It has been known that translation of CGA codon repeats in budding yeast is inefficient. This paper examines the reasons for inefficient translation, and the findings indicate unexpected complexity revealing that inhibition of translation by CGA codons results from at least two independent defects.
Keywords: translation, yeast, genetic code, ribosome, codons, ubiquitin
Abstract
Translation of CGA codon repeats in the yeast Saccharomyces cerevisiae is inefficient, resulting in dose-dependent reduction in expression and in production of an mRNA cleavage product, indicative of a stalled ribosome. Here, we use genetics and translation inhibitors to understand how ribosomes respond to CGA repeats. We find that CGA codon repeats result in a truncated polypeptide that is targeted for degradation by Ltn1, an E3 ubiquitin ligase involved in nonstop decay, although deletion of LTN1 does not improve expression downstream from CGA repeats. Expression downstream from CGA codons at residue 318, but not at residue 4, is improved by deletion of either ASC1 or HEL2, previously implicated in inhibition of translation by polybasic sequences. Thus, translation of CGA repeats likely causes ribosomes to stall and exploits known quality control systems. Expression downstream from CGA repeats at amino acid 4 is improved by paromomycin, an aminoglycoside that relaxes decoding specificity. Paromomycin has no effect if native tRNAArg(ICG) is highly expressed, consistent with the idea that failure to efficiently decode CGA codons might occur in part due to rejection of the cognate tRNAArg(ICG). Furthermore, expression downstream from CGA repeats is improved by inactivation of RPL1B, one of two genes encoding the universally conserved ribosomal protein L1. The effects of rpl1b-Δ and of either paromomycin or tRNAArg(ICG) on CGA decoding are additive, suggesting that the rpl1b-Δ mutant suppresses CGA inhibition by means other than increased acceptance of tRNAArg(ICG). Thus, inefficient decoding of CGA likely involves at least two independent defects in translation.
INTRODUCTION
Ribosomes elongate translation at nonuniform rates and with unequal efficiency through different coding sequences. In bacteria, it has long been clear that the rates of elongation at different codons vary significantly, with up to 25-fold differences in the in vivo rate of peptide bond formation (Pedersen 1984; Varenne et al. 1984; Curran and Yarus 1989; Sorensen et al. 1989). Likewise, ribosomes in eukaryotes do not read all codons at equal rates. Ribosomes in both C. elegans and in HeLa cells spend more time decoding codons that require G:U wobble interactions than codons decoded by the same tRNA without wobble (Stadler and Fire 2011). Moreover, ribosomes in both mouse stem cells and bacteria pause for extended periods of time at specific sequences, pauses that may be due either to the nascent peptide or to a combination of the nascent peptide and codon choice (Tanner et al. 2009; Ingolia et al. 2011).
Ribosomes in both bacteria and eukaryotes also stall when they encounter various problems during translation elongation, and these events generally elicit specific responses from quality control systems. In bacteria, either the absence of a stop codon or the presence of rare codons causes the ribosome to recruit SsrA RNA, which behaves as a joint alanyl-tRNA-mRNA hybrid and results in incorporation of an amino acid sequence that targets the peptide for release and proteolysis (Roche and Sauer 1999; Moore and Sauer 2007). Similarly, in eukaryotes there are translational quality control systems that respond to any of the following features: the absence of a stop codon (Frischmeyer et al. 2002; van Hoof et al. 2002); the presence of an inhibitory mRNA structure that prevents continued elongation (Doma and Parker 2006); the presence of polybasic nascent-peptide sequences, which are thought to interact with the exit tunnel (Ito-Harashima et al. 2007); or the presence of premature stop codons (Isken and Maquat 2007). In each of these cases, the problem is resolved by releasing the ribosome and by removal of the mRNA (Doma and Parker 2006; Isken and Maquat 2007; Kuroha et al. 2010; Shoemaker and Green 2012), which, in at least some instances, is accompanied by endonucleolytic cleavage of the mRNA near the stalled ribosome (Doma and Parker 2006; Huntzinger et al. 2008; Tsuboi et al. 2012). In two examples in yeast, the nascent protein products are targeted for proteolytic degradation by either of two E3 ubiquitin ligases, Not4 or Ltn1 (also called Rkr1) (Wilson et al. 2007; Dimitrova et al. 2009; Bengtson and Joazeiro 2010). In fact, the stalled ribosome likely recruits a complex of proteins since Ltn1 is part of the Ribosome Quality Control (RQC) complex that also includes Rqc1, Tae2, and Cdc48 (and likely its associated factors Ufd1 and Npl4) (Brandman et al. 2012). Similar to elongation stalls caused by problems in the mRNA, defective ribosomes are frequently eliminated during numerous checks during biogenesis, but others which begin translation and stall during elongation are themselves targeted for degradation during translation (LaRiviere et al. 2006; Fujii et al. 2009).
Two genes, ASC1 and HEL2, have been implicated in the cessation of translation at polybasic sites, since deletion of either one of these genes results in increased read through into the downstream sequences (Kuroha et al. 2010; Brandman et al. 2012). Asc1, the yeast homolog of human RACK1, is a stoichiometric component of the small subunit of the ribosome (Gerbasi et al. 2004), that is implicated in numerous signaling events (Nilsson et al. 2004), in recruitment of the mRNA binding protein Scp150 (Coyle et al. 2009) and in P-body formation in response to hydroxyurea treatment (Tkach et al. 2012). Hel2 is an E3 ubiquitin ligase known to influence histone protein levels (Singh et al. 2012). It is unclear how these genes cause improved read-through of inhibitory sequences and if they work together, although inactivation of either gene results in a common phenotype, sensitivity to hydroxyurea (Singh et al. 2012; Tkach et al. 2012).
We reported previously that Arg CGA codon pairs are significantly refractory to efficient protein synthesis in the yeast Saccharomyces cerevisiae, causing a dose-dependent reduction in expression compared to identical polypeptides encoded with the optimal Arg AGA codons. Inhibitory effects of CGA codons are observed with CGA codons inserted at either amino acid 4 or 314 upstream of firefly luciferase or at amino acid 4 upstream of Renilla luciferase (Letzring et al. 2010); even two adjacent CGA codons at amino acid 6 upstream of green fluorescent protein (GFP) cause a substantial reduction in GFP expression (Dean and Grayhack 2012). The defect in efficient translation is due to I•A wobble decoding of CGA, rather than to tRNA abundance, since a single integrated copy of an anticodon-mutated tRNAArg(UCG) that exactly base pairs with CGA strongly suppresses the expression defect caused by CGA codons (Letzring et al. 2010). It is likely that this defect in CGA decoding efficiency is biologically relevant, since I•A wobble decoding of the Arg CGA codon is conserved across >20 million years of evolution in the Hemiascomycetes fungi (Grosjean et al. 2010), despite the fact that any of 17 independent point mutations in the anticodons of redundant Arg tRNA genes in S. cerevisiae could convert a tRNAArg(ICG) or tRNAArg(UCU) to an exact base-pairing mutant tRNAArg(UCG) that would efficiently decode CGA (Letzring et al. 2010). I•A wobble decoding of CGA is also used in many bacteria (Curran 1995; Grosjean et al. 2010). Thus, decoding CGA in yeast impedes an intrinsic aspect of translation elongation and is likely functionally important.
The mechanism by which ribosomes discriminate CGA codons and cause poor expression of sequences downstream from these codons is unknown. The observation that CGA codon repeats result in production of an RNA product of the expected size and hybridization properties to correspond to an mRNA cleaved just upstream of the CGA repeats (Chen et al. 2010; Letzring et al. 2010) supports the idea that eukaryotic ribosomes stall at CGA codon repeats. Thus, ribosomes stalled at these codons might be recognized by proteins implicated in known quality control mechanisms. To begin to understand why ribosomes fail to translate through CGA codons, we investigated the events that occur at CGA codon repeats. We find that translation of CGA codon repeats involves parts of known quality control systems and ribosomal protein L1, and find evidence for two distinct mechanisms regulating translation of CGA codons.
RESULTS
Protein synthesis is discontinued at CGA codon repeats
We and others (Chen et al. 2010; Letzring et al. 2010) have observed that transcripts bearing in-frame CGA repeats result in truncated RNAs, as has been seen for transcripts bearing a stalled ribosome, albeit in different contexts (Chen et al. 2010; Tsuboi et al. 2012). The 5′ ends of these truncated RNAs are just upstream of the CGA codon repeats, mapping 37 and 67 nucleotides upstream of the CGA codons (Chen et al. 2010), as expected if they occur by cleavage at a paused ribosome.
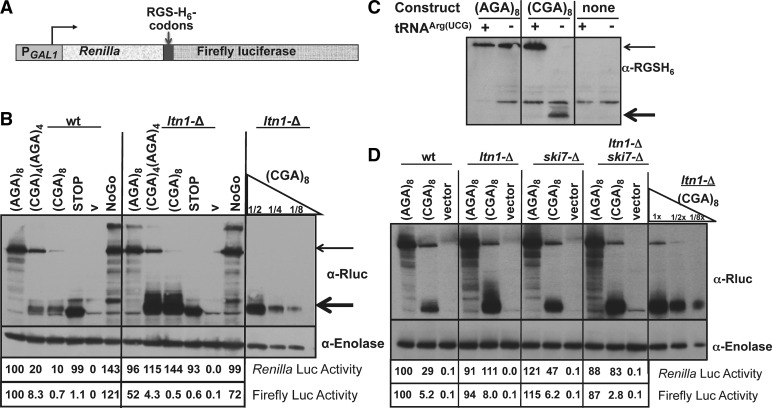
To determine if most ribosomes stop or stall at the CGA codon repeats, we examined the polypeptide products from Renilla luciferase-firefly luciferase fusion genes in which an Arg-Gly-Ser-(His)6-(Arg)8 sequence is inserted between the Renilla and firefly luciferase genes with the Arg-Gly-Ser (RGS) beginning at codon 315 and the Arg repeat beginning at codon 324 (Fig. 1A). Insertion of either four or eight CGA codons in a repeat with eight Arg residues, resulted in production of a small polypeptide, detected with an antibody directed at Renilla luciferase, whose size by SDS-PAGE is consistent with a C-terminal end at or near the CGA codons (Fig. 1B, heavy arrow). The migration of these polypeptides is similar to that of Renilla luciferase with a stop codon inserted at the position of the CGA codons. In contrast, only full-length polypeptide was seen from identical constructs containing eight Arg AGA codons (Fig. 1B, light arrow). As expected, the truncated polypeptide was also detected with antibody directed at the RGS-(His)6 epitope (Fig. 1C, heavy arrow). Furthermore, expression of the anticodon mutated tRNAArg(UCG) variant that suppresses CGA inhibitory effects (Letzring et al. 2010) restored expression of the full-length Renilla-firefly luciferase fusion polypeptide (Fig. 1C, light arrow) and reduced the truncated polypeptide below the detection limit (Fig. 1C), demonstrating that the truncated polypeptide derives from the Renilla-firefly luciferase fusion and is due to poor decoding of CGA.
FIGURE 1.
Polypeptides arrested by CGA codons are targeted for degradation by the E3 ubiquitin ligase, encoded by LTN1. (A) Schematic of the Renilla-firefly luciferase fusion protein in which RGS-(His)6-(Arg)8 sequences are inserted at amino acid 314, and expression is under control of the GAL1 promoter. (B) Analysis of Renilla-firefly luciferase fusion protein expressed from the Renilla-RGS-(His)6-(Arg)8-firefly luciferase-reporter constructs under control of the GAL1 promoter, in either wild-type or ltn1-Δ yeast strains. The (Arg)8 insertion is specified by (AGA)8, [(CGA)4(AGA)4], or (CGA)8, as indicated above the panel. Luciferase fusion protein was detected with antibody directed against Renilla luciferase; antibody to enolase served as a loading control. Dilutions were performed with crude extracts expressing the (CGA)8 reporter constructs (12.5 μg, 6.3 μg, 3.1 μg). Firefly and Renilla luciferase activities of various reporter constructs all containing RGS-(His)6-(Arg)8 insertions were normalized to the (AGA)8 reporter construct in the wild-type strain. (C) Analysis of Renilla-firefly luciferase fusion protein in strains expressing the exact base-pairing variant tRNAArg(UCG) or a vector control. Strains used in the first four lanes bear constructs that are similar to those described in A except that expression is driven by PGK1 promoter, as described previously (Letzring et al. 2010). Strains in the last two lanes have no fusion construct. The fusion protein was detected with antibody directed against the RGS-(His)6 epitope. (D) Analysis of Renilla-firefly luciferase protein in reporter constructs containing RGS-(His)6-(Arg)8 insertions under control of the PGK1 promoter, from wild-type, ltn1-Δ, ski7-Δ, and ltn1-Δ ski7-Δ yeast strains bearing the indicated Arg codon insertions. Antibody detection and luciferase assays were done as described in B.
The polypeptide ending near the CGA codons is targeted for proteolysis by Ltn1, the E3 ubiquitin ligase implicated in nonstop decay
We noted that both the Renilla luciferase activity and the corresponding polypeptide were diminished by the presence of CGA codons downstream from the Renilla luciferase gene (Fig. 1B). Polypeptides that are associated with stalled ribosomes due to lack of a stop codon are targeted for proteolysis by the E3 ubiquitin ligase, Ltn1 (Wilson et al. 2007; Bengtson and Joazeiro 2010). Consistent with a role for Ltn1 at CGA codon repeats, we found that the amount of both the CGA-dependent Renilla luciferase polypeptide and Renilla luciferase activity increased dramatically in an ltn1-Δ mutant (Fig. 1B). In contrast, deletion of LTN1 had almost no effect on constructs with AGA codons or stop codons. There was no increase in either the Renilla-firefly luciferase fusion polypeptide or in Renilla luciferase activity from the (AGA)8 construct, nor was there an increase in Renilla luciferase activity from the strain in which Renilla luciferase is followed by a stop codon (Fig. 1B). Thus, the CGA-dependent truncated polypeptide (and only this polypeptide) is targeted for proteolysis by the E3 ubiquitin ligase Ltn1, which is known to target polypeptides from ribosomes stalled due to the absence of a termination codon.
For ribosomes stalled due to lack of a stop codon or due to mRNA secondary structures that inhibit elongation, the mRNA 5′ to the stalled ribosome is targeted for degradation by Ski7, which interacts with the exosome (van Hoof et al. 2002). Indeed, we found that a ski7-Δ mutant showed a 1.6-fold increase in Renilla luciferase activity (from 29 to 47 units), as well as an increase in the truncated Renilla luciferase polypeptide (Fig. 1D), consistent with the idea that stalling ribosomes at CGA codons results in recruitment of mRNA decay systems. In examining the cellular responses to an mRNA lacking stop codons, Wilson et al. (2007) concluded that degradation of the protein and degradation of the mRNA were independent responses to the stalled ribosome because they found that combining mutations that affect the RNA decay pathway (ski7-Δ mutation) and that impair proteasome function (pre9-Δ mutation) had an additive effect on expression of a nonstop protein (although they did not examine an ltn1-Δ ski7-Δ mutant). Therefore it is intriguing that we did not observe an additive effect of the ski7-Δ ltn1-Δ double mutant on expression of Renilla luciferase upstream of the CGA codons (Fig. 1D), perhaps because deletion of LTN1 restores Renilla luciferase to wild-type levels.
Failure to translate through CGA codons is not due to proteolytic targeting of the upstream gene
We considered that proteolytic targeting of the upstream polypeptide might affect read-through of CGA codons into the downstream sequences; thus, we examined read-through of CGA codons in yeast with a mutation in the LTN1 gene. As shown in Figure 1, B and D, we found that expression of the firefly luciferase gene in these fusion constructs (which all have eight Arg codons) was not affected by deletion of LTN1. Since polybasic sequences themselves are reported to block the exit tunnel (Dimitrova et al. 2009), we considered that detection of the specific effects of LTN1 deletion on CGA read-through might be evident only with fewer Arg residues. Therefore, we made a Renilla-firefly luciferase fusion protein with four Arg residues (rather than eight) inserted between the Renilla and firefly luciferase genes. As shown in Figure 2A, Renilla luciferase activity from this CGA-containing construct was reduced in the wild-type (LTN1) strain—110.9 for the (AGA)4-construct compared to 38.1 for the (CGA)4-construct—and restored in strains bearing the ltn1-Δ mutation—to 94.6 for the (CGA)4-construct— an effect that was complemented by a plasmid-borne copy of LTN1. Thus, Ltn1 targets the (CGA)4-containing upstream polypeptide. However, there was no effect of the ltn1-Δ mutation on the expression of firefly luciferase downstream from the CGA codons (21.9 in the wild type compared to 22.2 in the ltn1-Δ) (Fig. 2B), although CGA-mediated inhibition of firefly luciferase was evident—63.4 in the (AGA)4-constructs compared to 21.9 in the (CGA)4 constructs in the wild-type strain (Fig. 2B). Thus, degradation initiated by Ltn1 is not responsible for the failure to efficiently read CGA codons; rather the failure to efficiently read CGA codons causes recruitment of Ltn1.
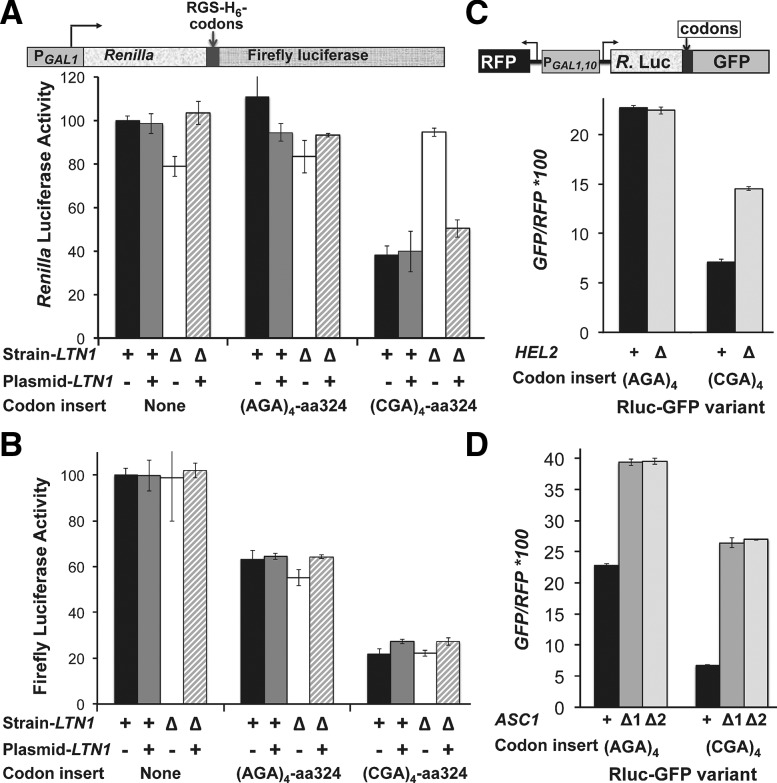
FIGURE 2.
Deletion of ASC1 or HEL2, but not LTN1, results in improved expression of genes downstream from CGA codons at amino acid 318. (A,B) Renilla (A) and firefly (B) luciferase activity of the Renilla-firefly luciferase fusion reporter constructs containing either no insert or the indicated RGS-(His)6-(Arg)4 insertions in the wild-type and ltn1-Δ strains. Strains were transformed with low copy plasmids with or without the LTN1 gene as indicated. Schematic of the reporter is included above panel A. (C,D) Mean GFP/RFP fluorescence (arbitrary units) of the Renilla-Arg4-GFP fusion (schematic) reporter in wild-type, hel2-Δ, or asc1-Δ strains. Two independent asc1-Δ mutant strains were constructed, confirmed by genomic PCR, and tested here; they are designated Δ1, Δ2. A diagram of the reporter is shown above panel C.
ASC1 and HEL2 exacerbate the effects of internal, but not N-terminal, CGA codons
Two other proteins, Asc1, the yeast homolog of RACK1, and Hel2, a putative E3 ubiquitin ligase, have been specifically implicated in translation through polybasic peptide sequences, since deletion of either ASC1 or HEL2 improves expression of sequences downstream from 12 amino acid polybasic repeats (Kuroha et al. 2010; Brandman et al. 2012). Since Ltn1 was also implicated in the inhibitory effects of polybasic peptide sequences (Wilson et al. 2007; Dimitrova et al. 2009; Bengtson and Joazeiro 2010; Brandman et al. 2012), we investigated the effects of ASC1 and HEL2 on CGA-mediated translation inhibition. For these experiments, we used a Renilla luciferase-(Arg)4-GFP reporter in which CGA codon repeats are inserted at residue 318 upstream of GFP (Fig. 2C). In this system, which is derived from the RNA-ID reporter (Dean and Grayhack 2012), a single bidirectional promoter (GAL1,10 promoter) drives expression of the Renilla luciferase-GFP fusion in one direction and red fluorescent protein (RFP) in the other direction. Standardizing measurements based on RFP expression reduces noise (Dean and Grayhack 2012) and facilitates comparisons between strains.
We found that deletion of either HEL2 or ASC1 resulted in an approximate twofold increase in read-through of the CGA codons based on the relative GFP/RFP values. With respect to HEL2, GFP/RFP expression from the Renilla luciferase-(CGA)4-GFP construct increased twofold (7.1 in the wild-type compared to 14.5 in the hel2-Δ mutant), but remained constant for the (AGA)4 construct (22.7 in the wild-type compared to 22.4 in the hel2-Δ mutant) (Fig. 2C). With respect to ASC1, both the (AGA)4 and (CGA)4 constructs yielded increased GFP/RFP levels in the asc1-Δ mutant (compared to the wild-type strain) but the relative increase was greater for the (CGA)4 construct. Thus, GFP/RFP increased 3.9-fold for the (CGA)4 construct (6.7 in the wild-type compared to 26.4 and 26.9 in two asc1-Δ mutants) (Fig. 2D) and only 1.7-fold for the (AGA)4 construct (22.8 in the wild-type compared to 39.3 and 39.5 in the asc1-Δ mutants) (Fig. 2D). In contrast, we observed no effect of either of these genes on CGA codon-mediated inhibition at amino acid four (data not shown). Thus, Asc1 and Hel2 may play some role in limiting expression at CGA codon repeats at amino acid 318.
Read-through of CGA codons is improved by treatment with paromomycin
We considered it possible that ribosomes might treat the CGA codon as a near-cognate mismatch for the tRNAArg(ICG), since the I•A interaction required to decode CGA requires an altered geometry of the anticodon to accommodate the purine–purine base pair within the decoding center of the Thermus thermophilus 30S subunits (Murphy and Ramakrishnan 2004). If so, then read-through of CGA codons might be improved by growth of yeast in the presence of the aminoglycoside paromomycin, which binds to the ribosome and is known to enhance stop codon read-through and acceptance of near-cognate tRNAs (Bonetti et al. 1995; Fan-Minogue and Bedwell 2008). To test this idea, we grew strains with the integrated GFP and RFP reporters in media containing increasing concentrations of translational inhibitors, paromomycin or cycloheximide. We used a range of concentrations for the inhibitors, in each case reaching a maximal concentration at which growth inhibition was evident, but in which >89% of the cells expressed RFP at ≥5 × 103 units, an arbitrary cutoff for robust induction of the GAL1,10 promoter, which was established with the nondrug treated sample (Dean and Grayhack 2012). For paromomycin, we have previously demonstrated that improved read-through of the TAA stop codon at amino acid 7 of GFP in a poor context is detectable with this GFP-RFP reporter (Dean and Grayhack 2012), even at the lowest concentration of paromomycin used here.
We found that expression of the (CGA)3-GFP reporter was specifically increased nearly twofold by inclusion of paromomycin at 100 μg/mL (mean GFP/RFP 21.0) compared to expression of the (CGA)3-GFP reporter in the absence of paromomycin (mean GFP/RFP 11.8) (Fig. 3A,B). The (CGA)3-GFP/RFP remained constant at this level as paromomycin was increased to 200 and 400 μg/mL. In contrast, expression of (AGA)3-GFP/RFP was slightly reduced with increasing concentrations of paromomycin (mean GFP/RFP decreased from 133.7 to 102.2 at 400 μg/mL paromomycin), as was expression of a construct that lacks any insert at amino acid 4 (data not shown). Thus, the (CGA)3-GFP/RFP relative to (AGA)3-GFP/RFP increased with increasing paromomycin (from 8.9% without paromomycin to 20.7% at 400 μg/mL paromomycin) (Fig. 3B). As we observed previously (Dean and Grayhack 2012), expression of the TAA-GFP construct increased from background expression levels (approximately 0.8) to nearly half the (CGA)3-GFP/RFP expression levels (6.9 GFP/RFP), and there was no change in GFP/RFP from a construct lacking the ATG initiation codon for GFP (data not shown). To determine if the effects of paromomycin on (CGA)3-GFP are specific or due to a general inhibition of translation, we examined the effects of cycloheximide on read-through of CGA codons. Cycloheximide appears to block the translocation step and possibly to inhibit eEF2 function (Schneider-Poetsch et al. 2010; Dang et al. 2011) but does not affect decoding interactions. We found no significant increase or decrease in expression of the (CGA)3-GFP reporter at concentrations of cycloheximide from 0.1 to 0.3 μg/mL (Fig. 3C).
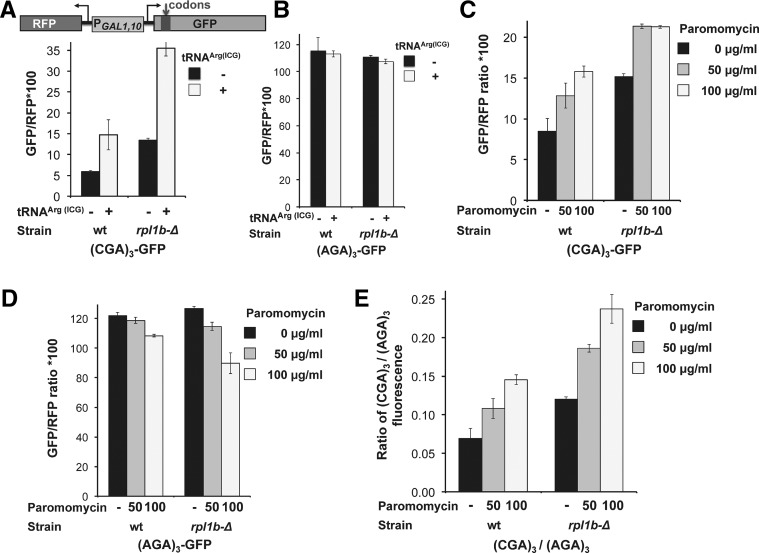
FIGURE 3.
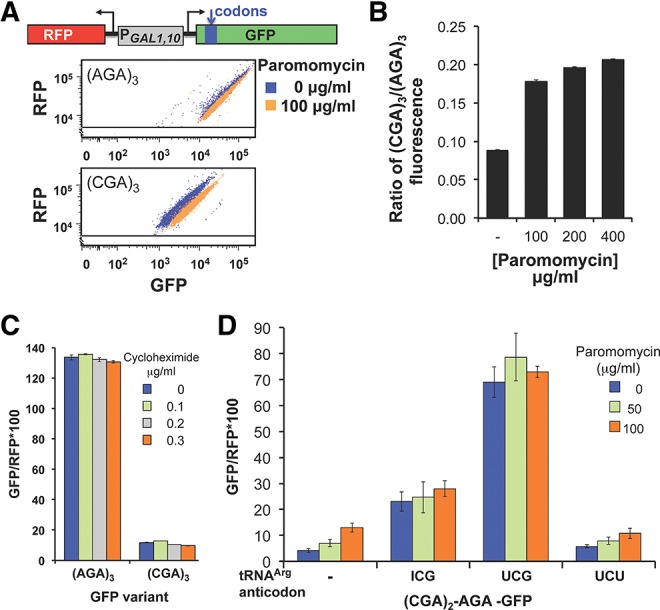
Paromomycin and increased amounts of native tRNAArg(ICG), but not cycloheximide, suppress expression of (CGA)3-GFP. (A) Addition of paromomycin results in increased GFP/RFP fluorescence of (CGA)3-GFP but not (AGA)3-GFP. Scatter plot of cells expressing (AGA)3-GFP or (CGA)3-GFP, grown with and without 100 μg/mL paromomycin. Schematic of the RNA-ID Arg4-GFP reporter used in A–D is included. The GFP variants each bear three codon insertions beginning at amino acid 4 or 6 (Dean and Grayhack 2012). (B) The expression of (CGA)3-GFP relative to that of (AGA)3-GFP increases mildly with increasing amounts of paromomycin. The average GFP/RFP is derived from the median GFP/RFP of at least three isolates of each construct. (C) Addition of cycloheximide has no effect on GFP/RFP fluorescence ratio of (CGA)3-GFP or (AGA)3-GFP variants. (D) Expression of either the native decoding tRNAArg(ICG) or the anticodon mutated tRNAArg(UCG) nearly abolish the effects of paromomycin. GFP/RFP fluorescence ratio of the [(CGA)2(AGA)1]-GFP variant as a function of increasing amounts of paromomycin is shown in strains bearing the indicated tRNAs on multicopy plasmids. tRNAArg species are indicated by the anticodon of the tRNA; the tRNA with the UCG anticodon was obtained by mutating the anticodon of tRNAArg(ICG) to UCG (Letzring et al. 2010).
If improved CGA decoding using paromomycin is the result of increased efficiency of acceptance of the tRNAArg(ICG) by the ribosome, then paromomycin and overproduction of this tRNAArg(ICG) would have the same effect. If so, we expect that paromomycin would have little or no additional effect on CGA decoding in cells that overproduce tRNAArg(ICG). As shown in Figure 3D, expression of tRNAArg(ICG) improved expression of [(CGA)2(AGA)1]-GFP, but there was no additional suppression by paromomycin.
Inactivating RPL1B, the dominant copy of ribosomal protein L1, improves CGA decoding
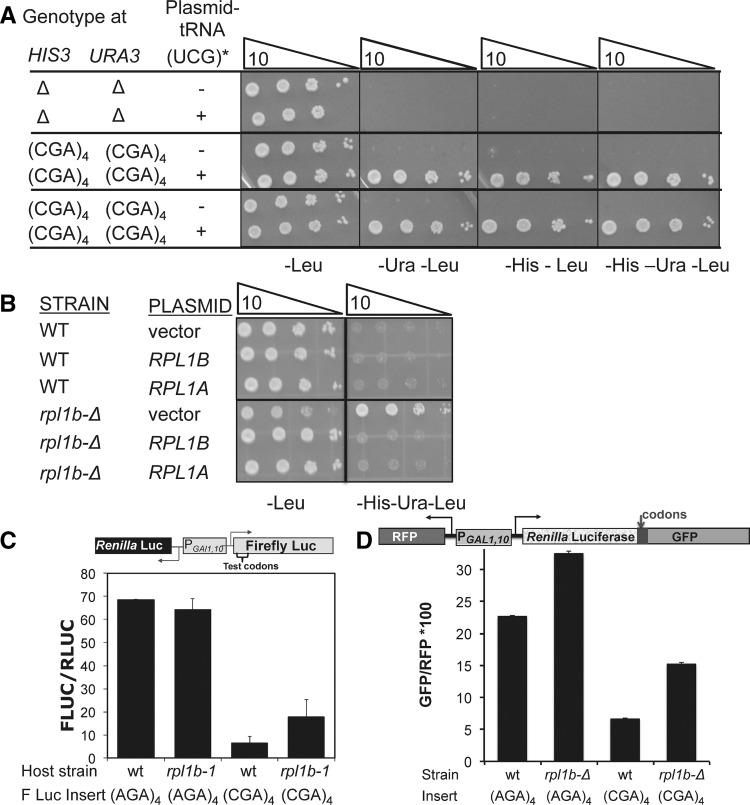
To identify genes whose products mediate inefficient decoding of CGA codons, we developed a selection for mutants that improve the expression of CGA codons. We reduced expression of both the HIS3 and URA3 genes by insertion of CGA codons near the 5′ end of each gene and demonstrated that insertion of four CGA codons upstream of the yeast URA3 gene or at amino acid 4 of the yeast HIS3 gene effectively silenced expression of these genes resulting in His−, Ura− yeast strains (Fig. 4A). Since expression of the exact base-pairing mutant tRNAArg(UCG) from a low copy plasmid suppressed these effects, converting the strains to a His+, Ura+ phenotype, the expression defect is likely primarily due to translation of the CGA codon (Fig. 4A). We also introduced a gcn4-Δ mutation into the strain to avoid transcriptional up-regulation of HIS3 by a mutation that confers a Gcd− phenotype, constitutively inducing general amino acid control (Hinnebusch 2005). We note, parenthetically, that in the presence of paromomycin, this strain grew on media lacking histidine and uracil, consistent with suppression of CGA-mediated inhibition by paromomycin (data not shown). We constructed both MATa and MATα haploid strains bearing these mutations, selected 15 independent His+ Ura+ suppressors in each mating type, and performed standard genetic analysis to identify recessive suppressors and define complementation groups. The recessive suppressors fall into two complementation groups.
FIGURE 4.
Mutation in RPL1B suppresses CGA-mediated inhibition of downstream gene expression. (A) Yeast strains bearing (CGA)4-ura3 and (CGA)4-his3 genes exhibit a His−Ura− phenotype that is suppressed by expression of the exact base-pairing mutant tRNAArg(UCG). The indicated strains bearing a CEN plasmid with or without mutant tRNAArg(UCG) were grown overnight in SD-leu media, and serial dilutions were spotted on the indicated media and grown at 25°C or 33°C. (B) Deletion of the RPL1B gene in the (CGA)4-ura3 and (CGA)4-his3 parent results in a His+, Ura+ phenotype, which is complemented by plasmid-borne copies of either RPL1B or RPL1A. (C) Expression of (CGA)4-firefly luciferase is increased nearly threefold in the rpl1b-1 mutant. (D) The inhibitory effects of CGA codons at amino acid 318 are suppressed in the rpl1b-Δ mutant. The mean GFP/RFP from a Renilla-(Arg)4-GFP fusion was compared with (AGA)4 and (CGA)4 insertions at amino acid 318 in the wild-type and rpl1b-Δ mutant.
We took advantage of a conditional glycerol− phenotype (indicative of a defect in respiration) to clone the gene for one suppressor, which proved to be an allele of RPL1B, a gene that encodes one of two copies of the universally conserved ribosomal protein L1 (Nikulin et al. 2003; Fei et al. 2008; Cornish et al. 2009). Three lines of evidence indicate that the phenotype of this mutant is due to a defect in the RBL1B gene. First, the suppressor phenotype was complemented by a single copy plasmid bearing the RPL1B gene (data not shown). Second, the RPL1B gene in the rpl1b-1 strain has an insertion of one base after codon 7, causing a frameshift and premature termination (data not shown). Third, independent construction of an rpl1b-Δ mutation in the starting strain resulted in a suppressor phenotype (His+ Ura+) (Fig. 4B). We infer that the phenotype is due to the reduced amounts of L1 protein since the phenotype is also complemented by a single copy plasmid bearing the RPL1A gene (Fig. 4B). Although RPL1A encodes the identical protein, it is expressed at much lower levels (Petitjean et al. 1995), and the rpl1a-Δ mutant does not exhibit this phenotype (data not shown).
We demonstrated directly that mutant strains bearing the rpl1b-1 allele exhibit improved CGA decoding. For these measurements, we used a reporter, expressing both firefly and Renilla luciferase on independent transcripts but driven by the bidirectional GAL1,10 promoter. Thus, we were able to normalize expression of firefly luciferase with the codon insertions to expression of Renilla luciferase, avoiding difficulties due to growth rate differences or differences in ribosome abundance between the wild-type and suppressor strains. Expression of firefly luciferase with 4 CGA codons inserted at amino acid 4 increased nearly threefold in the rpl1b-1 mutant compared to expression of this construct in the parent strain (Fig. 4C). In contrast, expression of firefly luciferase with four AGA codons was nearly identical in the mutant and wild-type strains (Fig. 4C). Similarly, we observed that CGA inhibition at amino acid 318 was also reduced in the rpl1b-Δ mutant; that is, expression of GFP/RFP from the Renilla luciferase-(CGA)4-GFP is increased from 29% of (AGA)4-GFP/RFP in the wild-type strain to 47% of the (AGA)4-GFP/RFP in the rpl1b-Δ mutant (Fig. 4D).
The effects of rpl1b-Δ and either tRNAArg(ICG) or paromomycin on CGA decoding are additive, suggesting that they affect different aspects of CGA decoding
To determine if these mutants have improved decoding of CGA by the native tRNAArg(ICG) or have relaxed the decoding specificity in a manner analogous to that caused by paromomycin, we examined the effects of both overproduction of tRNAArg(ICG) and of paromomycin on expression of (CGA)3 and (AGA)3 containing GFP reporters in the rpl1b-Δ mutant. To minimize differences between strains with respect to galactose induction or overall translation efficiency, we report the GFP/RFP ratio in all cases. As expected, the GFP/RFP value of GFP with (CGA)3 at amino acid 4 increased from 5.9 in the wild-type strain to 13.4 in the reconstructed rpl1b-Δ mutant in BY4741, whereas the GFP/RFP value of GFP with (AGA)3 was not affected (Fig. 5A,B, black bars). Remarkably, we observed that overproduction of tRNAArg(ICG) has nearly identical effects on improved CGA decoding in both the wild-type and rpl1b-Δ mutant (Fig. 5A,B, light gray bars). Thus, in the presence of a high copy plasmid encoding tRNAArg(ICG), (CGA)3-GFP/RFP increased 2.5-fold in the wild-type cell (from 5.9 to 14.7) and 2.7-fold in the rpl1b-Δ mutant (from 13.4 to 35.4), whereas (AGA)3-GFP/RFP was essentially constant (varies <3%) with or without the tRNA expression (Fig. 5A,B).
FIGURE 5.
CGA-mediated inhibition in rpl1b-Δ mutants is further suppressed by either tRNAArg(ICG) or paromomycin. (A) The mean (CGA)3-GFP/RFP is increased in the rpl1b-Δ strain and further improved by expression of tRNAArg(ICG). Schematic of the RNA-ID (Arg)3-GFP reporter used in A–E is included. (B) The mean (AGA)3-GFP/RFP is not affected by either the rpl1b-Δ mutation or expression of tRNAArg(ICG). (C) The mean (CGA)3-GFP/RFP in the rpl1b-Δ strain is further increased by growth in paromomycin. (D) The mean (AGA)3-GFP/RFP is slightly reduced by growth in paromomycin in either the wild-type or the rpl1b-Δ mutant. (E) The ratio of (CGA)3-GFP/(AGA)3-GFP expression increases as a function of increasing amounts of paromomycin.
Similarly, there was a substantial increase in (CGA)3-GFP expression when rpl1b-Δ mutants were grown in the presence of paromomycin. At 50 μg/mL, (CGA)3-GFP/RFP values increased 1.5-fold in wild-type cells (from 8.5 to 12.8) and 1.4-fold in the rpl1b-Δ mutant (from 15.2 to 21.3) (Fig. 5C). In contrast, expression of (AGA)3-GFP/RFP decreased as a function of increasing paromomycin, and this decrease was more severe in the rpl1b-Δ mutant than in wild-type cells (Fig. 5D). To obtain a more quantitative estimate of the effects of paromomycin on CGA read-through, we determined the ratio of (CGA)3-GFP/RFP fluorescence to (AGA)3-GFP/RFP fluorescence in each strain in each condition. As can be seen in Figure 5E, this analysis indicated that read-through of CGA codons in the rpl1b-Δ mutant increased with increasing paromomycin. Moreover, the 1.6-fold effect of 50 μg/mL paromomycin in the wild-type strain is almost identical to the 1.6-fold effect in the rpl1b-Δ mutant, and the 2.1-fold effect of 100 μg/mL paromomycin in the wild-type strain is very similar to the 2.0-fold effect in the rpl1b-Δ mutant. Thus, the rpl1b-Δ mutant responds both to overproduction of the native decoding tRNAArg(ICG) and to paromomycin treatment in a manner that is highly similar to a wild-type strain. These results suggest that suppression of CGA read-through defects in the rpl1b-Δ mutant occurs by a mechanism different than improved acceptance of the native tRNA at the A site.
DISCUSSION
Based on the observations reported here, we infer that ribosomes stalled at internal CGA codon repeats recruit components of known quality control systems to target peptides upstream of the CGA repeats for degradation and, in some cases, to reduce continued translation past an internal CGA repeat. These include the ubiquitin ligases Ltn1 and Hel2, the ribosome-associated scaffold protein Asc1, and most likely the other components of the RQC complex (Kuroha et al. 2010; Brandman et al. 2012). Ltn1 targets the polypeptide product upstream of the CGA codons, as well as the nascent polypeptide from a message lacking a stop codon (Wilson et al. 2007; Bengtson and Joazeiro 2010). Both Hel2 and Asc1 participate in blocking elongation through CGA codon repeats, in addition to their previously known role in reducing elongation through peptide sequences with 12 basic residues (Kuroha et al. 2010; Brandman et al. 2012). Thus, these two proteins may generally inhibit continued elongation by stalled ribosomes rather than acting specifically at ribosomes stalled by polybasic sequences. We note that, in all cases, the effects reported here are not due to amino acid composition since they are based on comparisons of identical polypeptides with Arg residues encoded with either CGA or AGA codons. Moreover, since the effects of the Asc1 and Hel2 proteins are only observed with internal CGA repeats, but not with CGA repeats at amino acid four, Asc1 and Hel2 may only act after the ribosome has translated some distance.
These observations reinforce and extend the idea enunciated by Shoemaker and Green (2012) that the cellular mechanisms that deal with stalled ribosomes are common to ribosomes stalled by any number of different events. The similarities between the responses to CGA codons and other translational stalls is also seen in the endonucleolytic cleavage of the mRNA immediately upstream of CGA codon repeats (Chen et al. 2010; Letzring et al. 2010). mRNA cleavage near the stall site has also been observed with ribosomes stalled by an inhibitory structure in the mRNA (Doma and Parker 2006), by a polybasic sequence (Kuroha et al. 2010), and by a ribosome that encounters a premature termination codon in higher eukaryotes (Gatfield and Izaurralde 2004; Eberle et al. 2009).
There are two obvious differences in CGA-mediated stalls from other systems in which ribosomes stall. First, there is not an actual lack of tRNAArg(ICG) to fill the A site, since there are six copies of the gene encoding tRNAArg(ICG) and since the CGU codon, which is also decoded by this tRNA, is clearly not inhibitory (Chen et al. 2010; Letzring et al. 2010; D Letzring and EJ Grayhack, unpubl.). However, there may be a perceived lack of this tRNA if it is rejected as a match in the A site of the ribosome; this interpretation is consistent with our results that expression downstream from CGA repeats is improved by high levels of the tRNAArg(ICG) or by paromomycin. Second, there is no obvious physical barrier that impedes continued elongation after the pause or when the A site is correctly filled. In many and perhaps most translation defects, there is a physical barrier to continued elongation including, for example, a peptide interaction with the exit tunnel, as occurs with the polybasic amino acids (Ito-Harashima et al. 2007) and the Arg attenuator peptide (Fang et al. 2004; Wu et al. 2012), or an mRNA structure that impedes ribosome translocation on the mRNA (Doma and Parker 2006). Thus, it is somewhat surprising that CGA codons elicit the same response as other ribosomal stalls, although it makes some sense for the cell to use a uniform set of components to respond to all stalls.
We infer that at least two independent defects in translation occur at CGA repeats since we observe additive effects of RPL1B depletion and either overproduction of tRNAArg(ICG) or paromomycin. As mentioned above, at least one defect in CGA read-through most likely involves the decoding interaction between tRNAArg(ICG) and the CGA codon in the A site, based on the improvement in decoding with either increased amounts of tRNAArg(ICG) or paromomycin.
The observation that reduced amounts of the universally conserved ribosomal L1 protein improve decoding of CGA codons is intriguing and puzzling. The effects of the rpl1b-1 mutation on CGA read-through might either be due to the general reduction in translating ribosomes known to occur in an rpl1b-Δ mutant (McIntosh et al. 2011) or to specific functions of L1 protein. For example, the L1 stalk, to which L1 protein binds, moves significantly during every elongation cycle, interacts with deacylated tRNA (Nikulin et al. 2003; Fei et al. 2009) and may play a role in the removal of tRNA from the E site (Wilson and Nierhaus 2006). Since ribosomes lacking the L1 protein are competent to translate messages (McIntosh et al. 2011), depletion of L1 protein might expedite removal of the tRNA from the E site, which is necessary for binding of the next aminoacyl tRNA in the A site. Thus, the explanation for the effects of the rpl1b-1 mutation on CGA read-through may lie either in general effects of reducing L1 protein concentration or in the specific functions of L1 on the ribosome.
MATERIALS AND METHODS
Strains and plasmids
The yeast strains BY4741 (MATa his3-Δ1, leu2-Δ0, met15-Δ0, ura3-Δ0) and BY4742 (MATα his3-Δ1, leu2-Δ0, lys2-Δ0, ura3-Δ0) were the parent strains for all yeast constructs. Deletions of yeast genes were performed by amplification of the gene from the corresponding knockout strain in the systematic deletion collection (OpenBiosystems) (Giaever et al. 2002). Deletions with the bleR cassette were obtained by amplification of pUG66 (Gueldener et al. 2002) with the indicated oligonucleotides. Yeast strains, plasmids, and oligonucleotides used in this study are listed in Supplemental Tables S1, S2, and S3. Plasmids containing the tRNAs tR(UCU)K [pDL866], tR(ACG)D [pDL867], and tR(UCG) [pDL869] on LEU2 2-µ plasmids have been described previously (Letzring et al. 2010), and tR(UCG) in a LEU2 Cen plasmid [pDL874] was cloned by ligation independent cloning (LIC) cloning of the PCR amplified tR(UCG) from DL869 into AVA0581 (Alexandrov et al. 2006).
Vector pDL485, in which Renilla and firefly luciferase fusion genes were expressed under PGAL1 control, was derived by PCR amplification of Renilla and firefly luciferase sequences in pDL202 (Letzring et al. 2010), which were cloned into the 2-µ URA3 vector BG2794 (Malkowski et al. 2007). Vector pEAW012, in which Renilla luciferase is fused to GFP under PGAL1 control, was obtained by PCR amplification of Renilla luciferase from pDL202 (Letzring et al. 2010) and insertion into the PacI site in pEKD1024 (Dean and Grayhack 2012) using LIC cloning. The LTN1, RPL1A, and RPL1B genes were amplified and inserted into the vector pAVA0581 using oligonucleotides listed in Supplemental Table S3 by LIC methods described previously (Aslanidis and de Jong 1990; Alexandrov et al. 2004).
Luciferase and GFP/RFP assays
Luciferase assays were performed as described (Letzring et al. 2010). For GFP and RFP fluorescence measurements, strains were grown and analytical flow cytometry was conducted as described previously (Dean and Grayhack 2012).
Western blotting
Transformants were grown in SD-uracil or SC-uracil with 2% raffinose, 2% galactose media (Sherman 1991) to O.D.600 between 0.5 and 1.0. Crude extract preparation and Western blot analysis were performed as described (Quartley et al. 2009). Membranes were incubated either with mouse anti-RGS-(His)6 (1:2500 dilution), mouse anti-RLuc (1:3125 dilution), or with rabbit anti-enolase (1:25,000 dilution), followed by washing as described previously and incubation with either HRP-conjugated Goat IgG anti-mouse (1:10,000 dilution, BioRad) for anti-RGS-(His)6 and anti-RLuc or HRP-conjugated Goat IgG anti-rabbit (1:10,000 dilution, Biorad) for anti-enolase, and development with ECL Plus western blotting detection system (GE Healthcare RPN2132).
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
We thank Eric Phizicky and Gloria Culver for advice during the course of this work and for comments on the manuscript; we also thank members of the Phizicky laboratories for helpful discussions. This work was supported by NSF grant MCB-0919658 awarded to E.J.G.
Glossary
Abbreviations: GFP, green fluorescent protein; RFP, red fluorescent protein; LIC, ligation independent cloning; RGS, Arg-Gly-Ser
REFERENCES
- Alexandrov A, Vignali M, LaCount DJ, Quartley E, de Vries C, De Rosa D, Babulski J, Mitchell SF, Schoenfeld LW, Fields S, et al. 2004. A facile method for high-throughput co-expression of protein pairs. Mol Cell Proteomics 3: 934–938 [DOI] [PubMed] [Google Scholar]
- Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM 2006. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell 21: 87–96 [DOI] [PubMed] [Google Scholar]
- Aslanidis C, de Jong PJ 1990. Ligation-independent cloning of PCR products (LIC-PCR). Nucleic Acids Res 18: 6069–6074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson MH, Joazeiro CA 2010. Role of a ribosome-associated E3 ubiquitin ligase in protein quality control. Nature 467: 470–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetti B, Fu L, Moon J, Bedwell DM 1995. The efficiency of translation termination is determined by a synergistic interplay between upstream and downstream sequences in Saccharomyces cerevisiae. J Mol Biol 251: 334–345 [DOI] [PubMed] [Google Scholar]
- Brandman O, Stewart-Ornstein J, Wong D, Larson A, Williams CC, Li GW, Zhou S, King D, Shen PS, Weibezahn J, et al. 2012. A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell 151: 1042–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Muhlrad D, Hauryliuk V, Cheng Z, Lim MK, Shyp V, Parker R, Song H 2010. Structure of the Dom34–Hbs1 complex and implications for no-go decay. Nat Struct Mol Biol 17: 1233–1240 [DOI] [PubMed] [Google Scholar]
- Cornish PV, Ermolenko DN, Staple DW, Hoang L, Hickerson RP, Noller HF, Ha T 2009. Following movement of the L1 stalk between three functional states in single ribosomes. Proc Natl Acad Sci 106: 2571–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle SM, Gilbert WV, Doudna JA 2009. Direct link between RACK1 function and localization at the ribosome in vivo. Mol Cell Biol 29: 1626–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran JF 1995. Decoding with the A:I wobble pair is inefficient. Nucleic Acids Res 23: 683–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran JF, Yarus M 1989. Rates of aminoacyl-tRNA selection at 29 sense codons in vivo. J Mol Biol 209: 65–77 [DOI] [PubMed] [Google Scholar]
- Dang Y, Schneider-Poetsch T, Eyler DE, Jewett JC, Bhat S, Rawal VH, Green R, Liu JO 2011. Inhibition of eukaryotic translation elongation by the antitumor natural product Mycalamide B. RNA 17: 1578–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean KM, Grayhack EJ 2012. RNA-ID, a highly sensitive and robust method to identify cis-regulatory sequences using superfolder GFP and a fluorescence-based assay. RNA 18: 2335–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova LN, Kuroha K, Tatematsu T, Inada T 2009. Nascent peptide-dependent translation arrest leads to Not4p-mediated protein degradation by the proteasome. J Biol Chem 284: 10343–10352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doma MK, Parker R 2006. Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature 440: 561–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle AB, Lykke-Andersen S, Muhlemann O, Jensen TH 2009. SMG6 promotes endonucleolytic cleavage of nonsense mRNA in human cells. Nat Struct Mol Biol 16: 49–55 [DOI] [PubMed] [Google Scholar]
- Fan-Minogue H, Bedwell DM 2008. Eukaryotic ribosomal RNA determinants of aminoglycoside resistance and their role in translational fidelity. RNA 14: 148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Spevak CC, Wu C, Sachs MS 2004. A nascent polypeptide domain that can regulate translation elongation. Proc Natl Acad Sci 101: 4059–4064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei J, Kosuri P, MacDougall DD, Gonzalez RL Jr 2008. Coupling of ribosomal L1 stalk and tRNA dynamics during translation elongation. Mol Cell 30: 348–359 [DOI] [PubMed] [Google Scholar]
- Fei JY, Bronson JE, Hofman JM, Srinivas RL, Wiggins CH, Gonzalez RL 2009. Allosteric collaboration between elongation factor G and the ribosomal L1 stalk directs tRNA movements during translation. Proc Natl Acad Sci 106: 15702–15707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischmeyer PA, van Hoof A, O’Donnell K, Guerrerio AL, Parker R, Dietz HC 2002. An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science 295: 2258–2261 [DOI] [PubMed] [Google Scholar]
- Fujii K, Kitabatake M, Sakata T, Miyata A, Ohno M 2009. A role for ubiquitin in the clearance of nonfunctional rRNAs. Genes Dev 23: 963–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatfield D, Izaurralde E 2004. Nonsense-mediated messenger RNA decay is initiated by endonucleolytic cleavage in Drosophila. Nature 429: 575–578 [DOI] [PubMed] [Google Scholar]
- Gerbasi VR, Weaver CM, Hill S, Friedman DB, Link AJ 2004. Yeast Asc1p and mammalian RACK1 are functionally orthologous core 40S ribosomal proteins that repress gene expression. Mol Cell Biol 24: 8276–8287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G, Chu AM, Ni L, Connelly C, Riles L, Veronneau S, Dow S, Lucau-Danila A, Anderson K, Andre B, et al. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418: 387–391 [DOI] [PubMed] [Google Scholar]
- Grosjean H, de Crécy-Lagard V, Marck C 2010. Deciphering synonymous codons in the three domains of life: Co-evolution with specific tRNA modification enzymes. FEBS Lett 584: 252–264 [DOI] [PubMed] [Google Scholar]
- Gueldener U, Heinisch J, Koehler GJ, Voss D, Hegemann JH 2002. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res 30: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG 2005. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol 59: 407–450 [DOI] [PubMed] [Google Scholar]
- Huntzinger E, Kashima I, Fauser M, Saulière J, Izaurralde E 2008. SMG6 is the catalytic endonuclease that cleaves mRNAs containing nonsense codons in metazoan. RNA 14: 2609–2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia NT, Lareau LF, Weissman JS 2011. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell 147: 789–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isken O, Maquat LE 2007. Quality control of eukaryotic mRNA: Safeguarding cells from abnormal mRNA function. Genes Dev 21: 1833–1856 [DOI] [PubMed] [Google Scholar]
- Ito-Harashima S, Kuroha K, Tatematsu T, Inada T 2007. Translation of the poly(A) tail plays crucial roles in nonstop mRNA surveillance via translation repression and protein destabilization by proteasome in yeast. Genes Dev 21: 519–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroha K, Akamatsu M, Dimitrova L, Ito T, Kato Y, Shirahige K, Inada T 2010. Receptor for activated C kinase 1 stimulates nascent polypeptide-dependent translation arrest. EMBO Rep 11: 956–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRiviere FJ, Cole SE, Ferullo DJ, Moore MJ 2006. A late-acting quality control process for mature eukaryotic rRNAs. Mol Cell 24: 619–626 [DOI] [PubMed] [Google Scholar]
- Letzring DP, Dean KM, Grayhack EJ 2010. Control of translation efficiency in yeast by codon–anticodon interactions. RNA 16: 2516–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkowski MG, Quartley E, Friedman AE, Babulski J, Kon Y, Wolfley J, Said M, Luft JR, Phizicky EM, DeTitta GT, et al. 2007. Blocking S-adenosylmethionine synthesis in yeast allows selenomethionine incorporation and multiwavelength anomalous dispersion phasing. Proc Natl Acad Sci 104: 6678–6683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh KB, Bhattacharya A, Willis IM, Warner JR 2011. Eukaryotic cells producing ribosomes deficient in Rpl1 are hypersensitive to defects in the ubiquitin-proteasome system. PLoS One 6: e23579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SD, Sauer RT 2007. The tmRNA system for translational surveillance and ribosome rescue. Annu Rev Biochem 76: 101–124 [DOI] [PubMed] [Google Scholar]
- Murphy FV, Ramakrishnan V 2004. Structure of a purine-purine wobble base pair in the decoding center of the ribosome. Nat Struct Mol Biol 11: 1251–1252 [DOI] [PubMed] [Google Scholar]
- Nikulin A, Eliseikina I, Tishchenko S, Nevskaya N, Davydova N, Platonova O, Piendl W, Selmer M, Liljas A, Drygin D, et al. 2003. Structure of the L1 protuberance in the ribosome. Nat Struct Biol 10: 104–108 [DOI] [PubMed] [Google Scholar]
- Nilsson J, Sengupta J, Frank J, Nissen P 2004. Regulation of eukaryotic translation by the RACK1 protein: A platform for signalling molecules on the ribosome. EMBO Rep 5: 1137–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S 1984. Escherichia coli ribosomes translate in vivo with variable rate. EMBO J 3: 2895–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean A, Bonneaud N, Lacroute F 1995. The duplicated Saccharomyces cerevisiae gene SSM1 encodes a eucaryotic homolog of the eubacterial and archaebacterial L1 ribosomal proteins. Mol Cell Biol 15: 5071–5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartley E, Alexandrov A, Mikucki M, Buckner FS, Hol WG, DeTitta GT, Phizicky EM, Grayhack EJ 2009. Heterologous expression of L. major proteins in S. cerevisiae: A test of solubility, purity, and gene recoding. J Struct Funct Genomics 10: 233–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche ED, Sauer RT 1999. SsrA-mediated peptide tagging caused by rare codons and tRNA scarcity. EMBO J 18: 4579–4589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Poetsch T, Ju J, Eyler DE, Dang Y, Bhat S, Merrick WC, Green R, Shen B, Liu JO 2010. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat Chem Biol 6: 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F 1991. Getting started with yeast. Methods Enzymol 194: 3–21 [DOI] [PubMed] [Google Scholar]
- Shoemaker CJ, Green R 2012. Translation drives mRNA quality control. Nat Struct Mol Biol 19: 594–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Gonzalez M, Kabbaj MH, Gunjan A 2012. Novel E3 ubiquitin ligases that regulate histone protein levels in the budding yeast Saccharomyces cerevisiae. PLoS One 7: e36295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen MA, Kurland CG, Pedersen S 1989. Codon usage determines translation rate in Escherichia coli. J Mol Biol 207: 365–377 [DOI] [PubMed] [Google Scholar]
- Stadler M, Fire A 2011. Wobble base-pairing slows in vivo translation elongation in metazoans. RNA 17: 2063–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner DR, Cariello DA, Woolstenhulme CJ, Broadbent MA, Buskirk AR 2009. Genetic identification of nascent peptides that induce ribosome stalling. J Biol Chem 284: 34809–34818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach JM, Yimit A, Lee AY, Riffle M, Costanzo M, Jaschob D, Hendry JA, Ou J, Moffat J, Boone C, et al. 2012. Dissecting DNA damage response pathways by analysing protein localization and abundance changes during DNA replication stress. Nat Cell Biol 14: 966–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Kuroha K, Kudo K, Makino S, Inoue E, Kashima I, Inada T 2012. Dom34:hbs1 plays a general role in quality-control systems by dissociation of a stalled ribosome at the 3′ end of aberrant mRNA. Mol Cell 46: 518–529 [DOI] [PubMed] [Google Scholar]
- van Hoof A, Frischmeyer PA, Dietz HC, Parker R 2002. Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science 295: 2262–2264 [DOI] [PubMed] [Google Scholar]
- Varenne S, Buc J, Lloubes R, Lazdunski C 1984. Translation is a non-uniform process. Effect of tRNA availability on the rate of elongation of nascent polypeptide chains. J Mol Biol 180: 549–576 [DOI] [PubMed] [Google Scholar]
- Wilson DN, Nierhaus KH 2006. The E-site story: The importance of maintaining two tRNAs on the ribosome during protein synthesis. Cell Mol Life Sci 63: 2725–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Meaux S, van Hoof A 2007. A genomic screen in yeast reveals novel aspects of nonstop mRNA metabolism. Genetics 177: 773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Wei J, Lin PJ, Tu L, Deutsch C, Johnson AE, Sachs MS 2012. Arginine changes the conformation of the arginine attenuator peptide relative to the ribosome tunnel. J Mol Biol 416: 518–533 [DOI] [PMC free article] [PubMed] [Google Scholar]