Abstract
Aims/Introduction
Diabetes mellitus and periodontitis are closely related. A huge number of reports has addressed the effect of periodontal intervention therapy on glycemic control, but no reports have addressed the effect of glycemic intervention therapy on periodontal disease in type 2 diabetic patients. The aim of this study was to examine the effect of improved glycemic control by glycemic intervention therapy on periodontitis in type 2 diabetic patients.
Materials and Methods
A total of 35 patients underwent intervention therapy to improve glycemic control without periodontal treatment. Glycohemoglobin (HbA1c), high‐sensitivity C‐reactive protein (hs‐CRP), bleeding on probing (BOP), probing pocket depth (PPD) and intraoral community periodontal index (CPI) codes of the World health Organization (WHO) were examined at baseline, and 2 and 6 months after the intervention therapy to improve glycemic control.
Results
After the improvement of glycemic control, BOP lesions improved, but deep PPD lesions and WHO CPI codes did not improve. Subanalyses showed that effective glycemic control (average HbA1c reduction 1.8%) improved BOP lesions, but did not affect deep PPD lesions and WHO CPI codes. In addition, high BOP lesions at baseline responded more effectively to glycemic intervention. Further analysis of CPI codes in all individual periodontal sites independent of WHO CPI codes in 35 patients showed that only gingival inflammation without a deep periodontal pocket improved after glycemic intervention.
Conclusions
Effective glycemic control improves BOP lesions in type 2 diabetic patients with periodontitis through ameliorating inflammation at the gingival sites of periodontal tissue. This trial was registered with the University Hospital Medical Information Network (no. UMIN000007670).
Keywords: Bleeding on probing, Periodontitis, Type 2 diabetes intervention therapy
Introduction
Periodontitis is a chronic infectious disease caused by periodontal bacterial infection. Chronic periodontal inflammation causes loss of alveolar bone and periodontal supportive tissue, eventually leading to loss of teeth1. Type 2 diabetes mellitus is mainly characterized by hyperglycemia as a result of impaired insulin action3. Although the etiology of the two diseases is different, there is a huge number of reports that have shown a close relationship between periodontitis and diabetes mellitus4.
Diabetes mellitus is considered one of the major risk factors for periodontitis, and, vice versa, periodontitis is considered to increase the risk of developing diabetes mellitus5. For many years, the effect of periodontal intervention therapy on glycemic control has been examined, and many intervention studies to validate these effects have been reported6. However, to the best of our knowledge, studies examining the effect of improved glycemic control by glycemic intervention therapy on periodontitis have never been reported. The aim of the present study was to examine whether improvement of glycemic control by glycemic intervention therapy affects periodontal disease in type 2 diabetic patients without treatment of periodontitis.
Methods
Participants
Type 2 diabetic patients were recruited from diabetic clinics at four institutions: Tokyo Medical University Hospital, Kyoto Prefecture Medical University Hospital, Aichi Gakuin University Hospital and Tokyo Teishin Hospital. At each institution, patients were selected based on the following inclusion criteria: age 40–75 years and glycohemoglobin (HbA1c) ≥7.4%. The exclusion criteria were: severe diabetic complications, evidence of systemic disease other than diabetes as a risk factor for periodontitis, systemic antibiotics taken during the preceding 3 months, pregnancy or lactation and periodontal treatment during the preceding 6 months. A total of 42 participants fulfilled the criteria, but seven chose not to participate. The study protocol was approved by the Ethics Committee of each university hospital, and written informed consent was obtained from each participant.
Intervention and Measurements
Based on the physician's diagnosis, each participant underwent a 6‐month treatment plan that ensured improvement in blood glucose levels, including dietary counseling, oral hypoglycemic agents and insulin administration. In each diabetic clinic, HbA1c and high‐sensitivity C‐reactive protein (hs‐CRP) measurements, and periodontal examinations were carried out at baseline and 2 and 6 months after the glycemic intervention therapy without receiving periodontal treatments. The white blood cell (WBC) count and serum creatinine were measured at baseline.
In each dental clinic, full‐mouth measurements of bleeding on probing (BOP) and probing pocket depth (PPD) were recorded using a manual probe (PCP‐UNC 15; Hu‐Friedy Manufacturing, Chicago, IL, USA) at all six sites of each tooth, simultaneously, at baseline and 2 and 6 months after the glycemic intervention therapy. World Health Organization (WHO) community periodontal index (CPI) codes were also recorded as an estimate of periodontitis11. In this examination, the right central incisor in the maxilla and the left central incisor in the mandible were considered to be the representative teeth in the anterior teeth block, and the first and second molar teeth in the right and left maxilla and mandible were similarly considered to be representative teeth. CPI codes of 0–4 were given for healthy cases (0), and those with BOP (1), with calculus (2) and with a probing pocket depth of 4–5 mm (3) or ≥6 mm (4), respectively. In this WHO classification of periodontitis, it has been determined that the highest CPI code should be selectively applied for each patient by determining all codes in six individual blocks in each periodontal patient.
Blood samples were analyzed for HbA1c, which was determined by high‐performance liquid chromatography (Kyotokagaku, Kyoto, Japan). The value for HbA1c (%) was described as a National Glycohemoglobin Standardization Program (NGSP) equivalent value (%) calculated by the formula HbA1c (%) = HbA1c (JDS) (%) + 0.4%, considering the relational expression of HbA1c (JDS) (%) measured by the previous Japanese standard substance and measurement methods, and referred to as HbA1c (NGSP)12. The serum hs‐CRP level was measured using a latex particle‐enhanced immunonephelometric method.
Physicians collected the medical data, and dentists carried out oral examinations. Dentists did not know patients' glycemic control when they carried out oral examinations, so that the dentists were blinded to glycemic control.
Statistical Analysis
Statistical analysis was carried out using the jmp® software program (SAS Institute, Cary, NC, USA). Changes in all parameters from baseline to 2 and 6 months were compared using the Wilcoxon signed‐rank test. For subanalyses, the Mann–Whitney U‐test and Fisher's exact test were used to compare the parameters between the HbA1c‐decreased (HbA1c‐D) and HbA1c‐no decrease or increased (HbA1c‐ND) groups, and the baseline bleeding on probing high (BOP‐H) and the baseline BOP low (BOP‐L) groups. The correlations between any two parameters were examined by Spearman's rank correlation coefficient. Participants with WBC >8,000 were regarded as having severe inflammation and were excluded from the analysis of hs‐CRP. P < 0.05 was regarded as significant.
Results
The results of 35 patients (18 males, 17 females) were analyzed. As shown in Table 1, the mean age of the participants was 59.4 ± 9.3 years. The mean BMI was 25.9 ± 5.0 kg/m2 at baseline. The mean number of present teeth was 23.1 ± 6.2. A total of 11 of the 35 patients were using insulin injections, 24 patients were using oral hypoglycemic drugs and none was being treated with diet alone. Creatinine in all participants was less than 1.0 mg/dL, meaning that no patients developed renal disfunction associated with diabetic nephropathy. The degree of BOP lesions was significantly reduced in parallel with the HbA1c reduction, but no significant changes in the degree of PPD, WHO CPI code for each patient and hs‐CRP were observed for 6 months.
Table 1. Diabetic and periodontal status at baseline and during follow up in all participants.
| All participants (n = 35) | Baseline | 2 months | 6 months | |
|---|---|---|---|---|
| Male/female | 18/17 | – | – | |
| Insulin/oral hypoglycemic | 11/24 | – | – | |
| HbA1c (%) | 9.5 ± 1.8 | 8.8 ± 1.3* | 8.3 ± 1.4* | |
| hs‐CRP (ng/mL) | 725 ± 699 | 963 ± 1318 | 956 ± 1055 | |
| PPD (mm) | 2.8 ± 0.9 | 2.8 ± .9 | 2.8 ± 0.7 | |
| BOP (%) | 37.7 ± 23.2 | 25.9 ± 16.4* | 24.7 ± 17.1* | |
| WHO classification of periodontitis CPI 1 or 2/CPI 3/CPI 4 | 3/10/22 | 2/12/21 | 2/14/19 |
Values are given as means ± SD. BOP, bleeding on probing; CPI, community periodontal index; HbA1c, glycohemoglobin; hs‐CRP, high‐sensitivity C‐reactive protein; PPD, probing pocket depth; WHO, World Health Organization.
Statistically significant decrease compared with baseline, *P < 0.01.
To further explore the effect of improved glycemic control by glycemic intervention therapy on periodontitis, subanalyses were carried out. First, in order to examine whether the reduction of HbA1c actually improved the BOP lesions, the patients were subdivided into two groups according to the change in HbA1c (Table 2). Reduction of HbA1c was observed in 25 patients at 6 months (HbA1c‐D group; average decrease 1.8%), and the HbA1c level was not decreased or increased in 10 patients (HbA1c‐ND groups). There were no significant differences in types of glycemic treatment, HbA1c, hs‐CRP, degree of PPD, BOP lesions, and WHO CPI codes at baseline between the HbA1c‐D and HbA1c‐ND groups. In the HbA1c‐D group, the degree of BOP lesions was significantly less at 2 and 6 months, which indicated that reduced HbA1c was actually related to the improvement of BOP lesions, but not related to the improvement of PPD, and WHO CPI codes did not change significantly over 6 months. No significant improvements were observed in BOP lesions, degree of PPD and WHO CPI codes in the HbA1c‐ND group throughout the study period. Hs‐CRP was not changed significantly during the study period in both groups.
Table 2. Diabetic and periodontal status at baseline and during follow up in glycohemoglobin decreased and glycohemoglobin no decrease or increased groups.
| HbA1c‐D and HbA1c‐ND groups | Baseline | 2 months | 6 months | |
|---|---|---|---|---|
| HbA1c decreased group (n = 25) | Male/female | 13/12 | – | – |
| Insulin/oral hypoglycemic | 8/17 | – | – | |
| HbA1c (%) | 9.7 ± 2.0 | 8.7 ± 1.3* | 7.9 ± 1.2* | |
| hs‐CRP (ng/mL) | 619 ± 447 | 649 ± 799 | 787 ± 843 | |
| PPD (mm) | 3.0 ± 0.9 | 2.9 ± 0.9 | 2.9 ± 0.8 | |
| BOP (%) | 42.6 ± 22.8 | 28.4 ± 17.3* | 26.8 ± 17.3* | |
| WHO classification of periodontitis CPI 1 or 2/CPI3/CPI4 | 1/6/18 | 0/7/18 | 1/9/15 | |
| HbA1c not decreased rather increased group (n = 10) | Male/female | 5/5 | – | – |
| Insulin/oral hypoglycemic | 3/7 | – | – | |
| HbA1c (%) | 9.0 ± 1.4 | 8.9 ± 1.6 | 9.5 ± 1.4† | |
| hs‐CRP (ng/mL) | 950 ± 1049 | 1591 ± 1903 | 1333 ± 1405 | |
| PPD (mm) | 2.5 ± 0.6 | 2.5 ± 0.6 | 2.6 ± 0.6 | |
| BOP (%) | 25.6 ± 20.2 | 18.4 ± 11.4 | 19.6 ± 16.4 | |
| WHO classification of periodontitis CPI 1 or 2/CPI 3/CPI 4 | 2/4/4 | 2/5/3 | 1/5/4 |
Values are given as means ± SD; CPI, community periodontal index; HbA1c‐D, glycohemoglobin decreased;HbA1c‐ND, glycohemoglobin no decrease or increased; hs‐CRP, high‐sensitivity C‐reactive protein; PPD, probing pocket depth; BOP, bleeding on probing; WHO, World Health Organization.
Statistically significant decrease compared with baseline; *P < 0.01. Statistically significant increase compared with baseline and difference compared with glycohemoglobin (HbA1c) decreased group.
†P < 0.01.
Second, in order to examine whether high BOP responds well to the improved glycemic control by the intervention therapy, the patients were subdivided into two groups based on the baseline degree of BOP (Table 3). Of the 35 patients, the percentage of BOP lesions at baseline was higher than the median percentage of BOP lesions (38.7%) in 17 patients (BOP‐H group), whereas the baseline percentage of BOP lesions was less than the median percentage of BOP lesions in the other 18 participants (BOP‐L group). In the BOP‐H group, BOP lesions were significantly reduced at 2 and 6 months with a significant reduction of HbA1c, whereas in the BOP‐L group, BOP lesions were not improved without a significant reduction in HbA1c at 2 months, but BOP lesions were significantly improved with a significant reduction in HbA1c at 6 months, which indicated that high BOP lesions responded more effectively to glycemic control by the intervention therapy, resulting in improvement of BOP lesions. However, no significant improvement was observed in the degree of PPD and WHO CPI codes throughout the study periods in the BOP‐H and BOP‐L groups. Hs‐CRP was not changed significantly during the study period in both of the groups.
Table 3. Diabetic and periodontal status at baseline and during follow up in bleeding on probing high and bleeding on probing low groups.
| BOP‐H and BOP‐L groups | Baseline | 2 months | 6 months | |
|---|---|---|---|---|
| Baseline BOP high group (n = 17) | Male/female | 10/7 | – | – |
| Insulin/oral hypoglycemic | 8/9 | – | – | |
| HbA1c (%) | 9.7 ± 1.9 | 8.7 ± 1.3** | 8.0 ± 1.5** | |
| hs‐CRP (ng/mL) | 831 ± 909 | 830 ± 1096 | 925 ± 1354 | |
| PPD (mm) | 3.0 ± 0.8 | 2.9 ± 0.7 | 3.0 ± 0.7 | |
| BOP (%) | 56.1 ± 16.9† | 36.3 ± 16.0**† | 36.5 ± 16.6**† | |
| WHO classification of periodontitis CPI 1 or 2/CPI3/CPI4 | 0/5/12 | 0/6/11 | 0/7/10 | |
| Baseline BOP low group (n = 18) | Male/female | 8/10 | – | – |
| Insulin/oral hypoglycemic | 3/15 | – | – | |
| HbA1c (%) | 9.3 ± 1.8 | 8.8 ± 1.4 | 8.6 ± 1.4* | |
| hs‐CRP (ng/mL) | 613 ± 368 | 1087 ± 1526 | 989 ± 648 | |
| PPD (mm) | 2.7 ± 0.9 | 2.7 ± 1.0 | 2.6 ± 0.7 | |
| BOP (%) | 20.4 ± 12.1 | 15.6 ± 8.6 | 13.6 ± 7.5* | |
| WHO classification of periodontitis CPI 1 or 2/CPI 3/CPI 4 | 3/5/10 | 2/6/10 | 2/7/9 |
Values are given as means ± SD. BOP, bleeding on probing; BOP‐H, bleeding on probing high; BOP‐L, bleeding on probing low; CPI, community periodontal index; HbA1c‐D, glycohemoglobin decreased; HbA1c‐ND, glycohemoglobin no decrease or increased; hs‐CRP, high‐sensitivity C‐reactive protein; PPD, probing pocket depth; WHO, World Health Organization.
Statistically significant decrease compared with baseline, *P < 0.05.
**P < 0.01.
Statistically significant difference compared with baseline BOP low group, †P < 0.01.
Third, in order to examine whether improvement of glycemic control by the intervention therapy affected individual periodontal sites independent of WHO classification of periodontitis in each patient, because the WHO CPI code can only identify the highest degree of periodontitis, changes of all individual CPI codes in all individual blocks were examined in 35 patients from baseline to 6 months. There were 45 blocks that showed CPI code 1 at baseline, and in 12 of the 45 code 1 blocks (26.7%) CPI code 1 returned to CPI code 0 after 6 months' glycemic intervention therapy. In contrast, there were 43 blocks with CPI code 4 at baseline, and only in one of the 43 code 4 blocks (2.3%) did CPI code 4 return to CPI code 0 after 6 months (Figure 1), which indicated that lesions of inflammation responded more effectively to the glycemic improvement by the intervention therapy.
Figure 1.
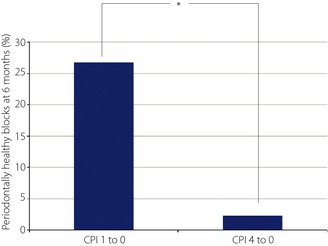
Percentage of periodontally healthy blocks at 6 months, * P < 0.05.
Discussion
There have been many studies that have addressed the effect of improved periodontitis by periodontal intervention therapy on glycemic control, and recently, Teeuw et al.13 carried out a meta‐analysis by selecting five reliable studies among 639 intervention studies, and they found a significant decline of 0.4% in HbA1c in type 2 diabetic patients after periodontal intervention therapy. Therefore, it is reasonable to consider that effective periodontal intervention therapy improves glycemic control in type 2 diabetic patients. However, to the best of our knowledge, no studies that addressed the effect of glycemic intervention therapy without periodontal treatment on periodontal status in type 2 diabetic patients have been reported.
In the present study, the effect of improved glycemic control by glycemic intervention therapy on periodontitis was investigated, and it was found for the first time that effective glycemic control with reduced HbA1c by glycemic intervention therapy improved BOP lesions without periodontal treatment over the 6‐month period. In addition, high BOP lesions at baseline responded more effectively to reduced HbA1c by the glycemic intervention therapy in type 2 diabetic patients with periodontitis. The results of two subanalyses strongly indicated that effective glycemic improvement by intervention therapy is able to ameliorate BOP lesions in periodontitis without periodontal treatment in type 2 diabetic patients. However, it was also found that even effective glycemic control did not improve deep PPD lesions in these patients.
These results were confirmed by analysis of all CPI codes in all individual blocks independent of WHO classification. Although mean WHO CPI codes did not change significantly, 26.7% of the blocks with CPI code 1 (periodontal inflammation without a deep periodontal pocket) returned to blocks with CPI code 0 (healthy periodontal tissue) after the glycemic intervention, but just 2.3% of the blocks with CPI code 4 (severe periodontitis with a deep periodontal pocket) returned to CPI code 0. This suggests that effective glycemic control can improve BOP lesions, but cannot improve deep PPD lesions in type 2 diabetes patients.
The etiological agent for deteriorating BOP lesions and degree of PPD is plaque biofilm, but the susceptibility to plaque biofilm differs among patients. Host inflammatory responses are the key factors for deteriorating BOP lesions, and destruction of periodontal tissue is a key factor for deteriorating PPD. BOP lesions are easily changeable within a short period of time by the accumulation of plaque biofilm in gingival tissues; however, deterioration of PPD usually takes a long period of time by chronic periodontal bacterial infection and host tissue reaction, and it takes time to restore PPD. It is well recognized that the absence of BOP predicts the absence of deterioration of PPD, suggesting that the deterioration of BOP lesions precedes deterioration of PPD14.
A large epidemiological study by Offenbacher et al.15 showed that diabetes augmented the gingival inflammatory responses against plaque biofilm, and the prevalence of diabetes was significantly higher in the gingivitis patients with high BOP lesions than in those with low BOP lesions. Accordingly, the amelioration of diabetes might improve the gingival inflammatory responses. Recession of the affected gingival tissue is dependent on gingival biotype (thickness of the periodontal tissue) and resolution of the inflammation. In contrast, deep PPD lesions are the result of irreversible destruction of the attachment of the gingival tissue and/or periodontal ligament on the root surface. Restoration of PPD is the clinical result seen from the attachment gain of the gingival tissue on the root and/or the recession of the gingival tissue. The diseased root surface is contaminated with bacterial plaque, and debridement of the contamination is required for the gingival tissue to gain the attachment. Therefore, diabetic treatment alone might not be enough to restore deep PPD though the recession of gingival tissue.
It has been established that bacterial microflora at periodontal disease sites in diabetic patients are similar to the microflora at similar disease sites in non‐diabetic subjects16, and thus, the causes of susceptibility and severity of periodontitis in type 2 diabetic patients come from host problems. Diabetic complications originate from complex abnormalities on the host side through chronic hyperglycemia18. These abnormalities involve many factors, such as inflammation19, advanced glycation end‐products (AGEs)20, oxidative stress21, microangiopathy22 and macroangiopathy23. We presume that the combination of these abnormalities makes diabetic patients susceptible to bacterial infection in the periodontal tissue.
Although BOP lesions in the HbA1c‐D group improved at 6 months, hs‐CRP was not significantly changed in these patients. Several reasons might explain why BOP lesions improved without reduction of hs‐CRP. First, improvement of BOP lesions in the HbA1c‐D group might not have had been strong enough to reduce circulatory hs‐CRP levels. Second, periodontitis is one of the complications in diabetes24, and gingival tissue might be susceptible to glycemic control compared with other parts of the human body. Chronic hyperglycemia might contribute to the simultaneous activation of many different pathological pathways, including oxidative stress and low‐grade inflammation. Improvement of glycemic control might ameliorate oxidative stress through the reduction of AGEs and oxygen radicals. Takeda et al. reported that AGEs, not hs‐CRP, were significantly related to the deterioration of periodontitis in type 2 diabetes patients25, indicating that gingival inflammation in type 2 diabetic patients might not necessarily be associated with the fluctuation of systemic low grade inflammation (hs‐CRP). Third, improvement of BOP was mainly observed in mild gingivitis lesions (CPI = 1), and severe periodontitis (CPI = 4) was not improved by glycemic control, implying that lack of improvement in the severe periodontal lesions might inhibit mean hs‐CRP reduction after glycemic control. A larger scale intervention study might be required to show a significant correlation between improvement of BOP and hs‐CRP levels.
Oral hygiene might also affect gingival conditions, such as BOP and PPD. The plaque control record of all patients was not available, and it is possible that improvement of oral hygiene might have affected BOP status even if the patients were not instructed in oral hygiene during the course of the study. In the present study, improvement of BOP lesions was observed only in the HbA1c‐D group; however, it is unlikely that HbA1c‐D group patients selectively improved their oral hygiene, resulting in the improvement of BOP lesions. The improvement of BOP lesions in the HbA1c‐D group strongly suggested that it was related to the improvement of HbA1c in the present study, as improvement of BOP lesions was not observed in the HbA1c‐ND group.
Many studies have shown that effective periodontal treatment in type 2 diabetic patients improves glycemic control6. Periodontitis is a chronic, subclinical, inflammatory disease that increases the risk of onset and progression of diabetes mellitus and arteriosclerosis. Taken with the present and previous results, it is conceivable that glycemic treatment could be carried out simultaneously with periodontal treatments in the cases of type 2 diabetic patients with periodontitis; it might facilitate glycemic control and, furthermore, it might prevent and inhibit the development of arteriosclerosis.
In conclusion, the present study showed, for the first time, that effective glycemic control ameliorated periodontitis in type 2 diabetic patients without periodontal treatment through ameliorating inflammation at the gingival site of periodontal tissue. Further large‐scale, multicenter studies are required to clarify the mechanisms by which improved glycemic control improves periodontitis in type 2 diabetic patients with periodontitis.
Acknowledgements
The authors are grateful to all of the study participants. This work was supported by a Grant‐in‐Aid from the Ministry of Health, Labour and Welfare of Japan (H16‐Iro‐020), a Grant‐in‐Aid for Young Scientists (B) (23792466), and the 8020 Promotion Foundation. The authors declare that there is no duality of interest associated with this manuscript.
(J Diabetes Invest, doi: 10.1111/jdi.12026, 2013)
References
- 1.Page RC, Offenbacher S, Schroeder HE, et al Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000 1997; 14: 216–248 [DOI] [PubMed] [Google Scholar]
- 2.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005; 366: 1809–1820 [DOI] [PubMed] [Google Scholar]
- 3.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58: 773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khader YS, Dauod AS, El‐Qaderi SS, et al Periodontal status of diabetics compared with nondiabetics: a meta‐analysis. J Diabetes Complications 2006; 20: 59–68 [DOI] [PubMed] [Google Scholar]
- 5.Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol 2006; 77: 1289–1303 [DOI] [PubMed] [Google Scholar]
- 6.Katagiri S, Nitta H, Nagasawa T, et al Multi‐center intervention study on glycohemoglobin (HbA1c) and serum, high‐sensitivity CRP (hs‐CRP) after local anti‐infectious periodontal treatment in type 2 diabetic patients with periodontal disease. Diabetes Res Clin Pract 2009; 83: 308–315 [DOI] [PubMed] [Google Scholar]
- 7.Jones JA, Miller DR, Wehler CJ, et al Does periodontal care improve glycemic control? The Department of Veterans Affairs Dental Diabetes Study. J Clin Periodontol 2007; 34: 46–52 [DOI] [PubMed] [Google Scholar]
- 8.Kiran M, Arpak N, Unsal E, et al The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol 2005; 32: 266–272 [DOI] [PubMed] [Google Scholar]
- 9.Promsudthi A, Pimapansri S, Deerochanawong C, et al The effect of periodontal therapy on uncontrolled type 2 diabetes mellitus in older subjects. Oral Dis 2005; 11: 293–298 [DOI] [PubMed] [Google Scholar]
- 10.Stewart JE, Wager KA, Friedlander AH, et al The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol 2001; 28: 306–310 [DOI] [PubMed] [Google Scholar]
- 11.WHO . Oral Health Surveys Basic Method, 4th edn. WHO, Geneva, 1997 [Google Scholar]
- 12.Seino Y, Nanjo K, Tajima N, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta‐analysis. Diabetes Care 2010; 33: 421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang NP, Adler R, Joss A, et al Absence of bleeding on probing. An indicator of periodontal stability. J Clin Periodontol 1990; 17: 714–721 [DOI] [PubMed] [Google Scholar]
- 15.Offenbacher S, Barros S, Singer R, et al Periodontal disease at the biofilm‐gingival interface. J Periodontol 2007; 78: 1911–1925 [DOI] [PubMed] [Google Scholar]
- 16.Zambon J, Reynolds H, Fisher J, et al Microbiological and immunological studies of adult periodontitis in patients with non‐insulin dependent diabetes mellitus. J Periodontol 1988; 59: 23–31 [DOI] [PubMed] [Google Scholar]
- 17.Sastrowijoto S, Hillemans P, Van Steenbergen T, et al Periodontal condition and microbiology of healthy and diseased periodontal pockets in type 1 diabetes mellitus patients. J Periodontol 1989; 16: 312–316 [DOI] [PubMed] [Google Scholar]
- 18.Shultis WA, Weil EJ, Looker HC, et al Effect of periodontitis on overt nephropathy and end‐stage renal disease in type 2 diabetes. Diabetes Care 2007; 30: 306–311 [DOI] [PubMed] [Google Scholar]
- 19.Esposito K, Nappo F, Marfella R, et al Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 2002; 106: 2067–2072 [DOI] [PubMed] [Google Scholar]
- 20.Nassar H, Kantarci A, Van Dyke TE. Diabetic periodontitis: a model for activated innate immunity and impaired resolution of inflammation. Periodontol 2000 2007; 43: 233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgartner‐Parzer SM, Wagner L, Pettermann M, et al High‐glucose–triggered apoptosis in cultured endothelial cells. Diabetes 1995; 44: 1323–1327 [DOI] [PubMed] [Google Scholar]
- 22.Roy S, Trudeau K, Behl Y, et al New insights into hyperglycemia‐induced molecular changes in microvascular cells. J Dent Res 2010; 89: 116–127 [DOI] [PubMed] [Google Scholar]
- 23.King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol 2008; 79: 1527–1534 [DOI] [PubMed] [Google Scholar]
- 24.Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 1993; 16: 329–334 [PubMed] [Google Scholar]
- 25.Takeda M, Ojima M, Yoshioka H, et al Relationship of serum advanced glycation end products with deterioration of periodontitis in type 2 diabetes patients. J Periodontol 2006; 77: 15–20 [DOI] [PubMed] [Google Scholar]


