Modification of tissue-specific inhibitory chromatin is critical for the regulation of plant developmental processes. Using a combination of functional genomics, gene network analysis, and genetic experiments, this study identified RLT2 and AIL5 proteins as essential regulators of seed-specific transcription, providing insight into the regulation of seed development.
Abstract
The complete lack of seed storage protein expression in vegetative tissues and robust expression during embryogenesis makes seed development an ideal system to study tissue-specific expression of genes. The promoter for the Phaseolin (phas) gene, which encodes the major seed storage protein in bean (Phaseolus vulgaris), is activated in two sequential steps: Phaseolus vulgaris ABI3-like factor (Pv-ALF)–dependent potentiation and abscisic acid–mediated activation. In this study, a heterologous in vivo Pv-ALF/phas-GUS (for β-glucuronidase) expression system in transgenic Arabidopsis thaliana leaves was used in conjunction with the powerful RNA-Seq approach to capture transcriptional landscapes of phas promoter expression. Remarkably, expression of over 1300 genes from 11 functional categories coincided with changes in the transcriptional status of the phas promoter. Gene network analysis of induced genes and artificial microRNA–mediated loss-of-function genetic assays identified transcriptional regulators RINGLET 2 (RLT2) and AINTEGUMENTA-LIKE 5 (AIL5) as being essential for phas transcription. Pv-ALF binding to the RLT2 and AIL5 promoter regions was confirmed by electrophoretic mobility shift assay. RLT2 and AIL5 knockdown lines displayed reduced expression of several endogenous seed genes, suggesting that these factors are involved in activation of endogenous Arabidopsis seed storage gene expression. Overall, the identification of these key factors involved in phas activation provides important insight into the two-step transcriptional regulation of seed-specific gene expression.
INTRODUCTION
Recently, much has been learned about the regulation of transcription from eukaryotic promoters, and it is now apparent that both epigenetic and genetic processes contribute to quantitative and spatial regulation of expression. Nevertheless, the exact molecular and cellular processes that activate specific promoters in particular tissues largely remain to be elucidated. In mammals, deregulation of transcription may result in neoplasia, the failure to express genes responsible for cellular differentiation or inappropriate expression of positively acting transcription factors (TFs) and nuclear oncogenes (Cox and Goding, 1991). Similarly, failure of the regulated gene transcription process in plants can cause disruption of normal functions (Finnegan et al., 1996). The plant networks responsible for the maintenance of transcriptional regulation are only now becoming apparent, and studies taking advantage of high-throughput methods to gain a genomic appraisal of this situation are urgently needed to better understand such processes. Because the seed represents such a vital stage of a plant’s life cycle and synthesis of seed storage proteins is intrinsic to seed development, seed development is a very attractive system to study such these plant networks.
Expression of Phaseolin (phas), the most abundant seed storage protein in the common bean Phaseolus vulgaris, is regulated by both genetic and epigenetic processes. It is stringently turned off during all vegetative stages of plant development by the presence of a nucleosome that is positioned over its TATA regions (Li et al., 1998). The bean TF Pv-ALF (for Phaseolus vulgaris ABI3-LIKE FACTOR) is orthologous to Arabidopsis thaliana ABA INSENSITIVE3 (ABI3) and is vital for transcription from the bean phas promoter (Van Der Geest et al., 1995). Expression of ALF, in combination with the plant growth regulator abscisic acid (ABA), has been shown to release transcriptional constraints on the phas promoter. Pv-ALF is a member of a B3 domain family of TFs, which are found uniquely in plants and serve as major components required for the expression of seed-specific genes involved in seed maturation. Amino acid sequence alignment of plant DNA binding B3 domains reveals a highly conserved globular domain with an all-β fold (Zhou et al., 2004). The Arabidopsis genome contains over a hundred genes encoding B3-type TFs, 14 of which form the seed-specific ABI3 subfamily (McCarty and Chory, 2000; Finkelstein et al., 2002; Carranco et al., 2004). Other proteins have been shown to act as key transcriptional regulators in seed maturation processes, including ABI3, Pv-ALF, their maize (Zea mays) ortholog Viviparous1 (VP1), and several other Arabidopsis B3-type proteins, such as Leafy cotyledon1 (Lec1), Lec2, and Fusca3 (Fus3) (Finkelstein et al., 2002; To et al., 2006; Suzuki and McCarty, 2008).
The contribution of chromatin to seed-specific expression in plants was originally illustrated by work with β-phaseolin (Li et al., 1998). Two discrete steps in expression from the phas promoter include (1) a potentiation step, which involves Pv-ALF–initiated remodeling of the chromatin structure at the TATA-box region and (2) an activation step driven by ABA that results in the synthesis of mRNA (Li et al., 1999). The chromatin remodeling step induced by Pv-ALF is accompanied by ordered histone modifications that are associated with transcriptional poising and activation of the phas promoter (Ng et al., 2006). Subsequent studies in other laboratories have complemented these findings, and it now appears that facilitating nucleosomal repositioning may be a general feature of B3-domain protein function (Suzuki et al., 2005; Yamamoto et al., 2010). In addition, other factors are likely to be needed to initiate chromatin changes mediated by Pv-ALF or other B3 TFs. Although their identity and means of recruitment are not yet known, possible candidates are proteins involved in chromatin remodeling and signal transduction, such as kinases and phosphatases (Chandrasekharan et al., 2003b; Ng et al., 2006).
The redundant nature of multiple seed-specific B3 TFs and the complexity of their transcriptional networks within the seed makes it technically challenging to identify the specific details of transcriptional activation of seed-specific genes (Braybrook and Harada, 2008; Suzuki and McCarty, 2008). Microarray techniques have previously been used to study the roles of B3 TFs in gene expression at specific stages of seed development (Suzuki et al., 2003; Le et al., 2010; Yamamoto et al., 2010). Currently, RNA-Seq has a higher sensitivity than microarrays, permitting detection of genes expressed at very low levels (Wang et al., 2009). In this study, we used the RNA-Seq technique to identify and dissect the molecular mechanisms involved in altering the chromatin structure of the phas promoter to achieve its transcriptional activation. Using a tightly controlled, inducible, heterologous Pv-ALF/phas-GUS (for β-glucuronidase) system, which includes estradiol-mediated expression of Pv-ALF and a phas-GUS reporter construct (Ng et al., 2006), which mimics the unique characteristics of seed-specific phaseolin expression, we characterized individual components of gene networks that are turned on by Pv-ALF to activate phas expression in transgenic Arabidopsis leaves. The roles of identified transcription regulators RLT2 and AIL5 in the transcriptional activation of the phas promoter were functionally confirmed using loss-of-function genetic assays. Our data provide key insight as to how B3 TFs orchestrate the initial steps of chromatin remodeling necessary for the expression of phas and related seed maturation genes.
RESULTS
Characteristics of the Inducible, Heterologous Pv-ALF/phas-GUS System
Under natural conditions in developing seeds, events associated with potentiation and activation of the phas promoter are inseparable. By contrast, in the estradiol-inducible heterologous Pv-ALF/phas-GUS system, these events can be temporarily separated (Ng et al., 2006). Therefore, this system provides an excellent means to obtain an improved understanding of chromatin remodeling associated with transcriptional potentiation and activation of the seed-specific phas promoter. The transcriptionally inhibitory chromatin at the promoter of the phas-GUS construct in Arabidopsis leaf tissue can be experimentally remodeled in two steps: (1) potentiation, in which the chromatin architecture is relaxed as a result of estradiol-induced expression of Pv-ALF; and (2) transcriptional activation, triggered by the exogenous application of ABA (see Supplemental Figure 1 online).
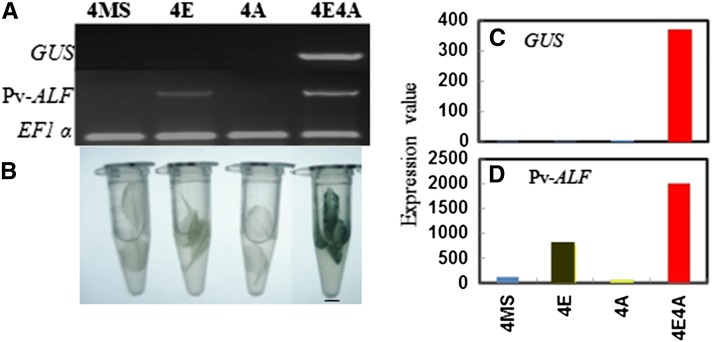
Rosette leaves of transgenic Arabidopsis plants were exposed to either 25 μM estradiol (4E treatment for the potentiation step) or 200 μM ABA (4A) or both estradiol and ABA (4E4A treatment for the activation step), as described in Methods. As a negative control for the potentiation step, leaves were also exposed to Murashige and Skoog (MS) medium only (4MS treatment). In accordance with previous results (Ng et al., 2006), RT-PCR analysis detected Pv-ALF mRNA expression in leaves during potentiation (4E) and activation (4E4A) steps, while GUS mRNA was observed only during the activation step (4E4A) (Figure 1A). Furthermore, GUS protein activity was detected by histochemical staining only in the presence of both Pv-ALF and ABA (4E4A) (Figure 1B).
Figure 1.
Expression of phas-GUS in Transgenic Arabidopsis Pv-ALF Leaves Is Dependent on Estradiol-Inducible Pv-ALF and Exogenous ABA.
(A) RT-PCR of Pv-ALF and GUS transcripts. Pv-ALF transcripts are expressed only during the potentiation (4E) and activation (4E4A) steps. GUS transcripts are only present during the activation step. EF1α is used as a control.
(B) GUS histochemical assay in uninduced Pv-ALF plants (4MS) or plants treated with 25 μM estradiol (4E), 200 μM ABA (4A), and with both 25 μM estradiol and 200 μM ABA (4E4A). Bar = 2 mm.
(C) and (D) RNA-Seq expression values for GUS (C) and Pv-ALF (D) transcripts under the same four experimental conditions are shown.
[See online article for color version of this figure.]
In order to identify the events and processes involved in the transition of the phas promoter from silent to active state, we analyzed two biological replicates via high-throughput sequencing. The RNA-Seq libraries were prepared from the four treatments (4MS, 4E, 4A, and 4E4A) and subjected to high-throughput sequencing using the Illumina GA3 platform. The Illumina reads were then aligned with the GUS marker gene and the Pv-ALF transgene. As expected from RT-PCR and histochemical staining data, GUS transcripts were only detected during the phas activation step (4E4A) (Figure 1C), whereas Pv-ALF transcripts were detected at very high levels during both potentiation (4E) and activation (4E4A) steps (Figure 1D), further illustrating the high stringency of the phas-GUS reporter system. These data further illustrate the remarkable power of the RNA-Seq approach to detect even the lowest abundance transcripts. An important aspect of the heterologous system used here is that Pv-ALF and the phas-GUS constructs are ectopically expressed in transgenic leaves, where the key endogenous Arabidopsis B3-type seed-specific TFs, such as FUS3, LEC1, LEC2, and ABI3 itself, are not present (Gao et al., 2009). Thus, we conclude that the RNA-Seq approach is uniquely suited to provide a detailed analysis of kinetic changes in transcription that occur at the phas promoter and throughout the leaf transcriptome in response to Pv-ALF expression (potentiation step) and exogenous application of ABA (activation step).
Identification of Differentially Expressed Genes
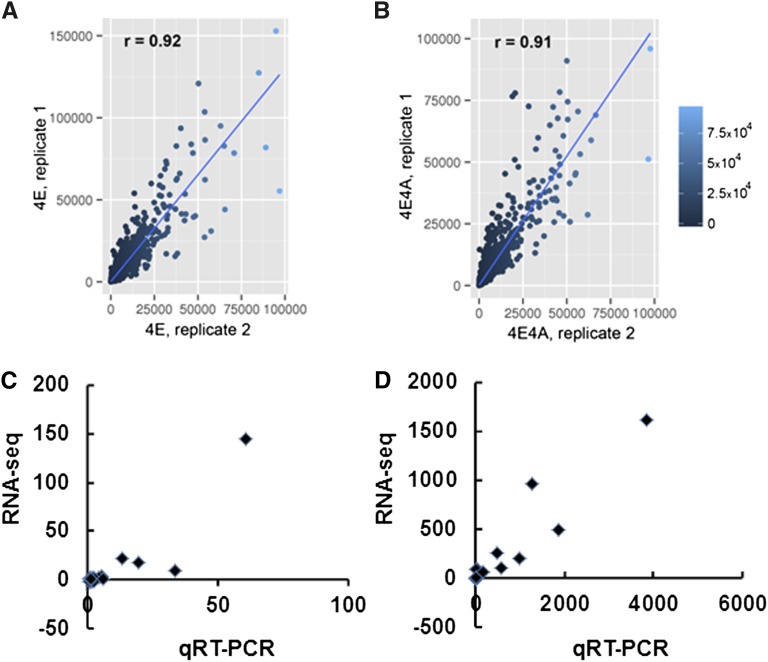
Data obtained by Illumina sequencing were processed, filtered, and normalized using the Illumina pipeline (http://www.illumina.com) to generate fast-q files, which were then analyzed using the RNA-Seq module of CLC Genomic Workbench (http://www.clcbio.com). For biological replicate 1, 16.8 to 22.9 million reads were obtained, and for biological replicate 2, 25.7 to 33.4 million reads were obtained. Of these reads, over 84% were uniquely mapped to the annotated Arabidopsis genome (TAIR10) (see Supplemental Table 1 online). The expression level of individual genes was quantified by counting the number of reads per kilobase per million mapped reads (RPKM), and Quantile normalization was applied to the RPKM values by taking the mean as the value to be normalized. A gene was considered to be expressed if it had at least one sequence read aligned with it. We detected expression of more than 16,000 genes in the 4MS, 4E, 4A, and 4E4A samples (see Supplemental Table 1 online). The shapes of distributions for average RPKM values were very similar among the four treatments of the two replicates (see Supplemental Figure 2A online). Correlation coefficients were calculated for RPKM values after eliminating genes with zero count in either of the two replicates. Between the two biological replicates, correlation coefficients for each condition ranged from 0.90 to 0.94, indicating a good statistical correlation between replicates (Figures 2A and 2B; see Supplemental Figures 2B and 2C online).
Figure 2.
Assessment of Reproducibility between the Two RNA-Seq Biological Replicates and Validation by qRT-PCR.
Correlation plots of the RPKM reads for each replicate for 4E (A) and 4E4A (B) treatments are shown. Coverage across transcripts was calculated by counting the number of reads on aligned transcripts generated by TopHat. All correlation analysis and plots were performed using the R statistical package. x and y axes show the number of counts generated with the program HTSeq-count. Values of Pearson’s correlation coefficient (r) are shown on each plot. For RNA-Seq data validation by qRT-PCR, Pearson correlation coefficients for 4E (r = 0.90) (C) and 4E4A (r = 0.94) (D) were obtained by plotting RNA-Seq data on y axis and qRT-PCR data on x axis. RNA-Seq data and qRT-PCR data are shown as fold change in gene expression compared with the 4MS control.
[See online article for color version of this figure.]
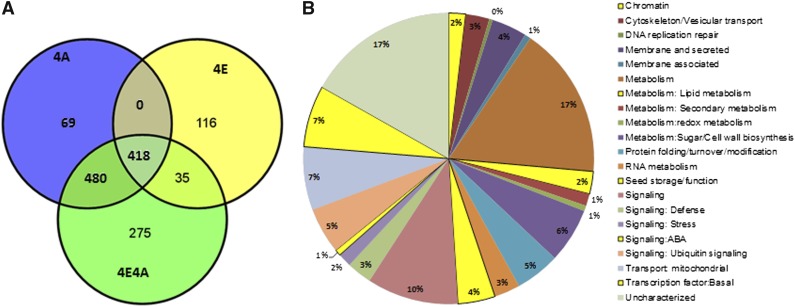
To identify for candidate genes involved in phas promoter activation, expression data from RNA-Seq experiments were imported into the GeneSpring GX12.5 (Agilent Technologies) analysis tool. Statistical significance of expression values for the two biological replicates with four conditions was analyzed using the analysis of variance test with multiple testing corrections by the Benjamini and Hochberg method (Benjamini and Hochberg, 1995) to correct false discovery rate, and asymptotic P value computation was performed to determine differential expression of genes. To judge the significance of differences in expression of most genes, we used the threshold of fold change ≥2 between treatment and control samples with corrected P value ≤ 0.05. To detect truly differentially expressed low-expressing TFs and chromatin-modifying genes, we used the corrected P value ≤ 0.2 (see below). Using this analysis, we identified 1208 genes that were upregulated by at least twofold (Figure 3A) and 2421 genes downregulated by at least twofold (see Supplemental Figure 3 online) in 4E4A condition compared with the 4MS control condition. Likewise, 569 and 809 genes were up- or downregulated by at least twofold in 4E treatment. To identify 4E-specific upregulated genes (genes uniquely activated by Pv-ALF or potentiation specific) and 4E4A-specific upregulated genes (ABA-dependent Pv-ALF–activated genes), three-way Venn diagrams were constructed based on the number of upregulated genes in any of the three conditions (Figure 3A). This diagram showed that 275 and 116 genes were specifically upregulated by at least twofold only in 4E4A and 4E, respectively (see Supplemental Data Sets 1 and 2 online). In addition, we identified 35 overlapping upregulated genes in both 4E and in 4E4A conditions and 480 overlapping upregulated genes in both 4A and 4E4A conditions. Similarly, 183 and 878 genes were substantially downregulated during the potentiation (4E) and ABA-mediated activation (4E4A) steps of phas-GUS expression (see Supplemental Figure 3 and Supplemental Data Sets 3A and 3B online). Importantly, many potentiation- and activation step–specific Arabidopsis promoters are strongly enriched for known or predicted Pv-ALF binding DNA elements (see Supplemental Table 2 online).
Figure 3.
Identification and Functional Classification of ABA-Dependent and -Independent Genes Induced in the Presence of Pv-ALF.
(A) Venn diagram of upregulated genes specific to the potentiation (4E) and activation (4E4A) steps of phas-GUS expression is shown.
(B) Functional classification of Pv-ALF–activated genes. The data consist of genes that have at least a twofold change in expression from two independent RNA-Seq experiments. The complete list is presented in Supplemental Data Set 4 online.
To confirm gene expression differences identified by RNA-Seq, we used real-time RT-PCR analysis to validate expression patterns of 20 genes that were upregulated either in 4E or 4E4A treatment conditions, including low-expressed chromatin remodeling genes detected in 4E and highly expressed LATE EMBRYOGENESIS ABUNDANT (LEA) family genes expressed in 4E4A treatment. Fold change differences in expression levels observed in RNA-Seq and quantitative RT-PCR (qRT-PCR) data for all 20 genes displayed similar patterns and also showed good correlations of 0.90 and 0.94 for 4E and 4E4A-specific genes, respectively (Figures 2C and 2D; see Supplemental Table 3 online).
Functional Classification and Annotation of Upregulated Genes
Of the 1324 genes detected in both biological replicates that were upregulated in 4E and 4E4A treatments, 1101 (∼83%) genes were annotated and functionally classified into 11 distinct categories that included chromatin proteins, TFs, and signaling and seed functions (Figure 3B; see Supplemental Data Set 4 online). Activation of genes from these diverse functional categories corresponds to a robust transcriptional response that occurs during ABA signaling and seed development. Indeed, as reported previously in studies on ABA signaling (Cutler et al., 2010), we observed coexpression of genes involved in seed function, stress response (including dehydration, drought, salinity, and other environmental stresses), sugar metabolism (e.g., genes involved in the biosynthesis of protective sugars like trehalose), polyamine metabolism (e.g., Arg decarboxylase involved in putrescine biosynthesis), enzymes that detoxify reactive oxygen species, transporters, lipid metabolism, pathogen response, and many distinct signaling pathways (Figure 3B). This suggests a strong crosstalk between these pathways and ABA signaling.
A comparison of the genes induced in our heterologous system with those reported in other related studies showed a substantial overlap (see Supplemental Figure 4 online and Supplemental Data Set 5 online). For example, in a study of genes induced upon heterologous expression of maize VP1 in Arabidopsis abi3 background (Suzuki et al., 2003), 194 genes were induced upon ABA treatment and VP1 expression, of which at least 140 genes (73%) are also induced in our heterologous system. Similarly, of 98 genes identified by Mönke et al. (2012) using ChIP-chip and transcriptome analysis as part of the Arabidopsis ABI3 regulon, 77 (78%) were also specifically upregulated in our system. By examining ectopic expression of seeds-specific genes in our heterologous system, we identified at least 50 genes (see below) that were previously reported as being expressed only in seeds (Le et al., 2010). Notably, only genes specifically upregulated in the 4E4A treatment overlapped with those detected in the above studies, strongly suggesting that the combined effect of Pv-ALF expression and ABA application recapitulates a subset of physiological responses that naturally occur during seed development.
Analysis of Seed-Specific Genes Turned on during Activation of the phas Promoter in Leaves
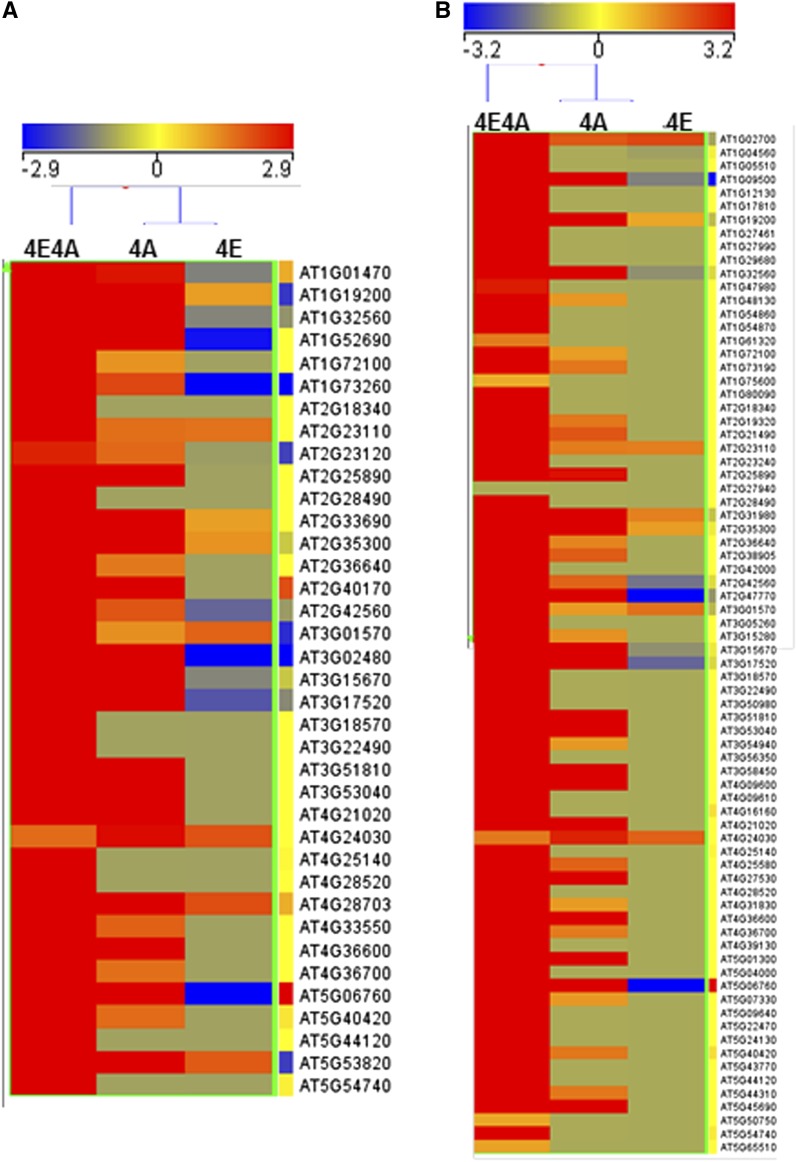
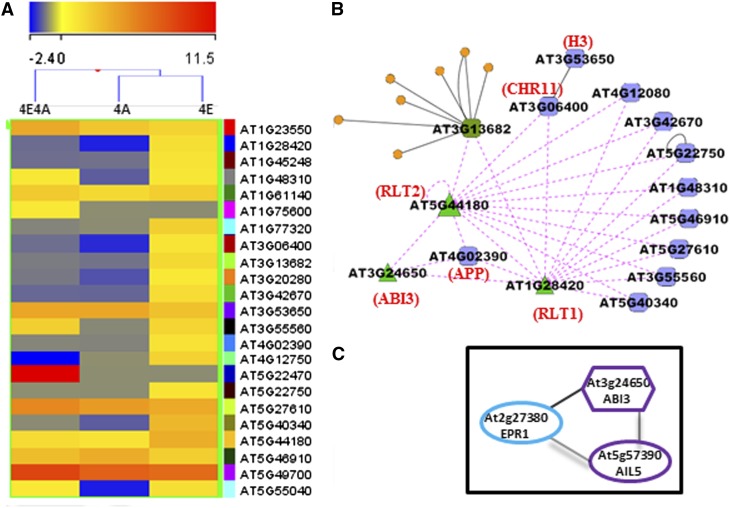
The list of genes expressed during activation of the phas promoter contains 37 genes (P value of ≤ 0.05) that, on the basis of domain composition, are annotated as being involved in seed storage (Figure 4A; see Supplemental Data Set 6 online). In addition to these, we have identified several other functional categories of genes that have been reported to be expressed in a seed-specific fashion (Becerra et al., 2006; Le et al., 2010; Mönke et al., 2012). Comparison of our data with the above-mentioned studies revealed that 76 Arabidopsis seed-specific genes (P value of ≤ 0.05) were upregulated in leaves during the activation step of phas expression (Figure 4B). These genes were functionally classified into 11 major categories, the largest of which includes 18 genes that are either involved in sequestration of seed lipids or serve as seed storage proteins, such as several cupins and oleosins (see Supplemental Figure 5 and Supplemental Data Set 7 online). The second largest category contains 10 seed-specific genes associated with dehydration and stress response, such as dehydrin-like and LEA proteins. Strikingly, only one Arabidopsis seed-specific TF was recovered (AIL7), and this was also found in a set of previously identified 48 seed-specific TFs (Le et al., 2010). Overall, the 4E4A data set shows that Pv-ALF and ABA trigger expression in Arabidopsis leaves of many endogenous experimentally verified seed-specific genes, which comprise a distinct subnetwork of the more complex natural transcriptional network in the seeds. Furthermore, our data suggest that the general pathway for activation of seed-specific gene networks is partially conserved between bean and Arabidopsis, such that a seed-specific TF from bean is capable of using components of the endogenous Arabidopsis transcriptional network to induce a comparable response.
Figure 4.
Analysis of Seed-Specific Arabidopsis Genes Expressed during Activation of the phas Promoter in Leaves.
Relative expression profiles of identified seed storage genes (A) and other seed-specific genes (B). The color indicates the degree of fold change: red, high; blue, low. Hierarchical clustering (heat map) was generated using the Euclidean-based method.
Differential Expression of a Unique Set of Core Genes Involved in ABA Signaling
Approximately 50 genes have been reported to be part of the core ABA signaling pathway (Cutler et al., 2010, and references therein), of which 30 genes are differentially regulated (P values of ≤ 7.7E-04 to 0.09) in our heterologous system (see Supplemental Figure 6A and Supplemental Data Set 8 online). Genes that are upregulated include three PP2C-like phosphatases (ABI2, HAB1, and AHG1) that function as negative regulators of the ABA signaling pathway, three kinases (OST1, CPK6, and CPK11) that positively regulate ABA signaling, and the TF ABI5, which regulates many of the genes involved in ABA signaling. By contrast, downregulated genes include two START-domain ABA receptors (PYL7 and PYL9), the kinases CPK3 and SNRK2.3, which positively regulate ABA signaling, and the farnesyltransferase β-subunit ERA1, which attenuates ABI3 expression by modifying ABI3 (Brady et al., 2003). The presence of this assortment of differentially regulated genes for a subset of the core ABA signaling pathway implies that expression of Pv-ALF (or its Arabidopsis ortholog ABI3) in conjunction with ABA hormone induces a distinct signaling response that specifically regulates a specific group of genes during seed development. This observation may enable further dissection of various phosphatases and kinases believed to operate at this stage of development (Zhang et al., 2012) and may also help in understanding how the ABA signaling pathway is fine-tuned for seed development as opposed to other stress responses.
Analysis of Specific TFs and Chromatin Proteins Regulated by Pv-ALF in the Presence of ABA
One of our key interests is to determine the identity of proteins that function in the transcriptional regulation of the phas promoter and their mechanism of action. Previous DNase I footprinting (Li et al., 1999) and chromatin immunoprecipitation (Ng et al., 2006) experiments established that changes in histone modification status and nucleosome positioning are required to initiate expression from the phas promoter and that chromatin-associated proteins are likely to have a major impact on phas expression. Furthermore, in vivo footprinting analysis of the phas promoter showed that over 20 cis-elements in the proximal part of the promoter are bound by potential TFs (Li and Hall, 1999). Whereas some of these factors are known or predicted, such as the RY/Sph-element binding Pv-ALF/ABI3, the ABRE element binding ABI5, and a CAAT-box binding protein, the precise identity of most other factors that bind these elements still remains to be elucidated. We therefore studied the expression patterns of TFs and chromatin proteins in our data set.
Although we detected several highly upregulated (fold change at least 2) TF and chromatin protein coding genes in the 4E and 4E4A condition, transcriptional regulators are often known to be expressed at very low levels (Alvarez et al., 2006; Vernimmen et al., 2011). We identified a total of 85 upregulated TFs and 22 chromatin proteins with P values from 0.05 to 0.2 (see Supplemental Data Sets 9 and 10 online). Overall, the identified TFs belong to 14 distinct protein superfamilies, with at least seven representatives from each of the NAC/NAM, MYB, AP2, WRKY, BZIP, and C2H2 zinc finger families dominating the landscape (see Supplemental Figure 6B and Supplemental Data Set 9 online). Genes showing the highest fold induction in 4E4A include those encoding BZIP factor ABI5 (63x), NAC domain–containing TFs, such as anac042 (27x), anac032 (17x), and RHL41 (20x), and the AP2 domain–containing TF DREB2 (10x). Of the highly expressed TFs, ABI5 has a major role in regulating seed genes regulated by ABA signaling, and it also regulates the phas promoter by binding to the G-box element (Ng and Hall, 2008). The homeodomain TF HB-7 regulates ABA signaling by regulating PP2C and ABA receptor expression (Valdés et al., 2012). At least 10 other known TFs induced in our system are either induced by or are regulators of various stress responses, including ABA response. A single member of the CAAT-box binding histone fold family, NF-YC2, is induced in the activation step. The CCAATT-box present in the phas promoter is responsible for uniform and high-level expression of the phas promoter in the embryo and is thus likely bound by this CAAT-box binding factor (Chandrasekharan et al., 2003a).
Of the 22 identified chromatin proteins, 12 and five were upregulated during potentiation and activation of the phas promoter, respectively. Chromatin-associated genes induced in this data set typically encode low abundance proteins showing up to 10-fold induction. These chromatin proteins include four nucleosome remodeling SWI2/SNF2 ATPases (CHR11, CHR18, and CHR38) and two demethylases (LDL2 and AT5G46910) (Figure 5A; see Supplemental Data Set 10 online). Other chromatin proteins upregulated in the 4E and/or 4E4A treatment include modified peptide binding adaptors containing the chromo family of domains that binds methylated peptides and two related proteins with a homeodomain, DDT domain, HARE-HTH domain, and WHIM motifs (RLT1 and RLT2) that are predicted to specifically interact with the ISWI class of SWI2/SNF2 ATPases (Aravind and Iyer, 2012). Of the structural components of chromatin, only histones H2B and H3 were upregulated upon activation. Of interest is the induction of chromatin genes involved in DNA repair. These include PARP-1 (APP) and related enzymes known to function in DNA repair, the RAD5 and HARP/SMARCAL-1-like (Ghosal et al., 2011) SWI2/SNF2 ATPases, and the MEI1-like multi-BRCT domain protein (Grelon et al., 2003). These data suggest that a robust DNA repair pathway is activated under these conditions. This observation supports the critical role of DNA repair proteins during seed imbibition (Balestrazzi et al., 2011) and suggests that the combined effect of ABI3/Pv-ALF and ABA activates this pathway during seed development.
Figure 5.
Expression Profiles of Chromatin Genes Identified by RNA-Seq in Leaves and Molecular Interactions of Candidate Genes with Pv-ALF/ABI3.
(A) Heat map of chromatin genes activated in 4E and 4E4A treatments. The color indicates the degree of fold change: red, very high; yellow, high; blue, low. Hierarchical clustering heat map was performed using Euclidean distance method.
(B) Map of molecular interactions between Pv-ALF/ABI3 and Pv-ALF–responding chromatin genes. Purple nodes denote regulatory edge. Green triangles define TFs. Orange circles on At3g13682 indicate small molecule modifications.
(C) Coexpression analysis of TFs regulated by Pv-ALF/ABI3.
Arabidopsis Gene Networks Involved in the Establishment of the Potentiation and Activation Steps of phas Expression
Although overexpression of several chromatin proteins and TFs correlates with phas promoter potentiation and activation, the challenge remains to identify exactly which regulators specifically target the phas promoter. We addressed this using two topical bioinformatic approaches, namely, VirtualPlant software (Katari et al., 2010) for chromatin related proteins and ATTED II (Obayashi et al., 2011) for TF interactions with ABI3/Pv-ALF. ABI3 was included in the input gene list in place of bean Pv-ALF, since the most parsimonious hypothesis is that exogenous Pv-ALF exerts its function through the Arabidopsis ABI3-dependent gene network. Results from the VirtualPlant network analysis revealed that ABI3/Pv-ALF bears a regulatory edge complementary only with 4E-specific PARP-1/APP, RLT1, and RLT2 genes (Figure 5B), suggesting that Pv-ALF initially regulates transcription of these genes during the potentiation step.
While VirtualPlant analysis indicated that ABI3/Pv-ALF interacts with several TFs, ATTED-II analysis revealed that the strongest coexpression connection emerged only for AINTEGUMENTA-LIKE5 (AIL5; At5g57390), a poorly characterized TF (Tsuwamoto et al., 2010), and for an extensin-like gene At2g27380 (Figure 5C; see Supplemental Figure 7 online). Importantly, the AIL5 gene was expressed upon addition of ABA and was also exceptionally highly expressed under 4E4A treatment, but not in other conditions. Furthermore, promoter analysis using the Known CIS Analyzer AGRIS software indicates that the AIL5 promoter harbors many Pv-ALF binding motifs (three ABRE-like, six G-box, and eight RAV1-A motifs). Taken together, these observations indicate that Pv-ALF may specifically upregulate expression of RLT2/RLT1/PARP-1(APP) and AIL5, which in turn may directly or indirectly regulate phas expression. Hence, these computational results provide a rationale for genetic tests aimed at understanding the functional role of these candidate genes in the two-step transcriptional activation of the phas promoter.
RLT2 and AIL5 Are Required for Efficient Activation of phas-GUS Expression in Transgenic Arabidopsis Leaves
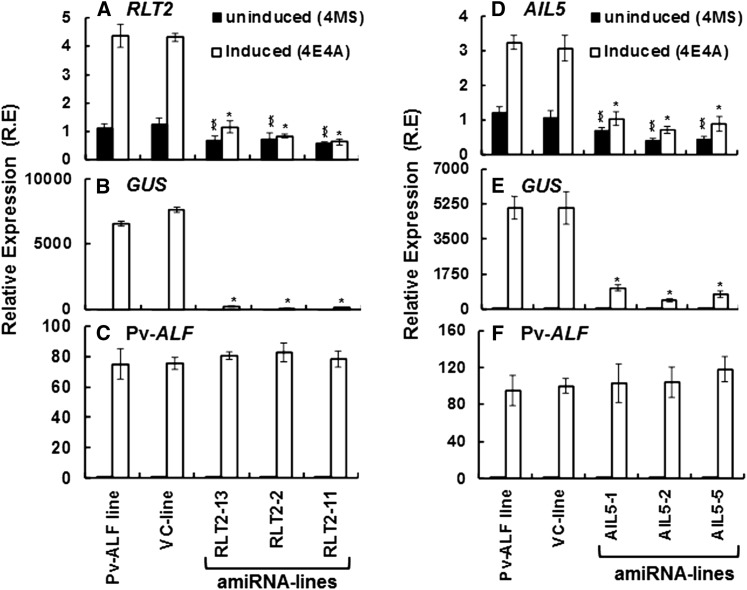
To dissect the putative network of genes involved in the activation of phas-GUS expression, we first focused on the two most likely candidates, RLT2 and AIL5, computationally predicted to be the key factors is the potentiation and activation steps of phas expression, respectively. To functionally characterize the role of these proteins in phas-GUS transcription, the Pv-ALF plants used to perform RNA-Seq experiments in this study were supertransformed with artificial miRNA (amiRNA) constructs targeting either RLT2 or AIL5 genes. This approach uses expression of an endogenous microRNA precursor engineered to yield a 21-nucleotide double-stranded amiRNAs complementary to and directed against either RLT2 or AIL5 (Alvarez et al., 2006; Schwab et al., 2006). Expression of RLT2 and AIL5 amiRNAs was driven by the constitutive cauliflower mosaic virus 35S promoter. Three knockdown lines for each gene were selected and used to investigate the function of the corresponding gene during the Pv-ALF–mediated phas-GUS expression in leaves. All experiments were performed with either untreated leaves (4MS condition) or leaves treated with estradiol and ABA as described above (4E4A condition).
In the uninduced 4MS condition, expression of RLT2 mRNA in the RTL2-13, RTL2-2, and RTL2-11 amiRNA lines decreased by 37 to 50% compared with the untransformed Pv-ALF control line (Figure 6A, black bars). The relatively low reduction of RLT2 expression by the gene-specific amiRNA could be partially due to difficulties in reducing the steady state level of certain housekeeping transcripts subject to negative feedback regulation (Ossowski et al., 2008). Nevertheless, no reduction in RLT2 expression was observed in the vector-only control line. As expected from the RNA-Seq data, upon induction (4E4A treatment), RLT2 mRNA levels increased over fourfold in the control Pv-ALF line (Figure 6A, white bars). By contrast, no induction of RLT2 gene expression was observed in the three amiRNA lines, and RLT2 mRNA levels in 4E4A condition stayed near the 4MS levels. Remarkably, while GUS mRNA can only be detected in the 4E4A-induced condition (white bars), its expression in all three RLT2 amiRNA lines was reduced by up to 98% in comparison to the Pv-ALF control line (Figure 6B). Thus, this drastic decrease in phas-GUS expression in the leaves of RLT2 amiRNA lines largely correlates with the lack of RLT2 induction in 4E4A condition. Importantly, knockdown of RLT2 expression did not affect the expression of the Pv-ALF transgene (Figure 6C), suggesting that RLT2 functions downstream of Pv-ALF to regulate the potentiation step of expression from the phas promoter. Taken together, these data point to a specific mechanism, in which the threshold RLT2 level in 4MS condition is not sufficient to activate phas-GUS expression in leaves, and suggest that Pv-ALF–dependent induction of RLT2 is necessary to trigger phas-GUS expression. A similar threshold mechanism for the basal level of p53 and toll like receptor 9 expression has recently been described for the activation of apoptosis and immune responses in human cells (Assaf et al., 2009; Kracikova et al., 2013).
Figure 6.
RLT2 and AIL5 Regulate phas Expression in Leaves.
Expression levels of RLT2 (A), AIL5 (D), GUS ([B] and [E]), and Pv-ALF ([C] and [F]) were analyzed in three independent supertransformed lines carrying either amiRNA-RLT2 ([A] to [C]) or amiRNA-AIL5 constructs ([D] to [F]), as well as in untransformed control Pv-ALF plants and plants supertransformed with vector only (VC) are shown. qRT-PCR reactions were performed using RNA samples isolated from uninduced 4MS (black bars) or induced 4E4A (white bars) samples. Expression values plotted are average of three biological replicates. Error bars represent sd. Double asterisks represent significant differences between expression in an uninduced Pv-ALF line and an uninduced amiRNA lines (P value < 0.05, Student’s t test). Single asterisks represent significant differences between the induced Pv-ALF line and induced amiRNA lines (P value < 0.001, Student’s t test). Expression levels of GUS and Pv-ALF in uninduced 4MS samples are below the detection limit.
As with the RLT2 amiRNA lines, real-time RT-PCR analysis revealed that, in the uninduced 4MS condition, AIL5 mRNA levels in the three independent AIL5 knockdown lines were reduced on average by 56%, compared with the untransformed Pv-ALF control line (Figure 6D, black bars). Upon induction (4E4A treatment), AIL5 mRNA levels increased 2.7-fold over 4MS levels in the control Pv-ALF line (Figure 6D, white bars). By contrast, the level of AIL5 gene expression in AIL5-1, AIL5-2, and AIL5-5 amiRNA lines, while slightly increased, did not exceed the 4MS levels in uninduced control Pv-ALF leaves. Importantly, GUS mRNA levels in 4E4A condition were 62 to 78% lower in the AIL5 amiRNA lines than in the control Pv-ALF plants (Figure 6E), indicating that AIL5 is required for efficient transcriptional activation of the phas-GUS transgene. Similar to the situation in the RLT2 amiRNA lines, Pv-ALF expression was not altered in AIL5 amiRNA lines (Figure 6F).
Interestingly, downregulation of the RLT2 and AIL5 genes also reduced the induction of several endogenous Arabidopsis seed genes in the 4E4A condition in transgenic leaves. Specifically, RLT2 downregulation resulted in a decrease of expression by 56 to 90% for LEA (At3g17520), 65 to 91% for cupin (At4g36700), and 86 to 90% for oleosin (At5g40420) compared with the control Pv-ALF line (see Supplemental Figure 8 online). Similarly, downregulation of AIL5 also resulted in decreased mRNA expression of cupin, oleosin, and LEA genes by up to 83, 85, and 69%, respectively (see Supplemental Figure 8 online). Taken together, these data not only provide functional evidence implicating RLT2 and AIL5 genes as key regulators of a gene network used by Pv-ALF for the transcriptional activation of phas-GUS in leaves, but also indicate that RLT2 and AIL5 can activate expression of several endogenous Arabidopsis seed-specific genes in the leaves of Pv-ALF plants.
Downregulation of RLT2 and AIL5 Affects Endogenous Seed Gene Expression in Seeds
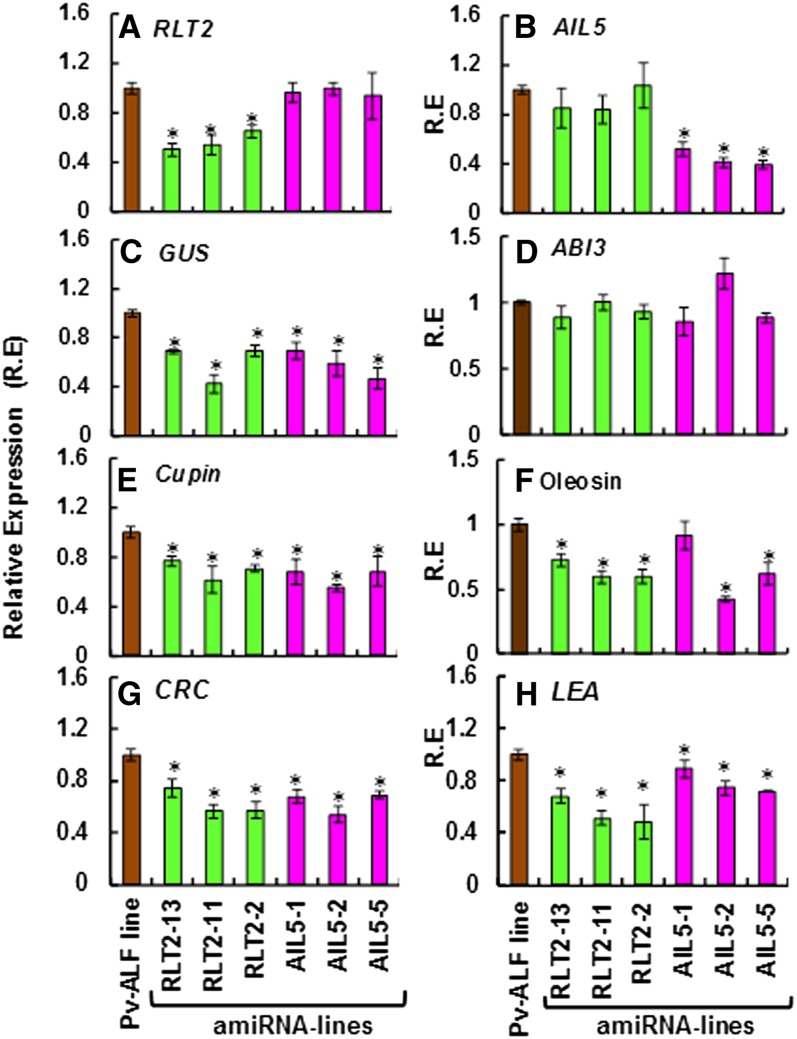
The apparent role of RLT2 and AIL5 in the activation of seed-specific gene expression in leaves indicates that these genes may also be involved in the transcriptional regulation of endogenous seed genes. To address this possibility, we examined expression of various genes involved in seed maturation processes in the mature seeds of Pv-ALF and amiRNA lines not subjected to any treatment. Similar to the situation in leaves, RLT2 expression in the seeds of RLT2 amiRNA lines was reduced by 34 to 49% (Figure 7A), whereas AIL5 expression in the seeds of AIL5 amiRNA lines was reduced by 47 to 60% compared with the seeds of control Pv-ALF plants (Figure 7B).
Figure 7.
Seeds of RLT2 and AIL5 amiRNA Knockdown Plants Show Reduced Expression of Endogenous Arabidopsis Seed Maturation Genes.
Expression of RLT2 (A), AIL5 (B), GUS (C), ABI3 (D), Cupin (E), Oleosin (F), CRC (G), and LEA (F) was analyzed by qRT-PCR using RNA isolated from fully developed seeds of three independent supertransformed lines carrying either amiRNA-RLT2 or amiRNA-AIL5 constructs, as well as from untransformed control Pv-ALF plants. Seeds were not treated with estradiol or exogenous ABA prior to analysis. Expression values plotted are average of three biological replicates. Error bars represent sd. Asterisks represent significant differences (P value < 0.023, Student’s t test).
[See online article for color version of this figure.]
As expected in the absence of estradiol, Pv-ALF transcripts were not detected in the mature seeds of Pv-ALF plants and all amiRNA lines. However, GUS expression was detected in all lines analyzed, even in the absence of induced Pv-ALF and exogenous ABA (Figure 7C). This is likely due to the expression of endogenous ABI3 protein (Figure 7D) and naturally elevated levels of intracellular ABA during the seed maturation processes in Arabidopsis. Nevertheless, GUS transcript levels in the seeds of amiRNA-RLT2 and amiRNA-AIL5 lines were reduced by 35 to 40% in comparison with the seeds from the control Pv-ALF line (Figure 7C). Furthermore, accumulation of maturation phase–specific mRNA transcripts that are necessary for seed development, such as cupin, oleosin, CRC, and LEA genes (Parcy et al., 1994; Crowe et al., 2000), was also decreased in seeds of all amiRNA knockdown lines (Figures 7E to 7H). By contrast, expression levels of endogenous Arabidopsis seed-specific B3 TFs ABI3 and FUS3, or proteins known to be major regulators of seed development, such as ABI4 and ABI5 (Brocard-Gifford et al., 2003), were not altered in RLT2 and AIL5 knockdown lines (Figure 7D; see Supplemental Figure 9 online). These data strongly suggest that RLT2 and AIL5 are involved in the regulatory processes that activate seed storage gene expression in Arabidopsis seeds. Interestingly, despite this reduction in expression of some seed storage genes, all individual RLT2 and AIL5 knockdown lines investigated here did not show abnormal changes in seed development. This is likely due to the fact that plants harbor multiple redundant pathways governing expression of seed-specific genes, which can compensate for the shortage of certain seed storage proteins with the increased accumulation of other similar proteins during seed filling. This scenario has recently been demonstrated in soybean (Glycine max), where RNA interference–mediated suppression of two major seed storage proteins, glycinin and conglycinin, resulted in rebalancing of soybean’s proteome, maintaining wild-type levels of seed protein and storage triglycerides (Schmidt et al., 2011).
Pv-ALF Binds to the Putative RLT2 and AIL5 Promoter Regions in Vitro
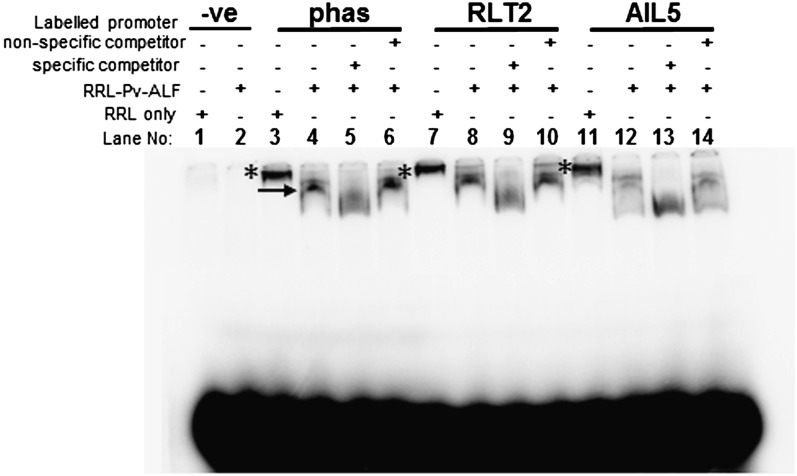
Having shown that RLT2 and AIL5 are required for phas-GUS expression, we next asked if Pv-ALF can directly bind putative RLT2 and AIL5 promoters in vitro. Recombinant Pv-ALF protein was expressed in the rabbit reticulocyte lysate expression system (RRL) and used in qualitative electrophoretic mobility shift assays (EMSAs) (Shakirov et al., 2009). PCR-amplified 32P-labeled 365-bp DNA fragments corresponding to the −340 to +25 regions of putative target promoters were used as probes. Although the DNA sequence specificity of B3-domain TFs is not well defined, Arabidopsis ABI3 protein is known to bind to ABRE motifs and RY/G-box elements (Ezcurra et al., 2000). As expected from previous studies (Carranco et al., 2004), a retarded band was observed when Pv-ALF was present in the binding mixture containing the phas promoter DNA (Figure 8, lane 4). Similar shifted bands of various intensities were also detected when the binding mixture contained the RLT2 or AIL5 promoters (Figure 8, lanes 8 and 12). The shifted complexes were only observed in the presence of Pv-ALF protein, as incubation of the target DNA probes with the reticulocyte extract alone did not yield a similarly shifted band (Figure 8, lanes 3, 7, and 11). Furthermore, incubation of RRL-expressed Pv-ALF protein with the −340 to +25 promoter region of At1g26680 gene (a negative control sequence harboring no known or predicted Pv-ALF binding motifs) did not yield a retarded band (Figure 8, lanes 1 and 2). Competition EMSAs were next used to further confirm sequence specificity of Pv-ALF binding to RLT2 and AIL5 promoters. As anticipated for a specific competitor, the addition of 100-fold excess of cold (unlabeled) phas, RLT2, and AIL5 PCR products resulted in complete disappearance of the corresponding shifted bands, which in all cases were replaced by a faster migrating diffuse smear, likely representing dynamic dissociation of the Pv-ALF-32P–labeled DNA complex in the presence of specific competitors (Figure 8, lanes 5, 9, and 13). By contrast, the addition of 100-fold excess of cold nonspecific At1g26680 probe did not result in any change in the profile of Pv-ALF–shifted band (Figure 8, lanes 6, 10, and 14), further confirming the specificity of Pv-ALF interaction with the phas, RLT2, and AIL5 promoters in vitro. Taken together, these results provide biochemical evidence for the ability of Pv-ALF to interact with RLT2 and AIL5 promoters in vitro and clearly suggest that Pv-ALF may directly stimulate expression of RLT2 and AIL5 genes in vivo.
Figure 8.
Qualitative Analysis of Pv-ALF Interaction with RLT2 and AIL5 Promoter Sequences in Vitro.
Equal amounts of Pv-ALF protein were incubated with the indicated 32P-labeled promoter sequences and the resulting protein-DNA complexes separated by native PAGE. Unprogrammed RRL reactions lacking ectopic DNA template for Pv-ALF protein were used as negative controls for each individual DNA probe (lanes 1, 3, 7, and 11). Specific Pv-ALF–DNA complexes (lanes 4, 8, and 12) are indicated by a black arrow. Asterisks designate a nonspecific band present in the negative RRL-only control reactions (lanes 3, 7, and 11). The promoter region of the At1g26680 gene (-ve probe), which harbors no known or predicted Pv-ALF binding motifs, was used as a negative control for Pv-ALF binding (lanes 1 and 2). Competition assays were performed with a 100-fold excess of unlabeled PCR products corresponding to either the specific phas (lane 5), RLT2 (lane 9), and AIL5 (lane 13) promoter regions or to nonspecific promoter region of the At1g26680 gene (lanes 6, 10, and 14).
DISCUSSION
Inducible, Heterologous Pv-ALF/phas-GUS System for the Discovery of Seed-Specific Transcriptional Networks in Arabidopsis
The development of the heterologous phas-GUS expression system, which includes estradiol-mediated expression of Pv-ALF and the phas-GUS reporter construct, provides a powerful strategy for dissecting the molecular events involved in expression of seed-specific genes (Ng et al., 2006). Transcriptional activation of the phas promoter is achieved through a series of changes in its chromatin architecture from repressed to potentiated to activated form. Importantly, the heterologous expression system described here has been shown to work not only in bean and Arabidopsis, but also in tobacco (Nicotiana tabacum) (Li et al., 1999). These findings indicate that, although some aspects of phas activation in different dicot lineages are expected to be species specific, the major players and pathways involved in the two-step expression of seed-specific genes are evolutionarily conserved among Fabales, Brassicales, and Solanales.
When constructing the likely Arabidopsis gene network responsible for the activation of phas-GUS expression, our approach relied on several key hypotheses, which were proposed in previous reports. First, because histone disposition and chromatin modifications are essential for phas-GUS expression (Li et al., 1999; Ng et al., 2006), our method centered on the analysis of chromatin genes (i.e., genes already known to be involved in chromatin biology). Second, we assumed that the mode of action of Pv-ALF in transcription of seed-specific genes is similar to that of Arabidopsis ABI3. Arabidopsis ABI3 and bean Pv-ALF are indeed orthologous and share over 46% amino acid identity and have the highest similarity among all B3-type Arabidopsis proteins (Bobb et al., 1995). Interestingly, the more distant maize VP1 (a monocot member of this orthologous group) can complement ABI3 defective Arabidopsis plants (Suzuki et al., 2001), further supporting the functional similarity between Pv-ALF and ABI3 action. In addition, Pv-ALF and ABI3 exhibit similar developmentally regulated expression and DNA binding patterns (Gao et al., 2009). Finally, while we cannot completely rule out the possibility that some of the genes uniquely present in 4E or 4E4A are irrelevant due to nonspecific Pv-ALF binding to their promoters, a majority of proteins identified in this analysis do have a presumed connection to ABI3.
Epigenetic Regulation by Pv-ALF–Mediated phas Expression
To dissect epigenetic mechanisms involved in Pv-ALF-mediated phas expression, we used a curated list previously derived comprehensive list of chromatin proteins (Iyer et al., 2008) and compared their transcription profiles across all treatments. Our RNA-Seq data demonstrated a remarkable enrichment of seed-specific proteins and many families of chromatin modifying and remodeling enzymes. These results support previous observations that dynamic changes in chromatin architecture are likely to be required for the highly regulated expression of seed-specific genes. This conclusion is also consistent with earlier reports that many genes involved in embryogenesis are repressed during vegetative growth or in vegetative tissues by several independent mechanisms of chromatin remodeling, which are dependent on Pickle (Li et al., 2005), Brahma (Tang et al., 2008), trihelix repressors (Gao et al., 2009), and polycomb group proteins (Makarevich et al., 2006). In addition, our results provide new insight and candidates for the developmentally programmed mechanisms required for changing the chromatin landscape in order to initiate the expression of the phas promoter.
Molecular Mechanism of Seed-Specific ABI3/Pv-ALF–Mediated phas Chromatin Remodeling
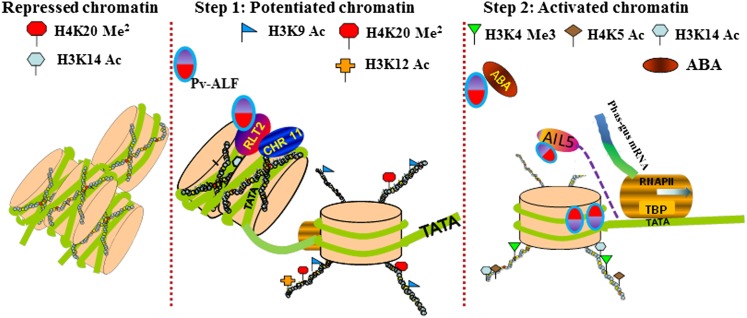
Results from amiRNA knockdown and EMSA experiments establish the requirement for RLT2 and AIL5 in Pv-ALF–dependent expression of the phas promoter. Based on these findings and the identification of potential regulators of histone modifications, we envision a specific model of Pv-ALF–dependent phas activation (Figure 9). Upon estradiol treatment, the binding of Pv-ALF to the phas promoter first triggers ordered histone modifications by as yet uncharacterized factors. Pv-ALF also induces expression of the homeobox chromatin protein RLT2 and the ISWI protein CHR11 (At3g06400). RLT2 is characterized by a homeodomain fused to a DDT domain, a HARE-HTH domain, and three WHIM motifs (Aravind and Iyer, 2012). The WHIM motifs have been computationally and experimentally shown to be absolutely essential for the interaction with the ISWI family of SWI2/SNF2 ATPases (Aravind and Iyer, 2012). CHR11 is the sole ISWI that is induced in our system, and its high expression during the potentiation (4E) step of phas expression supports its cofunctionality with RLT2 in the complex. A recent yeast two-hybrid study in Arabidopsis also confirms this interaction (Li et al., 2012). Thus, several lines of evidence suggest that RLT2 may promote the potentiated state of phas chromatin through its interaction with CHR11. ISWI-like ATPases like CHR11 use ATP hydrolysis to regulate nucleosome spacing by either optimizing it to facilitate repression or by randomizing it to facilitate transcriptional activation (Corona and Tamkun, 2004). Thus, it is likely that the rotationally and translationally positioned repressive nucleosome over the phas promoter, previously observed by our group (Li et al., 1998), might be randomized to facilitate its activation. We propose that the combined action of RLT2 and CHR11 proteins modifies phas chromatin, thereby providing a platform for the second step of phas expression (transcriptional activation).
Figure 9.
Model Depicting Sequential Changes in Chromatin Modifications over the phas Promoter during Potentiation and Activation.
In the repressed state during vegetative growth, the promoter is repressed by nucleosomes bearing dimethylated H4-K20. Pv-ALF–mediated potentiation (Step 1) is predicted to recruit RLT2, a component of ISWI chromatin-remodeling complex that also contains the CHR11-like SWI2/SNF2 ATPase. During this stage, ordered histone modifications occur by demethylation of histone H3-K4, acetylation of H3-K14 and H4-K5, and histone methylation (Ng et al., 2006). As illustrated in Step 1, this results in remodeling of the chromatin architecture over the TATA region of the phas promoter but does not lead to transcriptional activation in the absence of ABA. During the ABA-dependent activation illustrated in Step 2, Pv-ALF induces AIL5, which activates the expression of the phas promoter.
During the ABA-dependent activation step, Pv-ALF may bind several cis-elements present in the AIL5 promoter. AIL5 (also known as Embryomaker) contains two AP2 domains and has a documented role in the transition from vegetative to embryonic phase of development (Nole-Wilson et al., 2005; Tsuwamoto et al., 2010). Other Aintegumenta-like proteins implicated in embryo development include Baby Boom (promotes differentiation of Arabidopsis embryonic stem cells) (Boutilier et al., 2002) and Eg-AP2-1 protein in oil palm (Elaeis guineensis) (Morcillo et al., 2007). Most importantly, downregulation of AIL5 and RLT2 results in reduced expression of the GUS marker gene, as well as of endogenous seed-specific genes encoding cupin, oleosin, and LEA (this article). Thus, our data show that RLT2 and AIL5 act as important regulators of phas transcriptional activation.
In conclusion, we provide evidence that our heterologous Pv-ALF/phas-GUS expression system recapitulates a specific phase of seed development when the ABI3-like TFs interact with the ABA signaling pathway. In addition to providing a comprehensive transcriptional profile of the system during potentiation and activation, our analysis identifies a specific ABA-dependent signaling cascade and several transcriptional regulators that are active during this phase of seed development. In particular, the expression of a small yet diverse set of chromatin modifying and remodeling proteins supports previous observations that dynamic changes in chromatin architecture are likely to be required for the highly regulated expression of seed-specific genes. This conclusion is also consistent with earlier reports that many genes involved in embryogenesis are repressed during vegetative growth or in vegetative tissues by several independent mechanisms of chromatin remodeling (Li et al., 2005; Makarevich et al., 2006; Tang et al., 2008; Gao et al., 2009).
In combination with network analysis, our results provide insight and identify candidates for the developmentally programmed mechanisms required for changing the chromatin landscape to initiate expression from the phas promoter. Additionally, our analysis points toward several other distinct proteins and pathways that are potentially regulated in a manner similar to the phas promoter, such as a multitude of seed-specific proteins and DNA repair pathways.
METHODS
Plant Material and Treatments
Arabidopsis thaliana ecotype Columbia plants homozygous for both the estradiol-inducible XVE-HA-PvALF effector and the -1470phas-GUS reporter (Ng et al., 2006) (hereinafter referred to as the Pv-ALF line) were grown at 24°C/16°C day/night (16/8-h photoperiod and 70 to 80% humidity) in MetroMix 200 potting soil. Rosette leaves from 10 3- to 4-week-old plants were collected randomly and transferred to MS liquid medium (Murashige and Skoog, 1962) for four independent treatments with gentle agitation at room temperature in the continuous light. The treatments were as follows: uninduced control without the addition of estradiol or ABA (4MS), 25 μM estradiol (Sigma-Aldrich) treatment (4E), 200 μM ABA (Sigma-Aldrich) treatment (4A), and both 25 μM estradiol and 200 μM ABA (4E4A). For the 4E4A treatment only, samples were treated in sequential order. First, leaves were incubated for 4 h in MS liquid medium containing 25 μM β-estradiol, then rinsed with running distilled water to remove estradiol, and then incubated for another 4 h with MS liquid medium containing 200 μM ABA. After treatment, leaves were frozen in liquid N2 and stored at −80°C until further analysis. Histochemical staining for GUS activity was performed on the treated leaves using 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (Sigma-Aldrich) as substrate (Jefferson et al., 1987).
RNA Isolation and Library Preparation for RNA-Seq Transcriptome Analysis
Total RNA was extracted from leaves using the RNeasy mini kit (Qiagen) according to the manufacturer's protocol. RNA integrity was confirmed using the Agilent Bioanalyzer RNA NanoChip (Agilent Technologies). Samples were prepared for whole transcriptome analysis using mRNA-Seq kit according to manufacturer's protocol (Illumina). Briefly, mRNA was purified from total RNA (10 μg) using oligo(dT) magnetic beads (Illumina). Following purification, the mRNA was fragmented using divalent cations under elevated temperature, and cleaved RNA fragments were used for first-strand cDNA synthesis using reverse transcriptase (Invitrogen) and random primers. Second-strand cDNA synthesis was then performed using RNaseH and DNA polymerase I (Invitrogen). The resulting cDNA fragments were subjected to end repair and ligation of adapters. The ligated products were loaded on a 2% agarose gel and a gel slice corresponding to ∼250 bp was excised, purified, and enriched by PCR for use in creating the final cDNA library. Following a check of quantity and quality on the Agilent 1000 Chip (Agilent Technologies), the cDNA library was used for cluster generation. Single-end sequencing (72-bp reads) was performed on the Genome Analyzer II following the manufacturer’s protocol (Illumina) at Bioinformatics Core Facility, Texas A&M University.
Bioinformatic Analysis
Illumina GA pipeline 1.3 software was used for image deconvolution and quality value calculations. The raw reads were cleaned by removing adaptor sequences, empty reads, and low-quality sequences. TAIR10 annotations and sequence files for individual annotated genome features (genes, cDNAs, 3′ untranslated regions, 5′ untranslated regions, introns, exons, and intergenic regions) were downloaded from The Arabidopsis Information Resource ftp://ftp.Arabidopsis.org/home/tair/Sequences. CLC Genomics workbench version 4.0 was used to align the obtained Illumina reads with the Arabidopsis reference genome, allowing up to two mismatches. The RNA-Seq analysis tool (ERANGE software) of CLC Genomic Workbench (http://www.clcbio.com) was used to measure gene expression values in RPKM, a normalized measure of exon read density that allows transcript levels to be compared both within and between samples (Mortazavi et al., 2008). These values were used to calculate gene expression levels and to discover novel exons based on the annotated reference genome. Quantile normalization (Bolstad et al., 2003) was performed for all Illumina runs across the four treatments of two biological replicates. Genes with more than 1× median coverage with the RPKM value of more than 1.25 was considered for further analysis. Correlation plots of the RPKM reads for each replicate were used to assess the reproducibility of RNA-Seq experiments. Coverage across transcripts was calculated by counting the number of reads on aligned transcripts generated by TopHat (Trapnell et al., 2009). All correlation analysis and plots were performed in R.
Data analysis and subsequent statistical tests for the two biological replicates were performed using Genespring GX version 12.5 software (Agilent Technologies). Filtered expression data were imported into the GeneSpring Experimental Tool. To identify genes activated by Pv-ALF and ABA, entities were filtered based on a fold change of at least 2 between the conditions. One parameter with four experimental conditions (4MS, 4E, 4A, and 4E4A), each with two biological replicates, was used for the statistical analysis. The inbuilt statistical package of the GeneSpring software automatically applied the one-way analysis of variance with multiple testing corrections by the Benjamini and Hochberg method (Benjamini and Hochberg, 1995) to correct false discovery rate, and asymptotic P value computation was performed to determine the change in expression of a particular gene. Transcripts with absolute fold change values of at least 2 with the corrected P value of < 0.05 (or <0.2 where specified) were included in our analysis as differentially expressed genes. Hierarchical clustering of the transcriptome data was performed using the Ward criterion and the Euclidean method (Soukas et al., 2000).
Identification of putative Pv-ALF binding sites in the promoter regions (1000 bp; both DNA strands) of genes specifically upregulated in 4E and 4E4A was performed using the Motif Sampler bioinformatics tool (http://www.Arabidopsis.org/tools/bulk/motiffinder/index.jsp) at the TAIR10 genome database.
Annotation and Classification
For functional classification of genes, the following procedure was adopted. Domain architectures for the genes were first predicted by profile searches using HMMER software with a database of Pfam profiles in combination with locally compiled profiles not reported in Pfam (Iyer et al., 2008). Annotations from The Arabidopsis Information Resource (TAIR10 database, http://www.Arabidopsis.org/tools/bulk/go/index.jsp) (Berardini et al., 2004), Gene Ontology terms, and orthology with other model eukaryotes were used to manually refine our annotations. Identified transcriptional regulators were divided into chromatin proteins and TFs (Iyer et al., 2008), which can be differentiated based on their domain composition. Chromatin proteins are composed of domains involved in the posttranslational modification of histones and nuclear proteins and recognition of distinct histone or DNA modifications. In addition, chromatin proteins also include multiple types of ATP-dependent proteins or protein complexes that alter chromatin structure either on a local or chromosomal scale. TFs use DNA binding domains to regulate transcription, typically at promoter elements.
Network Analysis
Coexpression network analysis was performed using the Arabidopsis trans-factor and cis-element prediction database ATTED-II (Obayashi et al., 2009, 2011) to identify genes coexpressed with input genes. The extent of gene coexpression network view was calculated based on the Pearson’s correlation coefficient and mutual rank (MR; i.e., calculated as the geometric mean of the correlation rank of gene A and gene B and of gene B to gene A). Three types of edges (lines) are used to draw the networks: bold edges (MR < 5), normal edges (5 ≤ MR > 30), and thin edges (MR ≤ 30). The VirtualPlant software was used to visualize the known molecular interactions between the selected genes. This program uses a more extensive gene interactions database, which is based on known protein–protein interactions, enzymatic pathways, and transcriptional regulation pathways (Gutiérrez et al., 2005; Katari et al., 2010).
Transformation of Arabidopsis Pv-ALF Line with amiRNA Constructs
To generate AIL5 and RLT2 knockdown Arabidopsis plants, gene-specific amiRNAs were engineered by replacing the original miR319a sequence in the pRS300 plasmid with an artificial sequence (21-mer) specific to either AIL5 (5′-TGGAATGATTGTTATACCCAT-3′) or RLT2 (5′-TTGATATTCACGAATAGGCGT-3′), as previously described (Alvarez et al., 2006). The list of primers used for amiRNA cloning is provided in Supplemental Table 4 online. The constructs were cloned into the pCBK05 vector (Riha et al., 2002) behind the constitutive 35S promoter using the SpeI restriction site. The resulting AIL5 and RLT2 amiRNA constructs were introduced into Agrobacterium tumefaciens strain GV3101 by electroporation and supertransformed into the homozygous Pv-ALF line by the floral dip method (Clough and Bent, 1998). Arabidopsis supertransformants were selected on MS agar containing 20 mg/L phosphinotricine (Duchefa). Three knockdown lines against each gene were selected and used to investigate the function of the corresponding gene during the Pv-ALF–mediated phas-GUS expression. All experiments were performed after treating the leaves with estradiol and ABA as described above.
Real-Time Quantitative RT-PCR
Total RNA from leaf tissue was isolated and purified using RNAeasy mini kit (Qiagen) following the manufacturer's instructions. Total RNA from mature seed tissue was isolated as described previously (Oñate-Sánchez and Vicente-Carbajosa, 2008) and purified using an RNAeasy mini elute Qiagen RNA kit. In both cases, DNA was digested using Qiagen RNase-Free DNase set. DNA-free RNA was used to perform first-strand cDNA synthesis with oligo(dT) primers using the SuperScript III One-Step RT-PCR system (Invitrogen). Sequence-specific primers for real-time PCR were designed using the Primer-Blast online tool (Rozen and Skaletsky, 2000). Real-time qRT-PCR was conducted with the Power SYBR Green PCR Master Mix 7500 (Applied Biosystems) using the following conditions: 50°C for 2 min, 95°C for 10 min, followed by 45 cycles of 95°C for 30 s, and 60°C for 1 min, in a total reaction volume of 15 μL. Three biological replicates were performed for each gene. The relative quantities of PCR products were calculated using the equation 2(−ΔΔCT) at a threshold value of 0.02 (Livak and Schmittgen, 2001). Data were normalized to a control housekeeping gene, EF1α, which is stable under treatment conditions. Data were further calculated relative to the control Pv-ALF line or uninduced condition, which were designated as one. Reactions were performed in ABI PRISM 7000 (Applied Biosystems). Student’s t test was used to determine statistical significance between the uninduced and induced treatments, and the Pearson correlation coefficient (r) was used to determine statistical correlation between the RNA-Seq data and qRT-PCR data (see Supplemental Tables 5 and 6 online for the list of primers used for gene expression analysis).
In Vitro Translation and EMSA Assays
The bean Pv-ALF coding region was subcloned into the T7 expression vector pET28a (Novagen) for in vitro protein expression using the TnT-coupled RRL kit (Promega). Expression of Pv-ALF was verified using a separate control reaction performed in the presence of [35S]Met and was visualized following electrophoresis on SDS-PAGE and exposure to phosphor imager screens. The qualitative EMSA assays were conducted as described (Shakirov et al., 2009) with slight modifications. Briefly, each reaction (15 μL total volume) contained 4 μL of RRL-translated Pv-ALF protein (or 4 μL of Pv-ALF–free RRL lysate for negative controls), 0.5 pmol of 32P-labeled PCR product corresponding to the −340 to +25 promoter regions of either phas, RLT2, AIL5, or At1g26680 genes, 3 μL of 5 × DNA binding buffer (100 mM Tris-HCl, pH 8.0, 250 mM NaCl, 50 mM MgCl, 5 mM EDTA, 5 mM DTT, and 25% glycerol), and 1 μL each of nonspecific RNA and single-stranded DNA oligonucleotides, as described (Shakirov et al., 2009). Reactions were incubated at room temperature for 15 min. For competition assays, 100× of cold (unlabeled) competitor DNA was mixed with the radioactively labeled probe prior to the addition of the protein. The complexes were separated on 5% polyacrylamide gel (acrylamide:bisacrylamide 29:1) for 2 h at 150 V in 0.8× TBE (Tris Borate EDTA) at room temperature, dried, and exposed to phosphor imager screens. Screens were scanned by a Pharos FX Plus molecular imager and visualized with Quantity One v.4.6.5 software (Bio-Rad).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL database or the Arabidopsis Genome Initiative database under the following accession numbers: Pv-ALF, U28645; ABI3, At3g24650; RLT1, At1g28420; RLT2, AT5g44180; AIL5, At5g57390; ABI2, At5g57050; HAB1, At1g72770; AHG1, At5g51760; OST1, At4g33950; CPK6, At2g17290; CPK11, A1g35670; PYL7, At4g01026; PYL9, At1g01360; CPK3, At4g23650; SNRK2.3, At5g66880; ERA1, At5g40280; ABI5, At2g36270; Anac042, At2g43000; Anac032, At1g77450; RHL41, At5g05410; DREB2, At5g05410; CHR11, At3g06400; CHR18, At1g4830; CHR38, At3g42670; LDL2, At3g13682; JMJ, At5g46910; PARP1, At5g22470; RAD5, At5g22750; LEA, At3g17520; Cupin, At4g36700; Oleosin4, At5g40420; CRC, At4g28520; and FUS3, At3g26790.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Dynamic Two-Step System Used to Study Potentiation and Activation of the phas Promoter in Transgenic Arabidopsis Leaves.
Supplemental Figure 2. Assessment of Reproducibility between the Two RNA-Seq Biological Replicates.
Supplemental Figure 3. Analysis of Overlapping Genes That Are Downregulated during the Pv-ALF–Mediated phas Activation.
Supplemental Figure 4. Comparison of the Number of Genes Identified in This Study with Genes Detected in Related Studies.
Supplemental Figure 5. Functional Classification of Seed-Specific Arabidopsis Genes Expressed during Activation of the phas Promoter in Leaves.
Supplemental Figure 6. Expression Profiles of Genes Involved in the ABA Signaling Pathway and Transcription Factors Preferentially Expressed during Pv-ALF–Mediated phas Activation.
Supplemental Figure 7. Coexpression Analysis of Transcription Factors Regulated by Pv-ALF Expression.
Supplemental Figure 8. RLT2 and AIL5 Knockdown in a Pv-ALF Background Reduces Expression of Seed-Specific Genes in 4E4A-Treated Leaves.
Supplemental Figure 9. Seeds of RLT2 and AIL5 amiRNA Knockdown Plants Do Not Show Reduction in the Expression of Endogenous Arabidopsis ABI3-Related Genes.
Supplemental Table 1. RNA-Seq Sequence Summary.
Supplemental Table 2. Occurrence of Specific Binding Sites Enriched in the Promoters of ABA-Independent and ABA-Dependent Genes Upregulated upon Pv-ALF Induction.
Supplemental Table 3. Validation of RNA-Seq Data by qRT-PCR.
Supplemental Table 4. List of Primers Used for amiRNA Cloning.
Supplemental Table 5. List of Primers Used for RT-PCR Analysis.
Supplemental Table 6. List of Primers Used for qRT-PCR.
Supplemental Data Set 1. List of ABA-Independent Genes Activated by Pv-ALF Expression.
Supplemental Data Set 2. List of Genes Specifically Upregulated in Leaves upon Pv-ALF Induction and Addition of ABA (4E4A) Only.
Supplemental Data Set 3. List of ABA-Independent Genes Downregulated by Pv-ALF Expression.
Supplemental Data Set 4. Classification and Annotation of Genes Upregulated during Potentiation (4E) and Activation (4E4A) of the phas Promoter.
Supplemental Data Set 5. Comparison of Genes Identified in This Study with genes Detected in Related Studies.
Supplemental Data Set 6. List of Genes Encoding Seed-Related Proteins Expressed in the Pv-ALF/phas-gus Heterologous System during phas Activation.
Supplemental Data Set 7. List of Arabidopsis Seed-Specific Genes Expressed in Leaves of Pv-ALF Plants during Activation of the phas Promoter by Pv-ALF and ABA.
Supplemental Data Set 8. List of ABA Signaling Pathway Genes Differentially Regulated during Pv-ALF–Mediated phas Activation.
Supplemental Data Set 9. List of Transcription Factors That Are Overexpressed in the 4E and 4E4A Treatments.
Supplemental Data Set 10. List of Chromatin Proteins That Are Overexpressed in the 4E and 4E4A Treatments.
Acknowledgments
This work was supported by National Science Foundation Grants MCB 0843692 and MCB 1260947 to T.C.H., by MEYS-Czech Grant ME10038 to M.J., and by intramural funds of the National Institutes of Health-National Library of Medicine to L.M.I. We thank Charles Johnson and Eun-Gyu No (TX AgriLife Bioinformatics Service) for their help with Illumina GAII; Patricia Klien, Rodolfo Aramayo, and Phil Beremand for help with data analysis; Ginger Stuessy for plant culture; James Hardin and David Reed for computational services; and Gene Technologies Laboratory staff for sequencing.
AUTHOR CONTRIBUTIONS
T.C.H., S.S., and S.K. designed the project. S.S. and S.K. carried out research work. E.V.S performed gel shift assay. S.S. and L.M.I. analyzed the RNA-Seq data and interpreted the data. M.J. performed the comparative analysis using TopHAT. All authors contributed to writing the article.
Glossary
- TF
transcription factor
- ABA
abscisic acid
- MS
Murashige and Skoog
- RPKM
reads per kilobase per million mapped reads
- qRT-PCR
quantitative RT-PCR
- amiRNA
artificial miRNA
- RRL
rabbit reticulocyte lysate
- EMSA
electrophoretic mobility shift assay
- MR
mutual rank
References
- Alvarez J.P., Pekker I., Goldshmidt A., Blum E., Amsellem Z., Eshed Y. (2006). Endogenous and synthetic microRNAs stimulate simultaneous, efficient, and localized regulation of multiple targets in diverse species. Plant Cell 18: 1134–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L., Iyer L.M. (2012). The HARE-HTH and associated domains: Novel modules in the coordination of epigenetic DNA and protein modifications. Cell Cycle 11: 119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf A., Esteves H., Curnow S.J., Browning M.J. (2009). A threshold level of TLR9 mRNA predicts cellular responsiveness to CpG-ODN in haematological and non-haematological tumour cell lines. Cell. Immunol. 259: 90–99 [DOI] [PubMed] [Google Scholar]
- Balestrazzi A., Confalonieri M., Macovei A., Donà M., Carbonera D. (2011). Genotoxic stress and DNA repair in plants: Emerging functions and tools for improving crop productivity. Plant Cell Rep. 30: 287–295 [DOI] [PubMed] [Google Scholar]
- Becerra C., Puigdomenech P., Vicient C.M. (2006). Computational and experimental analysis identifies Arabidopsis genes specifically expressed during early seed development. BMC Genomics 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate - A practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B Met. 57: 289–300 [Google Scholar]
- Berardini T.Z., et al. (2004). Functional annotation of the Arabidopsis genome using controlled vocabularies. Plant Physiol. 135: 745–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb A.J., Eiben H.G., Bustos M.M. (1995). PvAlf, an embryo-specific acidic transcriptional activator enhances gene expression from phaseolin and phytohemagglutinin promoters. Plant J. 8: 331–343 [DOI] [PubMed] [Google Scholar]
- Bolstad B.M., Irizarry R.A., Astrand M., Speed T.P. (2003). A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185–193. [DOI] [PubMed] [Google Scholar]
- Boutilier, K., Offringa, R., Sharma, V.K., Kieft, H., Ouellet, T., Zhang, L., Hattori, J., Liu, C.M., van Lammeren, A.A., Miki, B.L., Custers, J.B., and van Lookeren Campagne, M.M. (2002). Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14: 1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady S.M., Sarkar S.F., Bonetta D., McCourt P. (2003). The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J. 34: 67–75 [DOI] [PubMed] [Google Scholar]
- Braybrook S.A., Harada J.J. (2008). LECs go crazy in embryo development. Trends Plant Sci. 13: 624–630 [DOI] [PubMed] [Google Scholar]
- Brocard-Gifford I.M., Lynch T.J., Finkelstein R.R. (2003). Regulatory networks in seeds integrating developmental, abscisic acid, sugar, and light signaling. Plant Physiol. 131: 78–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carranco R., Chandrasekharan M.B., Townsend J.C., Hall T.C. (2004). Interaction of PvALF and VP1 B3 domains with the β-phaseolin promoter. Plant Mol. Biol. 55: 221–237 [DOI] [PubMed] [Google Scholar]
- Chandrasekharan M.B., Bishop K.J., Hall T.C. (2003a). Module-specific regulation of the β-phaseolin promoter during embryogenesis. Plant J. 33: 853–866 [DOI] [PubMed] [Google Scholar]
- Chandrasekharan M.B., Li G., Bishop K.J., Hall T.C. (2003b). S phase progression is required for transcriptional activation of the β-phaseolin promoter. J. Biol. Chem. 278: 45397–45405 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Corona D.F., Tamkun J.W. (2004). Multiple roles for ISWI in transcription, chromosome organization and DNA replication. Biochim. Biophys. Acta 1677: 113–119 [DOI] [PubMed] [Google Scholar]
- Cox P.M., Goding C.R. (1991). Transcription and cancer. Br. J. Cancer 63: 651–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe A.J., Abenes M., Plant A., Moloney M.M. (2000). The seed-specific transactivator, ABI3, induces oleosin gene expression. Plant Sci. 151: 171–181 [DOI] [PubMed] [Google Scholar]
- Cutler S.R., Rodriguez P.L., Finkelstein R.R., Abrams S.R. (2010). Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant Biol. 61: 651–679 [DOI] [PubMed] [Google Scholar]
- Ezcurra I., Wycliffe P., Nehlin L., Ellerström M., Rask L. (2000). Transactivation of the Brassica napus napin promoter by ABI3 requires interaction of the conserved B2 and B3 domains of ABI3 with different cis-elements: B2 mediates activation through an ABRE, whereas B3 interacts with an RY/G-box. Plant J. 24: 57–66 [DOI] [PubMed] [Google Scholar]
- Finkelstein R.R., Gampala S.S.L., Rock C.D. (2002). Abscisic acid signaling in seeds and seedlings. Plant Cell 14 (Suppl): S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan E.J., Peacock W.J., Dennis E.S. (1996). Reduced DNA methylation in Arabidopsis thaliana results in abnormal plant development. Proc. Natl. Acad. Sci. USA 93: 8449–8454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M.J., Lydiate D.J., Li X., Lui H., Gjetvaj B., Hegedus D.D., Rozwadowski K. (2009). Repression of seed maturation genes by a trihelix transcriptional repressor in Arabidopsis seedlings. Plant Cell 21: 54–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal G., Yuan J., Chen J. (2011). The HARP domain dictates the annealing helicase activity of HARP/SMARCAL1. EMBO Rep. 12: 574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelon M., Gendrot G., Vezon D., Pelletier G. (2003). The Arabidopsis MEI1 gene encodes a protein with five BRCT domains that is involved in meiosis-specific DNA repair events independent of SPO11-induced DSBs. Plant J. 35: 465–475 Erratum. Plant J. 37: 460. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R.A., Shasha D.E., Coruzzi G.M. (2005). Systems biology for the virtual plant. Plant Physiol. 138: 550–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer L.M., Anantharaman V., Wolf M.Y., Aravind L. (2008). Comparative genomics of transcription factors and chromatin proteins in parasitic protists and other eukaryotes. Int. J. Parasitol. 38: 1–31 [DOI] [PubMed] [Google Scholar]
- Jefferson R.A., Kavanagh T.A., Bevan M.W. (1987). GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katari M.S., Nowicki S.D., Aceituno F.F., Nero D., Kelfer J., Thompson L.P., Cabello J.M., Davidson R.S., Goldberg A.P., Shasha D.E., Coruzzi G.M., Gutiérrez R.A. (2010). VirtualPlant: A software platform to support systems biology research. Plant Physiol. 152: 500–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracikova M., Akiri G., George A., Sachidanandam R., Aaronson S.A. (2013). A threshold mechanism mediates p53 cell fate decision between growth arrest and apoptosis. Cell Death Differ. 20: 576–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le B.H., et al. (2010). Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proc. Natl. Acad. Sci. USA 107: 8063–8070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Chandler S.P., Wolffe A.P., Hall T.C. (1998). Architectural specificity in chromatin structure at the TATA box in vivo: Nucleosome displacement upon β-phaseolin gene activation. Proc. Natl. Acad. Sci. USA 95: 4772–4777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Bishop K.J., Chandrasekharan M.B., Hall T.C. (1999). β-Phaseolin gene activation is a two-step process: PvALF-facilitated chromatin modification followed by abscisic acid-mediated gene activation. Proc. Natl. Acad. Sci. USA 96: 7104–7109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Hall T.C. (1999). Footprinting in vivo reveals changing profiles of multiple factor interactions with the β-phaseolin promoter during embryogenesis. Plant J. 18: 633–641 [DOI] [PubMed] [Google Scholar]
- Li G., Zhang J., Li J., Yang Z., Huang H., Xu L. (2012). Imitation Switch chromatin remodeling factors and their interacting RINGLET proteins act together in controlling the plant vegetative phase in Arabidopsis. Plant J. 72: 261–270 [DOI] [PubMed] [Google Scholar]
- Li H.C., Chuang K., Henderson J.T., Rider S.D., Jr, Bai Y., Zhang H., Fountain M., Gerber J., Ogas J. (2005). PICKLE acts during germination to repress expression of embryonic traits. Plant J. 44: 1010–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Makarevich G., Leroy O., Akinci U., Schubert D., Clarenz O., Goodrich J., Grossniklaus U., Köhler C. (2006). Different Polycomb group complexes regulate common target genes in Arabidopsis. EMBO Rep. 7: 947–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty D.R., Chory J. (2000). Conservation and innovation in plant signaling pathways. Cell 103: 201–209 [DOI] [PubMed] [Google Scholar]
- Mönke G., Seifert M., Keilwagen J., Mohr M., Grosse I., Hähnel U., Junker A., Weisshaar B., Conrad U., Bäumlein H., Altschmied L. (2012). Toward the identification and regulation of the Arabidopsis thaliana ABI3 regulon. Nucleic Acids Res. 40: 8240–8254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcillo F., Gallard A., Pillot M., Jouannic S., Aberlenc-Bertossi F., Collin M., Verdeil J.L., Tregear J.W. (2007). EgAP2-1, an AINTEGUMENTA-like (AIL) gene expressed in meristematic and proliferating tissues of embryos in oil palm. Planta 226: 1353–1362 [DOI] [PubMed] [Google Scholar]
- Mortazavi A., Williams B.A., McCue K., Schaeffer L., Wold B. (2008). Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5: 621–628 [DOI] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497. [Google Scholar]
- Ng, D.W., Chandrasekharan, M.B., and Hall, T.C. (2006). Ordered histone modifications are associated with transcriptional poising and activation of the phaseolin promoter. Plant Cell 18: 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, W.K., and Hall, T.C. (2008). PvALF and FUS3 activate expression from the phaseolin promoter by different mechanisms. Plant Mol. Biol. 66: 233–244 [DOI] [PubMed] [Google Scholar]
- Nole-Wilson S., Tranby T.L., Krizek B.A. (2005). AINTEGUMENTA-like (AIL) genes are expressed in young tissues and may specify meristematic or division-competent states. Plant Mol. Biol. 57: 613–628 [DOI] [PubMed] [Google Scholar]
- Obayashi T., Hayashi S., Saeki M., Ohta H., Kinoshita K. (2009). ATTED-II provides coexpressed gene networks for Arabidopsis. Nucleic Acids Res. 37 (Database issue): D987–D991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi T., Nishida K., Kasahara K., Kinoshita K. (2011). ATTED-II updates: Condition-specific gene coexpression to extend coexpression analyses and applications to a broad range of flowering plants. Plant Cell Physiol. 52: 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oñate-Sánchez L., Vicente-Carbajosa J. (2008). DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res. Notes 1: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski S., Schwab R., Weigel D. (2008). Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J. 53: 674–690 [DOI] [PubMed] [Google Scholar]
- Parcy F., Valon C., Raynal M., Gaubier-Comella P., Delseny M., Giraudat J. (1994). Regulation of gene expression programs during Arabidopsis seed development: Roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 6: 1567–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riha K., Watson J.M., Parkey J., Shippen D.E. (2002). Telomere length deregulation and enhanced sensitivity to genotoxic stress in Arabidopsis mutants deficient in Ku70. EMBO J. 21: 2819–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S., Skaletsky H. (2000). Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132: 365–386 [DOI] [PubMed] [Google Scholar]
- Schmidt M.A., Barbazuk W.B., Sandford M., May G., Song Z., Zhou W., Nikolau B.J., Herman E.M. (2011). Silencing of soybean seed storage proteins results in a rebalanced protein composition preserving seed protein content without major collateral changes in the metabolome and transcriptome. Plant Physiol. 156: 330–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab R., Ossowski S., Riester M., Warthmann N., Weigel D. (2006). Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18: 1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakirov E.V., Song X., Joseph J.A., Shippen D.E. (2009). POT1 proteins in green algae and land plants: DNA-binding properties and evidence of co-evolution with telomeric DNA. Nucleic Acids Res. 37: 7455–7467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukas A., Cohen P., Socci N.D., Friedman J.M. (2000). Leptin-specific patterns of gene expression in white adipose tissue. Genes Dev. 14: 963–980 [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Kao C.Y., Cocciolone S., McCarty D.R. (2001). Maize VP1 complements Arabidopsis abi3 and confers a novel ABA/auxin interaction in roots. Plant J. 28: 409–418 [DOI] [PubMed] [Google Scholar]
- Suzuki M., Ketterling M.G., Li Q.B., McCarty D.R. (2003). Viviparous1 alters global gene expression patterns through regulation of abscisic acid signaling. Plant Physiol. 132: 1664–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Ketterling M.G., McCarty D.R. (2005). Quantitative statistical analysis of cis-regulatory sequences in ABA/VP1- and CBF/DREB1-regulated genes of Arabidopsis. Plant Physiol. 139: 437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., McCarty D.R. (2008). Functional symmetry of the B3 network controlling seed development. Curr. Opin. Plant Biol. 11: 548–553 [DOI] [PubMed] [Google Scholar]
- Tang X.R., Hou A.F., Babu M., Nguyen V., Hurtado L., Lu Q., Reyes J.C., Wang A.M., Keller W.A., Harada J.J., Tsang E.W.T., Cui Y.H. (2008). The Arabidopsis BRAHMA chromatin-remodeling ATPase is involved in repression of seed maturation genes in leaves. Plant Physiol. 147: 1143–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To A., Valon C., Savino G., Guilleminot J., Devic M., Giraudat J., Parcy F. (2006). A network of local and redundant gene regulation governs Arabidopsis seed maturation. Plant Cell 18: 1642–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S.L. (2009). TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuwamoto R., Yokoi S., Takahata Y. (2010). Arabidopsis EMBRYOMAKER encoding an AP2 domain transcription factor plays a key role in developmental change from vegetative to embryonic phase. Plant Mol. Biol. 73: 481–492 [DOI] [PubMed] [Google Scholar]
- Valdés A.E., Overnäs E., Johansson H., Rada-Iglesias A., Engström P. (2012). The homeodomain-leucine zipper (HD-Zip) class I transcription factors ATHB7 and ATHB12 modulate abscisic acid signalling by regulating protein phosphatase 2C and abscisic acid receptor gene activities. Plant Mol. Biol. 80: 405–418 [DOI] [PubMed] [Google Scholar]
- Van Der Geest A., Frisch D.A., Kemp J.D., Hall T.C. (1995). Cell ablation reveals that expression from the Phaseolin promoter is confined to embryogenesis and microsporogenesis. Plant Physiol. 109: 1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernimmen D., Lynch M.D., De Gobbi M., Garrick D., Sharpe J.A., Sloane-Stanley J.A., Smith A.J.H., Higgs D.R. (2011). Polycomb eviction as a new distant enhancer function. Genes Dev. 25: 1583–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Gerstein M., Snyder M. (2009). RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 10: 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Kagaya Y., Usui H., Hobo T., Takeda S., Hattori T. (2010). Diverse roles and mechanisms of gene regulation by the Arabidopsis seed maturation master regulator FUS3 revealed by microarray analysis. Plant Cell Physiol. 51: 2031–2046 [DOI] [PubMed] [Google Scholar]
- Zhang K., Xia X., Zhang Y., Gan S.S. (2012). An ABA-regulated and Golgi-localized protein phosphatase controls water loss during leaf senescence in Arabidopsis. Plant J. 69: 667–678 [DOI] [PubMed] [Google Scholar]
- Zhou X.E., Wang Y., Reuter M., Mücke M., Krüger D.H., Meehan E.J., Chen L. (2004). Crystal structure of type IIE restriction endonuclease EcoRII reveals an autoinhibition mechanism by a novel effector-binding fold. J. Mol. Biol. 335: 307–319 [DOI] [PubMed] [Google Scholar]