Abstract
It has been reported that Golgi protein-73 (GP73), glypican-3 (GPC3), and des-γ-carboxy prothrombin (DCP) could serve as serum markers for the early detection of hepatocellular carcinoma (HCC). This study aimed to evaluate a panel of immunostaining markers (including GP73, GPC3, DCP, CD34, and CD31) as well as reticulin staining to distinguish HCC from the mimickers. Our results revealed that CD34 immunostaining and reticulin staining were highly sensitive for the diagnosis of HCC. A special immunoreaction pattern of GP73—a diffuse coarse-block pattern in a perinuclear region or a concentrated cluster-like or cord-like pattern in a certain part of the cytoplasm—was observed in HCC cells, in contrast to the cytoplasmic fine-granular pattern in surrounding non-tumor cells and non-malignant nodules. This coarse-block pattern correlated significantly with less differentiated HCC. In comparison, GPC3 displayed a good advantage in diagnosing well-differentiated HCC. In our study, DCP and CD31 showed little diagnostic value for HCC as an immunostaining marker. When GP73, GPC3, and CD34 were combined, the specificity improved to 96.6%. Our findings demonstrate for the first time that the immunohistochemical panel of GP73, GPC3, and CD34 as well as reticulin staining is highly specific for the pathological diagnosis of HCC.
Keywords: hepatocellular carcinoma, immunohistochemistry, GP73, GPC3, CD34
Hepatocellular carcinoma (HCC) is one of the most common human cancers worldwide, and the incidence is increasing. Most cases of HCC (>80%) occur in either Eastern Asia or sub-Saharan Africa and are strongly associated with hepatitis B or C virus (HBV or HCV) infection and liver cirrhosis (El-Serag and Rudolph 2007). Although the prognosis of patients with HCC has improved over the past two decades, the 5-year survival rate remains poor and currently stands at less than 10% (Farazi and DePinho 2006). More than two-thirds of patients present in advanced disease stages. Therefore, there is an urgent need to develop specific tumor markers to aid the early diagnosis of HCC.
Alpha-fetoprotein (AFP) is currently the only serum marker recommended for screening patients at high risk for HCC, although up to 40% of patients with HCC show normal serological AFP levels, particularly during the early disease stages (Farinati et al. 2006; Jia et al. 2007). Furthermore, AFP production can occur in other circumstances, especially during exacerbations of hepatitis, such as chronic liver disease and cirrhosis (Jing et al. 2010; Sterling et al. 2012). Immunohistochemistry provides a more intuitive approach for pathologists to directly observe the immunoreactions within tissues in the lesion, although the sensitivity is significantly lower than that of serological tests. This highlights the need for more sensitive and specific immunostaining markers for the diagnosis of HCC.
Well-differentiated HCC may closely mimic benign liver disease. Therefore, the overdiagnosis of a dysplastic nodule as a malignancy is one of the challenges for pathologists. A good immunostaining marker should readily differentiate tumor tissue from non-tumor tissue. The most widely used immunohistochemical marker for HCC is CD34. Benign hepatic sinusoids are different from other small blood vessels because they do not express endothelial cell markers such as von Willebrand factor or CD34 (Pusztaszeri et al. 2006; Akkiz et al. 2011). When sinusoidal cells of HCC tissues undergo a process of “capillarization,” CD34 shows diffuse positive staining (Sun et al. 1999; Coston et al. 2008). It has previously been reported that HCC tissues had no immunoreaction with CD34, whereas other non-malignant neoplastic lesions showed a positive immunoreaction (de Boer et al. 2000; El-Serag et al. 2005). However, in contrast to the low sensitivity of AFP, CD34 is a very sensitive but less specific marker for HCC.
A number of candidate biomarkers have recently been proposed for the differential diagnosis of hepatocellular nodules, including des-γ-carboxy prothrombin (DCP), Golgi protein-73 (GP73), and glypican-3 (GPC3). DCP, induced by the absence of vitamin K or antagonist II, is an abnormal prothrombin that lacks the ability to interact with other coagulation factors. It is currently used in Japan, together with AFP, as a serological tumor marker for the early detection of HCC (Beneduce et al. 2008; Yamamoto et al. 2010). GP73 was originally described as a Golgi type II transmembrane protein elevated in serum and tissue in the woodchuck model of HCC (Block et al. 2005). Recent studies of patients with liver disease, particularly with HCC, have identified that serum levels of GP73 are more than 30-fold greater than those in patients without the disease (Block et al. 2005; Marrero et al. 2005). GPC3 is a cell-surface glycoprotein that is absent in the hepatocytes of healthy subjects and patients with hepatitis but highly expressed in hepatocellular cancer cells (Sato and Mori 2011; Jin et al. 2012). Several studies have reported that these markers are superior to AFP for the early detection of HCC (Yamamoto et al. 2009; Mao et al. 2010; Tangkijvanich et al. 2010). However, all have varying amounts of detectable expression in cases of benign disease and non-malignant neoplastic nodules (Liu et al. 2011; Ozkan et al. 2011; Tian et al. 2011; Sterling et al. 2012). Currently, no single marker is sufficiently sensitive and specific for the diagnosis of HCC. There remains an urgent need to identify a panel of immunostaining markers for the accurate diagnosis of liver lesions.
In the present study, we aimed to determine the immunohistochemical expression profiles of GP73, GPC3, DCP, CD34, and CD31, together with reticulin staining, in HCCs and non-malignant neoplastic nodules, as well as to evaluate the diagnostic potential of these markers in differentiating HCC from other hepatic conditions.
Materials and Methods
Patients
A total of 124 surgically resected specimens, including 65 HCCs, 29 non-malignant neoplastic nodules (focal nodular hyperplasia, n=10; large regenerative nodules, n=6; low-grade dysplastic nodules, n=3; high-grade dysplastic nodules, n=7; and liver cell adenomas, n=3) and 30 hepatolithiasis (as controls) were collected from 2006 to 2010 at the Qilu Hospital of Shandong University. This study was approved by the Committee of Ethical Research at Shandong University, and written informed consent was obtained from all enrolled patients.
The specimens were fixed in 4% phosphate-buffered formaldehyde directly after resection and then embedded in paraffin. The diagnosis of hepatocellular nodules was performed according to criteria defined by the World Health Organization (2010) and the International Working Party on hematoxylin and eosin–stained sections (1995).
Immunohistochemistry
Immunohistochemical staining was performed according to our previous study (Li et al. 2011). Briefly, the sections were deparaffinized with xylene, rehydrated through a graded alcohol series, and then microwaved at 500 W for 2 × 5 min in 10 mM citrate buffer (pH 6.0). After rinsing in Tris-buffered saline (pH 7.6), the sections were immersed in a solution of H2O2 (3%) to block endogenous peroxidase activity followed by the addition of 5% fetal calf serum (1:20 dilution); samples were then incubated with a primary antibody overnight at 4C. After washing, the primary antibody was detected using an appropriate secondary antibody (Universal PV9000 Kit; Zhongshan Biotechnology, Beijing, China), incubated for 30 min at 37C, and then visualized using DAB. Counterstaining was carried out with hematoxylin. The antibodies used were as follows: GPC3 (9C2, ab129381, 1:100 dilution; Abcam, Cambridge, UK), GP73 (F-2, sc365817, 1:100 dilution; Santa Cruz Biotchnology, Santa Cruz, CA), DCP (MU-3, 1:100 dilution; Eisai, Tokyo, Japan), CD34 (QBEen10, sc52312, 1:100 dilution; Santa Cruz Biotechnology), and CD31 (JC/70A, ab9498, 1:200 dilution; Abcam).
Reticulin Staining
Reticulin staining was performed according to the Gomori method (Gomori 1947). Briefly, sections were cut at a 4-µm thickness from routinely processed paraffin blocks. These were deparaffinized, rehydrated, rinsed in distilled water, and immersed in 1% potassium permanganate (2 min). The sections were rinsed in the following solutions: 2.5% oxalic acid (1 min), 2% iron alum (1 min), Gomori’s solution (3 min), 10% formalin (2 min), gold chloride (1:500; 5 min), 3% potassium metabisulfite (1 min), and 3% sodium thiosulfate (1 min); sections were rinsed with distilled water before immersion in each solution. Then, the sections were dehydrated through a graded series of ethanol, cleared in xylene, and mounted with neutral gum.
Statistical Analysis
The data were analyzed by the Statistical Package for Social Sciences (SPSS for Windows, version 16.0; SPSS, Inc., an IBM Company, Chicago, IL). The comparison of positive rates of biomarkers between the groups was done using Chi-square or Fisher’s exact tests. All statistical analyses were two-sided, and p<0.05 was considered statistically significant.
Results
CD34
The patients’ clinical information and the immunostaining profiles of all biomarkers are listed in Suppl. Tables S1 and S2. Three immunoreaction patterns of CD34 were observed in hepatic lesions. The first pattern was a negative CD34 immunoreaction pattern, in which only blood vessels and bile ducts in portal tracts and/or rare sinusoidal spaces near portal tracts were positive (Fig. 1A). This pattern was observed in all control patients and in some non-malignant neoplastic nodules (Table 1), as well as in all non-tumor cells surrounding the hepatic mass; it was not observed in HCC cells. The second immunoreaction pattern showed diffuse CD34 expression, where most sinusoidal spaces throughout the mass were positive (Fig. 1B). This pattern was observed in most HCC tissues (62/65, 95.4%) and in 11 of 29 (37.9%) cases of non-malignant neoplastic nodules. The third observed pattern was the focal CD34 immunoreaction pattern in which only part of the sinusoidal spaces had immunoreactions with CD34 (Fig. 1C). This pattern was demonstrated in three (4.6%) of the HCC tissues and four (13.8%) cases with non-malignant neoplastic nodules.
Figure 1.
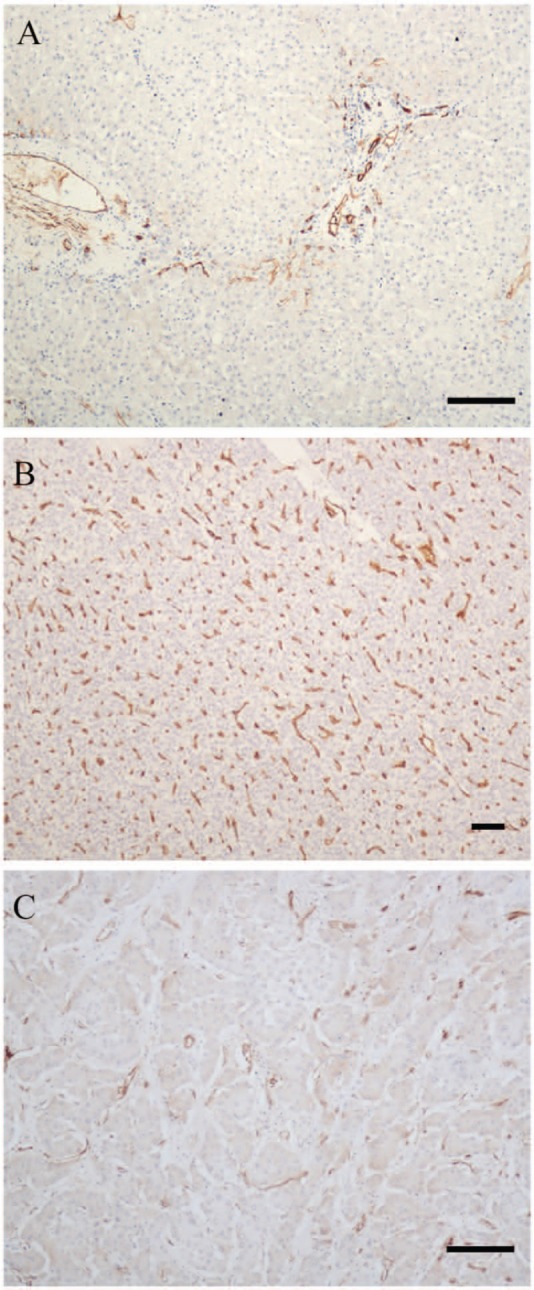
Three CD34 immunostaining patterns. (A) Negative CD34 immunoreactive pattern in a case of hepatolithiasis: only the blood vessels and bile ducts in portal tracts were positive, whereas most sinusoidal spaces were negative. (B) Diffuse CD34 immunostaining pattern in a case of hepatocellular carcinoma: most sinusoidal spaces were positive. (C) Focal CD34 immunostaining pattern in a case of focal nodular hyperplasia: some but not most sinusoidal spaces were positive for CD34. Bars = 100 µm.
Table 1.
Immunohistochemical Profiles of the Biomarkers and Reticulin Staining in HCCs and Non-malignant Neoplastic Nodules.
| HCCs, No. (%) |
||||||
|---|---|---|---|---|---|---|
| Immunostaining Markers | Expression Pattern | HCC1 (n=40) | HCC2-3 (n=25) | p Valuea | Non-malignant Neoplastic Nodules (n=29), No. (%) | p Valueb |
| CD34 | Diffuse + | 37 (92.5) | 25 (100.0) | NA | 11 (37.9) | <0.001* |
| Focal + | 3 (7.5) | 0 | 4 (13.8) | |||
| – | 0 | 0 | 14 (48.3) | |||
| GP73 | Diffuse coarse-block pattern + | 25 (62.5) | 24 (96.0) | 0.002* | 5 (17.2) | <0.001* |
| Focal coarse-block pattern + | 0 | 0 | 4 (13.8) | |||
| Diffuse fine-granular pattern + | 7 (17.5) | 0 | 16 (55.2) | |||
| – | 8 (20.0) | 1 (6.7) | 4 (13.8) | |||
| GPC3 | + | 35 (87.5) | 15 (60.0) | 0.016* | 8 (27.6) | <0.001* |
| – | 5 (12.5) | 10 (40.0) | 21 (72.4) | |||
| Reticulin staining | + | 40 (100.0) | 25 (100.0) | NA | 8 (27.6) | NA |
| – | 0 | 0 | 21 (72.4) | |||
HCC, hepatocellular carcinoma; HCC1, well-differentiated HCC; HCC2-3, moderately and poorly differentiated HCC; NA, not available (there is a “0” in the four-fold table, the Chi-square test could not be conducted because of the small sample size).
HCC1 vs. HCC2-3.
HCC vs. non-malignant neoplastic nodules.
These were statistically significant.
GP73
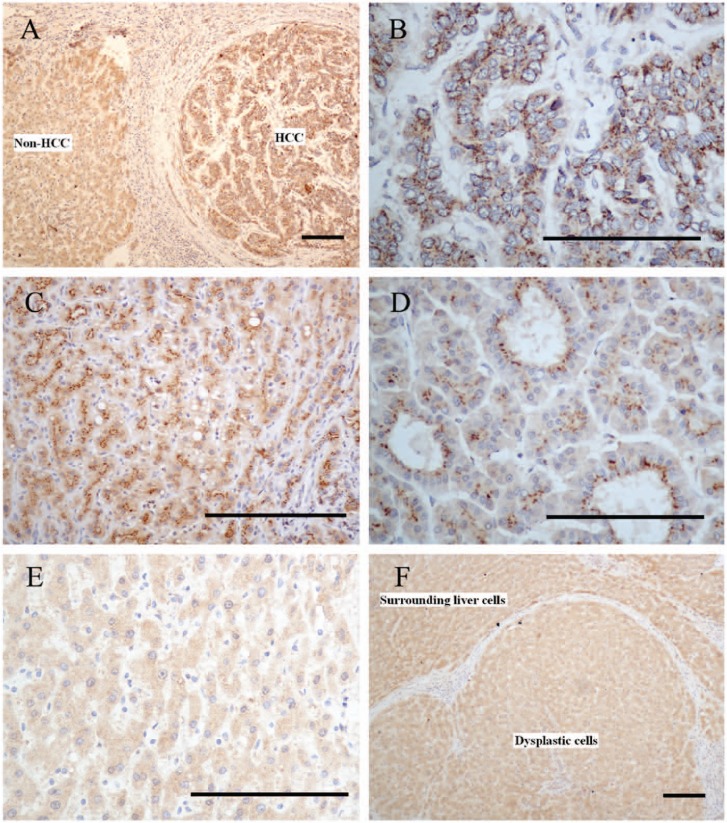
The immunoreaction of GP73 was seen in most HCC cases (49/65, 75.4%) (Table 1), including cases of small HCC (11/19, 57.9%). Interestingly, a large difference in the immunostaining patterns of GP73 was identified between the HCC cells and the surrounding non-tumor cells (Fig. 2A). HCC cells showed a diffuse coarse-block pattern, which was localized to the perinuclear region (Fig. 2B) or concentrated in a specific part of the cytoplasm, with the appearance of a cluster- or cord-like pattern (Fig. 2C). When the HCC cells formed a glandular structure, the coarse blocks were concentrated near the lumen (Fig. 2D). As distinct from HCC cells, the surrounding non-tumor cells showed a diffuse fine-granular pattern in the cytoplasm (Fig. 2E), and only 7 of 65 (10.8%) cases showed a focal coarse-block pattern. All moderately and poorly differentiated positive HCCs had a diffuse coarse-block pattern, and it appeared that the lesser the extent of differentiation, the larger the number of coarse blocks and the stronger the immunostaining intensity. This pattern was observed in 25 of 40 (62.5%) cases of well-differentiated HCCs, whereas 7 of 40 (17.5%) cases showed a diffuse fine-granular pattern, with no difference in the intensity of immunostaining. The remaining eight (20%) cases were negative. The level of GP73 immunostaining correlated significantly with less well-differentiated HCCs (p=0.002), whereas no correlation with other clinicopathological parameters was found.
Figure 2.
Different GP73 immuno-staining patterns in hepatocellular carcinoma (HCC) cells and non-HCC cells. (A) The immunostaining pattern of GP73 in HCC cells showed a diffuse coarse-block pattern. In contrast, the surrounding non-HCC cells showed a diffuse fine-granular pattern. (B) The coarse blocks were perinuclear. (C) The coarse blocks were concentrated in a certain part of the cytoplasm that gave a cluster- or cord-like appearance. (D) The coarse blocks were concentrated near the lumen of glandular structures. (E) The surrounding non-HCC cells showed a diffuse fine-granular pattern in the cytoplasm. (F) A case of a high-grade dysplastic nodule, which showed a diffuse fine-granular pattern with no difference in intensity between dysplastic cells and surrounding liver cells. Bars = 100 µm.
In cases with non-malignant neoplastic nodules, GP73 immunostaining with a diffuse coarse-block pattern was observed in 5 of 29 (17.2%) cases, with 4 (13.8%) cases displaying a focal coarse-block pattern. In the remaining 20 cases, 16 (55.2%) showed a diffuse fine-granular pattern with no difference in intensity between dysplastic cells and surrounding liver cells (Fig. 2F). Four (13.8%) cases were negative. However, in the control group, GP73 immunostaining had a diffuse fine-granular pattern in the cytoplasm (17/30; 56.7%) or was negative (13/30; 43.3%). Thus, we considered the diffuse coarse-block immunostaining pattern a useful indicator in the diagnosis of HCC. The sensitivity and specificity of GP73 for differentiating HCCs from non-malignant neoplastic nodules were 75.4% and 82.8%, respectively.
GPC3
The immunoreaction of GPC3 was observed in the vast majority of HCCs (50/65, 76.9%) (Table 1), including cases of small HCCs (14/19, 73.7%), which displayed a diffuse cytoplasmic and/or a membrane immunoreaction pattern (Fig. 3A). The data also revealed that not only HCC cells but also surrounding non-tumor cells were also positive for GPC3, including nine cases of well-differentiated HCC (9/40, 22.5%) that had an immunoreaction intensity greater than that of surrounding non-tumor cells (Fig. 3B). However, in non-malignant neoplastic nodules, 21 of 29 (72.4%) cases were negative for GPC3 (Fig. 3C). In the control group, 8 of 30 (26.7%) cases were GPC3 positive. It should be noted that in the well-differentiated HCC cases, 35 of 40 (87.5%) were GPC3 positive, and there was a statistically siginificant difference in the level of GPC3 immunostaining between well-differentiated and less well-differentiated HCCs (p=0.016). The sensitivity and specificity of GPC3 for differentiating HCC from non-malignant neoplastic nodules were 75.4% and 72.4%, respectively.
Figure 3.
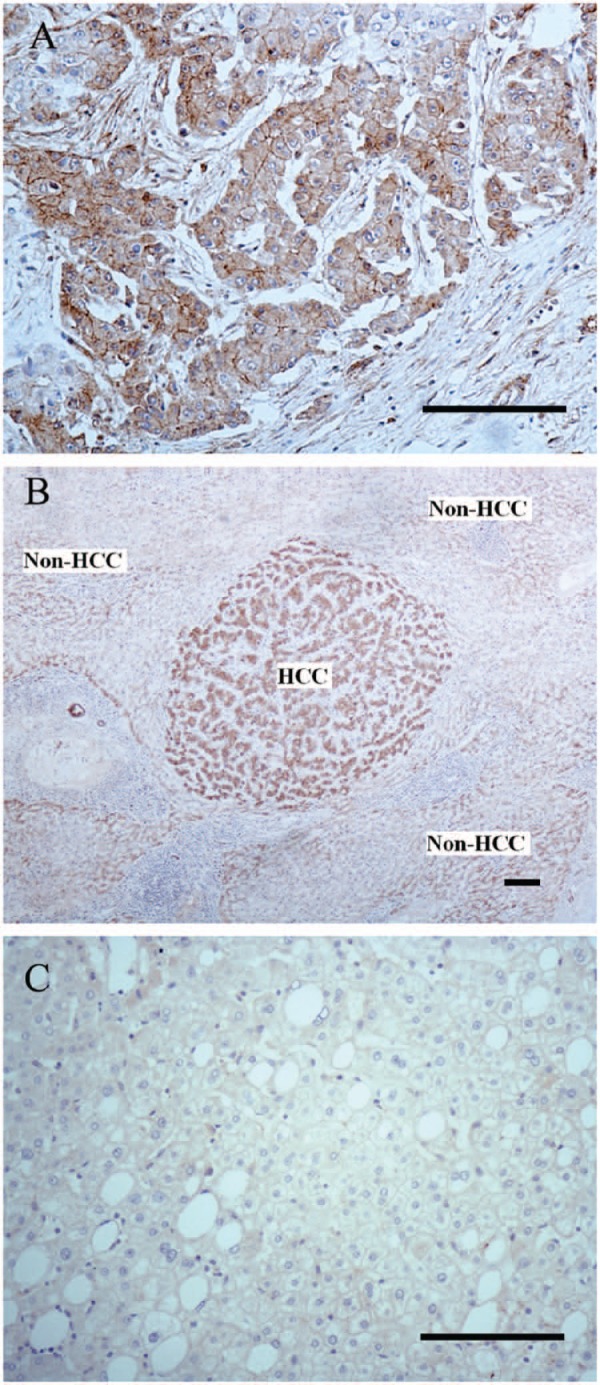
GPC3 immunostaining patterns in hepatocellular carcinoma (HCC) cells and non-HCC cells. (A) The immunoreactive pattern of GPC3 in HCC cells showed a diffuse cytoplasmic and membrane-associated pattern. (B) The intensity of GPC3 immunoreaction in HCC cells was greater than that of surrounding non-tumor cells in a case of well-differentiated HCC. (C) GPC3 staining was negative in a case of focal nodular hyperplasia. Bars = 100 µm.
DCP
A diffuse cytoplasmic immunostaining pattern of DCP was observed in the HCC cases (52/65, 80%). Similar to the immunostaining pattern of GPC3, the non-tumor cells surrounding HCCs were positive for DCP, with no difference in the pattern and intensity of the immunoreaction. Overall, 22 of 29 (75.9%) of the non-malignant neoplastic nodules cases were positive for DCP, and 19 of 30 (63.3%) cases from control patients also showed DCP immunoreaction. Thus, the data showed that DCP has a lower diagnostic value for HCC as an immunostaining marker.
CD31
No immunoreaction of CD31 was demonstrated in any case included in this study. The positive control for CD31 is shown in Suppl. Fig. S1.
Reticulin Staining
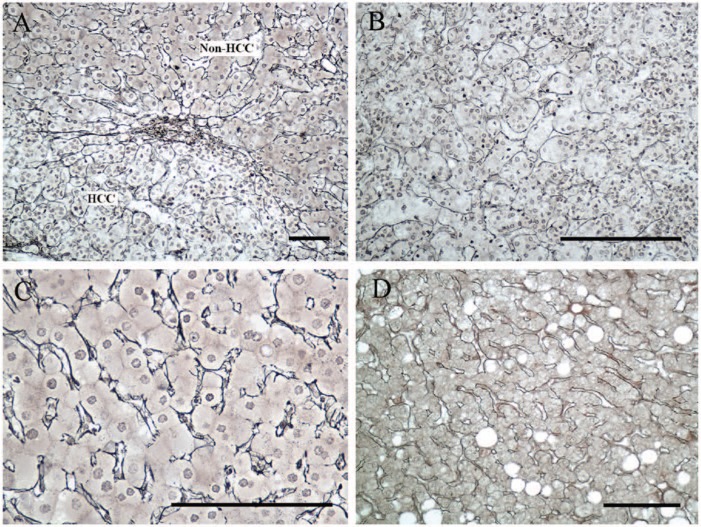
All HCCs showed an abnormal reticulin pattern, which was characterized by a thin layer of reticulin that surrounded each cell nest and was interconnected to form a complete network (Fig. 4A, B). All surrounding non-tumor liver cells and 21 of 29 (72.4%) cases of non-malignant neoplastic nodules showed a normal reticulin pattern. This was characterized by thick expression of reticulin along the liver sinusoids outlining the hepatic trabeculae that involved less than three cell layers (Fig. 4A, C, D); this was similar to the immunostaining pattern of CD34. It should be noted that in 8 of 29 (27.6%) cases of non-malignant neoplastic nodules, the reticulin pattern was similar to that of HCC.
Figure 4.
Reticulin staining patterns in hepatocellular carcinoma (HCC) cells and non-HCC cells. (A) HCC cells and non-HCC cells showed distinct patterns of reticulin staining. (B) The HCC cells showed an abnormal reticulin staining pattern, which was characterized by a thin layer of reticulin surrounding each cell nest and connected with each other to form a complete network. (C) The surrounding non-HCC cells displayed a normal reticulin staining pattern, which was characterized by thick reticulin expression along the liver sinusoids outlining the hepatic trabeculae, involving less than three cell layers. (D) The reticulin staining pattern in a case of a low-grade dysplastic nodule. Bars = 100 µm.
Combination of GP73, GPC3, CD34, and Reticulin Staining in the Pathological Diagnosis of HCC
The immunohistochemical profiles of all biomarkers in HCCs and non-malignant neoplastic nodules are listed in Table 1. The potential panels for the diagnosis of HCC are illustrated in Table 2. These data revealed that reticulin staining was the most specific marker (100%) for distinguishing HCC from non-malignant neoplastic nodules. Thus, to increase the specificity, we combined reticulin staining with the other three markers. Among the phenotypes of the two markers, the reticulin+/CD34+ panel was observed in 62 of 65 HCCs compared with 4 of 29 non-malignant neoplastic nodules, which demonstrated high levels of sensitivity (95.4%) and specificity (86.2%). In addition, the reticulin+/GP73+ phenotype was seen only in 2 of 29 (6.9%) non-malignant neoplastic nodules, showing the best specificity (93.1%), although the sensitivity was lower (49/65, 75.4%). When the three markers were combined, the specificity of the reticulin+/CD34+/GP73+ or reticulin+/CD34+/GPC3+ panels increased to 96.6%, retaining a sensitivity of 75.4%. When all four markers were used, the specificity was 100%, whereas the sensitivity dropped to 61.5%.
Table 2.
Sensitivity and Specificity of the Biomarkers under Study and Their Combination for HCC Diagnosis.
| Phenotype | HCCs (n=65) | Non-malignant Neoplastic Nodules (n=29), No. (%) | Sensitivity, % | Specificity, % |
|---|---|---|---|---|
| One marker positive | ||||
| CD34+a | 62 | 11 (37.9) | 95.4 | 62.1 |
| GP73+b | 49 | 5 (17.2) | 75.4 | 82.8 |
| GPC3+ | 50 | 8 (27.6) | 76.9 | 72.4 |
| Reticulin+ | 65 | 8 (27.6) | 100 | 72.4 |
| Two markers positive | ||||
| Reticulin+/CD34+ | 62 | 4 (13.8) | 95.4 | 86.2 |
| Reticulin+/GP73+ | 49 | 2 (6.9) | 75.4 | 93.1 |
| Reticulin+/GPC3+ | 50 | 3 (10.3) | 76.9 | 89.7 |
| CD34+/GP73+ | 49 | 3 (10.3) | 75.4 | 89.7 |
| CD34+/GPC3+ | 49 | 4 (13.8) | 75.4 | 86.2 |
| GP73+/GPC3+ | 40 | 2 (6.9) | 61.5 | 93.1 |
| Three markers positive | ||||
| Reticulin+/CD34+/GP73+ | 49 | 1 (3.4) | 75.4 | 96.6 |
| Reticulin+/CD34+/GPC3+ | 49 | 1 (3.4) | 75.4 | 96.6 |
| Reticulin+/GP73+/GPC3+ | 40 | 1 (3.4) | 61.5 | 96.6 |
| CD34+/GP73+/GPC3+ | 40 | 1 (3.4) | 61.5 | 96.6 |
| Four markers positive | ||||
| Reticulin+/CD34+/ GP73+/GPC3+ | 40 | 0 | 61.5 | 100 |
HCC, hepatocellular carcinoma.
CD34+: only diffuse + was considered positive.
GP73+: only diffuse coarse-block pattern + was considered positive.
Discussion
In liver disease, the differential diagnosis between HCC and benign conditions frequently confounds surgical pathologists. Several histological studies have examined the utility of CD34 in making a distinction between benign hepatic lesions and HCC (Heukamp et al. 2006; Li WC et al. 2006; Coston et al. 2008; Tatrai et al. 2009). In our study, we found that CD34 is still the most sensitive immunostaining marker for the diagnosis of HCC. Most HCC cells showed a diffuse CD34 staining pattern in contrast to the negative expression pattern in surrounding non-tumor liver cells (hepatitis cells, cirrhosis cells, or normal liver cells). Although 14 (48.3%) cases of non-malignant neoplastic nodules were negative for CD34 immunoreaction and 4 (13.8%) cases presented with focal CD34 immunostaining, 11 (37.9%) cases still mimicked the staining pattern of HCC cells. Thus, CD34 staining could be a useful marker to differentiate HCC from benign liver lesions, such as hepatitis or cirrhosis. It is also a sensitive, although not very specific, marker for differentiating HCC from non-malignant neoplastic nodules. Reticulin staining was also very useful for the differential diagnosis of HCC and benign lesions in our study. HCC cells showed a special pattern that was distinct from surrounding non-tumor cells and non-malignant neoplastic nodules, which delineated a thin layer around the HCC mass. However, as with CD34, its lower specificity limited its diagnostic value. As with cases that were positive for CD34, an abnormal reticulin pattern was observed in several liver cell adenoma nodules and in high- and low-grade dysplastic nodules. As a result of their high similarity with HCC cells and the limited number of cases, it was difficult to assess the differences between them.
Among the panel of five immunostaining markers in our study, CD31 was negative in all cases. Frachon et al. (2001) reported that although CD31 was negative in cirrhotic nodules and non-dysplastic regenerative macronodules, it was positive in 42.7% of dysplastic macronodules and 87% of HCCs. However, in their previous studies, they also found that CD31 was overexpressed along the sinusoids in liver fibrosis and inflammation (Couvelard et al. 1993; Scoazec and Feldmann 1994). These controversial results were explained by differences in tissues types (frozen materials vs. paraffin-embedded samples), and they proposed that variations in CD31 expression at the surface of sinusoidal endothelial cells were likely to be of a quantitative, rather than a qualitative, nature. In our study, the paraffin-embedded samples were collected several years ago, and the loss of CD31 antigen may be one of the reasons that it was undetectable. We agree with the conclusions of Frachon et al. that the loss of CD31 from the liver may hamper the value of CD31 as a marker of hepatic vascular changes.
DCP expression was detected in HCC cells but was also found in surrounding non-tumor cells and in most non-malignant neoplastic nodules and benign control liver tissues. This indicated that DCP was not a suitable immunostaining marker in combination with CD34 or reticulin staining for the diagnosis of HCC. However, several previous studies have shown that DCP is an effective serological tumor marker for HCC (Marrero et al. 2003; Lok et al. 2010; Takeji et al. 2013). In general, serological markers are more sensitive than immunostaining markers because of the more sensitive method used in serological detection. In a similar manner to AFP, DCP is more easily detected in serum but is more difficult to detect in tissues by immunochemistry. Some studies have shown that the overexpression of DCP is not only detected in HCC tissues but also in the surrounding non-HCC liver tissues; hence, these patients have significantly elevated serum DCP levels (Inagaki et al. 2011). Therefore, the use of DCP as an immunostaining marker for the diagnosis of HCC is not recommended, regardless of its value in the serum.
Compared with DCP, GP73 showed not only high sensitivity (75.4%) but also high specificity (82.8%) and correlated significantly with HCCs that had lower levels of differentiation. Many studies have demonstrated that GP73 could serve as a serum marker for the diagnosis of liver disease, but few studies have investigated the utility of GP73 as an immunostaining marker for the diagnosis of HCC. Riener et al. (2009) established that, in the normal human liver, GP73 expression was found in biliary epithelial cells and was relatively low in hepatocytes, but expression increases with liver damage, especially with HCV-induced hepatitis and HCC. They demonstrated that the GP73 immunostaining pattern was dot-like and localized in the perinuclear region toward the cell apex projecting to the Golgi apparatus, whereas there was no immunoreaction in the cytoplasm. However, although we observed a similar immunoreaction pattern of GP73 in HCC cells to that reported by Riener et al., it was not dot-like or confined to the Golgi. The pattern we observed was either a coarse block in the perinuclear region or a cluster-like or cord-like pattern in a specific part of the cytoplasm. If the HCC cells formed a glandular structure, the coarse blocks were concentrated near the lumen. The surrounding non-tumor tissues showed a diffuse fine-granular immunostaining pattern in the cytoplasm. Furthermore, a diffuse fine-granular immunoreaction pattern was demonstrated in seven well-differentiated HCCs. The underlying mechanism of the aberrant immunoreaction pattern of GP73 has yet to be explained. Bachert et al. (2007) had reported that GP73 is an integral membrane protein localized to the cis Golgi, which can be cleaved and released into the extracellular milieu. The use of a cleavage-specific antibody could be useful to understand its subcellular localization and better define its expression pattern. Kladney et al. (2012) reported that the increased expression of GP73 in hepatocytes appears to be a general feature of advanced liver disease (cirrhosis), regardless of the underlying etiology (e.g., HBV, HCV, alcohol or autoimmune disease induced). In our data, most cases of HCC were infected with HBV (60/65) and had a certain degree of cirrhosis. However, of the five HBV-negative HCC cases, three showed a diffuse coarse-block pattern for GP73 (Suppl. Table S1). Conversely, in 11 HBV+ non-malignant neoplastic nodules, only 2 cases had this pattern (Suppl. Table S2). This supports the opinion of Kladney et al. that the expression of GP73 in hepatocytes may be controlled by distinct virus-dependent and virus-independent mechanisms. Our results differed in that we also showed that GP73 could be a good immunostaining marker for differentiating HCC from cirrhosis and non-malignant neoplastic nodules. We hypothesize that GP73 may be initially present as a diffuse fine-granular pattern in the cytoplasm but then coalesces to form a coarse-block pattern during the progression of liver disease. The exact mechanisms of this change need to be further explored.
GPC3 is a member of the glypican family, a group of heparan sulfate proteoglycans linked to the cell surface through a glycosylphosphatidylinositol anchor (Filmus et al. 2008). It has been suggested GPC3 plays an important role in cell proliferation, differentiation, and migration to specific tissues (Viviano et al. 2005; Lai et al. 2008; Stigliano et al. 2009). Downregulation of this protein has been demonstrated in several cancers and cell lines, including mesothelioma and ovarian and breast cancers, suggesting a tumor-suppressive role in tumorigenesis (Murthy et al. 2000; Kandil and Cooper 2009; Zynger et al. 2010). However, it is highly expressed at both the messenger RNA and protein level in HCC (Capurro et al. 2003; Suzuki et al. 2010; Yan et al. 2011). In our study, positive immunoreactivity of GPC3 was found in 50 of 65 (76.9%) cases of HCC, especially in those with well-differentiated HCC (87.5%, 35/40). It should be noted that although HCC cells showed more intense staining of GPC3 than the surrounding non-tumor cells in nine cases of well-differentiated HCCs, GPC3 was mostly positive in both HCC cells and the surrounding non-tumor cells. Baumhoer et al. (2008) demonstrated a gradient of GPC3 expression in non-neoplastic (9.2%), preneoplastic (16%), and neoplastic (63.6%) liver tissue samples, which underlined the role of GPC3 in hepatocarcinogenesis. These findings were consistent with previously reported results (Zhu et al. 2001; Libbrecht et al. 2006; Wang et al. 2006). Shafizadeh et al. (2008) also found that HCCs that arose in cirrhotic livers were more likely to be GPC3 positive (91% vs. 57%). Therefore, we propose that the overregulation of GPC3 may occur in the very early stages of HCC, and some molecular changes may occur in the surrounding non-tumor liver cells that induce GPC3 expression. However, it is noteworthy that poorly differentiated tumor cells may lose certain immune markers during disease progression.
In conclusion, a panel that includes GP73, GPC3, CD34, and reticulin staining is very useful in making the diagnosis of HCC. The reticulin+/CD34+ panel was proven to be the best combination, showing a high sensitivity (95.4%) and a good specificity (86.2%). Moreover, the reticulin+/GP73+ panel showed a better specificity (93.1%) with a relatively lower sensitivity (75.4%). When using a panel of three markers, either the reticulin+/CD34+/GP73+ or the reticulin+/CD34+/GPC3+ panel is recommended, with an the increased specificity of 96.6% and a sensitivity maintained at 75.4%. The combination of four markers is not recommended because of its low specificity (61.5%).
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Nature Science Foundation of China (No. 81272277) and the Independent Innovation Foundation of Shandong University (No. 2012JC028).
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
References
- Akkiz H, Bayram S, Bekar A, Akgollu E, Uskudar O, Sandikci M. 2011. No association of pre-microRNA-146a rs2910164 polymorphism and risk of hepatocellular carcinoma development in Turkish population: a case-control study. Gene. 486:104–109 [DOI] [PubMed] [Google Scholar]
- Bachert C, Fimmel C, Linstedt AD. 2007. Endosomal trafficking and proprotein convertase cleavage of cis Golgi protein GP73 produces marker for hepatocellular carcinoma. Traffic. 8:1415–1423 [DOI] [PubMed] [Google Scholar]
- Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. 2008. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol. 129:899–906 [DOI] [PubMed] [Google Scholar]
- Beneduce L, Pesce G, Gallotta A, Zampieri F, Biasiolo A, Tono N, Boscato N, Gatta A, Pontisso P, Fassina G. 2008. Tumour-specific induction of immune complexes: DCP-IgM in hepatocellular carcinoma. Eur J Clin Invest. 38:571–577 [DOI] [PubMed] [Google Scholar]
- Block TM, Comunale MA, Lowman M, Steel LF, Romano PR, Fimmel C, Tennant BC, London WT, Evans AA, Blumberg BS, et al. 2005. Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proc Natl Acad Sci U S A. 102:779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. 2003. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 125:89–97 [DOI] [PubMed] [Google Scholar]
- Coston WM, Loera S, Lau SK, Ishizawa S, Jiang Z, Wu CL, Yen Y, Weiss LM, Chu PG. 2008. Distinction of hepatocellular carcinoma from benign hepatic mimickers using glypican-3 and CD34 immunohistochemistry. Am J Surg Pathol. 32:433–444 [DOI] [PubMed] [Google Scholar]
- Couvelard A, Scoazec JY, Feldmann G. 1993. Expression of cell-cell and cell-matrix adhesion proteins by sinusoidal endothelial cells in the normal and cirrhotic human liver. Am J Pathol. 143:738–752 [PMC free article] [PubMed] [Google Scholar]
- de Boer WB, Segal A, Frost FA, Sterrett GF. 2000. Can CD34 discriminate between benign and malignant hepatocytic lesions in fine-needle aspirates and thin core biopsies? Cancer. 90:273–278 [PubMed] [Google Scholar]
- El-Serag HB, Mallat DB, Rabeneck L. 2005. Management of the single liver nodule in a cirrhotic patient: a decision analysis model. J Clin Gastroenterol. 39:152–159 [PubMed] [Google Scholar]
- El-Serag HB, Rudolph KL. 2007. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 132:2557–2576 [DOI] [PubMed] [Google Scholar]
- Farazi PA, DePinho RA. 2006. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 6:674–687 [DOI] [PubMed] [Google Scholar]
- Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA, Benvegnu L, et al. 2006. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 101:524–532 [DOI] [PubMed] [Google Scholar]
- Filmus J, Capurro M, Rast J. 2008. Glypicans. Genome Biol. 9:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frachon S, Gouysse G, Dumortier J, Couvelard A, Nejjari M, Mion F, Berger F, Paliard P, Boillot O, Scoazec JY. 2001. Endothelial cell marker expression in dysplastic lesions of the liver: an immunohistochemical study. J Hepatol. 34:850–857 [DOI] [PubMed] [Google Scholar]
- Gomori G. 1947. Concerning methods of tissue staining. Am J Clin Pathol. 17:972. [PubMed] [Google Scholar]
- Heukamp LC, Fischer HP, Schirmacher P, Chen X, Breuhahn K, Nicolay C, Buttner R, Gutgemann I. 2006. Podocalyxin-like protein 1 expression in primary hepatic tumours and tumour-like lesions. Histopathology. 49:242–247 [DOI] [PubMed] [Google Scholar]
- Inagaki Y, Tang W, Makuuchi M, Hasegawa K, Sugawara Y, Kokudo N. 2011. Clinical and molecular insights into the hepatocellular carcinoma tumour marker des-gamma-carboxyprothrombin. Liver Int. 31:22–35 [DOI] [PubMed] [Google Scholar]
- International Working Party 1995. Terminology of nodular hepatocellular lesions. Hepatology. 22:983–993 [DOI] [PubMed] [Google Scholar]
- Jia HL, Ye QH, Qin LX, Budhu A, Forgues M, Chen Y, Liu YK, Sun HC, Wang L, Lu HZ, et al. 2007. Gene expression profiling reveals potential biomarkers of human hepatocellular carcinoma. Clin Cancer Res. 13:1133–1139 [DOI] [PubMed] [Google Scholar]
- Jin Y, Yu Q, Zhou D, Chen L, Huang X, Xu G, Huang J, Gao X, Gao Y, Shen L. 2012. The mitochondrial DNA 9-bp deletion polymorphism is a risk factor for hepatocellular carcinoma in the Chinese population. Genet Test Mol Biomarkers. 16:330–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X, Piao YF, Liu Y, Gao PJ. 2010. Beta2-GPI: a novel factor in the development of hepatocellular carcinoma. J Cancer Res Clin Oncol. 136:1671–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandil DH, Cooper K. 2009. Glypican-3: a novel diagnostic marker for hepatocellular carcinoma and more. Adv Anat Pathol. 16:125–129 [DOI] [PubMed] [Google Scholar]
- Kladney RD, Cui X, Bulla GA, Brunt EM, Fimmel CJ. 2002. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease. Hepatology. 35:1431–1440 [DOI] [PubMed] [Google Scholar]
- Lai JP, Sandhu DS, Yu C, Han T, Moser CD, Jackson KK, Guerrero RB, Aderca I, Isomoto H, Garrity-Park MM, et al. 2008. Sulfatase 2 up-regulates glypican 3, promotes fibroblast growth factor signaling, and decreases survival in hepatocellular carcinoma. Hepatology. 47:1211–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li J, Hao C, Zhang C, Mu K, Wang Y, Zhang T. 2011. Immunohistochemical evaluation of solid pseudopapillary tumors of the pancreas: the expression pattern of CD99 is highly unique. Cancer Lett. 310:9–14 [DOI] [PubMed] [Google Scholar]
- Li WC, Ye SL, Sun RX, Liu YK, Tang ZY, Kim Y, Karras JG, Zhang H. 2006. Inhibition of growth and metastasis of human hepatocellular carcinoma by antisense oligonucleotide targeting signal transducer and activator of transcription 3. Clin Cancer Res. 12:7140–7148 [DOI] [PubMed] [Google Scholar]
- Libbrecht L, Severi T, Cassiman D, Vander Borght S, Pirenne J, Nevens F, Verslype C, van Pelt J, Roskams T. 2006. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. Am J Surg Pathol. 30:1405–1411 [DOI] [PubMed] [Google Scholar]
- Liu X, Wan X, Li Z, Lin C, Zhan Y, Lu X. 2011. Golgi protein 73(GP73), a useful serum marker in liver diseases. Clin Chem Lab Med. 49:1311–1316 [DOI] [PubMed] [Google Scholar]
- Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, Morgan TR, Kim HY, Lee WM, Bonkovsky HL, et al. 2010. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 138:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, Zhao H, Chen W, Xu Y, Chi T, et al. 2010. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut. 59:1687–1693 [DOI] [PubMed] [Google Scholar]
- Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, Comunale MA, D’Amelio A, Lok AS, Block TM. 2005. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol. 43:1007–1012 [DOI] [PubMed] [Google Scholar]
- Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, Lok AS. 2003. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology. 37:1114–1121 [DOI] [PubMed] [Google Scholar]
- Murthy SS, Shen T, De Rienzo A, Lee WC, Ferriola PC, Jhanwar SC, Mossman BT, Filmus J, Testa JR. 2000. Expression of GPC3, an X-linked recessive overgrowth gene, is silenced in malignant mesothelioma. Oncogene. 19:410–416 [DOI] [PubMed] [Google Scholar]
- Ozkan H, Erdal H, Kocak E, Tutkak H, Karaeren Z, Yakut M, Koklu S. 2011. Diagnostic and prognostic role of serum glypican 3 in patients with hepatocellular carcinoma. J Clin Lab Anal. 25:350–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztaszeri MP, Seelentag W, Bosman FT. 2006. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 54:385–395 [DOI] [PubMed] [Google Scholar]
- Riener MO, Stenner F, Liewen H, Soll C, Breitenstein S, Pestalozzi BC, Samaras P, Probst-Hensch N, Hellerbrand C, Mullhaupt B, et al. 2009. Golgi phosphoprotein 2 (GOLPH2) expression in liver tumors and its value as a serum marker in hepatocellular carcinomas. Hepatology. 49:1602–1609 [DOI] [PubMed] [Google Scholar]
- Sato K, Mori M. 2011. Evolving molecular mechanism-based strategies for control of hepatocellular carcinoma. Curr Med Chem. 18:4375–4388 [DOI] [PubMed] [Google Scholar]
- Scoazec JY, Feldmann G. 1994. The cell adhesion molecules of hepatic sinusoidal endothelial cells. J Hepatol. 20:296–300 [DOI] [PubMed] [Google Scholar]
- Shafizadeh N, Ferrell LD, Kakar S. 2008. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Mod Pathol. 21:1011–1018 [DOI] [PubMed] [Google Scholar]
- Sterling RK, Wright EC, Morgan TR, Seeff LB, Hoefs JC, Di Bisceglie AM, Dienstag JL, Lok AS. 2012. Frequency of elevated hepatocellular carcinoma (HCC) biomarkers in patients with advanced hepatitis C. Am J Gastroenterol. 107:64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigliano I, Puricelli L, Filmus J, Sogayar MC, Bal de Kier Joffe E, Peters MG. 2009. Glypican-3 regulates migration, adhesion and actin cytoskeleton organization in mammary tumor cells through Wnt signaling modulation. Breast Cancer Res Treat. 114:251–262 [DOI] [PubMed] [Google Scholar]
- Sun HC, Tang ZY, Li XM, Zhou YN, Sun BR, Ma ZC. 1999. Microvessel density of hepatocellular carcinoma: its relationship with prognosis. J Cancer Res Clin Oncol. 125:419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Sugimoto K, Tanaka J, Tameda M, Inagaki Y, Kusagawa S, Nojiri K, Beppu T, Yoneda K, Yamamoto N, et al. 2010. Up-regulation of glypican-3 in human hepatocellular carcinoma. Anticancer Res. 30:5055–5061 [PubMed] [Google Scholar]
- Takeji S, Hirooka M, Koizumi Y, Tokumoto Y, Abe M, Ikeda Y, Nadano S, Hiasa Y, Onji M. 2013. Des-gamma-carboxy prothrombin identified by P-11 and P-16 antibodies reflects prognosis for patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 28:671–677 [DOI] [PubMed] [Google Scholar]
- Tangkijvanich P, Chanmee T, Komtong S, Mahachai V, Wisedopas N, Pothacharoen P, Kongtawelert P. 2010. Diagnostic role of serum glypican-3 in differentiating hepatocellular carcinoma from non-malignant chronic liver disease and other liver cancers. J Gastroenterol Hepatol. 25:129–137 [DOI] [PubMed] [Google Scholar]
- Tatrai P, Somoracz A, Batmunkh E, Schirmacher P, Kiss A, Schaff Z, Nagy P, Kovalszky I. 2009. Agrin and CD34 immunohistochemistry for the discrimination of benign versus malignant hepatocellular lesions. Am J Surg Pathol. 33:874–885 [DOI] [PubMed] [Google Scholar]
- Tian L, Wang Y, Xu D, Gui J, Jia X, Tong H, Wen X, Dong Z, Tian Y. 2011. Serological AFP/Golgi protein 73 could be a new diagnostic parameter of hepatic diseases. Int J Cancer. 129:1923–1931 [DOI] [PubMed] [Google Scholar]
- Viviano BL, Silverstein L, Pflederer C, Paine-Saunders S, Mills K, Saunders S. 2005. Altered hematopoiesis in glypican-3–deficient mice results in decreased osteoclast differentiation and a delay in endochondral ossification. Dev Biol. 282:152–162 [DOI] [PubMed] [Google Scholar]
- Wang XY, Degos F, Dubois S, Tessiore S, Allegretta M, Guttmann RD, Jothy S, Belghiti J, Bedossa P, Paradis V. 2006. Glypican-3 expression in hepatocellular tumors: diagnostic value for preneoplastic lesions and hepatocellular carcinomas. Hum Pathol. 37:1435–1441 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Imamura H, Matsuyama Y, Hasegawa K, Beck Y, Sugawara Y, Makuuchi M, Kokudo N. 2009. Significance of alpha-fetoprotein and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma undergoing hepatectomy. Ann Surg Oncol. 16:2795–2804 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Imamura H, Matsuyama Y, Kume Y, Ikeda H, Norman GL, Shums Z, Aoki T, Hasegawa K, Beck Y, et al. 2010. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol. 45:1272–1282 [DOI] [PubMed] [Google Scholar]
- Yan D, He Q, Chen Y, Wang L, Zhang X. 2011. Detection of alpha-fetoprotein and glypican-3 mRNAs in the peripheral blood of hepatocellular carcinoma patients by using multiple FQ-RT-PCR. J Clin Lab Anal. 25:113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZW, Friess H, Wang L, Abou-Shady M, Zimmermann A, Lander AD, Korc M, Kleeff J, Buchler MW. 2001. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 48:558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zynger DL, McCallum JC, Luan C, Chou PM, Yang XJ. 2010. Glypican 3 has a higher sensitivity than alpha-fetoprotein for testicular and ovarian yolk sac tumour: immunohistochemical investigation with analysis of histological growth patterns. Histopathology. 56:750–757 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.