Abstract
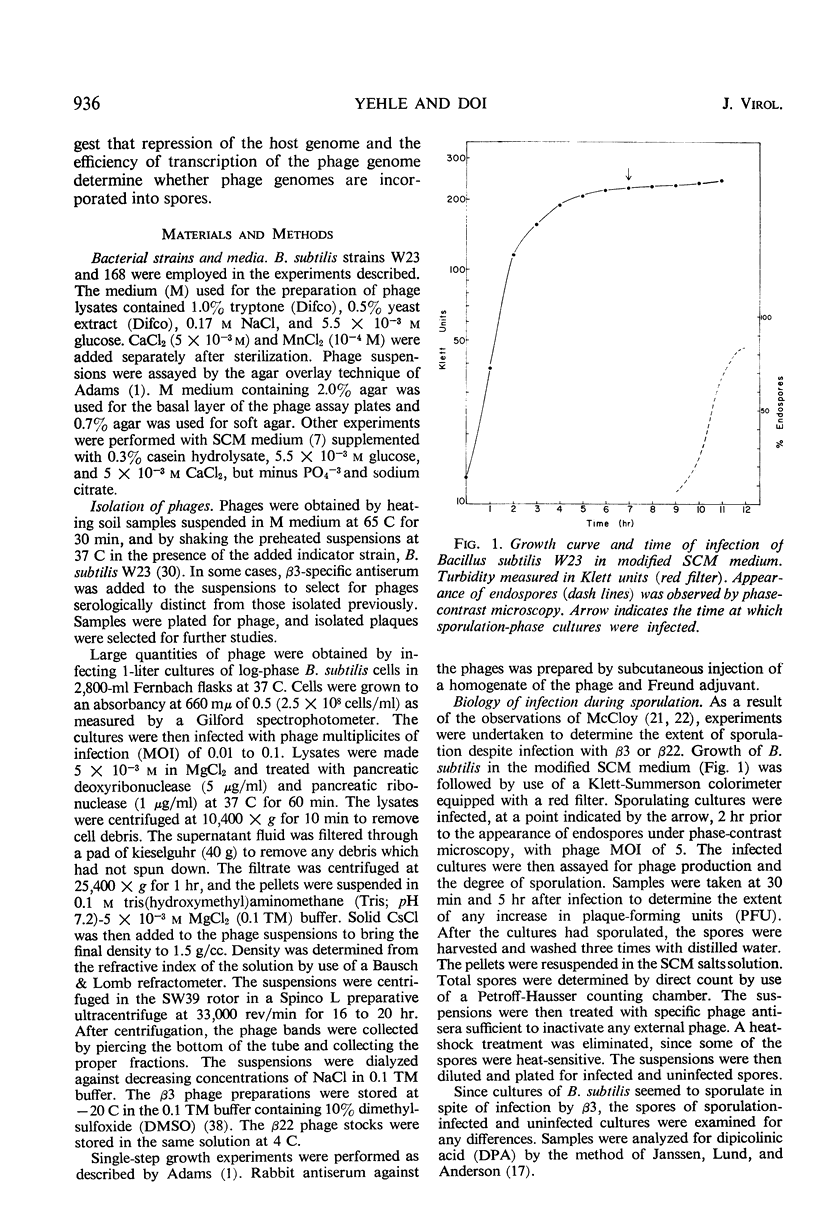
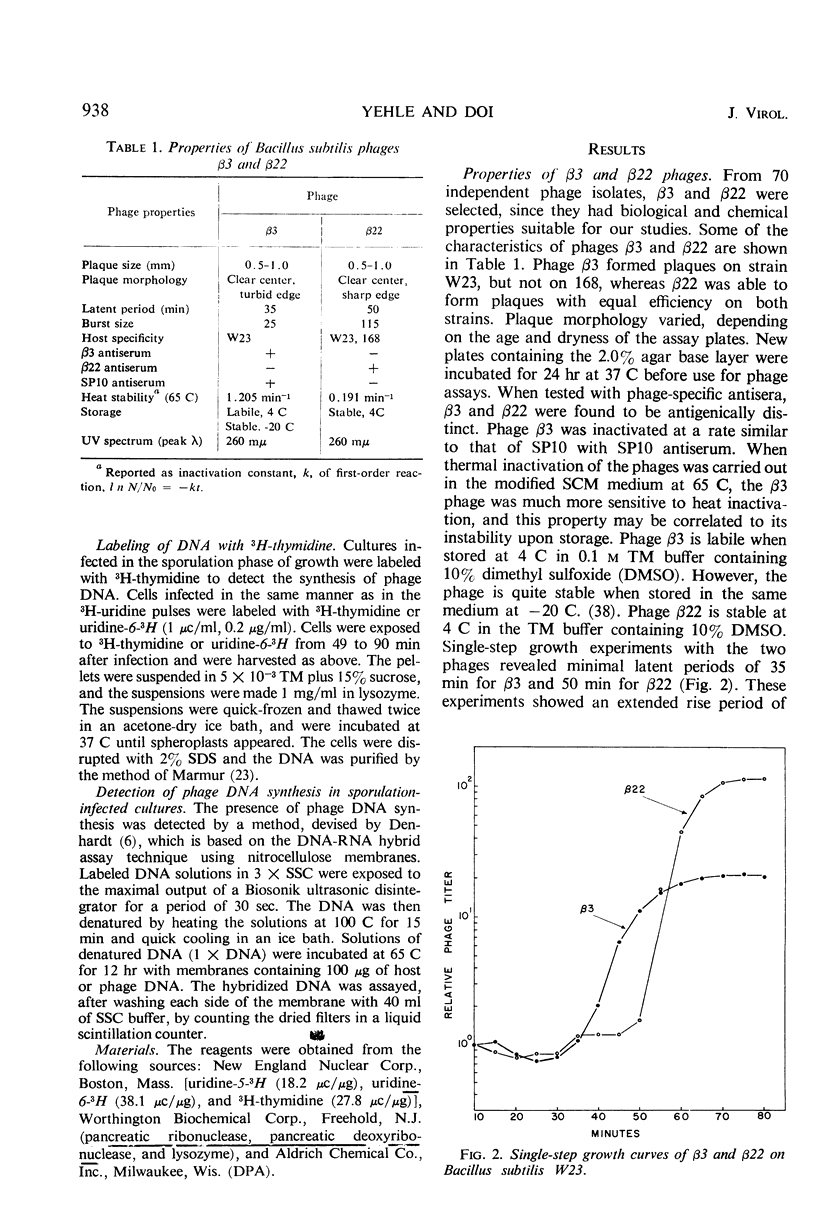
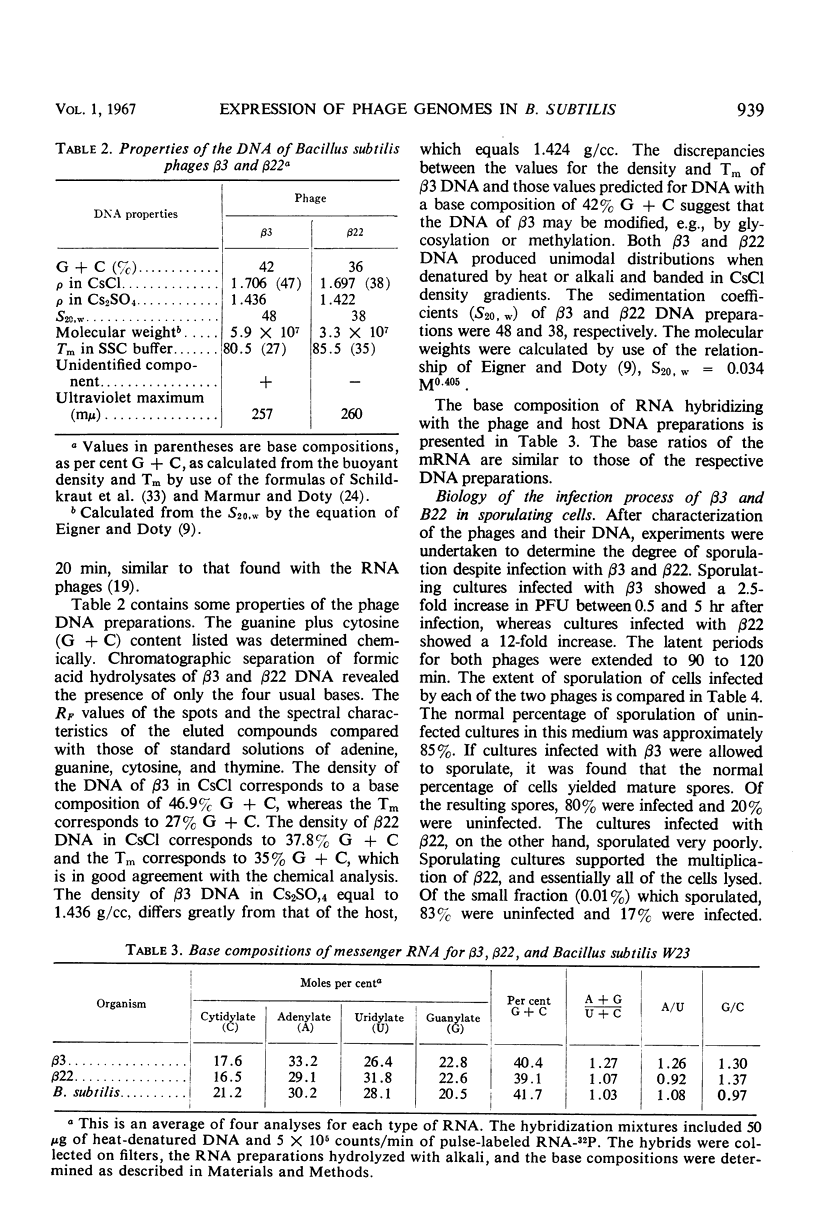
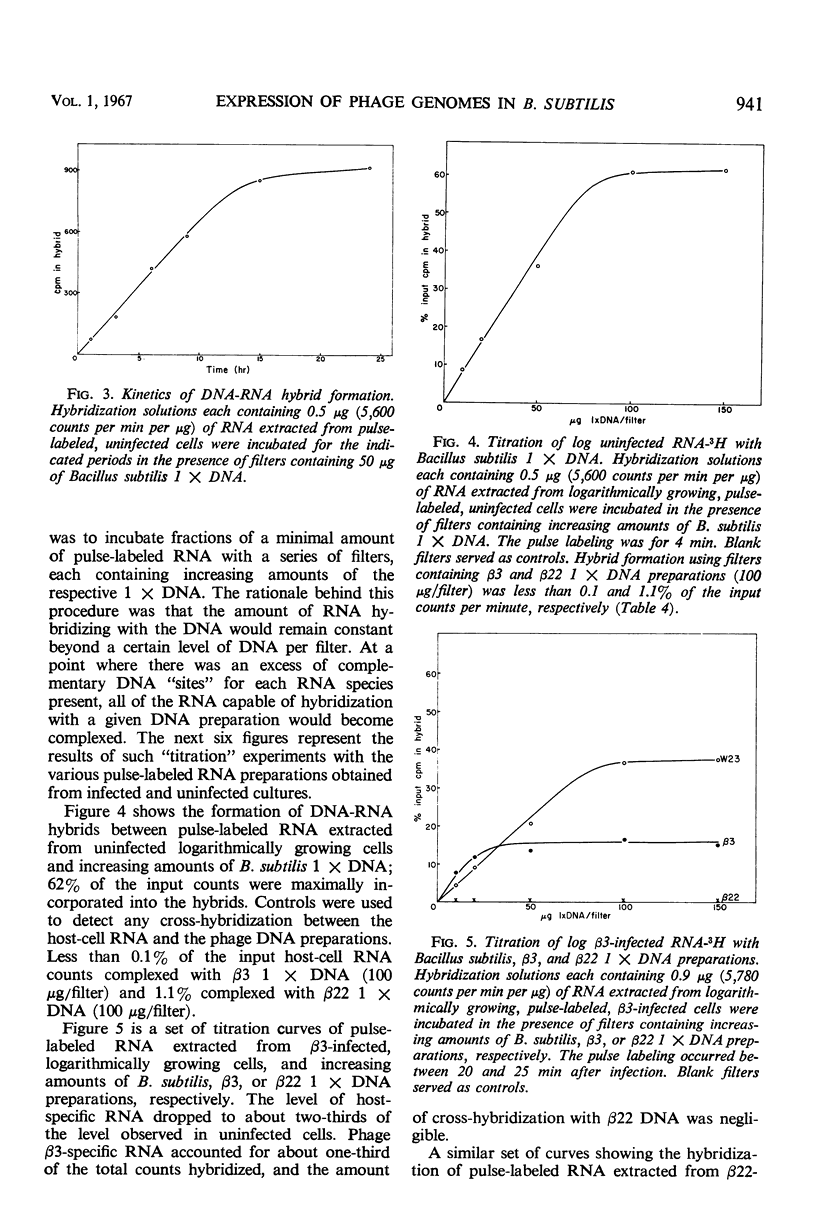
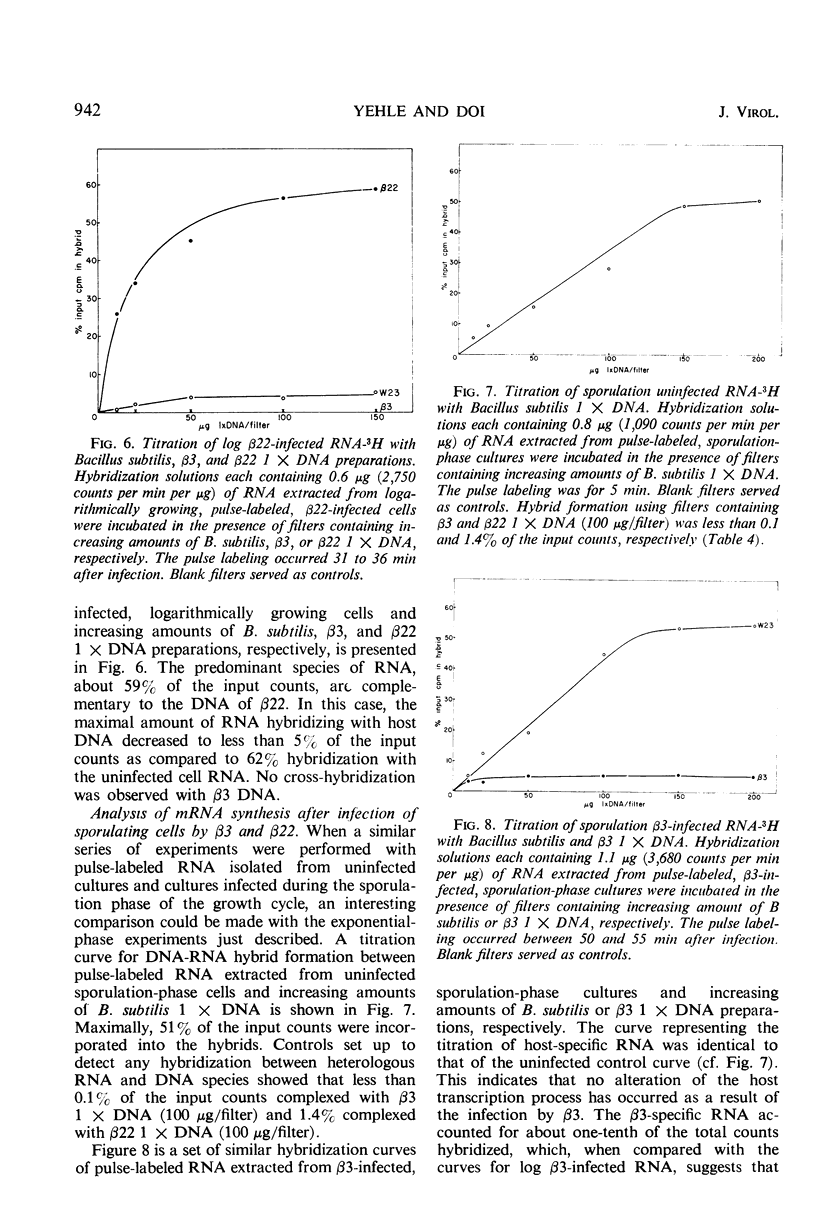
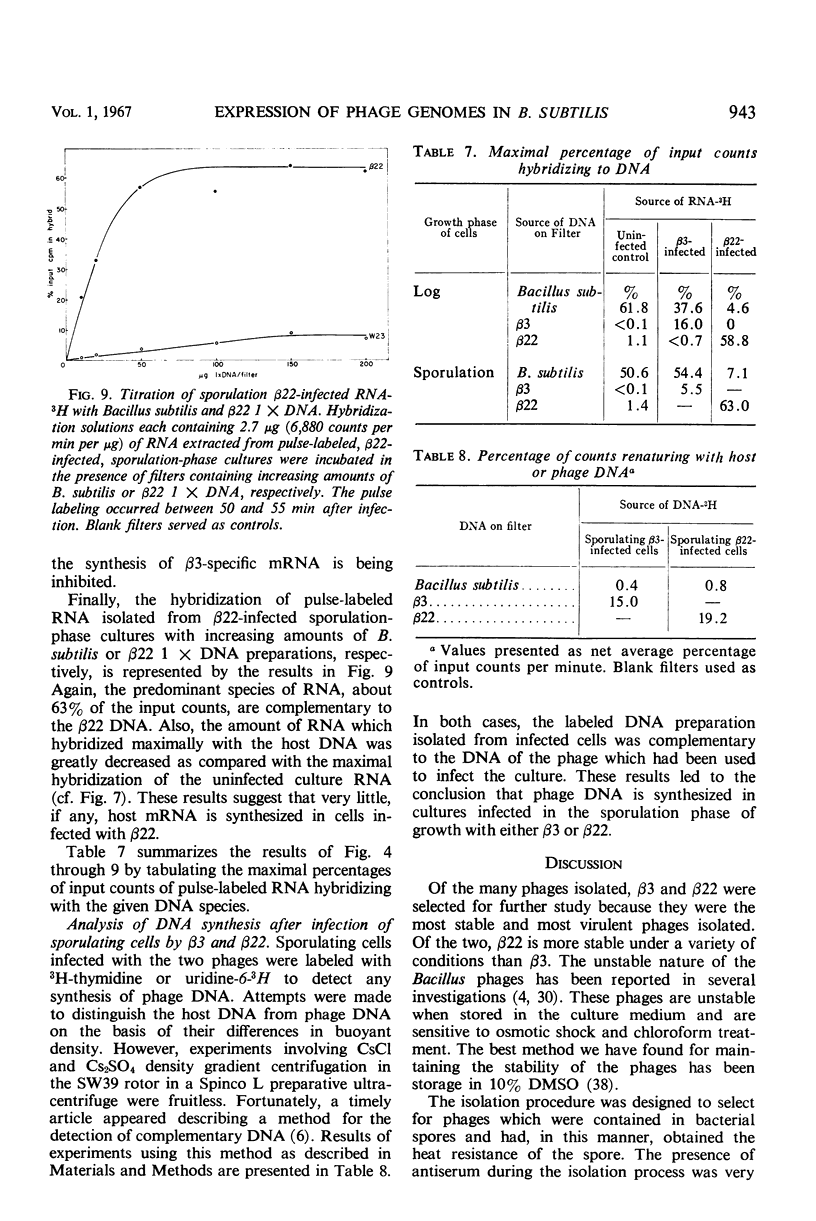
Two antigenically distinct bacteriophages, β3 and β22, have been isolated and characterized with Bacillus subtilis strain W23 as a host. They differ in plaque morphology, single-step growth characteristics, host range, and thermal stability. The deoxyribonucleic acids isolated from β3 and β22 differ in base composition, density in CsCl and Cs2SO4, sedimentation coefficient, molecular weight, and thermal denaturation temperature. These phages have been used to analyze the ability of B. subtilis to sporulate despite infection by virulent phages. When development of phages β3 and β22 in sporulating cultures was compared with that in log cultures, an increase in the latent periods of infection and a decrease in the burst sizes for the two phages were observed. Sporulating cultures infected with β3 yielded the usual percentage (85%) of mature spores; 80% of these contained phage determinants and 20% were uninfected. However, cultures infected with β22 lysed. Of the small fraction (0.01%) which sporulated, 83% were uninfected and 17% were infected. Phage β3-infected and uninfected spores were examined to distinguish any chemical or physical differences. Preparations of both types of spore contained 81.4 μg of dipicolinic acid per mg (dry weight), and examination by phase-contrast microscopy gave no evidence of any difference in outward appearance. A 20% decrease in infected spore count was observed upon heating at 80 C for 10 min. Differences in the infection processes of the two phages prompted an analysis of the transcription process after infection. Deoxyribonucleic acid-ribonucleic acid hybrid analysis of relative amounts of phage-specific and host-specific messenger ribonucleic acid (mRNA) present in infected cells suggested that β3 was unable to repress the synthesis of host mRNA and that β3-specific mRNA synthesis was repressed in sporulation-phase cultures. Phage β22, in contrast, was able to repress host-specific mRNA synthesis in both log-infected and sporulation-infected cells. The results suggest that the differential expression of the phage genomes is due to the relative ability of the phages to repress the host genome.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG R. L., BOEZI J. A. STUDIES OF ESCHERICHIA COLI RIBONUCLEIC ACID-DEOXYRIBONUCLEIC ACID COMPLEX. Biochim Biophys Acta. 1965 May 11;103:60–69. doi: 10.1016/0005-2787(65)90541-1. [DOI] [PubMed] [Google Scholar]
- BOTT K., STRAUSS B. THE CARRIER STATE OF BACILLUS SUBTILIS INFECTED WITH THE TRANSDUCING BACTERIOPHAGE SP10. Virology. 1965 Feb;25:212–225. doi: 10.1016/0042-6822(65)90200-x. [DOI] [PubMed] [Google Scholar]
- Brodetsky A. M., Romig W. R. Characterization of Bacillus subtilis bacteriophages. J Bacteriol. 1965 Dec;90(6):1655–1663. doi: 10.1128/jb.90.6.1655-1663.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVISON P. F., FREIFELDER D. The physical properties of the deoxyribonucleic acid from T7 bacteriophage. J Mol Biol. 1962 Dec;5:643–649. doi: 10.1016/s0022-2836(62)80092-8. [DOI] [PubMed] [Google Scholar]
- DOI R. H., IGARASHI R. T. CONSERVATION OF RIBOSOMAL AND MESSENGER RIBONUCLEIC ACID CISTRONS IN BACILLUS SPECIES. J Bacteriol. 1965 Aug;90:384–390. doi: 10.1128/jb.90.2.384-390.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOI R. H., IGARASHI R. T. RIBONUCLEIC ACIDS OF BACILLUS SUBTILIS SPORES AND SPORULATING CELLS. J Bacteriol. 1964 Feb;87:323–328. doi: 10.1128/jb.87.2.323-328.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Eigner J., Doty P. The native, denatured and renatured states of deoxyribonucleic acid. J Mol Biol. 1965 Jul;12(3):549–580. doi: 10.1016/s0022-2836(65)80312-6. [DOI] [PubMed] [Google Scholar]
- GIERER A., SCHRAMM G. Infectivity of ribonucleic acid from tobacco mosaic virus. Nature. 1956 Apr 14;177(4511):702–703. doi: 10.1038/177702a0. [DOI] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- HALL B. D., SPIEGELMAN S. Sequence complementarity of T2-DNA and T2-specific RNA. Proc Natl Acad Sci U S A. 1961 Feb 15;47:137–163. doi: 10.1073/pnas.47.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorson H. O., Vary J. C., Steinberg W. Developmental changes during the formation and breaking of the dormant state in bacteria. Annu Rev Microbiol. 1966;20:169–188. doi: 10.1146/annurev.mi.20.100166.001125. [DOI] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- KALLEN R. G., SIMON M., MARMUR J. The new occurrence of a new pyrimidine base replacing thymine in a bacteriophage DNA:5-hydroxymethyl uracil. J Mol Biol. 1962 Aug;5:248–250. doi: 10.1016/s0022-2836(62)80087-4. [DOI] [PubMed] [Google Scholar]
- LOEB T., ZINDER N. D. A bacteriophage containing RNA. Proc Natl Acad Sci U S A. 1961 Mar 15;47:282–289. doi: 10.1073/pnas.47.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUDLUM D. B., WARNER R. C. EQUILIBRIUM CENTRIFUGATION IN CESIUM SULFATE SOLUTIONS. J Biol Chem. 1965 Jul;240:2961–2965. [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- McCLOY E. W. Lysogenicity and immunity to Bacillus phage W. J Gen Microbiol. 1958 Feb;18(1):198–220. doi: 10.1099/00221287-18-1-198. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. A method for the detection of RNA-DNA complexes. Biochem Biophys Res Commun. 1963 Jul 18;12:98–104. doi: 10.1016/0006-291x(63)90242-0. [DOI] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. FORMATION AND PROPERTIES OF RNA-DNA COMPLEXES. J Mol Biol. 1964 Jul;9:125–142. doi: 10.1016/s0022-2836(64)80095-4. [DOI] [PubMed] [Google Scholar]
- OKUBO S., STODOLSKY M., BOTT K., STRAUSS B. SEPARATION OF THE TRANSFORMING AND VIRAL DEOXYRIBONUCLEIC ACIDS OF A TRANSDUCING BACTERIOPHAGE OF BACILLUS SUBTILIS. Proc Natl Acad Sci U S A. 1963 Oct;50:679–686. doi: 10.1073/pnas.50.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- ROMIG W. R., BRODETSKY A. M. Isolation and preliminary characterization of bacteriophages for Bacillus subtilis. J Bacteriol. 1961 Jul;82:135–141. doi: 10.1128/jb.82.1.135-141.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Sadoff H. L. The effect of gluconate in promoting sporulation in Bacillus cereus. Biochem Biophys Res Commun. 1966 Sep 8;24(5):691–695. doi: 10.1016/0006-291x(66)90379-2. [DOI] [PubMed] [Google Scholar]
- Saunders G. F., Campbell L. L. Characterization of a thermophilic bacteriophage for Bacillus stearothermophilus. J Bacteriol. 1966 Jan;91(1):340–348. doi: 10.1128/jb.91.1.340-348.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI I. INCORPORATION OF BACTERIOPHAGE GENOME BY SPORES OF BACILLUS SUBTILIS. J Bacteriol. 1964 Jun;87:1499–1502. doi: 10.1128/jb.87.6.1499-1502.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI I., MARMUR J. Replacement of thymidylic acid by deoxyuridylic acid in the deoxyribonucleic acid of a transducing phage for Bacillus subtilis. Nature. 1963 Feb 23;197:794–795. doi: 10.1038/197794a0. [DOI] [PubMed] [Google Scholar]
- WYATT G. R. The purine and pyrimidine composition of deoxypentose nucleic acids. Biochem J. 1951 May;48(5):584–590. doi: 10.1042/bj0480584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehle C. O., Doi R. H. Stabilization of Bacillus subtilis phage with dimethylsulfoxide. Can J Microbiol. 1965 Aug;11(4):745–746. doi: 10.1139/m65-099. [DOI] [PubMed] [Google Scholar]


