. Patients with severe LN were excluded from the BLISS-56 and BLISS-76 trials, but data from those patients with moderate nephritis raise hope that belimumab could also be efficient in severe LN.94 Such a trial is currently underway. Other B cell–directed strategies include atacicept (TACI-Ig), LY2127399 (anti-BAFF), and anti-BR3 (anti-BAFF-R).89
Dendritic cell–T cell interaction is a target of costimulatory ligand/receptor blockers such as CTLA-4-Ig (abatacept). This drug blocks the interaction of CD80 and CD86 on antigen-presenting cells with CD28 on T cells, which suppresses T cell activation.95 Abatacept suppressed lupus in mice but did not prevent flares in SLE patients.96 Three trials with anti-CD40L failed to demonstrate efficacy. Abetimus is a drug that modulates autoimmunity by altering antigen recognition by T cells. Abetimus is composed of a series of linked oligonucleotides, which block the binding of anti-dsDNA antibodies to their autoimmune targets and tolerize B cells with antigen specificity for DNA. Unfortunately, the results in clinical trials have been very modest. A similar approach is followed by edratide, a peptide derived from the antigen-binding region of a human monoclonal anti-dsDNA antibody. It has been proposed that this molecule can modulate the function of DNA-reactive B cells through idiotype–anti-idiotype interactions but again, the convincing data on efficacy are still lacking.
The concept of anti-dsDNA–specific therapeutic interventions is no longer preferred because it targets only a very small subset of B cells, and no longer seems sufficient in view of the failing B cell depletion trials and the fact that dsDNA antibodies certainly contribute to SLE but remain only one of many different pathogenic elements. Finally, plasma cells now appear as an attractive therapeutic target in SLE because they harbor the long-term memory of humoral immunity and produce the lupus autoantibodies.97 Rituximab does not deplete plasma cells, because these are negative for CD20.97 Massive antibody production in plasma cells involves the intracellular proteasome complex for protein processing. The proteasome inhibitor bortezomib was proven to be effective in mouse models of LN,98,99 but clinical trials with bortezomib in human LN is still pending.
Additional Innovative Therapeutic Strategies
The pseudo-antiviral immunity concept is based on the molecular mimicry of endogenous nucleic acids at the viral nucleic acid recognition receptors TLR7 and TLR9.100 Hence, blocking these TLRs and the subsequent IFN signaling are additive to established therapeutic targets in SLE.101 Current treatment guidelines recommend hydroxychloroquine treatment for all SLE patients, including all patients with LN.102–104 Antimalarial drugs like hydroxychloroquine inhibit lysosomal acidification, which blocks the adjuvant effect of endogenous nucleic acids by TLR7 and TLR9 during the lysosomal processing of nuclear particles in endolysosomal compartments of antigen-presenting cells.20 Meanwhile, more specific TLR7 and TLR9 agents have been developed that effectively suppressed LN in murine SLE models and are now being tested in clinical trials.21–23,105 Antagonism of IFN-α is feasible with IFN-α antibodies. A double-blind randomized study with sifalimumab, a fully human anti-IFN-α mAb, demonstrated that IFNα drives the overexpression of IFN-dependent genes in human SLE, which is reversed by sifalimumab.106 Other cytokine-directed innovative therapies include anti-Tweak (ATLAS trial) as well as anti-IL6R (tocilizumab). The current status for off-label use of these drugs was recently summarized.30,107 Finally, it remains an attractive notion to add anti-inflammatory agents to immunosuppressive drugs to reduce the degree of therapeutic immunosuppression and the risk of therapy-related infections. As a proof of concept, the combination therapy of the CCL2 chemokine antagonistic Spiegelmer, mNOX-E36, and 25% of full-dose cyclophosphamide was shown to be as effective as 100% cyclophosphamide to control severe SLE in MRLlpr/lpr mice108; thus, the cyclophosphamide-related T cell ablation and myelosuppression could be prevented, while maintaining treatment efficacy.108
Summary
The pathogenesis of LN involves extrarenal and intrarenal pathogenic mechanisms. The extrarenal factors include complex combinations of genetic variants that are different in each patient, which explains the variability of clinical manifestations. SLE develops when genetic variants compromise those mechanisms that normally assure immune tolerance for nuclear autoantigens. Loss of tolerance becomes clinically evident by the presence of antinuclear antibodies. The nucleic acid content of nuclear particles from netting or apoptotic neutrophils activates innate and adaptive immunity by TLR7 and TLR9, which triggers an IFN-α–mediated antiviral host defense program that accounts for many of the nonspecific SLE symptoms. As such, dendritic cells, T helper cells, B cells, and plasma cells all contribute to the aberrant polyclonal autoimmunity. The intrarenal etiology of LN involves antibody binding to intrarenal nuclear autoantigens, local complement, and FcR activation. Tertiary lymph follicles, to some degree, form inside the kidney, which include B cells with local proinflammatory effects as well as plasma cells that secrete autoantibody inside the kidney. These insights into the pathogenesis of lupus provide the rationale for a number of novel therapeutic targets.
Disclosures.
None.
Acknowledgments
This work was supported by the German Research Foundation (grants AN372/11-1 and GRK 1202).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Liu Z, Davidson A: Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med 18: 871–882, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsokos GC: Systemic lupus erythematosus. N Engl J Med 365: 2110–2121, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Goodnow CC: Multistep pathogenesis of autoimmune disease. Cell 130: 25–35, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Saxena R, Mahajan T, Mohan C: Lupus nephritis: Current update. Arthritis Res Ther 13: 240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Migliorini A, Anders HJ: A novel pathogenetic concept-antiviral immunity in lupus nephritis. Nat Rev Nephrol 8: 183–189, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Muñoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M: The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol 6: 280–289, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A: Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 107: 9813–9818, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, Shealy D, Denny MF, Plumas J, Chaperot L, Kretzler M, Bruce AT, Kaplan MJ: Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 187: 538–552, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V: Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 3: 73ra20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M: Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 3: 73ra19, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosch X: Systemic lupus erythematosus and the neutrophil. N Engl J Med 365: 758–760, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Hof D, Raats JM, Pruijn GJ: Apoptotic modifications affect the autoreactivity of the U1 snRNP autoantigen. Autoimmun Rev 4: 380–388, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Huck S, Deveaud E, Namane A, Zouali M: Abnormal DNA methylation and deoxycytosine-deoxyguanine content in nucleosomes from lymphocytes undergoing apoptosis. FASEB J 13: 1415–1422, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Karikó K, Ni H, Capodici J, Lamphier M, Weissman D: mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem 279: 12542–12550, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Savarese E, Chae OW, Trowitzsch S, Weber G, Kastner B, Akira S, Wagner H, Schmid RM, Bauer S, Krug A: U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood 107: 3229–3234, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Savarese E, Steinberg C, Pawar RD, Reindl W, Akira S, Anders HJ, Krug A: Requirement of Toll-like receptor 7 for pristane-induced production of autoantibodies and development of murine lupus nephritis. Arthritis Rheum 58: 1107–1115, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A: RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med 202: 1171–1177, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lech M, Kulkarni OP, Pfeiffer S, Savarese E, Krug A, Garlanda C, Mantovani A, Anders HJ: Tir8/Sigirr prevents murine lupus by suppressing the immunostimulatory effects of lupus autoantigens. J Exp Med 205: 1879–1888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lech M, Skuginna V, Kulkarni OP, Gong J, Wei T, Stark RW, Garlanda C, Mantovani A, Anders HJ: Lack of SIGIRR/TIR8 aggravates hydrocarbon oil-induced lupus nephritis. J Pathol 220: 596–607, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A: Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416: 603–607, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, Chan JH, Wright T, Punaro M, Bolland S, Soumelis V, Banchereau J, Coffman RL, Pascual V, Barrat FJ: TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature 465: 937–941, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patole PS, Zecher D, Pawar RD, Gröne HJ, Schlöndorff D, Anders HJ: G-rich DNA suppresses systemic lupus. J Am Soc Nephrol 16: 3273–3280, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Pawar RD, Ramanjaneyulu A, Kulkarni OP, Lech M, Segerer S, Anders HJ: Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J Am Soc Nephrol 18: 1721–1731, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ: Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity 33: 967–978, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theofilopoulos AN, Baccala R, Beutler B, Kono DH: Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol 23: 307–336, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ: A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med 189: 1639–1648, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zikherman J, Parameswaran R, Weiss A: Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature 489: 160–164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allam R, Sayyed SG, Kulkarni O, Lichtnekert J, Anders HJ: Murine double minute-2 drives systemic lupus erythematosus and lupus nephritis. J Am Soc Nephrol 22: 2016–2027, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z, Davidson A: BAFF and selection of autoreactive B cells. Trends Immunol 32: 388–394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gayed M, Gordon C: Novel treatments for systemic lupus erythematosus. Curr Opin Investig Drugs 11: 1256–1264, 2010 [PubMed] [Google Scholar]
- 31.Lech M, Weidenbusch M, Kulkarni OP, Ryu M, Darisipudi MN, Susanti HE, Mittruecker HW, Mak TW, Anders HJ: IRF4 deficiency abrogates lupus nephritis despite enhancing systemic cytokine production. J Am Soc Nephrol 22: 1443–1452, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caricchio R, McPhie L, Cohen PL: Ultraviolet B radiation-induced cell death: Critical role of ultraviolet dose in inflammation and lupus autoantigen redistribution. J Immunol 171: 5778–5786, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Cornacchia E, Golbus J, Maybaum J, Strahler J, Hanash S, Richardson B: Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J Immunol 140: 2197–2200, 1988 [PubMed] [Google Scholar]
- 34.Richardson B, Scheinbart L, Strahler J, Gross L, Hanash S, Johnson M: Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum 33: 1665–1673, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Hughes GC: Progesterone and autoimmune disease. Autoimmun Rev 11: A502–A514, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tojo T, Friou GJ: Lupus nephritis: Varying complement-fixing properties of immunoglobulin G antibodies to antigens of cell nuclei. Science 161: 904–906, 1968 [DOI] [PubMed] [Google Scholar]
- 37.Jacob N, Stohl W: Autoantibody-dependent and autoantibody-independent roles for B cells in systemic lupus erythematosus: Past, present, and future. Autoimmunity 43: 84–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu F, Wu LH, Tan Y, Li LH, Wang CL, Wang WK, Qu Z, Chen MH, Gao JJ, Li ZY, Zheng X, Ao J, Zhu SN, Wang SX, Zhao MH, Zou WZ, Liu G: Tubulointerstitial lesions of patients with lupus nephritis classified by the 2003 International Society of Nephrology and Renal Pathology Society system. Kidney Int 77: 820–829, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M, International Society of Nephrology Working Group on the Classification of Lupus Nephritis. Renal Pathology Society Working Group on the Classification of Lupus Nephritis : The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 65: 521–530, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Nowling TK, Gilkeson GS: Mechanisms of tissue injury in lupus nephritis. Arthritis Res Ther 13: 250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yung S, Chan TM: Autoantibodies and resident renal cells in the pathogenesis of lupus nephritis: Getting to know the unknown. Clin Dev Immunol 2012: 139365, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mortensen ES, Rekvig OP: Nephritogenic potential of anti-DNA antibodies against necrotic nucleosomes. J Am Soc Nephrol 20: 696–704, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Hedberg A, Fismen S, Fenton KA, Fenton C, Osterud B, Mortensen ES, Rekvig OP: Heparin exerts a dual effect on murine lupus nephritis by enhancing enzymatic chromatin degradation and preventing chromatin binding in glomerular membranes. Arthritis Rheum 63: 1065–1075, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Yung S, Cheung KF, Zhang Q, Chan TM: Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. J Am Soc Nephrol 21: 1912–1927, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Z, Deocharan B, Scherer PE, Ozelius LJ, Putterman C: Differential binding of cross-reactive anti-DNA antibodies to mesangial cells: The role of alpha-actinin. J Immunol 176: 7704–7714, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Mjelle JE, Rekvig OP, Van Der Vlag J, Fenton KA: Nephritogenic antibodies bind in glomeruli through interaction with exposed chromatin fragments and not with renal cross-reactive antigens. Autoimmunity 44: 373–383, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Fenton KA, Tømmerås B, Marion TN, Rekvig OP: Pure anti-dsDNA mAbs need chromatin structures to promote glomerular mesangial deposits in BALB/c mice. Autoimmunity 43: 179–188, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Sekine H, Ruiz P, Gilkeson GS, Tomlinson S: The dual role of complement in the progression of renal disease in NZB/W F(1) mice and alternative pathway inhibition. Mol Immunol 49: 317–323, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Sekine H, Kinser TT, Qiao F, Martinez E, Paulling E, Ruiz P, Gilkeson GS, Tomlinson S: The benefit of targeted and selective inhibition of the alternative complement pathway for modulating autoimmunity and renal disease in MRL/lpr mice. Arthritis Rheum 63: 1076–1085, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bao L, Haas M, Quigg RJ: Complement factor H deficiency accelerates development of lupus nephritis. J Am Soc Nephrol 22: 285–295, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niederer HA, Clatworthy MR, Willcocks LC, Smith KG: FcgammaRIIB, FcgammaRIIIB, and systemic lupus erythematosus. Ann N Y Acad Sci 1183: 69–88, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Allam R, Lichtnekert J, Moll A, Taubitz A, Vielhauer V, Anders HJ: Viral RNA and DNA trigger common antiviral responses in mesangial cells. J Am Soc Nephrol 20: 1986–1996, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fluer K, Allam R, Zecher D, Kulkarni O, Lichtnekert J, Schwarz M, Beutler B, Vielhauer V, Anders HJ: Viral RNA induces type I interferon-dependent cytokine release and cell death in mesangial cells via melanoma-differentiation-associated gene-5: Implications for viral infection-associated glomerulonephritis. Am J Pathol 175: 2014–2022, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hägele H, Allam R, Pawar RD, Anders HJ: Double-stranded RNA activates type I interferon secretion in glomerular endothelial cells via retinoic acid-inducible gene (RIG)-1. Nephrol Dial Transplant 24: 3312–3318, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Hägele H, Allam R, Pawar RD, Reichel CA, Krombach F, Anders HJ: Double-stranded DNA activates glomerular endothelial cells and enhances albumin permeability via a toll-like receptor-independent cytosolic DNA recognition pathway. Am J Pathol 175: 1896–1904, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fairhurst AM, Xie C, Fu Y, Wang A, Boudreaux C, Zhou XJ, Cibotti R, Coyle A, Connolly JE, Wakeland EK, Mohan C: Type I interferons produced by resident renal cells may promote end-organ disease in autoantibody-mediated glomerulonephritis. J Immunol 183: 6831–6838, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anders HJ, Lichtnekert J, Allam R: Interferon-alpha and -beta in kidney inflammation. Kidney Int 77: 848–854, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Triantafyllopoulou A, Franzke CW, Seshan SV, Perino G, Kalliolias GD, Ramanujam M, van Rooijen N, Davidson A, Ivashkiv LB: Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proc Natl Acad Sci U S A 107: 3012–3017, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anders HJ, Vielhauer V, Eis V, Linde Y, Kretzler M, Perez de Lema G, Strutz F, Bauer S, Rutz M, Wagner H, Gröne HJ, Schlöndorff D: Activation of toll-like receptor-9 induces progression of renal disease in MRL-Fas(lpr) mice. FASEB J 18: 534–536, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Rich SA: Human lupus inclusions and interferon. Science 213: 772–775, 1981 [DOI] [PubMed] [Google Scholar]
- 61.Allam R, Anders HJ: The role of innate immunity in autoimmune tissue injury. Curr Opin Rheumatol 20: 538–544, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Ito S, Chino Y, Goto D, Matsumoto I, Murata H, Tsutsumi A, Hayashi T, Uchida K, Usui J, Yamagata K, Sumida T: Laser microdissection-based analysis of cytokine balance in the kidneys of patients with lupus nephritis. Clin Exp Immunol 159: 1–10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kulkarni O, Anders HJ: Chemokines in lupus nephritis. Front Biosci 13: 3312–3320, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Kulkarni O, Pawar RD, Purschke W, Eulberg D, Selve N, Buchner K, Ninichuk V, Segerer S, Vielhauer V, Klussmann S, Anders HJ: Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J Am Soc Nephrol 18: 2350–2358, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Pérez de Lema G, Maier H, Franz TJ, Escribese M, Chilla S, Segerer S, Camarasa N, Schmid H, Banas B, Kalaydjiev S, Busch DH, Pfeffer K, Mampaso F, Schlöndorff D, Luckow B: Chemokine receptor Ccr2 deficiency reduces renal disease and prolongs survival in MRL/lpr lupus-prone mice. J Am Soc Nephrol 16: 3592–3601, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Anders HJ, Belemezova E, Eis V, Segerer S, Vielhauer V, Perez de Lema G, Kretzler M, Cohen CD, Frink M, Horuk R, Hudkins KL, Alpers CE, Mampaso F, Schlöndorff D: Late onset of treatment with a chemokine receptor CCR1 antagonist prevents progression of lupus nephritis in MRL-Fas(lpr) mice. J Am Soc Nephrol 15: 1504–1513, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Chong BF, Mohan C: Targeting the CXCR4/CXCL12 axis in systemic lupus erythematosus. Expert Opin Ther Targets 13: 1147–1153, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Bussolati B, Peri G, Salvidio G, Verzola D, Mantovani A, Camussi G: The long pentraxin PTX3 is synthesized in IgA glomerulonephritis and activates mesangial cells. J Immunol 170: 1466–1472, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, Cuccovillo I, Bastone A, Gobbi M, Valentino S, Doni A, Garlanda C, Danese S, Salvatori G, Sassano M, Evangelista V, Rossi B, Zenaro E, Constantin G, Laudanna C, Bottazzi B, Mantovani A: Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol 11: 328–334, 2010 [DOI] [PubMed] [Google Scholar]
- 70.Lech M, Römmele C, Kulkarni OP, Susanti HE, Migliorini A, Garlanda C, Mantovani A, Anders HJ: Lack of the long pentraxin PTX3 promotes autoimmune lung disease but not glomerulonephritis in murine systemic lupus erythematosus. PLoS ONE 6: e20118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, Kaverina N, Utset TO, Meehan SM, Quigg RJ, Meffre E, Clark MR: In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol 186: 1849–1860, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neusser MA, Lindenmeyer MT, Edenhofer I, Gaiser S, Kretzler M, Regele H, Segerer S, Cohen CD: Intrarenal production of B-cell survival factors in human lupus nephritis. Mod Pathol 24: 98–107, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Espeli M, Bökers S, Giannico G, Dickinson HA, Bardsley V, Fogo AB, Smith KG: Local renal autoantibody production in lupus nephritis. J Am Soc Nephrol 22: 296–305, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bekar KW, Owen T, Dunn R, Ichikawa T, Wang W, Wang R, Barnard J, Brady S, Nevarez S, Goldman BI, Kehry M, Anolik JH: Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum 62: 2443–2457, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, Davidson A: Selective blockade of BAFF for the prevention and treatment of systemic lupus erythematosus nephritis in NZM2410 mice. Arthritis Rheum 62: 1457–1468, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Apostolidis SA, Crispín JC, Tsokos GC: IL-17-producing T cells in lupus nephritis. Lupus 20: 120–124, 2011 [DOI] [PubMed] [Google Scholar]
- 77.Bethunaickan R, Berthier CC, Ramanujam M, Sahu R, Zhang W, Sun Y, Bottinger EP, Ivashkiv L, Kretzler M, Davidson A: A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J Immunol 186: 4994–5003, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Katsiari CG, Liossis SN, Sfikakis PP: The pathophysiologic role of monocytes and macrophages in systemic lupus erythematosus: A reappraisal. Semin Arthritis Rheum 39: 491–503, 2010 [DOI] [PubMed] [Google Scholar]
- 79.Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Gröne HJ, Floege J, Moeller MJ: Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1262–1274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sagrinati C, Mazzinghi B, Ronconi E, Becherucci F, Benigni A, Steenbergen E, Lasagni L, Remuzzi G, Wetzels J, Romagnani P: Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J Am Soc Nephrol 20: 2593–2603, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smeets B, Uhlig S, Fuss A, Mooren F, Wetzels JF, Floege J, Moeller MJ: Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol 20: 2604–2615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ryu M, Migliorini A, Miosge N, Gross O, Shankland S, Brinkkoetter PT, Hagmann H, Romagnani P, Liapis H, Anders HJ: Plasma leakage through glomerular basement membrane ruptures triggers the proliferation of parietal epithelial cells and crescent formation in non-inflammatory glomerular injury [published online ahead of print May 3, 2012]. J Pathol10.1002/path.4046 [DOI] [PubMed] [Google Scholar]
- 83.Croca SC, Rodrigues T, Isenberg DA: Assessment of a lupus nephritis cohort over a 30-year period. Rheumatology (Oxford) 50: 1424–1430, 2011 [DOI] [PubMed] [Google Scholar]
- 84.Houssiau F: Thirty years of cyclophosphamide: Assessing the evidence. Lupus 16: 212–216, 2007 [DOI] [PubMed] [Google Scholar]
- 85.Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, de Ramon Garrido E, Danieli MG, Abramovicz D, Blockmans D, Cauli A, Direskeneli H, Galeazzi M, Gül A, Levy Y, Petera P, Popovic R, Petrovic R, Sinico RA, Cattaneo R, Font J, Depresseux G, Cosyns JP, Cervera R: The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis 69: 61–64, 2010 [DOI] [PubMed] [Google Scholar]
- 86.Kulkarni OP, Sayyed SG, Kantner C, Ryu M, Schnurr M, Sárdy M, Leban J, Jankowsky R, Ammendola A, Doblhofer R, Anders HJ: 4SC-101, a novel small molecule dihydroorotate dehydrogenase inhibitor, suppresses systemic lupus erythematosus in MRL-(Fas)lpr mice. Am J Pathol 176: 2840–2847, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang HY, Cui TG, Hou FF, Ni ZH, Chen XM, Lu FM, Xu FF, Yu XQ, Zhang FS, Zhao XZ, Zhao MH, Wang GB, Qian JQ, Cai GY, Zhu TY, Wang YH, Jiang ZP, Li YN, Mei CL, Zou WZ, China Leflunomide Lupus Nephritis Study Group : Induction treatment of proliferative lupus nephritis with leflunomide combined with prednisone: A prospective multi-centre observational study. Lupus 17: 638–644, 2008 [DOI] [PubMed] [Google Scholar]
- 88.Chan OT, Madaio MP, Shlomchik MJ: The central and multiple roles of B cells in lupus pathogenesis. Immunol Rev 169: 107–121, 1999 [DOI] [PubMed] [Google Scholar]
- 89.Gregersen JW, Jayne DR: B-cell depletion in the treatment of lupus nephritis. Nat Rev Nephrol 8: 505–514, 2012 [DOI] [PubMed] [Google Scholar]
- 90.Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, Maciuca R, Zhang D, Garg JP, Brunetta P, Appel G, LUNAR Investigator Group : Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: The Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 64: 1215–1226, 2012 [DOI] [PubMed] [Google Scholar]
- 91.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, Utset TO, Gordon C, Isenberg DA, Hsieh HJ, Zhang D, Brunetta PG: Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: The randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 62: 222–233, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weidenbusch M, Römmele C, Schröttle A, Anders HJ: Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dial Transplant 28: 106–111, 2013 [DOI] [PubMed] [Google Scholar]
- 93.Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, León MG, Tanasescu C, Nasonov E, Lan JL, Pineda L, Zhong ZJ, Freimuth W, Petri MA, BLISS-52 Study Group : Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet 377: 721–731, 2011 [DOI] [PubMed] [Google Scholar]
- 94.Manzi S, Sánchez-Guerrero J, Merrill JT, Furie R, Gladman D, Navarra SV, Ginzler EM, D’Cruz DP, Doria A, Cooper S, Zhong ZJ, Hough D, Freimuth W, Petri MA, BLISS-52 and BLISS-76 Study Groups : Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: Combined results from two phase III trials. Ann Rheum Dis 71: 1833–1838, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mok CC: Abatacept for systemic lupus erythematosus: The outlook. Expert Opin Biol Ther 12: 1559–1561, 2012 [DOI] [PubMed] [Google Scholar]
- 96.Merrill JT, Burgos-Vargas R, Westhovens R, Chalmers A, D’Cruz D, Wallace DJ, Bae SC, Sigal L, Becker JC, Kelly S, Raghupathi K, Li T, Peng Y, Kinaszczuk M, Nash P: The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: Results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 62: 3077–3087, 2010 [DOI] [PubMed] [Google Scholar]
- 97.Hiepe F, Dörner T, Hauser AE, Hoyer BF, Mei H, Radbruch A: Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol 7: 170–178, 2011 [DOI] [PubMed] [Google Scholar]
- 98.Ichikawa HT, Conley T, Muchamuel T, Jiang J, Lee S, Owen T, Barnard J, Nevarez S, Goldman BI, Kirk CJ, Looney RJ, Anolik JH: Beneficial effect of novel proteasome inhibitors in murine lupus via dual inhibition of type I interferon and autoantibody-secreting cells. Arthritis Rheum 64: 493–503, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K, Wiethe C, Winkler TH, Kalden JR, Manz RA, Voll RE: The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med 14: 748–755, 2008 [DOI] [PubMed] [Google Scholar]
- 100.Anders HJ: Pseudoviral immunity - a novel concept for lupus. Trends Mol Med 15: 553–561, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Barrat FJ, Coffman RL: Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol Rev 223: 271–283, 2008 [DOI] [PubMed] [Google Scholar]
- 102.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, Karpouzas GA, Merrill JT, Wallace DJ, Yazdany J, Ramsey-Goldman R, Singh K, Khalighi M, Choi SI, Gogia M, Kafaja S, Kamgar M, Lau C, Martin WJ, Parikh S, Peng J, Rastogi A, Chen W, Grossman JM, American College of Rheumatology : American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 64: 797–808, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clowse ME, Magder L, Witter F, Petri M: Hydroxychloroquine in lupus pregnancy. Arthritis Rheum 54: 3640–3647, 2006 [DOI] [PubMed] [Google Scholar]
- 104.Pons-Estel GJ, Alarcón GS, McGwin G, Jr, Danila MI, Zhang J, Bastian HM, Reveille JD, Vilá LM, Lumina Study Group : Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum 61: 830–839, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barrat FJ, Meeker T, Chan JH, Guiducci C, Coffman RL: Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol 37: 3582–3586, 2007 [DOI] [PubMed] [Google Scholar]
- 106.Merrill JT, Wallace DJ, Petri M, Kirou KA, Yao Y, White WI, Robbie G, Levin R, Berney SM, Chindalore V, Olsen N, Richman L, Le C, Jallal B, White B, Lupus Interferon Skin Activity (LISA) Study Investigators : Safety profile and clinical activity of sifalimumab, a fully human anti-interferon α monoclonal antibody, in systemic lupus erythematosus: A phase I, multicentre, double-blind randomised study. Ann Rheum Dis 70: 1905–1913, 2011 [DOI] [PubMed] [Google Scholar]
- 107.Aringer M, Burkhardt H, Burmester GR, Fischer-Betz R, Fleck M, Graninger W, Hiepe F, Jacobi AM, Kötter I, Lakomek HJ, Lorenz HM, Manger B, Schett G, Schmidt RE, Schneider M, Schulze-Koops H, Smolen JS, Specker C, Stoll T, Strangfeld A, Tony HP, Villiger PM, Voll R, Witte T, Dörner T: Current state of evidence on ‘off-label’ therapeutic options for systemic lupus erythematosus, including biological immunosuppressive agents, in Germany, Austria and Switzerland—a consensus report. Lupus 21: 386–401, 2012 [DOI] [PubMed] [Google Scholar]
- 108.Kulkarni O, Eulberg D, Selve N, Zöllner S, Allam R, Pawar RD, Pfeiffer S, Segerer S, Klussmann S, Anders HJ: Anti-Ccl2 Spiegelmer permits 75% dose reduction of cyclophosphamide to control diffuse proliferative lupus nephritis and pneumonitis in MRL-Fas(lpr) mice. J Pharmacol Exp Ther 328: 371–377, 2009 [DOI] [PubMed] [Google Scholar]