Summary
Whereas intracellular carbon metabolism has emerged as an attractive drug target, the carbon sources of intracellularly replicating pathogens, such as the tuberculosis bacillus Mycobacterium tuberculosis, which causes long-term infections in one-third of the world’s population, remain mostly unknown. We used a systems-based approach—13C-flux spectral analysis (FSA) complemented with manual analysis—to measure the metabolic interaction between M. tuberculosis and its macrophage host cell. 13C-FSA analysis of experimental data showed that M. tuberculosis obtains a mixture of amino acids, C1 and C2 substrates from its host cell. We experimentally confirmed that the C1 substrate was derived from CO2. 13C labeling experiments performed on a phosphoenolpyruvate carboxykinase mutant revealed that intracellular M. tuberculosis has access to glycolytic C3 substrates. These findings provide constraints for developing novel chemotherapeutics.
Highlights
-
•
The intracellular metabolism of Mycobacterium tuberculosis was directly measured
-
•
A tool for analyzing metabolic interactions between host and pathogen was developed
-
•
Amino acids C1, C2, and C3 are intracellular substrates for M. tuberculosis
-
•
CO2 was identified as an intracellular carbon source for M. tuberculosis
Despite being a potential drug target, intracellular metabolism of Mycobacterium tuberculosis (Mtb) is one of the least understood aspects of host pathogen biology. Beste et al. probe the metabolism of the pathogen directly growing inside the macrophage and see that Mtb has access to a diverse diet and nutrients.
Introduction
Tuberculosis (TB) remains a major problem throughout the world and is responsible for 8.8 million cases of TB each year, resulting in 1.4 million deaths (World Health Organization, 2011). New drugs are urgently needed to combat the emergence of multidrug (MDR) and extensively resistant (XDR) TB (Sharma and Mohan, 2006; Velayati et al., 2009) strains of the pathogen. Intracellular metabolism of M. tuberculosis is an attractive target for development of novel anti-TB drugs; but despite more than a century of research, fundamental questions remain, such as the nature of the nutrients the pathogen obtains from its macrophage host cell. Mutagenesis studies (Muñoz-Elías and McKinney, 2005; Pandey and Sassetti, 2008) provide indirect evidence for a diet of fatty acids derived from host lipids, including cholesterol. However, definitive conclusions are compromised by the multiple roles of enzymes, the redundancy of metabolic pathways (Venugopal et al., 2011), and often contradictory data. More direct methods are therefore required to unravel the diet and metabolism of intracellular M. tuberculosis, which could illuminate novel treatment strategies against TB.
Recently, 13C-isotopologue profiling analysis (13C-IPA) based on mass spectrometry has been used to directly measure the intracellular metabolism of Listeria monocytogenes (Eisenreich et al., 2006) and several enterobacterial pathogens (Götz et al., 2010). This method involves using 13C-labeled substrates (13C-labeled substrates can either be provided during the infection or host cells can be labeled prior to infection) to track the intracellular metabolism of bacteria. The bacterial and host cells are then separated and the pattern of label in stable metabolites (proteinogenic amino acids) is measured using mass spectrometry. Model-free analysis is then used to infer the substrates transport reactions and central metabolic pathway utilization that are most consistent with the data. We previously applied the systems-based tool 13C-metabolic flux analysis (13C-MFA) (Wiechert et al., 2001) to directly measure metabolic fluxes of M. tuberculosis in vitro (Beste et al., 2011) and demonstrated the operation of an alternative pathway to the TCA cycle, the GAS pathway, which utilizes the Glyoxylate shunt and Anapleurotic reactions for oxidation of pyruvate, and Succinyl CoA synthetase for the generation of succinyl CoA and involves significant levels of CO2 fixation. The method is based on similar principles to 13C-IPA but uses in silico modeling to infer metabolic fluxes from the labeling patterns. Classical 13C-MFA can only be applied to metabolic systems in steady state. Thus, to examine the non-steady-state metabolism of intracellular TB bacilli, we developed a systems-based tool—13C-flux spectral analysis (13C-FSA)—and applied it to investigate the diet and metabolism of intracellular M. tuberculosis.
Results
Measuring the Intracellular Metabolism of M. tuberculosis
We used the THP-1 cell line as our model system for investigating the metabolic interaction between M. tuberculosis and macrophages. Although human alveolar macrophages (HAM-M) are the natural host for M. tuberculosis, they can only be collected via an invasive medical procedure and are impossible to obtain in large enough numbers for systems biology-type studies. Peripheral blood mononuclear cell-derived macrophages (PBMC-M) have been used as an alternative, but there are significant limitations of using PBMC-M cells, including large heterogeneity between donors and even within the population of cells and variable ex vivo differentiation procedures (Martinez et al., 2006), and it is not possible to obtain enough PBMC’s to perform the experiments presented here. THP-1 cells can be differentiated into macrophages following phorbol 12-myristate 13-acetate (PMA) stimulation and closely model the behavior of activated primary alveolar macrophage (Riendeau and Kornfeld, 2003) or peripheral blood mononuclear cell-derived macrophages (PMBC) following M. tuberculosis infection (Singhal et al., 2007). Differentiated THP-1 macrophages have been widely utilized as a model for M. tuberculosis infection in numerous studies, which furthered our knowledge on the interaction between M. tuberculosis and its host cell (for examples, see Kumar et al., 2010; Singh et al., 2012; Simeone et al., 2012; Fontán et al., 2008).
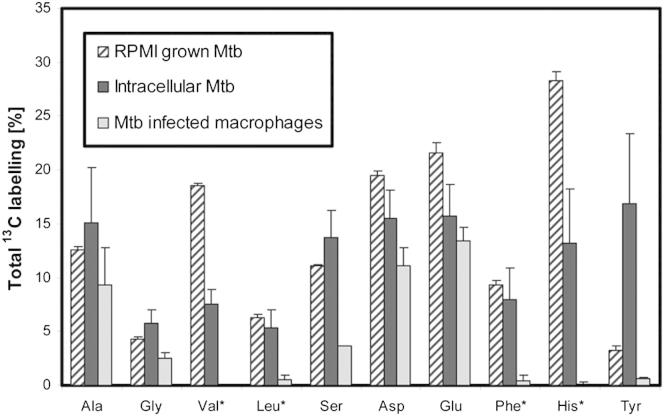
Human THP-1 macrophage-like cells were passaged three times in Roswell Park Memorial Institute (RPMI) media containing 100% uniformly labeled [U-13C6] glucose (13Cglucose-RPMI), before the cells were differentiated into macrophages by stimulation with phorbol 12-myristate 13-acetate (PMA), also in 13Cglucose-RPMI. After washing, the cells were infected with the H37Rv strain of M. tuberculosis (MOI = 5), incubated for 48 hr in unlabeled RPMI medium, and harvested. Differential centrifugation was used to separate cell lysates into intracellular bacterial and macrophage fractions. Cells were harvested at 48 hr as preliminary time course experiments demonstrated that M. tuberculosis was growing within macrophages at this time point (data not shown) and intracellular amino acids had attained a pseudoisotopic steady state (Table S1 available online). In parallel, control flasks of (1) uninfected labeled THP-1 cells and (2) M. tuberculosis were cultivated in 13Cglucose-RPMI medium for 48 hr. After acid hydrolysis, the isotopomer (with the same molecular formula but different isotopic composition) composition of proteinogenic amino acids was measured using gas chromatography-mass spectrometry (GC-MS). Using this method, ten amino acids were isolated in sufficient quantities for accurate measurement of the 13C enrichment. In the macrophage fraction, heavy isotopomer fractions (indicating incorporation of 13C) were detected in the nonessential amino acids, but not the essential amino acids, as expected (Figure 1; Table S2). In contrast, in the intracellular bacterial fraction, all amino acids were labeled with 13C. The distinct labeling patterns confirmed that differential centrifugation was successful in separating macrophage and intracellular M. tuberculosis fractions and demonstrated that the macrophages imported unlabeled (12C) essential amino acids from the RPMI medium but made nonessential amino acids (incorporating 13C) de novo, whereas M. tuberculosis amino acids were all synthesized from host-derived substrates (either de novo or directly incorporated from the host cell into protein). Perhaps surprisingly, there was no observed difference between the isotopologue profile of infected and uninfected macrophages (Table S2).
Figure 1.
Total 13C Labeling of Amino Acids Derived from Macrophages and M. tuberculosis Protein
GC-MS measured values for the total 13C incorporation into proteinogenic amino acids by RPMI-grown Mycobacterium tuberculosis (MTB), intracellular MTB, and infected THP-1 macrophages after 48 hr. The THP-1 cells were prelabeled by passaging with uniformly labeled [U-13C6] glucose prior to the infections. Essential amino acids are indicated (∗). Error bars indicate SD of triplicate or quadruplicate samples from independent macrophage infections.
See also Table S1.
13C-Flux Spectral Analysis
Because of the complexity of the data, we developed 13C-flux spectral analysis (FSA) to perform unsupervised systems-level analysis of the isotopologue profiles using an in silico model of metabolism. 13C-FSA utilizes similar fitting approaches as 13C-MFA, essentially to find the best fit of different hypotheses of nutrient uptake by M. tuberculosis to the measured data. Inputs to 13C-FSA are the isotopomer composition of macrophage amino acids (deconvoluted from the measured labeling patterns), the estimated isotopomer compositions of macrophage glucose, acetate, and glycerol pools (also obtained from the macrophage data and knowledge of the input 13C6 glucose), and the M. tuberculosis labeling data. Various hypotheses for nutrient uptake by M. tuberculosis were then tested in automated simulations (more than 600,000 optimization runs) to identify substrates and flux distributions that minimized the “residual value”, which is a measure of the quantity of the measured GC-MS data that could not be accounted for by the optimal in silico solution.
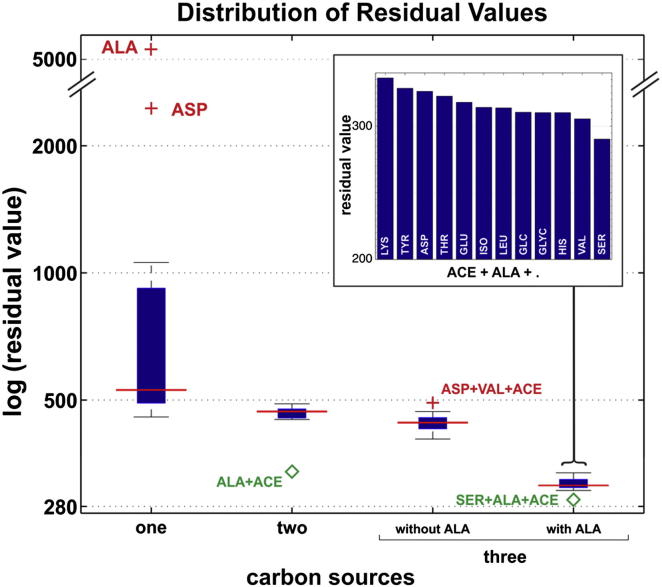
13C-FSA was used to scan a range of potential substrates (glucose, glycerol, acetate, or single amino acids) and combinations of substrates, which M. tuberculosis may obtain from the macrophage. All single substrates generated high residual values (poor fit to data), as shown in Figure 2, but pairs of substrates that included acetate generated lower residual values, with acetate and alanine providing the best fit. Adding a third substrate to the combination of alanine and acetate (Figure 2, inset) resulted in small improvements to the fit with the lowest residual value obtained with serine. 13C-FSA generated a flux solution with these substrates (Figure 3), which shares some features with the GAS pathway that we previously demonstrated operating in glycerol-limited chemostat-grown M. tuberculosis (Beste et al., 2011).
Figure 2.
Box Plot Representation of the Log Residual Values Obtained by 13C-FSA
Combinations of up to three intracellular carbon substrates were tested from 14 potential sources (alanine [ALA], acetate [ACE], glucose, glycerol, asparagine/aspartate [ASP], glutamate/glutamine [GLU], histidine [HIS], isoleucine [ISO], leucine [LEU], lysine [LYS], serine [SER], threonine [THR], tyrosine [TYR], and valine [VAL]). One and two carbon sources are represented by single bars, whereas three carbon sources are represented by two bars (with and without ALA). The inset histogram represents the logged residual values for combinations of three substrates, including ALA and ACE, showing that the optimal combination of substrates is ACE, ALA, and SER. The box plots depict the interquartile range (IQR) between the upper (Q3) and lower (Q1) quartiles (blue lines) and the median (red line). The length of the box represents the interquartile range (IQR). Whisker caps extend from the ends of the box within one IQR in each direction. Values more than one IQR from the upper whisker represent outliers, which are unlikely carbon sources (red cross), whereas values of less than one IQR from the lower whisker represent potential carbon substrates (green diamond) and are labeled with the name of the carbon source(s).
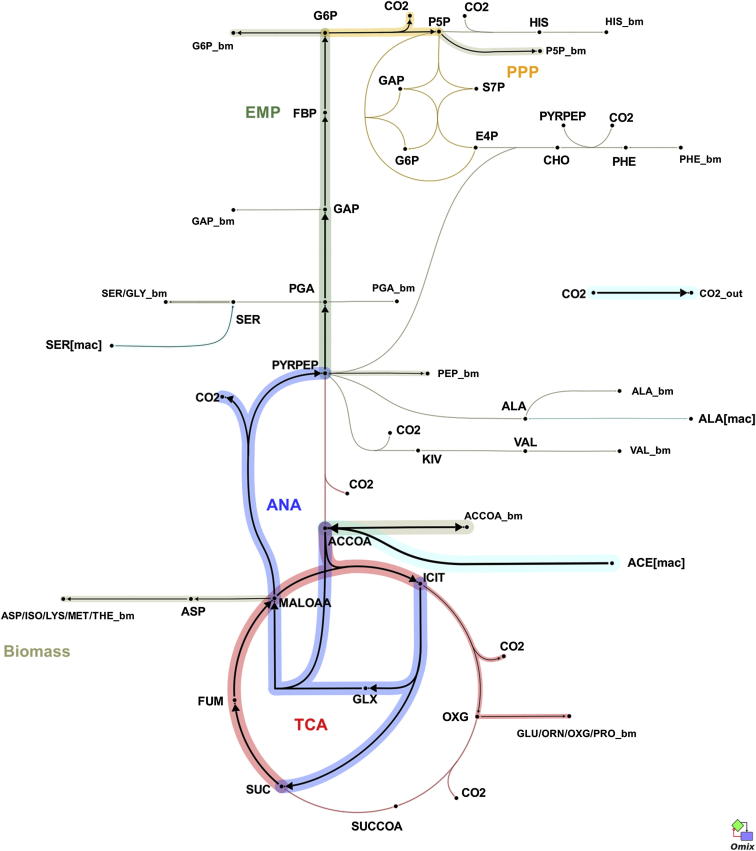
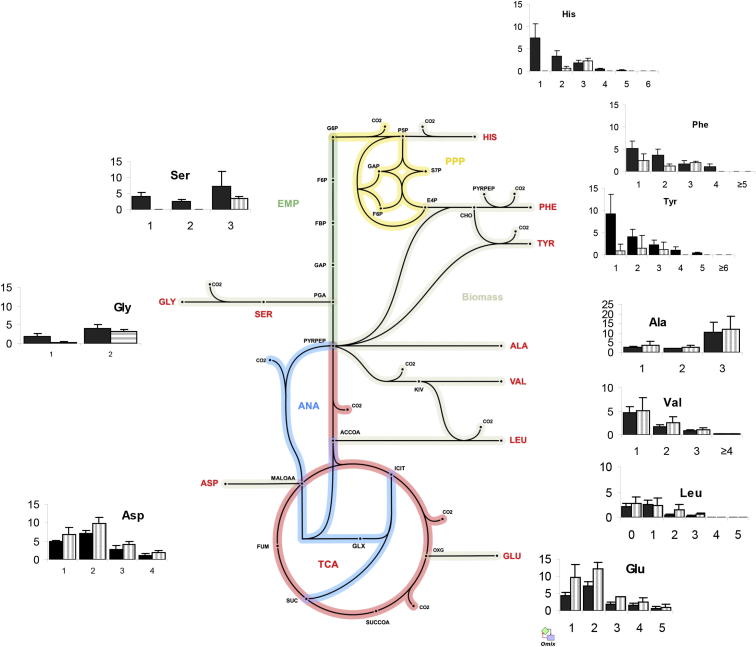
Figure 3.
Estimated Carbon Flux Distribution through Central Metabolism for Intracellular M. tuberculosis
Arrows point in the net flux direction, and the width of each line is proportional to the underlying flux value. Glycolysis/gluconeogenesis (TCA), oxidative pentose phosphate pathway (EMP), tricarboxylic acid cycle (TCA), anaplerotic reactions (ANA). Standard abbreviations are used for the amino acids. Metabolite abbreviations: ACCOA, acetyl-CoA; ACE, acetate; CHO, chorismate; E4P, d-erythrose 4-phosphate; F6P, d-fructose 6-phosphate; FBP, d-fructose 1,6-bisphosphate; FUM, fumarate; G6P, d-glucose 6-phosphate; GA3P, glyceraldehyde 3-phosphate; GLX, glyoxylate; GLYC, glycerol; ICIT, isocitrate/citrate; KIV, 2-oxoisovalerate; MALOAA, l-malate-oxaloacetate; OXG, 2-oxoglutarate; R5P,α-d-ribose 5-phosphate/l-ribulose 5-phosphate/l-xylulose 5-phosphate; PEP, phosphoenolpyruvate; PGA, 2-phospho-d-glycerate/3-phospho-d-glycerate; PYR, pyruvate; S7P, sedoheptulose 7-phosphate; SUC, succinate; SUCCOA, succinyl-CoA. Enzyme abbreviations: CS, citrate synthase; ENO, enolase; FBA, fructose-bisphosphate adolase; FBP, fructose-bisphosphatase; FUM:fumurase; GAPA, glyceraldehyde 3-phosphate dehydrogenase; GND, 6-phosphogluconate dehydrogenase; ICL, isocitrate lyase; ICDH, isocitrate dehydrogenase NADP-dependent; KOR/KGD, α-ketoglutarate ferredoxin oxidoreductase/α-ketoglutarate decarboxylase; MEZ/PCA, malic enzyme/pyruvate carboxylase: MDH, malate dehydrogenase; MS, malate synthase; PCK, phosphoenolpyruvate carboxykinase; PDH, pyruvate dehydrogenase; PGI, glucose phosphate isomerase; PYK, pyruvate kinase; SDH, succinate dehydrogenase; SCS, Succinyl CoA synthetase; TAL, transaldolase; TKT1/2, transketolase. Import from the macrophage is denoted as [mac], and export to biomass is labeled _bm.
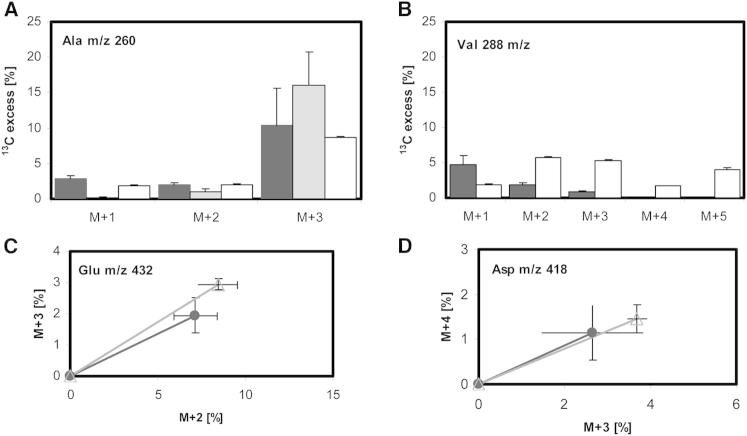
Manual inspection of the labeling profile confirmed many aspects of the 13C-FSA solution. For instance, valine and alanine, which are both formed from the same precursor (pyruvate), had discordant isotopomer profiles, indicating that they were not derived from the same precursor pool. The similarity between the composition of both bacterial and macrophage alanine (Figure 4A), but distinct patterns found for valine (Figure 4B), indicated that the M. tuberculosis alanine was directly imported from the macrophage, whereas valine was predominantly synthesized de novo in M. tuberculosis. The dominant isotope for both bacterial and macrophage alanine was M+3 (Figure 4A). In the macrophage, the metabolic precursor for 13C3 alanine is 13C3 pyruvate derived from [U-13C6] glucose. The 13C isotopologue profile of intracellular M.-tuberculosis-derived valine (produced by two pyruvate molecules with the removal of one carbon atom by decarboxylation) (Figure 4B) indicated that this amino acid was not derived from 13C3 pyruvate, owing to the absence of 13C5 labeling. By comparison, the labeling of alanine in the control cells grown in 13Cglucose-RPMI was predominantly 13C3, and as expected, significant levels of 13C5 valine were also detected. These data confirm that alanine was imported from the macrophage into M. tuberculosis and used for bacterial protein synthesis without any remodeling of the carbon skeleton. The lower but significant amounts of 13C1 and 13C2 alanine, however, indicate that, in addition to direct incorporation, some alanine is also synthesized de novo. The labeling profile of valine also indicates that 13C3-labeled host pyruvate is not a major carbon source for intracellular M. tuberculosis (Figure 4).
Figure 4.
Isotopomer Profiles Indicate that Alanine, ASP, and GLU Are Predominantly Taken Up from Macrophages and Incorporated Directly into Biomass, whereas Valine Is Predominantly Synthesized De Novo
(A–D) Proportion of 13C in isotopomers of alanine (A) and valine (B) derived from macrophage (gray bars), intracellular M. tuberculosis (black bars), and M. tuberculosis grown in labeled RPMI (white bars). Isotopomers ratios for GLU (C) and ASP (D) represented as line plots. The THP-1 cells were prelabeled by passaging with uniformly labeled [U-13C6] glucose prior to the infections. Closed symbols in black from intracellular M. tuberculosis protein; open symbols in gray from macrophage protein. The error bars indicate SD from 3–4 independent experiments.
See also Table S2.
Manual inspection of the isotopomer composition of asparagine/aspartate (ASP; the individual amino acids cannot be separated by GC-MS) and glutamate/glutamine (GLU) were indistinguishable between M. tuberculosis and macrophage fractions (Figures 4C and 4D), indicating that these amino acids were also imported from the host cell and incorporated directly into biomass.
CO2 Fixation
Although most carbon flux in intracellular M. tuberculosis appeared to be derived from acetate, significant 13C1 and 13C3 isotopomer signals in several amino acids (Figure 5; Table S2) were inconsistent with an exclusive C2 feed into central metabolism. A potential source for the C1 signal could be the anaplerotic reactions (phosphoenolpyruvate carboxykinase [PEPCK]/malic enzyme [MEZ]/pyruvate carboxylase [PYC]) operating in the direction of oxaloacetate/malate to fix carbon from CO2 as we previously demonstrated in vitro (Beste et al., 2011). Although the 13C-FSA solution showed a net gluconeogenic (decarboxylating rather than carbon-fixing) flux through these reactions (Figure 3), this result does not exclude the possibility that one or more of the contributing reactions could be operating in the reverse (anaplerotic) direction. To test this hypothesis, we infected unlabeled THP-1 macrophages with M. tuberculosis in RPMI medium containing sodium [13C] bicarbonate (13Cbicarbonate-RPMI). In accordance with expectations, there was no incorporation of CO2-derived 13C label into amino acids extracted from macrophages, whereas there was significant 13C incorporation into ten of the amino acids extracted from the intracellular M. tuberculosis fraction (Figure 6; Table S3), proving that M. tuberculosis fixes CO2 carbon during intracellular growth. In contrast, control experiments in which M. tuberculosis was grown directly in 13Cbicarbonate-RPMI showed significant 13C labeling in only three amino acids (glycine, ASP, and GLU), and the 13C excess was significantly lower than for intracellular M. tuberculosis (Table S3). These results demonstrated enhanced CO2 fixation during intracellular growth and a wider distribution of CO2-derived carbon throughout central metabolism as compared with in vitro growth.
Figure 5.
13C Isotopomer Distribution of Intracellular WT and ΔPEPCK M. tuberculosis Demonstrates Intracellular Consumption of Substrate(s), which Generate Significant 13C1 and 13C3 Intermediates
13C isotopomer distribution after infection of THP-1 macrophages prelabeled (with [U-13C6] glucose) with wild-type (black bars) and ΔPEPCK (striped bars) M. tuberculosis superimposed on a metabolic network of central metabolism with the positioning of each chart indicating the source of the carbon backbone of each amino acid. Error bars indicate SD of 3–4 samples from independent macrophage infections.
See also Table S4.
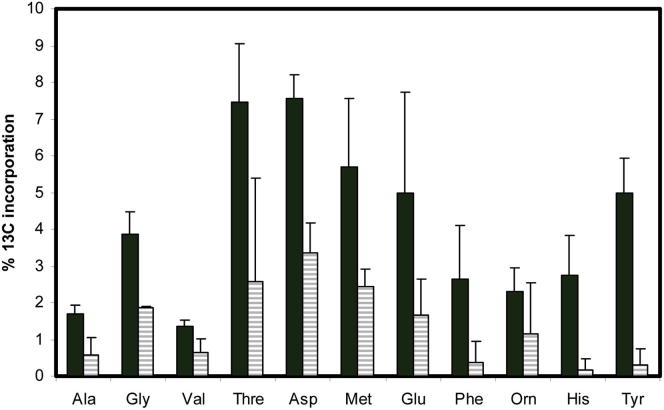
Figure 6.
Intracellular M. tuberculosis Utilizes Carbon from Fixed CO2, and the Enzyme PEPCK Is Contributing to the Observed Carbon Fixation
13C-incorporation into proteinogenic amino acids by wild-type M. tuberculosis (black bars) and ΔPEPCK (striped bars) after 48 hr of growing intracellularly within THP-1 macrophages in the presence of sodium [13C] bicarbonate. Error bars indicate SD of 3–4 samples from independent macrophage infections.
See also Table S3.
The predominance of 13C label from CO2 in ASP, threonine, and methionine during intracellular growth suggests that the carbon-fixing reaction is one or more of the anaplerotic reactions that generate their common precursor, oxaloactetate. Although anaplerotic fixation of CO2 could be performed by PEPCK, PYC, or MEZ, our previous in vitro experiments indicated that PEPCK was operating in the anaplerotic direction to fix CO2 (Beste et al., 2011). CO2 has also been found to induce the expression of the gene encoding PEPCK, pckA (Watanabe et al., 2011). To investigate whether PEPCK was fixing intracellular CO2, we repeated the 13C-bicarbonate labeling experiment in a pckA knockout (KO) strain (Marrero et al., 2010). The background strain for ΔpckA was Erdman, but control experiments detected no significant labeling differences between M. tuberculosis Erdman and H37Rv. Control in vitro experiments were also performed in 13C-bicarbonate-RPMI. Significant 13C labeling occurred in only four amino acids from the intracellular PEPCK mutant (glycine, ASP, MET, and GLU). In addition, incorporation into these amino acids was 50% less than the 13C enrichment measured for the same amino acids from the wild-type strain, indicating that whereas PEPCK was contributing to carbon fixation in M. tuberculosis ex vivo, it was not the sole enzyme involved (Figure 6; Table S3). The restriction of 13C labeling in ΔpckA to TCA cycle-derived amino acids demonstrated that carbon flux from fixed CO2 requires PEPCK to reach glycolysis- or pentose-phosphate-derived metabolites and confirmed the gluconeogenic role of this enzyme when M. tuberculosis is replicating within a macrophage.
C3 Substrate
The significant C3 signal in the labeling data (Figure 5; Table S2) could have been generated by combinations of C1 and C2 units derived from CO2 and acetate but could also indicate the presence of an additional carbon source feeding into central metabolism. The previous demonstration that gluconeogenic flux of acetate was abolished in vitro in a PEPCK KO (Marrero et al., 2010), together with our results, which show that there is a similar block in macrophages, prompted us to use this strain to probe for additional glycolytic substrates. We performed intracellular 13C isotopologue experiments with the PEPCK deletion mutant and respective parent Erdman strain using THP-1 macrophages that were prelabeled by passaging in 13Cglucose-RPMI (Table S4), using an identical protocol to the experiments described for intracellular M. tuberculosis H37Rv. As a control, M. tuberculosis Erdman and ΔPEPCK were also grown for 48 hr in 13Cglucose-RPMI media (Table S4). Again, we observed no significant difference between the profile of H37Rv and the Erdman strain of M. tuberculosis. These labeling experiments showed no significant differences in isotopomer composition between wild-type and ΔpckA for TCA cycle-derived amino acids, but in the case of amino acids derived from the pentose phosphate pathway and glycolysis/gluconeogenesis precursors (Figure 5; Table S4), there was a reduction in the 13C1 and 13C2 signal (presumably derived from acetate or CO2) and retention of 13C3 signal. The data clearly showed that, in addition to C2 and C1 substrates, M. tuberculosis is consuming substrate(s), which generate 13C3 glycolytic intermediates. Moreover, the labeling profile indicates that this substrate is entering at the midpoint of the glycolytic/gluconeogenic pathway and then is primarily being channeled into the pentose phosphate pathway.
Discussion
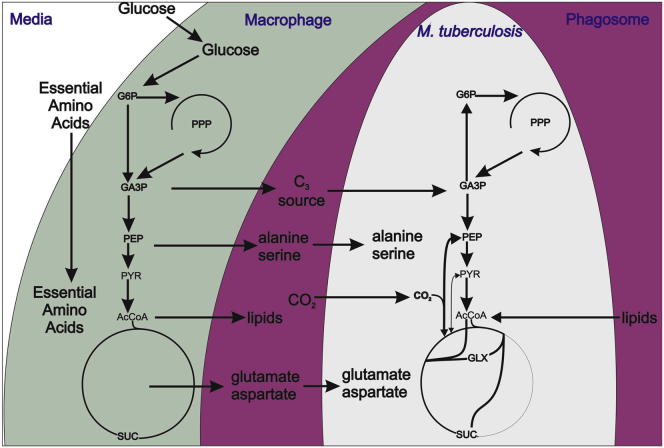
We (Beste et al., 2011) and others (de Carvalho et al., 2010) have demonstrated that M. tuberculosis cocatabolizes multiple carbon sources in vitro. Here, we show that intracellular M. tuberculosis also cocatabolizes several substrates, including amino acids, C1, C2, and C3 substrates (Figure 7), a finding that has considerable implication for efforts to develop novel antituberculosis drugs that target substrate uptake or intracellular metabolism. The major carbon source feeding central metabolism was shown to be a C2 compound that is highly likely to be acetate- or acetyl-CoA-derived from β-oxidation of host lipids, in agreement with previous indirect evidence (Muñoz-Elías and McKinney, 2005; Pandey and Sassetti, 2008) of a predominantly lipid diet for M. tuberculosis in vivo. However, our experiments with the PEPCK KO strain demonstrated that glycolytic C3 compounds are also carbon sources for intracellular M. tuberculosis, as has been shown for intracellular L. monocytogenes (Eylert et al., 2010). The identity of these substrate(s) could be one or all of the amino acids that we demonstrated were imported by intracellular M. tuberculosis, e.g., alanine, glutamate/glutamine, or asparagine/aspartate. However, glycerol 3-phosphate (derived from abundant membrane phospholipids) or glycerol is also a potential C3 source. Glycerol kinase, required for the conversion of glycerol to glycerol 3-phosphate, is dispensable for the growth of M. tuberculosis in a mouse model (Pethe et al., 2010), indicating that glycerol itself is not an essential carbon source.
Figure 7.
Substrates Consumed and Proposed Metabolic Pathways Employed by M. tuberculosis, Replicating in Macrophages
M. tuberculosis auxotrophic mutants of leucine, proline, tryptophan, and glutamine have previously been shown to be severely attenuated in vivo (Lee et al., 2006; Smith et al., 2001; Hondalus et al., 2000; Tullius et al., 2003), indicating that biosynthesis of some amino acids is required in the intracellular environment; but several auxotrophic strains (e.g., methionine, isoleucine, and valine) can successfully proliferate in macrophages, indicating that other amino acids are acquired from the macrophage (Awasthy et al., 2009; McAdam et al., 1995). The data presented here showed that the macrophage amino acids alanine, glutamate/glutamine, and asparagine/aspartate were contributing to the intracellular nutrition of M. tuberculosis. Alanine is a critical structural component of peptidoglycan, and it has been shown that impairing the ability of M. tuberculous to convert L-alanine into D-alanine severely restricts intracellular growth, both in vivo and ex vivo in macrophages (Awasthy et al., 2009). By accessing host cell alanine pools, M. tuberculosis may ensure intracellular cell wall homeostasis. The data were also consistent with additional uptake of either glutamate and/or glutamine. M. tuberculosis has only limited access to glutamine within a phagosome, and a glutamine auxotrophic strain (glnA1 mutant) is highly attenuated in macrophages (Tullius et al., 2003). These results, together with our data, indicate that glutamate, rather than glutamine, is more likely to be the substrate derived from the macrophage. No data are available for M. tuberculosis-auxotrophic mutants of aspartate and asparagine. However, in a transposon screen mutations in genes involved in asparagine uptake (Rv2127 and Rv0346c) were shown to have decreased intracellular fitness (Stewart et al., 2005).
CO2 is perhaps the most surprising carbon source demonstrated to be utilized by intracellular M. tuberculosis in this study. We previously established that glycerol-grown M. tuberculosis can incorporate CO2 carbon into biomass in vitro (Beste et al., 2011), and this was also demonstrated in a hypoxic model of in vitro growth (Watanabe et al., 2011). The current study provides direct evidence that M. tuberculosis not only fixes CO2 during intracellular growth but that fixation is at an increased level as compared with in vitro growth. The product of carbon fixation appears to be oxaloacetate generated partially, but not exclusively, by the action of PEPCK. The remainder of the CO2 fixation could be performed by one or more of the anaplerotic reactions catalyzed by PYC and MEZ or via the activity of propionyl CoA carboxylase of the methylmalonyl pathway (MMP). MMP is one route for metabolizing propionyl CoA, which can be generated from oxidation of odd and branched chain fatty acids and cholesterol when sufficient amounts of vitamin B12 are present (Savvi et al., 2008). It has also been shown that MMP can provide an anaplerotic feed to the TCA cycle (Savvi et al., 2008). However, in previous studies supplementation of vitamin B12 was required to activate the MMP when M. tuberculosis was growing intracellularly (Griffin et al., 2012; Lee et al., 2013). The role of the methyl malonyl pathway in CO2 fixation during intracellular growth is currently under investigation in our laboratory.
Cocatabolism of glucose, glycerol, and acetate in vitro has previously been reported to involve compartmentalized metabolism with each carbon source yielding distinct products (de Carvalho et al., 2010). Our data suggest a similar phenomenon operating inside macrophages, with the anaplerotic reactions operating in both the gluconeogenic (C2 substrate) and carbon-fixing anaplerotic (C3 substrate) direction simultaneously, despite the absence of a requirement for anaplerosis. It has previously been proposed that carbon fixation could play a role in maintaining redox balance in intracellular pathogens, acting as a redox sink in conditions of reduced oxygen availability (Srinivasan and Morowitz, 2006), a situation that may be relevant to the host environment of the TB bacillus. Carbon fixation provides a potentially attractive drug target, because macrophage cells are unable to fix CO2.
Overall, these studies describe the direct measurements of nutrient uptake and metabolism of M. tuberculosis growing inside its host macrophage. This was facilitated by the development of 13C-FSA, a powerful systems-based tool with utility in unraveling the complex metabolic interactions between host cells and their intracellular residents. The knowledge that M. tuberculosis cometabolizes a mixture of glycolytic and gluconeogenic carbon sources within a macrophage can be exploited in the design of suitable in vitro media for high-throughput drug screens, in addition to having implications for designing drugs that target nutrient uptake or intracellular metabolism of this and other intracellular pathogens.
Significance
Tuberculosis (TB) is a disease that plagued ancient Egyptians and remains one of the biggest killers in the world today. A key to the success of Mycobacterium tuberculosis, the bacterium that causes TB, is its ability to survive and grow in macrophages, the very cells that are equipped to eliminate bacteria from the body. In order to do this, M. tuberculosis has to acquire nutrients and energy from this isolated niche. Several studies have highlighted the fact that targeting nutrient utilization is a potentially productive route for drug development, yet the nutrients consumed by intracellular M. tuberculosis are currently unknown. We used 13C-labeled carbon sources to directly measure the metabolism of M. tuberculosis growing in macrophage host cells. Our data were analyzed using a bespoke mathematical model, which allowed us to identify that amino acids, C1 and C2 carbon sources were being obtained and metabolized by M. tuberculosis in the host cell. We have directly measured the intracellular metabolism of M. tuberculosis inside its host cell and used a mathematical approach—13C-flux spectral analysis (13C-FSA)—to scrutinize this type of complex interaction. We confirmed independently that the source of the C1 substrate was CO2. Using a mutant strain, we also demonstrated that C3 substrates were also contributing to the intracellular diet of M. tuberculosis. These studies demonstrate that M. tuberculosis has access to a diverse diet within its host cell, a finding which has significant implications for designing drugs against this and other intracellular pathogens, as well as advancing our knowledge of the pathogenesis of this globally important pathogen. We also describe an automated systems-level approach, which has utility in probing the intracellular metabolism of any microbe within their host cell.
Experimental Procedures
Bacterial Strains and Growth Conditions
Frozen stock of M. tuberculosis was cultivated using Middlebrook7H11 agar and Middlebrook 7H9 broth containing 5% (v/v) oleic acid-albumin-dextrose-catalase enrichment medium supplement (Becton Dickenson) and 0.5% (v/v) glycerol. The knockout strain of M. tuberculosis Erdman PCK and the parent Erdman strain (Marrero et al., 2010) were kindly provided by Sabine Erht.
Cultivation of Human THP-1 Macrophages
The THP-1 human monocytic cell line was obtained from ATCC TIB-202. Cells were grown in RPMI 1640 medium supplemented with 0.2% glucose, 0.2% sodium bicarbonate, and 10% heat-inactivated fetal calf serum (Sigma). Labeled RPMI medium was prepared from RPMI without glucose or sodium bicarbonate (Sigma) by the addition of 100% [U-13C6] glucose and unlabeled sodium bicarbonate (Cambridge Isotope Laboratories), followed by sterile filtration. For the generation of 13C-labeled THP-1 cells, RPMI media was prepared using 100% [U-13C6] glucose. Prelabeled monocytes were then generated by passaging the cells three times (12 days) in this media. RPMI was also prepared containing 0.2% sodium [13C]bicarbonate (Cambridge Isotope Laboratories) for the carbon fixation experiments. Cultures were passaged twice a week and maintained at a density below 106 cells ml−1.
Infection of Macrophages with M. tuberculosis
Bacterial infections were performed in 6–10 flasks (175 cm2). Each flask was seeded with 3 × 107 THP-1 cells and differentiated with 50 nM PMA for 72 hr at 37°C, 5% CO2, and 95% humidity. Cells were washed with PBS supplemented with 0.49 mM Mg2+ and 0.68 mM Ca2+ (PBS+). M. tuberculosis cultures were grown exponentially in 7H9 liquid medium to an optical density of 1.0 (1 × 108 CFU ml−1) for the infection and then washed in PBS and resuspended in RPMI with 20% FCS to obtain a bacterial suspension of 1.5 × 108 CFU ml−1. A total of 1 ml of bacterial suspension was added to each flask (multiplicity of infection: five) and incubated for 3–4 hr. After incubation the macrophages were washed three times with PBS+ and 30 ml of RPMI, plus 20% FCS was added to each flask. After incubation for 48 hr the floating cells were harvested by centrifugation at 300 × g for 5 min at 4°C. The adhered and floating cells were then washed with ice-cold PBS before being lysed with 0.1% Triton X-100. Mammalian cell debris was pelleted by centrifugation at 300 × g at 4°C. The supernatant containing the intracellular M. tuberculosis and the soluble host material was centrifuged at 4,000 × g for 20 min at 4°C to pellet the bacteria. The resulting supernatant was stored as a probe for the analysis of host cell amino acids. The resulting bacterial pellet was washed twice in RIPA buffer (Sigma) and was used as a probe for the analysis of 13C labeling of amino acids in intracellular M. tuberculosis.
13C Biomass Hydrolysate and Preparation of Amino Acid Derivatives
Amino acid derivatives were prepared from bacterial and host cell fractions as previously described (Beste et al., 2011). 13C excess and the isotopologue composition was determined using GC-MS analysis as previously described (Beste et al., 2011). 13C isotopologue abundances (i.e., 13C incorporation; 12Cn, 13C1, …, 13Cn) for each amino acid were determined for fragments containing the intact carbon skeleton for each amino acid, generally using the [M-57]+ ion mass spectra of the derivatized amino acids. These were corrected for the natural abundance of all stable isotopes.
Network Model
An isotopomer model of the central metabolism of M. tuberculosis (Beste et al., 2011) was modified to include 11 amino acids, acetate (ACE), glycerol (GLYC), and glucose (GLC) as potential carbon exchange pools (Table S5). Exchange of carbon between the macrophage environment and M. tuberculosis was modeled via unidirectional reaction routes. A biomass formula (Beste et al., 2007) was introduced to constrain metabolic flux rates. In total, 55 intracellular reactions (25 unidirectional, 11 reversible) and 43 metabolites were subject to 14 possible uptake fluxes in the model (Table S3). Flux rates (total of 33) were estimated using a total of 120 measured GC-MS isotopomer/ isotopologue measurements (Table S6). For each combination of potential carbon source, a network variant was built. In order to make simulation results comparable among these model variants, the sum of all carbon uptake fluxes was normalized to one (= 100%), as the ratio between biomass formation and carbon uptake is not known for intracellular M. tuberculosis.
13C-Flux Spectral Analysis
In order to reveal potential carbon sources (as isotopomers) for the intracellular mycobacterial cell, a multistage sampling-based 13C-MFA-type approach was implemented. First, the measured labeling patterns of macrophage amino acids were deconvoluted by generating random isotopomer labeling patterns for each potential substrate imitating the measured isotopologues. If necessary, the direction of transport fluxes was modified from a nominal efflux to a nominal uptake flux. Because of stoichiometric constraints, additional flux directions were also adapted to guarantee a nonempty flux solution space. Second, multistart flux estimations were run based on initial flux space sampling as described elsewhere (optimization library: NAG C; [http://www.nag.co.uk] with flux sampling rate = 100 and maximal iteration number max_iter = 150; Dalman et al., 2013) to account for locality pitfalls of the nonlinear weighted least-squares fitting problem. Results for all measured isotopologues and initial and optimal flux distributions were recorded and ranked using the residual valine, and the best 100 flux fits (lowest residual valine) were selected for further analysis. Combinations of up to three carbon sources were rigorously analyzed in a large-scale computational study (in excess of 600,000 optimization runs) performed with the high-performance software 13CFLUX2 (Weitzel et al., 2013).
Statistical Analysis
Statistical analysis was performed using the Statistics Toolbox in Matlab 2001b (The Mathworks), and flux maps were visualized using Omix (v. 1.5.8, http://www.13cflux.net/omix). Best flux estimates were calculated by minimizing the sum of squares (residual values) of the offsets between the measured and simulated labeling patterns. Flux estimations, including the calculation of residual valine, were performed using 13CFLUX2 module multifitfluxes compiled for openSUSE 11.4 64bit (http://www.13cflux.net).
Acknowledgments
We thank Sabine Erht for the PEPCK KO strain and Michael Weitzel for providing customized 13CFLUX2 scripts. This work was supported by a grant from the Wellcome Trust (088677). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Published: August 1, 2013
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information includes six tables and can be found with this article online at http://dx.doi.org/10.1016/j.chembiol.2013.06.012.
Supplemental Information
References
- Awasthy D., Gaonkar S., Shandil R.K., Yadav R., Bharath S., Marcel N., Subbulakshmi V., Sharma U. Inactivation of the ilvB1 gene in Mycobacterium tuberculosis leads to branched-chain amino acid auxotrophy and attenuation of virulence in mice. Microbiology. 2009;155:2978–2987. doi: 10.1099/mic.0.029884-0. [DOI] [PubMed] [Google Scholar]
- Beste D.J., Hooper T., Stewart G., Bonde B., Avignone-Rossa C., Bushell M.E., Wheeler P., Klamt S., Kierzek A.M., McFadden J. GSMN-TB: a web-based genome-scale network model of Mycobacterium tuberculosis metabolism. Genome Biol. 2007;8:R89. doi: 10.1186/gb-2007-8-5-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste D.J.V., Bonde B., Hawkins N., Ward J.L., Beale M.H., Noack S., Nöh K., Kruger N.J., Ratcliffe R.G., McFadden J. 13C metabolic flux analysis identifies an unusual route for pyruvate dissimilation in mycobacteria which requires isocitrate lyase and carbon dioxide fixation. PLoS Pathog. 2011;7:e1002091. doi: 10.1371/journal.ppat.1002091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalman T., Dörnemann T., Juhnke E., Weitzel M., Wiechert W., Nöh K., Freisleben B. Cloud MapReduce for Monte Carlo bootstrap applied to Metabolic Flux Analysis. Future Gener. Comput. Syst. 2013;29:582–590. [Google Scholar]
- de Carvalho L.P., Fischer S.M., Marrero J., Nathan C., Ehrt S., Rhee K.Y. Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chem. Biol. 2010;17:1122–1131. doi: 10.1016/j.chembiol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Eisenreich W., Slaghuis J., Laupitz R., Bussemer J., Stritzker J., Schwarz C., Schwarz R., Dandekar T., Goebel W., Bacher A. 13C isotopologue perturbation studies of Listeria monocytogenes carbon metabolism and its modulation by the virulence regulator PrfA. Proc. Natl. Acad. Sci. USA. 2006;103:2040–2045. doi: 10.1073/pnas.0507580103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylert E., Herrmann V., Jules M., Gillmaier N., Lautner M., Buchrieser C., Eisenreich W., Heuner K. Isotopologue profiling of Legionella pneumophila: role of serine and glucose as carbon substrates. J. Biol. Chem. 2010;285:22232–22243. doi: 10.1074/jbc.M110.128678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontán P., Aris V., Ghanny S., Soteropoulos P., Smith I. Global transcriptional profile of Mycobacterium tuberculosis during THP-1 human macrophage infection. Infect. Immun. 2008;76:717–725. doi: 10.1128/IAI.00974-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz A., Eylert E., Eisenreich W., Goebel W. Carbon metabolism of enterobacterial human pathogens growing in epithelial colorectal adenocarcinoma (Caco-2) cells. PLoS ONE. 2010;5:e10586. doi: 10.1371/journal.pone.0010586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J.E., Pandey A.K., Gilmore S.A., Mizrahi V., McKinney J.D., Bertozzi C.R., Sassetti C.M. Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem. Biol. 2012;19:218–227. doi: 10.1016/j.chembiol.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondalus M.K., Bardarov S., Russell R., Chan J., Jacobs W.R., Jr., Bloom B.R. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect. Immun. 2000;68:2888–2898. doi: 10.1128/iai.68.5.2888-2898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D., Nath L., Kamal M.A., Varshney A., Jain A., Singh S., Rao K.V.S. Genome-wide analysis of the host intracellular network that regulates survival of Mycobacterium tuberculosis. Cell. 2010;140:731–743. doi: 10.1016/j.cell.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Lee S., Jeon B.Y., Bardarov S., Chen M., Morris S.L., Jacobs W.R., Jr. Protection elicited by two glutamine auxotrophs of Mycobacterium tuberculosis and in vivo growth phenotypes of the four unique glutamine synthetase mutants in a murine model. Infect. Immun. 2006;74:6491–6495. doi: 10.1128/IAI.00531-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., VanderVen B.C., Fahey R.J., Russell D.G. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J. Biol. Chem. 2013;288:6788–6800. doi: 10.1074/jbc.M112.445056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero J., Rhee K.Y., Schnappinger D., Pethe K., Ehrt S. Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc. Natl. Acad. Sci. USA. 2010;107:9819–9824. doi: 10.1073/pnas.1000715107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F.O., Gordon S., Locati M., Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- McAdam R.A., Weisbrod T.R., Martin J., Scuderi J.D., Brown A.M., Cirillo J.D., Bloom B.R., Jacobs W.R., Jr. In vivo growth characteristics of leucine and methionine auxotrophic mutants of Mycobacterium bovis BCG generated by transposon mutagenesis. Infect. Immun. 1995;63:1004–1012. doi: 10.1128/iai.63.3.1004-1012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Elías E.J., McKinney J.D. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 2005;11:638–644. doi: 10.1038/nm1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A.K., Sassetti C.M. Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. USA. 2008;105:4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethe K., Sequeira P.C., Agarwalla S., Rhee K., Kuhen K., Phong W.Y., Patel V., Beer D., Walker J.R., Duraiswamy J. A chemical genetic screen in Mycobacterium tuberculosis identifies carbon-source-dependent growth inhibitors devoid of in vivo efficacy. Nat. Commun. 2010;1:57. doi: 10.1038/ncomms1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riendeau C.J., Kornfeld H. THP-1 cell apoptosis in response to Mycobacterial infection. Infect. Immun. 2003;71:254–259. doi: 10.1128/IAI.71.1.254-259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvi S., Warner D.F., Kana B.D., McKinney J.D., Mizrahi V., Dawes S.S. Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: implications for propionate metabolism during growth on fatty acids. J. Bacteriol. 2008;190:3886–3895. doi: 10.1128/JB.01767-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S.K., Mohan A. Multidrug-resistant tuberculosis: a menace that threatens to destabilize tuberculosis control. Chest. 2006;130:261–272. doi: 10.1378/chest.130.1.261. [DOI] [PubMed] [Google Scholar]
- Simeone R., Bobard A., Lippmann J., Bitter W., Majlessi L., Brosch R., Enninga J. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog. 2012;8:e1002507. doi: 10.1371/journal.ppat.1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V., Jamwal S., Jain R., Verma P., Gokhale R., Rao K.V. Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell Host Microbe. 2012;12:669–681. doi: 10.1016/j.chom.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Singhal A., Jaiswal A., Arora V.K., Prasad H.K. Modulation of gamma interferon receptor 1 by Mycobacterium tuberculosis: a potential immune response evasive mechanism. Infect. Immun. 2007;75:2500–2510. doi: 10.1128/IAI.01743-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.A., Parish T., Stoker N.G., Bancroft G.J. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect. Immun. 2001;69:1142–1150. doi: 10.1128/IAI.69.2.1142-1150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan V., Morowitz H.J. Ancient genes in contemporary persistent microbial pathogens. Biol. Bull. 2006;210:1–9. doi: 10.2307/4134531. [DOI] [PubMed] [Google Scholar]
- Stewart G.R., Patel J., Robertson B.D., Rae A., Young D.B. Mycobacterial mutants with defective control of phagosomal acidification. PLoS Pathog. 2005;1:269–278. doi: 10.1371/journal.ppat.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullius M.V., Harth G., Horwitz M.A. Glutamine synthetase GlnA1 is essential for growth of Mycobacterium tuberculosis in human THP-1 macrophages and guinea pigs. Infect. Immun. 2003;71:3927–3936. doi: 10.1128/IAI.71.7.3927-3936.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayati A.A., Masjedi M.R., Farnia P., Tabarsi P., Ghanavi J., Ziazarifi A.H., Hoffner S.E. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in iran. Chest. 2009;136:420–425. doi: 10.1378/chest.08-2427. [DOI] [PubMed] [Google Scholar]
- Venugopal A., Bryk R., Shi S., Rhee K., Rath P., Schnappinger D., Ehrt S., Nathan C. Virulence of Mycobacterium tuberculosis depends on lipoamide dehydrogenase, a member of three multienzyme complexes. Cell Host Microbe. 2011;9:21–31. doi: 10.1016/j.chom.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Zimmermann M., Goodwin M.B., Sauer U., Barry C.E., 3rd, Boshoff H.I. Fumarate reductase activity maintains an energized membrane in anaerobic Mycobacterium tuberculosis. PLoS Pathog. 2011;7:e1002287. doi: 10.1371/journal.ppat.1002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzel M., Nöh K., Dalman T., Niedenführ S., Stute B., Wiechert W. 13CFLUX2—high-performance software suite for (13)C-metabolic flux analysis. Bioinformatics. 2013;29:143–145. doi: 10.1093/bioinformatics/bts646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechert W., Möllney M., Petersen S., de Graaf A.A. A universal framework for 13C metabolic flux analysis. Metab. Eng. 2001;3:265–283. doi: 10.1006/mben.2001.0188. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva, Switzerland: 2011. Tuberculosis fact sheet. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.