Abstract
The attachment and eclipse of adenovirus have been studied with the aid of highly purified 14C-threonine and 32P-labeled adenovirus type 2 in KB cells in suspension cultures. Adenovirus particles and infectivity appear to attach at the same rate. The attachment rate appears to be highly dependent on the cell concentration and less dependent on virus concentration within the multiplicity range from 0.15 to 3 plaque-forming units per cell, probably corresponding to 4.5 to 90 particles per cell. Subsequent to attachment, 5 to 8% of the 14C label is eluted from the cell at a structure level, corresponding to free hexon. The 32P activity is rapidly associated with the cells and is converted within 20 to 30 min to 65 to 85% deoxyribonuclease-susceptible material. This process is unaffected by actinomycin and puromycin. The deoxyribonuclease-sensitive material is, however, associated with 14C label for an extended period after infection, and does not sediment as free deoxyribonucleic acid in sucrose gradients. The implications of these findings on the penetration mechanism of animal viruses are discussed.
Full text
PDF

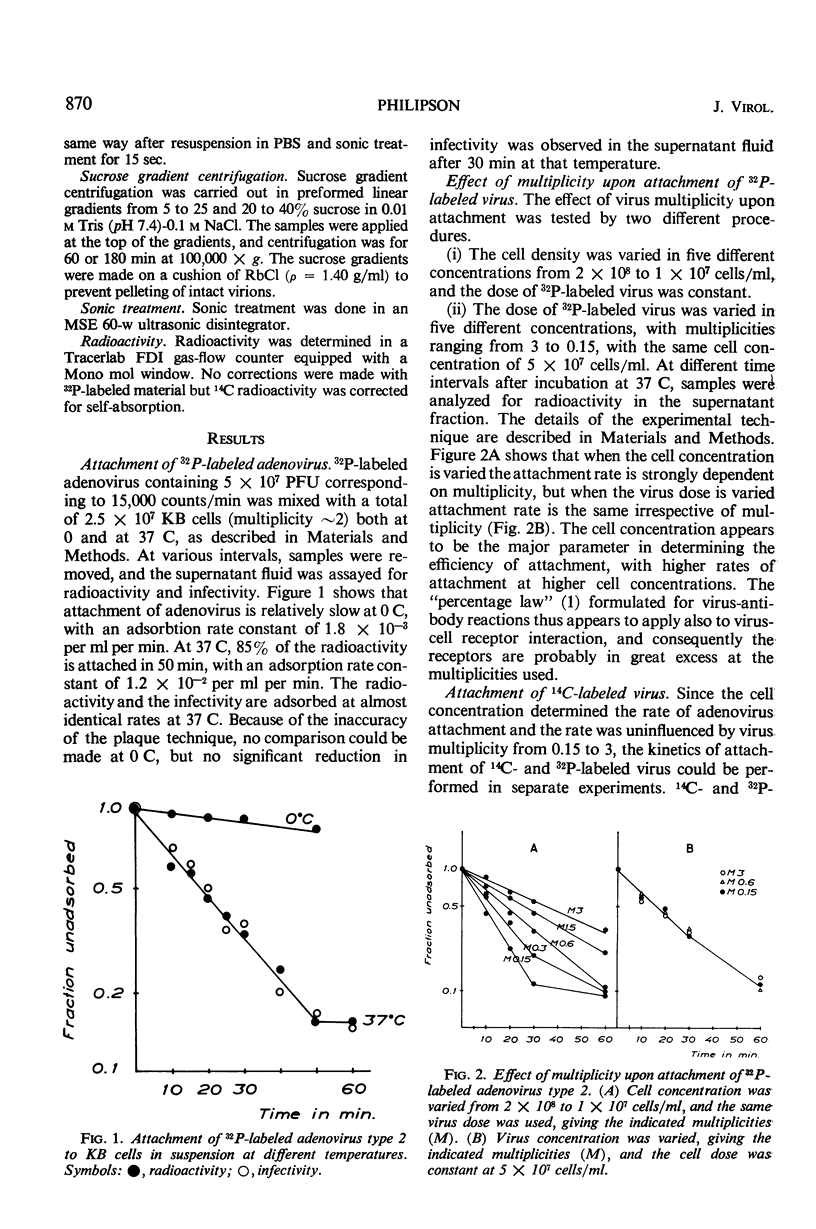

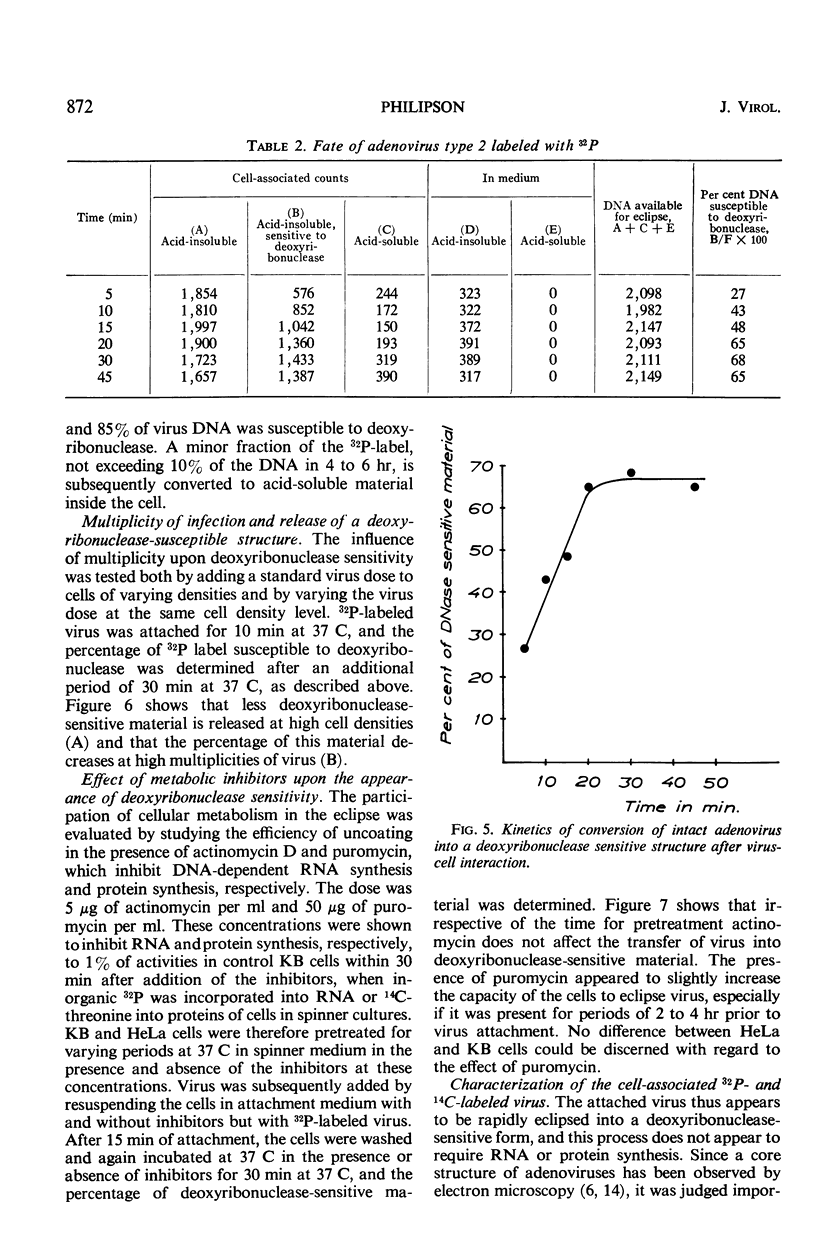



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohn Z. A. The regulation of pinocytosis in mouse macrophages. I. Metabolic requirements as defined by the use of inhibitors. J Exp Med. 1966 Oct 1;124(4):557–571. doi: 10.1084/jem.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. An electron microscope study of the early association between two mammalian viruses and their hosts. J Cell Biol. 1962 May;13:303–322. doi: 10.1083/jcb.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S. Pentration of animal viruses into cells. Prog Med Virol. 1965;7:1–43. [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., HOLT S. J., POWELL A. K. The fine structure and composition of type 5 adenovirus; an integrated electron microscopical and cytochemical study. Br J Exp Pathol. 1960 Dec;41:567–576. [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION, VI. PROPERTIES OF HIGHLY PURIFIED TUMORIGENIC HUMAN ADENOVIRUSES AND THEIR DNA. Proc Natl Acad Sci U S A. 1964 Jun;51:1251–1259. doi: 10.1073/pnas.51.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R. C. Biochemical studies on adenovirus multiplication. XII. Plaquing efficiencies of purified human adenoviruses. Virology. 1967 Mar;31(3):562–565. doi: 10.1016/0042-6822(67)90241-3. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D., CHASE M. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J Gen Physiol. 1952 May;36(1):39–56. doi: 10.1085/jgp.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., HOYER B. H. Early stages of enterovirus infection. Cold Spring Harb Symp Quant Biol. 1962;27:101–112. doi: 10.1101/sqb.1962.027.001.013. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR UNCOATING OF POXVIRUS DNA. I. THE FATE OF RADIOACTIVELY-LABELED RABBITPOX VIRUS. J Mol Biol. 1964 Feb;8:263–276. doi: 10.1016/s0022-2836(64)80136-4. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR UNCOATING OF POXVIRUS DNA. II. THE MOLECULAR BASIS OF THE UNCOATING PROCESS. J Mol Biol. 1964 Feb;8:277–288. doi: 10.1016/s0022-2836(64)80137-6. [DOI] [PubMed] [Google Scholar]
- Joklik W. K. The molecular basis of the viral eclipse phase. Prog Med Virol. 1965;7:44–96. [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Messenger RNA synthesis by a "coated" viral genome. Proc Natl Acad Sci U S A. 1967 Feb;57(2):314–320. doi: 10.1073/pnas.57.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILIPSON L. Adenovirus assay by the fluorescent cell-counting procedure. Virology. 1961 Nov;15:263–268. doi: 10.1016/0042-6822(61)90357-9. [DOI] [PubMed] [Google Scholar]
- PHILIPSON L., LIND M. ENTEROVIRUS ECLIPSE IN A CELL-FREE SYSTEM. Virology. 1964 Jul;23:322–332. doi: 10.1016/0042-6822(64)90254-5. [DOI] [PubMed] [Google Scholar]
- Woodson B. Vaccinia mRNA synthesis under conditions which prevent uncoating. Biochem Biophys Res Commun. 1967 Apr 20;27(2):169–175. doi: 10.1016/s0006-291x(67)80057-3. [DOI] [PubMed] [Google Scholar]


