Abstract
Mesenchymal stem cells (MSCs) although used for bone tissue engineering are limited by the requirement of isolation and culture prior to transplantation. Our recent studies have shown that biomaterial implants can be engineered to facilitate the recruitment of MSCs. In this study, we explore the ability of these implants to direct the recruitment and the differentiation of MSCs in the setting of a bone defect. We initially determined that both stromal derived factor-1alpha (SDF-1α) and erythropoietin (Epo) prompted different degrees of MSC recruitment. Additionally, we found that Epo and bone morphogenetic protein-2 (BMP-2), but not SDF-1α, triggered the osteogenic differentiation of MSCs in vitro. We then investigated the possibility of directing autologous MSC-mediated bone regeneration using a murine calvaria model. Consistent with our in vitro observations, Epo-releasing scaffolds were found to be more potent in bridging the defect than BMP-2 loaded scaffolds, as determined by Computed Tomography (CT) scanning, fluorescent imaging and histological analyses. These results demonstrate the tremendous potential, directing the recruitment and differentiation of autologous MSCs has in the field of tissue regeneration.
Keywords: Autologous stem cells, Scaffold, Erythropoietin, Stromal derived factor-1α, Bone morphogenetic protein-2, Bone, Calvaria, Bone Tissue Engineering
1. Introduction
A number of tissue regeneration applications have substantially improved over the years with the advent of stem cell technologies. Over the years, tissue engineering has emerged as a promising alternative with remarkable progress being made in understanding the role played by mesenchymal stem cells (MSC), scaffolds, growth factors and bioreactors.[1] MSCs are of great importance for regeneration of tissues of the mesenchyme, like bone.[2] Taking advantage of their potent tissue regenerative properties, MSCs have been employed extensively as the cell source for bone regeneration.[3-7] Almost all of these studies relied on the isolation, culture and seeding of MSCs onto scaffolds prior to implantation.[8-11] They have shown that the presence of MSCs substantially enhance the extent of mineralized bone regeneration.[9-11] Despite these impressive outcomes, the use of MSC-seeded scaffolds in a clinical setting, such as in the reconstruction of critical size bone defects, has been limited. Limiting the usefulness of these applications is the need to isolate and culture MSCs from the patients, which is, time consuming, expensive and cannot be mass produced.[12, 13] Therefore, there is a need for further development of new technologies for bone tissue engineering employing autologous MSCs.
It is well established that, shortly after fracture, MSCs are recruited to the injured site and differentiate into osteoblasts prior to bone regeneration. We hypothesized that bone regeneration can be achieved by recruiting and causing the differentiation of MSCs into osteoblasts with the use of scaffolds loaded with certain cytokines. Ideally, tissue regenerating scaffolds should be able to recruit and then cause the differentiation of MSCs. Ours and other groups’ studies have shown that large numbers of MSCs and hematopoietic stem cells (HSCs) are recruited to the biomaterial implant sites.[14, 15] The recruitment of MSCs is likely to be associated with the release of several inflammatory chemokines, including, granulocyte colony-stimulating factor, granulocyte macrophage-colony-stimulating factor, monocytes chemoattractant protein, macrophage inflammatory protein, matrix metalloproteinase-2, and tissue inhibitor of metalloproteinase-2.[16-18] However, these inflammatory chemokines also promote the accumulation of inflammatory cells.[19-22] Our recent studies have shown that the localized release of SDF-1α enhances MSC recruitment while reducing inflammatory cell recruitment to the implant sites.[23] Interestingly, localized stromal derived factor-1α (SDF-1α) treatment was also found to increase angiogenesis around the scaffolds.[23] Erythropoietin (Epo) has recently been shown to be a potent chemokine for both MSCs and HSCs recruitment as well as angiogenesis.[24-28] As a result, these data have stimulated significant interest in the role osteogenic and angiogenic cytokines can play in bone tissue engineering applications.[29, 30] Interestingly, a recent report has suggested that Epo triggers the differentiation of MSCs into osteoblasts.[31] Based on these reports and our own results, we have focused our investigation on the pro-osteogenic effects of SDF-1α and erythropoietin (Epo). In the past, bone morphogenetic protein-2 (BMP-2) has been used as an osteogenic agent in various clinical trials.[32, 33] It has been well established that incorporation of BMP-2 in tissue engineering scaffolds promotes bone mineralization both in vitro and in vivo.[32, 33] Therefore, in addition to the aforementioned individual chemokines, the effect of BMP-2 either alone, or in combination with Epo and SDF-1α was also investigated.
A series of studies were carried out to test the hypothesis that autologous stem cells can be recruited and caused to differentiate into bone forming cells in an effort to regenerate bone tissue. First, chemokines SDF-1α and Epo were tested specifically for their ability to recruit MSCs. To do this, these chemokines were loaded onto scaffolds produced using our established protein microbubble scaffold fabrication technique,[34] and the ability of these scaffolds to recruit stem cells was assessed in vivo. We then evaluated the ability of MSC chemokines and known osteogenic differentiation agent BMP-2, to differentiate MSCs into bone in vitro. Scaffolds loaded with these chemokines were tested for their ability to elicit bone regeneration using a murine calvarial defect model.
2. Materials and Methods
2.1 Materials
Poly (d, l-lactic-co-glycolic acid) PLGA (75:25) with a molecular weight of 113 kDa was purchased from Medisorb (Lakeshore Biomaterials, Birmingham, AL). The solvent 1, 4-dioxane was purchased from Aldrich (Milwaukee, WI), Gelatin from Sigma (St Louis, MO) and Masson’s trichrome kit was purchased from Sigma (St Louis, MO). Primary antibodies (1:50) against CD105, CD146, Stro-1, CD45 and osteocalcin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) while CD34 and CD56 antibodies were purchased from BD Biosciences Pharmingen (BD Biosciences, San Jose, CA). Secondary antibodies (1:100) labeled with FITC or Texas Red were from Jackson ImmunoResearch Laboratories, Inc (West Grove, PA). SDF-1α was purchased from Prospec-Tany TechnoGene Ltd (East Brunswick, NJ), Epo from Cell Sciences (Canton, MA) and BMP-2 from R&D Systems (Minneapolis, MN). Oyster800 used for scaffold drug release studies was purchased from Boca Scientific Inc., (Boca Raton, FL). All Balb/c mice used in this work were obtained from Taconic Farms (Germantown, NY) and were cared for in compliance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas at Arlington.
2.2 Role of chemokines in MSC migration
A transwell model was employed to compare the chemotaxis properties of various chemokines.[23] Briefly, Epo (200 IU) based on our pilot studies, or SDF-1α (10 ng/ml) based on our earlier publication,[23] was added in the lower chamber of 8μm pore membranes of 24-well plates (Corning Costar, Corning, NY). BM-MSCs was isolated from femurs and then cultured based on established procedures.[35] Murine MSCs (SSEA4+/CD45-) (~50000 cells/well) were seeded on to transwell inserts (six wells per group). After incubation for 8 hours at 37°C, the numbers of transmigrated cells were quantified under fluorescent microscope following nuclear staining with DAPI. BM-MSCs in media without any cytokine additives served as controls.
2.3 Tracking injected stem cells in vivo
Bone marrow MSC were isolated from Balb/c mice femurs as described earlier.[23] Cells from the second passage were incubated with 5 μM of near-infrared dye (X-sight, Rochester, NY) at 37°C for 24 hours. After washing the cells with PBS, cells were injected through the dorsal tail vein in Balb/c mice bearing microbubble (MB) scaffolds (see supplement) loaded with cytokines SDF-1α or Epo. MB scaffolds without any cytokines served as control. There were four animals per group. After 24 and 48 hours following implantation, the stem cell recruitment to the implants was determined using an in vivo imaging machine (Kodak Image System FX, excitation of 760 nm, emission of 830 nm, 45 s exposure, 8 × 8 binning, f-stop 2.5, field of view 120 mm) similar to our earlier studies.[15, 23]
2.4 In vivo autologous stem cell recruitment to chemokine loaded MB scaffolds
SDF-1α or Epo loaded MB scaffolds were implanted subcutaneously in Balb/c mice (four mice per group). At one week post-implantation, the scaffolds along with the surrounding tissues were explanted and histological analyses was performed to determine the presence of various stem cell surface markers (MSCs: CD105+CD45-CD34-CD56-; Multipotent stem cells: CD146+CD45-CD56-; Pre-osteogenic stem cells: Stro-1+CD45-CD56-). The positive markers were labeled red while the negative markers were labeled green. Cells that were exclusively red were quantified using ImageJ as described earlier.[15]
2.5 In vitro osteogenic differentiation of MSC
The osteogenic potential of bone marrow derived MSCs (BM-MSCs) was assessed as described earlier.[36] BMP-2 (200 ng/ml) was used in addition to Epo and SDF-1α. Briefly, BM-MSCs (3000 cells/mm2) were incubated with different growth factors/chemokines in the presence of osteogenic cocktail. After incubation (7 days), the numbers of variously treated cells (six wells per treatment) were quantified using MTS assay to assess the effect of treatment on cell growth.[34, 37] After incubation (21 days), cell differentiation into an osteogenic lineage was determined using Alizarin Red staining for calcified deposits.[36]
2.6 Calvarial defect in animal model
The animal use protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas at Arlington. A calvarial defect model was created as published earlier (six Balb/c mice per group).[38, 39] Briefly, following anesthesia, a defect measuring approximately 5 mm in diameter was created in the cranium with a drill taking care to avoid damage to the dura in a fashion similar to earlier publications.[29, 30] The periosteum surrounding the defect was left untouched. Different scaffolds were implanted into the defect prior to wound closure. Analyses of osteogenic activities at the defect sites were done at the end of 4 and 8 weeks. Systemic administration of antibiotics was not used as based on our earlier study, it could affect the stem cell responses at the implant site.[15]
2.7 CT Scan
After 8 weeks, cranial explants were examined with a Siemens Inveon CT/PET Multimodality system (Siemens Medical Solutions Inc., Knoxville, TN, USA) operating in the cone-beam method. Images were obtained at 80 kV and 500 mA with a focal spot of 58 μm. The total rotation of the gantry was 360° with 1080 rotation steps obtained at an exposure time of approximately 715 ms/frame. The images were attained using a bin factor of 1 and an average frame of 3. Under high magnification the effective pixel size was 11.34 μm. CT images were reconstructed with a down-sample factor of 1 using Cobra Reconstruction software. For each sample, 1760 tomographic slices were obtained. Three-dimensional reconstruction of the bone was analyzed with the manufacturer’s software. For each cranial bone sample, regions of interest (ROI) were drawn in the defect area. Fewer contours needed to be drawn since a routine facility calculated all the intermediary masks by interpolation. The ROI’s were interpolated yielding a final ROI encompassing approximately 5 slices. Bone volume fraction (BV/TV), which is the ratio of total bone volume (BV) to total volume (TV, whole defect area), was the parameter used to determine bone growth.
2.8 Histological evaluation of osteogenesis
The implants along with the surrounding tissues were embedded for histological evaluation at 1, 4 and 8 weeks. To assess the influence of various chemokines/growth factors on stem cell responses, we quantified the numbers of MSCs and pro-ostegenic stem cells in implants/surrounding tissues using their unique sets of markers. To investigate the effect of scaffold implants on osteoblast differentiation, we evaluated the expression of osteocalcin and osteopontin (osteoblast products) in scaffold implant and surrounding tissue. Some tissue sections were stained with Masson’s Trichrome blue for collagen in which nuclei stains blue-black and collagen stains blue. Stained sections were imaged using a Leica fluorescence microscope (Leica Microsystems, Wetzlar, GmbH) equipped with a CCD Camera (Retiga EXi, QImage). The fibrous tissue thickness was measured and quantified based on H&E staining. The area fraction of the Masson’s Trichrome Blue stained sections was quantified using ImageJ to determine collagen coverage similar to earlier publications.[34] The fluorescence intensity of the immunofluorescence stained images was determined using “integrated density” feature in ImageJ under Analyze – Set Measurements similar to earlier publications.[40-42] Background fluorescence was measured and the area of the tissue section was also determined. The fluorescence intensity was determined using the formula Corrected Fluorescence = Integrated Density – (Area of section × Background fluorescence) and expressed in terms of arbitrary units as in an earlier publication.[43]
2.9 Statistical Analyses
Data was expressed as mean ± SD and groups were compared using ANOVA and t-test. Differences were considered statistically significant when p < 0.05.
3. Results
3.1 In vitro stem cell chemotaxis and proliferation
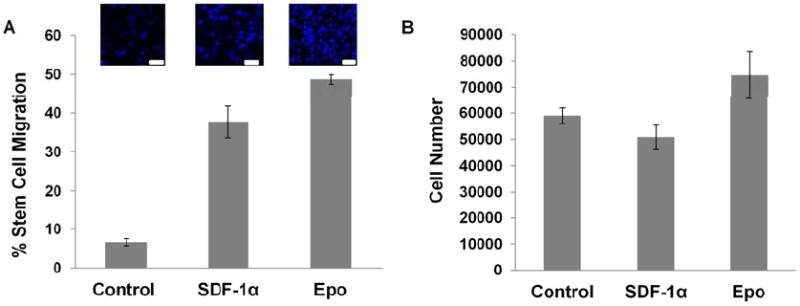
The ability of various cytokines and chemokines to induce stem cell chemotaxis was tested and then compared using a transwell migration system.[23] The membranes were stained with DAPI to locate the nucleus of migrated cells (Figure 1A). In comparison to controls SDF-1α was found to prompt a 4X increase of MSCs migration. Surprisingly, we found that Epo triggered an almost 8X increase of MSC migration which was significantly stronger than the chemotactic activity of SDF-1α. MSCs treated with Epo proliferated significantly more than untreated cells (Figure 1B).
Figure 1. MSC chemotaxis.
(A) MSCs exhibited chemotaxis in response to chemokines SDF-1α and Epo and the cell numbers were quantified. (B) MSC proliferation in response to various cytokines was assessed at the end of 1 week using Alamar Blue Assay. (n=6 per group; Scale bar 100 microns; Mag 200X)
3.2 Sustained cytokine release on autologous MSC recruitment in vivo
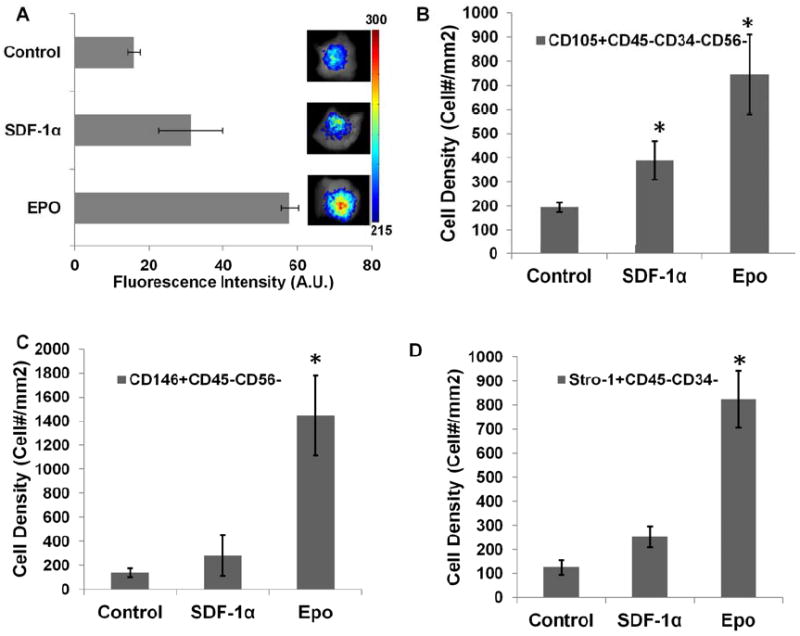
To investigate the ability of cytokine releasing scaffolds to recruit autologous MSCs, microbubble scaffolds loaded with SDF-1α, and Epo were implanted in the dorsal subcutaneous space of Balb/c mice. Microbubble scaffolds without any chemokines served as controls. MSCs tagged with NIR dye were injected through the dorsal tail vein and the animal was imaged using a real time imaging system. While SDF-1α loaded scaffolds were associated with an almost 2X increase in injected MSCs migration than controls, Epo loaded scaffolds were able to recruit almost 3X more MSCs than controls (Figure 2A).
Figure 2. Enhanced stem cell recruitment around chemokine loaded implants.
(A) Recruitment after 24 hours of injected NIR dye labeled MSCs to chemokine SDF-1α and Epo loaded microbubble scaffolds was imaged and quantified. MSC recruitment was compared based on (B) CD105+/ CD45-CD34-CD56-, (C) CD146+/ CD45-CD56- and (D) Stro-1+/ CD34-CD45- MSC markers using immunofluorescence labeling and quantified. (n=4; Mag 400X; significance of mean values with respect to control tested at p<0.05, *).
Having seen the migration of injected MSCs towards chemokine loaded scaffolds, we investigated the ability of such scaffolds to recruit autologous stem cells. After implantation for 1 week, the implants and surrounding tissues were analyzed for various groups of stem cells (Figure 2B-D). Specifically, CD105+CD34-CD45-CD56- was used as a general MSC marker.[44-48] CD146+CD45-CD56- has been utilized to identified multipotent stem cells while Stro-1+CD45-CD34- defines a subset of multipotent MSCs.[49-53] In agreement with the in vitro observations, we found that the localized release of SDF-1α slightly increased the recruitment of MSCs as compared with controls. However, the number of such cells found near Epo scaffolds was almost 4X more than control and 2X more than SDF-1α (Figure 2B). Interestingly, when we quantified CD146+CD45-CD56- stem cells and Stro-1+CD34-CD45-MSCs, Epo scaffolds were associated with almost a 7 - 8X more MSCs than controls (Figure 2C&D).
3.3 Effect of cytokines/growth factors in mediating stem cell differentiation in vitro
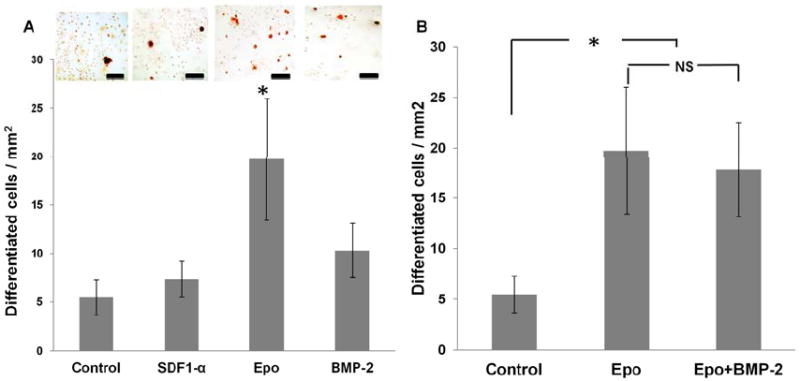
In addition to cell immigration, it is critical that tissue scaffolds be made to enhance BM-MSC proliferation and osteogenic differentiation. We thus tested the proliferation and osteogenic potential of different factors. Interestingly, among all factors tested, we found that only Epo, not SDF-1α nor BMP-2, promoted the proliferation of BM-MSC (Figure 3A). To assess the osteogenic potential, we found that, as expected, BMP-2 resulted in almost 2X more osteogenic differentiation than controls. On the other hand, SDF-1α treatment was not significantly better than controls in causing MSCs to differentiate into osteoblasts. Treatment with Epo produced almost 3X more osteoblasts than controls and was marginally significant than BMP-2 alone (Figure 3B). Interestingly, treatment with Epo and BMP-2 although significantly greater than control, was not more potent than Epo alone (Figure 3B).
Figure 3. In vitro stem cell differentiation in response to various chemokines.
(A) MSCs were cultured in osteogenic differentiation medium supplemented with various cytokines (SDF-1α, BMP-2, and Epo). Osteogenic differentiation was observed at the end of 21 days using Alizarin Red S staining and differentiated cells were quantified. (B) Osteogenic differentiation following Epo and Epo+BMP-2 treatment was compared with respect to control. (n=5 per group; Scale bar 50 microns; Mag 400X; (n=6 per group; Significance of mean values was tested with respect to control at p<0.05)
3.4 Cytokine releasing scaffolds in a critical size defect in mouse calvaria
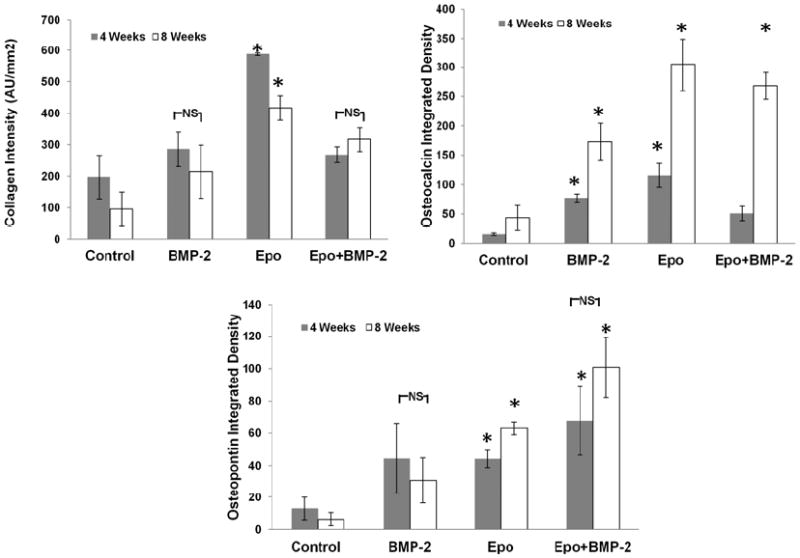
Until now we observed that Epo is a potent recruiter of MSCs with unexpected osteogenic differentiation capabilities. Using a calvarial defect model, we evaluated the ability of control microbubble scaffolds and microbubble scaffolds loaded with Epo alone, BMP-2 alone, or combined Epo+BMP-2 to prompt osteogenesis in the defect area. At the end of 4 and 8 weeks we performed immunohistological analyses to quantify collagen, osteocalcin and osteopontin in the implant. Masson’s Trichrome Blue staining was performed to assess collagen production at the implant sites. At 4 and 8 weeks, Epo group showed significantly higher collagen deposition than the other treatment groups which no significant difference between BMP-2 and Epo+BMP-2 (Figure 4A). Expression of osteocalcin, which is an osteoblast product and an indicator of bone formation, was analyzed. Epo was once again found to be associated with significantly greater osteocalcin than other groups. Interestingly, while Epo+BMP-2 group showed very little osteocalcin at week 4, it was comparable to Epo alone by 8 weeks and almost 2X better than BMP-2 (Figure 4B). The differences in osteopontin at 4 and 8 weeks, within various groups, were not significantly different. However, overall Epo+BMP-2 had significantly greater osteopontin than Epo alone, BMP-2 alone and controls. Interestingly, osteopontin intensities of Epo and BMP-2 treated groups were comparable (Figure 4C).
Figure 4. Immunohistological comparison of osteogenic activity at 4 and 8 weeks.
(A) Masson’s Trichrome Blue staining was done to examine collagen and the distribution was quantified. Immunofluorescence staining was performed for (B) osteocalcin and (C) osteopontin and quantified using ImageJ. (n=6 per group; Significance of mean values vs. control tested at p<0.05, *)
3.5 Micro-CT images and histological evaluation of mouse calvaria from scaffold-implanted animals
Long-term evaluation of the scaffolds in the defect area was performed at the end of 8 weeks. As seen in the CT scan images (Figure 5A), there was little bone regeneration in untreated controls. On the other hand, there was only partial bone formation in BMP-2 group. Consistent with our in vitro findings, the ectopic bone formation was observed with almost complete (more than 80%) bridging observed in the Epo and Epo+BMP-2 groups. This was confirmed by quantitative analysis of bone volume which showed significant increase in bone formation in Epo group and Epo+BMP-2 as compared to BMP-2 alone and control (Figure 5B).
Figure 5. Bridging calvarial defect using cytokine scaffolds after 8 weeks.
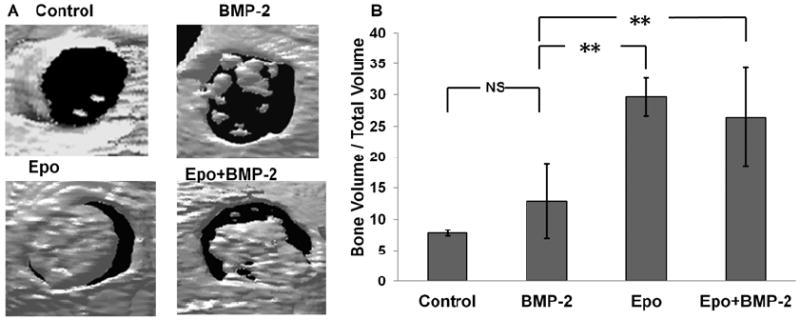
(A) CT scan images show incomplete to almost complete bridging of the defect in various treatment groups. (B) Bone mineral density was quantified. (n=6 per group; Significance of mean values vs. control tested at p<0.01, **)
4. Discussion
The objective of this study was to determine whether we could recruit and differentiate autologous stem cell into bone in vivo using such specific cytokine releasing scaffolds. To achieve the goal, we were specifically interested in the potential role of different cytokines in directing MSC recruitment and differentiation. In this study, we found that Epo is indeed a potent recruiter of stem cells in vitro followed by SDF-1α. Our observation of SDF-1α induced chemotaxis concurs with the findings reported in our recent publication.[23] Our finding on Epo is in agreement with an earlier study that also found that Epo was highly potent in inducing MSC chemotaxis.[54] It could be because Epo receptors are expressed by numerous cells including cardiovascular cells,[55-57] and bone cells.[58]
To determine whether this phenomenon holds true in vivo as well, based on established techniques,[34] we fabricated scaffolds using gelatin microbubble loaded with various cytokines including BMP-2, SDF-1α and Epo. These scaffolds were implanted in mice dorsal subcutaneous space to determine their ability to first recruit injected stem cells and subsequently autologous stem cells. Our observation of greater migration of injected MSCs to Epo load scaffolds followed by SDF-1α scaffolds provided credence for subsequent studies. Detection of autologous MSCs was done using a standard set of cell surface markers similar to ours and a number of earlier studies.[15, 45-48] The overall trend of multipotent MSC recruitment with Epo scaffolds eliciting the highest recruitment followed by SDF-1α was in agreement with our in vitro chemotaxis observations. The observation of lower MSC recruitment in response to SDF-1α scaffolds could be due to the fact that SDF-1α is associated with a reduction in the inflammatory cell responses as demonstrated by us earlier.[23] Such inflammatory cell responses are actually important for stem cell responses as shown in our recent study.[15]
Since in the absence of specific cues, recruited MSCs in the body can differentiate into myofibroblasts, it was imperative that we tested the effect of these cytokines on stem cell differentiation. It should be noted that the in vitro osteogenic differentiation of BM-MSCs by SDF-1α, Epo or BMP-2 requires the supplementation of osteogenic differentiation media. Osteogenic differentiation media is not required for causing in vivo osteogenic differentiation mediated by Epo, SDF-1α or BMP-2-scaffolds. It is possible that most of the scaffold-recruited stem cells are pre-osteoblasts in which the pre-differentiation stimulation such as osteogenic media is not required for launching their osteogenic activities. Furthermore, our observation of greater pro-osteogenic differentiation in the Epo group can be corroborated by recent observation of greater stem cell differentiation into osteoblasts by Epo as compared to BMP-2 alone.[31] However, it may be possible that poor solubility of BMP-2 in vitro could have reduced the in vitro differentiation ability. Surprisingly, we found that SDF-1α which is more chemotactic to MSCs than BMP-2, did not seem to wield any major influence on osteogenic differentiation of the cells.
Having seen that Epo is a potent MSC chemotactic, both in vitro and in vivo and associated with in vitro differentiation of stem cells into an osteogenic lineage, we explored the possibility of applying such scaffolds[34] in vivo in a critical size calvarial defect in the mice. Implantation of scaffolds delivering Epo, or BMP-2 or a combination of the two evoked varying extents of osteogenic activity as early as 4 weeks. The overall trend for osteogenic activity based on collagen, osteocalcin and osteopontin production was in favor of Epo loaded scaffolds. The differences between the treatment groups were more evident by 8 weeks as compared to 4 weeks.
Surprisingly, CT scanning at the end of 8 weeks unveiled some interesting findings. Almost complete (more than 80%) bridging of the defect area by new bone formation was observed along with higher percent bone volume for both Epo and Epo+BMP-2 groups as compared with BMP-2 alone. Interestingly, a previous study found that delivering an angiogenic agent like vascular endothelial growth factor (VEGF) along with BMP-2 enhanced the osteogenic activity,[29] while the same study and another recent finding showed that VEGF alone was not as effective as BMP-2 alone.[29, 30] The involvement of autologous MSCs by Epo could be a contributing factor behind the increased bone regenerative potential of Epo loaded scaffolds. At the same time, we did not see a synergistic effect when Epo and BMP-2 were delivered together. Whether, this is due to the fact that Epo inhibits Smad pathway,[59] one of the pathways used by BMP-2 to potentiate osteogenic responses as suggested by a few studies,[60-63] is yet to be ascertained. Nevertheless, in light of the current results, our findings bring forth a plethora of opportunities to harness the pro-osteogenic effect of Epo for biomaterial and bone tissue engineering research and product development.
5. Conclusions
An important aspect of this study is that it has further delineated the phenomena of autologous stem cell recruitment by chemokine loaded scaffolds and MSCs differentiation towards osteogenesis, using erythropoietin. This approach eliminates the need for extracting cells from the living system, expanding these cells in vitro, seeding them onto scaffolds and then reimplanting them in the bone defect. Instead, we have presented a system where autologous stem cell recruitment is synergized with the latest development in bone tissue engineering involving bioactive cytokine delivery and applied it to critical sized bone defect.
Supplementary Material
Acknowledgments
The authors would like to acknowledge NIH Grant EB007271. The authors would also like to thank and acknowledge Mr. Patrick Thomas for his help with CT scanning.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Betz RR. Limitations of autograft and allograft: new synthetic solutions. Orthopedics. 2002;25:s561–570. doi: 10.3928/0147-7447-20020502-04. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 4.Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, et al. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- 5.Meinel L, Karageorgiou V, Hofmann S, Fajardo R, Snyder B, Li C, et al. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J Biomed Mater Res A. 2004;71:25–34. doi: 10.1002/jbm.a.30117. [DOI] [PubMed] [Google Scholar]
- 6.Schneider RK, Neuss S, Knuchel R, Perez-Bouza A. Mesenchymal stem cells for bone tissue engineering. Pathologe. 2010;31(Suppl 2):138–146. doi: 10.1007/s00292-010-1329-7. [DOI] [PubMed] [Google Scholar]
- 7.Seong JM, Kim BC, Park JH, Kwon IK, Mantalaris A, Hwang YS. Stem cells in bone tissue engineering. Biomed Mater. 2010;5:062001. doi: 10.1088/1748-6041/5/6/062001. [DOI] [PubMed] [Google Scholar]
- 8.Diao H, Wang J, Shen C, Xia S, Guo T, Dong L, et al. Improved cartilage regeneration utilizing mesenchymal stem cells in TGF-beta1 gene-activated scaffolds. Tissue Eng Part A. 2009;15:2687–2698. doi: 10.1089/ten.TEA.2008.0621. [DOI] [PubMed] [Google Scholar]
- 9.Jukes JM, Both SK, Leusink A, Sterk LM, van Blitterswijk CA, de Boer J. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:6840–6845. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin M, Yoshimoto H, Vacanti JP. In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Eng. 2004;10:33–41. doi: 10.1089/107632704322791673. [DOI] [PubMed] [Google Scholar]
- 11.Wayne JS, McDowell CL, Shields KJ, Tuan RS. In vivo response of polylactic acid-alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng. 2005;11:953–963. doi: 10.1089/ten.2005.11.953. [DOI] [PubMed] [Google Scholar]
- 12.Jang S, Cho HH, Cho YB, Park JS, Jeong HS. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010;11:25. doi: 10.1186/1471-2121-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 14.Xu B, Zhang J, Brewer E, Tu Q, Yu L, Tang J, et al. Osterix enhances BMSC-associated osseointegration of implants. J Dent Res. 2009;88:1003–1007. doi: 10.1177/0022034509346928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair A, Shen J, Lotfi P, Ko CY, Zhang CC, Tang L. Biomaterial implants mediate autologous stem cell recruitment in mice. Acta Biomater. 2011;7:3887–3895. doi: 10.1016/j.actbio.2011.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 18.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 21.Wengner AM, Pitchford SC, Furze RC, Rankin SM. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 2008;111:42–49. doi: 10.1182/blood-2007-07-099648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wodnar-Filipowicz A, Heusser CH, Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989;339:150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- 23.Thevenot PT, Nair AM, Shen J, Lotfi P, Ko CY, Tang L. The effect of incorporation of SDF-1alpha into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials. 2010;31:3997–4008. doi: 10.1016/j.biomaterials.2010.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato S, Amano H, Ito Y, Eshima K, Aoyama N, Tamaki H, et al. Effect of erythropoietin on angiogenesis with the increased adhesion of platelets to the microvessels in the hind-limb ischemia model in mice. J Pharmacol Sci. 2010;112:167–175. doi: 10.1254/jphs.09262fp. [DOI] [PubMed] [Google Scholar]
- 25.Kertesz N, Wu J, Chen TH, Sucov HM, Wu H. The role of erythropoietin in regulating angiogenesis. Dev Biol. 2004;276:101–110. doi: 10.1016/j.ydbio.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Muller-Ehmsen J, Schmidt A, Krausgrill B, Schwinger RH, Bloch W. Role of erythropoietin for angiogenesis and vasculogenesis: from embryonic development through adulthood. Am J Physiol Heart Circ Physiol. 2006;290:H331–340. doi: 10.1152/ajpheart.01269.2004. [DOI] [PubMed] [Google Scholar]
- 27.Liu N, Tian J, Wang W, Cheng J, Hu D, Zhang J. Effect and mechanism of erythropoietin on mesenchymal stem cell proliferation in vitro under the acute kidney injury microenvironment. Exp Biol Med (Maywood) 2011;236:1093–1099. doi: 10.1258/ebm.2011.011001. [DOI] [PubMed] [Google Scholar]
- 28.Copland IB, Jolicoeur EM, Gillis MA, Cuerquis J, Eliopoulos N, Annabi B, et al. Coupling erythropoietin secretion to mesenchymal stromal cells enhances their regenerative properties. Cardiovasc Res. 2008;79:405–415. doi: 10.1093/cvr/cvn090. [DOI] [PubMed] [Google Scholar]
- 29.Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young S, Patel ZS, Kretlow JD, Murphy MB, Mountziaris PM, Baggett LS, et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15:2347–2362. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiozawa Y, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang Z, et al. Erythropoietin couples hematopoiesis with bone formation. PLoS One. 2010;5:e10853. doi: 10.1371/journal.pone.0010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008;2:81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 33.Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts) J Tissue Eng Regen Med. 2008;2:1–13. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- 34.Nair A, Thevenot P, Dey J, Shen J, Sun MW, Yang J, et al. Novel polymeric scaffolds using protein microbubbles as porogen and growth factor carriers. Tissue Eng Part C Methods. 2010;16:23–32. doi: 10.1089/ten.tec.2009.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S, et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol. 2009;183:993–1004. doi: 10.4049/jimmunol.0900803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thevenot P, Nair A, Dey J, Yang J, Tang L. Method to analyze three-dimensional cell distribution and infiltration in degradable scaffolds. Tissue Eng Part C Methods. 2008;14:319–331. doi: 10.1089/ten.tec.2008.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Z, Wang Z, Ge C, Krebsbach P, Franceschi RT. Healing cranial defects with AdRunx2-transduced marrow stromal cells. J Dent Res. 2007;86:1207–1211. doi: 10.1177/154405910708601213. [DOI] [PubMed] [Google Scholar]
- 39.Karageorgiou V, Tomkins M, Fajardo R, Meinel L, Snyder B, Wade K, et al. Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 in vitro and in vivo. J Biomed Mater Res A. 2006;78:324–334. doi: 10.1002/jbm.a.30728. [DOI] [PubMed] [Google Scholar]
- 40.Burgess A, Vigneron S, Brioudes E, Labbe JC, Lorca T, Castro A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci U S A. 2010;107:12564–12569. doi: 10.1073/pnas.0914191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell. 2010;18:533–543. doi: 10.1016/j.devcel.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potapova TA, Sivakumar S, Flynn JN, Li R, Gorbsky GJ. Mitotic progression becomes irreversible in prometaphase and collapses when Wee1 and Cdc25 are inhibited. Mol Biol Cell. 2011;22:1191–1206. doi: 10.1091/mbc.E10-07-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nissen L, Chingwaru W, Sgorbati B, Biavati B, Cencic A. Gut health promoting activity of new putative probiotic/protective Lactobacillus spp. strains: a functional study in the small intestinal cell model. Int J Food Microbiol. 2009;135:288–294. doi: 10.1016/j.ijfoodmicro.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 44.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 45.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 46.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 47.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Prockop DJ, Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: let’s not overlook some essential precautions. Blood. 2007;109:3147–3151. doi: 10.1182/blood-2006-03-013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Covas DT, Panepucci RA, Fontes AM, Silva WA, Jr, Orellana MD, Freitas MC, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 50.Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164–4173. [PubMed] [Google Scholar]
- 51.Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 52.Lin G, Liu G, Banie L, Wang G, Ning H, Lue TF, et al. Tissue distribution of mesenchymal stem cell marker Stro-1. Stem Cells Dev. 2011;20:1747–1752. doi: 10.1089/scd.2010.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20:587–591. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- 54.Zwezdaryk KJ, Coffelt SB, Figueroa YG, Liu J, Phinney DG, LaMarca HL, et al. Erythropoietin, a hypoxia-regulated factor, elicits a pro-angiogenic program in human mesenchymal stem cells. Exp Hematol. 2007;35:640–652. doi: 10.1016/j.exphem.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 55.Burger D, Lei M, Geoghegan-Morphet N, Lu X, Xenocostas A, Feng Q. Erythropoietin protects cardiomyocytes from apoptosis via up-regulation of endothelial nitric oxide synthase. Cardiovasc Res. 2006;72:51–59. doi: 10.1016/j.cardiores.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 56.Mudalagiri NR, Mocanu MM, Di Salvo C, Kolvekar S, Hayward M, Yap J, et al. Erythropoietin protects the human myocardium against hypoxia/reoxygenation injury via phosphatidylinositol-3 kinase and ERK1/2 activation. Br J Pharmacol. 2008;153:50–56. doi: 10.1038/sj.bjp.0707461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Lindern M, Parren-van Amelsvoort M, van Dijk T, Deiner E, van den Akker E, van Emst-de Vries S, et al. Protein kinase C alpha controls erythropoietin receptor signaling. J Biol Chem. 2000;275:34719–34727. doi: 10.1074/jbc.M007042200. [DOI] [PubMed] [Google Scholar]
- 58.Holstein JH, Menger MD, Scheuer C, Meier C, Culemann U, Wirbel RJ, et al. Erythropoietin (EPO): EPO-receptor signaling improves early endochondral ossification and mechanical strength in fracture healing. Life Sci. 2007;80:893–900. doi: 10.1016/j.lfs.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 59.Chen CL, Chou KJ, Lee PT, Chen YS, Chang TY, Hsu CY, et al. Erythropoietin suppresses epithelial to mesenchymal transition and intercepts Smad signal transduction through a MEK-dependent mechanism in pig kidney (LLC-PK1) cell lines. Exp Cell Res. 2010;316:1109–1118. doi: 10.1016/j.yexcr.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 60.Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Javed A, Bae JS, Afzal F, Gutierrez S, Pratap J, Zaidi SK, et al. Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J Biol Chem. 2008;283:8412–8422. doi: 10.1074/jbc.M705578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Washio A, Kitamura C, Morotomi T, Terashita M, Nishihara T. Possible involvement of Smad signaling pathways in induction of odontoblastic properties in KN-3 cells by bone morphogenetic protein-2: a growth factor to induce dentin regeneration. Int J Dent. 2012;2012:258469. doi: 10.1155/2012/258469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu B, Zhao X, Yang C, Crane J, Xian L, Lu W, et al. PTH induces differentiation of mesenchymal stem cells by enhancing BMP signaling. J Bone Miner Res. 2012;27:2001–2014. doi: 10.1002/jbmr.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


