Abstract
Background and Purpose
Risk factors for intracerebral hemorrhage (ICH) have been largely identified in case-control studies, with few longitudinal studies available.
Methods
Predictors of incident ICH among 27,760 African American (AA) and white participants from the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort study were assessed.
Results
There were 62 incident ICH events during an average follow-up of 5.7 years. The increase in risk with age differed substantially between AAs and whites (p = 0.006), with a 2.25-fold (95% CI: 1.63 – 3.12) increase per decade in whites, but no age association with ICH risk in AAs (HR = 1.09; 95% CI: 0.70 – 1.68). We observed increased risk among men, those with higher systolic blood pressure (SBP), and warfarin users.
Conclusions
The racial differences in the impact of age contributed to a risk of ICH that was over 5 times higher for AA than whites at age 45, but only about one-third as great by age 85. Confirming findings from other studies, men, participants with elevated SBP and warfarin users were also at greater risk. The contributors to the racial differences in ICH risk require additional investigation.
Introduction
The low incidence of intracerebral hemorrhage (ICH) events makes the assessment of risk factors challenging. A decade ago, Ariesen and colleagues reviewed largely case-control studies of ICH, concluding that age, male sex and hypertension were the largest risk factors for ICH.1 A case-control study by Woo and colleagues reported risk factors separately for lobar and non-lobar ICH, but also assessed risk factors for a pooled analysis finding increased risk with hypertension, frequent alcohol use, anticoagulant use, history of ischemic stroke and first-degree relative with ICH but not finding associations with diabetes, smoking, drug use, education, or Apo E2/E4.2 In a secondary analysis of a case-control study of ICH assessing a potential role of phenylpropanolamine, hypertension, diabetes, postmenopausal status, current cigarette smoking, alcohol use (>2/day), use of caffeinated drinks (>5/day) and use of drugs containing caffeine were associated with higher risk for ICH.3
In one of the very few prospective examinations of risk factors for ICH, Sturgeon and colleagues combined the data from the Atherosclerosis Risk in Communities (ARIC) study with the Cardiovascular Health Study (CHS) cohort, reporting that older age, black race, and hypertension were risk factors for incident ICH. This study also reported a race-by-age interaction, where at age 45, AAs had 5.8-times the risk of ICH compared to whites, a risk that was reduced to 0.94-times by age 75. The authors also reported a modest interaction between systolic blood pressure (SBP) and age. This report did not find associations of sex, smoking, diabetes, alcohol intake and measures of obesity with ICH risk.4
While surveillance studies are powerful for calculating incident event rates, with the exception of demographic factors (age, race and sex), they are not useful to assess clinical risk factors. However, a surveillance study was the first to show a differential impact of age on ICH risk by race, where between the ages of 55-74 the AA ICH risk was 1.8 times (95% CI: 1.0 – 3.2) greater than whites, but above the age of 75 the risk ratio was only 0.23 (95% CI: 0.1 – 0.8).5 The report of Sturgeon and colleagues is one of few confirmations of this age-by-race interaction, but that study included relatively few AAs, and confounded race with geographic disparities, as the majority of AAs age ≤65 were enrolled at the Jackson (Mississippi) ARIC center.4
While elevated cholesterol was shown to be protective of ICH in the Multiple Risk Factor Intervention Trial (MRFIT) screenee population,6 clinical trial evidence of the association of lipid lowering treatment is inconsistent, with some reports showing ICH risk increased7 and others decreased;8 further, a meta-analysis of 182,803 patients in 31 trials failed to show an association.9
Herein, we assess risk factors for ICH in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study, a longitudinal cohort study of white and African American (AA) community-dwelling participants.
Methods
The REGARDS goals are to advance the understanding of racial and geographic differences in stroke mortality, including assessing risk factors for ischemic stroke and ICH. The study recruited 30,239 community-dwelling participants across the US between 2003 and 2007. The study oversampled the stroke belt (56%) including North Carolina, South Carolina, Georgia, Tennessee, Alabama, Mississippi, Arkansas and Louisiana; with the remainder of the participants from the other 40 contiguous US states. The study also oversampled AAs (44%). Of the eligible participants contacted, the cooperation rate was 49%. A cardiovascular risk survey was completed by telephone and an in-home physical assessment conducted approximately 2 to 3 weeks later. Participants were followed at 6-month intervals by telephone, and medical records were retrieved and physician-adjudicated for suspected strokes. Details of the study methods are provided elsewhere.10
During the in-home assessment, two blood pressure measures were taken after the participant had been seated for five minutes, and average systolic blood pressure (SBP) was used in analyses. Hypertension was defined as SBP ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or self-reported use of antihypertensive medications. Diabetes was defined as fasting glucose ≥ 126 mg/dL (or ≥ 200 mg/dL for participants who failed to fast) or self-reported use of diabetic medications. Chronic kidney disease (CKD) was defined by estimated glomerular filtration rate (using the CKD Epi equation11) of less than 60 mL/min/1.73 m2 or albumin/creatinine ratio (ACR) greater than 30 mg/mmol. High sensitivity C-reactive protein (CRP), total cholesterol, high density lipoprotein and triglycerides were measured centrally, and low density lipoproteins calculated using the Friedewald formula.12 Smoking, current alcohol use, current aspirin use, and use of non-steroidal anti-inflammatory drugs were all defined from the telephone interview. Warfarin use was defined by medication inventory performed in the participant’s home.
Suspected stroke were identified by hospitalization for stroke or stroke-like symptoms that were solicited during follow-up telephone interviews. Medical records were retrieved for each suspected stroke, and stroke endpoints (including the determination of stroke subtype of infarction versus ICH) was determined by physician review. Details of this process are available elsewhere.13
Proportional hazards analysis was used to assess associations between risk factors and incident ICH events though April 1, 2012. Hypothesized interactions (age-race and age-SBP) were assessed. A priori, main effects were assessed at α = 0.05, while interactions were assessed at α = 0.10. Because some medical records could not be retrieved and other records remained in the adjudication process at the time of analysis (approximately 10% each), multiple imputation14 techniques were employed in the analysis to reduce the potential bias arising from undocumented stroke events. Details of the multiple imputation approach employed are provided elsewhere,15 and sensitivity analysis repeating analyses showed no differences in interpretation of the impact of any factors.
Results
Follow-up was available on 29,653 of the 30,239 (98%) study participants, of whom 27,760 (94%) were stroke-free at baseline (see Table 1). There were 62 ICH events over a follow-up averaging 5.7 years.
Table 1.
Description of the study population (n = 27,760)
| Age (years) | 64.6 ± 9.4 | |
| Black Race (%) | 40 | |
| Male Sex (%) | 45 | |
| Region | Belt | 34 |
| Buckle | 21 | |
| Rest of Nation | 44 | |
| Hypertension* (%) | 58 | |
| SBP (mmHg) | 127.3 ± 16.5 | |
| Diabetes† (%) | 21 | |
| CKD (%) | 22 | |
| Total Chol (mg/dL) | 192.5 ± 39.9 | |
| Triglycerides (mg/dL) | 131.8 ± 86.1 | |
| HDL (mg/dL) | 52.0 ± 16.3 | |
| LDL (mg/dL) | 131.8 ± 86.1 | |
| CRP | 4.6 ± 8.6 | |
| Smoking | Never | 46 |
| Past | 40 | |
| Current | 14 | |
| Alcohol Use | 38 | |
| Warfarin use | 3 | |
| Aspirin Use | 42 | |
| NSAID Use | 14 | |
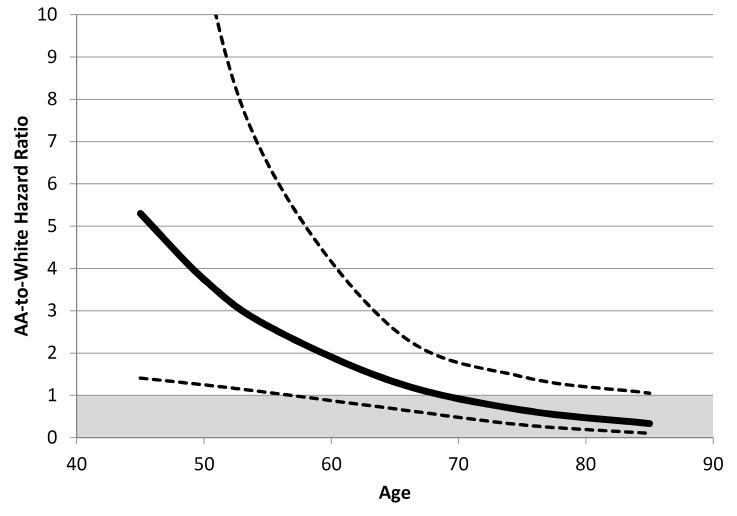
The crude event rates are shown for three age strata: 45-64 years, 65-74 years, and 75+ years (Table 2). For whites, there was a marked increased risk across the age strata (0.10% to 0.31% to 0.47%), but this was not seen for AAs across the age strata (0.21% to 0.17% to 0.25%). When age was modeled as a continuous variable (Table 3), the age-by-race interaction was highly significant (p = 0.0062), and not substantially mediated by other predictors of ICH risk. Because of this significant interaction, the remaining results, focus on the impact of race and age on ICH risk (Table 3). Table 4 displays the association of potential predictive variables. The estimated AA-to-white hazard ratio in the fully-adjusted model is also shown in Figure 1, where ICH risk in AAs is approximately 5-times greater than whites at age 45, but only one-third as great at age 85. For each 10-year difference in age, the risk of an ICH was 2.25 (95% CI: 1.63 – 3.12) times greater for whites, and was modestly attenuated to 2.03 (95% CI: 1.44 – 2.86) by adjustment for other significant factors (Table 3). In contrast, for AAs, for each 10-year difference in age, there was only a 1.09-times (95% CI: 0.70 – 1.68) increase in ICH risk, and this was almost completely attenuated (HR = 1.01; 95% CI: 0.65 – 1.58) by adjustment for other factors.
Table 2.
Number of Participants/Number (%) of Intracerebral Hemorrhage Events by Age Strata and Race; and the Event Rate per 100 000 Person-Years (With 95% Confidence Intervals)
| White | Black | |
|---|---|---|
| 45–64 y | 8129 | 6157 |
| 8 (0.10) | 13 (0.21) | |
| 21.5 (11.2–41.2) | 46.0 (26.5–79.7) | |
| 65–74 y | 5410 | 3472 |
| 17 (0.31) | 6 (0.17) | |
| 65.1 (39.1–108.4) | 40.1 (17.8–90.4) | |
| 75+ y | 3006 | 1586 |
| 14 (0.47) | 4 (0.25) | |
| 105.0 (64.3–171.3) | 65.8 (24.4–177.8) |
Table 3.
Hazard ratio for incident ICH assessing the age-by-race interaction (p-value), race-specific effects of age, and age-specific racial differences; all show in incremental models (race-age alone, addition of sex, addition of SBP, and the addition of warfarin) corresponding to Table 4
| Race-Age Alone |
After adjustment for Sex |
After further adjustment for SBP |
After further adjustment for warfarin |
||
|---|---|---|---|---|---|
| Race*age interaction p- value |
0.0062 | 0.0066 | 0.0071 | 0.010 | |
| Race- specific association of age (10 years) |
White | 2.25 (1.63 – 3.12) <0.0001 |
2.23 (1.60 – 3.09) <0.0001 |
2.12 (1.52 – 2.97) <0.0001 |
2.03 (1.44 – 2.86) <0.0001 |
| African American |
1.09 (0.70 – 1.68) 0.71 |
1.07 (0.69 – 1.66) 0.76 |
1.02 (0.66 – 1.60) 0.92 |
1.01 (0.65 – 1.58) 0.96 |
|
| Age- specific association of AA race |
45 | 5.41 (1.48 – 19.93) 0.011 |
6.20 (1.68 – 22.89) 0.0063 |
5.58 (1.49 – 20.90) 0.011 |
5.30 (1.41 – 19.91) 0.014 |
| 55 | 2.60 (1.08 – 6.29) 0.033 |
2.98 (1.23 – 7.20) 0.016 |
2.69 (1.10 – 6.61) 0.031 |
2.64 (1.07 – 6.48) 0.034 |
|
| 65 | 1.25 (0.66 – 2.40) 0.49 |
1.43 (0.75 – 2.73) 0.27 |
1.30 (0.67 – 2.51) 0.43 |
1.31 (0.68 – 2.54) 0.41 |
|
| 75 | 0.60 (0.28 – 1.31) 0.30 |
0.69 (0.32 – 1.50) 0.34 |
0.63 (0.29 – 1.38) 0.24 |
0.65 (0.30 – 1.44) 0.29 |
|
| 85 | 0.29 (0.09 – 0.92) 0.036 |
0.33 (0.10 – 1.05) 0.061 |
0.30 (0.09 – 0.97) 0.045 |
0.33 (0.10 – 1.05) 0.061 |
|
Table 4.
Hazard ratio, 95% confidence limits and p-value for predictors of ICH, shown in univariate, and after adjustment in incremental regression models; and final estimates from the most parsimonious model (also adjusted for age, race and age-race interaction)
| Univariate effects |
Adjustment for age-race |
Further adjustment for sex |
Further adjustment for SBP |
Further adjustment for warfarin use |
Most parsimonious model |
||
|---|---|---|---|---|---|---|---|
| Age (10 year) | Because of age-race interactions, estimates in these models is provided in Table 3 | ||||||
| Black Race | |||||||
| Male Sex | 2.83 (1.63 – 4.91) 0.0003 |
2.75 (1.60 – 4.73) 0.0003 |
2.60 (1.50 – 4.48) 0.0007 |
||||
| Region | Belt | 0.71 (0.41 – 1.23) 0.22 |
0.74 (0.43 – 1.27) 0.28 |
0.76 (0.44 – 1.32) 0.33 |
0.75 (0.43 – 1.30) 0.31 |
0.76 (0.44 – 1.31) 0.32 |
|
| Buckle | 0.90 (0.47 – 1.70) 0.74 |
0.94 (0.49 – 1.78) 0.84 |
1.01 (0.53 – 1.93) 0.77 |
1.01 (0.53 – 1.94) 0.97 |
1.01 (0.53 – 1.93) 0.97 |
||
| Hypertension | 1.57 (0.91 – 2.68) 0.10 |
1.35 (0.78 – 2.34) 0.28 |
1.38 (0.80 – 2.38) 0.25 |
1.08 (0.59 – 1.98) 0.78 |
1.06 (0.58 – 1.94) 0.85 |
||
| SBP (10 mmHg) | 1.24 (1.10 – 1.40) 0.0003 |
1.20 (1.06 – 1.36) 0.0053 |
1.18 (1.04 – 1.35) 0.011 |
1.19 (1.04 – 1.36) 0.0089 |
|||
| Diabetes | 1.28 (0.70 – 2.33) 0.43 |
1.29 (0.71 – 2.33) 0.40 |
1.23 (0.68 – 2.22) 0.50 |
1.17 (0.64 – 2.12) 0.61 |
1.14 (0.63 – 2.08) 0.66 |
||
| CKD | 2.26 (1.28 – 3.98) 0.0052 |
1.75 (0.98 – 3.13) 0.058 |
1.74 (0.97 – 3.10) 0.062 |
1.59 (0.89 – 2.86) 0.12 |
1.54 (0.85 – 2.77) 0.15 |
||
| Total Chol (10 mg/dL) | 0.95 (0.89 – 1.01) 0.12 |
0.96 (0.90 – 1.03) 0.28 |
0.99 (0.93 – 1.06) 0.82 |
0.99 (0.92 – 1.05) 0.70 |
0.99 (0.93 – 1.06) 0.75 |
||
| LN(Trig) | 0.82 (0.49 – 1.38) 0.45 |
0.84 (0.49 – 1.44) 0.52 |
0.84 (0.50 – 1.43) 0.53 |
0.80 (0.47 – 1.36) 0.41 |
0.79 (0.46 – 1.34) 0.38 |
||
| HDL (10 mg/dL) | 0.87 (0.75 – 1.02) 0.098 |
0.87 (0.74 – 1.02) 0.090 |
0.97 (0.82 – 1.15) 0.76 |
0.98 (0.83 – 1.15) 0.78 |
0.98 (0.83 – 1.16) 0.84 |
||
| LDL (10 mg/dL) | 0.97 (0.91 – 1.05) 0.49 |
0.99 (0.92 – 1.07) 0.83 |
1.00 (0.93 – 1.08) 0.90 |
1.00 (0.93 – 1.08) 1.00 |
1.00 (0.93 – 1.08) 0.94 |
||
| LN(CRP) | 1.06 (0.84 – 1.32) 0.63 |
1.06 (0.84 – 1.33) 0.60 |
1.14 (0.90 – 1.43) 0.28 |
1.12 (0.89 – 1.41) 0.34 |
1.10 (0.87 – 1.39) 0.43 |
||
| Smoking (vs. never-smoking) |
Past | 1.31 (0.77 – 2.21) 0.32 |
1.27 (0.75 – 2.15) 0.37 |
1.04 (0.60 – 1.78) 0.89 |
1.02 (0.60 – 1.75) 0.94 |
1.02 (0.60 – 1.76) 0.93 |
|
| Current | 1.22 (0.56 – 2.66) 0.61 |
1.53 (0.69 – 3.40) 0.29 |
1.38 (0.62 – 3.08) 0.43 |
1.32 (0.59 – 2.95) 0.50 |
1.33 (0.59 – 2.99) 0.48 |
||
| Smoking (versus non-smoking) | 1.07 (0.51 – 2.25) 0.86 |
1.36 (0.63 – 2.92) 0.43 |
1.35 (0.63 – 2.91) 0.44 |
1.30 (0.60 – 2.82) 0.50 |
1.32 (0.61 – 2.85) 0.48 |
||
| Alcohol Use | 1.05 (0.64 – 1.75) 0.84 |
1.16 (0.71 – 1.90) 0.55 |
0.97 (0.59 – 1.61) 0.91 |
0.97 (0.58 – 1.60) 0.89 |
0.97 (0.58 – 1.60) 0.90 |
||
| Warfarin use | 3.54 (1.63 – 7.67) 0.0014 |
2.40 (1.08 – 5.33) 0.032 |
2.15 (0.96 – 4.78) 0.062 |
2.24 (1.01 – 5.00) 0.048 |
2.24 (1.01 – 5.00) 0.048 |
||
| Aspirin Use | 1.51 (0.87 – 2.59) 0.14 |
1.32 (0.76 – 2.32) 0.32 |
1.19 (0.67 – 2.11) 0.55 |
1.19 (0.67 – 2.10) 0.55 |
1.27 (0.71 – 2.26) 0.41 |
||
| NSAID Use | 0.50 (0.17 – 1.43) 0.19 |
0.55 (0.19 – 1.58) 0.26 |
0.63 (0.22 – 1.80) 0.38 |
0.61 (0.21 – 1.77) 0.36 |
0.62 (0.22 – 1.79) 0.38 |
||
Figure 1.
AA-to-white hazard ratio (and 95% confidence interval) for intracerebral hemorrhage as a function of age after adjustment for sex, systolic blood pressure and warfarin use
Table 4 provides the outcome of the model-building, where univariately male sex was associated with a nearly three-times higher risk of ICH (HR = 2.83; 95% CI: 1.63 – 4.91). For each 10 mmHg higher difference in SBP there was a 24% increased risk of ICH (HR = 1.24; 95% CI: 1.10 – 1.40), with prevalent CKD more than a doubling of risk (HR = 2.26; 95% CI: 1.28 – 3.98), and with warfarin use more than three-times increased risk (HR = 3.54; 95% CI: 1.63 – 7.67). No other factors were significantly associated with ICH risk (p > 0.05), with a univariate association for CKD was mediated and becoming insignificant after adjustment for SBP (p = 0.12).
We were unable to demonstrate the age-by-SBP interaction as observed by Sturgeon and colleagues4 to be significant.
Discussion
Race played a major role in the ICH risk pattern with increasing age, where for white participants there was over a doubling of risk per decade of age, but virtually no change in risk across age periods for AAs. These race-specific differences had a profound impact on the AA-to-white relative risk of ICH, with AAs having over 5-times increased risk at age 45, but only one-third the risk at age 85. Our findings of a race-age interaction were strikingly similar to those reported by Sturgeon,4 who reported an AA-white relative risk of 5.8 at age 45 (compared to 5.41 in our data), a risk of 1.7-times at age 65 (compared to 1.25 in our data), and 0.94 at age 75 (compared to 0.60 in our data). These findings were also generally concordant with the surveillance study observations made by Broderick and colleagues, where the AA ICH risk was 1.8 times greater than whites between ages of 55 to 74, but was only 0.23 that of whites over the age of 75.5 We have previously reported that the relative risk at age 45 for “all stroke” events was 2.90-times (95% CI: 1.72 – 4.89) greater among AAs relative to whites.13, 16 With AA-to-white risk of ICH at age 45 being 5.41-times (95% CI: 1.48 – 19.93) greater, it seems likely that AA-to-white differences in ICH risk at young ages are larger than the overall excess of stroke events, and contributing to the higher “all stroke” risk.
We also confirmed SBP and male sex as powerful predictors of risk of ICH events. Unlike other reports,1-4 we failed to detect an association with hypertension status. We defined hypertension by elevated systolic and/or diastolic blood pressure levels or use of anti-hypertensive medications. Of the 16,017 hypertensive participants in this report, 13,867 (87%) were on current treatment. Compared to studies recruiting subjects during earlier years, it is possible we failed to show hypertension as a risk factor since at the time of our baseline visit (2003-2007) a larger proportion of hypertensive participants were on treatment, and those who were treated had better blood pressure control than study participants in earlier studies. Supporting this possibility, in the case-control study of Woo and colleagues, there was a higher odds ratio of 3.5 (95% CI: 2.3 – 5.2) for ICH risk with untreated hypertension, but a much more modest odds ratio of 1.4 (95% CI: 1.0 – 1.9) for treated hypertension.17
Bleeding is one of the expected side effects of warfarin use, with an estimated hazard ratio of 1.71 (95% CI: 1.21 – 2.41) for oral anticoagulation relative to aspirin therapy in a pooled analysis of 6 randomized clinical trials.18 Furthermore, recent results of the Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial confirmed excess cerebral hemorrhage risk among warfarin users (RR-2.05, CI: 1.36-3.12), though there was not a significant difference in risk of intracerebral or intracranial hemorrhage between the groups.19 However, literature documenting higher ICH risk for warfarin use in population-based studies is surprisingly sparse. There are two case-control studies reported by Woo and colleagues showing a univariate odds ratio three- to four-times greater for users of anticoagulants than non-users (p < 0.0001).2, 17 We also observed a univariate 3.54-times increased risk associated with warfarin use, a difference that was attenuated to 2.24 times greater by adjustment for other risk factors (including SBP). Warfarin use was not examined in the review papers by Ariesen and colleagues1 and Sturgeon and colleagues,4 and no association was seen in the large case-control series reported by Feldman.3
While CKD was strongly (HR = 2.26; p = 0.0052) associated with ICH risk in univariate models, adjustment for other risk factors substantially attenuated the association (HR = 1.54; p = 0.15) suggesting a confounding of CKD with these factors was responsible for the substantial univariate effect. Others have suggested higher ICH risk with heavy alcohol intake.1-3 Using the National Institute on Alcohol Abuse and Alcoholism (NIAAA) classification scheme,20 only 4% of REGARDS participants reported heavy drinking (7+ drinks/week for women, 14+ drinks/week for men). Because of the small proportion of participants reporting heavy drinking, we were compelled to examine “any alcohol use” rather than “heavy use,” and as such, an association with heavy drinking could not be ruled out. While we did observe a higher risk among current smokers and diabetic participants, the differences failed to reach statistical significance; however, the pattern for these risk factors was consistent with other studies.1, 3, 4
We failed to observe an association for total cholesterol or any of the subclasses (p > 0.10). Most reports assessing lipids as a risk factor for ICH focused on the association with total cholesterol, failing to find an association.9 A report by Ariesen showed significant evidence of heterogeneity (p = 0.001) of a lipid effect between studies and little evidence of a pooled effect (odds ratio = 1.22; 95% CI: 0.56 – 2.67).1 The studies included in that meta-analysis bridged the advent of neuroimaging, and one potential contributor to the heterogeneity (and hence to the lack of a finding) is the absence of advanced diagnostic imaging in the early studies included in his analysis. There was also no evidence of an association between total cholesterol and ICH risk in the subsequent report of Feldmann and colleagues3 or Sturgeon and colleagues.4 A meta-analysis of treatment for lipid lowering in randomized trials conducted primarily for secondary coronary risk reduction also failed to show a significant association with ICH risk.7 Our findings may suggest that the effect of lipids on ICH risk is absent or quite small.
In general, we suggest caution in the interpretation of risk factors where associations with ICH were not detected. While we had 156,876 person-years of exposure, we accrued only 62 ICH events, for a crude incidence rate of 39.5 events per 100,000 person-years. This incidence rate was only slightly lower than the crude rate of 51.2 per 100,000 person-years (135 events in 263,489 years of exposure) reported by Sturgeon and colleagues.3 However, the relatively small number of events in REGARDS limits statistical power. Specifically, for risk factors that are approximately 50% prevalent (such as hypertension or aspirin use), 62 events provides 80% power to detect a hazard ratio of 2.04. For risk factors such as diabetes or CKD that are 20% prevalent, a hazard ratio of 2.43 can be detected with 80% power. Hence, a risk factor would have to present at least a moderate impact on ICH risk to have a high probability of detection in our study.
As REGARDS continues to accrue stroke events, we look forward to having a sufficient number of ICH events to perform risk function analyses focusing on risk factors for ICH in specific brain regions and assess the racial differences in ICH risk in specific brain regions.
The study has several substantial strengths and a few limitations. A major strength of the REGARDS cohort is its substantial sample size and prospective design, allowing the assessment of risk factors for the relatively rare ICH events to be estimated in a single cohort under a single protocol. In addition, the oversampling of AAs in the cohort provides an unprecedented opportunity to evaluate racial differences in ICH risk. The study design of REGARDS, where participants are dispersed across approximately 60% of the counties in the continental US, makes the on-going monitoring of factors assessed at baseline (such as warfarin use) infeasible, and requires the analysis to depend on assessments made at baseline. Also, as noted above statistical power is limited by a relatively small number of events (62).
In conclusion, we report age and race have a dynamic interplay in the risk of ICH, with substantial increases in risk with increasing age for whites, a pattern that was not present in AAs. At young ages, AAs had over 5 times the risk of ICH compared with their white counterparts; however, at older ages their risk was only about one third that of whites. We also confirm male sex and elevated systolic blood pressure as major risk factors for ICH, and have confirmed the increased risk among warfarin users in the general population. Clearly, the contributors to the substantial age-by-race interaction need further investigation.
Footnotes
Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: A systematic review. Stroke; a journal of cerebral circulation. 2003;34:2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
- 2.Woo D, Sauerbeck LR, Kissela BM, Khoury JC, Szaflarski JP, Gebel J, et al. Genetic and environmental risk factors for intracerebral hemorrhage: Preliminary results of a population-based study. Stroke; a journal of cerebral circulation. 2002;33:1190–1195. doi: 10.1161/01.str.0000014774.88027.22. [DOI] [PubMed] [Google Scholar]
- 3.Feldmann E, Broderick JP, Kernan WN, Viscoli CM, Brass LM, Brott T, et al. Major risk factors for intracerebral hemorrhage in the young are modifiable. Stroke; a journal of cerebral circulation. 2005;36:1881–1885. doi: 10.1161/01.STR.0000177480.62341.6b. [DOI] [PubMed] [Google Scholar]
- 4.Sturgeon JD, Folsom AR, Longstreth WT, Jr., Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke; a journal of cerebral circulation. 2007;38:2718–2725. doi: 10.1161/STROKEAHA.107.487090. [DOI] [PubMed] [Google Scholar]
- 5.Broderick JP, Brott T, Tomsick T, Huster G, Miller R. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. The New England journal of medicine. 1992;326:733–736. doi: 10.1056/NEJM199203123261103. [DOI] [PubMed] [Google Scholar]
- 6.Iso H, Jacobs DR, Jr., Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. The New England journal of medicine. 1989;320:904–910. doi: 10.1056/NEJM198904063201405. [DOI] [PubMed] [Google Scholar]
- 7.Corvol JC, Bouzamondo A, Sirol M, Hulot JS, Sanchez P, Lechat P. Differential effects of lipid-lowering therapies on stroke prevention: A meta-analysis of randomized trials. Archives of internal medicine. 2003;163:669–676. doi: 10.1001/archinte.163.6.669. [DOI] [PubMed] [Google Scholar]
- 8.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. The New England journal of medicine. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 9.McKinney JS, Kostis WJ. Statin therapy and the risk of intracerebral hemorrhage: A meta-analysis of 31 randomized controlled trials. Stroke; a journal of cerebral circulation. 2012;43:2149–2156. doi: 10.1161/STROKEAHA.112.655894. [DOI] [PubMed] [Google Scholar]
- 10.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The reasons for geographic and racial differences in stroke study: Objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 13.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Annals of neurology. 2011;69:619–627. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin DB. Multiple imputation for nonresponse in surveys. J. Wiley & Sons; New York: 1987. [Google Scholar]
- 15.Howard G, McClure LA, Moy CS, Safford MM, Cushman M, Judd SE, et al. Imputation of incident events in longitudinal cohort studies. American journal of epidemiology. 2011;174:718–726. doi: 10.1093/aje/kwr155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, et al. Traditional risk factors as the underlying cause of racial disparities in stroke: Lessons from the half-full (empty?) glass. Stroke; a journal of cerebral circulation. 2011;42:3369–3375. doi: 10.1161/STROKEAHA.111.625277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo D, Haverbusch M, Sekar P, Kissela B, Khoury J, Schneider A, et al. Effect of untreated hypertension on hemorrhagic stroke. Stroke; a journal of cerebral circulation. 2004;35:1703–1708. doi: 10.1161/01.STR.0000130855.70683.c8. [DOI] [PubMed] [Google Scholar]
- 18.van Walraven C, Hart RG, Singer DE, Laupacis A, Connolly S, Petersen P, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: An individual patient meta-analysis. JAMA : the journal of the American Medical Association. 2002;288:2441–2448. doi: 10.1001/jama.288.19.2441. [DOI] [PubMed] [Google Scholar]
- 19.Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. The New England journal of medicine. 2012;366:1859–1869. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(NIAAA). NIoAAaA The physicians’ guide to helping patients with alcohol problems (revised) 2004. [DOI] [PubMed]