Abstract
Neural networks are more than the sum of their parts, but the properties of those parts are nonetheless important. For instance, neuronal properties affect the degree to which neurons receiving common input will spike synchronously, and whether that synchrony will propagate through the network. Stimulus-evoked synchrony can help or hinder network coding depending on the type of code. In this Perspective, we describe how spike initiation dynamics influence neuronal input-output properties, how those properties affect synchronization, and how synchronization affects network coding. We propose that synchronous and asynchronous spiking can be used to multiplex temporal (synchrony) and rate coding and discuss how pyramidal neurons would be well suited for that task.
The synaptic connectivity between neurons comprising a network is critical for the operation of that network but so too are the intrinsic properties of the constituent neurons. When it comes to studying network operation, focus on the former has often trumped consideration of the latter. We will, in this Perspective, shift the focus to neuronal properties and address how those properties affect the collective activity within a network, particularly with respect to synchrony (for review of network properties affecting synchrony, see Kumar et al., 2010). To be clear, we will not consider synchrony associated with network oscillations; instead, we will focus on the sort of stimulus-driven synchrony considered to be a “trivial reflection of anatomical connectivity” insofar as it arises in neurons receiving common input (Singer, 1999). Despite its humble origins, such synchrony has fundamentally important consequences for network coding and has been the focus of much debate (Brette, 2012; Bruno, 2011; de la Rocha et al., 2007; Diesmann et al., 1999; Ermentrout et al., 2008; Estebanez et al., 2012; Hong et al., 2012; Ikegaya et al., 2004; Josić et al., 2009; Kumar et al., 2008; Ostojic et al., 2009; Panzeri et al., 2010; Renart et al., 2010; Rossant et al., 2011; Salinas and Sejnowski, 2001; Sharafi et al., 2013; Stanley, 2013). Does this synchrony help or hinder network coding? Neuronal properties are a crucial yet underappreciated component of the answer.
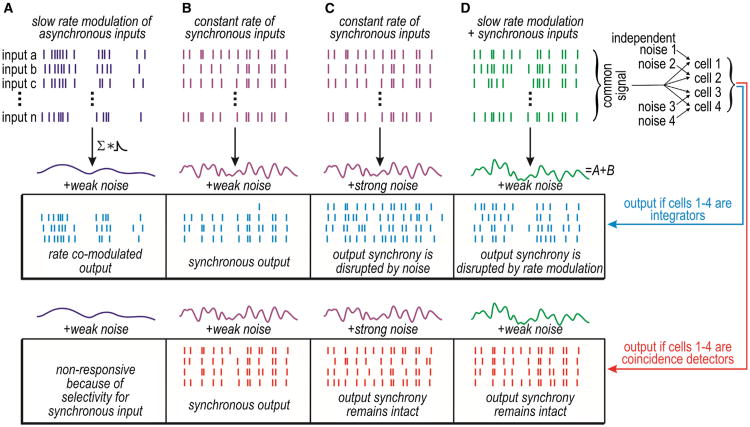
Neurons are often said to operate as integrators or as coincidence detectors based on how they process input (Abeles, 1982; König et al., 1996). Integrators can summate temporally dispersed (asynchronous) inputs, whereas coincidence detectors respond only to temporally coincident (synchronous) inputs. In other words, integrators and coincidence detectors are both sensitive to synchronous input, but coincidence detectors are selective for it. Selectivity is, as we will explain, derived from the dynamical mechanism responsible for transforming synaptic input into output spiking. Spike initiation dynamics also affect whether sets of neurons that receive common synchronous input spike synchronously and whether or not that output synchrony is easily disrupted (Figure 1). Spike initiation dynamics thus control synchrony transfer—the degree to which synchronous input elicits synchronous output. The precision and robustness of synchrony transfer has critical implications for both rate- and synchrony-based coding.
Figure 1. Synchrony Transfer Differs between Operating Modes.
Top: the generation of differently shaped cumulative inputs based on the summation of input spike trains convolved with a synaptic conductance waveform. Stimulus and/or noise conditions differ between (A)–(D). Bottom: responses within a set of integrators or coincidence detectors receiving common (shared) input and independent noise. Unlike integrators, coincidence detectors respond selectively to synchronous input (compare A and B). Both integrators and coincidence detectors receiving common synchronous input will spike synchronously (B), but synchrony transfer is more robust among coincidence detectors, i.e., their output synchrony is less easily disrupted by strong independent noise (C) or by rate-modulated input (D). The robustness of synchrony transfer is a distinguishing feature of coincidence detectors.
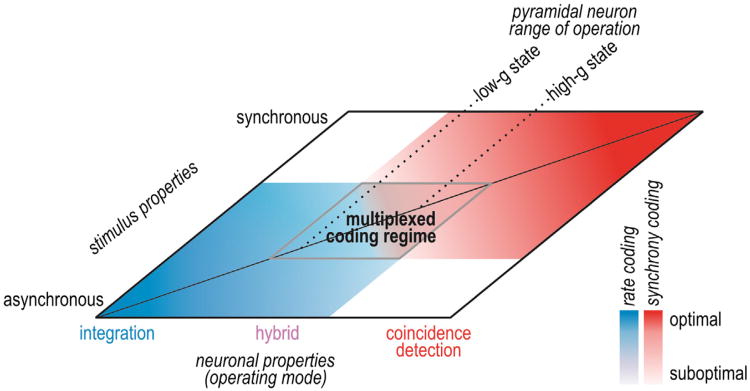
The terms integration and coincidence detection serve to highlight differences in neuronal operation—and we will use these terms for that purpose—but they do not accurately depict how an average neuron operates. Some neurons are exquisitely specialized to operate in one or the other mode but most, including the average pyramidal neuron, operate somewhere in between. In that respect, operating mode is best conceptualized not as a dichotomy, but rather as a continuum with “pure” integration and “pure” coincidence detection at either end (Figure 2). Neurons operating in the midrange may exhibit traits of both operating modes, with certain traits manifesting more strongly than others depending on stimulus properties. Indeed, although they are suboptimal for integration or coincidence detection, the lack of specialization may allow pyramidal neurons to simultaneously employ both operating modes so as to encode different stimulus features in concert, thus enabling rate- and synchrony-based coding to be multiplexed.
Figure 2. Neural Coding Depends Jointly on Neuronal Operating Mode and Stimulus Properties.
Neuronal operating mode is represented as a continuum on one axis. Pyramidal neurons tend to operate in the middle range and can shift where they operate based on factors like conductance state. Input synchrony is represented on the other axis. Neural coding strategies are represented in blue (rate coding) and red (synchrony coding), and deeper colors represent better coding than paler colors. Pale regions overlap, revealing a regime in which a hybrid operating mode and multiplexed coding are possible.
Beyond emphasizing that operating mode represents a continuum, we also propose to refocus its definition around the concept of synchrony transfer: coincidence detectors not only detect synchrony, they also transfer synchrony more precisely and robustly than do integrators (Figure 1). After establishing the importance of synchrony transfer, we will explain its biophysical basis by identifying the neuronal factors upon which synchrony transfer depends, namely, selectivity for synchronous input and capacity to produce robust synchronous output. By regulating synchrony transfer via these neuronal factors, spike initiation dynamics strongly influence whether a network encodes information by the timing of synchronous spikes and/or by the rate of asynchronous spikes.
Neural Coding Strategies: A Neuron's Perspective
Diverse candidate neural coding strategies have been identified (Perkel and Bullock, 1968). Those strategies are often divided into rate and temporal coding, but the division is not clear cut. The difference boils down to what timescale captures signal-dependent variations in spiking. The highest frequency (shortest timescale) encoded by the spike train can be inferred by analyzing the spike train with progressively smaller time windows to determine the window size at which mutual information between the spike train and the stimulus plateaus (Borst and Theunissen, 1999). The reciprocal of that time window represents the “sampling” rate, which, according to the Nyquist Theorem, should be at least twice the highest input frequency sampled by the neuron. Sampling rate relative to the spike rate determines whether the neural representation is sparse or dense, i.e., whether few (≤1) or many (>1) spikes can occur within each time window. Dense representations allow for spike counting, which is the basis for classic rate coding, whereas sparse representations do not (at least not within a single neuron on a single trial) and are thus often considered to imply temporal coding.
An important additional consideration for network coding is whether information is carried independently by each neuron or if information is available from the co-occurrence of spikes across two or more neighboring neurons—a correlation code (deCharms, 1998). We define synchrony as the co-occurrence of spikes within a time window narrow enough that only one spike per cell can occur within it (∼5 ms), whereas rate comodulation is the cross-cell correlation of spike counts within broader time windows. A synchrony code is, therefore, a subtype of correlation coding—one that depends on precise spike timing. A synchrony code is also a subtype of temporal coding—one that depends on spike timing in one neuron relative to spike timing in neighboring neurons. Notably, if synaptic transmission is weak and unreliable (as is the case for many central synapses), synchrony is necessary for enabling brief inputs to activate the postsynaptic neuron (Stevens, 1994; Wang et al., 2010), which implies that synchrony is necessary for temporal coding.
In a network that exclusively utilizes rate coding, optimal coding occurs when neighboring neurons spike independently because correlations constitute redundancy, and redundancy usually reduces information capacity (Barlow, 1961; Gawne and Richmond, 1993; Mazurek and Shadlen, 2002; Sompolinsky et al., 2001; for review see Averbeck et al., 2006). Proponents of rate coding thus tend to view correlations, including synchrony, as detrimental. Contrarily, synchrony between two neurons with overlapping receptive fields can lead to greater mutual information than if synchrony is ignored (Dan et al., 1998), meaning synchrony-encoded information can make up for, if not exceed, the reduction of rate-encoded information (Dan et al., 1998; Kenyon et al., 2004; Meister et al., 1995; Montani et al., 2007; Reich et al., 2001; Schnitzer and Meister, 2003), or so the proponents of synchrony coding would argue. Putting aside what proponents of either side think, we should ask what neurons think: to what inputs do they respond? Over what time window do they process input? After all, it is neurons that process information in the intact brain.
A single excitatory synaptic input typically causes only a small depolarization (<1 mV) in pyramidal and spiny stellate cells (Bruno and Sakmann, 2006; Mason et al., 1991; Sáez and Friedlander, 2009; Sayer et al., 1989; Song et al., 2005). Therefore, if a neuron sums input over a narrow time window (i.e., narrow enough that only one spike per presynaptic cell can occur within it), synchronous input from multiple presynaptic cells will be required to drive suprathreshold depolarization. On the other hand, if the neuron uses a broad time window (i.e., broad enough that multiple spikes per presynaptic cell can occur within it), suprathreshold depolarization can be driven by multiple inputs from just one presynaptic cell or via multiple presynaptic cells; the multicell input could be synchronous or asynchronous. Rewording earlier definitions, coincidence detectors can be said to sum their inputs using a narrow time window, whereas integrators use a broad window (König et al., 1996). An integrator receiving synchronous input may appear to use a narrow window, but the window size is really a property of the neuron, not of the stimulus, which supports a neuron-centric definition of operating mode as opposed to a stimulus-centric one (Rudolph and Destexhe, 2003). The importance of a neuron-centric definition becomes clear when comparing synchrony transfer: integrators respond to synchronous input, but they do not transfer that synchrony as robustly as coincidence detectors do (see Figure 1).
Before proceeding, itis worth noting that simply having aspike threshold endows the neuron with sensitivity to the derivative of the input current or membrane potential (Agüera y Arcas and Fairhall, 2003; Hong et al., 2007). In line with this, it has been shown that the simple threshold-and-fire model as well as leaky integrate-and-fire models can transfer synchrony under the appropriate stimulus conditions (Burak et al., 2009; Goedeke and Diesmann, 2008; Schultze-Kraft et al., 2013; Tchumatchenko et al., 2010). However, as Tchumatchenko et al. and Schultze-Kraft et al. note, this is true only for limited (and arguably unrealistic) stimulus conditions, i.e., high input synchrony driving large membrane potential fluctuations. In real neurons and in more sophisticated models whose spike initiation dynamics implement band-pass filtering, and which are therefore preferentially sensitive to relevant stimulus frequencies, the stimulus requirements for robust synchrony transfer are much less stringent (and more plausible).
Requirements for Synchrony Coding
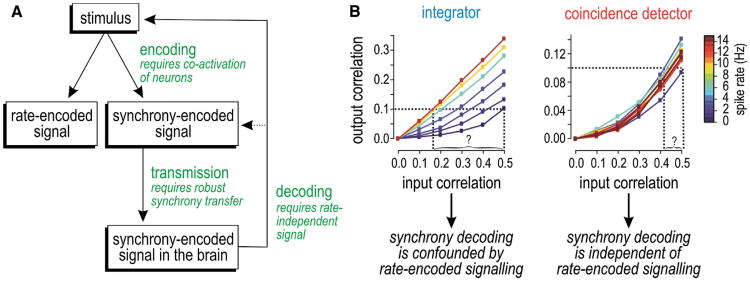
Rate coding is broadly accepted as the pre-eminent coding strategy in the brain; by comparison, synchrony coding is contentious and often considered applicable only to particular systems like the auditory midbrain. We contend that synchrony coding occurs more broadly based on several lines of evidence. We will organize our discussion of that evidence around the 3-fold requirements for synchrony coding (Figure 3A): (1) principal neurons must have coincidence detector traits (in order to reliably transfer synchrony under realistic stimulus conditions), (2) they must receive synchronous input that contains information, and (3) they must produce synchronous output that can be decoded. Note that rate coding and synchrony coding are not mutually exclusive even though factors that facilitate one often do so at the expense of the other. The feasibility and utility of each coding strategy should be gauged independently, contrary to many past debates.
Figure 3. Requirements for Synchrony Coding and the Robustness of Synchrony Transfer to Spike-Rate Variation.
(A) A synchrony-encoded signal arises from stimulus-dependent coactivation of neurons, which is not mutually exclusive of rate-encoded signaling. For synchrony-encoded signals to reach the CNS, they mustbe reliably transmitted across multiple synapses and must remain decodableinorder toprovide information about the original stimulus. Decodability relies on robust synchrony transfer.
(B) Graphs illustrate the challenge of decoding synchrony. Among integrators, the correlation input-output relationship varies with spike rate; consequently, for a given output correlation value, one cannot infer (decode) the input correlation without also knowing the spike rate. This suggests that synchrony coding cannot operate independently of rate coding and would necessitate a complicated decoding mechanism. However, among coincidence detectors, the input-output relationship is not confounded by variations in spike rate, meaning synchrony decoding from coincidence detectors is straightforward. (B) is modified from Hong et al. (2012).
Requirement 1 is satisfied insofar as principal neurons can and do operate as imperfect coincidence detectors. This is suggested by their highly irregular spike trains since integration of multiple asynchronous inputs tends to produce regular spiking (Softky and Koch, 1993); but this speaks more to the input (see below), since even integrators receiving irregularly timed synchronous inputs will spike irregularly (Salinas and Sejnowski, 2000; Shadlen and Newsome, 1998; Stevens and Zador, 1998). More importantly, pyramidal neurons in the intact brain are constantly bombarded by synaptic input, so much so that they are chronically depolarized and shunted (Bernander et al., 1991; Destexhe and Paré, 1999; for review see Destexhe et al., 2003). Moreover, sensory input causes concomitant (albeit momentarily unbalanced) increases in both excitatory and inhibitory drive (Borg-Graham et al., 1998; Haider et al., 2013; Pouille et al., 2009; for review see Isaacson and Scanziani, 2011), which implies further increases in total conductance. The reduction in input resistance (R = 1/g) decreases neuronal sensitivity to constant and slowly fluctuating (low-frequency) inputs, but the concomitant reduction in the membrane time constant (τ = RC) makes neurons relatively more sensitive to rapidly fluctuating (high-frequency) inputs. In addition, large membrane potential fluctuations driven by synaptic bombardment increase sensitivity to coincident inputs (Rossant et al., 2011). This tendency is enhanced by a nonlinear increase in adaptation that can further reduce sensitivity to slow input and thus enhance selectivity for fast input (Hong et al., 2012; Prescott et al., 2006, 2008b). The cumulative effect is that pyramidal neurons receiving realistic conductance-based background and stimulus-evoked inputs in vivo, and which therefore exist in a high-conductance state, behave more like coincidence detectors than is suggested by in vitro testing with artificial current-based stimuli (see also Azouz and Gray, 2000, 2003). To be clear, pyramidal neurons do not switch abruptly from one to the other operating mode but, instead, shift along a continuum (see Figure 2) and can exhibit reasonably strong coincidence detector traits.
Requirement 2 is satisfied insofar as principal neurons do receive synchronous input. For one, the cortex receives sensory input via synchronized activation of thalamocortical neurons (Alonso et al., 1996; Bruno and Sakmann, 2006) originating from the coactivation of primary sensory neurons (see below). Pyramidal neurons recorded in vivo exhibit irregular spiking (see above) driven by large fluctuations in membrane potential that, based on the small depolarization produced by unitary synaptic events, can only be accounted for by some degree of synchrony among presynaptic cells (Destexhe and Paré, 1999; DeWeese and Zador, 2006). Indeed, cross-cell correlations in membrane potential (Lampl et al., 1999; Poulet and Petersen, 2008; Yu and Ferster, 2010) and spiking (Cohen and Kohn, 2011; deCharms and Merzenich, 1996; Jadhav et al., 2009; Smith and Kohn, 2008) have been documented in vivo. Spike cross-correlations are typically measured through pairwise comparisons; however, a postsynaptic neuron experiences correlations across its entire set of presynaptic neurons, which means that correlation values measured through pairwise comparisons must be scaled in order to infer the total input correlation. Very small pairwise correlations that have been reported as evidence for asynchrony (e.g., Ecker et al., 2010) can in fact belie large total input correlation (Rossant et al., 2011; Schneidman et al., 2006).
The origins of synchronous spiking dictate whether synchrony represents signal or noise. Realistic stimuli have spatiotemporal structure that enables them to coactivate neurons with adjacent or overlapping receptive fields; consequently, coactivation patterns can contain information about the stimulus (Brette, 2012; Dan et al., 1998; Meister et al., 1995). If coactivation patterns contain information, synchrony represents part of the signal. Although this does not prove that synchrony-encoded signals are decoded, nor can synchrony be labeled noise simply because it reduces the information decodable from rateencoded signals; indeed, it would be equally unfair to label rate-encoded signals as noise because they compromise the decoding of synchrony-encoded signals (see below). That said, the aforementioned points do not rule out stimulus-independent synchrony that is truly noise (Mastronarde, 1989). What is arguably more important is that correlated spiking in higher brain areas has been observed to be stimulus dependent (Alonso et al., 1996; deCharms and Merzenich, 1996; Kohn and Smith, 2005; Temereanca et al., 2008), consistent with synchrony-encoded signals being successfully transmitted to the cortex.
Requirement 3 is satisfied insofar as synchrony-encoded signals are decodable depending on which type of cells carries the message. It has been suggested that synchrony decoding is implausible because of an “inextricable” link between output correlation and spike rate (de la Rocha et al., 2007). If synchrony transfer were to vary with spike rate, input correlation could not be unambiguously decoded from output correlation without that rate sensitivity being factored in, and indeed the synchronyencoded information could be lost unrecoverably. However, although synchrony transfer is rate dependent among integrators (except under more extreme stimulus conditions; Schultze-Kraft et al., 2013), the same is not true for coincidence detectors (Figure 3B) (Hong et al., 2012; Tchumatchenko et al., 2010), which argues that synchrony-encoded messages carried by coincidence detectors are decodable. Hence, pyramidal neurons with coincidence detector traits should be able to produce synchronous output that is decodable.
These three requirements reflect upon the encoding, transmission, and decoding of synchrony-based signals. Encoding requires the structured coactivation of neurons. Decoding requires that synchrony-encoded signals are not conflated with other signals; in that respect, decodability depends on reliable transmission. Reliable transmission requires robust synchrony transfer. We must, therefore, understand what makes synchrony transfer robust. We will deconstruct the biophysical basis for robust synchrony transfer by considering two factors: (1) the selectivity of neurons for synchronous input and (2) their capacity to produce synchronous output. We will explain each factor in turn, linking both to spike initiation dynamics.
Selectivity for Synchronous Input
According to our neuron-centric definition of operating mode, integrators can summate asynchronous inputs, whereas coincidence detectors are excited uniquely by synchronous inputs (see Figure 1). In other words, coincidence detectors are selective for (i.e., tuned to) synchrony, whereas integrators are relatively untuned with respect to synchrony. Synchrony is reflected in spectral properties of the input: synchronous input has greater power at high frequencies and less power at low frequencies compared with asynchronous input of equivalent magnitude (i.e., with equivalent total power) (Destexhe et al., 2001). Putting two and two together, one might (correctly) postulate that integrators are tuned to lower frequencies, akin to a low-pass filter, whereas coincidence detectors are tuned to higher frequencies, akin to a high-pass filter, although the end result is a band-pass filter when the high-pass filter implemented by spike initiation is combined with the low-pass filter implemented by membrane capacitance.
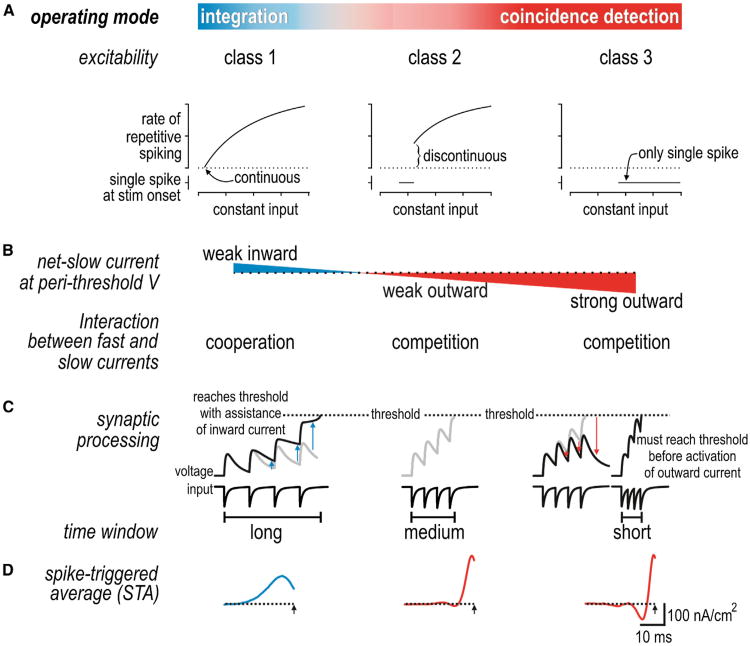
Differential tuning reflects differences in neuronal excitability. A simple yet invaluable classification of excitability was provided by Hodgkin (1948) who identified three spiking patterns in response to sustained depolarization: Class 1 neurons can spike repetitively at an arbitrarily low rate and thus have a continuous frequency-current (f-I) curve, class 2 neurons cannot spike repetitively below a certain rate and thus have a discontinuous f-I curve, and class 3 neurons fire only one or a few spikes at stimulus onset (Figure 4A). Each class of excitability is associated with differences in other response measures such as the phase response curve (Ermentrout, 1996) and spike-triggered average (Ermentrout et al., 2007; Mato and Samengo, 2008) (see below). In general, class 1 neurons exhibit integrator traits, whereas class 3 neurons and, to a lesser extent, class 2 neurons exhibit coincidence detector traits. Hodgkin's classification thus provides a useful starting point for relating neuronal excitability with operating mode.
Figure 4. Spike Initiation Dynamics Control Operating Mode.
(A) Classes 1, 2, and 3 of excitability are distinguished by the shape of the frequency-current curve defined by constant stimulation. Class 3 neurons (and class 2 neurons within a certain stimulus range) fire only one or a few spikes at stimulus onset. Those properties emerge from distinct nonlinear dynamical mechanisms that reflect whether fast and slow currents cooperate or compete during spike initiation.
(B) Differences in spike initiation dynamics can be ascribed to differences in the direction and magnitude of the net-slow current active at perithreshold potentials.
(C) Inward current helps sustain the depolarization caused by excitatory synaptic inputs, thereby lengthening the integration time window; outward current truncates depolarization, thereby shortening the integration time window.
(D) Differential processing is also evident in the shape of the spike-triggered stimulus average (STA).
Differences in excitability reflect differences in spike initiation dynamics (Izhikevich, 2007; Prescott et al., 2008a; Rinzel and Ermentrout, 1998). “Dynamics” refers to how fast and slow currents interact to control spike initiation. Notably, currents with similar kinetics sum linearly whereas those with different kinetics interact nonlinearly. Therefore, net-fast and net-slow currents interact nonlinearly, and ultra-slow processes like adaptation currents or cumulative inactivation of sodium current can be treated as modulating the fast-slow interaction. Net-fast current is necessarily inward (depolarizing) at spike threshold. In class 1 excitability, net-slow current is also inward at perithreshold voltages and thus cooperates with fast current during spike initiation (Figure 4B). In class 2 and 3 excitability, net-slow current is outward (hyperpolarizing) at perithreshold voltages and thus competes with fast current during spike initiation. Class 2 excitability exists if fast inward current overpowers slow outward current when constant stimulation exceeds threshold. Class 3 excitability exists if fast inward current overpowers slow outward current only during a stimulus transient, which precludes repetitive spiking during sustained stimulation. Thus, on the basis of whether fast and slow currents cooperate or compete at perithreshold voltages, three classes of excitability arise from a continuum in the strength and direction of net-slow current. The strength of net-fast current (which depends on leak current) affects its competition with net-slow current, thus influencing the boundary between class 2 and 3 excitability (Lundstrom et al., 2008; Prescott et al., 2008a). In dynamical terms, it is the cooperative versus competitive nature of the interaction controlling spike initiation that distinguishes integration and coincidence detection. To be clear, net current depends on both activation and inactivation of contributing ion channels, meaning inactivation of an outward current has effects comparable to activation of an inward current if the two processes occur with similar kinetics and voltage dependency. Accordingly, and especially given that pyramidal neurons express a multitude of different ion channels, there are several distinct channel combinations that can implement equivalent spike initiation dynamics. That said, the interaction between membrane currents also depends on the stimulus waveform because subthreshold membrane currents are differentially activated or inactivated by stimuli with different kinetics. This speaks to the joint dependence of spiking on neuronal properties and stimulus properties (see below for discussion on filtering).
With respect to synaptic input, subthreshold inward current helps sustain the depolarization caused by excitatory inputs, thereby encouraging temporal summation (integration) in class 1 neurons; contrariwise, subthreshold outward current truncates the depolarization caused by excitatory inputs, thereby discouraging summation and allowing only coincident inputs that drive fast suprathreshold depolarization (i.e., faster than outward current can activate) to elicit spiking in class 2 and 3 neurons (Figure 4C). In effect, the width of the integration time window is regulated by the strength and direction of subthreshold currents (Fricker and Miles, 2000; Gastrein et al., 2011; Prescott and De Koninck, 2005). Note that the delayed negative feedback implemented by voltage-dependent outward current in class 2 and 3 neurons has an effect very similar to that mediated by feed-forward synaptic inhibition, which is well recognized as a mechanism that limits the integration time window (e.g., Pouille and Scanziani, 2001; see also Ostojic et al., 2009). The difference lies in whether the negative feedback is a feature of the neuron or of the microcircuit.
An efficient way to assess signal processing characteristics, including the integration time window, is to measure the spike-triggered stimulus average (STA). This can be done by applying noisy stimulation comprising a range of input frequencies and calculating the average stimulus waveform that precedes each spike; the noisy input can be constructed to reasonably approximate synaptic bombardment (Destexhe et al., 2001) and avoids having to repeat testing across multiple single-frequency inputs and combinations thereof (Rieke et al., 1997). The STA differs between class 1 (integrator) and class 2/3 (coincidence detector) neurons, being broad and monophasic in the former versus narrow and biphasic in the latter (Hong et al., 2012; Mato and Samengo, 2008) (Figure 4D). Duration of the positive phase reflects the integration time window. More generally, the STA reflects the stimulus features that drive spiking based on the recruitment of subthreshold membrane currents: a broad monophasic STA represents low-passfiltering (which confers tuning to low frequencies), whereas a narrow biphasic STA represents band-pass filtering (which confers tuning to higher frequencies). The difference in signal processing is also evident in the spike-triggered stimulus correlation (STC) (Rieke et al., 1997) (see below).
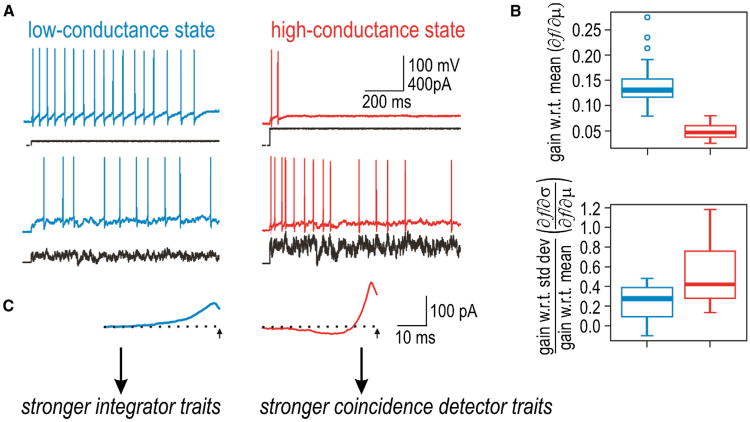
Where do pyramidal neurons fit into this classification? Regular spiking CA1 pyramidal neurons exhibit class 1 excitability when tested in brain slices (Prescott et al., 2006, 2008b), but the synaptic bombardment experienced in vivo (see above) is predicted to encourage class 2/3 excitability by biasing the net-slow current at threshold in the outward direction. Using dynamic clamp to mimic synaptic bombardment in brain slices, voltage threshold undergoes a depolarizing shift because greater depolarization is needed to activate enough fast sodium channels to overwhelm the increased outward leak current. This, in turn, allows activation of other voltage-dependent outward currents and slow inactivation of inward currents, thereby biasing the net-slow current in the outward direction and encouraging class 2 excitability (Prescott et al., 2006, 2008b). Consequently, the same neuron that spikes repetitively during constant current injection in the low-conductance state often spikes only transiently when retested in the high-conductance state, although fluctuating stimuli can elicit vigorous spiking in either conductance state (Figure 5A). The shift in excitability, from class 1 to class 2, is associated with quantifiable changes in tuning: neurons become less sensitive to the stimulus mean and relatively more sensitive to the stimulus variance (Hong et al., 2012) (Figure 5B). The shift in operating mode is paralleled by reshaping of the STA (Figure 5C). Similarly, neocortical pyramidal cells tested in vitro tend to operate near the division between class 1 and 2 excitability (Tateno et al., 2004; Tsubo et al., 2007) and can be made more class 2 excitable through enhanced adaptation (Stiefel et al., 2008). In general, adaptation currents and slow inactivation of inward currents can enhance sensitivity to the stimulus variance without completely nullifying responsiveness to the stimulus mean (Arsiero et al., 2007; Fernandez et al., 2011; Higgs et al., 2006; see also Lundstrom et al., 2009). These data show that pyramidal neurons exhibit coincidence detector traits and identify spike initiation dynamics as a key determinant of their operating mode.
Figure 5. Pyramidal Neuron Operating Mode Is Intermediate and Modulable.
(A) When tested in a low-conductance state, CA1 pyramidal neurons spike repetitively to constant stimulation and can maintain low spike rates, consistent with class 1 excitability. When the same neuron is tested in the high-conductance state (recreated via dynamic clamp), excitability is shifted toward class 2 excitability, as evidenced by a reduced tendency to maintain repetitive spiking during constant stimulation. Fluctuating stimuli can elicit vigorous spiking in either conductance state.
(B) The shift in excitability is accompanied by a shift in coding properties: neurons become less sensitive to the mean stimulus intensity (m) and relatively more sensitive to the amplitude of stimulus fluctuations (s), consistent with coincidence detector traits becoming more prominent in the high-conductance state.
(C) The shift is also accompanied by reshaping of the STA from a broad monophasic form to a narrower biphasic form. Modified from Hong et al. (2012).
Predicting Cross-Correlations through Reverse Correlation Analysis
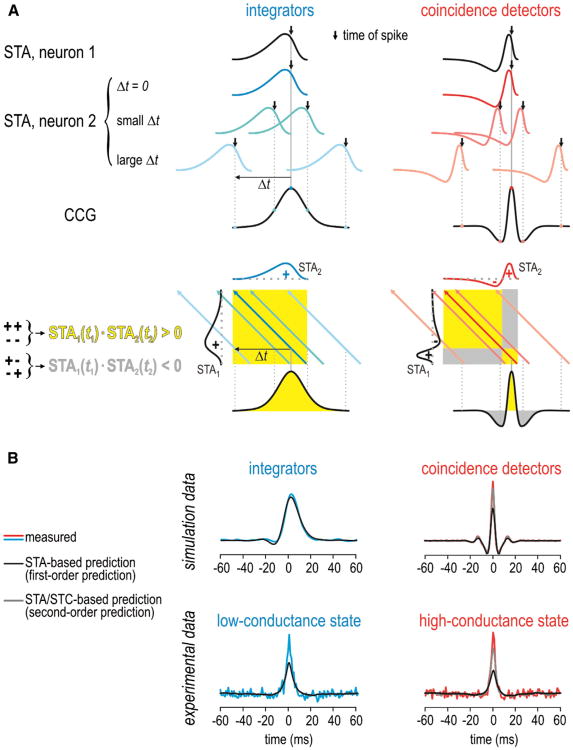
Given a neuron's output spike train and its STA, reverse correlation can be used to predict its input. Conversely, how the neuron encodes its input can be modeled using its STA. By extension, if two neurons receive common input, the STA can be used to predict the correlated spiking driven by that input, and thus it can predict the cross-correlogram (CCG) (Figure 6A). More precisely, the shape of the CCG can be inferred by convolving the STAs from each neuron (Goldberg et al., 2004). It follows from their differently shaped STAs that the CCG for a pair of coincidence detectors is narrow and multiphasic, whereas the CCG for a pair of integrators is broad and monophasic (Hong et al., 2012; see also Barreiro et al., 2010, 2012).
Figure 6. Predicting the Cross-Correlogram.
(A) The cross-correlogram (CCG) can be predicted by convolving the STAs of each neuron. Top: the STA in neuron 2 shifted by different Δt relative to the STA in neuron 1. For Δt = 0, the two STAs overlap perfectly, which corresponds to a high cross-correlation value. For large Δt, the cross-correlation drops to 0 as the STAs no longer overlap. For coincidence detectors, the cross-correlation can be negative for intermediate Δt if the STAs line up out of phase. Bottom: another depiction in which the STA in neuron 1 (STA1) is plotted against the STA in neuron 2 (STA2). Shading represents STA1(t1)·STA2(t2) with yellow corresponding to conditions in which the positive and negative components of STA1 are in phase with the positive and negative components of STA2, and gray corresponding to conditions in which those components are out of phase. Colored arrows are the projections of STA2 across STA1(t1)·STA2(t2) for the same Δt values shown in the top panel. The total cross-correlation represents the sum of STA1(t1)·STA2(t2) across that arrow.
(B) Examples of predicted CCGs for comparison with measured CCGs. The first-order prediction (based on the STA alone) provides a satisfactory fit to CCGs measured in the integrator model but does a poor job fitting the peak of CCGs measured from coincidence detector models. The “excess” synchrony was better accounted for by the second-order prediction (based on the STA and STC). For experimental data from CA1 pyramidal neurons, the second-order prediction becomes relatively more important when neurons are shifted toward the coincidence detector mode (i.e., in the high-conductance state) but is relevant even in the low-conductance state insofar as the first-order prediction is imperfect. This is consistent with pyramidal neurons operating inthe middlerange of the operating mode continuum.
However, the STA does not provide a sufficiently accurate description of neuronal response properties when the neuron is sensitive to multiple stimulus features. In this scenario, the information for building a good encoding model can be retrieved by the spike-triggered stimulus correlation (or equivalently the covariance; STC) (for details, see Schwartz et al., 2006). For reasons explained below, the STA-based encoding model provides a relatively good description of integrator response properties, whereas the multifeature model is needed to provide a similarly good description of coincidence detector response properties (Agüera y Arcas et al., 2003; Slee et al., 2005). By extension, the STC improves prediction of the CCG, but more so for coincidence detectors CCGs than for integrator CCGs (Hong et al., 2012). Notably, the multifeature model more accurately predicts the narrow central peak of the CCG that dominates the total correlation in coincidence detectors (Figure 6B).
Differential importance of the STC for predicting coincidence detector spiking compared with integrator spiking reflects upon the stimulus features that elicit spikes in each operating mode. In brief, integrators spike when the integrated stimulus intensity exceeds some threshold; the STA accurately captures that feature selectivity. Stimulus intensity is also important for spike initiation in coincidence detectors, but the competitive dynamics render the process additionally (and nonlinearly) sensitive to the rate of change of stimulus intensity. The shape of the coincidence detector STA hints at the importance of abrupt depolarizing input, but the STC more accurately captures the sensitivity to rate of change of stimulus intensity, including how that sensitivity varies with stimulus intensity (for more detailed explanation, see Agüera y Arcas et al., 2003). In short, the STA is sufficient to distinguish integrator and coincidence detector operating modes and it can be used to qualitatively predict the shape of the CCG for pairs of neurons operating in either mode, but higher-order stimulus properties such as the STC become important in the case of coincidence detectors and provide quantitatively more accurate predictions.
Capacity to Produce Robust Synchronous Output
Previous discussions of operating mode have emphasized how neurons process their input. But to explain synchrony transfer, we must also consider how neurons produce their output and, moreover, we must consider the output of multiple neurons in order to measure output synchrony. This would seem to require the difficult task of recording simultaneously from all the neurons whose output is to be cross-correlated; however, by replaying the same simulated synaptic input signal (along with different noise), one can collect many spike trains from individually recorded neurons and then cross-correlate their responses after alignment based on the common signal (de la Rocha et al., 2007; Hong et al., 2012; Reyes, 2003). We refer to this as a virtual network approach since the neurons, although not part of the same “real” network, are stimulated and analyzed as if they are part of the same “virtual” network. Notably, the input synchrony and the fraction of input that is shared across neurons are not only known, they are controlled by the experimenter. This approach is therefore very useful for studying how and why synchrony transfer differs between operating modes.
Synchronous spiking across a set of neurons requires that spike timing within each constituent neuron is temporally precise in relation to the input. Rapidly fluctuating input—the sort arising from presynaptic synchrony—drives more precisely timed spikes than constant or slowly fluctuating input (Bryant and Segundo, 1976; Cecchi et al., 2000; Galán et al., 2008; Mainen and Sejnowski, 1995; Nowak et al., 1997). Those data demonstrate that spike timing can be precise on the basis of input and thus support a stimulus-centric definition of operating mode (Schultze-Kraft et al., 2013), but neuronal properties are nonetheless critical. By being less sensitive to mean stimulus intensity, coincidence detectors exhibit better spike-timing precision than integrators firing at an equivalent average rate (Prescott et al., 2006; Prescott and Sejnowski, 2008). Indeed, several studies have linked stronger outward membrane current with increased precision (Berry and Meister, 1998; Billimoria et al., 2006; Schreiber et al., 2004; Svirskis and Rinzel, 2003), whereas inward currents or slowly inactivating outward currents have the opposite effect (Barreiro et al., 2012; Cudmore et al., 2010; Fricker and Miles, 2000). Specifically, band-pass filtering in coincidence detectors attenuates low-frequency input such that repetitive spiking is prevented or reduced and membrane potential is, in a sense, “clamped” below threshold. Rapid stimulus fluctuations elicit spikes (because they are not attenuated) and the timing of those spikes is very precise (see above). The critical point is this: because fluctuation-driven spikes are not superimposed on repetitive mean-driven spiking, spike timing is more tightly linked to stimulus fluctuation timing (Prescott and Sejnowski, 2008). Unlike in integrators, the rate of spiking in pure coincidence detectors reflects the rate of synchronous suprathreshold inputs, not the amplitude of a slow, rate-encoded signal (see Figure 1) (König et al., 1996)—this explains the rate insensitivity of synchrony transfer among coincidence detectors (Figure 3B). But once again bear in mind that pyramidal neurons operate in a middle range and can exhibit mean-driven and fluctuation-driven spiking. The two spike “types” can coexist so long as timing of the latter is not strongly corrupted by the former and so long as a decoder can ultimately separate the two. We will address both issues below.
Beyond being insensitive to spike rate, synchrony transfer must also be robust to noise. Indeed, it has been shown that a small perturbation can elicit an extra spike in the recipient cell, which in turn elicits extra spikes in multiple postsynaptic cells, resulting in large stimulus-independent (i.e., noisy) variations in membrane potential that disrupt spike timing (London et al., 2010). London et al. did not, however, demonstrate that perturbations elicit synchronous spikes; that would require that the perturbation occurs synchronously across multiple neurons (which is conceivable) and that the recipient neurons are all simultaneously close to threshold (which is doubtful) so that the input is not only received simultaneously, but it also elicits spikes simultaneously. Without synchronous activation of multiple presynaptic cells, postsynaptic coincidence detectors would not be activated, or at least a set of coincidence detectors would not be activated synchronously. As a result, asynchronous perturbation-driven spiking will be curtailed, not amplified, within a network of coincidence detectors. In this regard, it is noteworthy that London et al. used integrator-type model neurons in their simulations and that their experiments, although conducted in vivo, seemed to emphasize the low-conductance state (e.g., reported values of input resistance are comparable to those in Destexhe et al., 2001 before synaptic bombardment); this may reflect the inclusion of the down state that exists during anesthesia but that is absent during wakefulness (e.g., Constantinople and Bruno, 2011) and/or the exclusion of sensory evoked activity that would increase conductance (see above).
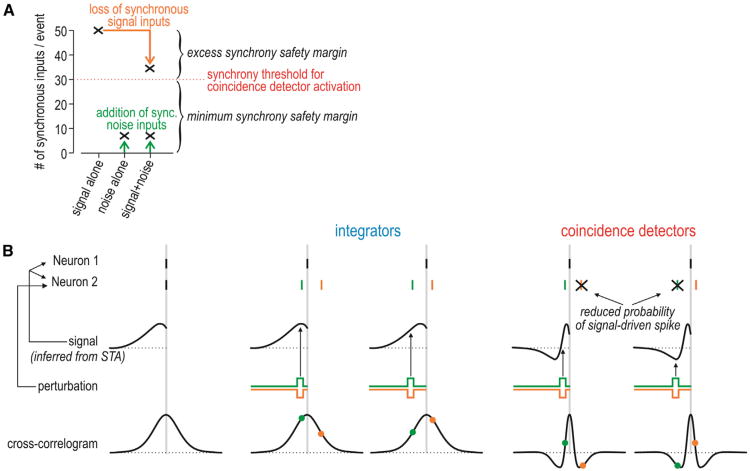
To better understand why synchrony transfer by coincidence detectors is robust, consider the following hypothetical scenario with numbers based loosely on published data (e.g., Wang et al., 2010). A set of 50 coactivated neurons synapse onto a postsynaptic coincidence detector that requires only 30 synchronous excitatory inputs to achieve suprathreshold depolarization. This means that only 30 of the 50 presynaptic neurons must spike simultaneously in order to excite the postsynaptic neuron. The other 20 presynaptic neurons need not be activated or their spikes could be lost to noise without compromising postsynaptic activation (Zador, 1998)—we refer to this as an excess synchrony safety margin (Figure 7A). By “lost spikes,” we mean spikes that which would have been elicited by the signal but are absent because of the effects of noise. On the other hand, the likelihood of noise simultaneously coactivating 30 presynaptic neurons is arguably quite low (see above)—we refer to this as the minimum synchrony safety margin. In other words, synchrony-driven spiking will not be easily disrupted or confused with noise-driven spiking in the presence of these safety margins. An important conclusion is that temporal coding is more robust when it uses synchronous spikes among multiple neurons rather than isolated spikes in single neurons—this seems obvious but is routinely overlooked.
Figure 7. Robustness of Synchrony Transfer to Noise.
(A) Graph depicts synchronous input to a hypothetical postsynaptic coincidence detector that requires 30 synchronous inputs for activation. Without noise, the signal coactivates 50 presynaptic neurons. This means that up to 20 synchronous inputs could fail to occur (e.g., because of the effects of noise) without compromising activation of the postsynaptic neuron—this constitutes the excess synchrony safety margin. On the other hand, noise would have to coactivate at least 30 presynaptic neurons in order to activate the postsynaptic neuron, which is unlikely—this constitutes the minimum synchrony safety margin. The former safety margin reduces false negatives, whereas the latter reduces false positives with respect to correctly detecting the input signal. Integrators, by definition, have a lower synchrony threshold, which implies a smaller minimum synchrony safety margin.
(B) If two neurons spike more synchronously than expected by chance, they probably receive common input (signal) and we can infer the shape of that input based on the STA. Furthermore, if one neuron spikes, the CCG tells us the probability that the other neuron will spike. If neuron 2 receives a brief perturbation, its spike (shown in the same color as the perturbation) is jittered relative to the spike in the other neuron. In an integrator, because the CCG peak is so broad, a jittered spike will still tend to fall near the peak of the CCG (as shown by colored dots). In a coincidence detector, by comparison, even moderate jittering can shift the timing of the anticipated spike such that it coincides with one of the troughs surrounding the narrow peak of the multiphasic CCG, which implies that the probability of spiking falls to below-chance levels and that the spike will probably be “lost.” Thus, the spike initiation dynamics that are characteristic of coincidence detectors implement a quality control mechanism, wherein precision is maintained at the expense of reliability. The CCG troughs also ensure that tightly synchronized spikes are clearly distinguishable from asynchronous spikes because the probability of loosely synchronized spiking is very low.
Beyond affecting the probability of signal-driven spikes, noise could also compromise synchrony by jittering the timing of signal-driven spikes. Intriguingly, spike timing in coincidence detectors is protected against jitter. This quality control mechanism can be understood from the shapes of the STA and CCG (Figure 7B). Consider another hypothetical scenario in which two neurons spike synchronously. The STA provides an estimate of the common signal that triggered those spikes. Next, consider what would happen if neuron 2 received a perturbation. The perturbation would almost certainly jitter spike timing in neuron 2, but it might also reduce the probability that neuron 2 even spikes. In an integrator, the timing of the perturbation relative to the broad monophasic STA is relatively unimportant in this regard; in a coincidence detector, on the other hand, timing of the perturbation relative to the narrow biphasic STA has important consequences. The reduced probability of signal-driven spiking is most easily understood from the CCG, which shows the probability that neuron 2 will spike at times shortly before or after the spike in neuron 1. If a perturbation in neuron 2 jitters the anticipated signal-driven spike such that its timing coincides with either trough (negative phase) of the CCG, the probability of that spike occurring will be reduced to below-chance levels. In other words, noise is more likely to cause “lost” spikes than to cause strongly jittered spikes in coincidence detectors; the signal-driven spikes that remain will be temporally precise and therefore well synchronized. This quality control mechanism, which trades off reliability for precision, makes sense if an excess synchrony safety margin can accommodate the lost spikes.
Compared with the broad CCG characteristic of integrators, the narrow peak of coincidence detector CCGs indicates more precise synchronization. Furthermore, the adjacent troughs seen in coincidence detector CCGs indicate correlated quiescence around the synchronous spikes; in other words, if neuron 2 does not spike within a couple of milliseconds of the spike in neuron 1 (during the CCG peak), it is less likely than chance to spike at slightly longer times (during the CCG troughs). Those troughs thus represent a boundary separating synchronous input-driven spikes from asynchronous input-driven spikes: the former are well synchronized, the latter are asynchronous, and there are few marginally synchronized spikes whose origin is ambiguous. Correctly identifying synchronous and asynchronous output spikes is important inasmuch as it can allow a decoder to distinguish spikes driven by a common signal from those driven by independent noise: the former are synchronous, whereas the latter are not. Similarly, it would allow a decoder to distinguish spikes driven by a common synchrony-encoded signal from those driven by a common rate-encoded signal: the former are synchronous, whereas the latter are not (which is not to exclude rate comodulation). The last point leads to the idea of multiplexing, but first, we must compare our claims against quantitative analysis of synchrony transfer.
When measured synchrony transfer is compared against the synchrony transfer predicted by reverse correlation analysis, output correlation among idealized integrators is accounted for by the first-order prediction (based on the STA), whereas coincidence detectors spike more synchronously than expected (Hong et al., 2012). “Excess” or unpredicted output correlation among coincidence detectors is concentrated at the center of the CCG (see Figure 6B), consistent with a failure of the STA to predict highly synchronized spiking that can be corrected by incorporating STC-based analysis. Those results speak to the importance of the rate of change of stimulus intensity in eliciting precisely synchronized spiking. Although rather obvious, that conclusion can be overlooked if oversimplified neuron models are used. Hong et al. (2012) found that pyramidal neurons were sensitive to stimulus variance in both the low- and high-conductance states and were simply more sensitive in the latter, consistent with operation in the midrange of the operating mode continuum. One should note that the comparison between predicted and measured cross-correlation was conducted using a broad range of stimulus intensities and noise conditions, the implication being that stimulus-dependent synchrony can persist despite stimulus-dependent modulation of the mean spike rate and can be properly analyzed for different stimulus parameters.
From Hybrid Operating Mode to Multiplexed Coding
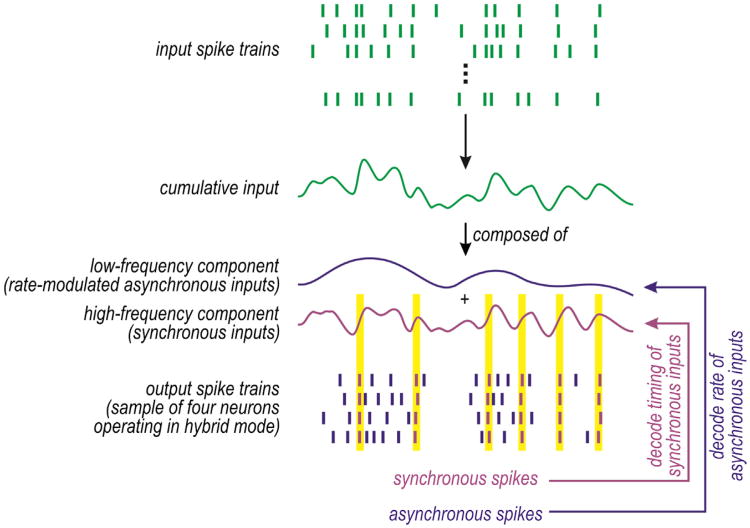
By not being optimized for integration or coincidence detection, pyramidal neurons exhibit traits of both operating modes and could, therefore, be said to use a hybrid mode. This raises the question of whether a hybrid operating mode conveys benefits that justify the lack of specialization. We propose that a hybrid operating mode allows rate and synchrony codes to be multiplexed (Figure 2). Multiplexing refers to the transmission of more than one signal via a single communication channel and can increase information capacity (Lathi and Ding, 2009). Single neurons in sensory systems have been shown to achieve multiplexing via temporal scale (frequency) division, wherein different signals are allocated to pass bands that span nonoverlapping frequencies (for review, see Panzeri et al., 2010). In the scenario considered here, synchrony-encoded signals (with power concentrated at high frequencies) are encoded by synchronous spiking, whereas asynchronous rateencoded signals (with power concentrated at lower frequencies) are encoded byasynchronous rate-modulated spiking (Figure 8). The distinctly represented signals can coexist if synchrony transfer is robust to rate-modulated spiking. The safety margins and spike timing quality control mechanism described in Figure 7 represent biologically straightforward ways to maintain the distinction between synchronous and asynchronous spikes; in engineering terms, those mechanisms could be said to implement guard bands that separate the two pass bands.
Figure 8. Multiplexed Coding.
Top rasters depict input comprising synchronous inputs plus rate-modulated asynchronous inputs. Bottom rasters depict output spike trains in four postsynaptic neurons operating in hybrid mode. Synchronous inputs elicit synchronous output spikes (purple), whereas rate-modulated asynchronous inputs elicit rate-modulated asynchronous output spikes (blue). By comparison, pure coincidence detectors would not respond to the asynchronous inputs (see Figure 1A) and pure integrators would not respond synchronously to synchronous inputs because of their rate-modulated asynchronous spiking (see Figure 1D).
Past studies have demonstrated rate coding multiplexed with temporal coding that depends on intrinsically generated network oscillations (Friedrich et al., 2004; Huxter et al., 2003; Mazzoni et al., 2011). Our proposed form of multiplexing more closely matches that described by Riehle et al. (1997) in the motor cortex and by Steinmetz et al. (2000) in the somatosensory cortex (see also Estebanez et al., 2012), where transient synchronization occurs independently of rate modulation but in relation to external and internal events, including attention. This form of multiplexing is also supported by our observation that precise synchrony can exist over a broad range of spike rates driven by different mean stimulus intensities (Hong et al., 2012). One potential argument against multiplexing is that recorded spike trains tend to exhibit only weak pairwise correlations. However, when cross-correlating the output spike trains of two neurons that are part of a multiplexing set—indeed, not all cross-correlated cell pairs will participate in the same set—synchronous spikes may occur only rarely compared with asynchronous spikes. This “dilution” will result in small cross-correlation values, but this does not rule out that precisely synchronized spikes occur, it simply means that those synchronous spikes are well hidden and necessitate careful analysis (Grün, 2009). We predict that synchrony-encoded signaling requires higher-order correlations— that synchrony among n neurons is greater than extrapolated from pairwise correlations—in order to support an excess synchrony safety margin. Indeed, despite being difficult to quantify (see Staude et al., 2010), such correlations do exist (Ohiorhenuan et al., 2010; Shimazaki et al., 2012; Yu et al., 2011).
A set of hybrid mode neurons can discriminate between a shared synchrony-encoded signal and independent noise by responding synchronously to the former and asynchronously to the latter. The same set can discriminate between a shared synchrony-encoded signal and a shared asynchronous rateencoded signal by, again, responding synchronously to the former and asynchronously to the latter. Distinguishing between the rate-encoded signal and noise relies onnoise being independent across the neurons so that it can be averaged out. Shared noise thus represents a problem for rate coding and synchrony coding; in that regard, both coding strategies could benefit from decorrelation mechanisms such as balanced excitation/inhibition (Renart et al., 2010) and inhibitory feedback (Tetzlaff et al., 2012), the only caveat being that signal-dependent correlations must persist while spurious correlations arising from noise are eliminated. This may come down to signal-dependent correlations being of higher order than noise-based correlations, consistent with the minimum synchrony safety margin.
Conclusions and Future Directions
Spike initiation dynamics differ between neurons and can be modulated within a given neuron, e.g., by changes in the total membrane conductance. Those dynamics represent how a multitude of membrane currents interact to control spike initiation. Although we have focused here on how spike initiation dynamics affect stimulus-driven synchrony within a feedforward network, these same dynamics are known to affect synchronization and oscillations within recurrently connected networks (Hansel et al., 1995). By restricting which inputs elicit spikes and which do not, spike initiation dynamics confer tuning to different stimulus parameters. Coincidence detectors function as band-pass filters that are tuned to high-input frequencies whose power represents the degree of input synchrony. Integrators function as low-pass filters tuned to lower frequencies and are thus relatively untuned with respect to synchrony. Real pyramidal neurons function somewhere in between.
Rapidly fluctuating (synchronous) inputs can produce precisely timed spikes within a single neuron, which translates into synchronous spiking across a set of neurons who share that input. In this respect, both integrators and coincidence detectors can respond to synchronous input with synchronous output. However, the two operating modes differ in how robust that output synchrony is to background noise (Figures 1C and 7) and to variations in firing rate (Figures 1D and 3B). Coincidence detectors transfer synchrony more robustly because of their spike initiation dynamics. For this reason, operating mode is best defined according to synchrony transfer.
Furthermore, operating mode must be treated as a continuum if we are to accurately describe the functioning of real neurons. Indeed, the longstanding debate over whether pyramidal neurons operate as integrators or coincidence detectors can be resolved by agreeing that they exhibit traits of both; moreover, those traits are modulated by factors like conductance state and are variably manifested by stimuli with different spectral properties. Once this is recognized, it becomes obvious that pyramidal neurons are suboptimal when it comes to integration or coincidence detection and, by extension, that they are suboptimal at rate and synchrony coding. However, a hybrid operating mode—one that exploits elements of both integration and coincidence detection—may enable multiplexing of rate and synchrony coding, thereby allowing pyramidal neurons to achieve higher total information capacity than if they used one or the other code optimally.
Several issues arise from this Perspective. For instance, which neuron models can capture the essential differences between integrator and coincidence detector operating mode? Conductance-based neuron models can exhibit either operating mode based on parameter values (Lundstrom et al., 2008; Prescott et al., 2008a). This is similarly true for more sophisticated integrate-and-fire (IF) models such as the adaptive exponential IF model (Brette and Gerstner, 2005; for review, see Brunel, 2010). In principle, stimulus-dependent variations in the voltage trajectory toward threshold can be replaced with stimulus-dependent variations in threshold (Yamauchi et al., 2011). What is important is that the model includes different time-scales so that intrinsic processes can interact with timescales present in the input, thus enabling inputs with power at lower or higher frequencies to preferentially elicit spikes. In this regard, the STA is invaluable in describing how stimulus properties and intrinsic neuron properties interact. Rather than pronouncing here on which models succeed or fail to capture different operating modes, we recommend that models be tested by measuring their STA under a broad range of stimulus conditions.
Beyond determining which models are most appropriate, it is important to experimentally determine where different types of neurons fall along the operating mode continuum, whether the population is tightly or broadly distributed along the continuum, etc. Like for models, the STA is a valuable descriptor of neuronal response properties. For neurons falling within the middle range, can they operate in a hybrid mode and achieve multiplexed coding under certain stimulus conditions? Under what stimulus conditions? Another broad and important set of questions includes how neurons operating in different modes function within different network architectures.
To conclude, spike initiation dynamics regulate synchrony transfer properties, and synchrony transfer properties regulate network coding strategies; therefore, spike initiation dynamics regulate network coding strategies. An accurate and complete understanding of network coding demands that we give greater consideration to neuronal properties, especially to spike initiation dynamics.
Acknowledgments
This work was support by NIH grant NS076706 (S.A.P) and the Okinawa Institute of Science and Technology School Corporation (E.D.S.). S.A.P. is also a Rita Allen Scholar in Pain and the 53rd Mallinckrodt Scholar. We thank Dan Simons for his constructive feedback.
References
- Abeles M. Role of the cortical neuron: integrator or coincidence detector? Isr J Med Sci. 1982;18:83–92. [PubMed] [Google Scholar]
- Agüera y Arcas B, Fairhall AL. What causes a neuron to spike? Neural Comput. 2003;15:1789–1807. doi: 10.1162/08997660360675044. [DOI] [PubMed] [Google Scholar]
- Agüera y Arcas B, Fairhall AL, Bialek W. Computation in a single neuron: Hodgkin and Huxley revisited. Neural Comput. 2003;15:1715–1749. doi: 10.1162/08997660360675017. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383:815–819. doi: 10.1038/383815a0. [DOI] [PubMed] [Google Scholar]
- Arsiero M, Lüscher HR, Lundstrom BN, Giugliano M. The impact of input fluctuations on the frequency-current relationships of layer 5 pyramidal neurons in the rat medial prefrontal cortex. J Neurosci. 2007;27:3274–3284. doi: 10.1523/JNEUROSCI.4937-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nat Rev Neurosci. 2006;7:358–366. doi: 10.1038/nrn1888. [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Dynamic spike threshold reveals a mechanism for synaptic coincidence detection in cortical neurons in vivo. Proc Natl Acad Sci USA. 2000;97:8110–8115. doi: 10.1073/pnas.130200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Adaptive coincidence detection and dynamic gain control in visual cortical neurons in vivo. Neuron. 2003;37:513–523. doi: 10.1016/s0896-6273(02)01186-8. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Possible principles underlying the transformation of sensory messages. In: Rosenblith W, editor. Sensory Communication. Cambridge, MA: MIT Press; 1961. [Google Scholar]
- Barreiro AK, Shea-Brown E, Thilo EL. Time scales of spiketrain correlation for neural oscillators with common drive. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;81:011916. doi: 10.1103/PhysRevE.81.011916. [DOI] [PubMed] [Google Scholar]
- Barreiro AK, Thilo EL, Shea-Brown E. A-current and type I/type II transition determine collective spiking from common input. J Neurophysiol. 2012;108:1631–1645. doi: 10.1152/jn.00928.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernander O, Douglas RJ, Martin KA, Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc Natl Acad Sci USA. 1991;88:11569–11573. doi: 10.1073/pnas.88.24.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MJ, 2nd, Meister M. Refractoriness and neural precision. J Neurosci. 1998;18:2200–2211. doi: 10.1523/JNEUROSCI.18-06-02200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billimoria CP, DiCaprio RA, Birmingham JT, Abbott LF, Marder E. Neuromodulation of spike-timing precision in sensory neurons. J Neurosci. 2006;26:5910–5919. doi: 10.1523/JNEUROSCI.4659-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg-Graham LJ, Monier C, Frégnac Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature. 1998;393:369–373. doi: 10.1038/30735. [DOI] [PubMed] [Google Scholar]
- Borst A, Theunissen FE. Information theory and neural coding. Nat Neurosci. 1999;2:947–957. doi: 10.1038/14731. [DOI] [PubMed] [Google Scholar]
- Brette R. Computing with neural synchrony. PLoS Comput Biol. 2012;8:e1002561. doi: 10.1371/journal.pcbi.1002561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brette R, Gerstner W. Adaptive exponential integrate-and-fire model as an effective description of neuronal activity. J Neurophysiol. 2005;94:3637–3642. doi: 10.1152/jn.00686.2005. [DOI] [PubMed] [Google Scholar]
- Brunel N. Modeling point neurons: from Hodgkin-Huxley to integrate-and-fire. In: De Schutter E, editor. Computational Modeling Methods for Neuroscientists. Cambridge, MA: MIT Press; 2010. [Google Scholar]
- Bruno RM. Synchrony in sensation. Curr Opin Neurobiol. 2011;21:701–708. doi: 10.1016/j.conb.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science. 2006;312:1622–1627. doi: 10.1126/science.1124593. [DOI] [PubMed] [Google Scholar]
- Bryant HL, Segundo JP. Spike initiation by transmembrane current: a white-noise analysis. J Physiol. 1976;260:279–314. doi: 10.1113/jphysiol.1976.sp011516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burak Y, Lewallen S, Sompolinsky H. Stimulus-dependent correlations in threshold-crossing spiking neurons. Neural Comput. 2009;21:2269–2308. doi: 10.1162/neco.2009.07-08-830. [DOI] [PubMed] [Google Scholar]
- Cecchi GA, Sigman M, Alonso JM, Martínez L, Chialvo DR, Magnasco MO. Noise inneurons is message dependent. Proc Natl Acad Sci USA. 2000;97:5557–5561. doi: 10.1073/pnas.100113597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Kohn A. Measuring and interpreting neuronal correlations. Nat Neurosci. 2011;14:811–819. doi: 10.1038/nn.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron. 2011;69:1061–1068. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudmore RH, Fronzaroli-Molinieres L, Giraud P, Debanne D. Spike-time precision and network synchrony are controlled by the homeostatic regulation of the D-type potassium current. J Neurosci. 2010;30:12885–12895. doi: 10.1523/JNEUROSCI.0740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Alonso JM, Usrey WM, Reid RC. Coding of visual information by precisely correlated spikes in the lateral geniculate nucleus. Nat Neurosci. 1998;1:501–507. doi: 10.1038/2217. [DOI] [PubMed] [Google Scholar]
- de la Rocha J, Doiron B, Shea-Brown E, Josic K, Reyes A. Correlation between neural spike trains increases with firing rate. Nature. 2007;448:802–806. doi: 10.1038/nature06028. [DOI] [PubMed] [Google Scholar]
- deCharms RC. Information coding in the cortex by independent or coordinated populations. Proc Natl Acad Sci USA. 1998;95:15166–15168. doi: 10.1073/pnas.95.26.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCharms RC, Merzenich MM. Primary cortical representation of sounds by the coordination of action-potential timing. Nature. 1996;381:610–613. doi: 10.1038/381610a0. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Paré D. Impact of network activity on the integrative propertiesof neocortical pyramidal neuronsinvivo. J Neurophysiol. 1999;81:1531–1547. doi: 10.1152/jn.1999.81.4.1531. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Fellous JM, Sejnowski TJ. Fluctuating synaptic conductances recreate in vivo-like activity in neocortical neurons. Neuroscience. 2001;107:13–24. doi: 10.1016/s0306-4522(01)00344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Paré D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci. 2003;4:739–751. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- DeWeese MR, Zador AM. Non-Gaussian membrane potential dynamics imply sparse, synchronous activity in auditory cortex. J Neurosci. 2006;26:12206–12218. doi: 10.1523/JNEUROSCI.2813-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesmann M, Gewaltig MO, Aertsen A. Stable propagation of synchronous spiking in cortical neural networks. Nature. 1999;402:529–533. doi: 10.1038/990101. [DOI] [PubMed] [Google Scholar]
- Ecker AS, Berens P, Keliris GA, Bethge M, Logothetis NK, Tolias AS. Decorrelated neuronal firing in cortical microcircuits. Science. 2010;327:584–587. doi: 10.1126/science.1179867. [DOI] [PubMed] [Google Scholar]
- Ermentrout B. Type I membranes, phase resetting curves, and synchrony. Neural Comput. 1996;8:979–1001. doi: 10.1162/neco.1996.8.5.979. [DOI] [PubMed] [Google Scholar]
- Ermentrout GB, Galán RF, Urban NN. Relating neural dynamics to neural coding. Phys Rev Lett. 2007;99:248103. doi: 10.1103/PhysRevLett.99.248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermentrout GB, Galán RF, Urban NN. Reliability, synchrony and noise. Trends Neurosci. 2008;31:428–434. doi: 10.1016/j.tins.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estebanez L, El Boustani S, Destexhe A, Shulz DE. Correlated input reveals coexisting coding schemes in a sensory cortex. Nat Neurosci. 2012;15:1691–1699. doi: 10.1038/nn.3258. [DOI] [PubMed] [Google Scholar]
- Fernandez FR, Broicher T, Truong A, White JA. Membrane voltage fluctuations reduce spike frequency adaptation and preserve output gain in CA1 pyramidal neurons in a high-conductance state. J Neurosci. 2011;31:3880–3893. doi: 10.1523/JNEUROSCI.5076-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker D, Miles R. EPSP amplification and the precision of spike timing in hippocampal neurons. Neuron. 2000;28:559–569. doi: 10.1016/s0896-6273(00)00133-1. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Habermann CJ, Laurent G. Multiplexing using synchrony in the zebrafish olfactory bulb. Nat Neurosci. 2004;7:862–871. doi: 10.1038/nn1292. [DOI] [PubMed] [Google Scholar]
- Galán RF, Ermentrout GB, Urban NN. Optimal time scale for spike-time reliability: theory, simulations, and experiments. J Neurophysiol. 2008;99:277–283. doi: 10.1152/jn.00563.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastrein P, Campanac E, Gasselin C, Cudmore RH, Bialowas A, Carlier E, Fronzaroli-Molinieres L, Ankri N, Debanne D. The role of hyperpolarization-activated cationic current in spike-time precision and intrinsic resonance in cortical neurons in vitro. J Physiol. 2011;589:3753–3773. doi: 10.1113/jphysiol.2011.209148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, Richmond BJ. How independent are the messages carried by adjacent inferior temporal cortical neurons? J Neurosci. 1993;13:2758–2771. doi: 10.1523/JNEUROSCI.13-07-02758.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedeke S, Diesmann M. The mechanism of synchronization in feed-forward neuronal networks. New J Phys. 2008;10:015007. [Google Scholar]
- Goldberg JA, Rokni U, Boraud T, Vaadia E, Bergman H. Spike synchronization in the cortex/basal-ganglia networks of Parkinsonian primates reflects global dynamics of the local field potentials. J Neurosci. 2004;24:6003–6010. doi: 10.1523/JNEUROSCI.4848-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grün S. Data-driven significance estimation for precise spike correlation. J Neurophysiol. 2009;101:1126–1140. doi: 10.1152/jn.00093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, Häusser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2013;493:97–100. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel D, Mato G, Meunier C. Synchrony in excitatory neural networks. Neural Comput. 1995;7:307–337. doi: 10.1162/neco.1995.7.2.307. [DOI] [PubMed] [Google Scholar]
- Higgs MH, Slee SJ, Spain WJ. Diversity of gain modulation by noise in neocortical neurons: regulation by the slow afterhyperpolarization conductance. J Neurosci. 2006;26:8787–8799. doi: 10.1523/JNEUROSCI.1792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL. The local electric changes associated with repetitive action in a non-medullated axon. J Physiol. 1948;107:165–181. doi: 10.1113/jphysiol.1948.sp004260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Agüera y Arcas B, Fairhall AL. Single neuron computation: from dynamical system tofeature detector. Neural Comput. 2007;19:3133–3172. doi: 10.1162/neco.2007.19.12.3133. [DOI] [PubMed] [Google Scholar]
- Hong S, Ratté S, Prescott SA, De Schutter E. Single neuron firing properties impact correlation-based population coding. J Neurosci. 2012;32:1413–1428. doi: 10.1523/JNEUROSCI.3735-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxter J, Burgess N, O'Keefe J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature. 2003;425:828–832. doi: 10.1038/nature02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, Yuste R. Synfire chains and cortical songs: temporal modules of cortical activity. Science. 2004;304:559–564. doi: 10.1126/science.1093173. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhikevich EM. Dynamical Systems in Neuroscience. Cambridge, MA: MIT Press; 2007. [Google Scholar]
- Jadhav SP, Wolfe J, Feldman DE. Sparse temporal coding of elementary tactilefeaturesduringactivewhisker sensation. Nat Neurosci. 2009;12:792–800. doi: 10.1038/nn.2328. [DOI] [PubMed] [Google Scholar]
- Josić K, Shea-Brown E, Doiron B, de la Rocha J. Stimulus-dependent correlations and population codes. Neural Comput. 2009;21:2774–2804. doi: 10.1162/neco.2009.10-08-879. [DOI] [PubMed] [Google Scholar]
- Kenyon GT, Theiler J, George JS, Travis BJ, Marshak DW. Correlated firing improves stimulus discrimination in a retinal model. Neural Comput. 2004;16:2261–2291. doi: 10.1162/0899766041941916. [DOI] [PubMed] [Google Scholar]
- Kohn A, Smith MA. Stimulus dependence of neuronal correlation in primary visual cortex of the macaque. J Neurosci. 2005;25:3661–3673. doi: 10.1523/JNEUROSCI.5106-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König P, Engel AK, Singer W. Integrator or coincidence detector? The role of the cortical neuron revisited. Trends Neurosci. 1996;19:130–137. doi: 10.1016/s0166-2236(96)80019-1. [DOI] [PubMed] [Google Scholar]
- Kumar A, Rotter S, Aertsen A. Conditions for propagating synchronous spiking and asynchronous firing rates in a cortical network model. J Neurosci. 2008;28:5268–5280. doi: 10.1523/JNEUROSCI.2542-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Rotter S, Aertsen A. Spiking activity propagation in neuronal networks: reconciling different perspectives on neural coding. Nat Rev Neurosci. 2010;11:615–627. doi: 10.1038/nrn2886. [DOI] [PubMed] [Google Scholar]
- Lampl I, Reichova I, Ferster D. Synchronous membrane potential fluctuations in neurons of the cat visual cortex. Neuron. 1999;22:361–374. doi: 10.1016/s0896-6273(00)81096-x. [DOI] [PubMed] [Google Scholar]
- Lathi BP, Ding Z. Modern Digital and Analog Communication Systems. Fourth Edition. Oxford: Oxford University Press; 2009. [Google Scholar]
- London M, Roth A, Beeren L, Häusser M, Latham PE. Sensitivity to perturbations in vivo implies high noise and suggests rate coding in cortex. Nature. 2010;466:123–127. doi: 10.1038/nature09086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom BN, Hong S, Higgs MH, Fairhall AL. Two computational regimes of a single-compartment neuron separated by a planar boundary in conductance space. Neural Comput. 2008;20:1239–1260. doi: 10.1162/neco.2007.05-07-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom BN, Famulare M, Sorensen LB, Spain WJ, Fairhall AL. Sensitivity of firing rate to input fluctuations depends on time scale separation between fast and slow variables in single neurons. J Comput Neurosci. 2009;27:277–290. doi: 10.1007/s10827-009-0142-x. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Reliability of spike timing in neocortical neurons. Science. 1995;268:1503–1506. doi: 10.1126/science.7770778. [DOI] [PubMed] [Google Scholar]
- Mason A, Nicoll A, Stratford K. Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. J Neurosci. 1991;11:72–84. doi: 10.1523/JNEUROSCI.11-01-00072.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN. Correlated firing of retinal ganglion cells. Trends Neurosci. 1989;12:75–80. doi: 10.1016/0166-2236(89)90140-9. [DOI] [PubMed] [Google Scholar]
- Mato G, Samengo I. Type I and type II neuron models are selectively driven by differential stimulus features. Neural Comput. 2008;20:2418–2440. doi: 10.1162/neco.2008.10-07-632. [DOI] [PubMed] [Google Scholar]
- Mazurek ME, Shadlen MN. Limits to the temporal fidelity of cortical spike rate signals. Nat Neurosci. 2002;5:463–471. doi: 10.1038/nn836. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Brunel N, Cavallari S, Logothetis NK, Panzeri S. Cortical dynamics during naturalistic sensory stimulations: experiments and models. J Physiol Paris. 2011;105:2–15. doi: 10.1016/j.jphysparis.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Meister M, Lagnado L, Baylor DA. Concerted signaling by retinal ganglion cells. Science. 1995;270:1207–1210. doi: 10.1126/science.270.5239.1207. [DOI] [PubMed] [Google Scholar]
- Montani F, Kohn A, Smith MA, Schultz SR. The role of correlations in direction and contrast coding in the primary visual cortex. J Neurosci. 2007;27:2338–2348. doi: 10.1523/JNEUROSCI.3417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak LG, Sanchez-Vives MV, McCormick DA. Influence of low and high frequency inputs on spike timing in visual corticalneurons. Cereb Cortex. 1997;7:487–501. doi: 10.1093/cercor/7.6.487. [DOI] [PubMed] [Google Scholar]
- Ohiorhenuan IE, Mechler F, Purpura KP, Schmid AM, Hu Q, Victor JD. Sparse coding and high-order correlations in fine-scale cortical networks. Nature. 2010;466:617–621. doi: 10.1038/nature09178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostojic S, Brunel N, Hakim V. How connectivity, background activity, and synaptic properties shape the cross-correlation between spike trains. J Neurosci. 2009;29:10234–10253. doi: 10.1523/JNEUROSCI.1275-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzeri S, Brunel N, Logothetis NK, Kayser C. Sensory neural codes using multiplexed temporal scales. Trends Neurosci. 2010;33:111–120. doi: 10.1016/j.tins.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Perkel DH, Bullock TH. Neural coding. Neurosci Res Prog Sum. 1968;3:405–527. [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Pouille F, Marin-Burgin A, Adesnik H, Atallah BV, Scanziani M. Input normalization by global feedforward inhibition expands cortical dynamic range. Nat Neurosci. 2009;12:1577–1585. doi: 10.1038/nn.2441. [DOI] [PubMed] [Google Scholar]
- Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Integration time in a subset of spinal lamina I neurons is lengthened by sodium and calcium currents acting synergistically to prolong subthreshold depolarization. J Neurosci. 2005;25:4743–4754. doi: 10.1523/JNEUROSCI.0356-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, Sejnowski TJ. Spike-rate coding and spiketime coding are affected oppositely by different adaptation mechanisms. J Neurosci. 2008;28:13649–13661. doi: 10.1523/JNEUROSCI.1792-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, Ratté S, De Koninck Y, Sejnowski TJ. Nonlinear interaction between shunting and adaptation controls a switch between integration and coincidence detection in pyramidal neurons. J Neurosci. 2006;26:9084–9097. doi: 10.1523/JNEUROSCI.1388-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y, Sejnowski TJ. Biophysical basis for three distinct dynamical mechanisms of action potential initiation. PLoS Comput Biol. 2008a;4:e1000198. doi: 10.1371/journal.pcbi.1000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, Ratté S, De Koninck Y, Sejnowski TJ. Pyramidal neurons switch from integrators in vitro to resonators under in vivo-like conditions. J Neurophysiol. 2008b;100:3030–3042. doi: 10.1152/jn.90634.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich DS, Mechler F, Victor JD. Independent and redundant information in nearby cortical neurons. Science. 2001;294:2566–2568. doi: 10.1126/science.1065839. [DOI] [PubMed] [Google Scholar]
- Renart A, de la Rocha J, Bartho P, Hollender L, Parga N, Reyes A, Harris KD. The asynchronous state in cortical circuits. Science. 2010;327:587–590. doi: 10.1126/science.1179850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes AD. Synchrony-dependent propagation of firing rate in iteratively constructed networks in vitro. Nat Neurosci. 2003;6:593–599. doi: 10.1038/nn1056. [DOI] [PubMed] [Google Scholar]
- Riehle A, Grün S, Diesmann M, Aertsen A. Spike synchronization and rate modulation differentially involved in motor cortical function. Science. 1997;278:1950–1953. doi: 10.1126/science.278.5345.1950. [DOI] [PubMed] [Google Scholar]
- Rieke F, Warland D, de Ruyter van Steveninck RR, Bialek W. Spikes: Exploring the Neural Code. Cambridge, MA: MIT Press; 1997. [Google Scholar]
- Rinzel J, Ermentrout GB. Analysis of neural excitability and oscillations. In: Koch C, Segev I, editors. Methods in Neuronal Modeling: From Ions to Networks. Cambridge, MA: MIT Press; 1998. pp. 251–291. [Google Scholar]
- Rossant C, Leijon S, Magnusson AK, Brette R. Sensitivity of noisy neurons to coincident inputs. J Neurosci. 2011;31:17193–17206. doi: 10.1523/JNEUROSCI.2482-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph M, Destexhe A. Tuning neocortical pyramidal neurons between integrators and coincidence detectors. J Comput Neurosci. 2003;14:239–251. doi: 10.1023/a:1023245625896. [DOI] [PubMed] [Google Scholar]
- Sáez I, Friedlander MJ. Synaptic output of individual layer 4 neurons in guinea pig visual cortex. J Neurosci. 2009;29:4930–4944. doi: 10.1523/JNEUROSCI.0046-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Impact of correlated synaptic input on output firing rate and variability in simple neuronal models. J Neurosci. 2000;20:6193–6209. doi: 10.1523/JNEUROSCI.20-16-06193.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer RJ, Redman SJ, Andersen P. Amplitude fluctuations in small EPSPs recorded from CA1 pyramidal cells in the guinea pig hippocampal slice. J Neurosci. 1989;9:840–850. doi: 10.1523/JNEUROSCI.09-03-00840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneidman E, Berry MJ, 2nd, Segev R, Bialek W. Weak pairwise correlations imply strongly correlated network states in a neural population. Nature. 2006;440:1007–1012. doi: 10.1038/nature04701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer MJ, Meister M. Multineuronal firing patterns in the signal from eye to brain. Neuron. 2003;37:499–511. doi: 10.1016/s0896-6273(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Fellous JM, Tiesinga P, Sejnowski TJ. Influence of ionic conductances on spike timing reliability of cortical neurons for suprathreshold rhythmic inputs. J Neurophysiol. 2004;91:194–205. doi: 10.1152/jn.00556.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze-Kraft M, Diesmann M, Grün S, Helias M. Noise suppression and surplus synchrony by coincidence detection. PLoS Comput Biol. 2013;9:e1002904. doi: 10.1371/journal.pcbi.1002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O, Pillow JW, Rust NC, Simoncelli EP. Spike-triggered neural characterization. J Vis. 2006;6:484–507. doi: 10.1167/6.4.13. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci. 1998;18:3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharafi N, Benda J, Lindner B. Information filtering by synchronous spikes in a neural population. J Comput Neurosci. 2013;34:285–301. doi: 10.1007/s10827-012-0421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki H, Amari S, Brown EN, Grün S. State-space analysis of time-varying higher-order spike correlation for multiple neural spike train data. PLoS Comput Biol. 2012;8:e1002385. doi: 10.1371/journal.pcbi.1002385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. 111–125. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Slee SJ, Higgs MH, Fairhall AL, Spain WJ. Two-dimensional time coding in the auditory brainstem. J Neurosci. 2005;25:9978–9988. doi: 10.1523/JNEUROSCI.2666-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Kohn A. Spatial and temporal scales of neuronal correlation in primary visual cortex. J Neurosci. 2008;28:12591–12603. doi: 10.1523/JNEUROSCI.2929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Softky WR, Koch C. The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J Neurosci. 1993;13:334–350. doi: 10.1523/JNEUROSCI.13-01-00334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky H, Yoon H, Kang K, Shamir M. Population coding in neuronal systems with correlated noise. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;64:051904. doi: 10.1103/PhysRevE.64.051904. [DOI] [PubMed] [Google Scholar]
- Song S, Sjöström PJ, Reigl M, Nelson S, Chklovskii DB. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 2005;3:e68. doi: 10.1371/journal.pbio.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley GB. Reading and writing the neural code. Nat Neurosci. 2013;16:259–263. doi: 10.1038/nn.3330. [DOI] [PubMed] [Google Scholar]
- Staude B, Grün S, Rotter S. Higher-order correlations in non-stationary parallel spike trains: statistical modeling and inference. Front Comput Neurosci. 2010;4:16. doi: 10.3389/fncom.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz PN, Roy A, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature. 2000;404:187–190. doi: 10.1038/35004588. [DOI] [PubMed] [Google Scholar]
- Stevens CF. Neuronal communication. Cooperativity of unreliable neurons Curr Biol. 1994;4:268–269. doi: 10.1016/s0960-9822(00)00062-2. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Zador AM. Input synchrony and the irregular firing of cortical neurons. Nat Neurosci. 1998;1:210–217. doi: 10.1038/659. [DOI] [PubMed] [Google Scholar]
- Stiefel KM, Gutkin BS, Sejnowski TJ. Cholinergic neuromodulation changes phase response curve shape and type in cortical pyramidal neurons. PLoS ONE. 2008;3:e3947. doi: 10.1371/journal.pone.0003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svirskis G, Rinzel J. Influence of subthreshold nonlinearities on signal-to-noise ratio and timing precision for small signals in neurons: minimal model analysis. Network. 2003;14:137–150. [PMC free article] [PubMed] [Google Scholar]
- Tateno T, Harsch A, Robinson HP. Threshold firing frequency-current relationships of neurons in rat somatosensory cortex: type 1 and type 2 dynamics. J Neurophysiol. 2004;92:2283–2294. doi: 10.1152/jn.00109.2004. [DOI] [PubMed] [Google Scholar]
- Tchumatchenko T, Malyshev A, Geisel T, Volgushev M, Wolf F. Correlations and synchrony in threshold neuron models. Phys Rev Lett. 2010;104:058102. doi: 10.1103/PhysRevLett.104.058102. [DOI] [PubMed] [Google Scholar]
- Temereanca S, Brown EN, Simons DJ. Rapid changes in thalamic firing synchrony during repetitive whisker stimulation. J Neurosci. 2008;28:11153–11164. doi: 10.1523/JNEUROSCI.1586-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff T, Helias M, Einevoll GT, Diesmann M. Decorrelation of neural-network activity by inhibitory feedback. PLoS Comput Biol. 2012;8:e1002596. doi: 10.1371/journal.pcbi.1002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubo Y, Takada M, Reyes AD, Fukai T. Layer and frequency dependencies of phase response properties of pyramidal neurons in rat motor cortex. Eur J Neurosci. 2007;25:3429–3441. doi: 10.1111/j.1460-9568.2007.05579.x. [DOI] [PubMed] [Google Scholar]
- Wang HP, Spencer D, Fellous JM, Sejnowski TJ. Synchrony ofthalamocortical inputs maximizes cortical reliability. Science. 2010;328:106–109. doi: 10.1126/science.1183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi S, Kim H, Shinomoto S. Elemental spiking neuron model for reproducing diverse firing patterns and predicting precise firing times. Front Comput Neurosci. 2011;5:42. doi: 10.3389/fncom.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Ferster D. Membrane potential synchrony in primary visual cortex during sensory stimulation. Neuron. 2010;68:1187–1201. doi: 10.1016/j.neuron.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Yang H, Nakahara H, Santos GS, Nikolić D, Plenz D. Higher-order interactions characterized in cortical activity. J Neurosci. 2011;31:17514–17526. doi: 10.1523/JNEUROSCI.3127-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zador A. Impact of synaptic unreliability on the information transmitted by spiking neurons. J Neurophysiol. 1998;79:1219–1229. doi: 10.1152/jn.1998.79.3.1219. [DOI] [PubMed] [Google Scholar]