Abstract
Pancreatic islet amyloid is a characteristic feature of type 2 diabetes. The major protein component of islet amyloid is the polypeptide hormone known as islet amyloid polypeptide (IAPP, or amylin). IAPP is stored with insulin in the β-cell secretory granules and is released in response to the stimuli that lead to insulin secretion. IAPP is normally soluble and is natively unfolded in its monomeric state, but forms islet amyloid in type 2 diabetes. Islet amyloid is not the cause of type 2 diabetes, but it leads to β-cell dysfunction and cell death, and contributes to the failure of islet cell transplantation. The mechanism of IAPP amyloid formation is not understood and the mechanisms of cytotoxicity are not fully defined.
Keywords: Islet Amyloid Polypeptide, Amylin, Amyloid, Type 2 Diabetes, IAPP
1. Introduction
The deposition of amyloid in the islets of Langerhans in the pancreas is a characteristic pathological feature of type 2 diabetes (T2D). Hyaline lesions in the pancreas were first described more than 110 years ago [1], and were later identified as amyloid. The deposits were initially assumed to be composed of insulin or pro-insulin or fragments of insulin, but in 1987 two groups independently showed that the major protein component of islet amyloid is a 37 residue polypeptide pancreatic hormone denoted as islet amyloid polypeptide (IAPP) or amylin [2–3]. IAPP has been found in all mammals studied to date. The molecule is stored together with insulin in the β-cell secretory granules and is released in response to the stimuli that lead to insulin secretion [4–6]. IAPP is normally soluble and is natively unfolded in its monomeric state, but forms islet amyloid in T2D [2–3,7]. IAPP can be readily induced to form amyloid in vitro and is one of the most amyloidogenic naturally occurring sequences known. Islet amyloid is not the cause of T2D, but it does lead to β-cell dysfunction and cell death, and contributes to loss of islet β-cell mass [8–10]. Rapid amyloid formation likely contributes to the failure of islet cell transplantation and prevention of amyloid formation can prolong graft survival [7,11–12].
In this review we briefly discuss the processing and normal function of IAPP, and then focus on amyloid formation by IAPP. There are a number of critical outstanding issues in the field. The mechanisms of IAPP amyloid formation in vivo and in vitro are still not understood, particularly in vivo. The site of initiation of amyloid formation in vivo is controversial. The nature of the toxic species generated during IAPP amyloid formation are not well defined, nor are the mechanisms of cell death completely understood. The mechanisms of clearance of IAPP amyloid in vivo and the role this may play in islet amyloid formation and cytotoxicity are not fully elucidated. Inhibitors of IAPP toxicity are less well developed than for other amyloidogenic proteins and most studies have made use of in vitro assays of toxicity.
2. The physiological role of IAPP
2.1 IAPP is synthesized as a pre-pro hormone
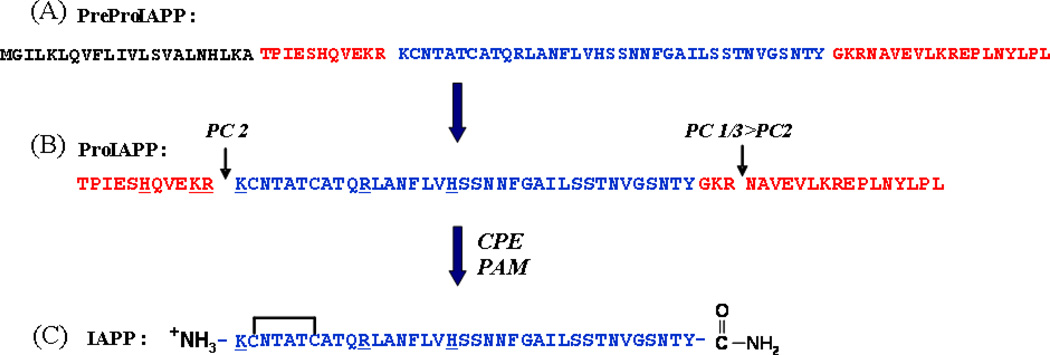
IAPP is synthesized as a 89 residue pre-pro form [13]. The 22 amino acid signal peptide is cleaved to give the 67 amino acid proform (proIAPP). ProIAPP is processed in the Golgi and in the insulin secretory granule [14]. The short C- and N- terminal flanking peptides of proIAPP are cleaved by the pro hormone convertases PC2 and PC1/3 [13]. The C-terminal cleavage leaves a Gly-Lys-Arg tri-peptide sequence at the C-terminus. The dibasic residues at the C-terminus are removed by carboxypeptidase E and the Gly serves as the nitrogen donor for amidation of the C-terminus by the peptidyl amidating mono-oxygenase complex (PAM). Amidation and disulfide bond formation lead to mature IAPP (Figure-1). Incorrect processing of proIAPP has been proposed to play a role in islet amyloid formation in vivo (see below).
Figure 1. Processing of human PreProIAPP to form mature IAPP.
(A) The primary sequence of human PreProIAPP, the peptide length is 89 residues. The 22 residue signal sequence is shown in black, the N- and C-terminal proIAPP flanking regions are shown in red, and the mature sequence in blue. (B) The primary sequence of the 67-residue human proIAPP. ProIAPP is cleaved by the prohormone convertases PC(1/3) and PC2 at the two dibasic sites, indicated by the arrows. Additional processing by CPE/PAM leads to an amidated C-terminus of IAPP. (C) The sequence of the mature 37-residue human IAPP. The biologically active peptide has an amidated C-terminus and a disulfide bridge between Cys-2 and Cys-7.
IAPP is stored in the insulin secretory granule where it is localized in the halo region while insulin is found in the dense core of the granule. The concentration of IAPP in the granule is about 1%–2% that of insulin, and this is much higher than the level required to promote rapid amyloid formation in vitro [15–16]. Thus, there must be factors which inhibit the premature, irreversible aggregation of IAPP in the granule. The low pH environment of the granule likely contributes since the rate of IAPP amyloid formation is strongly pH dependent and is slower at intragranule pH [17–19]. Soluble insulin is an inhibitor of IAPP aggregation and this may play a role in controlling intragranule aggregation, however insulin is found in a partially crystalline state in the granule [20–24].
2.2 IAPP receptors
IAPP binds the Calcitonin (CT) receptor with low affinity, but the affinity is significantly enhanced when the CT receptor forms a complex with receptor activity-modifying proteins (RAMPs). IAPP receptors are generated from co-expression of the CT receptor with one of three RAMPs [25]. Interaction with RAMPs changes the specificity of the CT receptor towards IAPP [26–27]. The CT receptor has two splice variants, so there could be six different subtypes of IAPP receptors. Despite the physiological importance of IAPP and its potential clinical relevance, it is not known whether different receptors are active in the peripheral tissue and CNS. It is also not known which receptor subtype(s) binds the FDA approved analog of IAPP, Pramlintide. Thus, a more detailed understanding of IAPP receptors is needed [28]. There are currently no approved small molecule agonists of IAPP receptors.
2.3 IAPP has multiple physiological roles
IAPP is co-secreted with insulin from the β-cells following nutrient influx. The circulating concentration of IAPP is 3 to 5 picomolar in rats, rising to 15 to 20 picomolar upon elevation of blood glucose [29]. The local concentration after release from the granule will be much higher and is the more relevant number for amyloid formation. The physiological roles of soluble IAPP are not completely understood, but IAPP is believed to play a role in controlling gastric emptying, in maintaining glucose homeostasis, in the suppression of glucagon release and in controlling satiety [7,30–31]. IAPP has been proposed to play a role in regulating blood glucose levels by inhibiting insulin secretion from the pancreas [32–33], but the main sites of action appear to be in the CNS [34–35]. IAPP has also been proposed to act as an adiposity signal [36]. The polypeptide has been reported to inhibit insulin-stimulated glucose uptake and the synthesis of glycogen in isolated rat skeletal muscle [37]. However, these effects were studied at concentrations of the polypeptide that are higher than physiological levels, thus the details of IAPP’s role are still not completely clear. Several recent reviews of the function of IAPP have recently appeared and provide a more in depth discussion [7,29,31].
3. Residue specific effects on amyloid formation
3.1 Differences in the primary sequence of IAPP correlate with amyloid formation in vitro and in vivo
IAPP is a member of the calcitonin related peptide family which consists of Calcitonin α- and β-Calcitonin gene-related peptide (CGRP), Adrenomedullin and Intermedin. The peptides share limited amino acid sequence identity, but have several important structural features in common (Figure-2). They all have an intramolecular disulfide bridge near the N-terminus and an amidated C-terminus.
Figure 2. Primary sequences of human CGRP and IAPP from different species.
Residues that differ from the human IAPP sequence are highlighted in blue. The biologically active mature sequences all have a disulfide bridge between Cys-2 and Cys-7 and an amidated C-terminus. Primates and cats have been reported to form islet amyloid while cows, rodents, and dogs do not. Ferret and porcine IAPP are reported to be significantly less amyloidogenic than human IAPP. Islet amyloid is found in the degu, but it is derived insulin, not IAPP. Only partial sequences are available for rabbit and hare.
IAPP is most similar to CGRP. Both are 37 residues in length, have a conversed disulfide bond between residues two and seven, contain an amidated aromatic residue at the C-terminus, and have a tendency to form low levels of transient helical structure over part of the sequence in their monomeric states [38–40]. Early studies showed that human IAPP (hIAPP) readily forms amyloid in vitro, but that CGRP does not. The two peptides have reasonable sequence similarity, with the greatest homology at the N- and C- terminal regions, but differ most between residues 20 and 29 [41]. These observations led to the hypothesis that the sequence within the 20 to 29 region determines the ability of IAPP to form amyloid. Only humans, nonhuman primates, and cats form islet amyloid in vivo, notably rats and mice do not [41–42]. Experiments with rat IAPP seemed to confirm the hypothesis that IAPP amyloidogenicity is controlled by the 20–29 segment. Rat IAPP and hIAPP differ at only six positions out of 37, five of which are located between residues 20–29. The rat sequence contains three Pro residues at positions 25, 28 and 29, while the human sequence has none. Pro is a well-known disrupter of secondary structure and is energetically unfavorable in a β-sheet. The inability of rat IAPP to form amyloid is attributed to the Pro substitutions [41]. These important early studies led to the view that the amyloidogenic properties of IAPP are dictated by the primary sequence in the 20–29 region, however the situation is more complex. Multiple Pro substitutions outside of the 20–29 region have been shown to abolish amyloid formation by hIAPP, as does replacement of Asn-14 or Asn-21 [43–44]. In contrast, substitution of the rat IAPP residues; Arg-18, Leu-23, and Val-26 by the residues found in hIAPP led to a weakly amyloidogenic polypeptide [45]. Thus, the 20–29 sequence is not the only factor governing in vitro amyloid formation, but there is no doubt that it is important.
The only polymorphism found in hIAPP that impacts amyloid formation in vivo is a Ser to Gly mutation at position 20. This mutation, which is found at low levels in certain Asian populations, has been proposed to lead to a slightly higher risk of diabetes, and has been shown to accelerate amyloid formation in vitro [7,46–49].
hIAPP contains six Asn residues and deamidation can alter the amyloidogenic properties of proteins. Spontaneous Asn deamidation is one of the most common non-enzymatic post translation modifications and is thought to play a role in amyloid formation by other polypeptides [50]. Deamidation proceeds via a cyclic succimide intermediate and, depending on how the ring is opened, will convert an Asn residue into L or D-Asp or L or D iso-Asp. In both cases a neutral residue is replaced by a negatively charged residue which reduces the net charge of hIAPP, and should thus reduce its solubility. Asn deamidation has been shown to accelerate hIAPP amyloid formation in vitro [51] and to allow amyloid formation by otherwise non amyloidogenic fragments of hIAPP [52]. Deamidation also leads to changes in the morphology of hIAPP amyloid fibrils [51].
3.2 Mutational analysis of amyloid formation by IAPP
Quantitative mutational studies of amyloid formation and amyloid fibril stability are more complicated than studies of the folding kinetics and stability of soluble globular proteins. Mutations can lead to the formation of different polymorphs and the determination of fibril stability can be difficult. There are well established methods for determining protein stability which are firmly grounded in theory, but this is not always the case for amyloid formation. Solubility measurements can yield apparent free energies, provided that the soluble phase is composed of monomers, and provided that activity effects can be ignored, but it is difficult to verify these assumptions. In addition, studies which report that a particular mutation abolishes amyloid formation may simply have not examined the protein for a long enough time. None-the-less, mutational analysis of amyloid formation has provided considerable insight and systematic studies, including proline scans, have been reported for a number of amyloidogenic proteins. No systematic analysis of all of the positions of IAPP has been reported.
A number of studies have examined the consequences of mutations on the amyloidogenicity of IAPP, but it is difficult to compare them since a range of conditions have been used and the rate of IAPP aggregation can be sensitive to seemingly small changes in buffer composition or pH. For example, some studies have used buffers that contain 1–2% (V/V) hexafluoroisoproponal (HFIP) and even this low level of HFIP accelerates significantly the rate of IAPP amyloid formation. pH is also an important variable and significant changes in the rate of amyloid formation are observed as a function of pH. These effects are due to changes in the protonation state of His-18 and-or the N-terminus. Further complicating matters, the rate of IAPP amyloid formation is strongly dependent on both the concentration of added salt and the identity of the anion, including common buffer components [53]. Another complication is that the majority of studies have made use of a truncated fragment of IAPP which lacks the first seven residues, (IAPP8–37). These residues are thought to be outside of the ordered amyloid core, but they could still affect the stability of the amyloid fibers by contributing to electrostatic repulsion (see below). High throughput screens of the solubility-aggregation behavior of IAPP are complicated by the fact that standard E.coli based expression systems lead to a free C-terminus instead of the physiologically relevant amidated C-terminus. Screens which involved fusing IAPP to a reporter protein can be powerful [54], but complications might arise since the reporter protein is much larger than IAPP.
Despite these potential complications, there is a growing body of mutation data on hIAPP and hIAPP8–37. Table-1 summarizes the available data from studies that have used C-terminally amidated hIAPP variants and which have reported direct tests of amyloid formation. Many of the substitutions that impact amyloid formation fall within the 20–29 segment reflecting the importance of this region. However, mutations in the putative helical region also alter the rate of amyloid formation, and a number of substitutions within the F15, L16, and V17 segment have noticeable effects. One model of the early stages in IAPP aggregation proposes that interactions near residue-15 are critical and are mediated by association of helical conformers. This model might rationalize the sensitivity of hIAPP amyloid formation to mutations at these positions [55].
Table 1.
| A: Mutational studies of amyloid formation by full length IAPP. Residues which differ from human IAPP are indicated in red. Citations to the primary literature are given. | ||
|---|---|---|
| Human IAPP | KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY | |
| I26P [82–83] | × | |
| G24P [82–83] | × | |
| 3XL [57–58] | ▼ | |
| S20G [49] | ▲ | |
| S20K [49] | ▼ | |
| Pramlintide [153] | × | |
| S28G [54] | × | |
| I26D [54] | × | |
| A13E [54] | × | |
| L16Q [54] | × | |
| F15L [59] | ▲ | |
| F23L [59] | ▼ | |
| Y37L [59] | ▼ | |
| F15LF23L [59] | ▼ | |
| F15LY37L [59] | ▼ | |
| F23LY37L [59] | ▼ | |
| F15NLe [59] | ▼ | |
| F15I [59] | ▼ | |
| F15TLe [59] | ▼ | |
| B: Mutational studies of amyloid formation by full length IAPP. | ||
|---|---|---|
| Human IAPP 8-37 | ATQRLANFLVHSSNNFGAILSSTNVGSNTY | |
| 3XP [43] | × | |
| H18R [67] | ▬ | |
| F23L [67] | ▼ | |
| H18RF23L [67] | ▼ | |
| H18RS28PS29P [67] | × | |
| H18RF23LA25PI26V [67] | × | |
| F15S [55] | ▲ | |
| F15A [55] | ▲ | |
| F15D [55] | ▲ | |
| F15K [55] | ▼ | |
| V17A [55] | ▲ | |
| V17K [55] | ▬ | |
| N14A [44] | × | |
| N14L [44] | × | |
| N14S [44] | × | |
| N21L [44] | × | |
| N21S [44] | × | |
| N22L [44] | ▬ | |
| N31L [44] | ▼ | |
| N35L [44] | ▼ | |
| L12NN14L [44] | × | |
| N14LL16N [44] | × | |
| A13C [44] | ▼ | |
| V17C [44] | ▲ | |
| A25C [44] | ▼ | |
| T30C [44] | ▼ | |
| T36C [44] | ▬ | |
“▬” no reported effect;
“▲” The mutation leads to faster amyloid formation;
“▼” The mutation leads to slower amyloid formation,
“ד The mutant is reported to abolish amyloid formation.
Aromatic-hydrophobic and aromatic-aromatic interactions have been proposed to play a critical role in amyloid formation by hIAPP. Experiments that made use of Ala scanning of short peptides supported this conjecture [56], but studies that employed more conservative aromatic to Leu substitutions revealed that aromatic residues are not required for amyloid formation by the full length polypeptide [57–59]. Aromatic-aromatic interactions may play a role in helping dictate the structure of the amyloid fibril and the kinetics of fibril formation, even though they are not required for amyloid formation. Replacement of the aromatic residues has been shown to alter the rate of self-assembly of IAPP: a triple mutant in which all three aromatic residues are replaced by Leu formed amyloid 5-fold slower than wild type hIAPP [58].
In the fiber the amide-containing Asn side chains are arranged in parallel arrays along the axis of the fiber, and are expected to both accept and donate hydrogen bonds to their equivalent residues in adjacent chains. A systematic examination of the role of different Asn side chains in hIAPP structure and assembly has been reported [44]. By replacing each Asn with the isosteric Leu, which occupies roughly the same volume, but has no hydrogen bonding ability, the authors found that different sites have drastically different consequences on amyloid kinetics. The truncated 8–37 hIAPP fragment was used as background in this study. Asn14Leu and Asn21Leu mutants did not form amyloid on the experimental timescale, and Asn14Leu could not be seeded by pre-formed wild type fibrils. Since both mutants lie in the region of predicted α-helical propensity, the disrupted amyloid formation kinetics can be rationalized based on different secondary structure propensities of the two side chains. Intriguingly, Asn14 is placed into the core of models of the amyloid fibril, and its desolvation would significantly enhance the strength of the hydrogen bonds made and received at this site, thus the Asn14Leu mutant might also impact fibril stability.
An interesting avenue for future exploration will be to use unnatural amino acids. Much more conservative changes can be made using non-genetically coded amino acids and, since IAPP is normally prepared by solid phase peptide synthesis, they can be readily incorporated. For example, analogs of aliphatic side chains can be incorporated which preserve hydrophobicity, but significantly alter secondary structure propensities. This approach has been proven useful in studies of protein folding transition states and seems ripe for exploitation in studies of IAPP [60].
4. The conformation of monomeric IAPP
4.1 Monomeric IAPP does not fold to a compact structure, but it is not a random coil
Proteins that form amyloid can be divided into two structural classes; those which fold to a compact globular structure in their unaggregated state and those which are natively unfolded. Important examples of the former include β2-microglobulin and TTR, while Aβ and IAPP are important examples of the latter. Unaggregated, monomeric IAPP does not fold to a globular structure, but it is not a classic random coil. The region encompassing residues 5–20 of hIAPP and rat IAPP has been shown via NMR to transiently sample helical φ, ψ angles in solution, but the level of persistent helical structure is low [38,61].
4.2 IAPP forms helical structure on model membranes
More persistent helical structure can be induced by negatively charged model membranes [39,62–63]. NMR studies have delineated the conformation of IAPP and IAPP fragments in membrane mimetic environments [62–63]. hIAPP adopts a helix-kink-helix structure on model membranes with the helices located between residues 5 to 17 and 20 to 27. Studies of peptide fragments have revealed interesting differences in the structure of hIAPP and rat IAPP in the presence of micelles. hIAPP1–19 and rat IAPP1–19 adopt very similar α-helical structures in the presence of detergent micelles, but they bind to membranes in different orientations [63]. The two peptides differ only at position 18, which is an Arg in rat IAPP and a His in hIAPP. hIAPP1–19 inserts deeply into the hydrophobic core of membranes, while rat IAPP1–19 binds near the surface. The differences are believed to be dependent on the charge of residue 18 and hIAPP1–19 binds near the surface, similar to rat IAPP1–19, at acidic pH when His-18 is protonated [63–64]. Membrane-bound structures of full length human and rat IAPP have also been reported and reveal structural similarities in the N-terminal half of the molecule, but significant differences in the C-terminal half. α-helical structure is formed in the N-terminal portion of both polypeptides [62–63,65]. The C-terminal region of rat IAPP is almost completely disordered [62], but hIAPP has a partially helical C-terminal region. The differences are almost certainly due to the multiple proline residues found in rat IAPP. The role of IAPP membrane interactions in amyloid formation and in toxicity is discussed in subsequent sections
5. The structure of IAPP amyloid fibrils
5.1 Models of the hIAPP protofibril reveal an in register, parallel β-sheet structure
Amyloid fibrils adopt a cross-β architecture in which the β-strands run perpendicular to the fibril long axis with the interstrand hydrogen bonds oriented parallel to the long axis. The first seven residues of hIAPP may not be part of the β-structure core due to conformational restrictions imposed by the disulfide bridge. Two atomic level models have been proposed for the hIAPP fibril and they share a number of features in common. One is derived from solid state NMR and the other from structural studies of hIAPP fragments. Both contain a parallel, in register arrangement of the β-strands. The protofibrils are made up of two columns of symmetry related hIAPP monomers with each polypeptide adopting a U-shaped structure. Each hIAPP monomer contains two β-strands connected by a loop. The β-strands form intermolecular hydrogen bonds with neighboring polypeptide chains within the same column, but there are no intrachain backbone hydrogen bonds. In the solid state NMR derived model, the first β-strand is made of residues 8–17 and the second encompasses residues 28–37, while the loop involves residues 18–27 [66]. Two structures were presented which were both consistent with the experimental NMR data. The main difference between the two had to do with the register of side-chain orientations. In one structure, all copies of Arg11 project into the monomer core, as do other odd-numbered residues (Ala13, Phe15, etc.); in the other structure, Arg11, Ala13 and Phe15 are all solvent-exposed. Burial of the charged Arg side chain is expected to be very unfavorable and thus the second structure seems more likely.
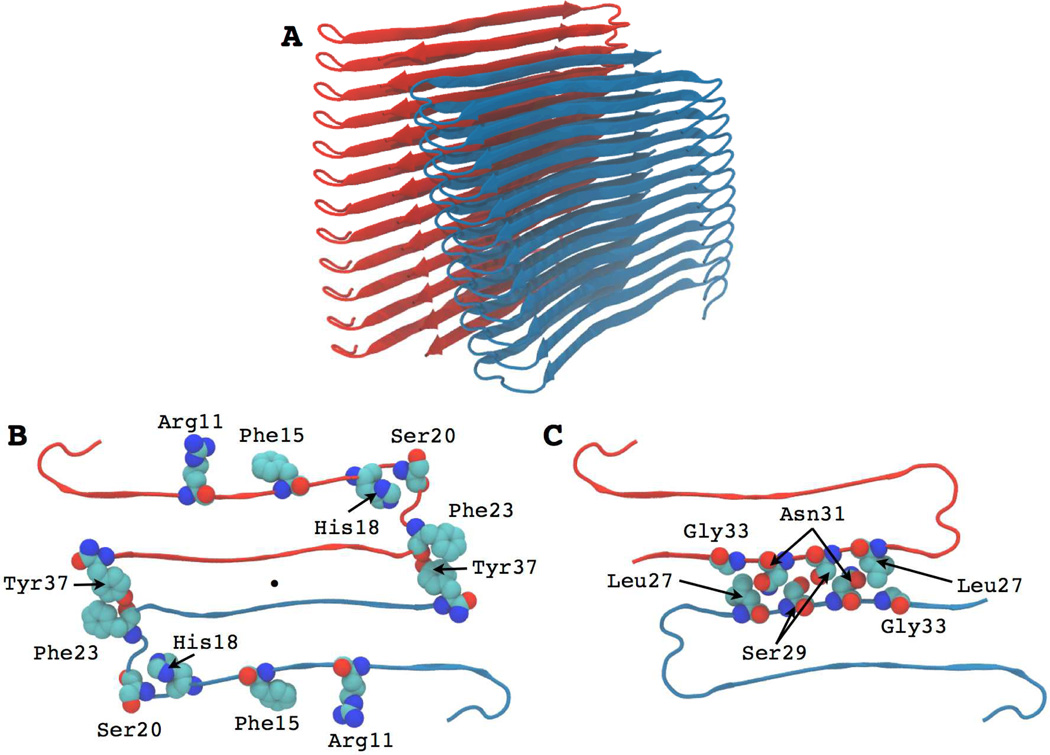
A second model has been developed based on X-ray crystallographic studies of two penta- or hexapeptide “steric zippers” derived from hIAPP (Figure-3) [67]. The crystallographic and solid state NMR derived models are similar, but differ in three features. There are differences in the details of the atomic packing in the core of each U-shaped monomer, differences at the bimolecular interface between the two hIAPP monomers, and differences in the register of side chain interdigitation at the bimolecular interface.
Figure 3. Structural model of the hIAPP fibril derived from studies of steric zippers.
(A) Ribbon diagram of the fibril structure. The two stacks of symmetry related monomers are shown. (B) Cross section of one fibril layer, looking down the fibril axis, showing several key residues. The aromatic residues Phe15, Phe23 and Tyr37 are shown in space filling format along with Arg11, His18 and Ser20. The color coding corresponds to that used in panel-A. (C) Cross section of one layer showing the tight “steric zipper” interface between two hIAPP monomers. Interdigitated residues Leu27, Ser29, Asn31 and Gly33 are shown in space filling representation.
Interestingly, the 20–29 segment is not part of a β-strand in either of the models, but instead adopts a partially ordered loop that connects the two strands. Is this compatible with the critical role the 20–29 region plays in modulating amyloidogenicity? Ser-28 and Ser-29 make key contacts in both models, arguing that the Pro substitutions in rat IAPP will disrupt the interface. Multiple Pro substitutions should also distort the bend structure due to the steric constraints imposed by the cyclic proline side chain. Thus, the importance of this region can be rationalized on structural grounds, but more work is required in order to understand the molecular basis of the significant effect of substitutions in this region of hIAPP. Formation of the loop may also be important for kinetic reasons; two dimensional IR (2D IR) spectroscopy studies have led to a model in which structure is formed early in this region based [68]. Along these lines, recent work has shown that stabilization of turn structures in the Alzheimer’s Aβ peptide can enhance significantly the rate of amyloid formation [69].
5.2 Models of amyloid fibril structure have important energetic implications
The in-register parallel β-sheet structure of amyloid has interesting implications for the energetics of amyloids. The structure generates quasi-infinite arrays of stacked identical residues. These in-register arrangements suggest the presence of significant ionic interactions in amyloids. In hIAPP both His-18 and Arg-11 are in the structured β-sheet core or immediately adjacent to it, suggesting that they could make net unfavorable contributions to the stability of the fibril. Electrostatic calculations performed at the level of the linearized Poisson Boltzmann (PB) equation show that the Arg residues make significant unfavorable interactions, but indicate that the His residues do not do so when the His side chains are neutral. In this case, the desolvation penalty can be overcome by specific interactions with the imidazole ring [53]. Of course, PB calculations may not be strictly valid for a strongly coupled system and thus they should be taken with a grain of salt. The problem of electrostatic interactions in amyloids is somewhat reminiscent of other systems with repetitive arrangements of like charges such as DNA.
The N-terminus of hIAPP is expected to make unfavorable electrostatic interactions in the amyloid fibril, even though it may not be well ordered, since the Lys side chains and N-termini on adjacent chains will be in close proximity. The importance of electrostatic interactions in hIAPP amyloid is reflected in the strong salt dependence of the kinetics of amyloid formation. The rate of hIAPP amyloid formation is significantly accelerated with increasing salt, as expected if charge repulsion is important. However, different salts have different effects, indicating that salts are involved in more than just simple electrostatic screening. A correlation with the electroselectivity series is observed for the anions at low to moderate salt concentrations, arguing that ion binding plays a role [53].
6. The role of early oligomeric intermediates in IAPP amyloid formation in vitro
6.1 The role of low order oligomers is not clear
There are conflicting reports on the importance of low molecular weight oligomers in IAPP amyloid formation. The nature of the early steps of aggregation and the nature of oligomer intermediates is of much more than academic interest. Oligomers have been proposed to be the toxic species for other amyloidogenic systems and the lack of knowledge about the nature of the toxic species produced during IAPP amyloid formation hinders rational drug development [70–71]. Many studies have made use of the conformation-specific polyclonal antibody A11 to detect oligomers, particularly in studies of Aβ, but its specificity toward non-Aβ oligomers has been called into question, since there are reports that it can give rise to false negatives and false positives under certain conditions [71–73].
Analytical ultracentrifugation experiments have failed to detect low order IAPP oligomers, however those studies were performed at low pH where IAPP aggregation is much slower and it is possible that the mechanism of aggregation is different at neutral pH [74]. 19F NMR studies of labeled IAPP also failed to detect lower order oligomers [75]. On the other hand, chemical cross linking studies have reported the presence of dimers, trimmers, tetramers and higher order oligomers, while mass spectroscopy measurements have provided evidence for dimers with a range of conformations [76–78]. CD studies of IAPP amyloid formation also give conflicting results. Some reports suggest the presence of an isodichroic point, consistent with lack of significantly populated intermediates, although an isodichroic point is a necessary, but not a sufficient condition for a two state process. In contrast, other studies show CD monitored transitions that lack an isodichroic point. It is clear that the presence or absence of low order oligomers in IAPP amyloid formation is still an open question.
6.2 The kinetics of hIAPP amyloid formation is very sensitive to conditions and sample preparation
An important practical issue that complicates studies of IAPP oligomers and the kinetics of IAPP amyloid formation is that a wide range of methods have been used to prepare the peptide for kinetic experiments. Many studies solubilize the peptide in fluoroalcohols or in DMSO and then dilute the resulting stock solutions into buffer. Unfortunately, even 1% by volume of these co-solvents has a significant effect upon the kinetics of amyloid formation. Fluoroalcohols also stabilize helical structure in IAPP, even at these low levels. Other investigations have relied upon adding buffer to dried peptide, but the procedure used to dry IAPP can impact the results. Some studies have prepared samples in organic solvents, typically HFIP, and then removed the solvent, either via lyophilization or by evaporation under nitrogen. Evaporation under a stream of nitrogen leads to a peptide film and it is not clear if the peptide will be monomeric when it is then dissolved in buffer. The presence of already aggregated material at the start of a kinetic experiment could significantly impact the results. Differences in the mode of preparation likely contribute to the wildly different lag times that are reported in the IAPP amyloid literature. Unfortunately, some studies do not provide detailed information about sample preparation, or about the methods used to initiate amyloid formation, and consequently they can be difficult to reproduce. One promising approach is to prepare the peptide in a “pro-form” that is soluble, but which can be rapidly converted to normal IAPP. The use of so called “switch peptides”, in which two residues are linked by an ester bond is one manifestation of this approach [79]. The variant is stable at acidic pHs, but a rapid conversion from the ester linkage to the more stable amide to regenerate IAPP is initiated by a simple pH jump.
6.3 Helical intermediates may be important for IAPP amyloid formation
hIAPP amyloid formation in vitro, in homogenous solution may involve a helical intermediate [38,55,61,80]. Self-association and helix formation are linked in many systems; examples include coiled coils, other peptides with a tendency to form amphiphilic helices and certain designed sequences. Helical wheel analysis reveals that hIAPP has the potential to form an amphiphilic helix between residues 5–20 [38] and NMR studies show that this region of the chain transiently samples α-helical φ, ψ angles. Initial aggregation might be driven by the energetic linkage between association and helix formation. Formation of an oligomeric helical intermediate with helical structure in the N-terminal portion of hIAPP will lead to a high local concentration of the amyloidogenic C-terminal segment. This could lead to intermolecular β-sheet formation which could then propagate through the sequence.
The crystal structure of a C-terminal truncated fragment of hIAPP fused to maltose binding protein (MBP) has been reported and offers suggestive, albeit indirect, evidence in support of the model [55]. Residues 8 to 18 and 22 to 27 form well ordered α-helices in the structure with a kink separating them. The MBP-IAPP fusion forms a dimer and the N-terminal helices from two hIAPP molecules pack against each other with key contacts being made near Phe-15. The consequences of replacement of Phe-15 with Ser, Ala, Asp and Lys were examined in the truncated 8–37 fragment as part of this work. The Ser, Ala and Asp substitutions were designed because they were predicted to promote early dimerization of hIAPP through the α-helical region [55]. All three substitutions accelerated amyloid formation. The Phe to Lys substitution was chosen because it was predicted to disrupt initial aggregation and it was found to slow amyloid formation.
Studies with inhibitors appear to support the helical model. Rat IAPP and some designed proline mutants of hIAPP are inhibitors of hIAPP amyloid formation which is consistent with the helical intermediate model [81–83]. These peptides should have a tendency to form amphiphilic helices similar to hIAPP, since the proline substitutions are not in the helical region. However, the prolines in the C-terminal portion of these variants should inhibit formation of β-sheet structure. This implies that rat IAPP and the proline mutants could function by binding to helical oligomers of hIAPP and inhibiting their conversion to β-structure [80–81]. The model is appealing, but it is important to remember that there is no direct structural data on the mode of inhibition, and the inhibitors also affect the growth phase suggesting they could have multiple effects. Insulin is a potent inhibitor of IAPP aggregation and IAPP-insulin interactions involve contacts between the helical B-chain of insulin and the putative helical region of hIAPP [24]. The proposed mode of interaction is consistent with helical conformers playing a role in IAPP amyloid formation. Small molecule inhibitors of hIAPP amyloid formation that are designed to target helical structure have also been reported [84].
6.4 Other models for early oligomers have been proposed
Ion mobility mass spectroscopy (IM-MS) in combination with MD simulations has led to a different model of early intermediates [76–77]. The model proposes formation of a set of conformers with helical structure and another set which contain side by side β-hairpin dimers. The β-hairpin dimers are postulated to lead to amyloid formation. The hairpin structure will require a significant rearrangement of the backbone hydrogen bonding to form the stacked column structures found in the amyloid fibril models. IM-MS has the important advantage that it can separate different conformers in a heterogeneous mixture, but has the potential disadvantage that one must assume that conformations detected in the gas phase are representative of those populated by the dynamic peptide in solution.
A third model has been proposed for early oligomers and is based on studies of a non-physiological variant of hIAPP with a free C-terminus. The free C-terminus reduces the net charge on the peptide and could introduce new intermolecular or intramolecular electrostatic interactions. Formation of an anti-parallel dimer was postulated with His-18 in one chain interacting with Tyr-37 in another. Interactions involving the side chain of His-18 and the C-terminal Tyr were observed via NMR. These included ring stacking interactions, but there could be a contribution from the free carboxylate at the C-terminus [85]. It remains to be seen if this interesting structure is formed in the biologically relevant version of hIAPP with its amidated C-terminus.
Studies that made use of Phe to Tyr FRET suggested that hIAPP adopts conformations in the lag phase in which one of the two Phe residues are close to the C-terminal Tyr. There is necessarily an ambiguity in the experiments since there are two Phe residues, F15 and F23. In apparent contrast, experiments that used the fluorescence analog p-cyanophenylanine (cyanoPhe) and cyanoPhe to Tyr FRET were interpreted to show that neither residue 15 nor residue 23 exhibits significant FRET to Tyr in the lag phase, suggesting that the positions-15 and 23 do not form close persistent contacts with Tyr37. Thus the role of the aromatic residues in oligomer formation is not completely clear [86–87].
7. In vivo amyloid deposits contain a range of components
7.1 Islet amyloid contains heparan sulfate proteoglycans and other factors
Islet amyloid contains serum amyloid P component (SAP), apolipoprotein E (apoE), and the heparan sulfate proteoglycan (HSPG) perlecan [88–89] as well as IAPP. There is no correlation between the presence of SAP and islet amyloid deposition. There is a correlation between levels of apoE and extent of amyloid formation by the Aβ peptide in Alzheimer’s disease, but this is not the case in T2D, and apoE knockouts do not affect islet amyloid formation [89]. However, there is growing evidence that implicates interactions with the glycosaminoglycan (GAG) component of HSPGs in IAPP amyloid formation, at least in vitro. This potentially important factor is discussed in the next section.
7.2 Model membranes and model glucosaminoglycans accelerate IAPP amyloid formation in vitro
hIAPP is a cationic polypeptide and has the potential to interact with negatively charged surfaces, anionic membranes and negatively charged biopolymers. Islet amyloid contains the HSPG perlecan. It is not known if HSPGs are associated with amyloids because in vivo amyloid fibers are stable long lived structures that present HSPG binding sites, or because HSPGs play a direct role in promoting amyloid formation, but it is clear that the glycosaminoglycan (GAG) chains of HSPGs can catalyze hIAPP amyloid formation in vitro [90]. Inhibition of GAG synthesis has been shown to reduce hIAPP amyloid deposition in cultured islets, as does over-expression of heparanse in a double transgenic mouse model that over-expresses hIAPP, suggesting that interactions with HSPGs may be important in vivo [91–92].
One model for the initiation of hIAPP amyloid formation in vivo invokes binding of proIAPP processing intermediates to the GAG chains of perlecan [93]. Secretion of an incompletely processed proIAPP intermediate, (NproIAPP), that includes the N-terminal prosequence has been reported to be increased in T2D [94–95]. The extension actually makes the polypeptide more soluble and less amyloidogenic, but it enhances its interactions with GAGs. Interactions with model GAGs accelerates amyloid formation by NproIAPP in vitro and the resulting amyloid is capable of seeding amyloid formation by fully processed hIAPP [96].
Anionic vesicles and other anionic model membranes promote hIAPP amyloid formation in vitro and more highly charged systems have a larger effect for high peptide to lipid ratios [97]. The mechanism of membrane catalyzed hIAPP aggregation is not completely understood, but helical intermediates have been proposed to be important [39,97–99]. Many of the studies of hIAPP-membrane interactions have used model membranes comprised of pure anionic lipids, such as phosphatidylglycerol (PG) or phosphatidylserine (PS), or mixtures of anionic lipids with zwitterionic lipids, such as phosphocholine (PC). The content of anionic lipid typically ranges from 50 to 20 mole %, which is noticeably higher than found in β-cells. β-cells have been reported to contain between 2.5 and 13.2 mole % anionic lipids [100]. The phospholipid composition of the β-cell is also very different from most model systems. In addition, β-cell membranes contain gangliosides and cholesterol. These considerations naturally lead to the question of how well model membranes mimic the in vivo environment. More complicated model membranes made up of the phospholipids found in β-cell membranes, but lacking cholesterol also accelerate hIAPP amyloid formation, as do anionic model membranes that are capable of forming lipid rafts [100–102].
8. hIAPP induced toxicity
8.1 Does islet amyloid formation have an extracellular or intracellular origin?
The in vivo origin of islet amyloid is controversial. Early histological studies with transgenic mice are consistent with extracellular deposition and amyloid deposits observed in T2D appear to be extracellular. However, studies that made use of rodent models in which IAPP was over expressed indicated that islet amyloid might have an intracellular origin [7,103–104]. Conversely, a recent study used a cultured islet model to show that secretion of IAPP is an important factor in islet amyloid formation and β-cell toxicity. That work used two sets of reagents: one that increased IAPP secretion, but did not increase the amount of IAPP produced, and a second that inhibited IAPP secretion, but maintained the level of production. Inhibition of IAPP secretion reduced amyloid formation, while increasing secretion increased amyloid formation and toxicity [104]. The results are consistent with an extracellular origin of islet amyloid, at least for the cultured islet model. The differences between the various studies might be related to the level at which IAPP is produced and to the methods used to detect amyloid [7,71,104]. Determining if islet amyloid has an intracellular or extracellular origin is important since it may impact therapeutic approaches.
8.2 Multiple mechanisms of hIAPP induced β-cell toxicity have been proposed
The decline in β-cell function in T2D has been attributed to a range of factors including islet inflammation, cholesterol accumulation, glucolipotoxicity and islet amyloid formation [105–108]. Amyloid formation by hIAPP induces apoptosis and β-cell dysfunction in isolated human islets [7–9,109–112]. The pathways that lead to hIAPP induced β-cell apoptosis are not completely characterized, but progress is being made [113–115]. The cJUN N-terminal kinase (JNK) pathway has been shown to mediate apoptosis in islets and in cultured β-cells that are exposed to high concentrations of hIAPP. The pathway has also been shown to do so in response to amyloid generated from endogenous hIAPP [114].
Even a brief reading of the literature strongly implies that there are multiple mechanisms of hIAPP induced cell death (Table-2). Here we provide an overview; more information can be found in the accompanying review article by Abedini and Schmidt in this issue. ER stress, defects in autophagy, the enhanced production of pro-inflammatory cytokines, mitochondrial membrane damage, permeabilization of cell membranes, activation of Calpain-2, receptor-mediated mechanisms linked to oxidative stress and the activation of cell death signaling pathways have all been proposed to contribute to IAPP toxicity [113–120].
Table 2.
Proposed mechanisms of IAPP-induced toxicity during amyloid formation.
| Mechanisms of IAPP Cytotoxicity |
|---|
| Cell membrane disruption |
| Cell membrane permeabilization |
| Binding to FAS receptor |
| Endoplasmic Reticulum (ER) Stress |
| Defects in Endoplasmic-reticulum-associated protein degradation (ERAD) |
| Unfolded protein response (UPR) |
| Mitochondrial dysfunction |
| Oxidative stress |
| Impairment of autophagy |
| Activation of inflammasome |
| Upregulation of IL–1β |
| Other alterations in signaling |
| Caspase 3 activation leading to apoptosis |
| Disruption of mitochondria membranes |
ER stress has been proposed to be an important contributor to hIAPP induced β-cell death and exogenously added hIAPP has been reported to induce ER stress [103,121]. However, the role of ER stress in hIAPP mediated toxicity in vivo is controversial. ER stress is important in transgenic models that overexpress hIAPP at high levels, but ER stress was not detected in studies of cultured islets that produce IAPP at lower levels [122].
Defects in autophagy play a role in the toxicity of other amyloidogenic proteins and overexpression of hIAPP in β-cells has been reported to lead to impaired autophagy [116,123]. Inhibiting autophagy-lysosomal degradation enhanced hIAPP induced β-cell apoptosis. In contrast, stimulation of autophagy protected against IAPP toxicity [116].
hIAPP aggregates may lead to β-cell dysfunction by triggering a localized inflammatory response [117,119]. Recent reports provide evidence that hIAPP can stimulate inflammasome activity [117]. Inflammasomes are multi protein complexes that recognize a range of pro-inflammatory stimuli and produce active caspase 1, which in turn produces the active cytokines IL-1β and IL-18 by cleaving their pro-forms. IL-1β is believed to play a part in hIAPP-induced β-cell dysfunction and cell death [117,119].
IAPP toxicity has also been proposed to be linked to its ability to perturb membranes [124–125]. hIAPP amyloid fibrils have been shown to cluster on or near membranes and there is very good evidence that exogenously added IAPP perturbs cell membranes [124–126]. However, the correlation between in vitro biophysical studies using model membranes and in vivo toxicity is less clear and caution should be employed when extrapolating from studies that utilize simple model membranes to the in vivo environment. Along these lines, variants of hIAPP which do not induce β-cell death in vivo can disrupt standard model membranes in vitro. It is also interesting to note that exogenously added IAPP has been reported to have very different effects on closely related cell types, arguing that non-specific membrane disruption is not the only mechanism of toxicity [127].
The ability of IAPP to permeabilize membranes depends on the lipid to peptide ratio, as well as on lipid composition, pH and ionic strength. IAPP interacts more strongly with model membranes that contain a high fraction of anionic lipids. Most model systems contain a much higher percentage of anionic lipids than found in the β-cell membrane [100], and usually lack gangliosides and cholesterol. This could be important since recent work has argued that gangliosides and cholesterol mediate hIAPP membrane interactions and may play a role in the uptake and clearance of hIAPP [101,126]. More physiologically relevant model membrane systems are starting to be employed in biophysical investigations and should provide new insights [100–102].
Mechanistic studies of IAPP induced model membrane disruption are an active area of investigation. Some studies have provided evidence for a pore like mechanism, while others have argued in favor of a detergent or carpeting mechanism. The process of fiber growth at the membrane surface has been demonstrated to contribute to membrane disruption in some cases, while other studies have shown that formation of β-structure is not required to disrupt membranes [125,128–133]. It is possible that multiple mechanisms may be operative and their relative importance might dependent on the specific membrane system under investigations. More information can be found in several recent reviews [97,134].
9. Inhibition of hIAPP amyloid formation: Progress is being made, but more work is required
Inhibition of amyloid formation by hIAPP has therapeutic potential. A large class of inhibitors decreases the final amount of amyloid fibrils without affecting the length of the lag phase. If oligomeric species are toxic, such inhibitors may not be particularly useful since they would only inhibit fibril production instead of oligomer formation. In the worst case, they could even be harmful since they could lead to the buildup of toxic species. A more valuable class of inhibitors are ones that interact with the monomers or very early oligomers and prevent them from forming toxic species. (–)-Epigallocatechin 3-Gallate (EGCG), a biologically active flavanol in green tea, is one such inhibitor. EGCG has been shown to bind to unaggregated polypeptides and has been proposed to redirect the pathway of amyloid formation to off-pathway non-toxic oligomers, although there is some debate on its mechanism [135–136]. The compound inhibits hIAPP amyloid formation and protects against hIAPP induced toxicity [137–138].
The mode of action of EGCG and other polyphenols with hIAPP is not known. Interactions with aromatic residues have been proposed to be critical, but this is not the case, at least for EGCG, since the compound effectively inhibits amyloid formation by a triple Leu mutant of hIAPP that lacks aromatic residues [138]. Schiff base formation with protein amino groups is another potentially important interaction, however the compound still inhibits mutants of hIAPP which lack amino groups, likewise interactions with thiols are not critical for EGCG’s effects on hIAPP [138]. One possibility is that the compound interacts with the protein backbone and also makes non-specific hydrophobic interactions with protein sidechains. Structure function studies of the interaction of EGCG with hIAPP have been reported [138].
Other inhibitors include sulfonated triphenyl methane derivatives. These compounds are potent inhibitors of hIAPP amyloid formation and of toxicity in cell culture, although they are unlikely drug candidates [139]. A lysine-specific molecular tweezers has been recently reported to have broad activity against a range of amyloid forming proteins and effectively inhibits hIAPP amyloid formation and toxicity [140]. A number of other small molecules containing aromatic groups and polyphenols have been demonstrated to inhibit hIAPP amyloid formation, although some of these have to be added in significant molar excess [78,141–146]. An interesting class of small molecule inhibitors has also been reported that targets helical intermediates [84,147]. These appear to be the first rationally designed small molecule inhibitors of IAPP amyloid formation.
Several rationally designed polypeptide inhibitors have been reported to inhibit hIAPP amyloid formation and toxicity. For example, certain single proline mutations in the 20–29 region convert hIAPP into a potent amyloid inhibitor [82–83] and a double N-methylated variant of hIAPP has been shown to be a very effective inhibitor of amyloid formation and hIAPP cytotoxicity [148]. As described above, these compounds might function by targeting helical oligomers, although their mode of action is not yet defined.
A range of protein based inhibitors of amyloid formation have been described, particularly for Aβ. Less work has been reported for IAPP, although two cases have been described recently. The calcium binding protein NUCB1 inhibits hIAPP amyloid formation by “capping off” fibers and protects cells from hIAPP toxicity [149]. A set of designed proteins have been developed that inhibit hIAPP amyloid formation. Segments of the hIAPP sequence were grafted into the loop region of a stable protein domain, in this case an IgG variable heavy domain. The resulting protein inhibited amyloid formation and protected cultured cells from hIAPP induced toxicity [150]. One advantage of this approach is that the target epitope of the amyloid binding domain is known, thus these molecules can be useful reagents for probing structure.
Although progress is being made, much work still clearly needs to be done in order to develop inhibitors of islet amyloid formation and toxicity that will be effective in vivo. One issue that can confound inhibitor studies is the use of thioflavin-T assays to follow amyloid formation. Many potential inhibitors can interfere with thioflavin-T assays, either by simple inner filter effects, or by quenching the fluorescence of bound thioflavin-T, or by displacing the bound dye. These effects can lead to false positives in inhibition assays and it is essential to support thioflavin-T studies with direct tests of amyloid formation [141,151]. There is a second potential complication with thioflavin-T assays related to the behavior of the system in the plateau region of the kinetic curve. It is possible that molecules could remodel amyloid fibrils without altering the thioflavin-T signal. An interesting example is provided by the behavior of mixtures of rat and hIAPP. As noted, rat IAPP slows amyloid formation by the human polypeptide, but the system eventually reaches a steady state in terms of thioflavin-T fluorescence and fibrils can be detected by electron microscopy [81]. However, 2D IR in combination with specific isotope labeling showed that the rat peptide actually disrupted the N-terminal external β-sheet of the hIAPP fibrils (Figure-3). Rat IAPP then templated onto the human fibrils and was induced to form β-structure [152]. Thioflavin-T assays can be blind to such processes. An important challenge in the field is to develop non-perturbing intrinsic probes of amyloid formation. Progress is being made with the use of minimally perturbing unnatural fluorescent amino acids [86] and by 19F NMR [75].
10. Concluding remarks
Despite considerable progress, there are important outstanding issues in the field of islet amyloid; these include defining the nature of the toxic species and identifying the initiation site(s) of amyloid formation in vivo, elucidating the mechanisms of islet amyloid formation in vivo and in vitro, and the development of effective, clinically relevant inhibitors. Advances in biophysical methods will aid our understanding of the process of IAPP amyloid formation in vitro, but a key challenge will be to connect biophysical studies performed on simplified model systems with the situation in vivo.
Acknowledgements
We thank Dr. S. Zraika for helpful discussions. This work was supported by grants from the United States National Institutes of Health GM078114 to D.P.R.; and F32 DK089734-02 to A.A.
References
- 1.Opie EL. The relation of diabetes mellitus to lesions of the pancreas. Hyaline degeneration of the islands of Langerhans. J. Exp. Med. 1901;5:527–540. doi: 10.1084/jem.5.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westermark P, Wernstedt C, Wilander E, Hayden DW, Obrien TD, Johnson KH. Amyloid fibrils in human insulinoma and islets of langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc. Natl. Acad. Sci. U. S. A. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper GJS, Willis AC, Clark A, Turner RC, Sim RB, Reid KBM. Purification and characterization of a peptide from amyloid-rich pancreases of type-2 diabetic-patients. Proc. Natl. Acad. Sci. U. S. A. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukinius A, Wilander E, Westermark GT, Engstrom U, Westermark P. Co-localization of islet amyloid polypeptide and insulin in the beta-cell secretory granules of the human pancreatic-islets. Diabetologia. 1989;32:240–244. doi: 10.1007/BF00285291. [DOI] [PubMed] [Google Scholar]
- 5.Kahn SE, Dalessio DA, Schwartz MW, Fujimoto WY, Ensinck JW, Taborsky GJ, Porte D. Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990;39:634–638. doi: 10.2337/diab.39.5.634. [DOI] [PubMed] [Google Scholar]
- 6.Stridsberg M, Sandler S, Wilander E. Cosecretion of islet amyloid polypeptide (IAPP) and insulin from isolated rat pancreatic-islets following stimulation or inhibition of beta-cell function. Regul. Pept. 1993;45:363–370. doi: 10.1016/0167-0115(93)90362-c. [DOI] [PubMed] [Google Scholar]
- 7.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol. Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 8.Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJS, Holman RR, Turner RC. Islet amyloid, increased alpha-cells, reduced beta-cells and exocrine fibrosis - quantitative changes in the pancreas in type-2 diabetes. Diabetes Res. Clin. Ex. 1988;9:151–159. [PubMed] [Google Scholar]
- 9.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic-islet cell toxicity of amylin associated with type-2 diabetes-mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 10.Konarkowska B, Aitken JF, Kistler J, Zhang S, Cooper GJ. The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. FEBS J. 2006;273:3614–3624. doi: 10.1111/j.1742-4658.2006.05367.x. [DOI] [PubMed] [Google Scholar]
- 11.Westermark GT, Westermark P, Berne C, Korsgren O, Transpla NNCI. Widespread amyloid deposition in transplanted human pancreatic islets. N. Engl. J. Med. 2008;359:977–979. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 12.Potter KJ, et al. Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4305–4310. doi: 10.1073/pnas.0909024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanke T, Bell GI, Sample C, Rubenstein AH, Steiner DF. An islet amyloid peptide is derived from an 89-amino acid precursor by proteolytic processing. J. Biol. Chem. 1988;263:17243–17246. [PubMed] [Google Scholar]
- 14.Marzban L, Trigo-Gonzalez G, Verchere CB. Processing of pro-islet amyloid polypeptide in the constitutive and regulated secretory pathways of beta cells. Mol. Endocrinol. 2005;19:2154–2163. doi: 10.1210/me.2004-0407. [DOI] [PubMed] [Google Scholar]
- 15.Jaikaran ETAS, Clark A. Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology. Biochim. Biophys. Acta, Mol. Basis Dis. 2001;1537:179–203. doi: 10.1016/s0925-4439(01)00078-3. [DOI] [PubMed] [Google Scholar]
- 16.Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: A critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocrinol. Metab. 2004;89:3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 17.Hutton JC. The internal pH and membrane-potential of the insulin-secretory granule. Biochem. J. 1982;204:171–178. doi: 10.1042/bj2040171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charge SBP, Dekoning EJP, Clark A. Effect of pH and insulin on fibrillogenesis of islet amyloid polypeptide in-vitro. Biochemistry. 1995;34:14588–14593. doi: 10.1021/bi00044a038. [DOI] [PubMed] [Google Scholar]
- 19.Abedini A, Raleigh DP. The role of His-18 in amyloid formation by human islet amyloid polypeptide. Biochemistry. 2005;44:16284–16291. doi: 10.1021/bi051432v. [DOI] [PubMed] [Google Scholar]
- 20.Westermark P, Li ZC, Westermark GT, Leckstrom A, Steiner DF. Effects of beta cell granule components on human islet amyloid polypeptide fibril formation. FEBS Lett. 1996;379:203–206. doi: 10.1016/0014-5793(95)01512-4. [DOI] [PubMed] [Google Scholar]
- 21.Janciauskiene S, Eriksson S, Carlemalm E, Ahren B. Beta cell granule peptides affect human islet amyloid polypeptide (IAPP) fibril formation in vitro. Biochem. Biophys. Res. Commun. 1997;236:580–585. doi: 10.1006/bbrc.1997.7014. [DOI] [PubMed] [Google Scholar]
- 22.Jaikaran ETAS, Nilsson MR, Clark A. Pancreatic beta-cell granule peptides form heteromolecular complexes which inhibit islet amyloid polypeptide fibril formation. Biochem. J. 2004;377:709–716. doi: 10.1042/BJ20030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson JL, Miranker AD. The mechanism of insulin action on islet amyloid polypeptide fiber formation. J. Mol. Biol. 2004;335:221–231. doi: 10.1016/j.jmb.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 24.Gilead S, Wolfenson H, Gazit E. Molecular mapping of the recognition interface between the islet amyloid polypeptide and insulin. Angew. Chem., Int. Ed. Engl. 2006;45:6476–6480. doi: 10.1002/anie.200602034. [DOI] [PubMed] [Google Scholar]
- 25.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 26.Muff R, Buhlmann N, Fischer JA, Born W. Amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1or-3. Endocrinology. 1999;140:2924–2927. doi: 10.1210/endo.140.6.6930. [DOI] [PubMed] [Google Scholar]
- 27.Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao YY, Fraser NJ, Main MJ, Foord SM, Sexton PM. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol. Pharmacol. 1999;56:235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- 28.Bailey RJ, Walker CS, Ferner AH, Loomes KM, Prijic G, Halim A, Whiting L, Phillips AR, Hay DL. Pharmacological characterization of rat amylin receptors: implications for the identification of amylin receptor subtypes. Br. J. Pharmacol. 2012;166:151–167. doi: 10.1111/j.1476-5381.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutz TA. The role of amylin in the control of energy homeostasis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R1475–R1484. doi: 10.1152/ajpregu.00703.2009. [DOI] [PubMed] [Google Scholar]
- 30.Montane J, Klimek-Abercrombie A, Potter KJ, Westwell-Roper C, Verchere CB. Metabolic stress, IAPP and islet amyloid. Diabetes Obes. Metab. 2012;14:68–77. doi: 10.1111/j.1463-1326.2012.01657.x. [DOI] [PubMed] [Google Scholar]
- 31.Lutz TA. Control of energy homeostasis by amylin. Cell. Mol. Life Sci. 2012;69:1947–1965. doi: 10.1007/s00018-011-0905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang ZL, Bennet WM, Ghatei MA, Byfield PG, Smith DM, Bloom SR. Influence of islet amyloid polypeptide and the 8–37 fragment of islet amyloid polypeptide on insulin release from perifused rat islets. Diabetes. 1993;42:330–335. doi: 10.2337/diab.42.2.330. [DOI] [PubMed] [Google Scholar]
- 33.Young A. Inhibition of insulin secretion. Adv. Pharmacol. 2005;52:173–192. doi: 10.1016/S1054-3589(05)52009-X. [DOI] [PubMed] [Google Scholar]
- 34.Potes CS, Lutz TA. Brainstem mechanisms of amylin-induced anorexia. Physiol. Behav. 2010;100:511–518. doi: 10.1016/j.physbeh.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Trevaskis JL, Parkes DG, Roth JD. Insights into amylin-leptin synergy. Trends Endocrinol. Metab. 2010;21:473–479. doi: 10.1016/j.tem.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Wielinga PY, Lowenstein C, Muff S, Munz M, Woods SC, Lutz TA. Central amylin acts as an adiposity signal to control body weight and energy expenditure. Physiol. Behav. 2010;101:45–52. doi: 10.1016/j.physbeh.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper GJS, Leighton B, Dimitriadis GD, Parrybillings M, Kowalchuk JM, Howland K, Rothbard JB, Willis AC, Reid KBM. Amylin found in amyloid deposits in human type-2 diabetes-mellitus may be a hormone that regulates glycogen-metabolism in skeletal-muscle. Proc. Natl. Acad. Sci. U. S. A. 1988;85:7763–7766. doi: 10.1073/pnas.85.20.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williamson JA, Miranker AD. Direct detection of transient alpha-helical states in islet amyloid polypeptide. Protein Sci. 2007;16:110–117. doi: 10.1110/ps.062486907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knight JD, Hebda JA, Miranker AD. Conserved and cooperative assembly of membrane-bound alpha-helical states of islet amyloid polypeptide. Biochemistry. 2006;45:9496–9508. doi: 10.1021/bi060579z. [DOI] [PubMed] [Google Scholar]
- 40.Breeze AL, Harvey TS, Bazzo R, Campbell ID. Solution structure of human calcitonin gene-related peptide by 1H-NMR and distance geometry with restrained molecular-dynamics. Biochemistry. 1991;30:575–582. doi: 10.1021/bi00216a036. [DOI] [PubMed] [Google Scholar]
- 41.Westermark P, Engstrom U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide - pinpointing amino-acid-residues linked to amyloid fibril formation. Proc. Natl. Acad. Sci. U. S. A. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Betsholtz C, Christmansson L, Engstrom U, Rorsman F, Svensson V, Johnson KH, Westermark P. Sequence divergence in a specific region of islet amyloid polypeptide (IAPP) explains differences in islet amyloid formation between species. FEBS Lett. 1989;251:261–264. doi: 10.1016/0014-5793(89)81467-x. [DOI] [PubMed] [Google Scholar]
- 43.Abedini A, Raleigh DP. Destabilization of human IAPP amyloid fibrils by proline mutations outside of the putative amyloidogenic domain: Is there a critical amyloidogenic domain in human IAPP? J. Mol. Biol. 2006;355:274–281. doi: 10.1016/j.jmb.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 44.Koo BW, Hebda JA, Miranker AD. Amide inequivalence in the fibrillar assembly of islet amyloid polypeptide. Protein Eng. Des. Sel. 2008;21:147–154. doi: 10.1093/protein/gzm076. [DOI] [PubMed] [Google Scholar]
- 45.Green J, Goldsbury C, Min T, Sunderji S, Frey P, Kistler J, Cooper G, Aebi U. Full-length rat amylin forms fibrils following substitution of single residues from human amylin. J. Mol. Biol. 2003;326:1147–1156. doi: 10.1016/s0022-2836(02)01377-3. [DOI] [PubMed] [Google Scholar]
- 46.Sakagashira S, Sanke T, Hanabusa T, Shimomura H, Ohagi S, Kumagaye KY, Nakajima K, Nanjo K. Missense mutation of amylin gene (S20G) in Japanese NIDDM patients. Diabetes. 1996;45:1279–1281. doi: 10.2337/diab.45.9.1279. [DOI] [PubMed] [Google Scholar]
- 47.Sakagashira S, Hiddinga HJ, Tateishi K, Sanke T, Hanabusa T, Nanjo K, Eberhardt NL. S20G mutant amylin exhibits increased in vitro amyloidogenicity and increased intracellular cytotoxicity compared to wild-type amylin. Am. J. Pathol. 2000;157:2101–2109. doi: 10.1016/S0002-9440(10)64848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma Z, Westermark GT, Sakagashira S, Sanke T, Gustavsson A, Sakamoto H, Engstrom U, Nanjo K, Westermark P. Enhanced in vitro production of amyloid-like fibrils from mutant (S20G) islet amyloid polypeptide. Amyloid. 2001;8:242–249. doi: 10.3109/13506120108993820. [DOI] [PubMed] [Google Scholar]
- 49.Cao P, Tu LH, Abedini A, Levsh O, Akter R, Patsalo V, Schmidt AM, Raleigh DP. Sensitivity of amyloid formation by human islet amyloid polypeptide to mutations at residue 20. J. Mol. Biol. 2012;421:282–295. doi: 10.1016/j.jmb.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abedini A, Gupta R, Marek P, Meng F, Raleigh DP, Taskent H, Tracz S. Role of posttranslational modifications in amyloid formation. In: Ramirez-Alvarado M, Kelly JW, Dobson CM, editors. In Protein Misfolding Diseases: Current and Emerging Principles and Therapies. Hoboken: John Wiley & Sons, Inc.; 2010. [Google Scholar]
- 51.Dunkelberger EB, Buchanan LE, Marek P, Cao P, Raleigh DP, Zanni MT. Deamidation accelerates amyloid formation and alters amylin fiber structure. J. Am. Chem. Soc. 2012;134:12658–12667. doi: 10.1021/ja3039486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nilsson MR, Driscoll M, Raleigh DP. Low levels of asparagine deamidation can have a dramatic effect on aggregation of amyloidogenic peptides: Implications for the study of amyloid formation. Protein Sci. 2002;11:342–349. doi: 10.1110/ps.48702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marek PJ, Patsalo V, Green DF, Raleigh DP. Ionic strength effects on amyloid formation by amylin are a complicated interplay among debye screening, ion selectivity, and hofmeister effects. Biochemistry. 2012;51:8478–8490. doi: 10.1021/bi300574r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fox A, Snollaerts T, Errecart Casanova C, Calciano A, Nogaj LA, Moffet DA. Selection for nonamyloidogenic mutants of islet amyloid polypeptide (IAPP) identifies an extended region for amyloidogenicity. Biochemistry. 2010;49:7783–7789. doi: 10.1021/bi100337p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiltzius JJW, Sievers SA, Sawaya MR, Eisenberg D. Atomic structures of IAPP (amylin) fusions suggest a mechanism for fibrillation and the role of insulin in the process. Protein Sci. 2009;18:1521–1530. doi: 10.1002/pro.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azriel R, Gazit E. Analysis of the structural and functional elements of the minimal active fragment of islet amyloid polypeptide (IAPP) - An experimental support for the key role of the phenylalanine residue in amyloid formation. J. Biol. Chem. 2001;276:34156–34161. doi: 10.1074/jbc.M102883200. [DOI] [PubMed] [Google Scholar]
- 57.Tracz SM, Abedini A, Driscoll M, Raleigh DP. Role of aromatic interactions in amyloid formation by peptides derived from human amylin. Biochemistry. 2004;43:15901–15908. doi: 10.1021/bi048812l. [DOI] [PubMed] [Google Scholar]
- 58.Marek P, Abedini A, Song BB, Kanungo M, Johnson ME, Gupta R, Zaman W, Wong SS, Raleigh DP. Aromatic interactions are not required for amyloid fibril formation by islet amyloid polypeptide but do influence the rate of fibril formation and fibril morphology. Biochemistry. 2007;46:3255–3261. doi: 10.1021/bi0621967. [DOI] [PubMed] [Google Scholar]
- 59.Tu LH, Raleigh DP. Role of aromatic interactions in amyloid formation by islet amyloid polypeptide. Biochemistry. 2013 doi: 10.1021/bi3014278. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anil B, Sato S, Cho JH, Raleigh DP. Fine structure analysis of a protein folding transition state; distinguishing between hydrophobic stabilization and specific packing. J. Mol. Biol. 2005;354:693–705. doi: 10.1016/j.jmb.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 61.Williamson JA, Loria JP, Miranker AD. Helix stabilization precedes aqueous and bilayer-catalyzed fiber formation in islet amyloid polypeptide. J. Mol. Biol. 2009;393:383–396. doi: 10.1016/j.jmb.2009.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nanga RP, Brender JR, Xu J, Hartman K, Subramanian V, Ramamoorthy A. Three-dimensional structure and orientation of rat islet amyloid polypeptide protein in a membrane environment by solution NMR spectroscopy. J. Am. Chem. Soc. 2009;131:8252–8261. doi: 10.1021/ja9010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nanga RP, Brender JR, Xu J, Veglia G, Ramamoorthy A. Structures of rat and human islet amyloid polypeptide IAPP(1–19) in micelles by NMR spectroscopy. Biochemistry. 2008;47:12689–12697. doi: 10.1021/bi8014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brender JR, Hartman K, Reid KR, Kennedy RT, Ramamoorthy A. A single mutation in the nonamyloidogenic region of islet amyloid polypeptide greatly reduces toxicity. Biochemistry. 2008;47:12680–12688. doi: 10.1021/bi801427c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nanga RP, Brender JR, Vivekanandan S, Ramamoorthy A. Structure and membrane orientation of IAPP in its natively amidated form at physiological pH in a membrane environment. Biochim. Biophys. Acta. 2011;1808:2337–2342. doi: 10.1016/j.bbamem.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luca S, Yau WM, Leapman R, Tycko R. Peptide conformation and supramolecular organization in amylin fibrils: Constraints from solid-state NMR. Biochemistry. 2007;46:13505–13522. doi: 10.1021/bi701427q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wiltzius JJW, Sievers SA, Sawaya MR, Cascio D, Popov D, Riekel C, Eisenberg D. Atomic structure of the cross-beta spine of islet amyloid polypeptide (amylin) Protein Sci. 2008;17:1467–1474. doi: 10.1110/ps.036509.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shim SH, Gupta R, Ling YL, Strasfeld DB, Raleigh DP, Zanni MT. Two-dimensional IR spectroscopy and isotope labeling defines the pathway of amyloid formation with residue-specific resolution. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6614–6619. doi: 10.1073/pnas.0805957106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doran TM, Anderson EA, Latchney SE, Opanashuk LA, Nilsson BL. Turn nucleation perturbs amyloid beta self-assembly and cytotoxicity. J. Mol. Biol. 2012;421:315–328. doi: 10.1016/j.jmb.2012.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr. Rev. 2008;29:303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zraika S, Hull RL, Verchere CB, Clark A, Potter KJ, Fraser PE, Raleigh DP, Kahn SE. Toxic oligomers and islet beta cell death: guilty by association or convicted by circumstantial evidence? Diabetologia. 2010;53:1046–1056. doi: 10.1007/s00125-010-1671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chimon S, Shaibat MA, Jones CR, Calero DC, Aizezi B, Ishii Y. Evidence of fibril-like beta-sheet structures in a neurotoxic amyloid intermediate of Alzheimer's beta-amyloid. Nat. Struct. Mol. Biol. 2007;14:1157–1164. doi: 10.1038/nsmb1345. [DOI] [PubMed] [Google Scholar]
- 73.Yoshiike Y, Minai R, Matsuo Y, Chen YR, Kimura T, Takashima A. Amyloid oligomer conformation in a group of natively folded proteins. PLoS One. 2008;3:e3235. doi: 10.1371/journal.pone.0003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vaiana SM, Ghirlando R, Yau WM, Eaton WA, Hofrichter J. Sedimentation studies on human amylin fail to detect low-molecular-weight oligomers. Biophys. J. 2008;94:L45–L47. doi: 10.1529/biophysj.107.125146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suzuki Y, Brender JR, Hartman K, Ramamoorthy A, Marsh ENG. Alternative pathways of human islet amyloid polypeptide aggregation distinguished by F-19 nuclear magnetic resonance-detected kinetics of monomer consumption. Biochemistry. 2012;51:8154–8162. doi: 10.1021/bi3012548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dupuis NF, Wu C, Shea JE, Bowers MT. Human islet amyloid polypeptide monomers form ordered beta-hairpins: a possible direct amyloidogenic precursor. J. Am. Chem. Soc. 2009;131:18283–18292. doi: 10.1021/ja903814q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dupuis NF, Wu C, Shea JE, Bowers MT. The amyloid formation mechanism in human IAPP: dimers have beta-strand monomer-monomer interfaces. J. Am. Chem. Soc. 2011;133:7240–7243. doi: 10.1021/ja1081537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng BA, Liu XR, Gong H, Huang LQ, Chen H, Zhang X, Li CZ, Yang MY, Ma BJ, Jiao LH, Zheng L, Huan K. Coffee components inhibit amyloid formation of human islet amyloid polypeptide in vitro: Possible link between coffee consumption and diabetes mellitus. J. Agric. Food Chem. 2011;59:13147–13155. doi: 10.1021/jf201702h. [DOI] [PubMed] [Google Scholar]
- 79.Cao P, Raleigh DP. Ester to amide switch peptides provide a simple method for preparing monomeric islet amyloid polypeptide under physiologically relevant conditions and facilitate investigations of amyloid formation. J. Am. Chem. Soc. 2010;132:4052–4053. doi: 10.1021/ja910763m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abedini A, Raleigh DP. A role for helical intermediates in amyloid formation by natively unfolded polypeptides? Phys. Biol. 2009;6:015005. doi: 10.1088/1478-3975/6/1/015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cao P, Meng F, Abedini A, Raleigh DP. The ability of rodent islet amyloid polypeptide to inhibit amyloid formation by human islet amyloid polypeptide has important implications for the mechanism of amyloid formation and the design of inhibitors. Biochemistry. 2010;49:872–881. doi: 10.1021/bi901751b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abedini A, Meng FL, Raleigh DP. A single-point mutation converts the highly amyloidogenic human islet amyloid polypeptide into a potent fibrillization inhibitor. J. Am. Chem. Soc. 2007;129:11300–11301. doi: 10.1021/ja072157y. [DOI] [PubMed] [Google Scholar]
- 83.Meng FL, Raleigh DP, Abedini A. Combination of kinetically selected inhibitors in trans leads to highly effective inhibition of amyloid formation. J. Am. Chem. Soc. 2010;132:14340–14342. doi: 10.1021/ja1046186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hebda JA, Saraogi I, Magzoub M, Hamilton AD, Miranker AD. A peptidomimetic approach to targeting pre-amyloidogenic states in type II diabetes. Chem. Biol. 2009;16:943–950. doi: 10.1016/j.chembiol.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei L, Jiang P, Xu WX, Li H, Zhang H, Yan LY, Chan-Park MB, Liu XW, Tang K, Mu YG, Pervushin K. The molecular basis of distinct aggregation pathways of islet amyloid polypeptide. J. Biol. Chem. 2011;286:6291–6300. doi: 10.1074/jbc.M110.166678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marek P, Mukherjee S, Zanni MT, Raleigh DP. Residue-specific, real-time characterization of lag-phase species and fibril growth during amyloid formation: a combined fluorescence and IR study of p-cyanophenylalanine analogs of islet amyloid polypeptide. J. Mol. Biol. 2010;400:878–888. doi: 10.1016/j.jmb.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Padrick SB, Miranker AD. Islet amyloid polypeptide: Identification of long-range contacts and local order on the fibrillogenesis pathway. J. Mol. Biol. 2001;308:783–794. doi: 10.1006/jmbi.2001.4608. [DOI] [PubMed] [Google Scholar]
- 88.Young ID, Ailles L, Narindrasorasak S, Tan R, Kisilevsky R. Localization of the basement membrane heparan sulfate proteoglycan in islet amyloid deposits in type II diabetes mellitus. Arch. Pathol. Lab. Med. 1992;116:951–954. [PubMed] [Google Scholar]
- 89.Vidal J, Verchere CB, Andrikopoulos S, Wang F, Hull RL, Cnop M, Olin KL, LeBoeuf RC, O'Brien KD, Chait A, Kahn SE. The effect of apolipoprotein E deficiency on islet amyloid deposition in human islet amyloid polypeptide transgenic mice. Diabetologia. 2003;46:71–79. doi: 10.1007/s00125-002-0984-5. [DOI] [PubMed] [Google Scholar]
- 90.Jha S, Patil SM, Gibson J, Nelson CE, Alder NN, Alexandrescu AT. Mechanism of amylin fibrillization enhancement by heparin. J. Biol. Chem. 2011;286:22894–22904. doi: 10.1074/jbc.M110.215814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hull RL, Zraika S, Udayasankar J, Kisilevsky R, Szarek WA, Wight TN, Kahn SE. Inhibition of glycosaminoglycan synthesis and protein glycosylation with WAS-406 and azaserine result in reduced islet amyloid formation in vitro. Am. J. Physiol. Cell Physiol. 2007;293:C1586–C1593. doi: 10.1152/ajpcell.00208.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Westermark GT, Westermark P. Localized amyloids important in diseases outside the brain - lessons from the islets of Langerhans and the thoracic aorta. FEBS J. 2011;278:3918–3929. doi: 10.1111/j.1742-4658.2011.08298.x. [DOI] [PubMed] [Google Scholar]
- 93.Park K, Verchere CB. Identification of a heparin binding domain in the N-terminal cleavage site of pro-islet amyloid polypeptide - Implications for islet amyloid formation. J. Biol. Chem. 2001;276:16611–16616. doi: 10.1074/jbc.M008423200. [DOI] [PubMed] [Google Scholar]
- 94.Paulsson JF, Westermark GT. Aberrant processing of human proislet amyloid polypeptide results in increased amyloid formation. Diabetes. 2005;54:2117–2125. doi: 10.2337/diabetes.54.7.2117. [DOI] [PubMed] [Google Scholar]
- 95.Marzban L, Rhodes CJ, Steiner DF, Haataja L, Halban PA, Verchere CB. Impaired NH2-terminal processing of human proislet amyloid polypeptide by the prohormone convertase PC2 leads to amyloid formation and cell death. Diabetes. 2006;55:2192–2201. doi: 10.2337/db05-1566. [DOI] [PubMed] [Google Scholar]
- 96.Meng F, Abedini A, Song B, Raleigh DP. Amyloid formation by pro-islet amyloid polypeptide processing intermediates: Examination of the role of protein heparan sulfate interactions and implications for islet amyloid formation in type 2 diabetes. Biochemistry. 2007;46:12091–12099. doi: 10.1021/bi7004834. [DOI] [PubMed] [Google Scholar]
- 97.Hebda JA, Miranker AD. The interplay of catalysis and toxicity by amyloid intermediates on lipid bilayers: insights from type II diabetes. Annu. Rev. Biophys. 2009;38:125–152. doi: 10.1146/annurev.biophys.050708.133622. [DOI] [PubMed] [Google Scholar]
- 98.Nath A, Miranker AD, Rhoades E. A membrane-bound antiparallel dimer of rat islet amyloid polypeptide. Angew. Chem., Int. Ed. 2011;50:10859–10862. doi: 10.1002/anie.201102887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jayasinghe SA, Langen R. Lipid membranes modulate the structure of islet amyloid polypeptide. Biochemistry. 2005;44:12113–12119. doi: 10.1021/bi050840w. [DOI] [PubMed] [Google Scholar]
- 100.Seeliger J, Weise K, Opitz N, Winter R. The effect of abeta on IAPP aggregation in the presence of an isolated beta-cell membrane. J. Mol. Biol. 2012;421:348–363. doi: 10.1016/j.jmb.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wakabayashi M, Matsuzaki K. Ganglioside-induced amyloid formation by human islet amyloid polypeptide in lipid rafts. FEBS Lett. 2009;583:2854–2858. doi: 10.1016/j.febslet.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 102.Weise K, Radovan D, Gohlke A, Opitz N, Winter R. Interaction of hIAPP with model raft membranes and pancreatic beta-cells: cytotoxicity of hIAPP oligomers. ChemBioChem. 2010;11:1280–1290. doi: 10.1002/cbic.201000039. [DOI] [PubMed] [Google Scholar]
- 103.Gurlo T, Ryazantsev S, Huang CJ, Yeh MW, Reber HA, Hines OJ, O'Brien TD, Glabe CG, Butler PC. Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway. Am. J. Pathol. 2010;176:861–869. doi: 10.2353/ajpath.2010.090532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aston-Mourney K, Hull RL, Zraika S, Udayasankar J, Subramanian SL, Kahn SE. Exendin-4 increases islet amyloid deposition but offsets the resultant beta cell toxicity in human islet amyloid polypeptide transgenic mouse islets. Diabetologia. 2011;54:1756–1765. doi: 10.1007/s00125-011-2143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poitout V, Robertson RP. Minireview: Secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–342. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- 106.Brunham LR, Kruit JK, Hayden MR, Verchere CB. Cholesterol in beta-cell dysfunction: the emerging connection between HDL cholesterol and type 2 diabetes. Curr. Diab. Rep. 2010;10:55–60. doi: 10.1007/s11892-009-0090-x. [DOI] [PubMed] [Google Scholar]
- 107.Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 108.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 109.Saafi EL, Konarkowska B, Zhang SP, Kistler J, Cooper GJS. Ultrastructural evidence that apoptosis is the mechanism by which human amylin evokes death in RINm5F pancreatic islet beta-cells. Cell Biol. Int. 2001;25:339–350. doi: 10.1006/cbir.2000.0643. [DOI] [PubMed] [Google Scholar]
- 110.Butler AE, Janson J, Soeller WC, Butler PC. Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes - Evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes. 2003;52:2304–2314. doi: 10.2337/diabetes.52.9.2304. [DOI] [PubMed] [Google Scholar]
- 111.Cooper GJS, Aitken JF, Zhang S. Is type 2 diabetes an amyloidosis and does it really matter (to patients)? Diabetologia. 2010;53:1011–1016. doi: 10.1007/s00125-010-1715-y. [DOI] [PubMed] [Google Scholar]
- 112.Matveyenko AV, Butler PC. Beta-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes. 2006;55:2106–2114. doi: 10.2337/db05-1672. [DOI] [PubMed] [Google Scholar]
- 113.Zhang S, Liu J, Dragunow M, Cooper GJ. Fibrillogenic amylin evokes islet beta-cell apoptosis through linked activation of a caspase cascade and JNK1. J. Biol. Chem. 2003;278:52810–52819. doi: 10.1074/jbc.M308244200. [DOI] [PubMed] [Google Scholar]
- 114.Subramanian SL, Hull RL, Zraika S, Aston-Mourney K, Udayasankar J, Kahn SE. cJUN N-terminal kinase (JNK) activation mediates islet amyloid-induced beta cell apoptosis in cultured human islet amyloid polypeptide transgenic mouse islets. Diabetologia. 2012;55:166–174. doi: 10.1007/s00125-011-2338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park YJ, Lee S, Kieffer TJ, Warnock GL, Safikhan N, Speck M, Hao Z, Woo M, Marzban L. Deletion of Fas protects islet beta cells from cytotoxic effects of human islet amyloid polypeptide. Diabetologia. 2012;55:1035–1047. doi: 10.1007/s00125-012-2451-2. [DOI] [PubMed] [Google Scholar]
- 116.Rivera JF, Gurlo T, Daval M, Huang CJ, Matveyenko AV, Butler PC, Costes S. Human-IAPP disrupts the autophagy/lysosomal pathway in pancreatic beta-cells: protective role of p62-positive cytoplasmic inclusions. Cell Death Differ. 2011;18:415–426. doi: 10.1038/cdd.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masters SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat. Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zraika S, Hull RL, Udayasankar J, Aston-Mourney K, Subramanian SL, Kisilevsky R, Szarek WA, Kahn SE. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52:626–635. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Westwell-Roper C, Dai DL, Soukhatcheva G, Potter KJ, van Rooijen N, Ehses JA, Verchere CB. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J. Immunol. 2011;187:2755–2765. doi: 10.4049/jimmunol.1002854. [DOI] [PubMed] [Google Scholar]
- 120.Huang CJ, Gurlo T, Haataja L, Costes S, Daval M, Ryazantsev S, Wu X, Butler AE, Butler PC. Calcium-activated calpain-2 is a mediator of beta cell dysfunction and apoptosis in type 2 diabetes. J. Biol. Chem. 2010;285:339–348. doi: 10.1074/jbc.M109.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Casas S, Gomis R, Gribble FM, Altirriba J, Knuutila S, Novials A. Impairment of the ubiquitin-proteasome pathway is a downstream endoplasmic reticulum stress response induced by extracellular human islet amyloid polypeptide and contributes to pancreatic beta-cell apoptosis. Diabetes. 2007;56:2284–2294. doi: 10.2337/db07-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hull RL, Zraika S, Udayasankar J, Aston-Mourney K, Subramanian SL, Kahn SE. Amyloid formation in human IAPP transgenic mouse islets and pancreas, and human pancreas, is not associated with endoplasmic reticulum stress. Diabetologia. 2009;52:1102–1111. doi: 10.1007/s00125-009-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morita S, Sakagashira S, Shimajiri Y, Eberhardt NL, Kondo T, Kondo T, Sanke T. Autophagy protects against human islet amyloid polypeptide-associated apoptosis. J. Diabetes Invest. 2011;2:48–55. doi: 10.1111/j.2040-1124.2010.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–498. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 125.Mirzabekov TA, Lin MC, Kagan BL. Pore formation by the cytotoxic islet amyloid peptide amylin. J. Biol. Chem. 1996;271:1988–1992. doi: 10.1074/jbc.271.4.1988. [DOI] [PubMed] [Google Scholar]
- 126.Trikha S, Jeremic AM. Clustering and internalization of toxic amylin oligomers in pancreatic cells require plasma membrane cholesterol. J. Biol. Chem. 2011;286:36086–36097. doi: 10.1074/jbc.M111.240762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Law E, Lu S, Kieffer TJ, Warnock GL, Ao Z, Woo M, Marzban L. Differences between amyloid toxicity in alpha and beta cells in human and mouse islets and the role of caspase-3. Diabetologia. 2010;53:1415–1427. doi: 10.1007/s00125-010-1717-9. [DOI] [PubMed] [Google Scholar]
- 128.Last NB, Rhoades E, Miranker AD. Islet amyloid polypeptide demonstrates a persistent capacity to disrupt membrane integrity. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9460–9465. doi: 10.1073/pnas.1102356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brender JR, Lee EL, Cavitt MA, Gafni A, Steel DG, Ramamoorthy A. Amyloid fiber formation and membrane disruption are separate processes localized in two distinct regions of IAPP, the type-2-diabetes-related peptide. J. Am. Chem. Soc. 2008;130:6424–6429. doi: 10.1021/ja710484d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Engel MFM, Khemtemourian L, Kleijer CC, Meeldijk HJD, Jacobs J, Verkleij AJ, de Kruijff B, Killian JA, Hoppener JWM. Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6033–6038. doi: 10.1073/pnas.0708354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sparr E, Engel MFM, Sakharov DV, Sprong M, Jacobs J, de Kruijff B, Hoppener JWM, Killian JA. Islet amyloid polypeptide-induced membrane leakage involves uptake of lipids by forming amyloid fibers. FEBS Lett. 2004;577:117–120. doi: 10.1016/j.febslet.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 132.Porat Y, Kolusheva S, Jelinek R, Gazit E. The human islet amyloid polypeptide forms transient membrane-active prefibrillar assemblies. Biochemistry. 2003;42:10971–10977. doi: 10.1021/bi034889i. [DOI] [PubMed] [Google Scholar]
- 133.Anguiano M, Nowak RJ, Lansbury PT. Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry. 2002;41:11338–11343. doi: 10.1021/bi020314u. [DOI] [PubMed] [Google Scholar]
- 134.DeToma AS, Salamekh S, Ramamoorthy A, Lim MH. Misfolded proteins in Alzheimer's disease and type II diabetes. Chem. Soc. Rev. 2012;41:608–621. doi: 10.1039/c1cs15112f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, Engemann S, Pastore A, Wanker EE. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 136.Hudson SA, Ecroyd H, Dehle FC, Musgrave IF, Carver JA. (−)-Epigallocatechin-3-Gallate (EGCG) maintains kappa-casein in its pre-fibrillar state without redirecting its aggregation pathway. J. Mol. Biol. 2009;392:689–700. doi: 10.1016/j.jmb.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 137.Meng F, Abedini A, Plesner A, Verchere CB, Raleigh DP. The flavanol (−)-epigallocatechin 3-gallate inhibits amyloid formation by islet amyloid polypeptide, disaggregates amyloid fibrils, and protects cultured cells against IAPP-induced toxicity. Biochemistry. 2010;49:8127–8133. doi: 10.1021/bi100939a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cao P, Raleigh DP. Analysis of the inhibition and remodeling of islet amyloid polypeptide amyloid fibers by flavanols. Biochemistry. 2012;51:2670–2683. doi: 10.1021/bi2015162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meng F, Abedini A, Plesner A, Middleton CT, Potter KJ, Zanni MT, Verchere CB, Raleigh DP. The sulfated triphenyl methane derivative acid fuchsin is a potent inhibitor of amyloid formation by human islet amyloid polypeptide and protects against the toxic effects of amyloid formation. J. Mol. Biol. 2010;400:555–566. doi: 10.1016/j.jmb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sinha S, et al. Lysine-specific molecular tweezers are broad-spectrum inhibitors of assembly and toxicity of amyloid proteins. J. Am. Chem. Soc. 2011;133:16958–16969. doi: 10.1021/ja206279b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Noor H, Cao P, Raleigh DP. Morin hydrate inhibits amyloid formation by islet amyloid polypeptide and disaggregates amyloid fibers. Protein Sci. 2012;21:373–382. doi: 10.1002/pro.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zelus C, Fox A, Calciano A, Faridian BS, Nogaj LA, Moffet DA. Myricetin inhibits islet amyloid polypeptide (IAPP) aggregation and rescues living mammalian cells from IAPP toxicity. Open Biochem J. 2012;6:66–70. doi: 10.2174/1874091X01206010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cheng BA, Gong H, Li XC, Sun Y, Zhang X, Chen H, Liu XR, Zheng L, Huang K. Silibinin inhibits the toxic aggregation of human islet amyloid polypeptide. Biochem. Biophys. Res. Commun. 2012;419:495–499. doi: 10.1016/j.bbrc.2012.02.042. [DOI] [PubMed] [Google Scholar]
- 144.Rigacci S, Guidotti V, Bucciantini M, Parri M, Nediani C, Cerbai E, Stefani M, Berti A. Oleuropein aglycon prevents cytotoxic amyloid aggregation of human amylin. J. Nutr. Biochem. 2010;21:726–735. doi: 10.1016/j.jnutbio.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 145.Porat Y, Mazor Y, Efrat S, Gazit E. Inhibition of islet amyloid polypeptide fibril formation: A potential role for heteroaromatic interactions. Biochemistry. 2004;43:14454–14462. doi: 10.1021/bi048582a. [DOI] [PubMed] [Google Scholar]
- 146.Mishra R, Sellin D, Radovan D, Gohlke A, Winter R. Inhibiting islet amyloid polypeptide fibril formation by the red wine compound resveratrol. ChemBioChem. 2009;10:445–449. doi: 10.1002/cbic.200800762. [DOI] [PubMed] [Google Scholar]
- 147.Saraogi I, Hebda JA, Becerril J, Estroff LA, Miranker AD, Hamilton AD. Synthetic alpha-helix mimetics as agonists and antagonists of islet amyloid polypeptide aggregation. Angew. Chem., Int. Ed. 2010;49:736–739. doi: 10.1002/anie.200901694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yan LM, Tatarek-Nossol M, Velkova A, Kazantzis A, Kapurniotu A. Design of a mimic of nonamyloidogenic and bioactive human islet amyloid polypeptide (IAPP) as nanomolar affinity inhibitor of IAPP cytotoxic fibrillogenesis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2046–2051. doi: 10.1073/pnas.0507471103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gupta R, Kapoor N, Raleigh DP, Sakmar TP. Nucleobindin 1 caps human islet amyloid polypeptide protofibrils to prevent amyloid fibril formation. J. Mol. Biol. 2012;421:378–389. doi: 10.1016/j.jmb.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ladiwala AA, Bhattacharya M, Perchiacca JM, Cao P, Raleigh DP, Abedini A, Schmidt AM, Varkey J, Langen R, Tessier PM. Rational design of potent antibody inhibitors of amyloid fibril assembly. Proc. Natl. Acad. Sci. U. S. A. 2012 doi: 10.1073/pnas.1208797109. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Meng FL, Marek P, Potter KJ, Verchere CB, Raleigh DP. Rifampicin does not prevent amyloid fibril formation by human islet amyloid polypeptide but does inhibit fibril thioflavin-T interactions: Implications for mechanistic studies beta-cell death. Biochemistry. 2008;47:6016–6024. doi: 10.1021/bi702518m. [DOI] [PubMed] [Google Scholar]
- 152.Middleton CT, Marek P, Cao P, Chiu CC, Singh S, Woys AM, de Pablo JJ, Raleigh DP, Zanni MT. Two-dimensional infrared spectroscopy reveals the complex behaviour of an amyloid fibril inhibitor. Nat. Chem. 2012;4:355–360. doi: 10.1038/nchem.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ratner RE, Dickey R, Fineman M, Maggs DG, Shen L, Strobel SA, Weyer C, Kolterman OG. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in Type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet. Med. 2004;21:1204–1212. doi: 10.1111/j.1464-5491.2004.01319.x. [DOI] [PubMed] [Google Scholar]