A calcium signaling module composed of a calcium sensor and its interacting protein kinase participate in programmed cell death in plant immune responses, likely linking Ca2+ and reactive oxygen species signaling through phosphorylation events.
Abstract
Ca2+ signaling is an early and necessary event in plant immunity. The tomato (Solanum lycopersicum) kinase Pto triggers localized programmed cell death (PCD) upon recognition of Pseudomonas syringae effectors AvrPto or AvrPtoB. In a virus-induced gene silencing screen in Nicotiana benthamiana, we independently identified two components of a Ca2+-signaling system, Cbl10 (for calcineurin B-like protein) and Cipk6 (for calcineurin B-like interacting protein kinase), as their silencing inhibited Pto/AvrPto-elicited PCD. N. benthamiana Cbl10 and Cipk6 are also required for PCD triggered by other plant resistance genes and virus, oomycete, and nematode effectors and for host susceptibility to two P. syringae pathogens. Tomato Cipk6 interacts with Cbl10 and its in vitro kinase activity is enhanced in the presence of Cbl10 and Ca2+, suggesting that tomato Cbl10 and Cipk6 constitute a Ca2+-regulated signaling module. Overexpression of tomato Cipk6 in N. benthamiana leaves causes accumulation of reactive oxygen species (ROS), which requires the respiratory burst homolog RbohB. Tomato Cbl10 and Cipk6 interact with RbohB at the plasma membrane. Finally, Cbl10 and Cipk6 contribute to ROS generated during effector-triggered immunity in the interaction of P. syringae pv tomato DC3000 and N. benthamiana. We identify a role for the Cbl/Cipk signaling module in PCD, establishing a mechanistic link between Ca2+ and ROS signaling in plant immunity.
INTRODUCTION
Plants respond to pathogen infection by activating a two-part immune response, including pattern-triggered immunity (PTI) and effector-triggered immunity (ETI; Jones and Dangl, 2006). PTI results from the recognition of microbe- or pathogen-associated molecular patterns (PAMPs), whereas ETI occurs when cytoplasmic resistance (R) proteins detect specific pathogen effectors. ETI is more robust than PTI and is often accompanied by a localized programmed cell death (PCD) event known as the hypersensitive response (HR), which is believed to limit pathogen establishment and spread by killing both the pathogen and host cell (Greenberg, 1997).
Both PTI and ETI share early signaling events, including changes in protein phosphorylation status, cytosolic Ca2+ elevation, production of reactive oxygen species (ROS) in the oxidative burst, and activation mitogen-activated protein kinase (MAPK) cascades that lead to common downstream defense responses differing mainly in their kinetics and magnitude (Boller and Felix, 2009; Tsuda and Katagiri, 2010). Protein phosphorylation and Ca2+ and ROS signaling were described as sequential events (Blume et al., 2000; Grant et al., 2000; Lecourieux et al., 2002; Garcia-Brugger et al., 2006) that influence each other differently depending on the pathosystem. For instance, in Nicotiana benthamiana plants treated with flagellin, Ca2+ influx was required for the ROS burst, which negatively regulated Ca2+ influx (Segonzac et al., 2011), whereas in the cryptogein/tobacco cells system, ROS burst stimulated extracellular Ca2+ influx to the cytosol (Lecourieux et al., 2002).
The main enzymatic source for the oxidative bursts in Arabidopsis thaliana, the membrane-bound NADPH oxidases (also known as respiratory burst homolog [RBOH] proteins), are synergistically regulated by Ca2+ and phosphorylation (Torres, 2010). RBOHs possess an N-terminal cytoplasmic regulatory domain, which contains Ca2+ binding EF hands and phosphorylation sites by Ca2+-dependent protein kinases (CDPKs or CPKs) that are necessary for RBOH function (Kobayashi et al., 2007; Ogasawara et al., 2008; Dubiella et al., 2013). Thus, a complex spatio-temporal Ca2+/ROS crosstalk exists in early immune signaling. To date, it is still unclear what are the signaling components (proteins and second messengers) that link initial PAMP or effector recognition with Ca2+, ROS, and MAPK signaling and how these signaling events influence each other. A current challenge is to understand how the cytoplasmic Ca2+ increase is interpreted and turned into a functional immune response (Segonzac et al., 2011).
The bacterial pathogen Pseudomonas syringae pv tomato (Pst) causes bacterial speck disease in tomato (Solanum lycopersicum), which affects tomato fruit quality and yield resulting in important economic losses (Pedley and Martin, 2003). Pto, a tomato Ser/Thr kinase, recognizes Pst effectors AvrPto and AvrPtoB and together with Prf, a protein containing a nucleotide binding site and a region of leucine rich repeats elicit an ETI resistance response against Pst, which includes activation of a complex array of signaling cascades and involves PCD (Mucyn et al., 2006; Oh and Martin, 2011). AvrPto and AvrPtoB are delivered into the host cell through a type III secretion system where they suppress PTI and promote plant disease susceptibility (Anderson et al., 2006; Xiao et al., 2007; Shan et al., 2008; Hann et al., 2010). Several components (∼25) of the Pto-mediated signaling pathway have been identified in tomato, tobacco (Nicotiana tabacum), and N. benthamiana using different loss-of-function approaches (Oh and Martin, 2011). Still, relevant aspects of early signaling after Pto/Prf activation remain to be characterized, like the participation of Ca2+ and ROS as second messengers.
A virus-induced gene silencing (VIGS) screen using randomly selected cDNA fragments from N. benthamiana identified several genes that caused an alteration in Pto/AvrPto-induced PCD upon silencing. Among the candidates identified, MAPKKKα, a positive regulator of PCD, was further characterized and found to regulate both ETI and disease-associated PCD (del Pozo et al., 2004). Here, we report the identification and characterization of two components of a Ca2+ signaling module also identified in this VIGS screen: a calcium sensor (calcineurin B-like protein [CBL]) and its putative target, a calcineurin B-like interacting protein kinase (CIPK). In the general context of R protein/effector signaling, few proteins that sense and relay Ca2+ signals have been described, among them the CDPKs/CPKs, which have been shown to play a role in defense activation in different pathosystems (Boudsocq et al., 2010; Kobayashi et al., 2012; Dubiella et al., 2013). Ca2+-related downstream signaling events include salicylic acid synthesis, nitric oxide generation, and transcriptional gene activation (Ma et al., 2008; Du et al., 2009; Boudsocq et al., 2010; Ma et al., 2012). In Pto-mediated ETI, APR134, a tomato Calmodulin-like protein, has been the only Ca2+-signaling component characterized in this pathway (Chiasson et al., 2005).
Calcium plays an important role as a universal second messenger in adaptation of cells to environmental changes. After pathogen perception, apoplastic Ca2+ enters the cytoplasm presumably via cyclic nucleotide-gated channels and Glu receptor-like channels (Ma et al., 2009; Kwaaitaal et al., 2011; Tapken et al., 2013). Specificity is achieved by the stimulus-specific spatial and temporal Ca2+ influx pattern (calcium signature) and by the local presence of Ca2+ binding proteins or sensors (CBLs, CaMs, Calmodulin-like proteins, and CDPKs), which bind Ca2+ with high affinity through EF hands, a helix-loop-helix structural motif (Harper et al., 2004). Ca2+ binding changes EF-hand protein conformation, resulting in activity changes in the sensor itself or modulating the function of downstream target proteins (sensor relays), transforming Ca2+ changes into cellular responses.
CBLs bind Ca2+ through four EF hands and relay the signal by interacting specifically with a C-terminal regulatory domain (NAF or FISL) of CIPKs, Ser/Thr protein kinases belonging to Snf1-RELATED KINASE3 family (Suc nonfermenting 1-related kinases, group 3; SnRK3) also known as PKS (protein kinase related to SOS2) regulating their kinase activity (Ishitani et al., 2000; Albrecht et al., 2001; Gong et al., 2002; Hrabak et al., 2003). Thus, it is assumed that CBL/CIPKs modules relay Ca2+ signals into target protein(s) phosphorylation. To date, few CIPK phosphorylation targets have been described and most of them are membrane proteins (Quintero et al., 2002; Li et al., 2006; Ho et al., 2009). Database analysis identified 10 CBLs and at least 26 CIPKs in Arabidopsis (www.arabidopsis.org). Because a specific CBL can interact with several CIPKs and a specific CIPK with several CBLs, CBL/CIPK module combinatorial possibilities are vast, thus conferring an extraordinary diversity and versatility of Ca2+ responsiveness to this signaling system, and their roles are only beginning to emerge. Most CBLs and CIPKs described so far are involved in different aspects of abiotic stress signaling and plant nutrient acquisition (Quintero et al., 2002; Kim et al., 2007; Quan et al., 2007; Ho et al., 2009; Weinl and Kudla, 2009). Their involvement in biotic stress signaling has been speculated about (Kudla et al., 2010), but their role in defense responses remains unclear.
We report the identification and characterization of two tomato and N. benthamiana participants, Cbl10 and Cipk6, in PCD events associated with ETI mediated by Pto/AvrPto and by different R proteins and effectors from oomycetes, fungus, and virus, thus constituting a convergent signaling node in ETI. We demonstrate that tomato Cbl10 and Cipk6 interact in planta and that Cipk6 kinase activity is positively regulated in the presence of Ca2+ and Cbl10, indicating that Cbl10/Cipk6 constitutes a functional signaling module regulated by Ca2+. In an attempt to explore Cipk6 downstream signaling connections, we found that Cipk6 overexpression led to ROS generation, which was dependent on Cipk6 kinase activity and required respiratory burst homolog NbRbohB. Importantly, both Cbl10 and Cipk6 interact with RbohB and the complexes localize at the plasma membrane. Finally, we demonstrate that N. benthamiana Cbl10 and Cipk6 contribute to the ROS peak generated during ETI in N. benthamiana in response to Pst infection. Altogether, we present Cbl10/Cipk6 as participants in ETI PCD, likely integrating Ca2+ and ROS signaling events via phosphorylation.
RESULTS
N. benthamiana Cbl10 and Cipk6 Play a Role in Pto-Mediated PCD
In a VIGS screen of randomly selected gene fragments from N. benthamiana directed at identifying components of Pto-mediated PCD, several genes were identified (del Pozo et al., 2004). N. benthamiana plants silenced with Potato virus X (PVX)–based constructs cNbME25F4 or cNbME30H4 showed compromised Pto-mediated PCD in all of the events examined (for cNbME25F4, four out of four events showed complete PCD inhibition, whereas for cNbME30H4, two out of four events showed partial and two no PCD).
A BLAST search with the cNbME25F4 and cNbME30H4 cDNAs in the tomato EST databases (www.sgn.cornell.edu and www.tigr.org) identified two tomato contigs representing partial sequences for each clone (SGN-U229233 and SGN-E250261 for cNbME25F4; SGN-U566705 and SGN-E339676 for cNbME30H4) sharing 95.6 and 92.3% identity, respectively, at the protein level with the N. benthamiana identified clones. A potato (Solanum tuberosum) contig, SGN-U271168, spanning the complete coding sequence for St-Cipk6 was later identified (see Supplemental Figure 1 online). BLAST performed with both sequences in the Arabidopsis database (www.tair.org) established that cNbME25F4 was a partial clone of the N. benthamiana ortholog for At-CBL10 (also known as SOS3-LIKE Ca2+ BINDING PROTEIN8 [SCaBP8]), a CBL protein (Kim et al., 2007; Quan et al., 2007), and that cNbME30H4 corresponded to a partial N. benthamiana ortholog clone for At-CIPK6, a CIPK, a target for CBLs (Shi et al., 1999; Tripathi et al., 2009).
Tomato cDNA clones having the complete open reading frame were obtained, and we refer to them as Sl-Cbl10 and Sl-Cipk6 (see Supplemental Figure 1 online). Because both N. benthamiana and tomato displayed a high degree identity at the nucleotide level (91.2 and 92.4%, with several stretches of more than 21 to 24 nucleotides with 100% identity), either Sl or Nb constructs were used for effective silencing in both species, and in silencing experiments we refer to them generally as Cbl10 and Cipk6.
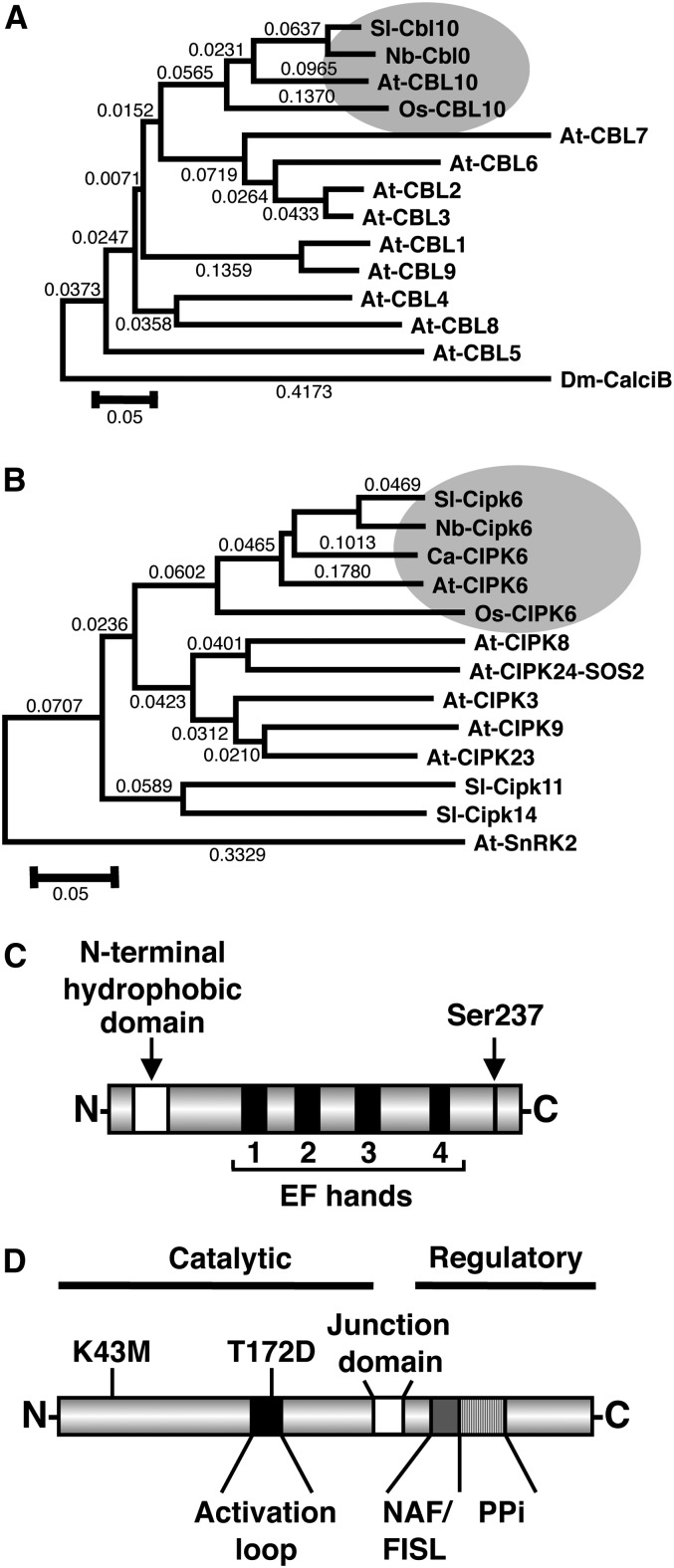
Protein sequences were derived and aligned for all 10 CBLs present in Arabidopsis, along with Sl-Cbl10, Nb-Cbl10, and a putative rice (Oryza sativa) Os-CBL10 protein (GenBank DQ201203), and the alignment used to build a phylogenetic tree (Figure 1A; see Supplemental Data Set 1 and Supplemental Figure 2 online). As expected, cNbME25F4 appears to be the ortholog of At-CBL10 (Figure 1A). Sl-Cbl10 has four EF hands and an extended N-terminal hydrophobic domain characteristic of At-CBL10 necessary for membrane association, which is also present in the putative rice ortholog Os-CBL10 but absent in other CBLs (Quan et al., 2007). Ser-241 residue in At-CBL10, phosphorylated by SOS2, is also conserved in Sl-Cbl10 (Ser-237) (Figure 1C; see Supplemental Figure 2 online) (Lin et al., 2009).
Figure 1.
Tomato and N. benthamiana Cbl10 and Cipk6 Phylogenetic Analysis and Protein Structure.
(A) and (B) Phylogenetic trees of CBL amino acid sequences from tomato, Sl-Cbl10; N. benthamiana, Nb-Cbl10; rice, Os-CBL10; Arabidopsis CBLs; and Calcineurin B protein from Drosophila melanogaster used as the outgroup (A) as well as CIPK6 amino acid sequences from tomato, Sl-Cipk6; N. benthamiana, Nb-Cipk6; chickpea, Ca-CIPK6; Arabidopsis, At-CIPK6; rice, Os-CIPK6; Arabidopsis CIPKs, including At-CIPK8, At-CIPK24 (SOS2), At-CIPK3, At-CIPK9, and At-CIPK23; tomato Sl-Cipk11 and Sl-Cipk14 (B). At-SnRK2 was used as the outgroup. Phylogenetic trees were done using the neighbor-joining method (ClustalW program; Thompson et al., 1994) and MEGA 4 software. The scale represents amino acid substitutions, and numbers on the tree represent bootstrap scores.
(C) Schematic structure of tomato Cbl10 protein representing conserved Ca2+ binding EF-hands, N-terminal hydrophobic domain, and putative phosphorylatable Ser-237 residue.
(D) Schematic structure of tomato Cipk6 protein representing N-terminal catalytic, junction, and C-terminal regulatory domains, protein phosphatase interaction, NAF/FISL, and activation loop domains. Residues mutagenized for Cipk6 characterization Lys-43 to Met (K43M) and Thr-172 to Asp (T172D) are marked.
The closest sequence for cNbME30H4 in the Arabidopsis database was At-CIPK6. Protein sequences of putative At-CIPK6 orthologs from tomato, chickpea (Cicer arietinum; GenBank accession number EU492906), and rice (Q6Z9F4) were aligned, together with a representation of other CIPKs with assigned functions (see Supplemental Data Set 2), and a phylogenetic tree was developed (Figure 1B; see Supplemental Figure 3 online). Indeed, cNbME30H4 and its putative tomato ortholog were located in the same clade as At-CIPK6, Ca-CIPK6, and Os-CIPK6 and therefore were designated as Nb-Cipk6 and Sl-Cipk6, respectively. Sl-Cipk6 has a typical SnRK3 structure, displaying an N-terminal kinase catalytic domain and a C-terminal regulatory domain separated by a junction region (Hrabak et al., 2003). The regulatory domain contains the NAF/FISL region, which binds CBLs and physically overlaps with a protein phosphatase interaction binding domain (Liu et al., 2000; Albrecht et al., 2001; Ohta et al., 2003). In the catalytic domain, the conserved ATP binding Lys (Lys-43), and within the activation loop, a putative trans-phosphorylatable Thr residue (Thr-172) was observed (Figure 1D).
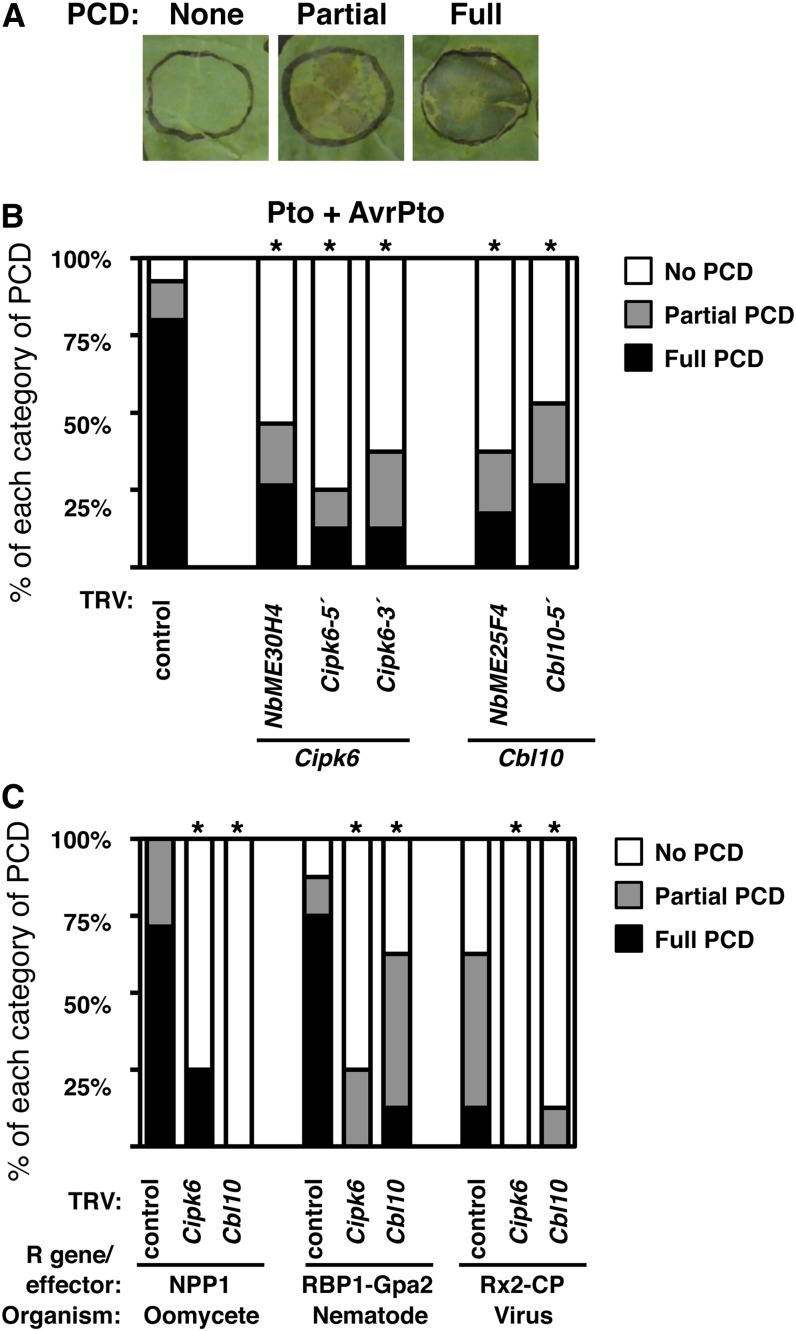
To confirm and further characterize the phenotype associated with the loss of function of Cbl10 and Cipk6 in plant immunity, we cloned cDNA fragments from tomato (SlME25F4 and SlME30H4, respectively) corresponding to those originally identified as N. benthamiana VIGS clones into a tobacco rattle virus vector (TRV; Liu et al., 2002), which causes a more uniform and persistent silencing phenotype than PVX. In order to substantiate the specificity of their silencing phenotypes, we generated additional nonoverlapping silencing constructs in TRV for Cbl10 (Sl-Cbl10-5′) and Cipk6 (Sl-Cipk6-5′and Sl-Cipk6-3′) (see Supplemental Figure 1 online) and used them for VIGS. PCD was monitored visually and classified according to its extent (Figure 2A). Similar loss of PCD phenotypes were obtained using either the originally identified clones or each of the alternative silencing constructs for either N. benthamiana Cbl10 or Cipk6 gene, indicating that the phenotype is due to specific silencing of Cipk6 or Cbl10 (Figure 2B). Full Pto/AvrPto-mediated PCD developed in TRV control plants in 80% of the cases, whereas it ranged between 15 and 25% in TRV-Sl-Cipk6-5′, TRV-Sl-Cipk6-3′, or TRV-Nb-Cipk6–silenced N. benthamiana leaves and between 15 and 25% in TRV-Sl-Cbl10-5′ or TRV-Nb-Cbl10–silenced N. benthamiana leaves. The degree of silencing was assessed by RT-PCR, which showed that N. benthamiana Cbl10 and Cipk6 transcript abundances were reduced ∼90% compared with TRV control plants (see Supplemental Table 1 online).
Figure 2.
Cbl10 or Cipk6 Silencing in N. benthamiana Compromises PCD Mediated by Different R Gene/Effector Gene Interactions.
(A) PCD was monitored visually and classified according to its extent: full PCD, >80%; partial PCD, 20 to 80%; no PCD, <10%.
(B) PCD mediated by Pto is greatly reduced in Cipk6- or Cbl10-silenced N. benthamiana leaves using the originally identified fragments in the screen or additional silencing constructions for Cipk6 (TRV-Nb-ME30H4, TRV-Sl-Cipk6-5′, and TRV-Sl-Cipk6-3′) and for Cbl10 (TRV-Nb-ME25F4 and TRV-Sl-Cbl10-5′) compared with TRV control leaves agroinfiltrated with Pto or AvrPto. For each silencing construction, we used five plants with two leaves infiltrated per plant. The experiment was repeated six times with similar results.
(C) Suppression of PCD elicited by other R genes/elicitors is also observed in Cbl10- or Cipk6-silenced N. benthamiana leaves compared with TRV control leaves after agroinfiltration of NPP1, RBP-1/Gpa2, and Rx2/CP. Four plants per line were tested, and PCD was observed 5 d later for NPP1, 7 d later for RBP-1/Gpa2, and 6 d later for Rx2/CP. TRV-Nb-ME25F4 and TRV-Nb-ME30H4 were used for silencing. Asterisks indicate significant reduction in PCD (Student’s t test; P < 0.05).
[See online article for color version of this figure.]
Silencing specificity was determined by checking possible changes in transcript abundances of the closest genes found in the databases (see Supplemental Table 1 online). The tomato genome sequence was then released and BLAST analysis identified a sequence, SGN-U583600, which shared three stretches of 23, 26, and 25 nucleotides 100% identical to Cipk6 located toward the 3’ end of the putative gene sequence. Two of them overlapped with the TRV-Sl-Cipk6-3′ silencing construct, whereas none overlapped with TRV-Sl-Cipk6-5′ or TRV-Nb-Cipk6 silencing constructs. RT-PCR performed with specific oligonucleotides for SGN-U583600 and cDNA from infected or noninfected tomato and N. benthamiana leaves did not amplify any band, suggesting that this putative gene is not transcribed in leaves (see Supplemental Table 1 online). From these experiments, we conclude that Cbl10 and Cipk6 participate in Pto/AvrPto-triggered PCD.
N. benthamiana Cbl10 and Cipk6 Contribute to PCD Triggered by Different Elicitors
Next, we asked whether Cbl10 and Cipk6 expression was also required in other R/Avr- or general elicitor-triggered PCD. For that purpose, we used Agrobacterium tumefaciens–mediated transient transformation of leaves (agroinfiltration) to express NPP-1 (for necrosis-inducing Phytophthora protein1; Fellbrich et al., 2002), potato Gpa2/RBP-1 from potato cyst nematode (Sacco et al., 2009), and potato Rx2/coat protein (CP) from PVX (Bendahmane et al., 2000) in N. benthamiana leaves silenced for Cbl10, Cipk6, and TRV control-infected leaves. PCD triggered by NPP-1, Gpa2/RBP-1, and Rx2/CP was substantially suppressed in TRV-Cipk6– and TRV-Cbl10–silenced plants compared with TRV control plants (Figure 2C). These results demonstrate that Cbl10 and Cipk6 are required by different R genes/effectors in N. benthamiana, thereby constituting a convergent signaling node in plant immunity shared by different pathogen-responsive pathways.
Tomato Cbl10 and Cipk6 Transcript Abundances Increase upon Pst Infection
Cipk6 and Cbl10 were both identified through VIGS in a leaf PCD assay in N. benthamiana. Therefore, we wished to confirm their expression in tomato leaves and other tissues. Tomato Cbl10 and Cipk6 were moderately expressed in all the tissues analyzed with the exception of stems, where both transcripts were in low abundance, especially Cipk6 (see Supplemental Figure 4A online). Cbl10 and Cipk6 transcripts were also detectable in roots. Cbl10 and Cipk6 expression in roots and stems contrasts with their low abundance reported in roots for Arabidopsis SCABP8/CBL10 and chickpea CIPK6 and their high level of expression detected in stems for both Arabidopsis orthologs CIPK6 and CBL10 (Quan et al., 2007; Kim et al., 2007; Tripathi et al., 2009). These differences might underlie divergent functions for CBLs and CIPKs orthologs in different plant species.
We next examined whether transcript abundance of tomato Cbl10 and Cipk6 changed during ETI and susceptible responses of tomato to Pst DC3000 (which expresses AvrPto and AvrPtoB) (see Supplemental Figures 4B and 4C online). Both responses in tomato are accompanied by PCD, associated with the HR or with bacterial speck lesion formation, respectively, and are separated in the timing of their appearance (∼16 to 18 h for HR and 3 to 4 d for specks). For ETI, resistant tomato plants expressing Pto (RG-PtoR) were infiltrated with a high titer of Pst DC3000 (1 × 108 colony-forming units [cfu]/mL), which generated visible PCD 16 h later, and Cipk6 and Cbl10 mRNA accumulation was determined 0, 4, 8, and 16 h after. Cipk6 transcript abundance sharply increased 4 h after Pst DC3000 inoculation and decreased 8 h after. By contrast, Cbl10 transcript abundance was only slightly increased 8 h after inoculation and decreased thereafter.
For the disease interaction, susceptible tomato plants (RG-PtoS) were inoculated with Pst DC3000 (5 × 105 cfu/mL), and tissue was collected 0, 1, 2, 3, and 4 d later. Specks were first noticeable 3 d after infection (DAI). Both Cipk6 and Cbl10 transcript accumulation increased during the infection process, albeit showing different patterns. Cipk6 transcript increase could be detected 1 DAI and continued steadily until it reached its peak accumulation at 3 DAI. By contrast, Cbl10 transcript accumulation doubled at 3 DAI and increased at 4 DAI (see Supplemental Figure 4C online).
Tomato Cipk6 and Cbl10 Are Required for Pto/AvrPto ETI Triggered by Pst DC3000
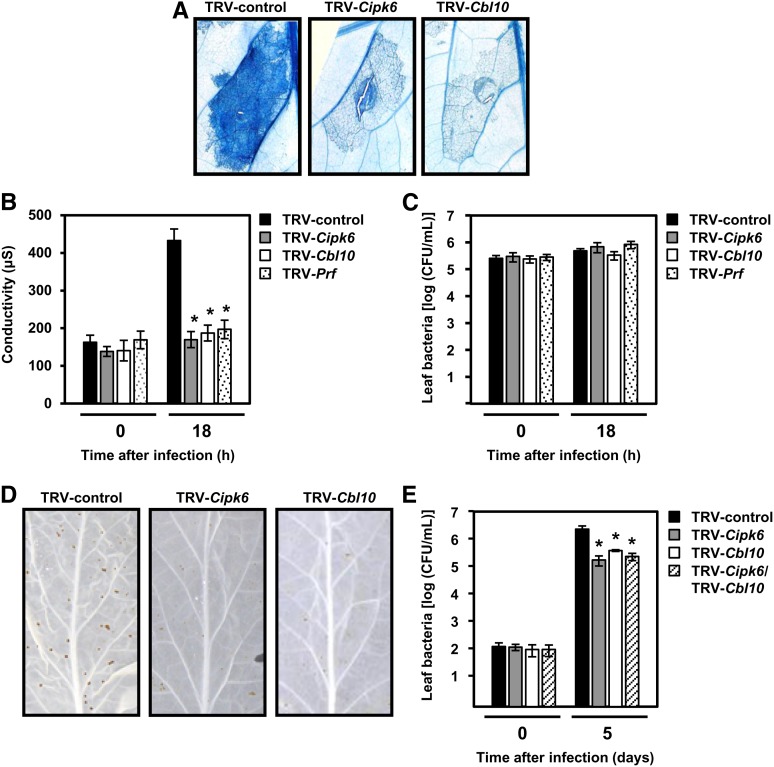
Next, we examined Cipk6 and Cbl10 function in tomato host responses upon Pst DC3000 infection. To test their role in ETI, we used VIGS to silence Cbl10 and Cipk6 in resistant tomato plants (RG-PtoR). Tomato leaves were syringe infiltrated with a PCD-inducing titer of Pst DC3000 (0.5 × 107 cfu/mL), along with TRV-Prf and TRV control-inoculated plants, and PCD was visualized 18 h after infiltration. Infiltrated areas were excised and stained with trypan blue. PCD did not develop in TRV-Cbl10, TRV-Cipk6 (Figure 3A), or TRV-Prf plants (data not shown; see del Pozo et al., 2004), as trypan blue staining was greatly reduced in comparison to TRV control plants, which showed confluent PCD with uniform trypan blue staining covering the entire infiltrated area. These results were supported by a conductivity assay, which measures ion leakage in leaves undergoing PCD (Figure 3B). No significant differences were found in bacterial populations 18 h after infiltration of the RG-PtoR leaves when comparing TRV control plants with TRV-Cbl10 and TRV-Cipk6 plants (Figure 3C).
Figure 3.
Cipk6 and Cbl10 Are Required for Both Immunity and Disease Associated PCD in Tomato after Pst DC3000 Infection.
(A) RG-PtoR–resistant tomato leaves plants were syringe inoculated with Pst DC3000 (0.5 × 107 cfu/mL) and stained for PCD after 18 h with trypan blue.
(B) and (C) PCD shown in (A) was quantified by measurement of conductivity (B), and growth of Pst DC3000 was assessed 18 h after inoculation (C). Data presented are means of three to five plants/line, and error bars represent sd. The experiment was repeated three times with similar results.
(D) and (E) TRV control-infected, Cbl10- and/or Cipk6-silenced susceptible RG-Prf3 tomato plants were infected with Pst DC3000 (1 × 104 cfu/mL) (D). Five days later, leaves were destained to visualize bacterial specks. TRV-Cbl10 or TRV-Cipk6 leaves showed a clear reduction in the number of specks compared with TRV control leaves as well as reduced growth of Pst DC3000 in TRV-Cipk6 and/or TRV-Cbl10 tomato leaves (RG-Prf3) compared with TRV control leaves 5 d after Pst DC3000 inoculation (2.5 × 104 cfu/mL) (E). Experiments were performed with an average of five to seven silenced plants for each silencing construct and repeated at least six times, leading to similar results in five out of seven experiments. Asterisks mark significant differences from TRV control (Student’s t test; P ≤ 0.025).
[See online article for color version of this figure.]
N. benthamiana and Tomato Cipk6 and Cbl10 Also Participate in Disease Lesion Formation Caused by Two P. syringae Strains
P. syringae is a hemibiotrophic pathogen that suppresses PCD early during infection, but later in the infection process, induces cell death leading to disease lesions that may contribute to pathogen dissemination. Lesion-associated cell death is considered a PCD that shares molecular mechanisms with ETI PCD, differing mainly in the timing and number of cells undergoing this response (Tao et al., 2003; del Pozo et al., 2004). We hypothesized that N. benthamiana Cipk6 and Cbl10 might also play a role in disease-associated PCD as was observed previously for MAPKKKα (del Pozo et al., 2004). We analyzed the disease symptoms caused by 5 × 105 cfu/mL P. syringae pv tabaci infection in TRV-Cipk6, TRV-Cbl10, TRV-Cipk6/TRV-Cbl10–silenced, and TRV control N. benthamiana leaves (see Supplemental Figure 5 online). Four days after P. syringae pv tabaci infection, fully collapsed tissue due to successful disease progression was observed in TRV control plants (see Supplemental Figure 5A online). However, no disease-associated PCD was apparent in 50 to 60% of the infiltrated areas in single or cosilenced plants for Cipk6 and/or Cbl10 (see Supplemental Figures 5A and 5B online). Bacterial growth was reduced in TRV-Cbl10, TRV-Cipk6, and to the same extent in TRV-Cipk6/TRV-Cbl10 plants (see Supplemental Figure 5C online). These observations were substantiated by a conductivity assay (see Supplemental Figure 5D online).
To characterize Cipk6/Cbl10 role in disease-associated PCD in tomato caused by Pst DC3000, we silenced Cipk6 and Cbl10 individually or together in susceptible tomato plants (RG-prf3) and inoculated the resulting plants with 2.5 × 104 cfu/mL of Pst DC3000. Five days later, mild disease symptoms, consisting of few and weak specks, could be observed in TRV-Cipk6 and TRV-Cbl10 plants, whereas in TRV control plants, numerous well-defined speck lesions were present (Figure 3D). Bacterial growth was reduced in TRV-Cipk6, TRV-Cbl10, and TRV-Cipk6/TRV-Cbl10 RG-prf3 plants compared with the TRV control (Figure 3E). TRV-Cipk6/TRV-Cbl10 plants showed similar reduction in bacterial specks and growth as single silenced TRV-Cipk6 or TRV-Cbl10, suggesting that Cipk6 and Cbl10 genetically function in the same pathway. These results were observed in five independent experiments. Thus, both N. benthamiana and tomato Cbl10 and Cipk6 are involved in PCD signaling that occurs during both ETI and disease and contributes to disease susceptibility to two P. syringae pathovars.
Tomato Cipk6 Kinase Activity Is Enhanced by SlCbl10 and Ca2+
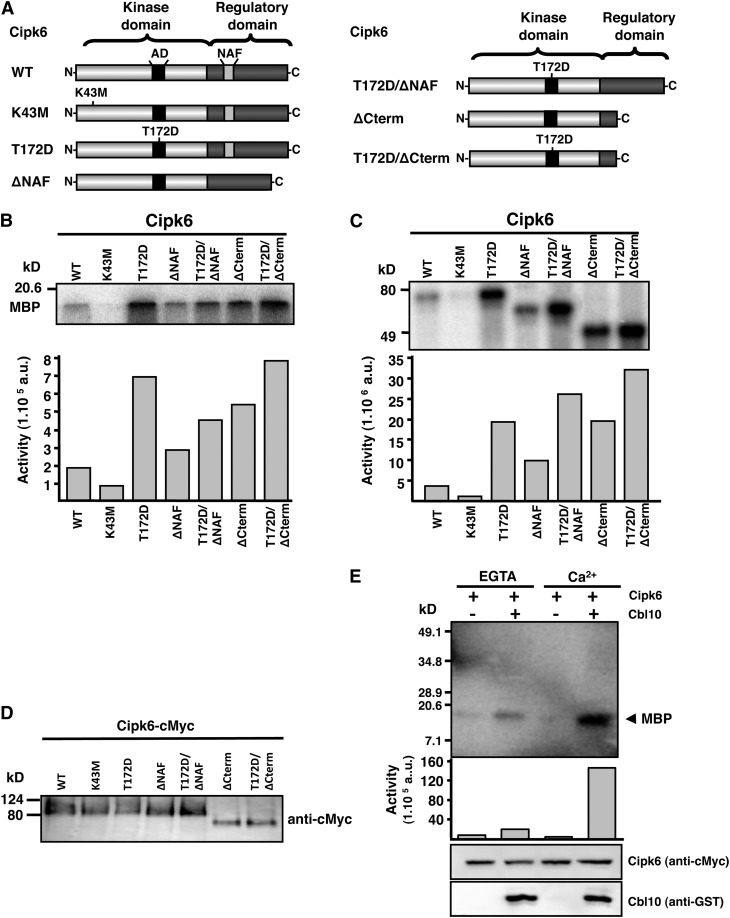
Despite the central role attributed to Ca2+ signaling in plant immunity, little is known about how plants decode Ca2+ signals into cellular responses leading to plant immunity. Cbl10/Cipk6 participation in immunity-associated PCD prompted us to test if tomato Cipk6 could participate as a Ca2+ relay through phosphorylation events. We set up to test and characterize Cipk6 kinase activity (Figure 4). As a negative control, a kinase inactive version was generated, where the conserved catalytic Lys within the kinase domain was substituted by Met (Sl-Cipk6[K43M]) (Liu et al., 2000). We also generated different Cipk6 versions mimicking previously described mutations in other CIPKs that conferred enhanced kinase activity (Gong et al., 2002). Initial yeast two-hybrid analysis followed by structural information has demonstrated that the N-terminal kinase domain and the C-terminal regulatory domain of SOS2 (Arabidopsis CIPK24) interact intramolecularly through the NAF/FISL domain, inhibiting kinase activity possibly by blocking substrate access to the catalytic site (Albrecht et al., 2001; Sánchez-Barrena et al., 2007; Akaboshi et al., 2008). We generated a deletion mutant version that lacked the regulatory domain (Sl-Cipk6ΔCterm) (Figure 4A). A Sl-Cipk6ΔNAF version, lacking the NAF/FISL binding site was previously generated (see above). It has been also described that substitution of a conserved Thr within the activation loop to Asp, mimics phosphorylation (and possibly activation) by unknown upstream kinases (Guo et al., 2001; Gong et al., 2002). We generated, through site-directed mutagenesis, a version with this substitution, Sl-Cipk6(T172D), and obtained double mutants by combining this point mutation with deletion mutants described above (Figure 4A).
Figure 4.
Tomato Cipk6 Is an Active Kinase and Is Regulated by Cbl10 and Ca2+.
(A) Tomato Cipk6 schematic diagram showing kinase and regulatory domains and mutant derivatives. NAF, CBL interaction domain; AD, activation domain. Mutagenized residues and deleted domains are marked. WT, full-length Cipk6. K43M and T172D, single amino acid substitution of Lys-43 to Met and Thr-172 to Asp, respectively. ΔNAF (NAF/FISL amino acids 302 to 321) and ΔCterm (amino acids 302 to 432) deletions. (T172D)/ΔNAF and (T172D)/ΔCterm combine both mutations within the same protein.
(B) For the phosphorylation assays, Cipk6 and mutant versions were incubated with 1 µg of MBP in the presence [γ-32P]ATP and kinase buffer, electrophoresed on SDS-polyacrylamide gel, and autoradiographed. a.u., arbitrary units.
(C) For the autophosphorylation assays, proteins were incubated with [γ-32P]ATP, and samples were processed as in (B).
(D) Equimolecular levels of protein corresponding to Sl-Cipk6 and mutant versions were confirmed by immunoblot using anti-cMyc antibodies.
(E) Cipk6 kinase activity is regulated by Cbl10 in a Ca2+-dependent manner. MBP substrate was incubated in the presence of [γ-32P]ATP and Ca2+ or EGTA in kinase buffer with Cipk6 and with or without GST-Cbl10.
To determine whether tomato Cipk6 is an active kinase, we cloned Cipk6 and mutant versions into the pYL436 tandem affinity purification (TAP) vector (Rubio et al., 2005), agroinfiltrated them into N. benthamiana leaves, and purified the tagged proteins. Cipk6 and mutant versions were observed at the expected molecular masses, and an in vitro kinase assay was performed with the recombinant proteins to determine their kinase activity in the presence of [γ-32P]ATP and myelin basic protein (MBP) as a substrate as well as their autophosphorylation activity (Figures 4B to 4D). Wild type Sl-Cipk6 was observed to have a weak transphosphorylation and autophosphorylation activity, whereas Sl-Cipk6(K43M) (kinase inactive) had no activity (Figures 4B and 4C). Altogether, the Cipk6 variants (Sl-Cipk6[T172D], Sl-Cipk6ΔNAF, and Sl-Cipk6ΔCterm) showed noticeably increased kinase and autophosphorylation activity, with Sl-Cipk6(T172D) displaying the highest kinase activity (approximately threefold above the wild type).
Interestingly, Cipk6 variants resulting from the addition of the single substitution, T172D, to the deletion mutants (Sl-Cipk6[T172D]/ΔNAF and Sl-Cipk6[T172D]/ΔCterm) increased both kinase activity and most noticeably autophosphorylation activity with Sl-Cipk6(T172D)/ΔCterm displaying highest autophosphorylation activity compared with single mutant versions. Kinase and autophosphorylation assays were performed in parallel with identical sample loadings, along with an immunoblot to assess that similar amount of protein was used (Figure 4D). Thus, it appears that the highest kinase activity is achieved through transactivation and highest autophosphorylation through release of the catalytic domain of the inhibitory effect of the regulatory domain.
To test whether tomato Cipk6 kinase activity was modulated by Cbl10 and Ca2+, in vitro transphosphorylation assays were performed using MBP as substrate. Cipk6 kinase activity increased significantly (≥16×) in the presence of both Cbl10 and Ca2+, therefore demonstrating that Cipk6 kinase activity is regulated by Cbl10 in a Ca2+-dependent manner (Figure 4E). No Cbl10 phosphorylation by Cipk6 could be observed in the conditions assayed (Figure 4E). Arabidopsis CBL10 was found previously to be phosphorylated by SOS2 (CIPK24) (Lin et al., 2009).
Tomato Cipk6 Kinase Activity Is Associated with ROS Production
An increase in cytoplasmic Ca2+ is among the earliest responses upon pathogen recognition and blocking this influx with inhibitors demonstrated that Ca2+-derived signals are necessary for downstream signaling processes, including ROS production and PCD (Blume et al., 2000; Grant et al., 2000; Segonzac et al., 2011). Therefore, given the participation of the Cbl10/Cipk6 module in ETI-associated PCD, we set out to investigate whether tomato Cipk6 kinase activity could also affect ROS production.
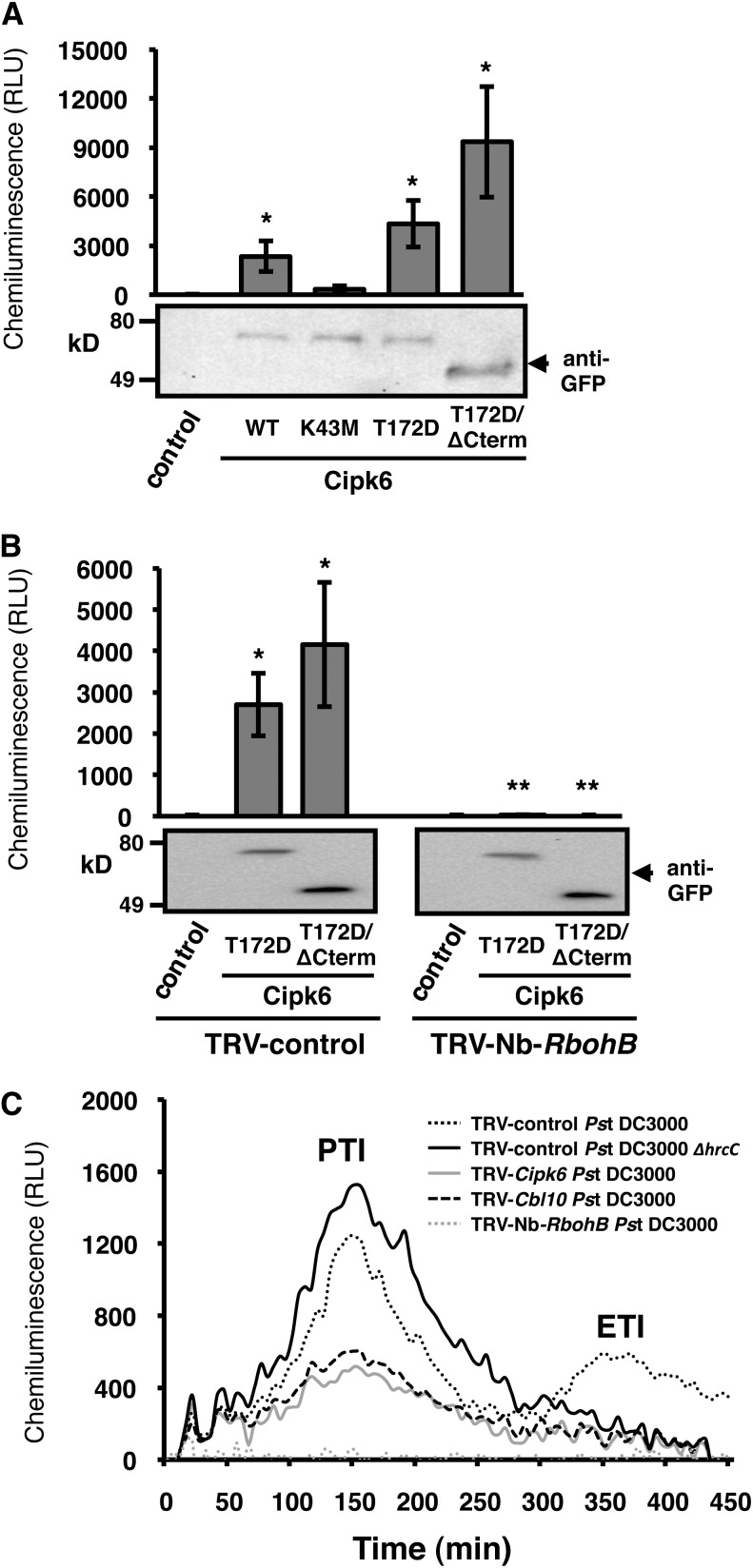
Tomato Cipk6 and its mutant versions were agroinfiltrated using an estradiol-inducible system in N. benthamiana leaves, and production of ROS was quantified in the presence of luminol in a luminometer (Figure 5A). ROS were detected in samples expressing Sl-Cipk6 and increased more than two- and fourfold in samples expressing enhanced kinase activity versions Sl-Cipk6(T172D) and Sl-Cipk6(T172D)/ΔCterm, respectively, whereas ROS was much reduced after expression of the kinase-inactive version Sl-Cipk6(K43M), indicating that Sl-Cipk6 kinase activity is necessary for ROS generation. The highest ROS accumulation was detected in Sl-Cipk6(T172D)/ΔCterm-expressing samples, thus correlating with highest kinase and autophosphorylation activity (Figures 4B and 4C) likely as a result of combining both derepression and mimicking-transactivation of kinase activity. Cipk6 and all the mutant versions proteins were expressed similarly (Figure 5A).
Figure 5.
Tomato Cipk6 Expression Results in ROS Generation: Cbl10 and Cipk6 Contribute to ROS Production in Response to Pst DC3000.
(A) Luminol-enhanced chemiluminescent assay in leaf discs agroexpressing Sl-Cipk6, Sl-Cipk6 mutant versions, or control (empty vector) in pER8 XVE vector after 17β-estradiol treatment. ROS was quantified as relative light units (RLU) 2 to 3 h after. Data presented are means of 8/10 measures of independent discs. The experiment was repeated five times with similar results. Asterisks indicate a significant increase compared with control (Student’s t test; P < 0.05). WT, the wild type.
(B) ROS mediated by Sl-Cipk6(T172D) or Sl-Cipk6(T172D)ΔCterm was drastically reduced in TRV-Nb-RbohB plants. Experiment was repeated twice with similar results, with eight biological replicas and three technical replicates. Single asterisks, significant increase compared with control (empty vector-agroinfiltrated). Double asterisks, significant decrease compared with TRV control plants (Student’s t test; P < 0.01).
(C) ROS is greatly diminished in TRV-Cbl10 and TRV-Cipk6 and almost undetectable in TRV-Nb-RbohB Nb plants compared with TRV control plants after Pst DC3000 (2.5 × 108 cfu/mL) inoculation. ROS were measured for 7 h as in (A). Pst DC3000 ΔhrcC was employed as a control for the ETI (2.5 × 108 cfu/mL). The experiment was repeated three times with similar results.
Membrane-bound RBOHs play a key role as ROS-producing enzymes in plants. N. benthamiana RbohB silenced plants were susceptible to an avirulent pathogen and showed reduced HR (Yoshioka et al., 2003). RbohB was also required for ROS accumulation during PTI in response to PAMPs (Segonzac et al., 2011). In order to determine if RbohB was required for ROS generation mediated by Cipk6, we expressed Sl-Cipk6(T172D) and Sl-Cipk6(T172D)/ΔCterm and measured ROS as described in Figure 5A in N. benthamiana plants silenced for RbohB and nonsilenced control plants (Figure 5B). Silencing RbohB completely abolished Sl-Cipk6(T172D)– and Sl-Cipk6(T172D)/ΔCterm–mediated ROS generation. ROS were detected in samples expressing Sl-Cipk6(T172D) and Sl-Cipk6(T172D)/ΔCterm in TRV control plants. This result clearly indicates that N. benthamiana RbohB is required for tomato Cipk6-mediated ROS generation.
We also determined if tomato Cbl10 and Cipk6 contributed to ROS generation during the response of N. benthamiana to Pst DC3000 infection. Using the in vivo luminol-based assay, TRV control plants infected with Pst DC3000 showed two ROS peaks approximately at 2.5 and 6 h after infection (Figure 5C). The early ROS burst is associated with PTI, whereas the later one corresponds to ETI, since TRV control plants infected with Pst DC3000 hrcC (deficient in the type III secretion system and therefore eliciting PTI but not ETI) showed the first but not the second peak. Both ROS peaks were substantially reduced in TRV-Cipk6 or TRV-Cbl10 but not completely abrogated, as observed in TRV-RbohB N. benthamiana plants (Figure 5C). Collectively, these data demonstrate that Cbl10 and Cipk6 contribute to ROS generation during the immune response to Pst DC3000 in N. benthamiana.
Cipk6 and Cbl10 Interact in Vivo and Constitute a Signaling Module
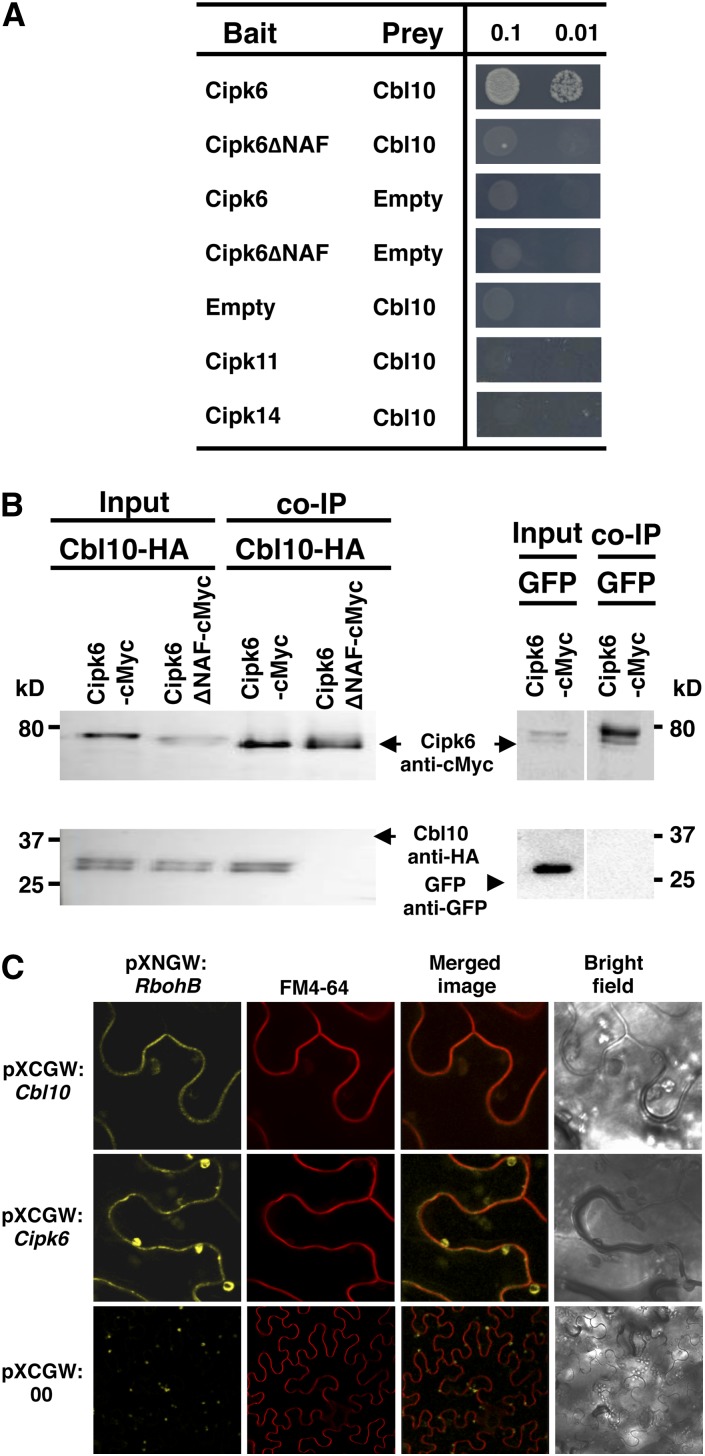
The fact that plants cosilenced for Cipk6/Cbl10 did not show an additive loss of PCD or reduced bacterial growth compared with plants silenced individually for these genes suggested that their proteins might act together as a PCD-inducing signaling module. To test if these two proteins interact physically, we first performed a yeast two-hybrid assay. Yeast two-hybrid analysis and in vitro binding assays have demonstrated that CBLs often bind a number of CIPKs with different affinity (Albrecht et al., 2001; Batistic and Kudla, 2009). We found that tomato Cbl10 interacted with Cipk6, whereas, as expected, Sl-Cipk6ΔNAF, an altered protein lacking the NAF/FISL domain necessary for CBL binding, did not interact (Figure 6A). Cbl10 did not interact with Cipk11 or Cipk14, tomato CIPK proteins with high amino acid similarity to Cipk6, therefore suggesting that the Cipk6/Cbl10 interaction has a high level of specificity (Figure 6A). Cbl10, Cipk6, Cipk6ΔNAF, Cipk11, and Cipk14 were expressed in yeast (see Supplemental Figure 6 online).
Figure 6.
Tomato Cbl10 and Cipk6 Interact in Yeast and in Planta and with RbohB at the Plasma Membrane.
(A) EGY48 yeast strain cultures containing either Sl-Cipk6, Sl-Cipk6ΔNAF, Sl-Cipk11, or Sl-Cipk14 in the bait pEG202 vector and Sl-Cbl10 in the prey vector pJG4-5 were spotted at OD600 = 0.1 and 0.01 on selective medium. Growth indicates Cipk6/Cbl10 interaction.
(B) Plant protein extracts from leaves agroexpressing Sl-Cipk6-Myc and Sl-Cbl10-HA proteins were incubated with IgG agarose beads, and proteins eluted were immunoblotted using anti-cMyc and anti-HA Sl-Cipk6, which did not co-IP with GFP (right panel, lanes derive from same blots but from no correlative lanes).
(C) Sl-Cipk6-YN and Sl-Cbl10-YC interact with Sl-RbohB in vivo in N. benthamiana by BiFC. YFP fluorescence was monitored 24 h after agroinfiltration using confocal laser scanning microscopy. Plasma membrane was stained with FM4-64.
To further examine the Cipk6 and Cbl10 interaction and to test if the presence of Ca2+ influenced their binding, we attempted pull-down experiments with tomato Cipk6 and Cbl10 proteins isolated from Escherichia coli. We successfully expressed Cbl10 fused to glutathione S-transferase (GST), Sl-Cbl10-GST, and because Cipk6 protein production was limiting, we produced Sl-Cipk6-MetS35–labeled protein in vitro. Pull-down experiments demonstrated direct interaction between Cipk6 and Cbl10, which was not dependent on the presence of Ca2+ in our in vitro assay (see Supplemental Figure 7 online). The interaction of Arabidopsis CIPK24 (SOS2) and CBL10 (SCaBP8) is also not affected by Ca2+ (Quan et al., 2007).
In order to examine the likelihood that tomato Cipk6 and Cbl10 constitute a signaling module in vivo and to further test the specificity of their interaction, we performed coimmunoprecipitation (co-IP) experiments. We transiently expressed by agroinfiltration wild-type Sl-Cipk6 or Sl-Cipk6ΔNAF fused with a c-Myc tag and Sl-Cbl10 fused with a HA tag in N. benthamiana leaves (Figure 6B). Sl-Cipk6ΔNAF accumulated to lower levels; therefore, we adjusted the input of Sl-Cipk6ΔNAF to be the same as Sl-Cipk6 to minimize the effect on the co-IP experiments with Cbl10. Tomato Cbl10 coimmunoprecipitated with Cipk6, whereas no co-IP was detected with Sl-Cipk6ΔNAF, as expected. Also, Cipk6 did not co-IP with another negative control, green fluorescent protein (GFP) (Figure 6B).
In order to relay Ca2+ signals into target phosphorylation, CBL/CIPK modules require specific and coordinated subcellular localization with their targets. To examine the likelihood that Cbl10 and Cipk6 could regulate RbohB directly, in vivo interaction between tomato Cbl10 or Cipk6 with RbohB was tested by bimolecular fluorescence complementation (BiFC) experiments in N. benthamiana leaves (Walter et al., 2004). Fluorescence due to the reconstitution of the yellow fluorescent protein (YFP) was observed by confocal microscopy and localized at the periphery of the cell when the combinations of Sl-Cbl10-CFPC and Sl-RbohB-YFPN or Sl-Cipk6-CFPC and Sl-RbohB-YFPN were agroinfiltrated into N. benthamiana leaves (Figure 6C). No fluorescence was detected when SlRbohB-YFPN was agroinfiltrated with empty vector pXCGW (Figure 6C). Reconstituted YFP signal overlapped with the plasma membrane stained with the plasma membrane marker FM4-64 (Figure 6C). These results indicate that tomato Cbl10 and Cipk6 interact with RbohB at the plasma membrane in N. benthamiana cells, thus raising the possibility that RbohB is a phosphorylation target of Cbl10/Cipk6.
DISCUSSION
Several CBL/CIPK complexes have been previously identified as regulators of different stress responses, including salinity, drought, cold, and in response to abscisic acid. In this study, we report the identification of tomato Cbl10 and Cipk6, from a large VIGS fast-forward genetic screen (del Pozo et al., 2004), and their functional characterization as positive regulators of PCD events in ETI mediated by different R genes/effectors from bacteria, oomycete, nematode, and virus and in susceptible responses to two pathogenic strains of P. syringae. We propose that tomato Cbl10 and Cipk6 act in a convergent regulatory node in ETI- and disease-associated PCD signaling in plant immunity.
Biochemical characterization indicated that tomato Cipk6 is an active kinase, whose activity is positively regulated by Cbl10 and Ca2+. Because Cbl10 and Cipk6 interact in planta, we conclude that Cbl10/Cipk6 constitute a signaling module regulated by Ca2+. In an attempt to investigate Cipk6 downstream signaling connections, we found that Cipk6 overexpression results in ROS generation in planta, which required Cipk6 kinase activity and NADPH oxidase RbohB. Both Cbl10 and Cipk6 interacted in vivo with RbohB at the plasma membrane, thus suggesting that RbohB is a target of Cbl10/Cipk6. Importantly, Cbl10 or Cipk6 loss-of-function analysis in N. benthamiana revealed their contribution to ROS bursts occurring during both PTI and ETI in response to Pst DC3000 infection, thus validating their physiological role in the plant immune response.
Our studies reveal a functional role for a CBL/CIPK module in ETI PCD and resistance and provide molecular insights into the mechanistic conversion of Ca2+ signaling into ROS signaling in plant immunity through still unknown phosphorylation events in plants. Based on these results, we propose a model where Ca2+ influx after pathogen perception might be detected by Cbl10, which interacts with Cipk6, thereby derepressing its kinase activity, which in turn phosphorylates RbohB, resulting in ROS production contributing to PCD and resistance responses.
Ca2+ seems not to be necessary for the Cbl10/Cipk6 interaction, at least in our in vitro assay. However, Cipk6 kinase activity increased notably in the presence of Cbl10 and Ca2+, demonstrating the relevance of Ca2+ regulation in the system. Similarly, SOS2/SOS3 and Arabidopsis CBL2/CIPK14 displayed Ca2+-independent interaction and Ca2+-dependent kinase activity (Halfter et al., 2000; Akaboshi et al., 2008). Among the single mutants, Sl-Cipk6(T172D) displayed the greatest kinase and autophosphorylation activity. Thr-172 is a phosphorylatable residue located in the activation loop and is conserved at the same position in all SnRK3s. Substitution of this residue to Asp, to mimic Thr phosphorylation, gives CIPKs enhanced kinase activity (Guo et al., 2001; Gong et al., 2002). Because this activated state was observed in several cases in which Ser/Thr was mutated to an Asp, it has been proposed that CIPKs could also be activated by a phosphorylation cascade (Harper et al., 2004). Changes in the phosphorylation state of proteins are among the earliest events in plant immunity (Dietrich et al., 1990; Felix et al., 1991). In the future, identification of a kinase(s) that could regulate Cipk6 activity could fill in knowledge gaps in early immune signaling in plants between early phosphorylation events and Ca2+-mediated responses. One possible candidate is Pto. However, the participation of N. benthamiana Cipk6 in diverse R gene/effector-elicited PCD events would argue for the involvement of a convergent kinase playing this role.
In plants, cell death induced by pathogen infection is associated with both disease resistance and susceptibility. In the tomato/Pst DC3000 interaction, both ETI and susceptibility are associated with PCD events, which seem to share some common regulators and physiological changes but their development occurs at different timing and involves different number of cells (del Pozo et al., 2004; López-Solanilla et al., 2004; Tao et al., 2003). In contrast with HR PCD, the molecular basis of disease-associated PCD that takes place during compatible interactions is largely unknown. In this work, we observed that tomato and N. benthamiana Cbl10 and Cipk6 positively regulate both immunity-associated PCD and disease-associated PCD and contribute to disease susceptibility in a fashion similar as described previously for MAPKKKα (del Pozo et al., 2004). This raises the possibility that Cbl10/Cipk6 and MAPKKKα act in the same pathway or that crosstalk between them might exist. We did not detect any interaction between MAPKKKα (full-length or kinase domain) and tomato Cipk6 (wild-type or constitutive active derivatives) by yeast two-hybrid assays (O. del Pozo, unpublished data). MAPKKKα overexpression results in PCD, whereas overexpression of tomato Cipk6 (wild-type or enhanced kinase activity versions) in the presence or absence of Cbl10 did not result in PCD (O. del Pozo, unpublished data). Possibly, an increase of cytosolic Ca2+ is required for full Cipk6 kinase activation. Alternatively, Cipk6 kinase activity and ROS generation might be necessary but not sufficient for PCD execution. In this regard, it is known that H2O2 can diffuse and activate PCD; however, it must be accompanied by suppression of ROS detoxification mechanisms and by a balanced concentration of nitric oxide (Mittler et al., 1999; Delledonne et al., 2001). Because Cbl10 and Cipk6 loss-of-function phenotypes are reminiscent of those previously described for MAPKKKα (del Pozo et al., 2004), it will be important to explore in the future possible crosstalk between Cipk6 and MAPKKKα or other MAPK cascades in PCD regulation. However, as we have stated previously for MAPKKKα (del Pozo et al., 2004), we cannot rule out that Cbl10/Cipk6 might regulate other susceptibility-related pathways in addition to controlling cell death. Perturbation of these pathways could also lead to inhibition of bacterial growth as there is no direct link between lesion formation and virulence or ETI PCD and increased resistance as shown in Pst DC3000 mutant strains that affect both forms of PCD (López-Solanilla et al., 2004; Gassmann, 2005).
We discovered evidence of a direct and positive connection between tomato Cipk6 kinase activity and ROS production in planta; more importantly, we provide genetic and physiological in vivo evidence that Cbl10 and Cipk6 contribute to ROS generated during both PTI and ETI peaks in the N. benthamiana/Pst DC3000 interaction. These results indicate that ROS generation in ETI and PTI might be mechanistically linked at the Cbl10/Cipk6 signaling node. Because Cbl10 and Cipk6 are interacting partners in yeast and in planta (using co-IP) and both interact directly with RbohB in N. benthamiana plasma membrane (by BiFC), we propose that RbohB is a target of the Cbl10/Cipk6 module, thus providing a mechanistic link between Ca2+-derived signals, phosphorylation, and ROS signaling during the plant immune response to bacterial pathogens.
Work performed in elicitor-treated suspension cultures indicated that phosphorylation and Ca2+ signaling are required for ROS generation (Blume et al., 2000; Grant et al., 2000, Kimura et al., 2012), whereas other studies demonstrated that ROS production was necessary for Ca2+ influx or played a positive feedback role (Van Breusegem et al., 2008). These studies revealed a specific and complex crosstalk between Ca2+ and ROS signaling in early immune responses in different plant–pathogen interactions. An oxidative burst is a hallmark in nearly all plant responses to pathogen attack and plays an important signaling role in PCD (Torres, 2010). Different enzymatic sources of ROS have been described (Daudi et al., 2012; Ishiga et al., 2012; Rojas et al., 2012); however, the membrane-bound RBOH complex seems to play a central role in generating apoplastic ROS in PTI and ETI and possibly in amplifying ROS generated in different cellular compartments (Torres, 2010). The role of specific RBOH enzymes in pathogen-elicited PCD and resistance is complex, and they play distinct roles depending on the pathogen and the plant species. For instance, Arabidopsis rbohF plants are more resistant to Peronospora parasitica, displaying enhanced HR, but are impaired in resistance to compatible bacteria, whereas N. benthamiana plants silenced for RbohB (Arabidopsis RBOHF ortholog) are more susceptible to Phytophtora infestans and present reduced HR, but appear not to contribute to bacterial resistance (Yoshioka et al., 2003; Segonzac et al., 2011; Chaouch et al., 2012). A role for Arabidopsis RBOHs in signaling has been proposed, as they regulate the spread of salicylic acid–mediated cell death and the synthesis of salicylic acid and defense-related metabolites (Torres et al., 2005; Chaouch et al., 2012). Recently, a model for CPK5 and RBOHD in a self-activating circuit that activates distal plant defense has been proposed (Dubiella et al., 2013). In the future, it will be interesting to determine if Cipk6 regulation of RbohB contributes to defense-related metabolite production and if it affects the outcome of defense responses regulation in both tomato and N. benthamiana.
Plant RBOH proteins are regulated by Ca2+ at different levels. They contain EF hands that can bind Ca2+ (Keller et al., 1998) and are phosphorylated during pathogen responses by CDPKs or CPKs (Kobayashi et al., 2007; Dubiella et al., 2013). Additional events of RBOH phosphorylation had been described in proteomic analysis performed in Arabidopsis cells after elicitor treatment (Benschop et al., 2007; Nühse et al., 2007). These results highlight the importance of Ca2+ and phosphoregulation of RBOHs in early immune signaling, and we propose that CIPKs are candidates for such phosphorylation events in plant responses to pathogen attack, in addition to CDPKs or CPKs.
Recent reports indicate that at least in vitro, Arabidopsis RBOH proteins might be also regulated by different Ca2+-regulated kinases that play a role in abiotic stress responses (Sirichandra et al., 2009; Drerup et al., 2013). Arabidopsis CIPK6, CBL10, and orthologs have been shown to participate in different processes like salt stress tolerance, development, regulation of potassium channel AKT2 activity or modulation, or abscisic acid signaling (Kim et al., 2007; Quan et al., 2007; Tripathi et al., 2009; Held et al., 2011; Chen et al., 2012; Ren et al., 2013). We did not observe any abnormal growth or development in N. benthamiana or tomato plants silenced for either Cbl10, Cipk6, or both, growing under standard greenhouse conditions. Because N. benthamiana and tomato plants become fully silenced when they are over 1 month old, such early development-associated phenotypes might not have been apparent in our silenced plants. Interestingly, ROS are also generated during salt stress and during development (Borsani et al., 2001; Hernández et al., 2001), and RBOHs participate in initial phases of salt stress (Jiang et al., 2012). It will be interesting to determine whether Arabidopsis CIPK6 also mediates ROS production and its possible contribution to immunity, salt tolerance, or development.
Earlier, a connection between CIPKs and reactive oxygen signaling was suggested, as different SOS2-interacting proteins (NUCLEOSIDE DIPHOSPHATE KINASE2 and two catalases [CAT2 and CAT3]) involved in ROS signaling and decomposition, respectively, were identified (Verslues et al., 2007). SOS1, a Na+/H+ antiporter, which is a target of the SOS3/SOS2 complex, has been reported to be posttranscriptionally regulated by ROS and to interact with oxidative responses regulators (Katiyar-Agarwal et al., 2006; Chung et al., 2008). In rice cultured cells treated with PAMPs, CIPK14/15 appear to mediate ROS production (Kurusu et al., 2010), and, more recently, Arabidopsis CIPK26 was shown to interact with RBOHF in planta and positively and negatively regulate its ROS-producing activity in human cultured cells (HEK293T) (Kimura et al., 2012; Drerup et al., 2013). In planta validation of such results in the context of a specific physiological response will consolidate these promising results.
CBLs and CIPKs have been described to interact specifically with protein phosphatases, the chaperone-like protein DNAJ and a 14-3-3 protein (Fuglsang et al., 2007; Yang et al., 2010). Understanding the regulation of the formation of the Cbl10/Cipk6 complex, identification of additional regulatory components, together with transcriptional regulation of their genes under developmental-, tissue-, and cell-specific and environmental or biotic stress conditions will be important for understanding how downstream Cbl10/Cipk6 signaling specificity is achieved from upstream Ca2+-derived signals in abiotic and biotic stress signaling.
Our results and other recent reports that suggest the participation of rice CIPK14/15 in regulation of defense gene expression and phytoalexin production in rice cultured cells and the possible regulation of NPR1 by PKS5 (Arabidopsis CIPK24) phosphorylation (Kurusu et al., 2010; Xie et al., 2010) support a role for CBL/CIPK participation in different aspects of plant defense. Thus, CBL/CIPK complexes are important players in modulating outputs during biotic interactions. In the future, we will investigate the molecular basis of tomato Cbl10/Cipk6 regulation of RbohB ROS production. For that purpose, we will determine if RbohB is a Cipk6 phosphorylation substrate, identify the putative residues phosphorylated, and analyze the contribution of these putative phosphorylation events to ROS generation, PCD, and resistance in the plant immune responses to pathogenic bacteria through functional analysis. Alternatively, phosphorylation-independent regulation of RbohB by Cipk6 also could be plausible, in a similar fashion to SOS2 (CIPK24) regulation of the H+/Ca2+ antiporter CAX1 (Cheng et al., 2004). Thus, characterization of RbohB regulation by Cipk6 and identification and characterization of additional Cipk6 phosphorylation targets involved in plant immunity will be the next important steps to gain understanding about the cellular processes regulated by this key regulator of PCD.
METHODS
Plant Material and Bacterial Strains
Resistant tomato (Solanum lycopersicum) line Rio Grande-PtoR (RG-PtoR; Pto/Pto and Prf/Prf), susceptible lines Rio Grande-PtoS (RG-PtoS; ptp/pto and Prf/Prf), and Rio Grande-prf3 (RG-prf3; Pto/Pto and prf/prf) and Nicotiana benthamiana were used for VIGS. Agrobacterium tumefaciens strains GV2260 and GV3101 were used for VIGS in N. benthamiana and tomato, respectively, and C58C1 for Agrobacterium-mediated transient expression in N. benthamiana. Pseudomonas syringae pv tomato strain DC3000 and P. syringae pv tomato strain DC3000 ΔhrcC- (Pst DC3000 ΔhrcC) were used in tomato and N. benthamiana infection analysis, and P. syringae pv tabaci strain 11528R was used for pathogen assays in N. benthamiana.
cDNA Cloning for Tomato Cipk6, Cbl10, and RbohB
Tomato Cipk6, Cbl10, and RbohB cDNAs containing the complete open reading frames were obtained by RT-PCR using as a template a tomato cDNA library obtained from Pst DC3000-infected leaves using Gateway-adapted oligonucleotides OPS-13/OPS-14 for Cipk6, OPS-56/OPS-64 for Cbl10, and OPS-637/OPS-638 for RbohB and cloned into the pDONR207 vector (Invitrogen; Supplemental Table 2).
Site-Directed Mutagenesis and Deletion Mutants
PCR-based site mutagenesis was performed to generate the substitutions K43M and T172D in Sl-Cipk6 using the QuickChange kit (Stratagene) using the following oligonucleotides: OPS-40/OPS-41 for Sl-Cipk6(K43M) and OPS-42/OPS-43 for Sl-Cipk6(T172D). For Sl-Cipk6ΔNAF and Sl-Cipk6ΔCterm, OPS-44/OPS-45 and OPS-13/OPS-98 were used, respectively (Supplemental Table 2).
Electrolyte Leakage Assay and PCD Staining
Trypan blue staining in tomato was performed as described by del Pozo et al. (2004). Ion leakage assays were performed with three discs per plant (1 cm diameter) floated in 3 mL of MilliQ water for 2 h at room temperature with gentle shaking, and conductivity was measured with an Acorn Con 6 meter (Oakton Instruments).
VIGS
PVX clones containing N. benthamiana partial cDNAs corresponding to Cipk6 (cNbME30H4) and Cbl10 (cNbME35F4) were identified previously (del Pozo et al., 2004). Tomato identical clones were amplified and transferred into the TRV silencing vector pYL215 (Liu et al., 2002) to obtain TRV-Cipk6 and TRV-Cbl10. TRV-Cipk6-5′, a 403-bp Cipk6 fragment, Cipk6-3′, a 463-bp fragment, and TRV-Cbl10-5′, a 205-bp fragment, were PCR amplified using Gateway-adapted oligonucleotides OPS-15/OPS-16, OPS-19/OPS-20, and OPS-22/OPS-24, respectively. PCR products were gel purified, inserted into the TRV vector by the Gateway reaction (Invitrogen), sequenced, and transformed into Agrobacterium strain GV2260. TRV-Nb-RbohB silencing construct was kindly provided by Cecile Segonzac (Segonzac et al., 2011). For silencing in tomato and N. benthamiana, seedlings with two emerging leaflets and 14-d-old plants, respectively, were syringe infiltrated with cultures containing pYL215 derived constructs and mixed with pTRV1, both with an OD600 of 0.075, according to procedures described by Liu et al. (2002).
Agrobacterium-Mediated Transient Transformation Assay
Agrobacterium-mediated transient expression of proteins in N. benthamiana leaves was performed as described by He et al. (2004). Agrobacterium GV2260 cultures carrying Pto, avrPto, Cf9, avr9, PtoY207D, and Bax were infiltrated at OD600 = 0.2; for cultures carrying RBP1 or Gpa2, OD600 = 0.1; and for cultures carrying NPP1, RX2, or CP, OD600 = 0.04 was used. OD600 was adjusted for each culture to synchronize PCD development in N. benthamiana and allow parallel scoring. Tomato Cipk6 and mutant derivatives were cloned into the estradiol-inducible pER8-XVE vector (Zuo et al., 2000), with a GFP fusion tag at the C terminus (oligonucleotides OPS-51, OPS-178, and OPS-404), and into the pYL436 TAP-tag vector (Rubio et al., 2005) (oligonucleotides OPS-189, OPS-190, and OPS-191). Agrobacterium strain C58C1 carrying XVE, pYL436 TAP-tag, or the tomato bushy stunt virus p19 gene constructs was adjusted to an OD600 = 0.4 (Supplemental Table 2).
Yeast Two-Hybrid Assay
Tomato Cbl10 tagged with an HA epitope was cloned into pJG4-5 vector under control of the Gal-inducible promoter and tomato Cipk6 gene and derivatives (fused to the LexA DNA binding domain) into pEG202 vector and transformed in the yeast strain EGY48 (ura3, his3, trp1, lexAPo-leu2) following the LiAc/PEG method (Yeast Protocols Handbook, Clontech). Two-hybrid tests were performed on indicator plates supplemented with 2% Gal and 1% raffinose to induce prey fusion protein expression. Activation of the chromosomal LEU2 reporter was tested by scoring growth on minimal medium lacking uracil, His, Trp, and Leu (Golemis and Khazak, 1997). Expression of fusion proteins was verified by immunoblot using monoclonal antibodies mouse anti-LexA SC-7544 (Santa Cruz Biotechnology) or rat anti-HA 3F10 (Roche Applied Science).
Preparation and Purification of Tomato Cipk6 Recombinant Proteins
Tomato Cipk6 and derivatives were PCR amplified with LIC-compatible primer OPS-189 (forward) and OPS-190 or OPS-191 (reverse) oligonucleotides for full-length or Cipk6ΔCterm, respectively, and cloned as described by Popescu et al. (2007 Supplemental Table 2). Six-week-old N. benthamiana leaves were coinfiltrated with a mix of Agrobacterium C58C1 cultures carrying pYL436-Cipk6 or derivatives and p19. Three days later, leaves were collected, frozen in liquid nitrogen, ground, and mixed 1:2 (v/v) with extraction buffer (100 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 10% glycerol, 0.1% Triton X-100, 1× Complete protease inhibitors [Roche], and 1 mM phenylmethylsulfonyl fluoride) and centrifuged for 20 min at 14,000g. Supernatants were collected and filtered through four layers of Miracloth (Calbiochem). Ten milliliters was incubated with 50 μL IgG beads (Amersham Biosciences) for 2 h at 4°C with gentle rotation. After centrifugation at 150g for 3 min at 4°C, the IgG beads were recovered and washed three times with 10 mL of washing buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10% glycerol, and 0.1% Triton X-100) and once with 10 mL of cleavage buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10% glycerol, 0.1% Triton X-100, and 1 mM DTT) at 4°C. Elution from the IgG beads was performed by incubation with 10 μL (20 units) of Prescission protease (Amersham Biosciences) in 1 mL of cleavage buffer at 4°C with gentle rotation.
Preparation and Purification of Tomato Cbl10 Recombinant Protein
To produce GST-Cbl10 recombinant protein, GST coding region was cloned in frame in the NdeI and NcoI sites of pET30a expression vector. Then, Cbl10 was cloned in frame into the EcoRI site, sequenced, and transformed into Escherichia coli strain BL21-codonPlus-(DE3)-RIL (Stratagene). Cells were grown at 37°C with shaking in Luria Bertani broth containing 50 µg/mL kanamycin and 34 µg/mL chloramphenicol until OD600 = 0.6; 1 mM isopropyl-β-d-thiogalactoside was then added and incubated for 14 h at 10°C with shaking. Cells were pelleted by centrifugation at 4000g, resuspended in PBS buffer, pH 8.0, lysed by sonication, and centrifuged at 22,000g, and the recombinant proteins in soluble phase were purified by affinity chromatography using GST MicroSpin columns (Amersham).
Kinase Assays
For autophosphorylation assays, purified tomato Cipk6 and mutant version proteins were incubated in kinase buffer (20 mM Tris, pH 7.5, 15 mM MgCl2, 1 mM DTT, 50 µM ATP, and 10 µCi of [γ-32P]ATP [Amersham; 3000 Ci/mmol, 1 Ci = 37 GBq]) in a final volume of 30 μL and incubated 45 min at 30°C; 1 mM CaCl2 or 10 mM EGTA was included in the mixture or in the kinase buffer, respectively. Reactions were terminated by adding 10 μL of 4× Laemmli loading buffer, and samples were separated on 12% SDS-PAGE gels. Gels were stained with Coomassie Brilliant Blue (Bio-Rad) and visualized by autoradiography. Cipk6 kinase transactivity was determined in the presence or absence of 100 ng of GST-Cbl10 fusion protein, and 3 µg of MBP (Roche), 5 µCi of [γ-32P]ATP, and 50 µM ATP were added to the kinase reaction mixture and incubated at 30°C for 45 min. Samples were separated on 15% SDS-PAGE gels. Kinase activity was visualized by autoradiography.
ROS Detection and Measurement
N. benthamiana leaf discs (0.28 cm2) were floated on 100 μL of distilled water in a 96-well white-bottom plate for 8 to 12 h at room temperature at dark. Water was replaced with 100 μL solution containing 100 µM luminol (Sigma-Aldrich) and 1 µg of horseradish peroxidase. Expression was induced by adding 5 nM 17β-estradiol and 0.1% Silwet L-77. For ROS detection after bacterial infection, 2.5 × 108 cfu/mL Pst DC3000 or Pst DC3000 ΔhrcC was added to the wells, and ROS were measured in vivo as luminescence using a Varioskan Flash Multimode Reader (Thermo Scientific) every 30 s up to 8 h.
BiFC Assay
Tomato Cipk6 and Cbl10 were Gateway cloned into the pXCGW vector, using oligonucleotides OPS13/OPS63 and OPS56/OPS64, respectively; tomato RbohB was cloned into the pXNGW vector (OPS-637/OPS-638). For the BiFC assay, Agrobacterium C58C1 cultures (OD600 = 0.5) carrying pXCGW-Sl-Cipk6 or pXCGW-Sl-Cbl10, pXNGW-Nb-RbohB and tomato bushy stunt virus p19, were mixed at 1:1:1 v/v/v ratio, were coinfiltrated in N. benthamiana plants and kept in the greenhouse for 48 h. YFP reconstitution was visualized by confocal microscopy. FM4-64 was infiltrated (20 µM) and observed immediately by confocal microscopy. See Supplemental Table 2.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: cNbME25F4 (JF974260), cNbME30H4 (JF974261), Nb-Cipk6 (EB449468), Sl-Cbl3 (SGN-U585014), Sl-Cbl4 (SGN-U587071), Sl-Cbl10 (JF831199; Solyc08g065330), Sl-Cipk3 (SGN-U580739), Sl-Cipk6 (JF831200; Solyc12g010130), Sl-Cipk11 (JF831201; Solyc06g082440), Sl-Cipk14 (JF831202; Solyc10g085450), Sl-RbohB (Solyc03g117980), Os-Cbl10 (DQ201204), Os-Cipk6 (Q6Z9F4), Ca-Cipk6 (ACC96114), and Dm-CalciB (NM_080002). From Arabidopsis, they are as follows: At-CIPK6 (AT4g30960), At-CIPK8 (AT4g24400), At-CIPK24 (SOS2, AT5g35410), At-CIPK3 (AT2g26980), At-CIPK9 (AT1g01140), At-CIPK23 (AT1g30270), At-SnRK2 (At1g10940), At-CBL1 (AT4G17615), At-CBL2 (AT5G55990), At-CBL3 (AT4G26570), At-CBL4 (AT5G24270), At-CBL5 (AT4G01420), At-CBL6 (AT4G16350), At-CBL7 (AT4G26560), At-CBL8 (AT1G64480), At-CBL9 (AT5G47100), and At-CBL10 (AT4g33000).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. ORF Cloning of Sl-Cbl10 and Sl-Cipk6 and Derivative VIGS Constructs.
Supplemental Figure 2. Cbl10 Protein Sequence Alignment.
Supplemental Figure 3. SlCipk6 Protein Sequence Alignment.
Supplemental Figure 4. Cipk6 and Cbl10 Genes Are Expressed in All Tissues Analyzed and Their Expression Is Induced after Pathogen Infection.
Supplemental Figure 5. Reduction of Disease Symptoms in N. benthamiana Plants Silenced for Cipk6 or Cbl10.
Supplemental Figure 6. Tomato Cipk and Cbl10 Proteins Were Expressed in Yeast.
Supplemental Figure 7. Cbl10/Cipk6 Interaction Is Not Calcium Dependent in in Vitro Pull-Down Assays.
Supplemental Table 1. TRV-Cbl10 and TRV-Cipk6 Constructs Specifically Target Their Corresponding mRNAs in Tomato.
Supplemental Table 2. Oligonucleotides Used in This Work.
Supplemental Data Set 1. Text File of Alignment Corresponding to Phylogenetic Analysis in Figure 1A.
Supplemental Data Set 2. Text File of Alignment Corresponding to Phylogenetic Analysis in Figure 1B.
Acknowledgments
We thank Peter Moffett for providing Rx2/CP and Gpa2/RBP-1 constructs and the co-IP protocol, Cecile Segonzac for TRV-Nb-RbohB construct and helping with the ROS assay, and Mary Beth Mudgett for Gateway-compatible BiFC vectors. We thank Jose Manuel Pardo for critically reading the article and for suggesting fruitful experiments. This work was funded in part by the European Regional Development Fund through the Ministerio de Ciencia e Innovación (BIO2005-02136 and BIO2009-08648), Junta de Andalucía (P07-CVI-03171), Marie Curie Programme through the International Reintegration grants (MIRG-CT-2005-031174) (O.D.); National Science Foundation IOS-0841807 and (IOS-1025642) (G.B.M.). O.D. was supported in part by the Junta de Andalucía (Programa de Retorno de Investigadores), F.D. by a Juan de la Cierva contract (Ministerio de Ciencia e Innovación, Spain), and Y.P.-J. by Grant BIO2009-08648. E.G.-B. was a recipient of an Formación de Personal Investigador fellowship (Ministerio de Educación, Spain).
AUTHOR CONTRIBUTIONS
F.D. and O.D. designed experiments and analyzed the data. O.D. wrote most of the article, and F.D. and G.B.M. helped with the writing. F.D. performed most of experiments. O.D. performed initial VIGS assays. E.G.-B. performed yeast two-hybrid and RT-PCR analyses. Y.P.-J. performed tomato RbohB cloning and BiFC. S.C. performed in planta experiments using oomycete, viral, and nematode effectors. G.B.M. assisted with data analysis.
Glossary
- PTI
PAMP-triggered immunity
- ETI
effector-triggered immunity
- PAMP
pathogen-associated molecular pattern
- PCD
programmed cell death
- HR
hypersensitive response
- ROS
reactive oxygen species
- MAPK
mitogen-activated protein kinase
- CDPK
Ca2+-dependent protein kinase
- Pst
Pseudomonas syringae pv tomato
- VIGS
virus-induced gene silencing
- PVX
Potato virus X
- TRV
tobacco rattle virus
- cfu
colony-forming units
- DAI
days after infection
- TAP
tandem affinity purification
- co-IP
coimmunoprecipitation
- GFP
green fluorescent protein
- BiFC
bimolecular fluorescence complementation
- YFP
yellow fluorescent protein
References
- Akaboshi M., Hashimoto H., Ishida H., Saijo S., Koizumi N., Sato M., Shimizu T. (2008). The crystal structure of plant-specific calcium-binding protein AtCBL2 in complex with the regulatory domain of AtCIPK14. J. Mol. Biol. 377: 246–257 [DOI] [PubMed] [Google Scholar]
- Albrecht V., Ritz O., Linder S., Harter K., Kudla J. (2001). The NAF domain defines a novel protein-protein interaction module conserved in Ca2+-regulated kinases. EMBO J. 20: 1051–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.C., Pascuzzi P.E., Xiao F., Sessa G., Martin G.B. (2006). Host-mediated phosphorylation of type III effector AvrPto promotes Pseudomonas virulence and avirulence in tomato. Plant Cell 18: 502–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistic O., Kudla J. (2009). Plant calcineurin B-like proteins and their interacting protein kinases. Biochim. Biophys. Acta 1793: 985–992 [DOI] [PubMed] [Google Scholar]
- Bendahmane A., Querci M., Kanyuka K., Baulcombe D.C. (2000). Agrobacterium transient expression system as a tool for the isolation of disease resistance genes: application to the Rx2 locus in potato. Plant J. 21: 73–81 [DOI] [PubMed] [Google Scholar]
- Benschop J.J., Mohammed S., O’Flaherty M., Heck A.J., Slijper M., Menke F.L. (2007). Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol. Cell. Proteomics 6: 1198–1214 [DOI] [PubMed] [Google Scholar]
- Blume B., Nürnberger T., Nass N., Scheel D. (2000). Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12: 1425–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Felix G. (2009). A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Borsani O., Valpuesta V., Botella M.A. (2001). Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol. 126: 1024–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M., Willmann M.R., McCormack M., Lee H., Shan L., He P., Bush J., Cheng S.H., Sheen J. (2010). Differential innate immune signalling via Ca(2+) sensor protein kinases. Nature 464: 418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouch S., Queval G., Noctor G. (2012). AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J. 69: 613–627 [DOI] [PubMed] [Google Scholar]
- Chen L., Ren F., Zhou L., Wang Q.Q., Zhong H., Li X.B. (2012). The Brassica napus calcineurin B-Like 1/CBL-interacting protein kinase 6 (CBL1/CIPK6) component is involved in the plant response to abiotic stress and ABA signalling. J. Exp. Bot. 63: 6211–6222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N.H., Pittman J.K., Zhu J.-K., Hirschi K.D. (2004). The protein kinase SOS2 activates the Arabidopsis H(+)/Ca(2+) antiporter CAX1 to integrate calcium transport and salt tolerance. J. Biol. Chem. 279: 2922–2926 [DOI] [PubMed] [Google Scholar]
- Chiasson D., Ekengren S.K., Martin G.B., Dobney S.L., Snedden W.A. (2005). Calmodulin-like proteins from Arabidopsis and tomato are involved in host defense against Pseudomonas syringae pv. tomato. Plant Mol. Biol. 58: 887–897 [DOI] [PubMed] [Google Scholar]
- Chung J.S., Zhu J.K., Bressan R.A., Hasegawa P.M., Shi H. (2008). Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. Plant J. 53: 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudi A., Cheng Z., O’Brien J.A., Mammarella N., Khan S., Ausubel F.M., Bolwell G.P. (2012). The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24: 275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delledonne M., Zeier J., Marocco A., Lamb C. (2001). Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc. Natl. Acad. Sci. USA 98: 13454–13459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo O., Pedley K.F., Martin G.B. (2004). MAPKKKalpha is a positive regulator of cell death associated with both plant immunity and disease. EMBO J. 23: 3072–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A., Mayer J.E., Hahlbrock K. (1990). Fungal elicitor triggers rapid, transient, and specific protein phosphorylation in parsley cell suspension cultures. J. Biol. Chem. 265: 6360–6368 [PubMed] [Google Scholar]
- Drerup M.M., Schlücking K., Hashimoto K., Manishankar P., Steinhorst L., Kuchitsu K., Kudla J. (2013). The Calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol. Plant 6: 559–569 [DOI] [PubMed] [Google Scholar]
- Du L., Ali G.S., Simons K.A., Hou J., Yang T., Reddy A.S., Poovaiah B.W. (2009). Ca(2+)/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 457: 1154–1158 [DOI] [PubMed] [Google Scholar]
- Dubiella, U., Seybold, H., Durian, G., Komander, E., Lassig, R., Witte, C.P., Schulze, W.X., and Romeis, T. (2013). Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 110: 8744–8749. [DOI] [PMC free article] [PubMed]
- Felix G., Grosskopf D.G., Regenass M., Boller T. (1991). Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc. Natl. Acad. Sci. USA 88: 8831–8834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellbrich G., Romanski A., Varet A., Blume B., Brunner F., Engelhardt S., Felix G., Kemmerling B., Krzymowska M., Nürnberger T. (2002). NPP1, a Phytophthora-associated trigger of plant defense in parsley and Arabidopsis. Plant J. 32: 375–390 [DOI] [PubMed] [Google Scholar]
- Fuglsang A.T., Guo Y., Cuin T.A., Qiu Q., Song C., Kristiansen K.A., Bych K., Schulz A., Shabala S., Schumaker K.S., Palmgren M.G., Zhu J.K. (2007). Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. Plant Cell 19: 1617–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Brugger A., Lamotte O., Vandelle E., Bourque S., Lecourieux D., Poinssot B., Wendehenne D., Pugin A. (2006). Early signaling events induced by elicitors of plant defenses. Mol. Plant Microbe Interact. 19: 711–724 [DOI] [PubMed] [Google Scholar]
- Gassmann W. (2005). Natural variation in the Arabidopsis response to the avirulence gene hopPsyA uncouples the hypersensitive response from disease resistance. Mol. Plant Microbe Interact. 18: 1054–1060 [DOI] [PubMed] [Google Scholar]
- Golemis E.A., Khazak V. (1997). Alternative yeast two-hybrid systems. The interaction trap and interaction mating. Methods Mol. Biol. 63: 197–218 [DOI] [PubMed] [Google Scholar]
- Gong D., Guo Y., Jagendorf A.T., Zhu J.K. (2002). Biochemical characterization of the Arabidopsis protein kinase SOS2 that functions in salt tolerance. Plant Physiol. 130: 256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M., Brown I., Adams S., Knight M., Ainslie A., Mansfield J. (2000). The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 23: 441–450 [DOI] [PubMed] [Google Scholar]
- Greenberg J.T. (1997). Programmed cell death in plant–pathogen interactions. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48: 525–545 [DOI] [PubMed] [Google Scholar]
- Guo Y., Halfter U., Ishitani M., Zhu J.K. (2001). Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 13: 1383–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter U., Ishitani M., Zhu J.K. (2000). The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA 97: 3735–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann D.R., Gimenez-Ibanez S., Rathjen J.P. (2010). Bacterial virulence effectors and their activities. Curr. Opin. Plant Biol. 13: 388–393 [DOI] [PubMed] [Google Scholar]
- Harper J.F., Breton G., Harmon A. (2004). Decoding Ca(2+) signals through plant protein kinases. Annu. Rev. Plant Biol. 55: 263–288 [DOI] [PubMed] [Google Scholar]
- He X., Anderson J.C., del Pozo O., Gu Y.Q., Tang X., Martin G.B. (2004). Silencing of subfamily I of protein phosphatase 2A catalytic subunits results in activation of plant defense responses and localized cell death. Plant J. 38: 563–577 [DOI] [PubMed] [Google Scholar]
- Held K., Pascaud F., Eckert C., Gajdanowicz P., Hashimoto K., Corratgé-Faillie C., Offenborn J.N., Lacombe B., Dreyer I., Thibaud J.B., Kudla J. (2011). Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 21: 1116–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández J.A., Ferrer M.A., Jiménez A., Barceló A.R., Sevilla F. (2001). Antioxidant systems and O(2)(.-)/H(2)O(2) production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 127: 817–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabak E.M., et al. (2003). The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 132: 666–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C.H., Lin S.H., Hu H.C., Tsay Y.F. (2009). CHL1 functions as a nitrate sensor in plants. Cell 138: 1184–1194 [DOI] [PubMed] [Google Scholar]
- Ishiga Y., Ishiga T., Wangdi T., Mysore K.S., Uppalapati S.R. (2012). NTRC and chloroplast-generated reactive oxygen species regulate Pseudomonas syringae pv. tomato disease development in tomato and Arabidopsis. Mol. Plant Microbe Interact. 25: 294–306 [DOI] [PubMed] [Google Scholar]
- Ishitani M., Liu J., Halfter U., Kim C.S., Shi W., Zhu J.K. (2000). SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell 12: 1667–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Belfield E.J., Mithani A., Visscher A., Ragoussis J., Mott R., Smith J.A., Harberd N.P. (2012). ROS-mediated vascular homeostatic control of root-to-shoot soil Na delivery in Arabidopsis. EMBO J. 31: 4359–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.D., Dangl J.L. (2006). The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Katiyar-Agarwal S., Zhu J., Kim K., Agarwal M., Fu X., Huang A., Zhu J.K. (2006). The plasma membrane Na+/H+ antiporter SOS1 interacts with RCD1 and functions in oxidative stress tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 103: 18816–18821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T., Damude H.G., Werner D., Doerner P., Dixon R.A., Lamb C. (1998). A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10: 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.G., Waadt R., Cheong Y.H., Pandey G.K., Dominguez-Solis J.R., Schültke S., Lee S.C., Kudla J., Luan S. (2007). The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J. 52: 473–484 [DOI] [PubMed] [Google Scholar]
- Kimura S., Kaya H., Kawarazaki T., Hiraoka G., Senzaki E., Michikawa M., Kuchitsu K. (2012). Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim. Biophys. Acta 1823: 398–405 [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Ohura I., Kawakita K., Yokota N., Fujiwara M., Shimamoto K., Doke N., Yoshioka H. (2007). Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19: 1065–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Yoshioka M., Asai S., Nomura H., Kuchimura K., Mori H., Doke N., Yoshioka H. (2012). StCDPK5 confers resistance to late blight pathogen but increases susceptibility to early blight pathogen in potato via reactive oxygen species burst. New Phytol. 196: 223–237 [DOI] [PubMed] [Google Scholar]
- Kudla J., Batistic O., Hashimoto K. (2010). Calcium signals: The lead currency of plant information processing. Plant Cell 22: 541–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu T., Hamada J., Hamada H., Hanamata S., Kuchitsu K. (2010). Roles of calcineurin B-like protein-interacting protein kinases in innate immunity in rice. Plant Signal. Behav. 5: 1045–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaaitaal M., Huisman R., Maintz J., Reinstädler A., Panstruga R. (2011). Ionotropic glutamate receptor (iGluR)-like channels mediate MAMP-induced calcium influx in Arabidopsis thaliana. Biochem. J. 440: 355–365 [DOI] [PubMed] [Google Scholar]
- Lecourieux D., Mazars C., Pauly N., Ranjeva R., Pugin A. (2002). Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 14: 2627–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Kim B.G., Cheong Y.H., Pandey G.K., Luan S. (2006). A Ca(2)+ signaling pathway regulates a K(+) channel for low-K response in Arabidopsis. Proc. Natl. Acad. Sci. USA 103: 12625–12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Yang Y., Quan R., Mendoza I., Wu Y., Du W., Zhao S., Schumaker K.S., Pardo J.M., Guo Y. (2009). Phosphorylation of SOS3-LIKE CALCIUM BINDING PROTEIN8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis. Plant Cell 21: 1607–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Ishitani M., Halfter U., Kim C.S., Zhu J.K. (2000). The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA 97: 3730–3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Schiff M., Dinesh-Kumar S.P. (2002). Virus-induced gene silencing in tomato. Plant J. 31: 777–786 [DOI] [PubMed] [Google Scholar]
- López-Solanilla E., Bronstein P.A., Schneider A.R., Collmer A. (2004). HopPtoN is a Pseudomonas syringae Hrp (type III secretion system) cysteine protease effector that suppresses pathogen-induced necrosis associated with both compatible and incompatible plant interactions. Mol. Microbiol. 54: 353–365 [DOI] [PubMed] [Google Scholar]
- Ma W., Smigel A., Tsai Y.C., Braam J., Berkowitz G.A. (2008). Innate immunity signaling: Cytosolic Ca2+ elevation is linked to downstream nitric oxide generation through the action of calmodulin or a calmodulin-like protein. Plant Physiol. 148: 818–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Smigel A., Verma R., Berkowitz G.A. (2009). Cyclic nucleotide gated channels and related signaling components in plant innate immunity. Plant Signal. Behav. 4: 277–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Walker R.K., Zhao Y., Berkowitz G.A. (2012). Linking ligand perception by PEPR pattern recognition receptors to cytosolic Ca2+ elevation and downstream immune signaling in plants. Proc. Natl. Acad. Sci. USA 109: 19852–19857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R., Herr E.H., Orvar B.L., van Camp W., Willekens H., Inzé D., Ellis B.E. (1999). Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyperresponsive to pathogen infection. Proc. Natl. Acad. Sci. USA 96: 14165–14170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucyn T.S., Clemente A., Andriotis V.M., Balmuth A.L., Oldroyd G.E., Staskawicz B.J., Rathjen J.P. (2006). The tomato NBARC-LRR protein Prf interacts with Pto kinase in vivo to regulate specific plant immunity. Plant Cell 18: 2792–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse T.S., Bottrill A.R., Jones A.M., Peck S.C. (2007). Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J. 51: 931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara Y., et al. (2008). Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J. Biol. Chem. 283: 8885–8892 [DOI] [PubMed] [Google Scholar]
- Oh C.S., Martin G.B. (2011). Effector-triggered immunity mediated by the Pto kinase. Trends Plant Sci. 16: 132–140 [DOI] [PubMed] [Google Scholar]
- Ohta M., Guo Y., Halfter U., Zhu J.K. (2003). A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2. Proc. Natl. Acad. Sci. USA 100: 11771–11776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley K.F., Martin G.B. (2003). Molecular basis of Pto-mediated resistance to bacterial speck disease in tomato. Annu. Rev. Phytopathol. 41: 215–243 [DOI] [PubMed] [Google Scholar]
- Popescu S.C., Popescu G.V., Bachan S., Zhang Z., Seay M., Gerstein M., Snyder M., Dinesh-Kumar S.P. (2007). Differential binding of calmodulin-related proteins to their targets revealed through high-density Arabidopsis protein microarrays. Proc. Natl. Acad. Sci. USA 104: 4730–4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan R., Lin H., Mendoza I., Zhang Y., Cao W., Yang Y., Shang M., Chen S., Pardo J.M., Guo Y. (2007). SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 19: 1415–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero F.J., Ohta M., Shi H., Zhu J.K., Pardo J.M. (2002). Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc. Natl. Acad. Sci. USA 99: 9061–9066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X.L., Qi G.N., Feng H.Q., Zhao S., Zhao S.S., Wang Y., Wu W.H. (2013). Calcineurin B-like protein CBL10 directly interacts with AKT1 and modulates K+ homeostasis in Arabidopsis. Plant J. 74: 258–266 [DOI] [PubMed] [Google Scholar]
- Rojas C.M., Senthil-Kumar M., Wang K., Ryu C.M., Kaundal A., Mysore K.S. (2012). Glycolate oxidase modulates reactive oxygen species-mediated signal transduction during nonhost resistance in Nicotiana benthamiana and Arabidopsis. Plant Cell 24: 336–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio V., Shen Y., Saijo Y., Liu Y., Gusmaroli G., Dinesh-Kumar S.P., Deng X.W. (2005). An alternative tandem affinity purification strategy applied to Arabidopsis protein complex isolation. Plant J. 41: 767–778 [DOI] [PubMed] [Google Scholar]
- Sacco M.A., Koropacka K., Grenier E., Jaubert M.J., Blanchard A., Goverse A., Smant G., Moffett P. (2009). The cyst nematode SPRYSEC protein RBP-1 elicits Gpa2- and RanGAP2-dependent plant cell death. PLoS Pathog. 5: e1000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Barrena M.J., Fujii H., Angulo I., Martínez-Ripoll M., Zhu J.K., Albert A. (2007). The structure of the C-terminal domain of the protein kinase AtSOS2 bound to the calcium sensor AtSOS3. Mol. Cell 26: 427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonzac C., Feike D., Gimenez-Ibanez S., Hann D.R., Zipfel C., Rathjen J.P. (2011). Hierarchy and roles of pathogen-associated molecular pattern-induced responses in Nicotiana benthamiana. Plant Physiol. 156: 687–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L., He P., Li J., Heese A., Peck S.C., Nürnberger T., Martin G.B., Sheen J. (2008). Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe 4: 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Kim K.N., Ritz O., Albrecht V., Gupta R., Harter K., Luan S., Kudla J. (1999). Novel protein kinases associated with calcineurin B-like calcium sensors in Arabidopsis. Plant Cell 11: 2393–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichandra C., Gu D., Hu H.C., Davanture M., Lee S., Djaoui M., Valot B., Zivy M., Leung J., Merlot S., Kwak J.M. (2009). Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 583: 2982–2986 [DOI] [PubMed] [Google Scholar]
- Tao Y., Xie Z., Chen W., Glazebrook J., Chang H.S., Han B., Zhu T., Zou G., Katagiri F. (2003). Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell 15: 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapken D., Anschütz U., Liu L.H., Huelsken T., Seebohm G., Becker D., Hollmann M. (2013). A plant homolog of animal glutamate receptors is an ion channel gated by multiple hydrophobic amino acids. Sci. Signal. 6: ra47. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M.A. (2010). ROS in biotic interactions. Physiol. Plant. 138: 414–429 [DOI] [PubMed] [Google Scholar]
- Torres M.A., Jones J.D., Dangl J.L. (2005). Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 37: 1130–1134 [DOI] [PubMed] [Google Scholar]
- Tripathi V., Parasuraman B., Laxmi A., Chattopadhyay D. (2009). CIPK6, a CBL-interacting protein kinase is required for development and salt tolerance in plants. Plant J. 58: 778–790 [DOI] [PubMed] [Google Scholar]
- Tsuda K., Katagiri F. (2010). Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol. 13: 459–465 [DOI] [PubMed] [Google Scholar]
- Van Breusegem F., Bailey-Serres J., Mittler R. (2008). Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiol. 147: 978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues P.E., Batelli G., Grillo S., Agius F., Kim Y.S., Zhu J., Agarwal M., Katiyar-Agarwal S., Zhu J.K. (2007). Interaction of SOS2 with nucleoside diphosphate kinase 2 and catalases reveals a point of connection between salt stress and H2O2 signaling in Arabidopsis thaliana. Mol. Cell. Biol. 27: 7771–7780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M., Chaban C., Schütze K., Batistic O., Weckermann K., Näke C., Blazevic D., Grefen C., Schumacher K., Oecking C., Harter K., Kudla J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Weinl S., Kudla J. (2009). The CBL-CIPK Ca(2+)-decoding signaling network: Function and perspectives. New Phytol. 184: 517–528 [DOI] [PubMed] [Google Scholar]
- Xiao F., He P., Abramovitch R.B., Dawson J.E., Nicholson L.K., Sheen J., Martin G.B. (2007). The N-terminal region of Pseudomonas type III effector AvrPtoB elicits Pto-dependent immunity and has two distinct virulence determinants. Plant J. 52: 595–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Zhou X., Deng X., Guo Y. (2010). PKS5, a SNF1-related kinase, interacts with and phosphorylates NPR1, and modulates expression of WRKY38 and WRKY62. J. Genet. Genomics 37: 359–369 [DOI] [PubMed] [Google Scholar]
- Yang Y., Qin Y., Xie C., Zhao F., Zhao J., Liu D., Chen S., Fuglsang A.T., Palmgren M.G., Schumaker K.S., Deng X.W., Guo Y. (2010). The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. Plant Cell 22: 1313–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H., Numata N., Nakajima K., Katou S., Kawakita K., Rowland O., Jones J.D., Doke N. (2003). Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15: 706–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J., Niu Q.W., Chua N.H. (2000). Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24: 265–273 [DOI] [PubMed] [Google Scholar]