Abstract
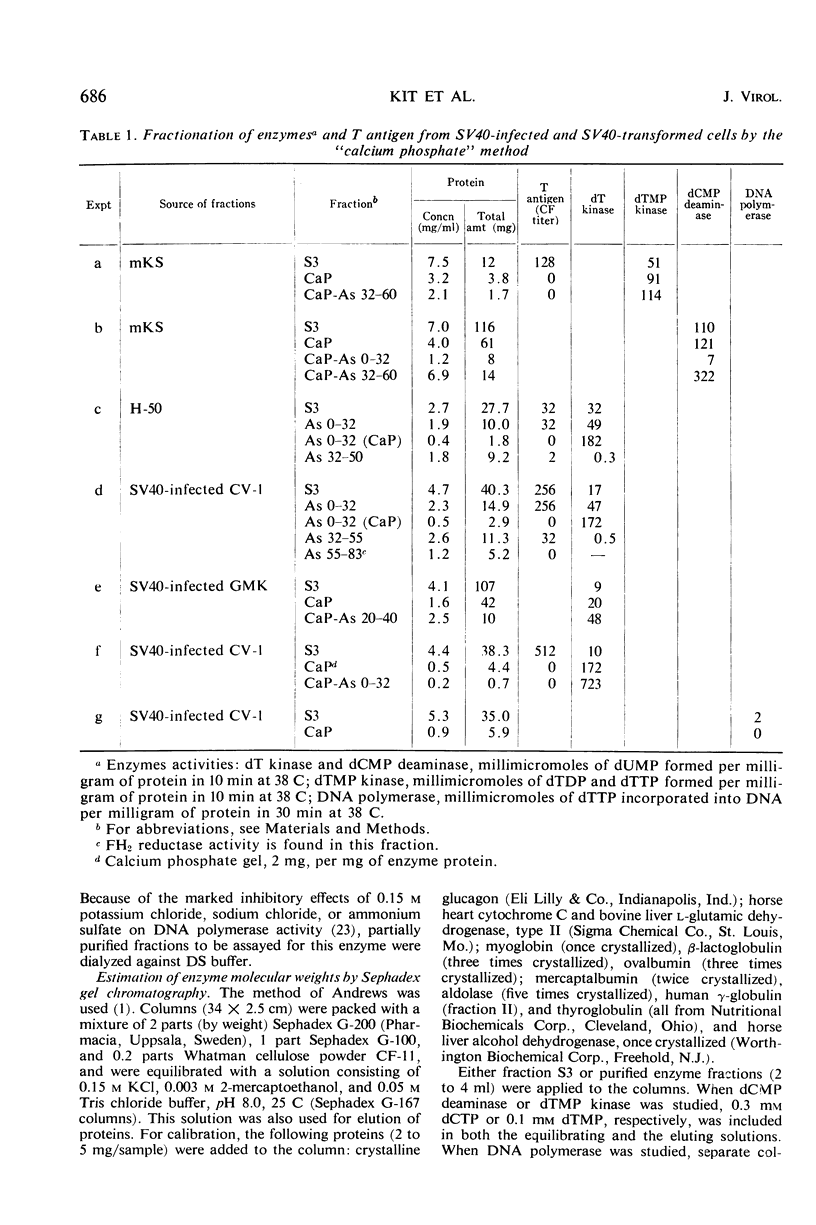
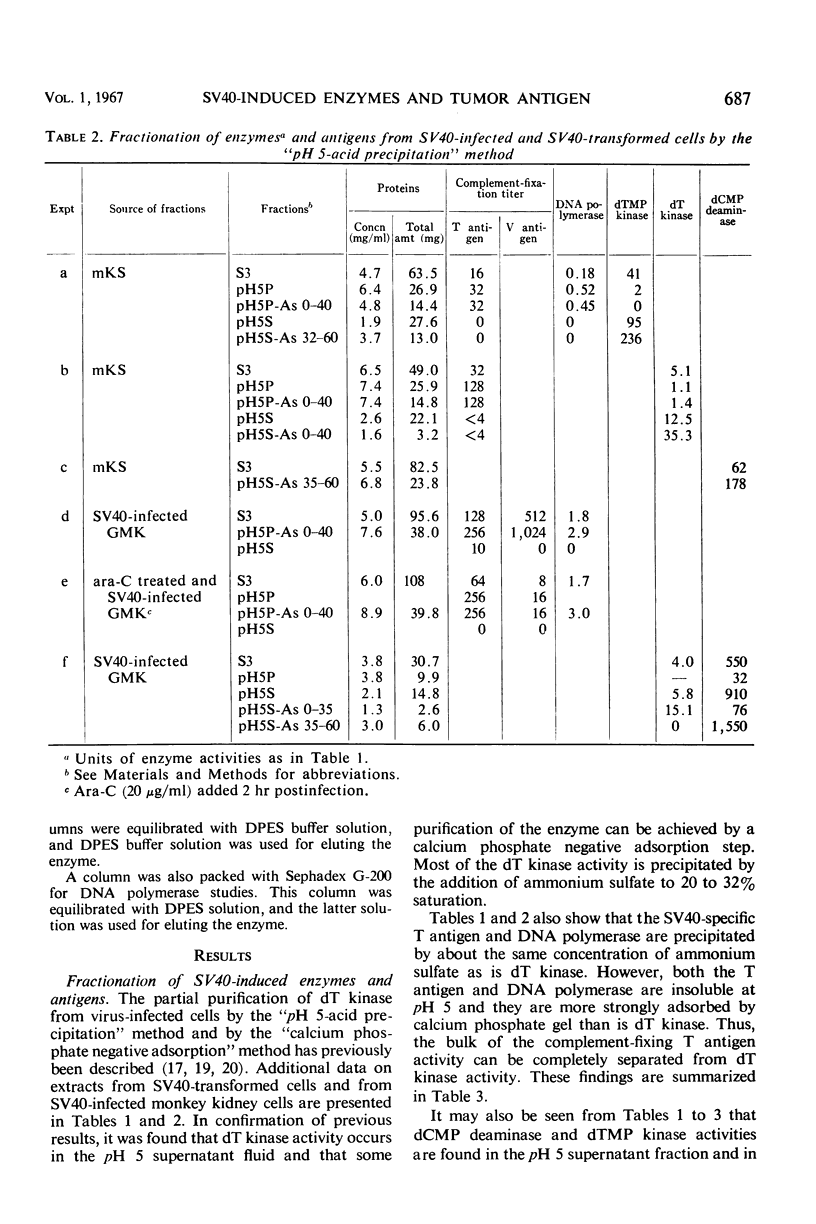
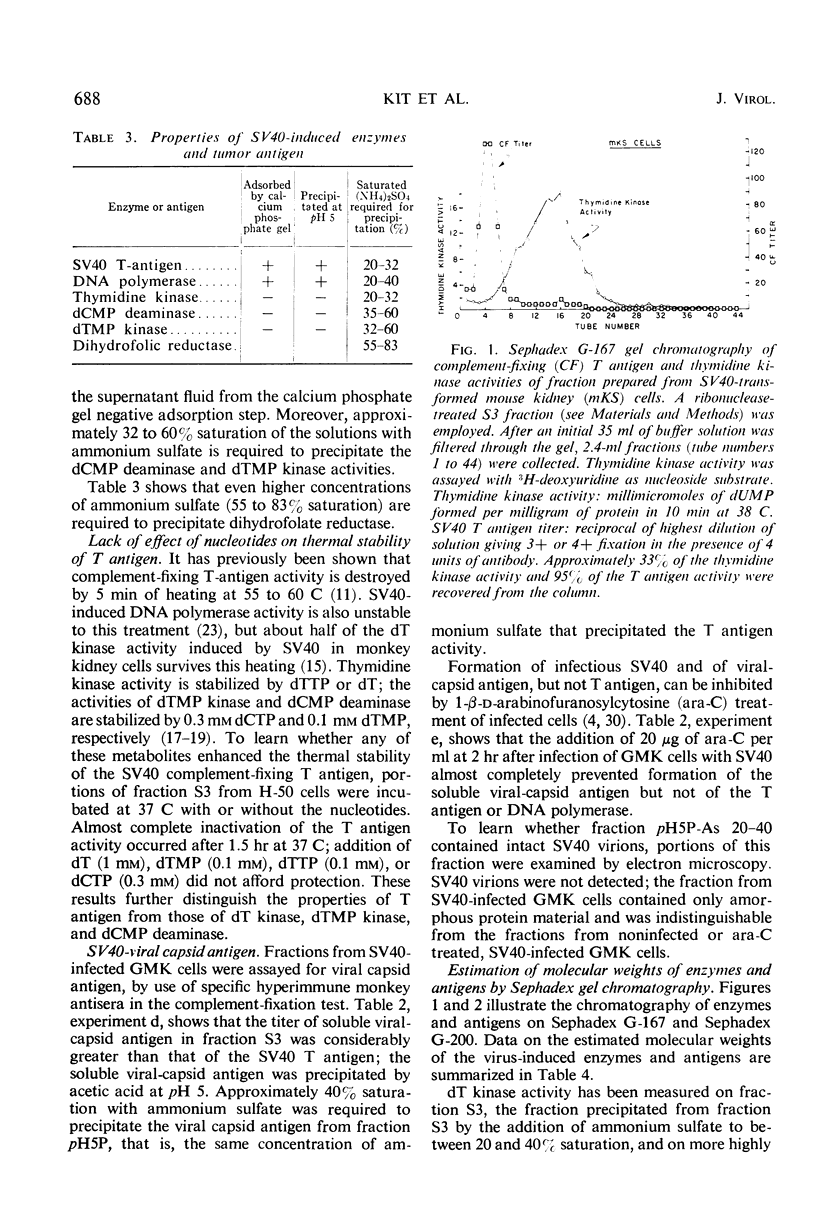
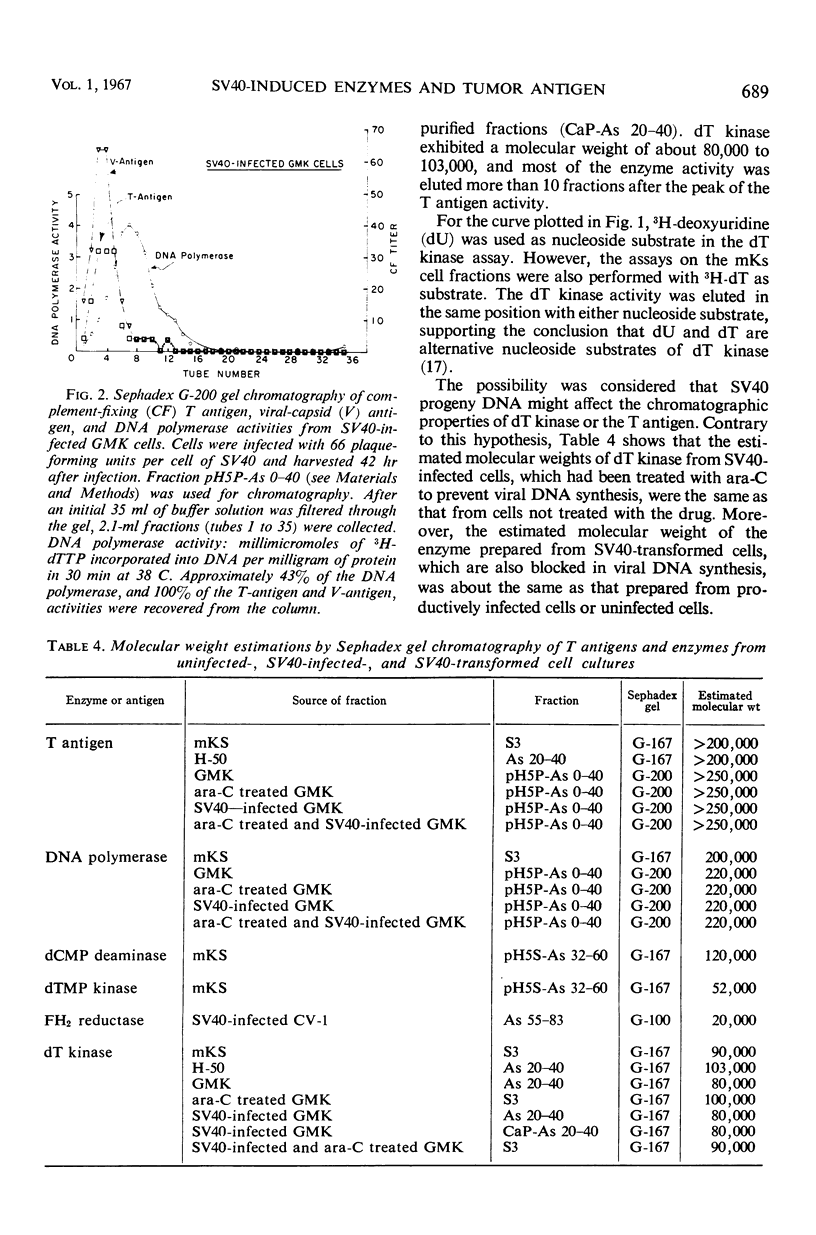
The complement-fixing tumor (T) antigen induced by simian virus 40 (SV40) has been prepared from SV40-infected cell cultures, from infected cell cultures treated at the time of infection with 1-β-d-arabinofuranosylcytosine (ara-C), and from SV40-transformed cells. Upon partial purification, the T antigen exhibited the following properties: it was tightly adsorbed by calcium phosphate gel, it was precipitated by acetic acid at pH 5 or by ammonium sulfate at about 20 to 32% saturation, and it had a molecular weight greater than 250,000, as estimated by Sephadex G-200 gel chromatography. In contrast, deoxycytidylate (dCMP) deaminase, thymidylate (dTMP) kinase, and thymidine (dT) kinase were less strongly bound to calcium phosphate and were not precipitated at pH 5; these enzymes also had much lower molecular weights than the T antigen, as did dihydrofolic (FH2) reductase. Furthermore, higher ammonium sulfate concentrations were required to precipitate dCMP deaminase, dTMP kinase, and FH2 reductase activities than to precipitate the T antigen. Another difference was that the T antigen was not stabilized, but dCMP deaminase, dTMP kinase, and dT kinase, were stabilized, respectively, by dCTP, dTMP, and dT or dTTP. Deoxyribonucleic acid (DNA) polymerase activity resembled the T antigen in adsorption to calcium phosphate, in precipitation by ammonium sulfate or at pH 5, and in the rate of inactivation when incubated at 38 C. However, the polymerase activity could be partly separated from the T antigen by Sephadex G-200 gel chromatography. The cell fraction containing partially purified T antigen also contained a soluble complement-fixing antigen (presumably a subunit of the viral capsid) which reacted with hyperimmune monkey sera. The latter antigen was present in very low titers or absent from cell extracts prepared from SV40-infected monkey kidney cell cultures which had been treated with ara-C at the time of infection, or from SV40-transformed mouse kidney (mKS) or hamster tumor (H-50) cells. The T antigen, however, was present in usual amounts in SV40-transformed cells or ara-C treated, infected cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHKENAZI A., MELNICK J. L. Tumorigencity of simian papovavirus SV40 and of virus-transformed cells. J Natl Cancer Inst. 1963 Jun;30:1227–1265. [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Rapp F. The effect of arabinofuranosylcytosine on the growth cycle of simian virus 40. Virology. 1965 Dec;27(4):490–495. doi: 10.1016/0042-6822(65)90174-1. [DOI] [PubMed] [Google Scholar]
- CREETH J. M., NICHOL L. W. Evidence for the chemical interaction of urease in solution. Biochem J. 1960 Nov;77:230–239. doi: 10.1042/bj0770230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp R. I., Gilden R. V. A comparison of the replication cycles of simian virus 40 in human diploid and African green monkey kidney cells. Virology. 1966 Jan;28(1):150–162. doi: 10.1016/0042-6822(66)90316-3. [DOI] [PubMed] [Google Scholar]
- Carp R. I., Kit S., Melnick J. L. The effect of ultraviolet light on the infectivity and enzyme-inducing capacity of papovavirus SV40. Virology. 1966 Jul;29(3):503–509. doi: 10.1016/0042-6822(66)90231-5. [DOI] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. THE EFFECT OF TEMPERATURE ON INDUCTION OF DEOXYTHYMIDINE KINASE ACTIVITY BY HERPERS SIMPLEX MUTANTS. Virology. 1965 Feb;25:256–270. doi: 10.1016/0042-6822(65)90204-7. [DOI] [PubMed] [Google Scholar]
- Frearson P. M., Kit S., Dubbs D. R. Induction of dihydrofolate reductase activity by SV40 and polyoma virus. Cancer Res. 1966 Aug;26(8):1653–1660. [PubMed] [Google Scholar]
- GILDEN R. V., CARP R. I., TAGUCHI F., DEFEND V. THE NATURE AND LOCALIZATION OF THE SV 40-INDUCED COMPLEMENT-FIXING ANTIGEN. Proc Natl Acad Sci U S A. 1965 Mar;53:684–692. doi: 10.1073/pnas.53.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden R. V., Carp R. I. Effects of cycloheximide and puromycin on synthesis of simian virus 40 T antigen in green monkey kidney cells. J Bacteriol. 1966 Mar;91(3):1295–1297. doi: 10.1128/jb.91.3.1295-1297.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGGAN M. D., ROWE W. P., BLACK P. H., HUEBNER R. J. PRODUCTION OF "TUMOR-SPECIFIC" ANTIGENS BY ONCOGENIC VIRUSES DURING ACUTE CYTOLYTIC INFECTIONS. Proc Natl Acad Sci U S A. 1965 Jan;53:12–19. doi: 10.1073/pnas.53.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. PROPERTIES OF DEOXYTHYMIDINE KINASE PARTIALLY PURIFIED FROM NONINFECTED MOUSE FIBROBLAST CELLS. Virology. 1965 May;26:16–27. doi: 10.1016/0042-6822(65)90021-8. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Relationship between protein synthesis and viral deoxyribonucleic acid synthesis. J Virol. 1967 Feb;1(1):110–114. doi: 10.1128/jvi.1.1.110-114.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., De Torres R. A., Dubbs D. R. Arabinofuranosylcytosine-induced stimulation of thymidine kinase and deoxycytidylic deaminase activities of mammalian cultures. Cancer Res. 1966 Sep;26(9):1859–1866. [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M. Enzymes of nucleic acid metabolism in cells infected with polyoma virus. Cancer Res. 1966 Apr;26(4):638–646. [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M., Melnick J. L. Enzyme induction in SV40-infected green monkey kidney cultures. Virology. 1966 May;29(1):69–83. doi: 10.1016/0042-6822(66)90197-8. [DOI] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Piekarski L. J., de Torres R. A., Melnick J. L. Acquisition of enzyme function by mouse kidney cells abortively infected with papovavirus SV40. Proc Natl Acad Sci U S A. 1966 Aug;56(2):463–470. doi: 10.1073/pnas.56.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Piekarski L. J., Dubbs D. R. DNA polymerase induced by Simian virus 40. J Gen Virol. 1967 Apr;1(2):163–173. doi: 10.1099/0022-1317-1-2-163. [DOI] [PubMed] [Google Scholar]
- Kitahara T., Melnick J. L. Thermal separation of the synthesis of papovavirus SV40 tumor and virus antigens. Proc Soc Exp Biol Med. 1965 Dec;120(3):709–712. doi: 10.3181/00379727-120-30632. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lark K. G. Regulation of chromosome replication and segregation in bacteria. Bacteriol Rev. 1966 Mar;30(1):3–32. doi: 10.1128/br.30.1.3-32.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinthal J. D., Wicker R., Cerottini J. C. Study of intracellular SV40 antigens by indirect immunoferritin technique. Virology. 1967 Mar;31(3):555–558. doi: 10.1016/0042-6822(67)90239-5. [DOI] [PubMed] [Google Scholar]
- MELNICK J. L., STINEBAUGH S. E., RAPP F. INCOMPLETE SIMIAN PAPOVAVIRUS SV40. FORMATION OF NON-INFECTIOUS VIRAL ANTIGEN IN THE PRESENCE OF FLUOROURACIL. J Exp Med. 1964 Feb 1;119:313–326. doi: 10.1084/jem.119.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., BUTEL J. S., FELDMAN L. A., KITAHARA T., MELNICK J. L. DIFFERENTIAL EFFECTS OF INHIBITORS ON THE STEPS LEADING TO THE FORMATION OF SV40 TUMOR AND VIRUS ANTIGENS. J Exp Med. 1965 Jun 1;121:935–944. doi: 10.1084/jem.121.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., BUTEL J. S., MELNICK J. L. VIRUS-INDUCED INTRANUCLEAR ANTIGEN IN CELLS TRANSFORMED BY PAPOVAVIRUS SV40. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:1131–1135. doi: 10.3181/00379727-116-29472. [DOI] [PubMed] [Google Scholar]
- RAPP F., KITAHARA T., BUTEL J. S., MELNICK J. L. SYNTHESIS OF SV40 TUMOR ANTIGEN DURING REPLICATION OF SIMIAN PAPOVAVIRUS (SV40). Proc Natl Acad Sci U S A. 1964 Nov;52:1138–1142. doi: 10.1073/pnas.52.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABIN A. B., KOCH M. A. SOURCE OF GENETIC INFORMATION FOR SPECIFIC COMPLEMENT-FIXING ANTIGENS IN SV40 VIRUS-INDUCED TUMORS. Proc Natl Acad Sci U S A. 1964 Nov;52:1131–1138. doi: 10.1073/pnas.52.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGEL L. M., MONTY K. J. DETERMINATION OF MOLECULAR WEIGHTS AND FRICTIONAL RATIOS OF MACROMOLECULES IN IMPURE SYSTEMS: AGGREGATION OF UREASE. Biochem Biophys Res Commun. 1965 May 3;19:494–499. doi: 10.1016/0006-291x(65)90152-x. [DOI] [PubMed] [Google Scholar]
- SMITH K. O., MELNICK J. L. A method for staining virus particles and identifying their nucleic acid type in the electron microscope. Virology. 1962 Jul;17:480–490. doi: 10.1016/0042-6822(62)90143-5. [DOI] [PubMed] [Google Scholar]


